A Facile Single-Phase-Fluid-Driven Bubble Microfluidic Generator for Potential Detection of Viruses Suspended in Air
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Setup of SPFM Device
2.3. Preparation of Microbubbles/Droplets in the SPFM Device
2.4. Detection of the Fluorescent Microparticles in Ambient Air
3. Results and Discussion
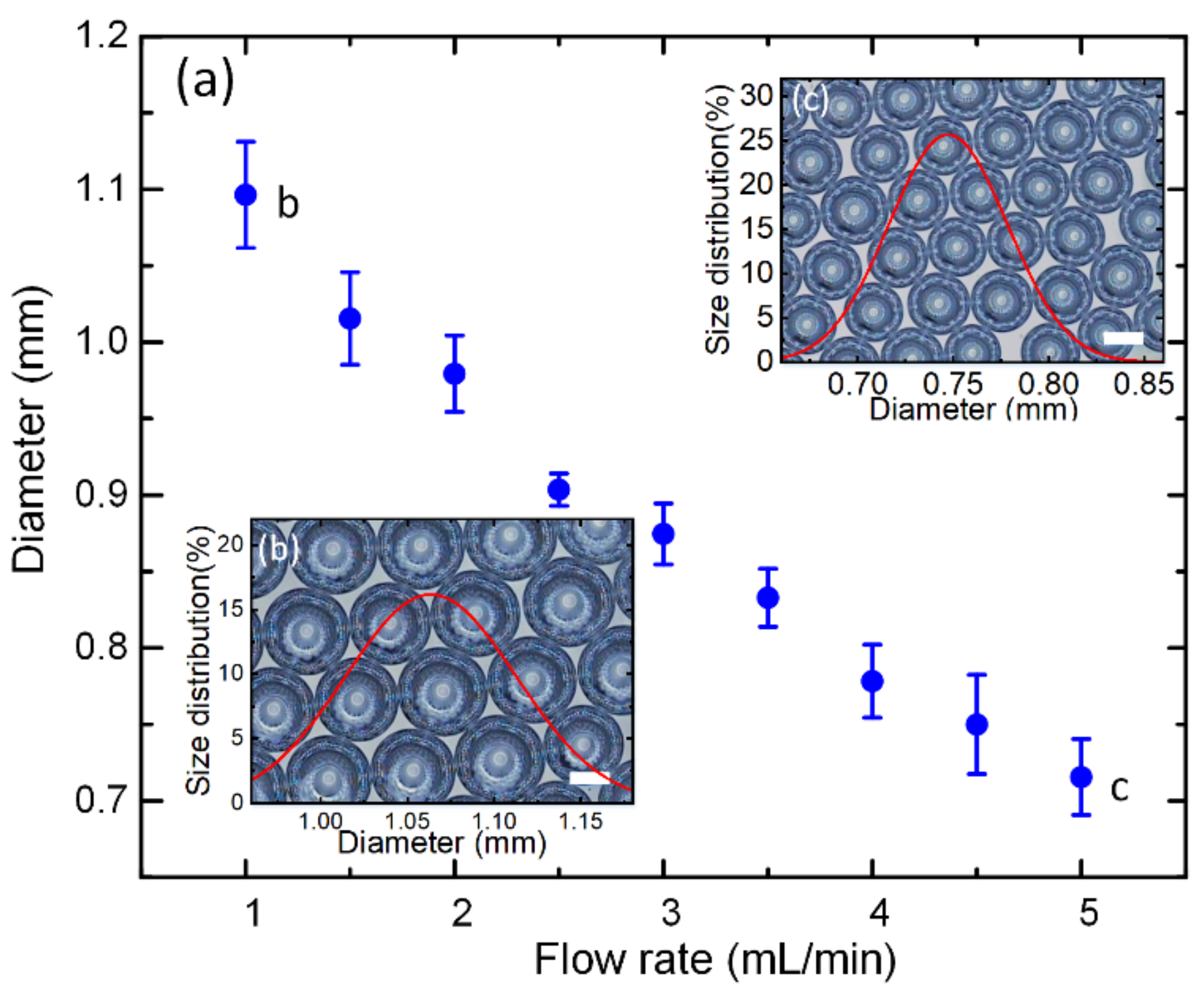
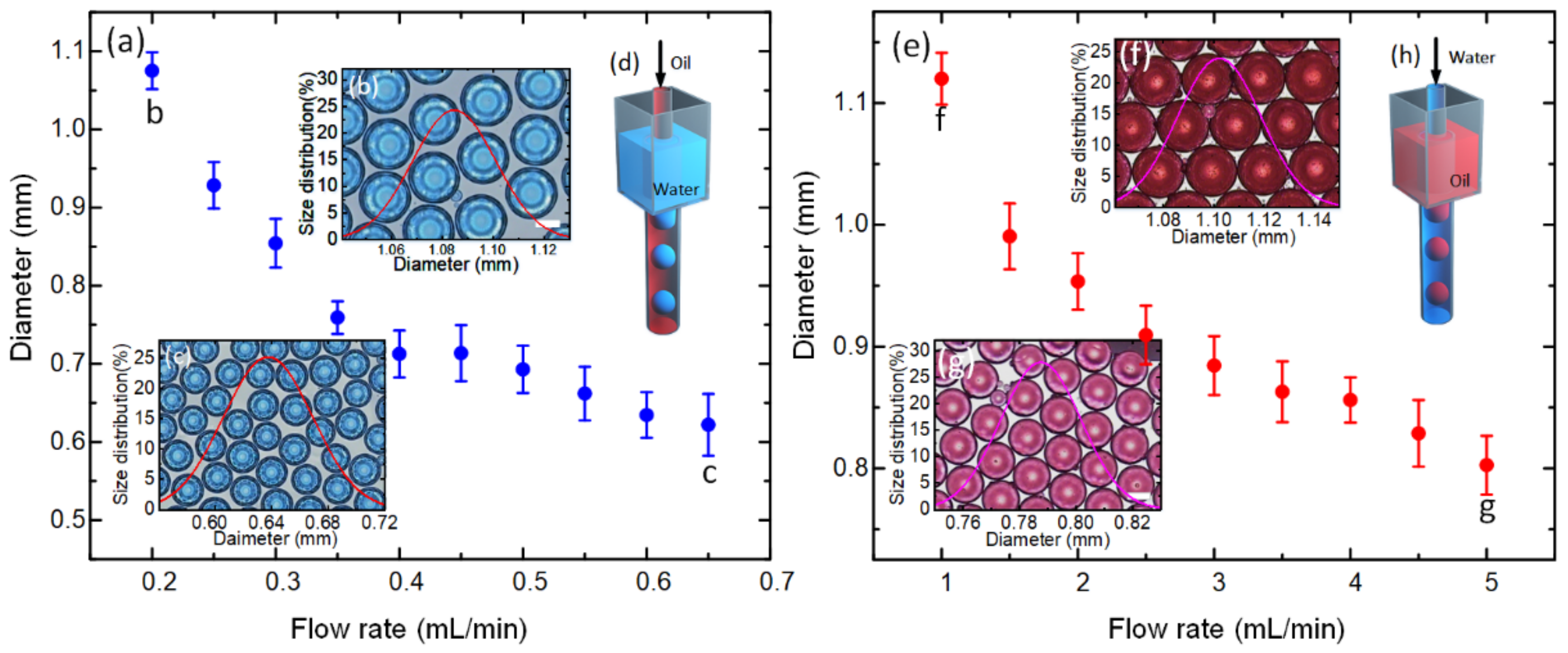
3.1. Microbubble Formation and Size Control
3.2. Mechanism of Microbubble Formation
3.3. Droplet Generation in the SPFM Device
3.4. Potential Application in Virus Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hernandez, R.M.; Orive, G.; Murua, A.; Luis Pedraz, J. Microcapsules and microcarriers for in situ cell delivery. Adv. Drug Deliv. Rev. 2010, 62, 711–730. [Google Scholar] [CrossRef] [PubMed]
- Bin, X.; Kangning, R.; Yiwei, S.; Yin, C.; Bo, S.; Hongkai, W. Recent developments in microfluidics for cell studies. Adv. Mater. 2014, 26, 5525–5532. [Google Scholar]
- Zhao, C.X. Multiphase flow microfluidics for the production of single or multiple emulsions for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1420–1446. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.K.; Yuet, K.; Hwang, D.K.; Bong, K.W.; Doyle, P.S.; Hatton, T.A. Synthesis of Nonspherical Superparamagnetic Particles: In Situ Coprecipitation of Magnetic Nanoparticles in Microgels Prepared by Stop-Flow Lithography. J. Am. Chem. Soc. 2012, 134, 7337–7343. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, H.; Xie, Z.; Shum, H.C.; Wang, B.; Gu, Z. Bioinspired Multifunctional Janus Particles for Droplet Manipulation. J. Am. Chem. Soc. 2013, 135, 54–57. [Google Scholar] [CrossRef]
- Wang, X.Y.; Hou, Y.; Yao, L.; Gao, M.Y.; Ge, M.F. Generation, Characterization, and Application of Hierarchically Structured Self-Assembly Induced by the Combined Effect of Self-Emulsification and Phase Separation. J. Am. Chem. Soc. 2016, 138, 2090–2093. [Google Scholar] [CrossRef]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef]
- Hatch, A.C.; Fisher, J.S.; Tovar, A.R.; Hsieh, A.T.; Lin, R.; Pentoney, S.L.; Yang, D.L.; Lee, A.P. 1-Million droplet array with wide-field fluorescence imaging for digital PCR. Lab Chip 2011, 11, 3838–3845. [Google Scholar] [CrossRef]
- Yin, H.; Wu, Z.; Shi, N.; Qi, Y.; Jian, X.; Zhou, L.; Tong, Y.; Cheng, Z.; Zhao, J.; Mao, H. Ultrafast multiplexed detection of SARS-CoV-2 RNA using a rapid droplet digital PCR system. Biosens. Bioelectron. 2021, 188, 11382. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.G.; Han, D.H.; Lee, J.-S.; Lee, S.J.; Park, J.-K. Pushbutton-activated microfluidic dropenser for droplet digital PCR. Biosens. Bioelectron. 2021, 181, 113159. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.; Nunes, J.; Hao, S.; Liu, B.; Chen, H. Droplet Micro-Reactor for Internal Gelation to Fabricate ZrO2 Ceramic Microspheres. J. Am. Ceram. Soc. 2017, 100, 41–48. [Google Scholar] [CrossRef]
- Liang, S.S.; Shen, L.J.; Zhou, C.C.; Chen, H.S.; Li, J. Scalable preparation of hollow ZrO2 microspheres through a liquid-liquid phase reunion assisted sol-gel method. Ceram. Int. 2020, 46, 14188–14194. [Google Scholar] [CrossRef]
- Zhou, C.; Man, J.; Yu, H.; Xia, H.; Man, L.; Qi, B.; Li, J. Fabrication of crescent-shaped ceramic microparticles based on single emulsion microfluidics. Ceram. Int. 2021, 47, 10866–10872. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Man, J.; Li, J.; Cui, X.; Zhang, C.; Shi, W.; Li, D.; Zhang, S.; Li, J. Histone Deacetylase Inhibitor-loaded Calcium Alginate Microspheres for Acute Kidney Injury Treatment. ACS Appl. Bio Mater. 2020, 3, 6457–6465. [Google Scholar] [CrossRef]
- Xia, H.; Li, A.; Man, J.; Li, J.; Li, J. Fabrication of Multi-Layered Microspheres Based on Phase Separation for Drug Delivery. Micromachines 2021, 12, 723. [Google Scholar] [CrossRef]
- Kwan, J.J.; Myers, R.; Coviello, C.M.; Graham, S.M.; Shah, A.R.; Stride, E.; Carlisle, R.C.; Coussios, C.C. Ultrasound-Propelled Nanocups for Drug Delivery. Small 2015, 11, 5305–5314. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, Z.; Wang, C.; Li, X.; Liu, J.; Xu, M.; Xu, S.; Xie, X.; Jiang, Q.; Wang, W.; et al. Highly Uniform Perfluoropropane-Loaded Cerasomal Microbubbles As a Novel Ultrasound Contrast Agent. ACS Appl. Mater. Interfaces 2016, 8, 15024–15032. [Google Scholar] [CrossRef]
- Dixon, A.J.; Dhanaliwala, A.H.; Chen, J.L.; Hossack, J.A. Enhanced intracellular delivery of a model drug using microbubbles produced by a microfluidic device. Ultrasound Med. Biol. 2013, 39, 1267–1276. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Zhang, X.; Zhao, C.; Fu, X.; Zhang, W.; Kong, W.; Zhang, B.; Zhao, Y. Ultrasound-Responsive Microfluidic Microbubbles for Combination Tumor Treatment. Adv. Ther. 2021, 4, 2100050. [Google Scholar] [CrossRef]
- Wang, J.T.; Wang, J.; Han, J.J. Fabrication of advanced particles and particle-based materials assisted by droplet-based microfluidics. Small 2011, 7, 1728–1754. [Google Scholar] [CrossRef]
- Guo, S.; Yao, T.; Ji, X.; Zeng, C.; Wang, C.; Zhang, L. Versatile Preparation of Nonspherical Multiple Hydrogel Core PAM/PEG Emulsions and Hierarchical Hydrogel Microarchitectures. Angew. Chem. Int. Ed. 2014, 53, 7504–7509. [Google Scholar] [CrossRef]
- Wang, J.; Shang, L.; Cheng, Y.; Ding, H.; Zhao, Y.; Gu, Z. Microfluidic Generation of Porous Particles Encapsulating Spongy Graphene for Oil Absorption. Small 2015, 11, 3890–3895. [Google Scholar] [CrossRef]
- Abate, A.R.; Weitz, D.A. High-Order Multiple Emulsions Formed in Poly(dimethylsiloxane) Microfluidics. Small 2009, 5, 2030–2032. [Google Scholar] [CrossRef]
- Sattari, A.; Hanafizadeh, P.; Hoorfar, M. Multiphase flow in microfluidics: From droplets and bubbles to the encapsulated structures. Adv. Colloid Interface Sci. 2020, 282, 102208. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Li, J.Y.; Man, J.; Man, L.M.; Zhang, S.G.; Li, J.F. Recent progress in preparation of functional microparticles based on microfluidic technique. Mater. Today Commun. 2021, 29, 102740. [Google Scholar] [CrossRef]
- Wan, J.; Bick, A.; Sullivan, M.; Stone, H.A. Controllable Microfluidic Production of Microbubbles in Water-in-Oil Emulsions and the Formation of Porous Microparticles. Adv. Mater. 2008, 20, 3314–3318. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Wan, J.; Weitz, D.A.; Stone, H.A. Gas-core triple emulsions for ultrasound triggered release. Soft Matter 2013, 9, 38–42. [Google Scholar] [CrossRef]
- Chen, H.S.; Man, J.; Li, Z.N.; Li, J. Microfluidic Generation of High-Viscosity Droplets by Surface-Controlled Breakup of Segment Flow. ACS Appl. Mater. Interfaces 2017, 9, 21059–21064. [Google Scholar] [CrossRef]
- Man, J.; Li, Z.; Li, J.; Chen, H. Phase inversion of slug flow on step surface to form high viscosity droplets in microchannel. Appl. Phys. Lett. 2017, 110, 81601. [Google Scholar]
- Man, J.; Chien, S.; Liang, S.; Li, J.; Chen, H. Size-Dependent Phase Separation in Emulsion Droplets. ChemPhysChem 2018, 19, 1995–1998. [Google Scholar] [CrossRef]
- Moon, B.-U.; Hwang, D.K.; Tsai, S.S.H. Shrinking, growing, and bursting: Microfluidic equilibrium control of water-in-water droplets. Lab Chip 2016, 16, 2601–2608. [Google Scholar] [CrossRef]
- Utada, A.S.; Fernandez-Nieves, A.; Stone, H.A.; Weitz, D.A. Dripping to Jetting Transitions in Coflowing Liquid Streams. Phys. Rev. Lett. 2007, 99, 094502. [Google Scholar] [CrossRef]
- Marusic-Paloka, E.; Pazanin, I. Effects of boundary roughness and inertia on the fluid flow through a corrugated pipe and the formula for the Darcy-Weisbach friction coefficient. Int. J. Eng. Sci. 2020, 152, 103293. [Google Scholar] [CrossRef]
- Massalha, T.; Digilov, R.M. The shape function of a free-falling laminar jet: Making use of Bernoulli’s equation. Am. J. Phys. 2013, 81, 733–737. [Google Scholar] [CrossRef]
- Yao, H.P.; Song, Y.T.; Chen, Y.; Wu, N.P.; Xu, J.L.; Sun, C.J.; Zhang, J.X.; Weng, T.H.; Zhang, Z.Y.; Wu, Z.G.; et al. Molecular Architecture of the SARS-CoV-2 Virus. Cell 2020, 183, 730–738. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, J.; Man, L.; Zhou, C.; Li, J.; Liang, S.; Zhang, S.; Li, J. A Facile Single-Phase-Fluid-Driven Bubble Microfluidic Generator for Potential Detection of Viruses Suspended in Air. Biosensors 2022, 12, 294. https://doi.org/10.3390/bios12050294
Man J, Man L, Zhou C, Li J, Liang S, Zhang S, Li J. A Facile Single-Phase-Fluid-Driven Bubble Microfluidic Generator for Potential Detection of Viruses Suspended in Air. Biosensors. 2022; 12(5):294. https://doi.org/10.3390/bios12050294
Chicago/Turabian StyleMan, Jia, Luming Man, Chenchen Zhou, Jianyong Li, Shuaishuai Liang, Song Zhang, and Jianfeng Li. 2022. "A Facile Single-Phase-Fluid-Driven Bubble Microfluidic Generator for Potential Detection of Viruses Suspended in Air" Biosensors 12, no. 5: 294. https://doi.org/10.3390/bios12050294
APA StyleMan, J., Man, L., Zhou, C., Li, J., Liang, S., Zhang, S., & Li, J. (2022). A Facile Single-Phase-Fluid-Driven Bubble Microfluidic Generator for Potential Detection of Viruses Suspended in Air. Biosensors, 12(5), 294. https://doi.org/10.3390/bios12050294




