Hydrophobic Wafer-Scale High-Reproducibility SERS Sensor Based on Silicon Nanorods Arrays Decorated with Au Nanoparticles for Pesticide Residue Detection
Abstract
:1. Introduction
2. Materials and Methods
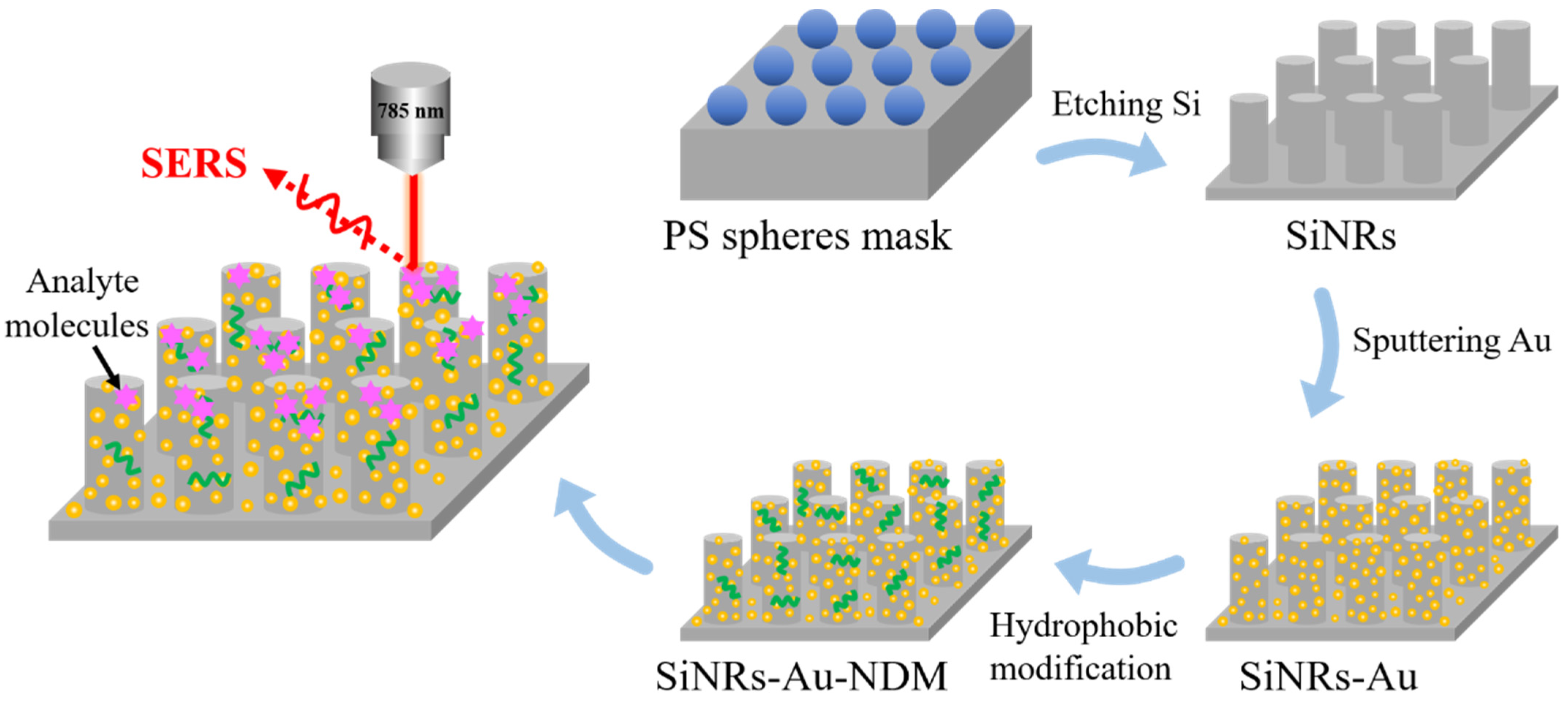
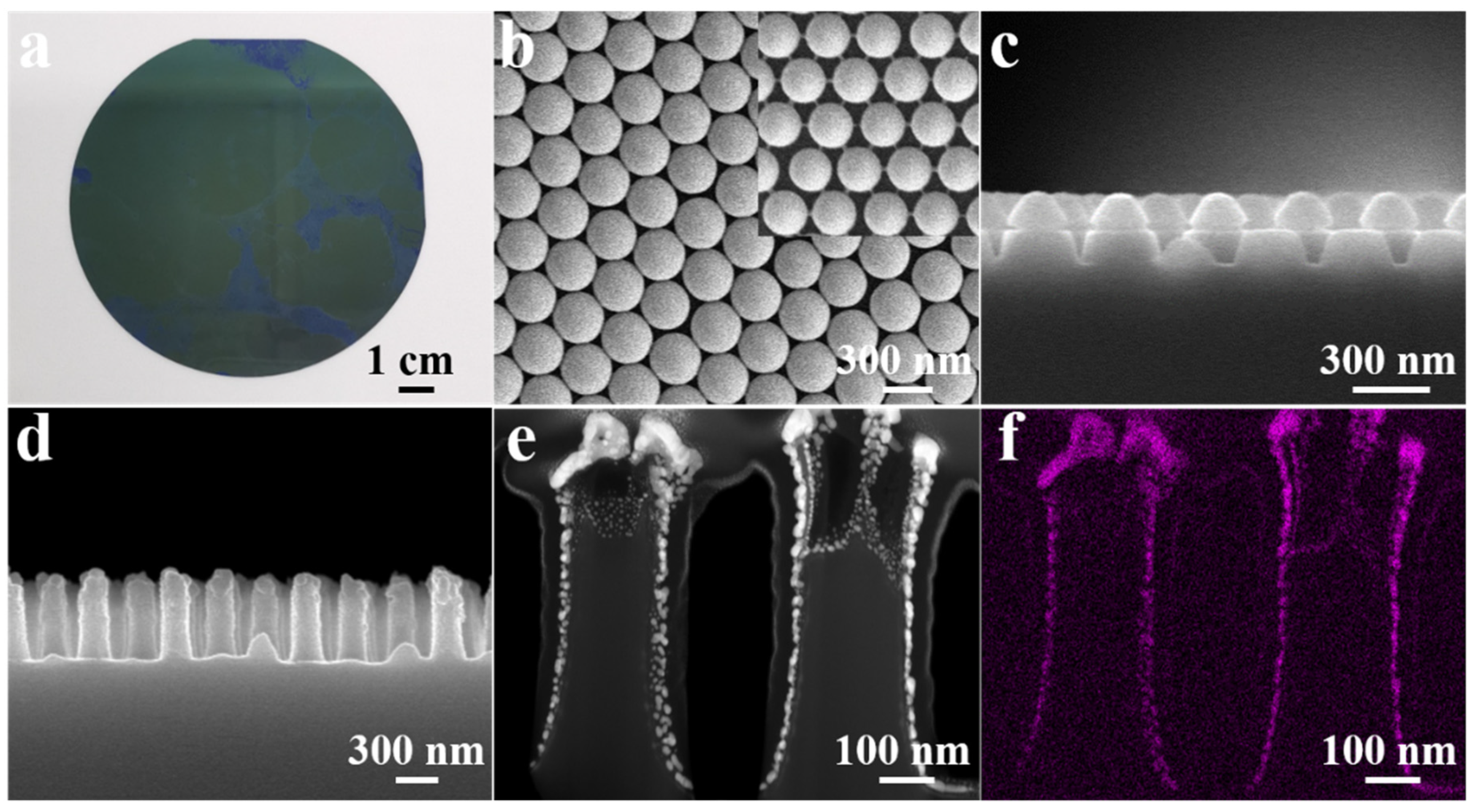
2.1. Preparation and Characterization of the SERS Substrate
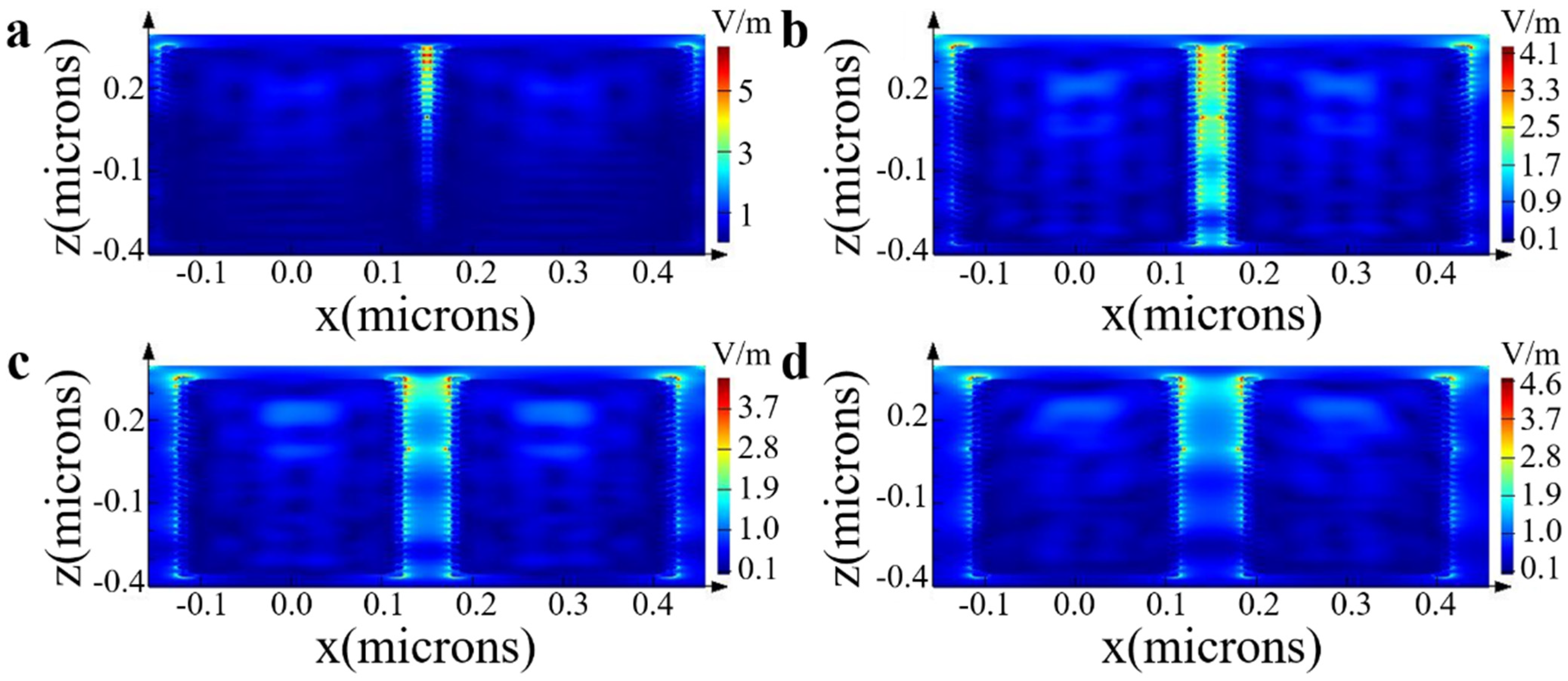
2.2. FDTD Simulation
2.3. Raman Analysis of the SERS Substrate
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, Y.; Lin, C.; Li, Y.; Gao, Y.; Wang, J.; He, J.; Huang, Z.; Liu, J.; Luo, X.; Yang, Y. Identifying infectiousness of SARS-CoV-2 by ultra-sensitive SnS2 SERS biosensors with capillary effect. Matter 2022, 5, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, W.; Li, Q.; Xing, W.; Luo, H.; Ji, H.; Fang, X.; Luo, Z.; Zhang, L. CRISPR/Cas12a-Derived electrochemical aptasensor for ultrasensitive detection of COVID-19 nucleocapsid protein. Biosens. Bioelectron. 2022, 200, 113922. [Google Scholar] [CrossRef] [PubMed]
- Poore, G.D.; Kopylova, E.; Zhu, Q.; Carpenter, C.; Fraraccio, S.; Wandro, S.; Kosciolek, T.; Janssen, S.; Metcalf, J.; Song, S.J.; et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 2020, 579, 567–574. [Google Scholar] [CrossRef]
- Leong, S.X.; Leong, Y.X.; Tan, E.X.; Sim, H.Y.F.; Koh, C.S.L.; Lee, Y.H.; Chong, C.; Ng, L.S.; Chen, J.R.T.; Pang, D.W.C.; et al. Noninvasive and Point-of-Care Surface-Enhanced Raman Scattering (SERS)-Based Breathalyzer for Mass Screening of Coronavirus Disease 2019 (COVID-19) under 5 min. ACS Nano 2022, 16, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Trojanowska, A.; Marciniak, L.; Binczyk, M.; Runka, T.; Tylkowski, B.; Jastrzab, R. Preparation and characterization of long-term stable SERS active materials as potential supports for medical diagnostic. Appl. Surf. Sci. 2019, 472, 93–98. [Google Scholar] [CrossRef]
- Ben-Jaber, S.; Peveler, W.J.; Quesada-Cabrera, R.; Cortes, E. Sotelo-Vazquez, C.; Abdul-Karim, N.; Maier, S.A.; Parkin, I.P., Photo-induced enhanced Raman spectroscopy for universal ultra-trace detection of explosives, pollutants and biomolecules. Nat. Commun. 2016, 7, 12189. [Google Scholar] [CrossRef] [Green Version]
- Krivitsky, V.; Filanovsky, B.; Naddaka, V.; Patolsky, F. Direct and Selective Electrochemical Vapor Trace Detection of Organic Peroxide Explosives via Surface Decoration. Anal. Chem. 2019, 91, 5323–5330. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernandez-Abedul, M.T.; Merkoci, A.; Manz, A.; Urban, G.A.; Guder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, e1806739. [Google Scholar] [CrossRef]
- Yu, Z.; Jung, D.; Park, S.; Hu, Y.; Huang, K.; Rasco, B.A.; Wang, S.; Ronholm, J.; Lu, X.; Chen, J. Smart traceability for food safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 905–916. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.J.; Wei, T.; Ma, X.; Zheng, X.S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Zhan, C.; Chen, X.-J.; Yi, J.; Li, J.-F.; Wu, D.-Y.; Tian, Z.-Q. From plasmon-enhanced molecular spectroscopy to plasmon-mediated chemical reactions. Nat. Rev. Chem. 2018, 2, 216–230. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Lai, K.; Fan, Y.; Rasco, B.A. Trace analysis of organic compounds in foods with surface-enhanced Raman spectroscopy: Methodology, progress, and challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 622–642. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Yang, Z.W.; Meng, L.Y.; Lin, J.S.; Yang, W.M.; Radjenovic, P.; Shen, S.X.; Xu, Q.C.; Yang, Z.L.; Tian, Z.Q.; Li, J.F. 3D Hotspots Platform for Plasmon Enhanced Raman and Second Harmonic Generation Spectroscopies and Quantitative Analysis. Adv. Opt. Mater. 2019, 7, 1901010. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Yi, J.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Panneerselvam, R.; Tian, Z.-Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Wang, X.; Guo, L. SERS Activity of Semiconductors: Crystalline and Amorphous Nanomaterials. Angew. Chem. Int. Ed. Engl. 2020, 59, 4231–4239. [Google Scholar] [CrossRef]
- Ran, P.; Jiang, L.; Li, X.; Li, B.; Zuo, P.; Lu, Y. Femtosecond Photon-Mediated Plasma Enhances Photosynthesis of Plasmonic Nanostructures and Their SERS Applications. Small 2019, 15, e1804899. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, H.; Tian, Y.; Du, Y.; Ma, Y.; Zeng, S.; Gu, C.; Jiang, T.; Zhou, J. In Situ Recyclable Surface-Enhanced Raman Scattering-Based Detection of Multicomponent Pesticide Residues on Fruits and Vegetables by the Flower-like MoS2@Ag Hybrid Substrate. ACS Appl. Mater. Interfaces 2020, 12, 14386–14399. [Google Scholar] [CrossRef]
- Huang, Z.; Meng, G.; Hu, X.; Pan, Q.; Huo, D.; Zhou, H.; Ke, Y.; Wu, N. Plasmon-tunable Au@Ag core-shell spiky nanoparticles for surface-enhanced Raman scattering. Nano Res. 2018, 12, 449–455. [Google Scholar] [CrossRef]
- Wang, C.; Xu, X.; Qiu, G.; Ye, W.; Li, Y.; Harris, R.A.; Jiang, C. Group-Targeting SERS Screening of Total Benzodiazepines Based on Large-Size (111) Faceted Silver Nanosheets Decorated with Zinc Oxide Nanoparticles. Anal. Chem. 2021, 93, 3403–3410. [Google Scholar] [CrossRef]
- Liu, C.; Yang, M.; Yu, J.; Lei, F.; Wei, Y.; Peng, Q.; Li, C.; Li, Z.; Zhang, C.; Man, B. Fast multiphase analysis: Self-separation of mixed solution by a wettability-controlled CuO@Ag SERS substrate and its applications in pollutant detection. Sens. Actuators B Chem. 2020, 307, 127663. [Google Scholar] [CrossRef]
- Guang, Y.; Peng, Y.; Yan, Z.; Liu, Y.; Zhang, J.; Zeng, X.; Zhang, S.; Zhang, S.; Burn, D.M.; Jaouen, N.; et al. Electron Beam Lithography of Magnetic Skyrmions. Adv. Mater. 2020, 32, e2003003. [Google Scholar] [CrossRef]
- Probst, C.; Meichner, C.; Kreger, K.; Kador, L.; Neuber, C.; Schmidt, H.W. Athermal Azobenzene-Based Nanoimprint Lithography. Adv. Mater. 2016, 28, 2624–2628. [Google Scholar] [CrossRef]
- Politano, G.G.; Cazzanelli, E.; Versace, C.; Vena, C.; De Santo, M.P.; Castriota, M.; Ciuchi, F.; Bartolino, R. Graphene oxide on magnetron sputtered silver thin films for SERS and metamaterial applications. Appl. Surf. Sci. 2018, 427, 927–933. [Google Scholar] [CrossRef]
- Demirel, G.; Gieseking, R.L.M.; Ozdemir, R.; Kahmann, S.; Loi, M.A.; Schatz, G.C.; Facchetti, A.; Usta, H. Molecular engineering of organic semiconductors enables noble metal-comparable SERS enhancement and sensitivity. Nat. Commun. 2019, 10, 5502. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Ren, B.; Li, J.F.; Yang, Z.L. Expanding generality of surface-enhanced Raman spectroscopy with borrowing SERS activity strategy. Chem. Commun. (Camb) 2007, 34, 3514–3534. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Yang, M.; Li, Z.; Liu, C.; Wei, Y.; Zhang, C.; Man, B.; Lei, F. Hierarchical Particle-In-Quasicavity Architecture for Ultratrace In Situ Raman Sensing and Its Application in Real-Time Monitoring of Toxic Pollutants. Anal. Chem. 2020, 92, 14754–14761. [Google Scholar] [CrossRef]
- Qi, Q.; Liu, C.; Liu, L.; Meng, Q.; Wei, S.; Ming, A.; Zhang, J.; Wang, Y.; Wu, L.; Zhu, X.; et al. Fabrication, Characterization, and Application of Large-Scale Uniformly Hybrid Nanoparticle-Enhanced Raman Spectroscopy Substrates. Micromachines 2019, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Cao, M.; Bai, H.; Zhao, T.; Ning, Y.; Wang, X.; Liu, K.; Jiang, L. Unidirectional Liquid Manipulation Via an Integrated Mesh with Orthogonal Anisotropic Slippery Tracks. Adv. Funct. Mater. 2019, 29, 1904446. [Google Scholar] [CrossRef]
- Niu, Z.Z.; Gao, F.Y.; Zhang, X.L.; Yang, P.P.; Liu, R.; Chi, L.P.; Wu, Z.Z.; Qin, S.; Yu, X.; Gao, M.R. Hierarchical Copper with Inherent Hydrophobicity Mitigates Electrode Flooding for High-Rate CO2 Electroreduction to Multicarbon Products. J. Am. Chem. Soc. 2021, 143, 8011–8021. [Google Scholar] [CrossRef]
- Li, F.; Wu, W.; Wang, S. Pore network simulations of liquid water and oxygen transport in gas diffusion layers with spatially variable wettability. J. Power Sources 2021, 506, 230207. [Google Scholar] [CrossRef]
- Huang, C.J.; Fang, W.F.; Ke, M.S.; Chou, H.Y.; Yang, J.T. A biocompatible open-surface droplet manipulation platform for detection of multi-nucleotide polymorphism. Lab Chip 2014, 14, 2057–2062. [Google Scholar] [CrossRef]
- Xu, T.; Xu, L.P.; Zhang, X.; Wang, S. Bioinspired superwettable micropatterns for biosensing. Chem. Soc. Rev. 2019, 48, 3153–3165. [Google Scholar] [CrossRef]
- Zhou, B.; Mao, M.; Cao, X.; Ge, M.; Tang, X.; Li, S.; Lin, D.; Yang, L.; Liu, J. Amphiphilic Functionalized Acupuncture Needle as SERS Sensor for In Situ Multiphase Detection. Anal. Chem. 2018, 90, 3826–3832. [Google Scholar] [CrossRef]
- Li, R.; Mao, H.; Shi, M.; Zhao, Q.; Chen, D.; Xiong, J. Parahydrophobic 3D nanohybrid substrates with two pathways of molecular enrichment and multilevel plasmon hybridization. Sens. Actuators B Chem. 2020, 320, 128357. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Hou, J.; Li, Y.; Li, M.; Song, Y. Bioinspired Micropatterned Superhydrophilic Au-Areoles for Surface-Enhanced Raman Scattering (SERS) Trace Detection. Adv. Funct. Mater. 2018, 28, 1800448. [Google Scholar] [CrossRef]
- Chen, F.; Qi, Q.; Zhao, Y.; Zhang, S.; Zhao, Y.; Ming, A.; Mao, C. Large-scale Uniformly Hybrid Micro-nano Structure Wetting Solid Substrate for Surface-enhanced Raman Spectroscopy. In Proceedings of the 2021 IEEE 16th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Xiamen, China, 25–29 April 2021; pp. 1142–1145. [Google Scholar]
- Lin, D.; Wu, Z.; Li, S.; Zhao, W.; Ma, C.; Wang, J.; Jiang, Z.; Zhong, Z.; Zheng, Y.; Yang, X. Large-Area Au-Nanoparticle-Functionalized Si Nanorod Arrays for Spatially Uniform Surface-Enhanced Raman Spectroscopy. ACS Nano 2017, 11, 1478–1487. [Google Scholar] [CrossRef]
- Lotito, V.; Zambelli, T. Approaches to self-assembly of colloidal monolayers: A guide for nanotechnologists. Adv. Colloid Interface Sci. 2017, 246, 217–274. [Google Scholar] [CrossRef]
- Guo, Q.; Xu, M.; Yuan, Y.; Gu, R.; Yao, J. Self-Assembled Large-Scale Monolayer of Au Nanoparticles at the Air/Water Interface Used as a SERS Substrate. Langmuir 2016, 32, 4530–4537. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Z.; Li, Y.; Wang, C.; Sun, J.; Ye, W.; Wang, X.; Shao, B. Durable and flexible Ag-nanowire-embedded PDMS films for the recyclable swabbing detection of malachite green residue in fruits and fingerprints. Sens. Actuators B Chem. 2021, 347, 130602. [Google Scholar] [CrossRef]
- Yang, W.; Tang, J.; Ou, Q.; Yan, X.; Liu, L.; Liu, Y. Recyclable Ag-Deposited TiO2 SERS Substrate for Ultrasensitive Malachite Green Detection. ACS Omega 2021, 6, 27271–27278. [Google Scholar] [CrossRef] [PubMed]

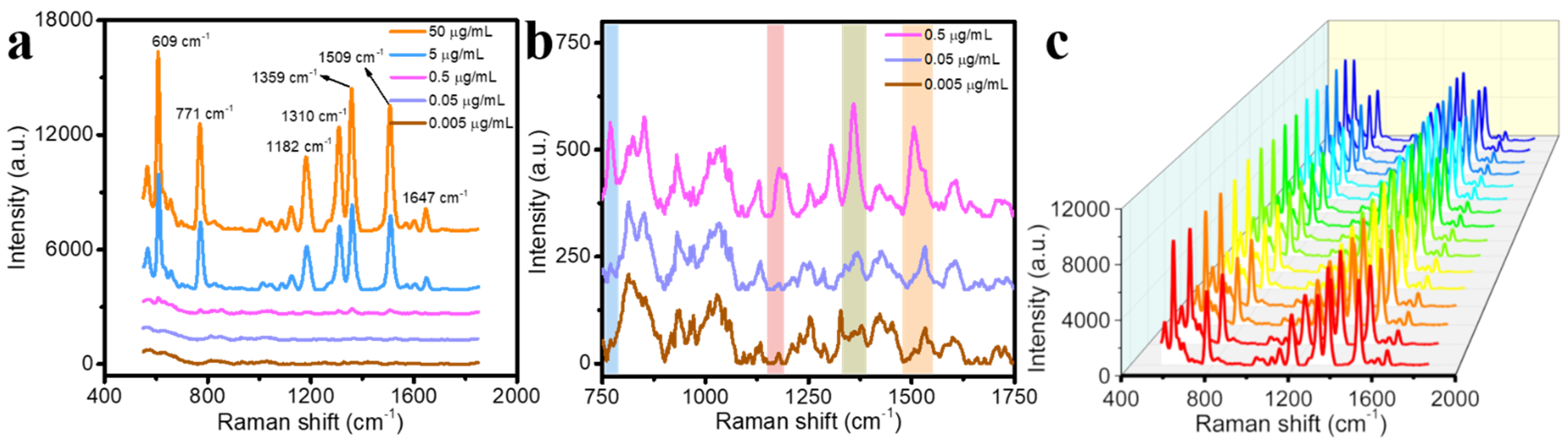
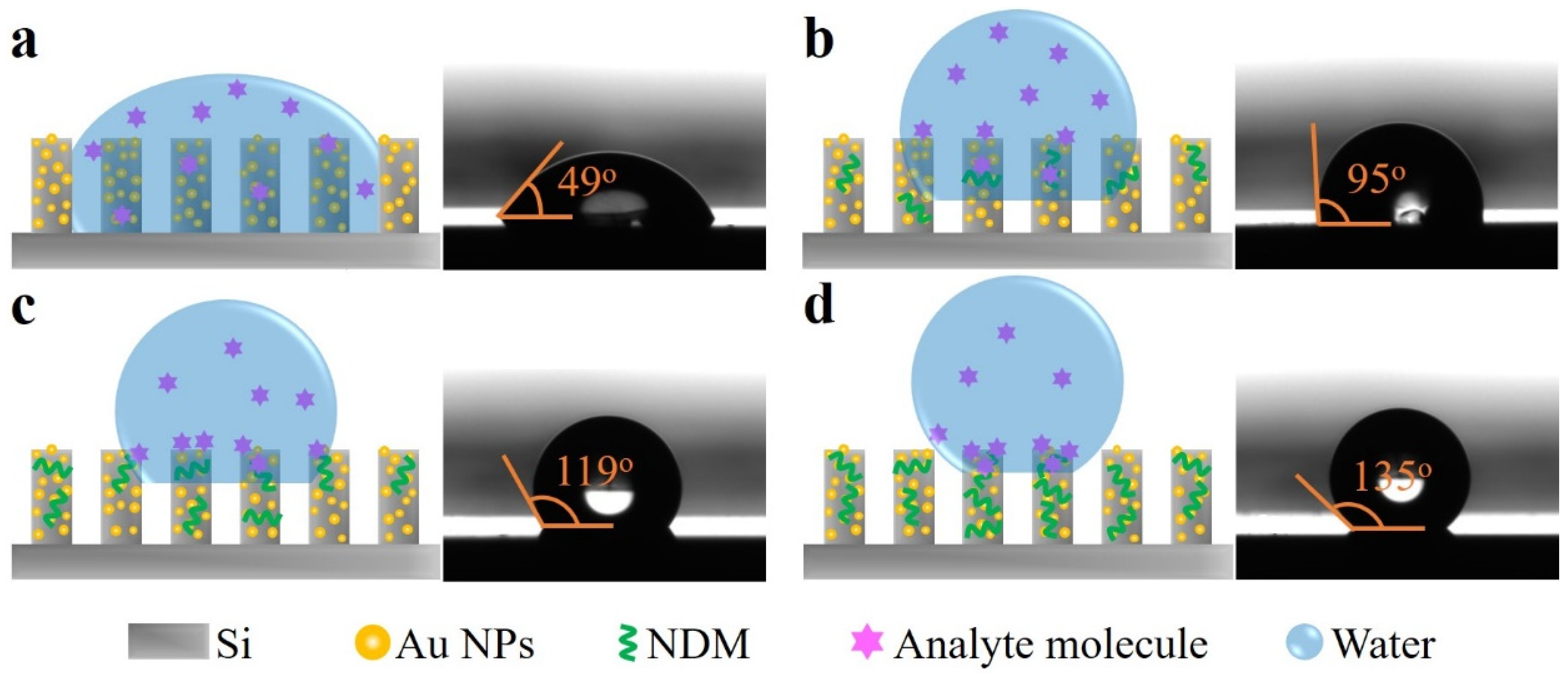
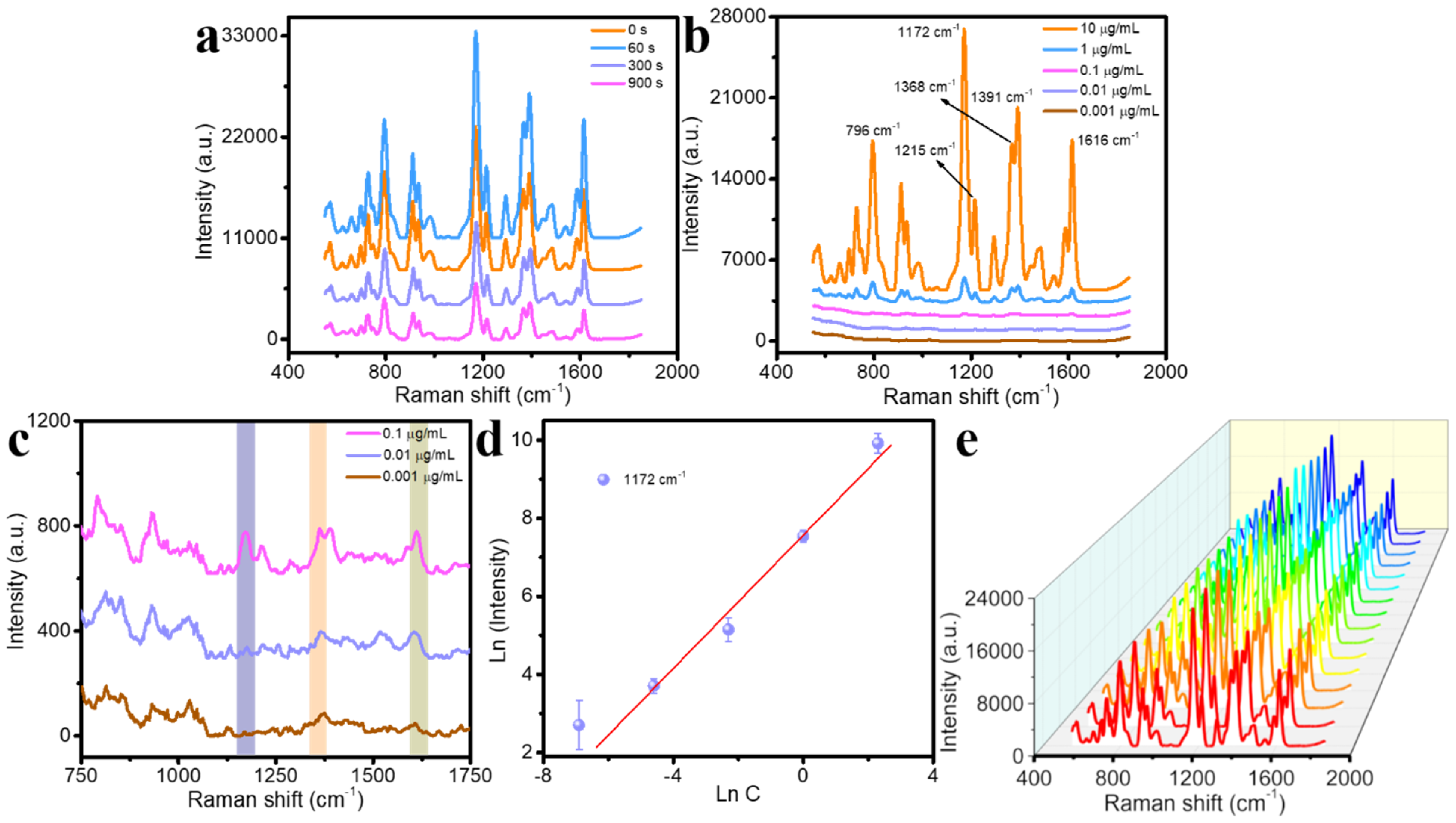
| Raman Shift | 796 cm−1 | 1172 cm−1 | 1215 cm−1 | 1368 cm−1 | 1391 cm−1 | 1616 cm−1 |
| RSD | 6.14% | 4.04% | 6.08% | 4.51% | 4.24% | 5.51% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Zhao, Y.; Zhang, S.; Wei, S.; Ming, A.; Mao, C. Hydrophobic Wafer-Scale High-Reproducibility SERS Sensor Based on Silicon Nanorods Arrays Decorated with Au Nanoparticles for Pesticide Residue Detection. Biosensors 2022, 12, 273. https://doi.org/10.3390/bios12050273
Chen F, Zhao Y, Zhang S, Wei S, Ming A, Mao C. Hydrophobic Wafer-Scale High-Reproducibility SERS Sensor Based on Silicon Nanorods Arrays Decorated with Au Nanoparticles for Pesticide Residue Detection. Biosensors. 2022; 12(5):273. https://doi.org/10.3390/bios12050273
Chicago/Turabian StyleChen, Fanhong, Yupeng Zhao, Shaoxun Zhang, Shuhua Wei, Anjie Ming, and Changhui Mao. 2022. "Hydrophobic Wafer-Scale High-Reproducibility SERS Sensor Based on Silicon Nanorods Arrays Decorated with Au Nanoparticles for Pesticide Residue Detection" Biosensors 12, no. 5: 273. https://doi.org/10.3390/bios12050273
APA StyleChen, F., Zhao, Y., Zhang, S., Wei, S., Ming, A., & Mao, C. (2022). Hydrophobic Wafer-Scale High-Reproducibility SERS Sensor Based on Silicon Nanorods Arrays Decorated with Au Nanoparticles for Pesticide Residue Detection. Biosensors, 12(5), 273. https://doi.org/10.3390/bios12050273




