Rapid Detection of Anti-SARS-CoV-2 Antibodies with a Screen-Printed Electrode Modified with a Spike Glycoprotein Epitope
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Samples and Project Approval
2.2. Chemicals and Reagents
2.3. Solid-Phase Peptide Synthesis
2.4. Modification of the SPE’s Working Electrode
2.5. Electrochemical Assay to Detect Antibodies COVID-19 Antibody IgG
2.6. Analysis of Blood Serum Samples
2.7. Statistical Analysis
3. Results
3.1. Development of Electrochemical Immunosensor
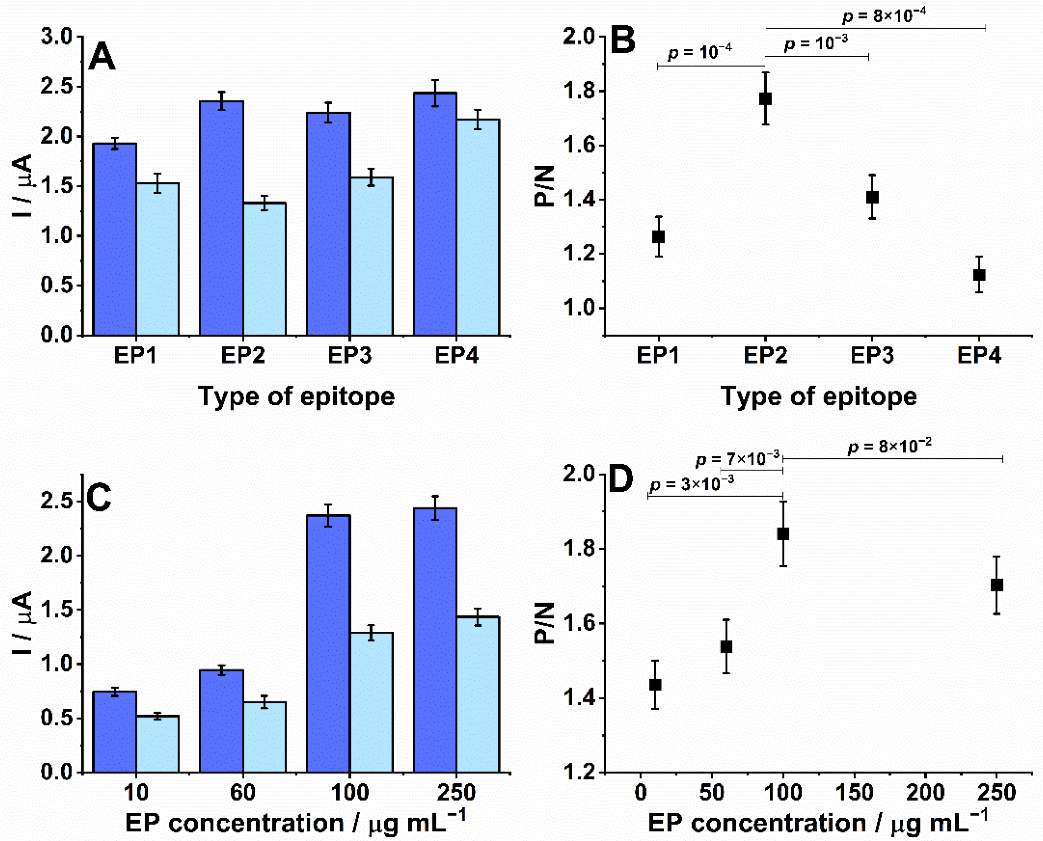
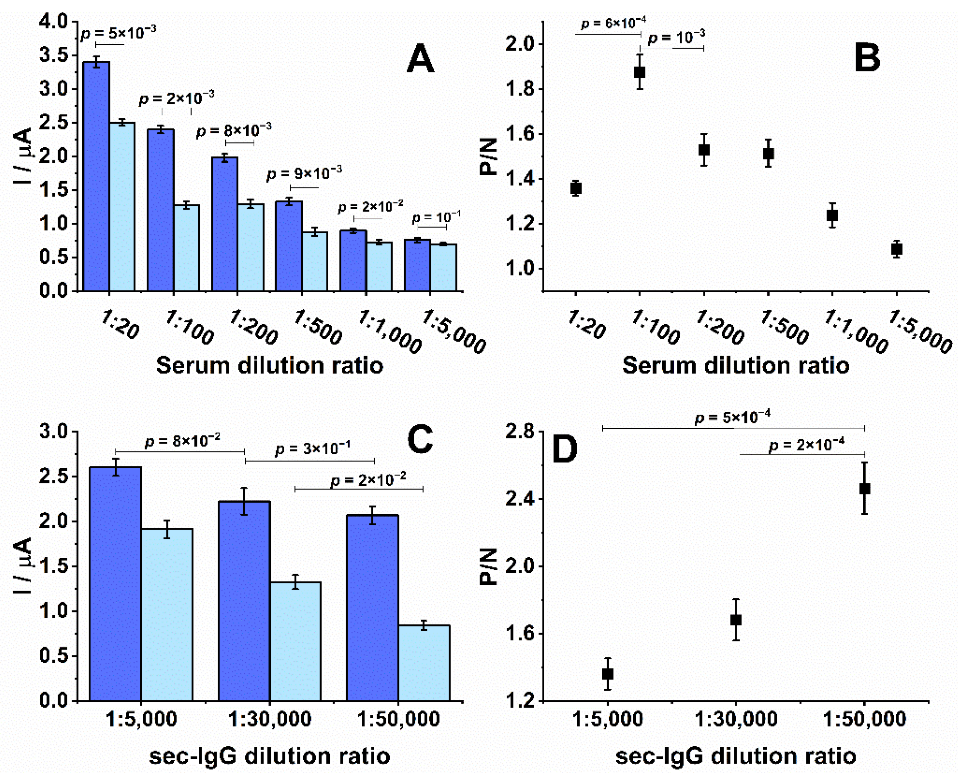
3.2. Optimization of Experimental Parameters, Reproducibility, and Stability
3.3. Biosensor Performance
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ejazi, S.A.; Ghosh, S.; Ali, N. Antibody detection assays for COVID-19 diagnosis: An early overview. Immunol. Cell Biol. 2021, 99, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Kudr, J.; Michalek, P.; Ilieva, L.; Adam, V.; Zitka, O. COVID-19: A challenge for electrochemical biosensors. Trends Anal. Chem. 2021, 136, 116192. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Santoro, M.L.; Marrone, G.; D’Agostini, C.; Amelio, I.; Duggento, A.; Tesauro, M.; Di Daniele, N. Serological determinants of COVID-19. Biol. Direct 2020, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Harvala, H.; Mehew, J.; Robb, M.L.; Ijaz, S.; Dicks, S.; Patel, M.; Watkins, N.; Simmonds, P.; Brooks, T.; Johnson, R.; et al. Blood and transplant convalescent plasma testing group. convalescent plasma treatment for SARS-CoV-2 infection: Analysis of the first 436 donors in England, 22 April to 12 May 2020. Euro Surveill. 2020, 25, 2001260, Erratum in Euro Surveill. 2021, 26, 210325d. https://doi.org/10.2807/1560-7917.ES.2021.26.12.210325d. [Google Scholar] [CrossRef] [PubMed]
- Romanholo, P.V.V.; Razzino, C.A.; Raymundo-Pereira, P.A.; Prado, T.M.; Machado, S.A.S.; Sgobbi, L.F. Biomimetic electrochemical sensors: New horizons and challenges in biosensing applications. Biosens. Bioelectron. 2021, 185, 113242. [Google Scholar] [CrossRef]
- Ameku, W.A.; Ataide, V.N.; Costa, E.T.; Gomes, L.R.; Napoleão-Pêgo, P.; William Provance, D.; Paixão, T.R.L.C.; Salles, M.O.; De-Simone, S.G. A pencil-lead immunosensor for the rapid electrochemical measurement of anti-diphtheria toxin antibodies. Biosensors 2021, 11, 489. [Google Scholar] [CrossRef]
- Hughes, G.; Westmacott, K.; Honeychurch, K.C.; Crew, A.; Pemberton, R.M.; Hart, J.P. Recent advances in the fabrication and application of screen-printed electrochemical (bio)sensors based on carbon materials for biomedical, agri-food, and environmental analyses. Biosensors 2016, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Ameku, W.A.; De Araujo, W.R.; Rangel, C.J.; Ando, R.A.; Paixão, T.R.L.C. Gold nanoparticle paper-based dual-detection device for forensics applications. ACS Appl. Nano Mater. 2019, 9, 5460–5468. [Google Scholar] [CrossRef]
- Orzari, L.O.; Cristina de Freitas, R.; Aparecida de Araujo Andreotti, I.; Gatti, A.; Janegitz, B.C. A novel disposable self-adhesive inked paper device for electrochemical sensing of dopamine and serotonin neurotransmitters and glucose biosensing. Biosens. Bioelectron. 2019, 138, 111310. [Google Scholar] [CrossRef]
- Núnez-Bajo, E.; Carmen Blanco-López, M.; Costa-García, A.; Teresa Fernández-Abedul, M. Integration of gold-sputtered electrofluidic paper on wire-included analytical platforms for glucose biosensing. Biosens. Bioelectron. 2017, 91, 824–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, M.D.T.; de Araujo, W.R.; de Lima, L.F.; Ferreira, A.L.; de la Fuente-Nunez, C. Low-cost biosensor for rapid detection of SARS-CoV-2 at the point-of-care. Matter 2021, 4, 2403–2416. [Google Scholar] [CrossRef] [PubMed]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-based electrochemical biosensor for diagnosing COVID-19: Detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef] [PubMed]
- Ataide, V.N.; Ameku, W.A.; Bacil, R.P.; Angnes, L.; De Araujo, W.R.; Paixão, T.R.L.C. Enhanced performance of pencil-drawn paper-based electrodes by laser-scribing treatment. RSC Adv. 2021, 11, 1644–1653. [Google Scholar] [CrossRef]
- de Araujo, W.R.; Frasson, C.M.R.; Ameku, W.A.; Silva, J.R.; Angnes, L.; Paixão, T.R. Single-step reagentless laser scribing fabrication of electrochemical paper-based analytical devices. Angew. Chem. Int. 2017, 129, 15113–15117. [Google Scholar] [CrossRef] [PubMed]
- Bottino, C.G.; Gomes, L.P.; Pereira, J.B.; Coura, J.R.; Provance, D.W.; De-Simone, S.G. Chagas disease-specific antigens: Characterization of epitopes in CRA/FRA by synthetic peptide mapping and evaluation by ELISA-peptide assay. BMC Infect. Dis. 2013, 13, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, A.L.; Faria, R.X.; Calabrese, K.S.; Hardoim, D.J.; Taniwaki, N.; Alves, L.A.; De-Simone, S.G. Temporizin and temporizin-1 peptides as novel candidates for eliminating Trypanosoma cruzi. PLoS ONE 2016, 11, e0157673. [Google Scholar] [CrossRef]
- Doughty, P.T.; Hossain, I.; Gong, C.; Ponder, K.A.; Pati, S.; Arumugam, P.U.; Murray, T.A. Novel microwire-based biosensor probe for simultaneous real-time measurement of glutamate and GABA dynamics in vitro and in vivo. Sci. Rep. 2020, 10, 12777. [Google Scholar] [CrossRef]
- Ahmad, H.M.N.; Dutta, G.; Csoros, J.; Si, B.; Yang, R.; Halpern, J.M.; Seitz, W.R.; Song, E. Stimuli-responsive templated polymer as a target receptor for a conformation-based electrochemical sensing platform. ACS Appl. Polym. Mater. 2021, 3, 329–341. [Google Scholar] [CrossRef]
- Ternynck, T.; Avrameas, S. Polymerization and immobilization of proteins using ethyl chloroformate and glutaraldehyde. Scand. J. Immunol. 1976, 5, 29–35. [Google Scholar] [CrossRef]
- Lardeux, F.; Torrico, G.; Aliaga, C. Calculation of the ELISA’s cut-off based on the change-point analysis method for detection of Trypanosoma cruzi infection in Bolivian dogs in the absence of controls. Mem. Inst. Oswaldo Cruz 2016, 111, 501–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dopico, E.; Del-Rei, R.P.; Espinoza, B.; Ubillos, I.; Zanchin, N.I.T.; Sulleiro, E.; Moure, Z.; Celedon, P.A.F.; Souza, W.V.; Da Silva, E.D.; et al. Immune reactivity to Trypanosoma cruzi chimeric proteins for Chagas disease diagnosis in immigrants living in a non-endemic setting. BMC Infect. Dis. 2019, 19, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, F.; Shen, H.; Zhang, G.; Jiang, X.; Kang, X. Chemiluminescence immunoassay based on microfluidic chips for α-fetoprotein. Clin. Chim. Acta 2014, 431, 113–117. [Google Scholar] [CrossRef]
- Michel, M.; Bouam, A.; Edouard, S.; Fenollar, F.; Di Pinto, F.; Mège, J.L.; Drancourt, M.; Vitte, J. Evaluating ELISA, immunofluorescence, and lateral flow assay for SARS-CoV-2 serologic assays. Front. Microbiol. 2020, 11, 597529. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, S.; Shabani, E.; Abdekhodaie, M.J. Serological assays and host antibody detection in coronavirus-related disease diagnosis. Arch. Virol. 2021, 166, 715–731. [Google Scholar] [CrossRef] [PubMed]
- De Lima, L.F.; Ferreira, A.L.; Torres, M.D.T.; Araujo, W.R. Minute-scale detection of SARS-CoV-2 using a low-cost biosensor composed of pencil graphite electrodes. Proc. Natl. Acad. Sci. USA 2021, 118, e2106724118. [Google Scholar] [CrossRef]
- Nicol, T.; Lefeuvre, C.; Serri, O.; Pivert, A.; Joubaud, F.; Dubée, V.; Kouatchet, A.; Ducancelle, A.; Lunel-Fabiani, F.; Le Guillou-Guillemette, H. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech). J. Clin. Virol. 2020, 129, 104511. [Google Scholar] [CrossRef]
- Beavis, K.G.; Matushek, S.M.; Abeleda, A.P.F.; Bethel, C.; Hunt, C.; Gillen, S.; Moran, A.; Tesic, V. Evaluation of the EUROIMMUN anti-SARS-CoV-2 ELISA assay for detection of IgA and IgG antibodies. J. Clin. Virol. 2020, 129, 104468. [Google Scholar] [CrossRef]
- Guevara-Hoyer, K.; Fuentes-Antrás, J.; De la Fuente-Muñoz, E.; Rodríguez de la Peña, A.; Viñuela, M.; Cabello-Clotet, N.; Estrada, V.; Culebras, E.; Delgado-Iribarren, A.; Martínez-Novillo, M.; et al. Serological tests in the detection of SARS-CoV-2 Antibodies. Diagnostics 2021, 11, 678. [Google Scholar] [CrossRef]
- Mekonnen, D.; Mengist, H.M.; Derbie, A.; Nibret, E.; Munshea, A.; He, H.; Li, B.; Jin, T. Diagnostic accuracy of serological tests and kinetics of severe acute respiratory syndrome coronavirus 2 antibody: A systematic review and meta-analysis. Rev. Med. Virol. 2021, 31, e2181. [Google Scholar] [CrossRef]
- Gutiérrez-Cobos, A.; Gómez de Frutos, S.; Domingo García, D.; Navarro Lara, E.; Yarci Carrión, A.; Fontán García-Rodrigo, L.; Fraile Torres, A.M.; Cardeñoso Domingo, L. Evaluation of diagnostic accuracy of 10 serological assays for detection of SARS-CoV-2 antibodies. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.R.; Durans, A.M.; Napole, P.; Waterman, J.A.; Freitas, M.S.; De Sá, N.B.; Pereira, L.V.; Furtado, J.S.; Aquino, G.; Machado, M.C.R.; et al. Multiepitope proteins for the differential detection of IgG antibodies against RBD of the spike protein and non-RBD regions of SARS-CoV-2. Vaccines 2021, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Liu, Z.; Wang, G.; Guo, X.; Akbar Khan, S.; Lai, C.; Chen, H.; Huang, S.; Xia, S.; Chen, B.; et al. Detection of COVID-19: A review of the current literature and future perspectives. Biosens Bioelectron. 2020, 166, 112455. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameku, W.A.; Provance, D.W.; Morel, C.M.; De-Simone, S.G. Rapid Detection of Anti-SARS-CoV-2 Antibodies with a Screen-Printed Electrode Modified with a Spike Glycoprotein Epitope. Biosensors 2022, 12, 272. https://doi.org/10.3390/bios12050272
Ameku WA, Provance DW, Morel CM, De-Simone SG. Rapid Detection of Anti-SARS-CoV-2 Antibodies with a Screen-Printed Electrode Modified with a Spike Glycoprotein Epitope. Biosensors. 2022; 12(5):272. https://doi.org/10.3390/bios12050272
Chicago/Turabian StyleAmeku, Wilson A., David W. Provance, Carlos M. Morel, and Salvatore G. De-Simone. 2022. "Rapid Detection of Anti-SARS-CoV-2 Antibodies with a Screen-Printed Electrode Modified with a Spike Glycoprotein Epitope" Biosensors 12, no. 5: 272. https://doi.org/10.3390/bios12050272
APA StyleAmeku, W. A., Provance, D. W., Morel, C. M., & De-Simone, S. G. (2022). Rapid Detection of Anti-SARS-CoV-2 Antibodies with a Screen-Printed Electrode Modified with a Spike Glycoprotein Epitope. Biosensors, 12(5), 272. https://doi.org/10.3390/bios12050272






