Recent Progress in Non-Enzymatic Electroanalytical Detection of Pesticides Based on the Use of Functional Nanomaterials as Electrode Modifiers
Abstract
:1. Introduction
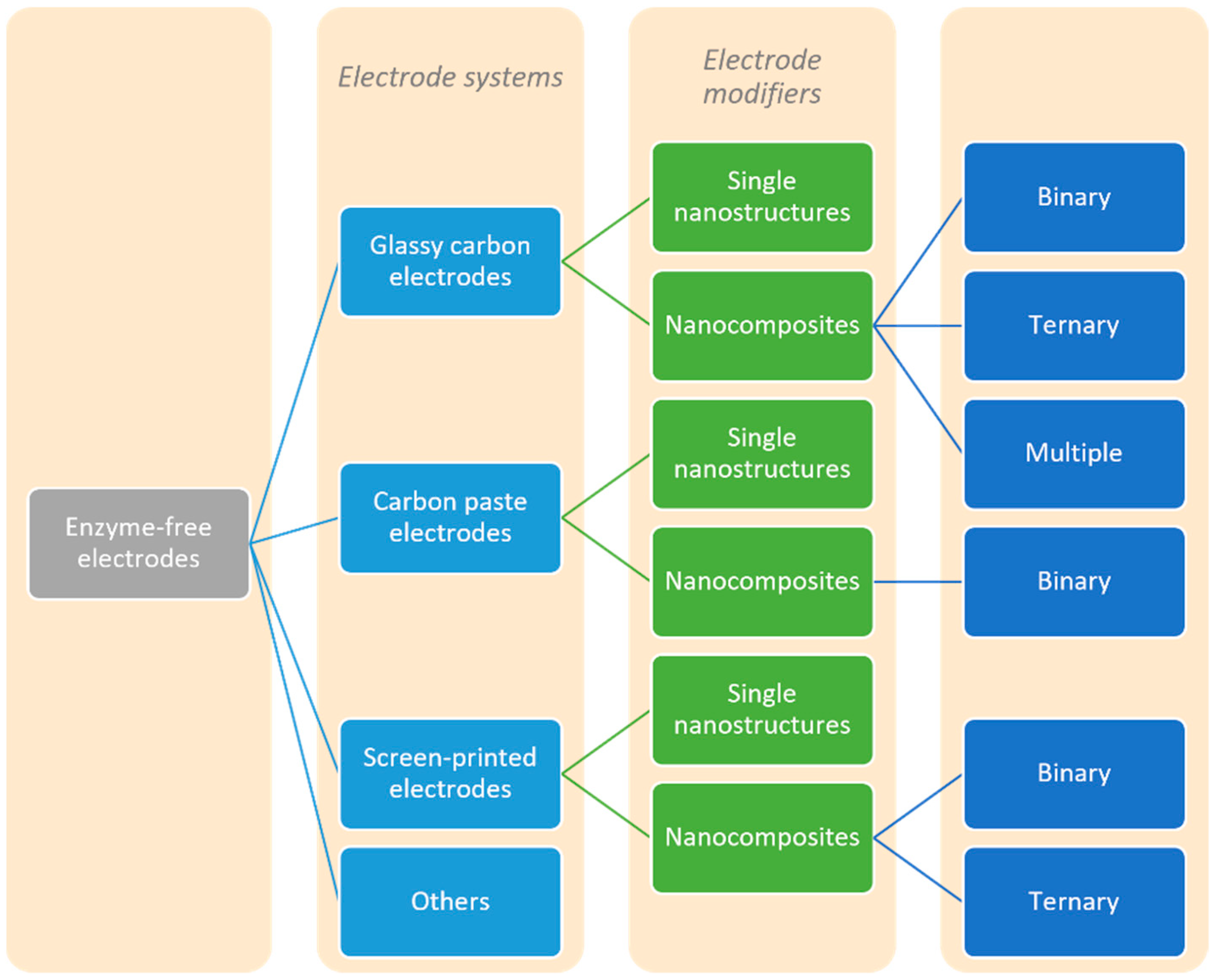
2. The Use of Different Enzyme-Free Electrodes
2.1. Glassy Carbon Electrode
2.1.1. Modification of the Glassy Carbon Electrodes Using Single Nanostructures-Based Modifiers
2.1.2. Modification of the Glassy Carbon Electrodes Using Binary Nanocomposites-Based Modifiers
2.1.3. Modification of the Glassy Carbon Electrodes Using Ternary Nanocomposites-Based Modifiers
2.1.4. Modification of the Glassy Carbon Electrodes Using Multiple Nanocomposites-Based Modifiers
2.2. Carbon Paste Electrode
2.2.1. Modification of the Carbon Paste Electrodes Using Single Nanostructures-Based Modifiers
2.2.2. Modification of the Carbon Paste Electrodes Using Binary Nanocomposites-Based Modifiers
2.3. Screen-Printed Electrode
2.3.1. Modification of the Screen-Printed Electrodes Using Single Nanostructures-Based Modifiers
2.3.2. Modification of the Screen-Printed Electrodes Using Binary Nanocomposites-Based Modifiers
2.3.3. Modification of the Screen-Printed Electrodes Using Ternary Nanocomposites-Based Modifiers
2.4. Other Electrodes
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABIN | azobisisobutyronitrile |
| AC | activated carbon |
| AChE | Acetylcholinesterase |
| AC | atomic cluster |
| BDD | boron-doped diamond |
| BET | Brunauer–Emmett–Teller |
| BTC | benzene-1,3,5-tricarboxylic acid |
| B-R | Britton-Robinson |
| CBF | carbofuran |
| CBM | carbendazim |
| CBR | carbaryl |
| CMC | carboxymethyl cellulose |
| CMCh | carboxymethyl chitosan |
| CNH | carbon nanohorn |
| CNT | carbon nanotube |
| CPE | carbon paste electrode |
| CSS | carbon spherical shells |
| CTAB | cetyltrimethylammonium bromide |
| DMF | dimethylformamide |
| DPASV | differential pulse anodic stripping voltammetry |
| DPSV | differential pulse stripping voltammetry |
| DPV | differential pulse voltammetry |
| EGMRA | ethylene glycol maleic rosinate acrylate |
| EIS | electrochemical impedance spectroscopy |
| FEN | fenitrothion |
| FNM | fenamiphos |
| FS | fumed silica |
| GC | gas chromatography |
| GCE | glassy carbon electrode |
| GC-MS | gas chromatography-mass spectrometry |
| GNS | graphene nanosheets |
| GO | graphene oxide |
| g-C3N4 | graphitic carbon nitride |
| HCNT | hydroxylated multiwall carbon nanotubes |
| HF | hollow fibre |
| HPLC | high-performance liquid chromatography |
| HS | headspace |
| IF | imprinting factor |
| IL | ionic liquid |
| ISO | isoproturon |
| ITO | indium tin oxide |
| LC | liquid chromatography |
| LOD | limit of detection |
| LOQ | limit of quantification |
| LPME | Liquid-phase microextraction |
| LSV | linear sweep voltammetry |
| MAA | methyl acrylic acid |
| MIL(Fe) | the iron-carboxylate nano metal-organic framework |
| MIP | molecularly imprinted polymer |
| MNP | magnetic nanoparticles |
| MP | methyl parathion |
| MUA | 11-mercaptoundecanoic acid |
| MWCNT | Multi-wall carbon nanotubes |
| MWCNTPE | Multi-wall carbon nanotube paste electrode |
| NIP | Non-imprinted polymer |
| NP | nanoparticle |
| NPG | nanoporous gold |
| NR | not reported |
| NS | nanosheets |
| NT | nanotubes |
| NW | nanowires |
| OP | organophosphorous pesticides |
| PANI | polyaniline |
| PCL | poly(ε-caprolactone) |
| PCNB | Printex carbon nanoballs |
| PDA | polydopamine |
| PET | polyethylene terephthalate |
| POT | potentiometry |
| PPy | polypyrrole |
| PTH | polythiophene |
| QD | quantum dot |
| rGO | reduced graphene oxide |
| R | resistance |
| Rct | charge transfer resistance |
| SCE | saturated calomel electrode |
| SEM | scanning electron microscope |
| SPCE | screen-printed carbon electrode |
| SPE | screen-printed electrode |
| SPME | solid-phase microextraction |
| SS | stainless steel |
| SWASV | anodic stripping square-wave voltammetry |
| SWCNT | single-wall carbon nanotubes |
| SWV | square-wave voltammetry |
| SBET | specific surface area calculated using the BET method |
| TBOZ | zirconium n-butoxide |
| TEM | transmission electron microscope |
| TEOS | tetraethoxysilane |
| UiO | 66-metal-organic framework ([Zr6O4(OH)4] clusters with 1,4-benzodicarboxylic acid struts) |
| 4,4′-DDT | dichlorodiphenyltrichloroethane |
References
- WHO; FAO. International Code of Conduct on Pesticide Management. In Guidelines on Highly Hazardous Pesticides; Food & Agriculture Org.: Rome, Italy; WHO: Geneve, Switzerland, 2016; 37p. [Google Scholar]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of Surface Area, Primary Particle Size, and Crystal Phase on Titanium Dioxide Nanoparticle Dispersion Properties. Nanoscale Res. Lett. 2010, 6, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. International Code of Conduct on the Distribution and Use of Pesticides. In Guidance on Pest and Pesticide Management Policy Development; Food & Agriculture Org.: Rome, Italy, 2010. [Google Scholar]
- Saeedi Saravi, S.S.; Shokrzadeh, M. Role of Pesticides in Human Life in the Modern Age: A Review. In Pesticides in the Modern World—Risks and Benefits; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011; Volume 3, pp. 3–12. [Google Scholar]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pino, F.; Merkoçi, A. Nanomaterials for Sensing and Destroying Pesticides. Chem. Rev. 2012, 112, 5317–5338. [Google Scholar] [CrossRef] [PubMed]
- Goicolea, M.A.; Gómez-Caballero, A.; Barrio, R.J. New Materials in Electochemical Sensors for Pesticides Monitoring. In Pesticides—Strategies for Pesticides Analysis; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Villaverde, J.J.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L.; Sandín-España, P. Trends in analysis of pesticide residues to fulfil the European Regulation (EC) No. 1107/2009. TrAC Trends Anal. Chem. 2016, 80, 568–580. [Google Scholar] [CrossRef]
- van der Hoff, G.R.; van Zoonen, P. Trace analysis of pesticides by gas chromatography. J. Chromatogr. A 1999, 843, 301–322. [Google Scholar] [CrossRef]
- Geerdink, R.B.; Niessen, W.M.A.; Brinkman, U.A.T. Trace-level determination of pesticides in water by means of liquid and gas chromatography. J. Chromatogr. A 2002, 970, 65–93. [Google Scholar] [CrossRef]
- Alamgir Zaman Chowdhury, M.; Fakhruddin, A.N.M.; Nazrul Islam, M.; Moniruzzaman, M.; Gan, S.H.; Khorshed Alam, M. Detection of the residues of nineteen pesticides in fresh vegetable samples using gas chromatography–mass spectrometry. Food Control 2013, 34, 457–465. [Google Scholar] [CrossRef]
- Pico, Y.; Alfarhan, A.H.; Barcelo, D. How recent innovations in gas chromatography-mass spectrometry have improved pesticide residue determination: An alternative technique to be in your radar. TrAC Trends Anal. Chem. 2020, 122, 115720. [Google Scholar] [CrossRef]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of pesticide residue analysis in fruits and vegetables. Pre-treatment, extraction and detection techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef]
- Sharma, D.; Nagpal, A.; Pakade, Y.B.; Katnoria, J.K. Analytical methods for estimation of organophosphorus pesticide residues in fruits and vegetables: A review. Talanta 2010, 82, 1077–1089. [Google Scholar] [CrossRef]
- Zamora-Sequeira, R.; Starbird-Pérez, R.; Rojas-Carillo, O.; Vargas-Villalobos, S. What are the Main Sensor Methods for Quantifying Pesticides in Agricultural Activities? A Review. Molecules 2019, 24, 2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.-P.; Huang, S.-D. Determination of organochlorine pesticides in water using solvent cooling assisted dynamic hollow-fiber-supported headspace liquid-phase microextraction. J. Chromatogr. A 2007, 1176, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Afshar Mogaddam, M.R.; Mohebbi, A.; Pazhohan, A.; Khodadadeian, F.; Farajzadeh, M.A. Headspace mode of liquid phase microextraction: A review. TrAC Trends Anal. Chem. 2019, 110, 8–14. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Bakirhan, N.K.; Uslu, B.; Ozkan, S.A. Chapter 5—The Detection of Pesticide in Foods Using Electrochemical Sensors. In Food Safety and Preservation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 91–141. [Google Scholar]
- Uniyal, S.; Sharma, R.K. Technological advancement in electrochemical biosensor based detection of Organophosphate pesticide chlorpyrifos in the environment: A review of status and prospects. Biosens. Bioelectron. 2018, 116, 37–50. [Google Scholar] [CrossRef]
- Hanrahan, G.; Patil, D.G.; Wang, J. Electrochemical sensors for environmental monitoring: Design, development and applications. J. Environ. Monit. 2004, 6, 657–664. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical sensors. In Analytical Electrochemistry; Wang, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Volume 3, pp. 201–243. [Google Scholar]
- Barry, S.; O’Riordan, A. Electrochemical nanosensors: Advances and applications. Rep. Electrochem. 2016, 6, 1–14. [Google Scholar]
- Díaz-González, M.; Gutiérrez-Capitán, M.; Niu, P.; Baldi, A.; Jiménez-Jorquera, C.; Fernández-Sánchez, C. Electrochemical devices for the detection of priority pollutants listed in the EU water framework directive. TrAC Trends Anal. Chem. 2016, 77, 186–202. [Google Scholar] [CrossRef] [Green Version]
- Cardenas-Riojas, A.A.; Cornejo-Herrera, A.F.; Muedas-Taipe, G.; La Rosa-Toro, A.; Sotomayor, M.D.P.T.; Ponce-Vargas, M.; Baena-Moncada, A.M. Electrochemical sensor based on 1,8-dihydroxyanthraquinone adsorbed on a glassy carbon electrode for the detection of [Cu(CN)3](aq)2− in alkaline cyanide copper plating baths waste. J. Electroanal. Chem. 2021, 880, 114909. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Kurbanoglu, S.; Erkmen, C.; Uslu, B. Frontiers in electrochemical enzyme based biosensors for food and drug analysis. TrAC Trends Anal. Chem. 2020, 124, 115809. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Gai, P.; Liu, X.; Li, F. Degradable metal-organic framework/methylene blue composites-based homogeneous electrochemical strategy for pesticide assay. Sens. Actuators B Chem. 2020, 323, 128701. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, M.; Pu, L.; Gai, P.; Li, F. Nitrogen-Enriched Conjugated Polymer Enabled Metal-Free Carbon Nanozymes with Efficient Oxidase-Like Activity. Small 2022, 18, 2104993. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, Q.; Li, Q.; Li, H.; Li, F. Two-Dimensional MnO2 Nanozyme-Mediated Homogeneous Electrochemical Detection of Organophosphate Pesticides without the Interference of H2O2 and Color. Anal. Chem. 2021, 93, 4084–4091. [Google Scholar] [CrossRef]
- Zhou, T.; Ding, L.; Che, G.; Jiang, W.; Sang, L. Recent advances and trends of molecularly imprinted polymers for specific recognition in aqueous matrix: Preparation and application in sample pretreatment. TrAC Trends Anal. Chem. 2019, 114, 11–28. [Google Scholar] [CrossRef]
- Boulanouar, S.; Mezzache, S.; Combès, A.; Pichon, V. Molecularly imprinted polymers for the determination of organophosphorus pesticides in complex samples. Talanta 2018, 176, 465–478. [Google Scholar] [CrossRef] [Green Version]
- Tonle, K.I.; Ngameni, E. Voltammetric Analysis of Pesticides. In Pesticides in the Modern World—Trends in Pesticides Analysis; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Rhouati, A.; Majdinasab, M.; Hayat, A. A perspective on non-enzymatic electrochemical nanosensors for direct detection of pesticides. Curr. Opin. Electrochem. 2018, 11, 12–18. [Google Scholar] [CrossRef]
- Majdinasab, M.; Daneshi, M.; Louis Marty, J. Recent developments in non-enzymatic (bio)sensors for detection of pesticide residues: Focusing on antibody, aptamer and molecularly imprinted polymer. Talanta 2021, 232, 122397. [Google Scholar] [CrossRef]
- Rassaei, L.; Marken, F.; Sillanpää, M.; Amiri, M.; Cirtiu, C.M.; Sillanpää, M. Nanoparticles in electrochemical sensors for environmental monitoring. TrAC Trends Anal. Chem. 2011, 30, 1704–1715. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, Z.; Hu, X. Multivariate nanocomposites for electrochemical sensing in the application of food. TrAC Trends Anal. Chem. 2019, 118, 759–769. [Google Scholar] [CrossRef]
- Chauhan, C. Contemporary voltammetric techniques and its application to pesticide analysis: A review. Mater. Today: Proc. 2021, 37, 3231–3240. [Google Scholar] [CrossRef]
- Rawtani, D.; Khatri, N.; Tyagi, S.; Pandey, G. Nanotechnology-based recent approaches for sensing and remediation of pesticides. J. Environ. Manag. 2018, 206, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sato, H. Some Physical Properties of Glassy Carbon. Nature 1962, 193, 261–262. [Google Scholar] [CrossRef]
- Van der Linden, W.E.; Dieker, J.W. Glassy carbon as electrode material in electro-analytical chemistry. Anal. Chim. Acta 1980, 119, 1–24. [Google Scholar] [CrossRef]
- Zittel, H.E.; Miller, F.J. A Glassy-Carbon Electrode for Voltammetry. Anal. Chem. 1965, 37, 200–203. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Sundari, P.L.A.; Palaniappan, S.P.; Manisankar, P. Enhanced Sensing of Carbendazim, a Fungicide on Functionalized Multiwalled Carbon Nanotube Modified Glassy Carbon Electrode and Its Determination in Real Samples. Anal. Lett. 2010, 43, 1457–1470. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, T.; Zhang, D.; Li, M. Preparation of Gd2O3 Hollow Nanospheres for Electrochemical Detection of Methyl Parathion. J. Electrochem. Soc. 2019, 166, H669–H675. [Google Scholar] [CrossRef]
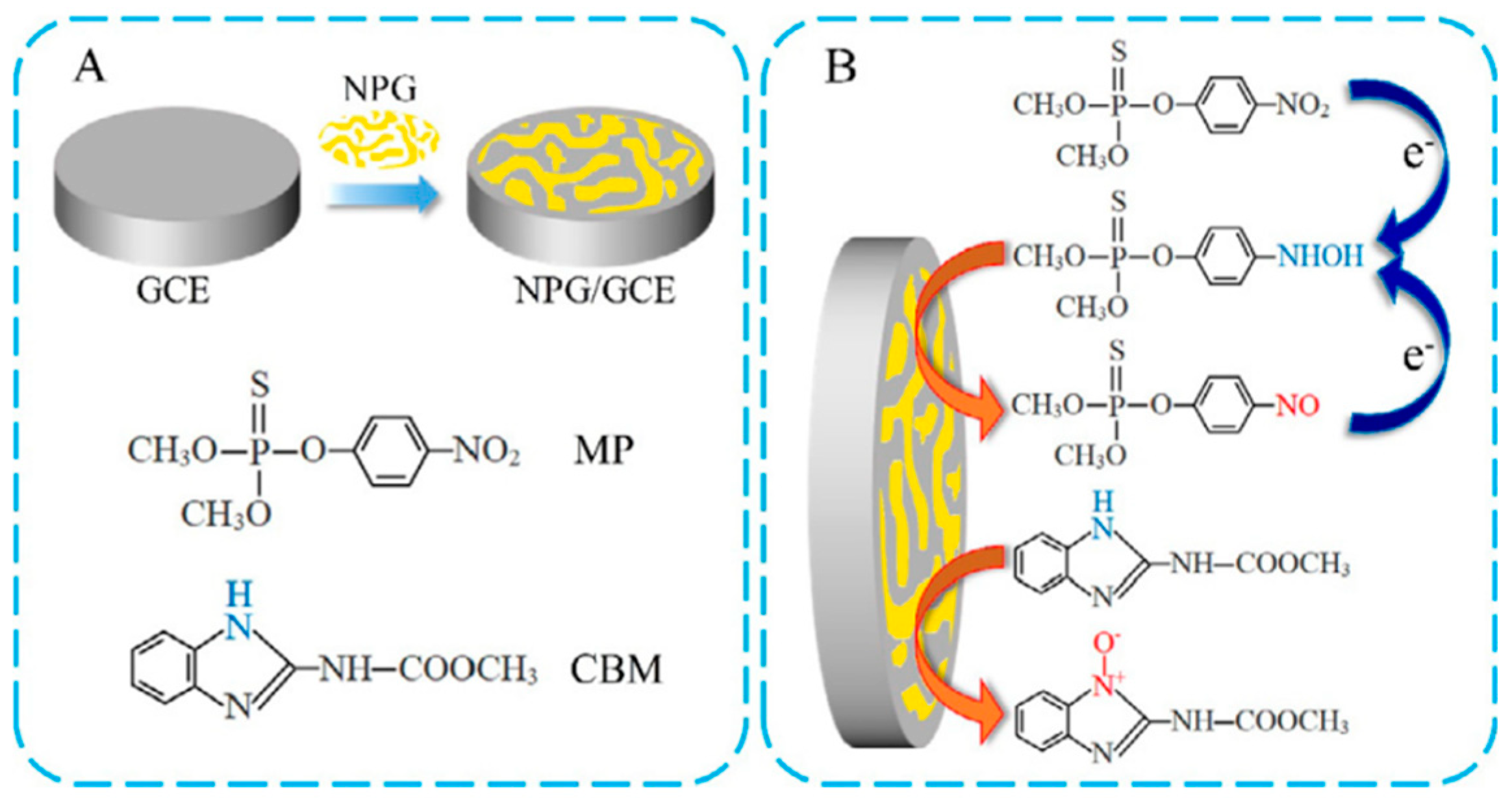
- Gao, X.; Gao, Y.; Bian, C.; Ma, H.; Liu, H. Electroactive nanoporous gold driven electrochemical sensor for the simultaneous detection of carbendazim and methyl parathion. Electrochim. Acta 2019, 310, 78–85. [Google Scholar] [CrossRef]
- Govindasamy, M.; Chen, S.-M.; Mani, V.; Akilarasan, M.; Kogularasu, S.; Subramani, B. Nanocomposites composed of layered molybdenum disulfide and graphene for highly sensitive amperometric determination of methyl parathion. Microchim. Acta 2017, 184, 725–733. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, Y.; Lu, L.; Ma, X.; Gong, L.; Huang, X.; Liu, G.; Yu, Y. CuO nanoparticles decorated 3D graphene nanocomposite as non-enzymatic electrochemical sensing platform for malathion detection. J. Electroanal. Chem. 2018, 812, 82–89. [Google Scholar] [CrossRef]
- Aghaie, A.; Khanmohammadi, A.; Hajian, A.; Schmid, U.; Bagheri, H. Nonenzymatic Electrochemical Determination of Paraoxon Ethyl in Water and Fruits by Graphene-Based NiFe Bimetallic Phosphosulfide Nanocomposite as a Superior Sensing Layer. Food Anal. Methods 2019, 12, 1545–1555. [Google Scholar] [CrossRef]
- Ghodsi, J.; Rafati, A.A. A voltammetric sensor for diazinon pesticide based on electrode modified with TiO2 nanoparticles covered multi walled carbon nanotube nanocomposite. J. Electroanal. Chem. 2017, 807, 1–9. [Google Scholar] [CrossRef]
- Amatatongchai, M.; Sroysee, W.; Sodkrathok, P.; Kesangam, N.; Chairam, S.; Jarujamrus, P. Novel three-Dimensional molecularly imprinted polymer-coated carbon nanotubes (3D-CNTs@MIP) for selective detection of profenofos in food. Anal. Chim. Acta 2019, 1076, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Aruna, P.; Prabhakar, K.; Sreedhar, N.Y. Effective SWCNTs/Nafion Electrochemical Sensor for Detection of Dicapthon Pesticide in Water and Agricultural Food Samples. Chem. Methodol. 2018, 2, 277–290. [Google Scholar] [CrossRef]
- Wang, H.; Pan, L.; Liu, Y.; Ye, Y.; Yao, S. Electrochemical sensing of nitenpyram based on the binary nanohybrid of hydroxylated multiwall carbon nanotubes/single-wall carbon nanohorns. J. Electroanal. Chem. 2020, 862, 113955. [Google Scholar] [CrossRef]
- Liao, X.; Huang, Z.; Huang, K.; Qiu, M.; Chen, F.; Zhang, Y.; Wen, Y.; Chen, J. Highly Sensitive Detection of Carbendazim and Its Electrochemical Oxidation Mechanism at a Nanohybrid Sensor. J. Electrochem. Soc. 2019, 166, B322–B327. [Google Scholar] [CrossRef]
- Zhang, X.; Du, J.; Wu, D.; Long, X.; Wang, D.; Xiong, J.; Xiong, W.; Liao, X. Anchoring Metallic MoS2 Quantum Dots over MWCNTs for Highly Sensitive Detection of Postharvest Fungicide in Traditional Chinese Medicines. ACS Omega 2021, 6, 1488–1496. [Google Scholar] [CrossRef]
- Razzino, C.A.; Sgobbi, L.F.; Canevari, T.C.; Cancino, J.; Machado, S.A.S. Sensitive determination of carbendazim in orange juice by electrode modified with hybrid material. Food Chem. 2015, 170, 360–365. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, F.; Tu, X.; Ma, X.; Dai, R.; Peng, G.; Yu, Y.; Lu, L. Flake-like neodymium molybdate wrapped with multi-walled carbon nanotubes as an effective electrode material for sensitive electrochemical detection of carbendazim. J. Electroanal. Chem. 2019, 855, 113468. [Google Scholar] [CrossRef]
- Yazhen, W.; Hongxin, Q.; Siqian, H.; Junhui, X. A novel methyl parathion electrochemical sensor based on acetylene black–chitosan composite film modified electrode. Sens. Actuators B Chem. 2010, 147, 587–592. [Google Scholar] [CrossRef]
- Xie, Y.; Tu, X.; Ma, X.; Fang, Q.; Liu, G.; Dai, R.; Qu, F.; Yu, Y.; Lu, L.; Huang, X. A CuO-CeO2 composite prepared by calcination of a bimetallic metal-organic framework for use in an enzyme-free electrochemical inhibition assay for malathion. Microchim. Acta 2019, 186, 567. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xiao, B.; Cui, L. Electrochemical analysis of carbaryl in fruit samples on graphene oxide-ionic liquid composite modified electrode. J. Food Compos. Anal. 2015, 40, 14–18. [Google Scholar] [CrossRef]
- Kumaravel, A.; Chandrasekaran, M. Electrochemical Determination of Chlorpyrifos on a Nano-TiO2/Cellulose Acetate Composite Modified Glassy Carbon Electrode. J. Agric. Food Chem. 2015, 63, 6150–6156. [Google Scholar] [CrossRef] [PubMed]
- Ranđelović, M.S.; Momčilović, M.Z.; Milićević, J.S.; Đurović-Pejčev, R.D.; Mofarah, S.S.; Sorrel, C.C. Voltammetric sensor based on Pt nanoparticles suported MWCNT for determination of pesticide clomazone in water samples. J. Taiwan Inst. Chem. Eng. 2019, 105, 115–123. [Google Scholar] [CrossRef]
- Moraes, F.C.; Mascaro, L.H.; Machado, S.A.S.; Brett, C.M.A. Direct Electrochemical Determination of Glyphosate at Copper Phthalocyanine/Multiwalled Carbon Nanotube Film Electrodes. Electroanalysis 2010, 22, 1586–1591. [Google Scholar] [CrossRef]
- Miao, J.; Liu, A.; Wu, L.; Yu, M.; Wei, W.; Liu, S. Magnetic ferroferric oxide and polydopamine molecularly imprinted polymer nanocomposites based electrochemical impedance sensor for the selective separation and sensitive determination of dichlorodiphenyltrichloroethane (DDT). Anal. Chim. Acta 2020, 1095, 82–92. [Google Scholar] [CrossRef]
- Kumaravel, A.; Murugananthan, M.; Mangalam, R.; Jayakumar, S. A novel, biocompatible and electrocatalytic stearic acid/nanosilver modified glassy carbon electrode for the sensing of paraoxon pesticide in food samples and commercial formulations. Food Chem. 2020, 323, 126814. [Google Scholar] [CrossRef]
- Soltani-Shahrivar, M.; Karimian, N.; Fakhri, H.; Hajian, A.; Afkhami, A.; Bagheri, H. Design and Application of a Non-enzymatic Sensor Based on Metal-organic Frameworks for the Simultaneous Determination of Carbofuran and Carbaryl in Fruits and Vegetables. Electroanalysis 2019, 31, 2455–2465. [Google Scholar] [CrossRef]
- Tawade, A.K.; Mohan Kumar, D.; Talele, P.; Sharma, K.K.K.; Tayade, S.N. Flower-Like ZnO-Decorated Polyaniline–Graphene Oxide Nanocomposite for Electrochemical Oxidation of Imidacloprid: A Hybrid Nanocomposite Sensor. J. Electron. Mater. 2019, 48, 7747–7755. [Google Scholar] [CrossRef]
- Ramachandran, T.; Dhayabaran, V.V. Utilization of a MnO2/polythiophene/rGO nanocomposite modified glassy carbon electrode as an electrochemical sensor for methyl parathion. J. Mater. Sci. Mater. Electron. 2019, 30, 12315–12327. [Google Scholar] [CrossRef]
- Gao, N.; He, C.; Ma, M.; Cai, Z.; Zhou, Y.; Chang, G.; Wang, X.; He, Y. Electrochemical co-deposition synthesis of Au-ZrO2-graphene nanocomposite for a nonenzymatic methyl parathion sensor. Anal. Chim. Acta 2019, 1072, 25–34. [Google Scholar] [CrossRef]
- Gong, J.; Miao, X.; Zhou, T.; Zhang, L. An enzymeless organophosphate pesticide sensor using Au nanoparticle-decorated graphene hybrid nanosheet as solid-phase extraction. Talanta 2011, 85, 1344–1349. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, W.-D.; Chen, C.-H.; Yu, Y.-X. Electrochemical determination of methyl parathion at a Pd/MWCNTs-modified electrode. Microchim. Acta 2010, 171, 57–62. [Google Scholar] [CrossRef]
- Canevari, T.C.; Prado, T.M.; Cincotto, F.H.; Machado, S.A.S. Immobilization of ruthenium phthalocyanine on silica-coated multi-wall partially oriented carbon nanotubes: Electrochemical detection of fenitrothion pesticide. Mater. Res. Bull. 2016, 76, 41–47. [Google Scholar] [CrossRef] [Green Version]
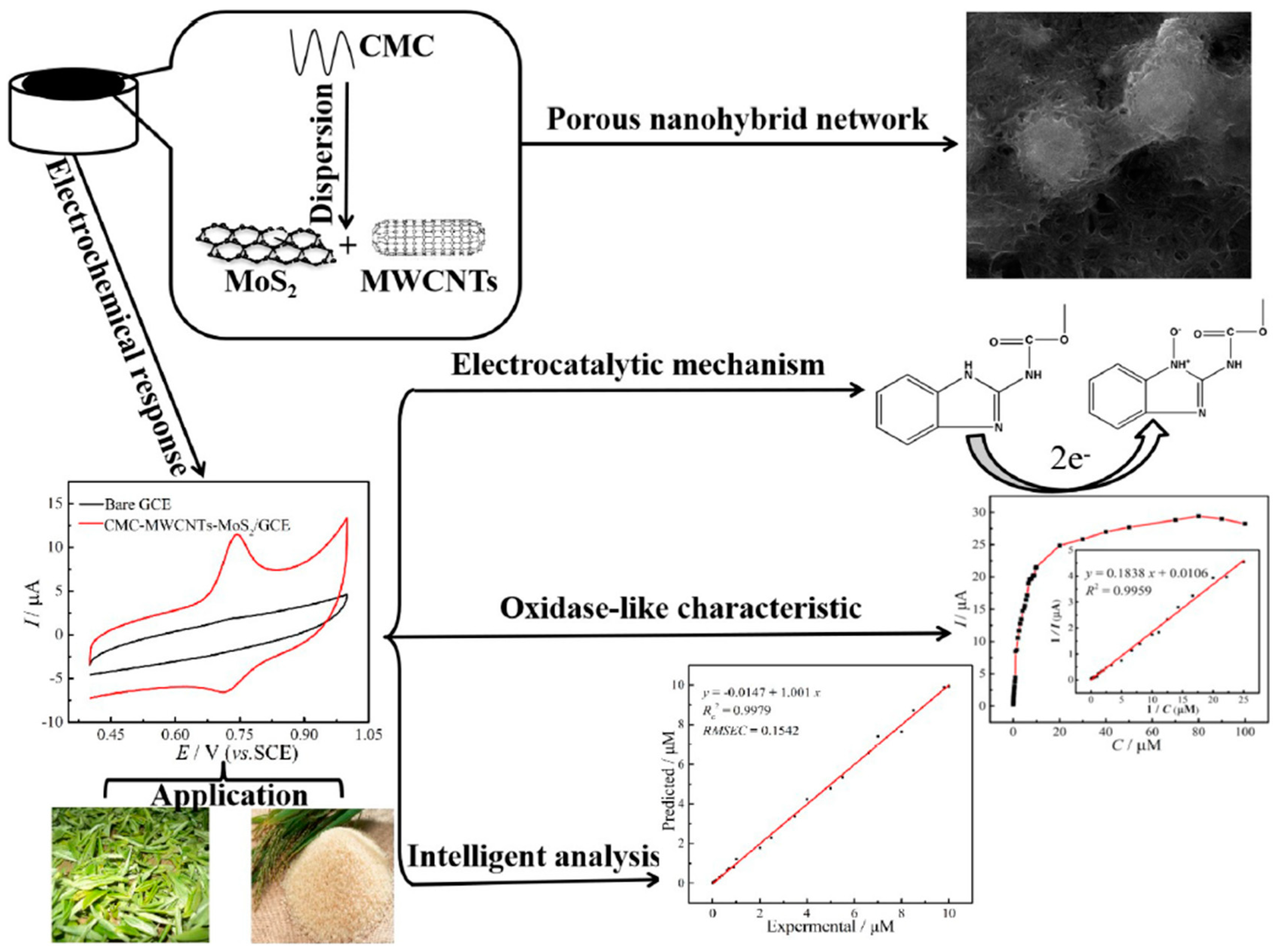
- Zhu, X.; Liu, P.; Ge, Y.; Wu, R.; Xue, T.; Sheng, Y.; Ai, S.; Tang, K.; Wen, Y. MoS2/MWCNTs porous nanohybrid network with oxidase-like characteristic as electrochemical nanozyme sensor coupled with machine learning for intelligent analysis of carbendazim. J. Electroanal. Chem. 2020, 862, 113940. [Google Scholar] [CrossRef]
- Teadoum, D.N.; Noumbo, S.K.; Arnaud, K.T.; Ranil, T.T.; Mvondo Zé, A.D.; Tonle, I.K. Square Wave Voltammetric Determination of Residues of Carbendazim Using a Fullerene/Multiwalled Carbon Nanotubes/Nafion/Coated Glassy Carbon Electrode. Int. J. Electrochem. 2016, 2016, 7839708. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Yang, J.; Liu, X.; Zhang, L. Ionic Liquids-Modified CaFe2O4/MWCNTs Nano-Hybrid as an Electrode Material for Detection of Carbendazim. J. Electrochem. Soc. 2016, 163, B652–B658. [Google Scholar] [CrossRef]
- Yang, Y.; Tu, H.; Zhang, A.; Du, D.; Lin, Y. Preparation and characterization of Au–ZrO2–SiO2 nanocomposite spheres and their application in enrichment and detection of organophosphorus agents. J. Mater. Chem. 2012, 22, 4977–4981. [Google Scholar] [CrossRef]
- Tian, X.; Liu, L.; Li, Y.; Yang, C.; Zhou, Z.; Nie, Y.; Wang, Y. Nonenzymatic electrochemical sensor based on CuO-TiO2 for sensitive and selective detection of methyl parathion pesticide in ground water. Sens. Actuators B Chem. 2018, 256, 135–142. [Google Scholar] [CrossRef]
- Gai, K.; Qi, H.; Xiao, L.; Liu, X. Detection of Residual Methomyl in Vegetables with an Electrochemical Sensor based on a glassy carbon electrode modified with Fe3O4/Ag composite. Int. J. Electrochem. Sci. 2019, 14, 1283–1292. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, L.; Zhang, L. Simultaneous Electrochemical Detection of Benzimidazole Fungicides Carbendazim and Thiabendazole Using a Novel Nanohybrid Material-Modified Electrode. J. Agric. Food Chem. 2017, 65, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, J.; Wang, M.; Zhang, D.; Chen, J. Electrochemical nonenzymatic sensor based on CoO decorated reduced graphene oxide for the simultaneous determination of carbofuran and carbaryl in fruits and vegetables. Food Chem. 2014, 151, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Fakhri, H.; Amidi, S.; Hajian, A.; Arduinie, F.; Bagheri, H. A novel sensing layer based on metal–organic framework UiO-66 modified with TiO2–graphene oxide: Application to rapid, sensitive and simultaneous determination of paraoxon and chlorpyrifos. New J. Chem. 2019, 43, 2600–2609. [Google Scholar] [CrossRef]
- Tan, X.; Hu, Q.; Wu, J.; Li, X.; Li, P.; Yu, H.; Li, X.; Lei, F. Electrochemical sensor based on molecularly imprinted polymer reduced graphene oxide and gold nanoparticles modified electrode for detection of carbofuran. Sens. Actuators B Chem. 2015, 220, 216–221. [Google Scholar] [CrossRef]
- Wei, X.-P.; Luo, Y.-L.; Xu, F.; Chen, Y.-S.; Yang, L.-H. In-situ non-covalent dressing of multi-walled carbon nanotubes@titanium dioxides with carboxymethyl chitosan nanocomposite electrochemical sensors for detection of pesticide residues. Mater. Des. 2016, 111, 445–452. [Google Scholar] [CrossRef]
- Suresh, I.; Selvaraj, S.; Nesakumar, N.; Rayappan, J.B.B.; Kulandaiswamy, A.J. Nanomaterials based non-enzymatic electrochemical and optical sensors for the detection of carbendazim: A review. Trends Environ. Anal. Chem. 2021, 31, e00137. [Google Scholar] [CrossRef]
- Collinson, M.M. Nanoporous Gold Electrodes and Their Applications in Analytical Chemistry. ISRN Anal. Chem. 2013, 2013, 692484. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Chen, S.-M. Design and Construction of the Gadolinium Oxide Nanorod-Embedded Graphene Aerogel: A Potential Application for Electrochemical Detection of Postharvest Fungicide. ACS Appl. Mater. Interfaces 2020, 12, 16216–16226. [Google Scholar] [CrossRef]
- Wei, P.; Gan, T.; Wu, K. N-methyl-2-pyrrolidone exfoliated graphene as highly sensitive analytical platform for carbendazim. Sens. Actuators B Chem. 2018, 274, 551–559. [Google Scholar] [CrossRef]
- Pham, T.S.H.; Fu, L.; Mahon, P.; Lai, G.; Yu, A. Fabrication of β-Cyclodextrin-Functionalized Reduced Graphene Oxide and Its Application for Electrocatalytic Detection of Carbendazim. Electrocatalysis 2016, 7, 411–419. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, S.; Wang, H.; Chen, C.; Han, Z.; Chen, M.; Zhu, Y.; Cui, R.; Zhang, G. Three-dimensional nanoporous copper and reduced graphene oxide composites as enhanced sensing platform for electrochemical detection of carbendazim. J. Electroanal. Chem. 2019, 847, 113243. [Google Scholar] [CrossRef]
- Luo, S.; Wu, Y.; Gou, H. A voltammetric sensor based on GO–MWNTs hybrid nanomaterial-modified electrode for determination of carbendazim in soil and water samples. Ionics 2013, 19, 673–680. [Google Scholar] [CrossRef]
- Westmacott, K.; Weng, B.; Wallace, G.G.; Killard, A.J. 7—Nanostructured conducting polymers for electrochemical sensing and biosensing. In Nanosensors for Chemical and Biological Applications; Honeychurch, K.C., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 150–194. [Google Scholar]
- Tsakova, V.; Seeber, R. Conducting polymers in electrochemical sensing: Factors influencing the electroanalytical signal. Anal. Bioanal. Chem. 2016, 408, 7231–7241. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Li, Q.; Zhang, Y.; Hou, C.; Lei, Y. A highly efficient organophosphorus pesticides sensor based on CuO nanowires–SWCNTs hybrid nanocomposite. Sens. Actuators B Chem. 2014, 199, 410–417. [Google Scholar] [CrossRef]
- Liu, W.; Yin, X.-B. Metal–organic frameworks for electrochemical applications. TrAC Trends Anal. Chem. 2016, 75, 86–96. [Google Scholar] [CrossRef]
- Sherigara, B.S.; Kutner, W.; D’Souza, F. Electrocatalytic Properties and Sensor Applications of Fullerenes and Carbon Nanotubes. Electroanalysis 2003, 15, 753–772. [Google Scholar] [CrossRef]
- Švancara, I.; Vytřas, K.; Kalcher, K.; Walcarius, A.; Wang, J. Carbon Paste Electrodes in Facts, Numbers, and Notes: A Review on the Occasion of the 50-Years Jubilee of Carbon Paste in Electrochemistry and Electroanalysis. Electroanalysis 2009, 21, 7–28. [Google Scholar] [CrossRef]
- Adams, R.N. Carbon Paste Electrodes. Anal. Chem. 1958, 30, 1576. [Google Scholar] [CrossRef]
- Švancara, I.; Vytřas, K.; Barek, J.; Zima, J. Carbon Paste Electrodes in Modern Electroanalysis. Crit. Rev. Anal. Chem. 2001, 31, 311–345. [Google Scholar] [CrossRef]
- Švancara, I.; Walcarius, A.; Kalcher, K.; Vytřas, K. Carbon paste electrodes in the new millennium. Open Chem. 2009, 7, 598–656. [Google Scholar] [CrossRef]
- Oliveira, P.C.; Maximiano, E.M.; Oliveira, P.A.; Camargo, J.S.; Fiorucci, A.R.; Arruda, G.J. Direct electrochemical detection of glyphosate at carbon paste electrode and its determination in samples of milk, orange juice, and agricultural formulation. J. Environ. Sci. Health Part B 2018, 53, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Brycht, M.; Łukawska, A.; Frühbauerová, M.; Pravcová, K.; Metelka, R.; Skrzypek, S.; Sýs, M. Rapid monitoring of fungicide fenhexamid residues in selected berries and wine grapes by square-wave voltammetry at carbon-based electrodes. Food Chem. 2021, 338, 127975. [Google Scholar] [CrossRef] [PubMed]
- Arruda, G.J.; Lima, F.D.; Cardoso, C.A.L. Ultrasensitive determination of carbendazim in water and orange juice using a carbon paste electrode. J. Environ. Sci. Health Part B 2016, 51, 534–539. [Google Scholar] [CrossRef] [PubMed]
- de Lima, F.; Gozzi, F.; Fiorucci, A.R.; Cardoso, C.A.L.; Arruda, G.J.; Ferreira, V.S. Determination of linuron in water and vegetable samples using stripping voltammetry with a carbon paste electrode. Talanta 2011, 83, 1763–1768. [Google Scholar] [CrossRef]
- Zahirifar, F.; Rahimnejad, M.; Abdulkareem, R.A.; Najafpour, G. Determination of Diazinon in fruit samples using electrochemical sensor based on carbon nanotubes modified carbon paste electrode. Biocatal. Agric. Biotechnol. 2019, 20, 101245. [Google Scholar] [CrossRef]
- Mercan, H.; İnam, R.; Aboul-Enein, H.Y. Square Wave Adsorptive Stripping Voltammetric Determination of Cyromazine Insecticide with Multi-Walled Carbon Nanotube Paste Electrode. Anal. Lett. 2011, 44, 1392–1404. [Google Scholar] [CrossRef]
- Nurdin, M.; Maulidiyah, M.; Salim, L.O.A.; Muzakkar, M.Z.; Umar, A.A. High performance cypermethrin pesticide detection using anatase TiO2-carbon paste nanocomposites electrode. Microchem. J. 2019, 145, 756–761. [Google Scholar] [CrossRef]
- Parham, H.; Rahbar, N. Square wave voltammetric determination of methyl parathion using ZrO2-nanoparticles modified carbon paste electrode. J. Hazard. Mater. 2010, 177, 1077–1084. [Google Scholar] [CrossRef]
- Hunde, T.; Berhe, M.; Tadese, A.; Tirfu, M.; Woldu, A.; Menasbo, B.; Saini, R.C. Nano Fe3O4–graphite paste modified electrochemical sensor for phosphatic pesticide-chlorpyrifos. Momona Ethiop. J. Sci. 2017, 9, 76–89. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Cui, R.; Dang, Y.; Li, Y.; Zou, Y. Doping controlled oxygen vacancies of ZnWO4 as a novel and effective sensing platform for carbendazim and biomolecule. Sens. Actuators B Chem. 2019, 296, 126680. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Han, P.; Dang, Y.; Zhu, M.; Li, Q.; Fu, Y. A novel low-dimensional heteroatom doped Nd2O3 nanostructure for enhanced electrochemical sensing of carbendazim. New J. Chem. 2019, 43, 14009–14019. [Google Scholar] [CrossRef]
- Đorđević, J.S.; Maksimović, V.M.; Gadžurić, S.B.; Trtić-Petrović, T.M. Determination of Carbendazim by an Ionic Liquid-Modified Carbon Paste Electrode. Anal. Lett. 2017, 50, 1075–1090. [Google Scholar] [CrossRef]
- Khadem, M.; Faridbod, F.; Norouzi, P.; Rahimi Foroushani, A.; Ganjali, M.R.; Shahtaheri, S.J.; Yarahmadi, R. Modification of Carbon Paste Electrode Based on Molecularly Imprinted Polymer for Electrochemical Determination of Diazinon in Biological and Environmental Samples. Electroanalysis 2017, 29, 708–715. [Google Scholar] [CrossRef]
- Khadem, M.; Faridbod, F.; Norouzi, P.; Foroushani, A.R.; Ganjali, M.R.; Shahtaheri, S.J. Biomimetic electrochemical sensor based on molecularly imprinted polymer for dicloran pesticide determination in biological and environmental samples. J. Iran. Chem. Soc. 2016, 13, 2077–2084. [Google Scholar] [CrossRef]
- Wong, A.; Foguel, M.V.; Khan, S.; Oliveira, F.M.d.; Tarley, C.R.T.; Sotomayor, M.D.P.T. Development of an electrochemical sensor modified with MWCNT-COOH and MIP for detection of diuron. Electrochim. Acta 2015, 182, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, K.; Malode, S.J.; Shetti, N.P.; Kulkarni, R.M. Analysis of herbicide and its applications through a sensitive electrochemical technique based on MWCNTs/ZnO/CPE fabricated sensor. Chemosphere 2022, 287, 132086. [Google Scholar] [CrossRef] [PubMed]
- Malode, S.J.; Keerthi, P.K.; Shetti, N.P.; Kulkarni, R.M. Electroanalysis of Carbendazim using MWCNT/Ca-ZnO Modified Electrode. Electroanalysis 2020, 32, 1590–1599. [Google Scholar] [CrossRef]
- Demir, E.; Göktug, Ö.; İnam, R.; Doyduk, D. Development and characterization of iron (III) phthalocyanine modified carbon nanotube paste electrodes and application for determination of fluometuron herbicide as an electrochemical sensor. J. Electroanal. Chem. 2021, 895, 115389. [Google Scholar] [CrossRef]
- Nurdin, M.; Prabowo, O.A.; Arham, Z.; Wibowo, D.; Maulidiyah, M.; Saad, S.K.M.; Umar, A.A. Highly sensitive fipronil pesticide detection on ilmenite (FeO.TiO2)-carbon paste composite electrode. Surf. Interfaces 2019, 16, 108–113. [Google Scholar] [CrossRef]
- Nurdin, M.; Arham, Z.; Rahayu, S.; Salim, L.O.A.; Maulidiyah, M. Electroanalytical performance of graphene paste electrode modified Al(III)-TiO2 nanocomposites in fipronil solution. J. Rekayasa Kim. Dan Lingkung. (J. Chem. Eng. Environ.) 2020, 15, 71–78. [Google Scholar] [CrossRef]
- Amra, S.; Bataille, T.; Bourouina Bacha, S.; Bourouina, M.; Hauchard, D. Nanostructured Modified Carbon Paste Electrode as Voltrammetric Sensor for Isoproturon Trace Analysis in Water. Electroanalysis 2020, 32, 1346–1353. [Google Scholar] [CrossRef]
- Özcan, A.; Hamid, F.; Özcan, A.A. Synthesizing of a nanocomposite based on the formation of silver nanoparticles on fumed silica to develop an electrochemical sensor for carbendazim detection. Talanta 2021, 222, 121591. [Google Scholar] [CrossRef] [PubMed]
- Ilager, D.; Shetti, N.P.; Reddy, K.R.; Tuwar, S.M.; Aminabhavi, T.M. Nanostructured graphitic carbon nitride (g-C3N4)-CTAB modified electrode for the highly sensitive detection of amino-triazole and linuron herbicides. Environ. Res. 2022, 204, 111856. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, B.; Bigdeli, S.A.; Banan, K.; Afsharara, H.; Hatamabadi, D.; Mousavi, P.; Hussain, C.M.; Keçili, R.; Ghorbani-Bidkorbeh, F. Molecularly imprinted polymer-carbon paste electrode (MIP-CPE)-based sensors for the sensitive detection of organic and inorganic environmental pollutants: A review. Trends Environ. Anal. Chem. 2021, 32, e00144. [Google Scholar] [CrossRef]
- Mohamed, H.M. Screen-printed disposable electrodes: Pharmaceutical applications and recent developments. TrAC Trends Anal. Chem. 2016, 82, 1–11. [Google Scholar] [CrossRef]
- Domínguez Renedo, O.; Alonso-Lomillo, M.A.; Martínez, M.J.A. Recent developments in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202–219. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Della Pelle, F.; Angelini, C.; Sergi, M.; Del Carlo, M.; Pepe, A.; Compagnone, D. Nano carbon black-based screen printed sensor for carbofuran, isoprocarb, carbaryl and fenobucarb detection: Application to grain samples. Talanta 2018, 186, 389–396. [Google Scholar] [CrossRef]
- Khairy, M.; Ayoub, H.A.; Banks, C.E. Non-enzymatic electrochemical platform for parathion pesticide sensing based on nanometer-sized nickel oxide modified screen-printed electrodes. Food Chem. 2018, 255, 104–111. [Google Scholar] [CrossRef]
- Petroni, J.M.; Lucca, B.G.; Fogliato, D.K.; Ferreira, V.S. Sensitive Approach for Voltammetric Determination of Carbendazim Based on the Use of an Anionic Surfactant. Electroanalysis 2016, 28, 1362–1369. [Google Scholar] [CrossRef]
- Govindasamy, M.; Umamaheswari, R.; Chen, S.-M.; Mani, V.; Su, C. Graphene Oxide Nanoribbons Film Modified Screen-Printed Carbon Electrode for Real-Time Detection of Methyl Parathion in Food Samples. J. Electrochem. Soc. 2017, 164, B403–B408. [Google Scholar] [CrossRef]
- Topsoy, O.K.; Muhammad, F.; Kolak, S.; Ulu, A.; Güngör, Ö.; Şimşek, M.; Köytepe, S.; Ateş, B. Fabrication of electrospun polycaprolactone/chitosan nanofiber-modified screen-printed electrode for highly sensitive detection of diazinon in food analysis. Measurement 2022, 187, 110250. [Google Scholar] [CrossRef]
- Gopi, P.K.; Ngo, D.B.; Chen, S.-M.; Ravikumar, C.H.; Surareungchai, W. High-performance electrochemical sensing of hazardous pesticide Paraoxon using BiVO4 nano dendrites equipped catalytic strips. Chemosphere 2021, 288, 132511. [Google Scholar] [CrossRef]
- Noyrod, P.; Chailapakul, O.; Wonsawat, W.; Chuanuwatanakul, S. The simultaneous determination of isoproturon and carbendazim pesticides by single drop analysis using a graphene-based electrochemical sensor. J. Electroanal. Chem. 2014, 719, 54–59. [Google Scholar] [CrossRef]
- Govindasamy, M.; Mani, V.; Chen, S.-M.; Chen, T.-W.; Sundramoorthy, A.K. Methyl parathion detection in vegetables and fruits using silver@graphene nanoribbons nanocomposite modified screen printed electrode. Sci. Rep. 2017, 7, 46471. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, S.; Veerakumar, P.; Chen, S.-M.; Lin, K.-C. Three-dimensional zinc oxide nanostars anchored on graphene oxide for voltammetric determination of methyl parathion. Microchim. Acta 2019, 187, 17. [Google Scholar] [CrossRef]
- Rajaji, U.; Murugan, K.; Chen, S.-M.; Govindasamy, M.; Chen, T.-W.; Lin, P.H.; Lakshmi Prabha, P. Graphene oxide encapsulated 3D porous chalcopyrite (CuFeS2) nanocomposite as an emerging electrocatalyst for agro-hazardous (methyl paraoxon) detection in vegetables. Compos. Part B Eng. 2019, 160, 268–276. [Google Scholar] [CrossRef]
- Govindasamy, M.; Rajaji, U.; Chen, S.-M.; Kumaravel, S.; Chen, T.-W.; Al-Hemaid, F.M.A.; Ali, M.A.; Elshikh, M.S. Detection of Pesticide Residues (Fenitrothion) in Fruit Samples Based On Niobium Carbide@Molybdenum Nanocomposite: An Electrocatalytic Approach. Anal. Chim. Acta 2018, 1030, 52–60. [Google Scholar] [CrossRef]
- Akkarachanchainon, N.; Rattanawaleedirojn, P.; Chailapakul, O.; Rodthongkum, N. Hydrophilic graphene surface prepared by electrochemically reduced micellar graphene oxide as a platform for electrochemical sensor. Talanta 2017, 165, 692–701. [Google Scholar] [CrossRef]
- Sundaresan, P.; Fu, C.-C.; Liu, S.-H.; Juang, R.-S. Facile synthesis of chitosan-carbon nanofiber composite supported copper nanoparticles for electrochemical sensing of carbendazim. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126934. [Google Scholar] [CrossRef]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Reduced graphene oxide nanosheets modified with nickel disulfide and curcumin nanoparticles for non-enzymatic electrochemical sensing of methyl parathion and 4-nitrophenol. Microchim. Acta 2019, 186, 704. [Google Scholar] [CrossRef] [PubMed]
- Annu; Sharma, S.; Jain, R.; Raja, A.N. Review—Pencil Graphite Electrode: An Emerging Sensing Material. J. Electrochem. Soc. 2020, 167, 037501. [Google Scholar] [CrossRef]
- Kariuki, J.K. An Electrochemical and Spectroscopic Characterization of Pencil Graphite Electrodes. J. Electrochem. Soc. 2012, 159, H747–H751. [Google Scholar] [CrossRef]
- David, I.G.; Popa, D.-E.; Buleandra, M. Pencil Graphite Electrodes: A Versatile Tool in Electroanalysis. J. Anal. Methods Chem. 2017, 2017, 1905968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolat, G.; Abaci, S. Non-Enzymatic Electrochemical Sensing of Malathion Pesticide in Tomato and Apple Samples Based on Gold Nanoparticles-Chitosan-Ionic Liquid Hybrid Nanocomposite. Sensors 2018, 18, 773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholivand, M.-B.; Akbari, A.; Norouzi, L. Development of a novel hollow fiber- pencil graphite modified electrochemical sensor for the ultra-trace analysis of glyphosate. Sens. Actuators B Chem. 2018, 272, 415–424. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; da Fonseca Alves, R.; Vicentini, F.C.; Fatibello-Filho, O.; Del Pilar Taboada Sotomayor, M. Simultaneous determination of direct yellow 50, tryptophan, carbendazim, and caffeine in environmental and biological fluid samples using graphite pencil electrode modified with palladium nanoparticles. Talanta 2021, 222, 121539. [Google Scholar] [CrossRef]
- Anandhakumar, S.; Dhanalakshmi, K.; Mathiyarasu, J. Non-enzymatic organophosphorus pesticide detection using gold atomic cluster modified electrode. Electrochem. Commun. 2014, 38, 15–18. [Google Scholar] [CrossRef]
- Gai, K.; Qi, H.; Zhu, X.; Wang, M. Preparation of Ag-Fe3O4 nanoparticles sensor and application in detection of methomyl. E3S Web Conf. 2019, 118, 01002. [Google Scholar] [CrossRef] [Green Version]
- Taşaltın, N.; Karakuş, S.; Taşaltın, C.; Baytemir, G. Highly sensitive and selective rGO based Non-Enzymatic electrochemical sensor for propamocarb fungicide pesticide detection. Food Chem. 2022, 372, 131267. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, G.M.; Simões, F.R. Self-assembled films based on polypyrrole and carbon nanotubes composites for the determination of Diuron pesticide. J. Solid State Electrochem. 2018, 22, 1439–1448. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Shen, C.; Wang, C.; Hu, X.; Wang, G. An electrochemical sensor on the hierarchically porous Cu-BTC MOF platform for glyphosate determination. Sens. Actuators B Chem. 2019, 283, 487–494. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, W.; Liu, T.; Zhang, L. Facile fabrication of chitosan–calcium carbonate nanowall arrays and their use as a sensitive non-enzymatic organophosphate pesticide sensor. Nanoscale 2011, 3, 3123–3131. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Abroa-Nemeir, I.; Ben Halima, H.; Gallardo-Gonzalez, J.; El Alami El Hassani, N.; Alcacer, A.; Bausells, J.; Jaffrezic-Renault, N.; et al. Electrochemical impedance spectroscopy determination of glyphosate using a molecularly imprinted chitosan. Sens. Actuators B Chem. 2020, 309, 127753. [Google Scholar] [CrossRef]
- Sakdarat, P.; Chongsuebsirikul, J.; Thanachayanont, C.; Prichanont, S.; Pungetmongkol, P. Development of a Nonenzymatic Electrochemical Sensor for Organophosphate Pesticide Detection Using Copper (II) Oxide Nanorod Electrodes. J. Nanomater. 2021, 2021, 6623668. [Google Scholar] [CrossRef]
- França, R.F.; de Oliveira, H.P.M.; Pedrosa, V.A.; Codognoto, L. Electroanalytical determination of carbendazim and fenamiphos in natural waters using a diamond electrode. Diam. Relat. Mater. 2012, 27–28, 54–59. [Google Scholar] [CrossRef]
- Pop, A.; Manea, F.; Flueras, A.; Schoonman, J. Simultaneous Voltammetric Detection of Carbaryl and Paraquat Pesticides on Graphene-Modified Boron-Doped Diamond Electrode. Sensors 2017, 17, 2033. [Google Scholar] [CrossRef] [Green Version]
- Bakytkarim, Y.; Tursynbolat, S.; Zeng, Q.; Huang, J.; Wang, L. Nanomaterial ink for on-site painted sensor on studies of the electrochemical detection of organophosphorus pesticide residuals of supermarket vegetables. J. Electroanal. Chem. 2019, 841, 45–50. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Shimizu, F.M.; Machado, S.A.S.; Oliveira, O.N. Selective and sensitive multiplexed detection of pesticides in food samples using wearable, flexible glove-embedded non-enzymatic sensors. Chem. Eng. J. 2021, 408, 127279. [Google Scholar] [CrossRef]
- Oliveira, A.C.M.; Araújo, D.A.G.; Pradela-Filho, L.A.; Takeuchi, R.M.; Santos, A.L. A robust and versatile micropipette tip-based miniaturized electrochemical cell for determination of carbendazim. Sens. Actuators B Chem. 2021, 327, 128880. [Google Scholar] [CrossRef]

| Analyte | Modification | Supporting Electrolyte, pH | Detection Technique | LOD | LOQ | Linear Concentration Range | Sensitivity | Repeatability: RSD at Certain Concentration (%) | Special Observation (Real Sample Analysis, Interferences, …) | Recovery at Certain Concentration (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As Reported | Calculated (µM) | As Reported | Calculated (µM) | ||||||||||
| Glassy Carbon Electrode (GCE) | |||||||||||||
| Single Nanomaterial | |||||||||||||
| CBM | MWCNT | 0.1 M H2SO4, pH 1.0 | DPSV | 0.01 µg L−1 | 5.23·10−5 | NR | / | 0.01–5·104 µg L−1 | 0.8326 µA µg L−1 | 2.3 (NR) | Real samples: soil, water, interferences: Cl−, Br−, SO42−, NO3−, phenol, o-Chloro phenol, endosulfan, MP, malathion | 82.10–93.73 (10–300 µg L−1) | [44] |
| MP | Gd2O3 hollow nanospheres | 0.05 M phosphate buffer, pH 7 | DPV | 0.03 µM | 3.00·10−2 | NR | / | 0.05–100 µM | 0.1834 µA µM−1 | NR | Real samples: cabbage, tap water, paddy field water, interferences: ascorbic acid, hydroquinone, glucose, M-nitrophenol, Imidacloprid, Pyrazosulfuron, 4-nitrobenzaldehyde, nitrobenzene, PO43−, SO42−, NO3−, Fe2+, Ni2+, K+ | 95.5–106 (1–5 µM) | [45] |
| MP | Nanoporous Au | 100 mM HAC-NaAC solution, pH 4.0 | DPV | 0.02 µM | 2.00·10−2 | NR | / | 0.5–150 µM | 186.53 µA mM−1 cm−2 | NR | NR | NR | [46] |
| CBM | 0.24 μM | 24.00·10−2 | NR | / | 3.0–120 µM | 484.51 µA mM−1 cm−2 | NR | NR | NR | [46] | |||
| MP and CBM simultaneously | 0.085 μM (MP) 0.27 μM (CBM) | 8.50·10−2 (MP) 27.00·10−2 (CBM) | NR | / | 3–25 μM (MP) 10–70 μM (CBM) | 629.68 µA mM−1 cm−2 (MP) 20.53 µA mM−1 cm−2 (CBM) | <2.6 (20 µM MP, 20 µM CBM) | Real sample: wastewater and seawater, interferences: Mg2+, K+, Na+, NH4+, SO42−, PO43−, CO32−, NO3−, thiabendazole, methomyl, chlorpyrifos, tebuconazole, benomyl | 94.93–104.73 (3.0–25.0 μM MP) 94.92–103.48 (10.0–70.0 μM CBM) | [46] | |||
| Binary Nanocomposites | |||||||||||||
| MP | MoS2-graphene NS | 0.1 M phosphate buffer, pH 7 | amperometry | 3.23 µM | 3.23 | NR | / | 10 nM–1.9 mM | 0.457 µA µM−1 cm−2 | 3.9 (200 µM) | Real samples: apple, kiwi, tomato, cabbage, interferences: Cl−, I−, Zn2+, NO32−, Cu2+, Ba2+, Ca2+, dopamine, uric acid, ascorbic acid, glucose, diuron, fenuron, SO42−, NO32−, nitrobenzene, 4-nitrophenol, 2-aminophenol, 4-aminophenol, 4-nitroaniline, 4-acetamidophenol and chloramphenicol | NR | [47] |
| Malathion | CuO NP-3D graphene | 0.1 M Na2HPO4-citrate buffer, pH 5 | DPV | 0.01 nM | 1.00·10−5 | NR | / | 0.03–1.5 nM | 31.96%/nM | 3.25 (at 1 nM) | Real sample: lake water, interferences: Na+, K+, Ca2+, Mg2+, Zn2+, Cl−, NO3−, PO43−, SO42−, glucose, carbentazim, lindane, trichlorphon | 95.4–102.4 (at 0.3–1.5 nM) | [48] |
| Paraoxon ethyl | Graphene-NiFeSP | 0.1 M phosphate buffer, pH 7 | SWV | 3.7 nmol L−1 | 3.70·10−3 | NR | / | 0.01–1.00 µM and 1.00–10.00 µM | 10.243 µA L µmol−1 and 2.6267 µA L µmol−1 | 5.2 (at 8.0 μmol L−1) | Real samples: tap water, tomato juice, cucumber juice, interferences: PO43−, SO42−, NO3−, 4-nitrophenol, carbaryl, fenamiphos, MP | 98–102.3 (at 150–1000 nmol L−1) | [49] |
| Diazinon | CNTs-TiO2 | 0.05 M phosphate buffer, pH 7 | SWV | 3 nM | 3.00·10−3 | 10 nM | 10.00·10−3 | 11–8360 nM | 1.1753 µA µM−1 | 3.8 (NR) | Real samples: agricultural well water, city piped water | 97.5–105.5 (at 1.0–2.0 µM) | [50] |
| Profenofos | 3D CNTs-MIP | 0.1 M phosphate buffer, pH 7 | amperometry | 0.002 μM | 2.00·10−3 | 0.007 μM | 0.007 | 0.01–200 μM | 0.573 µA µM−1 | 4.8 (at 0.5 μM) | Real samples: Spring onion, tomato, Chinese cabbage, cabbage, green pepper, chili pepper, interferences: chlorpyrifos, carbofuran, hydroquinone, caffeine, phenol, MgSO4, NaCl | 100.1–105.4 (at 0.05–0.1 μM) | [51] |
| Dicapthon | SWCNTs-Nafion | 0.01 M B-R buffer, pH 5.0 | DPV | 0.036 µg L−1 | 1.21·10−4 | 0.054 µg L−1 | 1.81·10−4 | 0.2–60 µg mL−1 | 0.8535 µA cm−2 µg−1 mL | 3.2 (NR) | Real samples: tap and well water, rice, corn, interferences: Pb2+, Cd2+, Mn2+, Cu2+, Co2+, Fe2+, Zn2+, Ca2+, Mg2+, ascorbic acid, dopamine | 98.00–99.50 (at 10–40 µg mL−1) | [52] |
| Nitenpyram | HMWCNT-CNH | 0.1 M phosphate buffer, pH 11 | DPV | 4.0 nM | 4.00·10−3 | NR | / | 20–2000 nM | 0.0158 µA nM−1 | 5.19 (at 1000 nM) | Real samples: corn, river water, interferences: ascorbic acid, fipronil, glucose, vitamin A | 93.41–109.73 (at 20–200 nM) | [53] |
| CBM | CMC-MWCNT | 0.1 M phosphate buffer, pH 7.0 | DPV | 0.015 µM | 15.00·10−3 | NR | / | 0.03–10 µM | 6.588 µA µM−1 | 1.68 (NR) | Real samples: peer and kiwifruit, interferences: Na+, Cl−, K+, NO−3, fructose, sucrose | 97.67–100.5 (at 1.000–4.000 μM) | [54] |
| CBM | MoS2 QD-MWCNTs | 0.1 M phosphate buffer, pH 7.0 | SWV | 0.026 µM | 26.00·10−3 | NR | / | 0.04–1.00 µM | 12.0171 µA µM−1 | NR | Real samples: platycodon grandiflorum, pears, interferences: MgCl2, CaCl2, KCl, Pb(NO3)2, ascorbic acid, carotene | 97.31−105.57 (at 0.3–1.0 µM) | [55] |
| CBM | SiO2-MWCNT | 0.1 M phosphate buffer, pH 8.0 | SWV | 0.056 µM | 56.00·10−3 | 0.187 µM | 0.187 | 0.2–4.0 μM | 0.485 A mol L−1 | 1.4 (at 2.0 μM) | Real samples: commercial orange juice, interferences: methomyl, carbaryl, ascorbic acid, citric acid | 94.6–104 (at 0.5–5.0 μM) | [56] |
| CBM | Nd2Mo3O9-MWCNTs | 0.1 M phosphoric acid buffer, pH 7.0 | DPV | 0.0167 nM | 1.67·10−5 | NR | / | 5.0·10−5–9.0 µM | 6.227 µA µmol L−1 | NR | Real sample: water, interferences: Na+, K+, NH4+, Cu2+, Cd2+, Al3+, Cl−, CO32−, SO42+, PO43−, MP, fenitrothion, malathion, dichlorophenol, benomyl, thiabendazole, thiophanate, thiophanate-methyl, fuberidazole, glucose, ascorbic acid, vitamin B, C, E, dopamine, serine | 96.7–102.0 (at 0.006–8.00 µM) | [57] |
| MP | Acetylene black-chitosan | Mcllvaine buffer, pH 5.6 | DPV | 2·10−9 mol L−1 | 2.00·10−3 | NR | / | 2·10−8–1·10−4 M | 0.2528 µA L/µmol | 1.49 (at 1·10−5 M) | Real sample: cabbage, interferences: Na+, K+, Ca2+, Mg2+, Cu2+, Cl−, NO3−, PO43−, SO42−, CO32−, amino acid, glucose, sucrose, malathion, ascorbic acid, uric acid, p-aminophenol, o-, m- and p-phenylenediamine, nitrobenzene | 95.4–105.1 (at 0.8–2.0 µM) | [58] |
| Malathion | CuO-CeO2 | 0.1 M phosphate buffer, pH 5.0 | DPV | 3.3 fM | 3.00·10−9 | NR | / | 10 fM–100 nM | 2.07 μA nM−1 cm−2 | 3.9 (at 1 nM) | Real samples: lake water, garlic, apple, interferences: chlorpyrifos, parathion, paraoxon, malaoxon, carberidazim, thiabendazole, cysteine, glutathione, mercaptoethanol, glucose, nitrobenzene, nitrophenol, Na+, K+, Fe2+, Fe3+, Al3+, Cl−, NO3−, SO42−, PO43− | 96.2– 103.5 (at 0.02–1.8 nM) | [59] |
| Carbaryl | GO-[Bmim]PF6 | B-R buffer, (pH 5.0)-methanol-water | SWV | 0.02 µM | 0.02 | NR | / | 0.10–12 µM | 1.1 µA µM−1 | 3.2 (at 2 µM) | Real sample: grape, tomato, interferences: K2SO4, MgCl2, Ca(NO3)2, hydroquinone, guanine, phenol, catechol, glucose, ascorbic acid | 90.0–96.7 (at 0.5–1.5 µM) | [60] |
| Chlorpyrifos | TiO2-cellulose acetate | 0.05 M tetra-n-butyl ammoniumbromide in methanol/water | CV | 4.4 µM | 4.40 | 14.7 µM | 14.7 | 10–30 µM | NR | 2.54 (at 50 µM) | Real samples: tap water, commercial sample, soil, interferences: other pesticides: MP, fenitrothion, chlorophenol, chloroaniline, chlorobenzene, Ca2+, Mg2+, Na+, NH4+, K+ | 91.84 (at 100 µM) | [61] |
| DPV | 3.5 µM | 3.50 | 11.7 µM | 11.7 | 20–110 µM | NR | NR | Real samples: tap water, commercial sample, soil | 96.28 (at 100 µM) | ||||
| amperometry | 11.8 µM | 11.80 | 39.2 µM | 39.2 | 20–340 µM | NR | NR | Real samples: tap water, commercial sample, soil | 96.46 (at 100 µM) | ||||
| Clomazone | Pt NPs-MWCNTs | 0.1 M phosphate buffer, pH 7.0 | DPASV | 0.38 ng cm−3 | 1.59·10−3 | 0.61 ng cm−3 | 2.54·10−3 | 0.61–20.56 ng cm−3 | 1.09 nA ng−1 mL | NR | Interferences: Ca2+, Na+, Ag+, K+, Cl−, HCO3−, CO32−, NO3−, linuron, imidacloprid, tebufenozide | NR | [62] |
| Glyphosate | MWCNT-CuPc | 0.1 M phosphate buffer, pH 7.4 | DPV | 12.2 nmol L−1 | 12.2·10−3 | NR | / | 0.83–9.90 µM | 6.14 µA cm−2 µM−1 | NR | NR | NR | [63] |
| Dichlorodiphenyltrichloroethane | PDA-Fe3O4-MIP NPs | 5.5 mM [Fe(CN)6]3− 0.1 M KCl | EIS | 6·10−12 M | 6.00·10−6 | NR | / | 1·10−11–1·10−3 M | 19.33 Ω pmol−1 L | 3.28 (at 1·10−3 M) | Real sample: radish juice, interferences: tetrabromobisphenol A, 3,4-dihydroxybenzoic acid, hydroquinone solution, p-methoxychlor | 89–102 (at 0.01–100 µM) | [64] |
| Paraoxon | Stearic acid-nanosilver | Phosphate buffer, pH 7 | DPV | 0.1 nM | 0.10·10−3 | NR | / | 0.1–5 nM | NR | 2.7 (at 50 µM) | Real samples: onion, paddy grains, interferences: Na+, Ca2+, Mg2+, Fe2+, NH4+, K+, lindane, chlorpyrifos, imidacloprid, fenitrothion, thiamethoxam, monocrotophos, malathion | 100.00 (at 0.2–0.5 nM) | [65] |
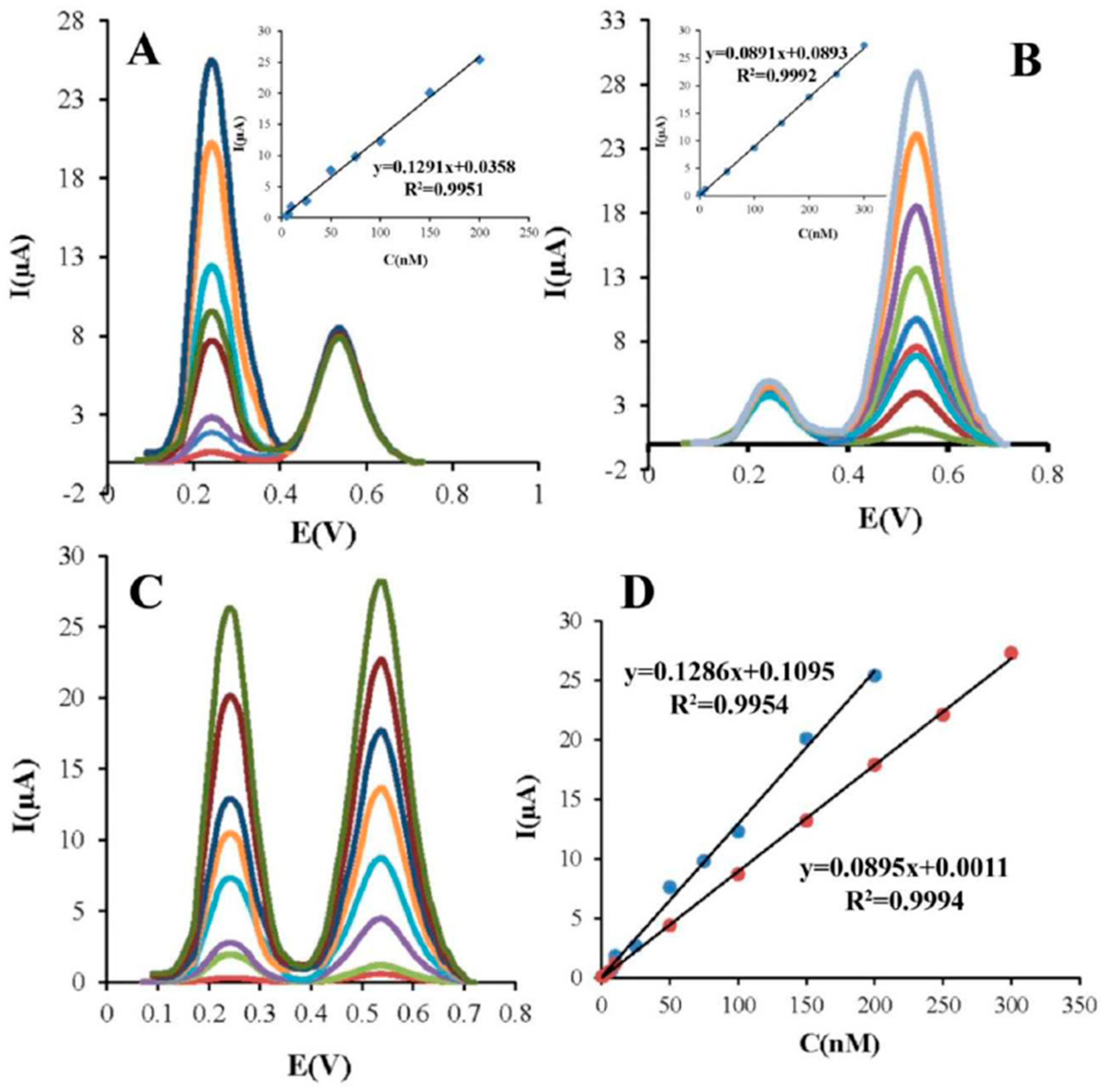
| Carbofuran (CBF) and carbaryl (CBR) simultaneously | 35MIL(Fe)-101-rGO | 0.1 M B-R buffer/ Acetonitrile, pH 4.0 | DPV | 0.52 nM (CBF) 0.11 nM (CBR) | 0.52·10−3 (CBF)0.11·10−3 (CBR) | NR | / | 5.0–200.0 nM (CBF) 1.0–300.0 nM (CBR) | 0.1286 µA nM−1 (CBF) 0.0895 µA nM−1 (CBR) | 2.9 CBF 3.2 CBR (at 100 nM CBF and CBR) | Real samples: cucumber, tomatoes, oranges, cabbages, interferences: Co2+, Ni2+, Cu2+, Cd2+, K+, Ca2+, Mg2+, Fe3+, Al3+, Ni2+, Zn2+, Cu2+, F−, Cl−, Br−, SO42−, PO43−, NO3−, CO32−, diazinon, malathion, paraoxon, parathion, fenamiphos | 98.0–104.7 (at 100–160 nM) | [66] |
| Ternary Nanocomposites | |||||||||||||
| Imidacloprid | ZnO-PANI-GO | 0.1 M phosphate buffer, pH 5.8 | CV | 1.3·10−8 M | 1.30·10−2 | 1.3·10−7 M | 0.13 | 1.25·10−7–2.12·10−6 M | 1.5604 A M−1 | NR | Real samples: chilli, tomato, potato | 98.23–104.37 (at 1.00·10−6–1.75·10−6 M) | [67] |
| MP | MnO2-PTH-rGO | 0.1 M phosphate buffer, pH 7.0 | amperometry | 5.72 nM | 5.72·10−3 | NR | / | 10 nM–1 µM | 0.0498 µA µM−1 cm−2 | NR | Real samples: human urine and serum | 88.5–97.2 (at 0.5–10 µM) | [68] |
| MP | Au-ZrO2-GNS | 0.1 M phosphate buffer, pH 5.6 | SWV | 1 ng mL−1 | 3.80·10−3 | NR | / | 1–100 ng mL−1 and 100–2400 ng mL−1 | 0.00351 µA ng−1 mL and 0.01136 µA ng−1 mL | NR | Real sample: chinese cabbage, interferences: p-nitrophenol, p-nitroaniline, trinitrotoluene, NO3−, PO43−, SO42− | 96.2–102.1 (at 300–1500 ng mL−1) | [69] |
| MP | Au NP-chitosan-GNS | 0.1 M phosphate buffer, pH 5.7 | SWASV | 0.6 ng mL−1 | 2.28·10−3 | NR | / | 0.001–0.1 and 0.2–1.0 µg mL−1 | 256.3 µA µg−1 mL and 11.7 µA µg−1 mL | 5.6 (at 0.1 µg mL−1) | Real samples: garlic, cabbage, tea, interferences: as p-nitrophenol, nitrobenzene, p-nitroaniline, trinitrotoluene, PO43−, SO42−, NO3− | 96.2–105 (at 5.86–6.17 ng mL−1) | [70] |
| MP | Pd-MWCNTs-Nafion | 0.1 M phosphate buffer, pH 7.0 | DPV | 0.05 μg mL−1 | 19.00·10−2 | NR | / | 0.10–14 μg mL−1 | 18.30 µA μg−1 mL | 4.6 (at 2.0 μg mL−1) | Interferences: Cl−, PO43−, SO42− and NO3− | NR | [71] |
| Fenitrothion | SiO2-MWCNTs-RuPc | 0.1 M acetate buffer, pH 4.5 | DPV | 1.62 µM | 1.62 | NR | / | 3·10−6–6·10−5 M | 0.0822 µA µmol−1 L | 2.3 (at 16.6 µmol L−1) | Real sample: fresh orange juice, interferences: malathion, chlorpyrifos, ascorbic acid | 91.6–98.8 (at 6.10–24.98 µmol L−1) | [72] |
| CBM | CMC-MWCNTs-MoS2 | 0.1 M phosphate buffer, pH 7.0 | DPV | 7.4 nM | 74.00 10−2 | NR | / | 0.04–9 µM | NR | 0.57 (NR) | Real samples: tea, rice, interferences: vitamin C, vitamin B2, imidacloprid, glyphosate, endosulfan, buprofezin, fructose, sucrose, L-arginine, L-serine | 89.18–105.56 (0.45–4.2 µM) | [73] |
| CBM | Fullerene-MWCNTs-Nafion | 0.1 M ammoniacal buffer, pH 9 | SWV | 1.7·10−8 M | 1.70·10−2 | 5.57·10−8 | 5.57·10−2 | 2·10−8–3.5·10−7 M | 419.69 A mol−1 L | 3.12 (at 5·10−7 M) | Real sample: soil, interferences: K+, Na+, Ca2+, Mg2+, Fe3+ | 37.8–38.4 (at 5·10−5 M) | [74] |
| CBM | IL-CaFe2O4-MWCNTs | 0.2 M phosphate buffer, pH 4.0 | DPV | 9.41 nM | 9.41·10−3 | NR | / | 3.14·10−8–1.05·10−5 M and1.05·10−5–1.05·10−4 M | 2.009 µA µmol−1 L and 0.297 µA µmol−1 L | 3.5 (at 5.23·10−5 M) | Real samples: paddy water, apple, tomato, interferences: K+, Na+, Mg2+, Zn2+, Ni2+, PO43−, Cl−, NO3 −, CO32−, HCO32−, SO42−, thiabendazole, tricyclazole, pyrimethanil, paranitrophenol | 94.7–105.5 (at 4.18·10−6–7.23·10−5 M) | [75] |
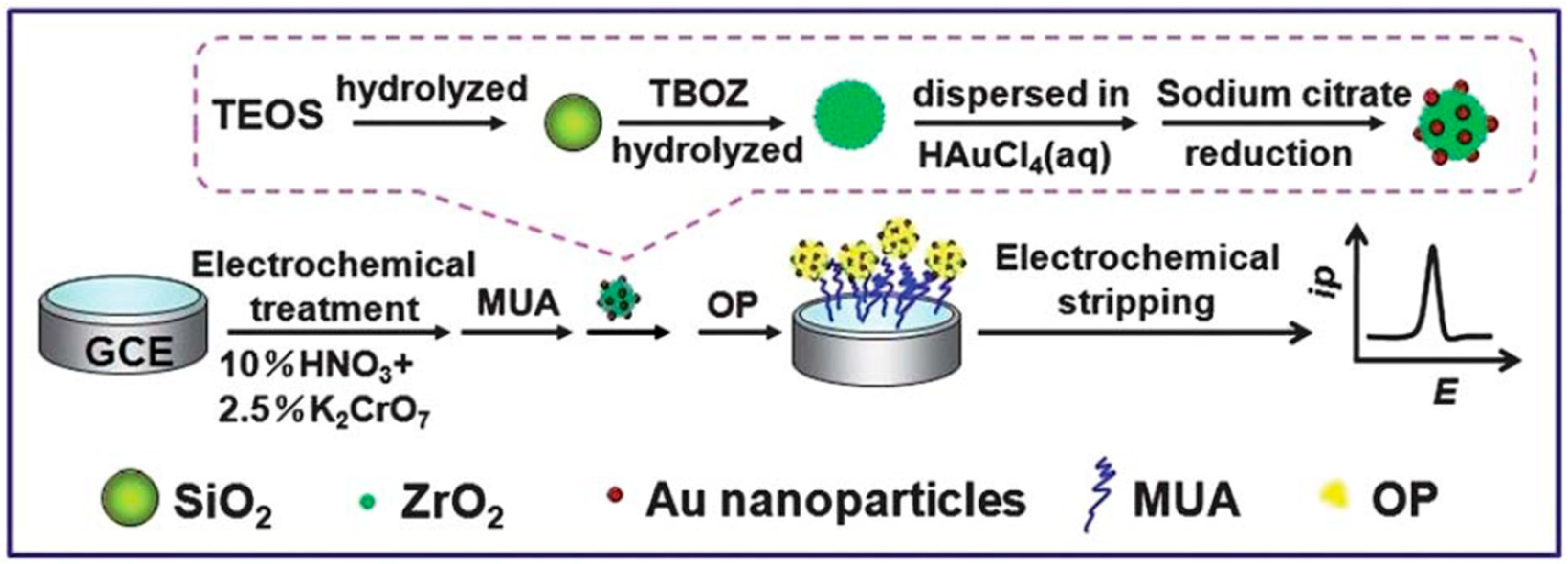
| Paraoxon ethyl | Au-ZrO2-SiO2 | 0.2 M acetate buffer, pH 5.2 | SWV | 0.5 ng mL 1 | 1.82·10−3 | NR | / | 1.0–500 ng mL−1 | NR | NR | Interferences: nitrobenzene, nitrophenol, PO43−, SO42−, NO3− | NR | [76] |
| MP | CuO-TiO2-Nafion | 0.1 M phosphate buffer, pH 6 | DPV | 1.21 ppb | 4.60·10−3 | NR | / | 10–500 ppb | 0.0412% ppb−1 | 2.9 (at 10 ppb) | Real sample: ground water, interferences: trichlorphon, caeberidazim, carbaryl, 4-nitrobenzaldehyde, nitrobenzene, PO43−, SO42−, NO3−, Fe2+, Ni2+, K+ | 98.80–106.20 (at 40–200 ppb) | [77] |
| Methomyl | Ag-Fe3O4-chitosan | 0.2 M phosphate buffer, pH 6.9 | CV | 2.97·10−5 M | 29.70 | NR | / | 3.47·10−5–3.47·10−4 M | 0.009166 A mol−1 L | NR | Real sample: lettuce, rape, spinach, interferences: | 93.08–96.45 (at 0.0121–0.0325 mg·kg−1) | [78] |
| CBM and thiabendazole (TBZ) simultaneously | ZnFe2O4-SWCNTs-Nafion | 0.2 M phosphate-buffered saline, pH 7.0 + 10.0 µg/mL CTAB | DPV | 0.09 µM (CBM) 0.05 µM (TBZ) | 9.00·10−2 (CBM) 5.00·10−2 (TBZ) | NR | / | 1.0–100.0 µM (CBM) 1.0–100.0 µM (TBZ) | 1.039 µA µmol L−1 (CBM) 0.798 µA µmol L−1 (TBZ) | NR | Real samples: apple, leek, tomato, paddy water, sea water, interferences: Na+, K+, NH4+, Cl−, NO3−, H2PO4−, HCO3−, CO32−, SO42−, Mg2+, Pb2+, Cu2+, Zn2+, Cd2+, ascorbic acid, catechin, anthocyanin, triadimenol, tricyclazole, paranitrophenol, Pyrimethanil | 88.2–104.4 (at 5.0–50.0 µM) | [79] |
| Carbofuran (CBF) and carbaryl (CBR) simultaneously | CoO-rGO-Nafion | 0.1 M B-R buffer/acetonitrile, pH 4 | DPV | 4.2 μg/L (CBF) 7.5 μg/L | 1.90·10−2 (CBF) 3.73·10−2 (CBR) | NR | / | 0.2–70 µM (CBF) 0.5–200 µM (CBR) | 0.07045 µA cm2/µM (CBF) 0.01952 µA cm2/µM (CBR) | 2.9 (at 30 µM CBF, 70 µM CBR) | Real samples: grapes, oranges, tomato, cabbages, interferences: Na+, K+, Mg2+, Ca2+, Zn2+, Al3+, F−, Cl−, CO32−, SO42−, NO−, isoprocarb, methiocarb, propoxur, hydroquinone, xanthine, guanine, phenol, catechol, caffeine. | 96.0–104.0 (at 0.50–1.00 µM CBF) 96.6–102.6 (at 5.00–10.00 µM CBR) | [80] |
| Paraoxon and chlorpyrifossimultaneously | TiO2-GO-UiO-66 | 0.1 M B-R buffer/ acetonitrile, pH 5 | SWV | 0.22 nM (paraoxon) 1.20 nM (chlorp.) | 2.20·10−4 (paraoxon) 1.20·10−3 (chlorp.) | NR | / | 1.0–100.0 nM (paraoxon) 5.0–300.0 nM (chlorpyrifos) | 0.3393 µA nM−1 (paraoxon) 0.091 µA nM−1 (chlorpyrifos) | 2.6 (at 50 nM) (paraoxon) 2.2 (at 50 nM) (chlorpyrifos) | Real samples: tap water, celery, lettuce, cabbage, interferences:Cl−, SO42−, CO32−, NO3−, PO43−, Cu2+, Zn2+, Pb2+, Fe2+, Cd2+ | 97.0–106.4 (at 50–70 nM) | [81] |
| Multiple Nanocomposites | |||||||||||||
| Carbofuran | MAA-EGMRA-ABIN-rGO-Au NP | 0.1 M KCl, pH 7.0 | DPV | 0.02 µM | 2.00·10−2 | NR | / | 5·10−8–2·10−5 M | 0.04917 µA µmol−1 L | 1.1 (at 1.0·10−7 M) | Real samples: cabbage, cucumber, inferferences: carbaryl, metolcarb, 3,5-xylyl methylcarbamate | 97.7–110.6 (at 1–20·10−6 M) | [82] |
| Trichlorfon | MWCNT-TiO2-CMCh-Nafion | 0.2 M phosphate buffer, pH 7.0 | DPV | 4·10−7 M | 40.00·10−2 | NR | / | 1·10−11–1·10−5 M | 0.5077 µA µM−1 | 1.57 (at 1·10−6 M) | Real samples: apple, mushroom, cucumber | 72.0–98.0 (at 0.5–4.0·10−10 M) | [83] |
| Analyte | Modifier | Supporting Electrolyte, pH | Detection Technique | LOD | LOQ | Linear Concentration Range | Sensitivity | Repeatability: RSD at Certain Concentration (%) | Special Observation (Real Sample Analysis, Interferences, …) | Recovery at Certain Concentration (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As Reported | Calculated (µM) | As Reported | Calculated (µM) | ||||||||||
| Carbon Paste Electrodes (CPEs) | |||||||||||||
| Glyphosate | None | 0.2 M B-R buffer, pH 5.0 | SWV | 2.0 nM | 2.0·10−3 | 7.0 nM | 7.00·10−3 | 4.40·10−8–2.80·10−6 M | 27.14 μA µM−1 | NR | Real samples: orange juice, milk and agricultural formulations, interferences: Na+, NH4+, Ca2+, Mg2+, Al3+, Cu2+, Cl−, OH−, NO3−, SO42−, atrazine, linuron, thiamethoxam, trifluralin, dichlorophenoxyacetic acid, trifloxystrobin, ascorbic acid | 98.31–103.75 (at 21.10–84.40 nM) | [100] |
| Fenhexamid | None | 0.1 M B-R buffer, pH 4, 10 vol.% MeOH | SWV | 0.97 µM | 0.97 | NR | / | 3.22–44.60 µM | 0.120 μA µM−1 | NR | Real samples: blueberries, strawberries, red wine grapes, white wine grapes | 92.9–99.8 (at 5–50 µM) | [101] |
| CBM | None | 0.1 M C6H8O7-Na2HPO4 buffer, pH 5.0 | DPV | 0.96 µg L−1 | 5.02·10−3 | NR | / | 2.84–45.44 µg L−1 | 0.101 μA L µg−1 | 1.05 (at 2.84 µg L−1) | Real sample: water, orange juice, interferences: orange juice, CuSO4, glyphosate, thiamethoxam, endosulfan | 99.12–101.41 (at 2.84–22.72 µg L−1) | [102] |
| Linuron | None | 0.2 M B-R buffer, pH 5.5 | SWV | 23.00 µg L−1 | 9.23·10−2 | NR | / | 25.75–309.02 µg L−1 | 0.01627 A L µg−1 | NR | Real sample: natural water, distilled water, carrot, potato, onion, interferences | 96.00–103.00 (at 50.25–59.80 µg L−1) | [103] |
| Single Nanomaterial | |||||||||||||
| Diazinon | MWCNTs | Acetate buffer, pH 5.25 | DPV | 4.5·10−10 M | 4.50·10−4 | NR | / | 1·10−10–6·10−8 M | 18.973 μA µM−1 | NR | Real samples: tomato, apple, cucumber, spinach, sweet peppers, lettuce, cabbage, eggplant, interferences: K+, Ca2+, Mg2+, Ni2+ | NR | [104] |
| Cyromazine | MWCNTs | 0.1 M H2SO4 | SWV | 0.12 µg mL−1 | 7.22·10−1 | 0.41 µg mL−1 | 2.47 | 0.41–83.30 µg mL−1 | 2.26 µA mL µg−1 | NR | Real samples: river and tap water, agrochemical pesticide formulation Trigard®, interferences: Zn2+, Mg2+, Ni2+, Co2+, Na+, Cl−, Cu2+, Pb2+, cyanazine, atrazine, cymoxanil | 96.7–101.5 (at 5.0–25.0 µg mL−1) | [105] |
| Fenhexamid | MWCNTs | 0.1 M B-R buffer, pH 4, 10 vol.% MeOH | SWV | 0.52 µM | 52.00·10−2 | NR | / | 1.74–157.48 µM | 0.108μA µM−1 | NR | NR | NR | [101] |
| Cypermethrin | TiO2 NP | Citrate buffer, pH 5 | DPV | 0.0978 ppm | 24.00·10−2 | NR | / | 0.1–1 ppm | 8.4865 µA cm−2 ppm−1 | 0.37 (at 1 ppm) | NR | NR | [106] |
| MP | ZrO2 NP | Acetate buffer, pH 5.0 | SWV | 2 ng mL−1 | 7.60·10−3 | 5 ng mL−1 | 1.90·10−3 | 5–3000 ng mL−1 | 1.3461 µA mL µg−1 | 4.5 (at 0.050 µg mL−1) | Real samples: tap and river water, interferences: Na+, K+, NH4+, SO42−, NO3−, Cl−, Ca2+, Mg2+, Ni2+, Co2+, Fe2+, Fe3+, Hg2+, Cr3+, Pb2+, Cd2+, Cu2+, nitrophenol, phenol | 94.0–102.0 (at 0.050–0.800 µg mL−1) | [107] |
| Chlorpyrifos | Fe3O4 | 0.1 M phosphate buffer, pH 7.5 | DPV | 2.8·10−6 M | 2.80 | NR | / | 1–100 µM | 0.587 μA μM−1 | 3.42 (at 2.5 mM) | NR | 92.9–99.8 (at 5–50 µM) | [108] |
| CBM | Ce-ZnWO4 | 0.1 M phosphate buffer, pH 7.0 | DPV | 0.003 μM | 3.00·10−3 | NR | / | 0.01–~5.5 μM | 3.5781 μA μM−1 | ±5 (at 5.0·10−5 M) | Real samples: dopamine, uric acid | (at 5.0·10−5 M) | [109] |
| CBM | La-Nd2O3 | 0.1 M phosphate buffer, pH 7.0 | DPV | 0.027 µM | 0.27·10−2 | NR | / | 0.08–15 µM 15–50 µM | 2.1760 μA μM−1 0.8466 μA μM−1 | 2.94 (at 5 µM) | Interferences: NaCl, Mg(NO3)2, CuSO4, glucose, sucrose, ascorbic acid, pheno | NR | [110] |
| CBM | 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | 0.1 M B-R buffer, pH 5.0 | DPASV | 1.7 µg L−1 | 8.89·10−3 | 5.7 µg L−1 | 2.98·10−2 | 0.010–0.247 mg L−1 | NR | 1.3 (at 0.010 mg L−1) | Real sample: tap water, interferences: linuron, imidacloprid, acetamiprid | 104.1 (NR) | [111] |
| Binary Nanocomposite | |||||||||||||
| Diazinon | MIP-MWCNTs | 0.1 M acetate buffer, pH 4.0 | SWV | 4.1·10−10 M | 4.10·10−4 | 1·10−9 M | 1.00·10−3 | 5·10−10–1·10−6 M | 0.9418 µA nM−1 0.0942 µA nM−1 | 3.16 (NR) | Real sample: urine, tap water, river water, interferences: coumachlor, dicloran, dichlrofention, dimethoate, Cd2+, Ca2+, Mg2+, Pb2+, NO3− | 92.00–97.50 (at 20–2000 ng mL−1) | [112] |
| Dicloran | MIP-MWCNTs | 0.04 M KCl pH 8.0 | SWV | 4.8·10−10 M | 4.80·10−4 | 9.4·10−10 M | 9.40·10−10 | 5·10−9–1·10−6 M | 0.1055 µA nM−1 | NR | Real samples: tap water, river water, urine, interferences: carbofuran, diazinon, dichlrofention, dimethoate | 89.70–100.30 (at 20–2000ng mL−1) | [113] |
| Diuron | MIP-MWCNTs-COOH | 0.1 M phosphate buffer, pH 8.0 | SWV | 9.0·10−9 M | 9.00·10−3 | NR | / | 5.2·10−8–1.25·10−6 M | 5.1·105 µA M−1 | NR | Real sample: river water, interferences: metribuzin, 2,4-D, CBF, CBM | 96.1–99.5 (at 5.2·10−8 M) | [114] |
| Linuron | MWCNTs-ZnO | 0.2 M phosphate buffer, pH 6.0 | SWV | 5.83·10−9 M | 5.83·10−3 | 1.94·10−8 M | 1.94·10−2 | 0.02–0.34 µM | 2.4239 μA μM−1 | NR (0.1 mM) | Real samples: black soil, lake soil, agricultural soil, brick soil, red soil, water (pond, dam, tap, reverse osmosis, lake), interferences: CaCl2, CuSO4, MnSO4, KNO3, FeSO4, ZnCl2 | 96.2–99.42 (at 0.1·10−5–1.0·10−4 M) | [115] |
| CBM | MWCNT-Ca-ZnO | 0.2 M phosphate buffer, pH 7.0 | SWV | 4.68·10−9 M | 4.68·10−3 | 1.75·10−8 M | 1.75·10−2 | 0.01–0.45 µM | 2.2776 μA μM−1 | NR | Real samples: soil, water | 81.0–96.2 (at 0.2·10−5 –1.0·10−4 M) | [116] |
| Fluometuron | FePc-MWCNT | B-R buffer, pH 6.0 | DPV | 69.8 µg L−1 | 3.01·10−1 | 233 µg L−1 | 1.00 | 0.40–15.0 mg L−1 | 4.596 µA mg−1 L | 3.83 (at 0.75 mg L−1) | Real samples: tap water, commercial herbicide formulations, interferences: captan, halosulfuron methyl, monocrotophos, pencycuron, tolclofos-methyl, teflubenzuron pesticides, Cu2+, Fe2+, Pb2+, Zn2+ | 96.0 ± 2.7 (at 0.75 mg L−1) | [117] |
| Fipronil | FeO-TiO2 | 1.0 M MgSO4 | CV | 0.0012 μM | 12.00·10−4 | NR | / | 1.0·10−3–1.0·10−2 µM | NR | 0.17 (at 1 µM) | Interference: Cu2+ | NR | [118] |
| Fipronil | Al-TiO2 | 0.1 M HCl and Na2SO4 | CV | 0.0164 µg L−1 | 3.75·10−5 | NR | / | 0.01–0.09 µg L−1 | 325 µA L µg−1 | NR | Interferences: Cd2+, Pb2+ | NR | [119] |
| Isoproturon | CuO-CNTs | 0.5 M H2SO4 | CV | 5·10−10 M | 5.00·10−4 | 1.5·10−9 M | 1.50·10−3 | 1·10−8–1·10−6 M | 1.328 A M−1 | 2.0 (at 9·10−8 M) | Real sample: tap water, interferences: linuron, propazine, tetrazine, metazachlore, chlordecone | 96.4–101.7 (at 0.2–0.6 µM) | [120] |
| CBM | FS-Ag NPs | 0.1 M phosphate buffer, pH 7.4 | DPV | 9.4·10−10 M | 9.40·10−4 | NR | / | 5.0·10−8–3.0·10−6 M 3.0·10−6–1.0·10−5 M | 7.001 μA μM−1 1.895 μA μM−1 | NR | Real samples: river water, tomatoes juice, commercial apple and orange juices | 92.1–105.6 (at 1.5·10−5 and 3.0·10−5 M) | [121] |
| Amino-triazole | g-C3N4-CTAB | Phosphate buffer, pH 4.2 | SWV | 6.41·10−8 M | 6.41·10−2 | 2.14·10−7 M | 2.14·10−1 | 3.0·10−7–4.5·10−5 M | 13.645 μA μM−1 | NR (at 0.1 mM) | Real samples: black soil, lake soil, agricultural soil, brick soil, red soil, water (pond, dam, tap, reverse osmosis, lake), interferences: CaCl2, MgSO4, FeSO4, ZnCl2, KCl, NaCl | 95.50–99.50 (at 0.1·10−5 and 0.2·10−5 M) | [122] |
| Linuron | g-C3N4-CTAB | Phosphate buffer, pH 4.2 | SWV | 2.47·10−8 M | 2.47·10−2 | 8.23·10−8 M | 8.23·10−2 | 1.2·10−7–3.0·10−4 M | 6.7148 μA μM−1 | NR (at 0.1 mM) | Real samples: black soil, lake soil, agricultural soil, brick soil, red soil, water (pond, dam, tap, reverse osmosis, lake), interferences: CaCl2, MgSO4, FeSO4, ZnCl2, KCl, NaCl | 89.20–98.00 (at 0.4·10−5 and 0.5·10−5 M) | [122] |
| Analyte | Modification | Supporting Electrolyte, pH | Detection Technique | LOD | LOQ | Linear Concentration Range | Sensitivity | RSD at Certain Concentration (%) | Special Observation (Real Sample Analysis, Interferences, …) | Recovery at Certain Concentration (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As Reported | Calculated (µM) | As Reported | Calculated (µM) | ||||||||||
| Screen-Printed Electrodes (SPEs) | |||||||||||||
| Single Nanomaterial | |||||||||||||
| Carbaryl | Nano carbon black | MeOH:phosphate buffer, pH 7.0 | DPV | 4.8·10−8 M | 4.80·10−2 | NR | / | 1.0·10−7–1.0·10−4 M | 4.94·10−1 A M−1 cm−2 | NR | Real samples: durum wheat, organic durum wheat, soft wheat, organic soft wheat, maize | 78–102 (at 0.25–0.75 mg kg−1) | [127] |
| Isoprocarb | 7.9·10−8 M | 7.90·10−2 | NR | / | 1.0·10−7–1.0·10−4 M | 3.98·10−1 A M−1 cm−2 | NR | ||||||
| Fenobucarb | 8.0·10−8 M | 8.00·10−2 | NR | / | 1.0·10−7–1.0·10−4 M | 3.90·10−1 A M−1 cm−2 | NR | ||||||
| Carbofuran | 4.9·10−8 M | 4.90·10−2 | NR | / | 1.0·10−7–1.0·10−4 M | 4.86·10−1 A M−1 cm−2 | NR | ||||||
| Parathion | NiO NPs | B-R buffer, pH 6 | DPV | 24 nmol L−1 | 2.40·10−2 | NR | / | 0.1–5 and 5–30 µmol L−1 | 0.51 µA µM and 0.24 µA µM | 2.87 (at 20 µM) 3.54 (at 1.0 µM) | Real samples: tap water, urine, tomato juice, interferences: CaCl2, FeCl3, KI, NaNO3, Na2SO4, durspan, imidacloprid, p-nitrophenol | 94–103 (at 1–2 µM) | [128] |
| CBM | MWCNT | 0.04 M B-R buffer, pH 4.00 | SWV | 1.40·10−8 M | 1.40·10−2 | 4.21·10−8 M | 4.21·10−2 | 4.00·10−8–4.01·10−7 M | 19.2 µA M−1 | 3.1 (at 3.05·10−6 M) | Real sample: orange juice | 100–103.2 (at 15.6 ppb) | [129] |
| MP | GO nanoribbons | 0.1 M phosphate buffer, pH 7.0 | Amperometry | 0.5 nM | 0.50·10−3 | NR | / | 0.1–100 µM 100–2500 µM | 1.804 µA µM cm2 0.8587 µA µM cm2 | 3.95 (at 0.1 µM) | Real samples: ugli fruit, tomato, beetroot, broccoli, interferences: Ni2+, Cu2+, Mn2+, Zn2+, Ca2+, Ba2+, NO3−, malathion, 4-nitrophenol, nitrobenzene, aminophenol, 2-nitro aniline, 4-nitro aniline, 4-acetamidophenol | NR | [130] |
| Diazinon | PCL-chitosan nanofibers | 0.1 M acetate buffer, pH 5.25 | DPV | 2.888 nM | 2.88·10−3 | NR | / | 3–100 nM | 0.2041 µA µM | 3.12 (at 10 nM) | Real sample: tomato juice, interferences: Ca2+, K+, Mg2+, Ni2+ | 93.27–108.30 (at 20–60 nM) | [131] |
| Paraoxon | BiVO4 | 0.1 M phosphate buffer, pH 7.0 | DPV | 0.034 µM | 3.40·10−2 | 0.115 µM | 1.15·10−1 | 0.2–1.96 µM | 0.345 μA μM−1 cm−2 | NR | Real sample: river water, interferences: glucose, dopamine, urea, uric acid, Ca2+, Zn2+, Mg2+, Na2+ | 95.01–98.42 (at 1–5 µM) | [132] |
| Isoproturon (ISO) and CBM simultaneously | Graphene | 1.0 M HClO4, pH 2 | SWV | 0.02 mg L−1 (ISO) 0.11 mg L−1 (CBM) | 9.70·10−2 (ISO) 5.75·10−1 (CBM) | 0.07 mg L−1 (ISO) 0.38 mg L−1 (CBM) | 3.39·10−1 (ISO) 1.99 (CBM) | 0.02–10.0 mg L−1 (ISO) 0.50–10.0 mg L−1 (CBM) | 0.4294 µA L mg−1 (ISO) 0.2417 µA L mg−1 (CBM) | 9.2 (at 0.02 mg L−1 ISO) 10 (at 0.50 mg L−1 CBM) | Real samples: river water, rice-field water, rice-field soil, tomatoes, lettuce, interferences: CN−, CO32−, NO3−, PO43−, SO42−, Ca2+, Cd2+, Co2+, Cu2+, K+, Mg2+, Na+, Ni2+, Pb2+, Zr4+, Zn2+, disulfiram, thiram | 77.9–107 (at 2.00 mg L−1 ISO and CBM) | [133] |
| Binary Nanocomposites | |||||||||||||
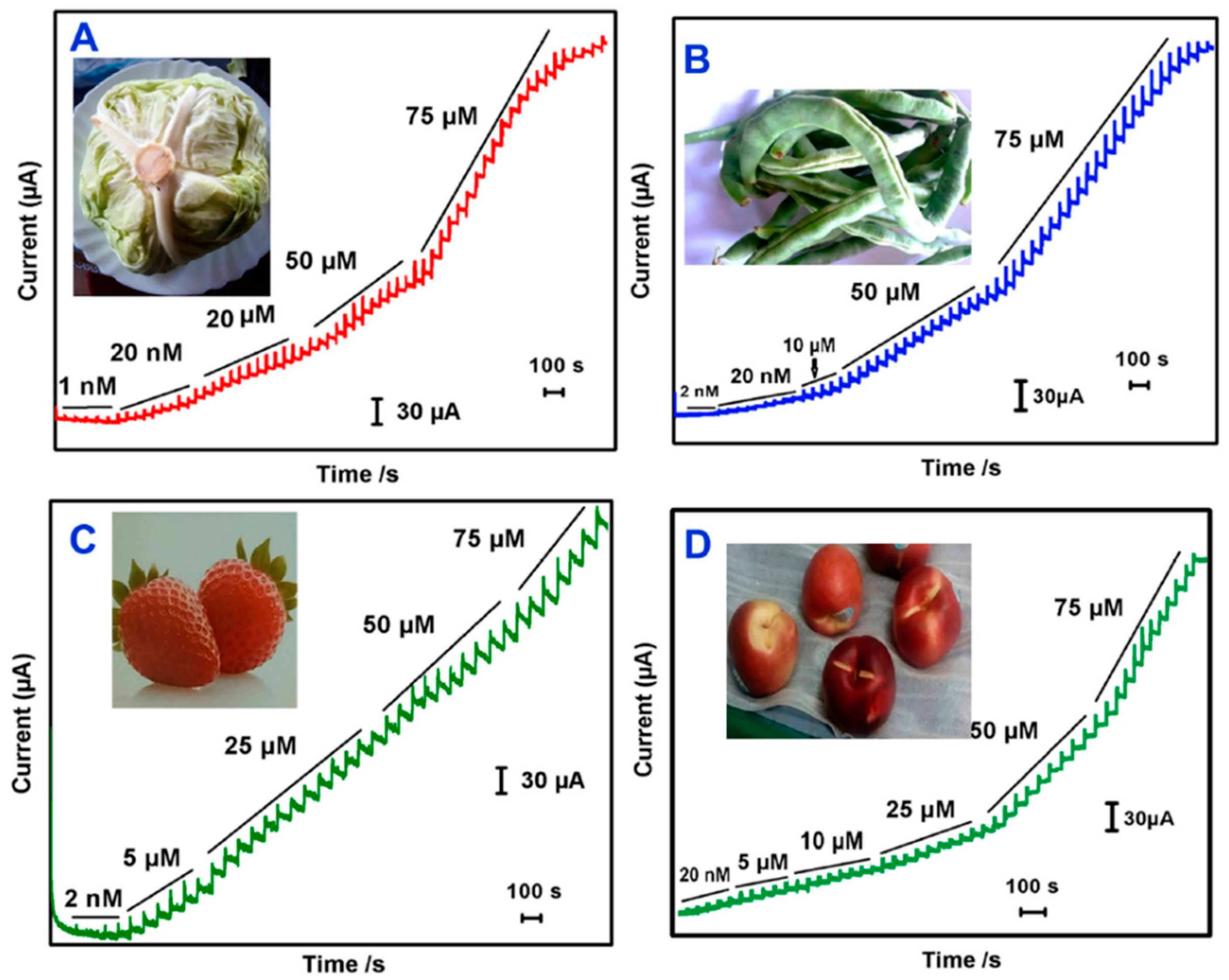
| MP | Ag NP- graphene nanoribbons | Phosphate buffer, pH 7.0 | amperometry | 0.5 nM | 5.00·10−4 | NR | / | 0.005–2780 µM | 0.5940 µA µM−1 cm−2 | 4.51 (at 100 nM) | Real samples: cabbage, green beans, strawberry, nectarine, interferences: Ca2+, Cu2+, Mn2+, Ba2+, Ni2+, Zn2+, NO3−, 4-Acetaminophenol, 4-Nitrophenol, 4-Nirobenzene, 4-Aminophenol, 2-Nitro aniline, 4-Nitro Aniline, 4-acetamido phenol. | NR | [134] |
| MP | GO NS-ZnO | 0.1 M phosphate buffer, pH 7.0 | DPV | 1.23 nM | 1.23·10−3 | 8.61 nM | 8.61·10−3 | 0.03–669.65 μM | 16.5237 μA μM−1 cm−2 | 3.75 (at 50 µM) | Real samples: apple, broccoflower, collard greens interferences: fenitrothion, ethyl parathaion, thiamethoxam, imidacloprid, catechol, hydroquinone, resorcinol, tannic acid, NaCl | 98.00–98.50 (2–5 µM) | [135] |
| Methyl paraoxon | GO NS-CuFeS2 | Phosphate buffer, pH 7 | DPV | 4.5 nM | 4.50·10−3 | NR | / | 0.073–801.5 µM | 17.97 µA µM−1 cm−2 | 3.72 (at 50 µM) | Real samples: lettuce, cherry tomato, interferences: 2,4 di-tert-butylphenol, fructose, butylated hydroxyl anisole, propylgallate, ascorbic acid, folic acid, Ca2+, glucose, caffeic acid | 96.36–99.68 (at 10–20 µM) | [136] |
| Fenitrothion | NbC-Mo | 0.1 M phosphate buffer, pH 7.0 | DPV | 0.15 nM | 1.50·10−4 | NR | / | 0.01–1889 µM | 0.355 µA µM−1 cm−2 | 3.23 (at 50 µM) | Real samples: grapes and cranberry extracts, interferences: ascorbic acid, catechol, glucose, caffeic acid, uric acid hydroquinone, dopamine, Ca2+, K+, Zn2+, Fe2+, Ba2+, Cu2+, NO2−, SO42−, NO3−, I−, Br−, Cl−, urea, 4-nitrophenol, 4-nitrobenzene, fenamiphos, carbofuran, azathioprine | NR | [137] |
| CBF and CBM simultaneously | GO-CTAB | 0.1 M phosphate buffer, pH 7 | SWV | 10 µg L−1 (CBF) 5 µg L−1 (CBM) | 4.52·10−2 (CBF) 2.62·10−2 (CBM) | NR | / | 40–20,000 µg L−1 (CBF) 25–5000 µg L−1 (CBM) | 0.0003 µA L µg−1 (CBF) 0.002 µA L µg−1 (CBM) | NR | Real samples: soybeans, rice, tomatoes | 95.7–105.5 (at 50–4000 CBF, CBM) | [138] |
| Ternary Nanocomposites | |||||||||||||
| CBM | Chitosan-fC-Cu | 0.05 M phosphate buffer, pH 7.0 | LSV | 0.028 μM | 2.80·10−2 | NR | / | 0.8–277.0 μM | 0.0981 μA μM | NR | Real samples: environmental water, interferences: diuron, bentazon, diphenylamine, carbofuran | 97.0–98.5 (5–40 µM) | [139] |
| MP | NiS2-rGO NS-curcumin NP | Phosphate buffer, pH 7.4 | DPV | 8.7 nM | 8.70·10−3 | NR | / | 0.25–5 μM 5–80 μM | 7.165 μA μM−1 cm−2 2.796 μA μM−1 cm−2 | 2.1 (at 40 µM) | Real samples: tomato and apple juices, river water, interferences (investigated with AMP): dinotefuran, H2O2, tannic acid, NaSO4, catechol, hydroquinone, 2,4-dinitrobenzene | 96.5–100.6 (at 20 µM) | [140] |
| Analyte | Modifier | Supporting Electrolyte, pH | Detection Technique | LOD | LOQ | Linear Concentration Range | Sensitivity | RSD at Certain Concentration (%) | Special Observation (Real Sample Analysis, Interferences, …) | Recovery at Certain Concentration (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As Reported | Calculated (µM) | As Reported | Calculated (µM) | ||||||||||
| Pencil Graphite Electrodes | |||||||||||||
| Malathion | IL-chitosan-Au NP | 0.2 M B-R buffer, pH 7 | SWV | 0.68 nM | 0.68·10−3 | NR | / | 0.89–5.94 nM 5.94–44.6 nM | 3.3123 µA nM−1 0.5287 µA nM−1 | NR | Real samples: tomato, apples, interferences: K+, Na+, Bi3+, SO42−, NO3−, Cl−, fenitrothion | NR | [144] |
| Glyphosate | Hollow fibers-CuO-MWCNTs-IL | 0.1 M phosphate buffer, pH 7 | DPV | 1.3 nM | 1.30·10−3 | 4.3 nM | 4.30·10−3 | 5 nM–1.1 µM | 10.256 µA µM−1 | NR | Real sample: river water, soil, interferences: Zn2+, Cd2+, Ca2+, Mg2+, Na+, NH4+, Br−, NO3−, SO42−, PO43−, glufosinate, bialaphos, tridemorph, chlorpyrifos, cypermethrin, (aminomethyl) phosphonic acid | 92.19–103.25 (at 30–90 nM) | [145] |
| CBM simultaneously with yellow 50, tryptophan and caffeine | Pd NPs | 0.1 M H2SO4 | SWV | 1.8·10−8 | 1.8·10−2 | NR | / | 0.2–1.6 µM | 173 µA µM−1 | 6.9 (at 5.0·10−7 M) | Real samples: synthetic urine, river water, interferences: ascorbic acid, urea, NaCl, catechol, hydroquinone, Pd2+, Cd2+, uric acid, ranitidine, captopril | 92.0–104 (at 2.5·10−7–5.0·10−7 M) | [146] |
| Gold-Based Electrodes | |||||||||||||
| MP | Au atomic clusters | 0.1 M KCl | SWV | 0.65 nM | 0.65·10−3 | NR | / | 1–10 nM 10–80 µM | 0.1468 µA nM−1 1.8153 µA µM−1 | 2.5 (NR) | Real sample: water from bore wells, interferences: Cl−, NO3−, PO43−, nitrophenol, nitrobenzene, nitroaniline | 97 (at 10 nM) | [147] |
| Glyphosate | MIP chitosan | [Fe(CN)6]3−/4−, PBS | EIS | 0.005 pg mL−1 | 2.96·10−8 | NR | / | 0.31 pg/mL–50 ng/mL | 0.087 fg−1 mL | NR | Real samples: river water, interferences: gluphosinate-ammonium, chlorpyrifos, phosmet | NR | [153] |
| Methomyl | Au NP-Fe3O4 NP-chitosan | 0.1 M B-R buffer, pH 6.9 | amperometry | 2.08·10−5 M | 20.80 | NR | / | 2.97·10−5–3.47·10−4 M | 0.03973 A M−1 | NR | Real samples: lettuce, oilseed rape, spinach | 90.02–98.26 (at 1.04·10−3 mol L−1) | [148] |
| Propamocarb | rGO | NR | CV | 0.6 µM | 0.60 | NR | / | 1–5 µM | 101.1 µA µM−1 cm−2 | NR | Real sample: cucumber, interferences: malathion, deltamethrin, cypermethrin | NR | [149] |
| Glass-Based Electrodes | |||||||||||||
| Diuron | PPy-ITO | 0.1 M B-R buffer, pH 2.0 | SWV | 6.4·10−7 M | 0.64 | 2.2·10−6 M | 2.20 | 8.58·10−7–4.29·10−5 M | 0.022 µA µM−1 | NR | NR | NR | [150] |
| Diuron | PPy-MWCNT- ITO | 0.1 M B-R buffer, pH 2.0 | SWV | 2.6·10−7 M | 0.26 | 8.6·10−7 M | 0.86 | 8.58·10−7–4.29·10−5 M | 0.231 µA µM−1 | NR | NR | NR | [150] |
| Others | |||||||||||||
| Glyphosate | Cu-BTC | 0.1 M phosphate buffer, pH 5.5 | DPV | 1.4·10−13 M | 1.4·10−7 | NR | / | 1.0·10−12–1.0·10−9 M 1.0·10−9–1.0·10−5 M | 2.4767 µA M−1 0.782 µA M−1 | NR | Real sample: soybean, interferences: aminomethylphosphonic acid, Trichlorfon, CBM, Acetochlor, Thiram, K+, Ca2+, Zn2+, NO3−, Cl−, SO42− | 98.0–105.0 (at 0.10–1.00 µM) | [151] |
| MP | CaCO3-chitosan nanowall arrays | 0.1 M phosphate buffer, pH 7.0 | SWV | 0.8 ng mL−1 | 3.04·10−3 | NR | / | 0.001–0.1 µg mL−1 | 591.8 µA mL µg−1 | 4.5 (at 0.1 µg mL−1) | Real sample: garlic, interferences: nitrobenzene, nitrophenol, PO42−, SO43−, NO3− | 98.3–105.0 (at 0.002–0.050 µg mL−1) | [152] |
| Pirimiphos | CuO nanorods | 0.25 M NaOH | CV | 0.294 µM | 2.94·10−1 | NR | / | NR | 2.833 µA mL ng−1 | NR | Interferences: carbaryl, paraquat, sodium nitrate, sodium sulphate, toluene | NR | [154] |
| Paraoxon | CuO nanorods | 0.25 M NaOH | CV | 0.557 µM | 5.57·10−1 | NR | / | NR | 1.657 µA mL ng−1 | NR | Interferences: carbaryl, paraquat, sodium nitrate, sodium sulphate, toluene | NR | [154] |
| Parathion | CuO nanorods | 0.25 M NaOH | CV | 0.612 µM | 6.12·10−1 | NR | / | NR | 1.425 µA mL ng−1 | NR | Interferences: carbaryl, paraquat, sodium nitrate, sodium sulphate, toluene | NR | [154] |
| Chlorpyrifos | CuO nanorods | 0.25 M NaOH | CV | 0.571 µM | 5.71·10−1 | NR | / | NR | 1.269 µA mL ng−1 | NR | Interferences: carbaryl, paraquat, sodium nitrate, sodium sulphate, toluene | NR | [154] |
| CBM | BDD | 0.1 M Na2HPO4, pH 2.0 | SWV | 1.2·10−7 M | 1.2·10−1 | 4.0·10−7 M | 4.0·10−1 | 0.5·10−6–15·10−6 M | 0.08 A M−1 | 2.0 (5.0·10−6 M) | Real samples: pure and river water | 90.0–96.0 (at 0.5·10−6 –40·10−6 M) | [155] |
| Fenamiphos | BDD | 0.1 M Na2HPO4, pH 2.0 | SWV | 1.0·10−7 M | 1.0·10−1 | 3.0·10−7 M | 3.0·10−1 | 0.5·10−6–25·10−6 M | 0.14 A M−1 | 3.1 (5.0·10−6 M) | Real samples: pure and river water | 96.0–107.5 (at 0.5·10−6 –40·10−6 M) | [155] |
| CBM and fenamiphos (FNP) simultaneously | BDD | 0.1 M Na2HPO4, pH 2.0 | SWV | 9.2 µg L−1 (CBM) | 4.81·10−2 (CBM) | 125 µg L−1 (CBM) | 6.54·10−1 (CBM) | 1·10−6–15·10−6 M (CBM) 0.5·10−6–7.0·10−6 M (FNP) | NR | NR | Real samples: pure and river water | NR | [155] |
| Carbaryl | Graphene-BDD | Acetate buffer, pH 5.6 | CV | 0.14 µM | 0.14 | 0.46 µM | 0.46 | 10–60 µM | 1.85 µA µM−1 cm−2 | NR | NR | NR | [156] |
| Carbaryl | DPV | 0.07 µM | 0.07 | 0.23 µM | 0.23 | 1–12 µM | 30.5 µA µM−1 cm−2 | NR | NR | NR | |||
| Paraquat | CV | 0.01 µM | 0.01 | 0.04 µM | 0.04 | 0.2–1.2 µM | 46.12 µA µM−1 cm−2 | NR | NR | NR | |||
| Paraquat | DPV | 0.04 µM | 0.04 | 0.13 µM | 0.13 | 1–6 µM | 30.8 µA µM−1 cm−2 | NR | Real sample: fresh apple juice | NR | |||
| Carbaryl (CBR) and paraquat (PQ) simultaneously | DPV | 0.07 µM (CBR) | 0.07 | 0.23 µM | 0.23 | 1–6 µM | 33.27 µA µM−1 cm−2 | 2.5 (8·10−6 M) | Real sample: fresh apple juice | NR | |||
| 0.01 µM (PQ) | 0.01 | 0.02 µM | 0.02 | 0.2–1.2 µM | 31.83 µA µM−1 cm−2 | 1.2 (1·10−6 M) | |||||||
| Parathion | SiC NPs-MWCNTs–chitosan | 0.2 M phosphate buffer, pH 6 | DPV | 20 ng mL−1 | 6.87·10−2 | NR | / | 50–2000 ng mL−1 2000–10,000 ng mL−1 | 0.00198 µA ng−1 mL and 0.0006975 µA ng−1 mL | NR | Real samples: sweet potato leaf, Chinese cabbage, cucumber, interferences: NaNO3, MnSO4, CaCl2, citric acid, glucose, ascorbic acid | 76.0–96.2 (at 1000–5000 ng mL−1) | [157] |
| CBM | CSS | 0.1 M phosphate buffer, pH 7.0 | DPV | 4.7·10−8 M | 4.7·10−2 | NR | / | 0.1–1.0 µM | 0.18 A M−1 | NR | Real samples: cabbages, apples, orange juice, interferences: chlorpyrifos, CBR, metomyl, atrazine, trifluralin, glyphosate, chloranil | 96–101 (at 0.3–200.0 µM) | [158] |
| Diuron | PCNB | 0.1 M phosphate buffer, pH 7.0 | DPV | 9.2·10−7 M | 9.2·10−1 | NR | / | 1–10 µM | 0.04 A M−1 | NR | Real samples: cabbages, apples, orange juice, interferences: chlorpyrifos, CBR, metomyl, atrazine, trifluralin, glyphosate, chloranil | 103–110 (at 2.0–796.0 µM) | [158] |
| Paraquat (PQ) and fenitrothion (FEN) simultaneously | Carbon ink | 0.1 M phosphate buffer, pH 7.0 | SWV | 2.4·10−8 M (PQ) 6.4·10−7 M (FEN) | 2.4·10−2 (PQ) 6.4·10−1 (FEN) | NR | / | 0.1–1.0 µM (PQ) 1–10 µM (FEN) | 2.47 A M−1 (PQ) 0.42 A M−1 (FEN) | NR | Real samples: cabbages, apples, orange juice, interferences: chlorpyrifos, CBR, metomyl, atrazine, trifluralin, glyphosate, chloranil | 88.5–108 (at 0.2–7.96 µM, PQ) 93–107 (at 1.99–79.6 µM, FEN) | [158] |
| CBM | MWCNTs | 0.04 M B-R buffer, pH 4.0 | DPASV | 0.049 µM | 4.9·10−2 | NR | / | 0.25–2.50 µM | 8.53 µA µM−1 | 12 (at 0.75 µM) | Real samples: mineral water, orange juice, interferences: | 90–99 (at 0.8 µM) | [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrabelj, T.; Finšgar, M. Recent Progress in Non-Enzymatic Electroanalytical Detection of Pesticides Based on the Use of Functional Nanomaterials as Electrode Modifiers. Biosensors 2022, 12, 263. https://doi.org/10.3390/bios12050263
Vrabelj T, Finšgar M. Recent Progress in Non-Enzymatic Electroanalytical Detection of Pesticides Based on the Use of Functional Nanomaterials as Electrode Modifiers. Biosensors. 2022; 12(5):263. https://doi.org/10.3390/bios12050263
Chicago/Turabian StyleVrabelj, Tanja, and Matjaž Finšgar. 2022. "Recent Progress in Non-Enzymatic Electroanalytical Detection of Pesticides Based on the Use of Functional Nanomaterials as Electrode Modifiers" Biosensors 12, no. 5: 263. https://doi.org/10.3390/bios12050263
APA StyleVrabelj, T., & Finšgar, M. (2022). Recent Progress in Non-Enzymatic Electroanalytical Detection of Pesticides Based on the Use of Functional Nanomaterials as Electrode Modifiers. Biosensors, 12(5), 263. https://doi.org/10.3390/bios12050263






