Two-Dimensional Quantum Dot-Based Electrochemical Biosensors
Abstract
:1. Introduction
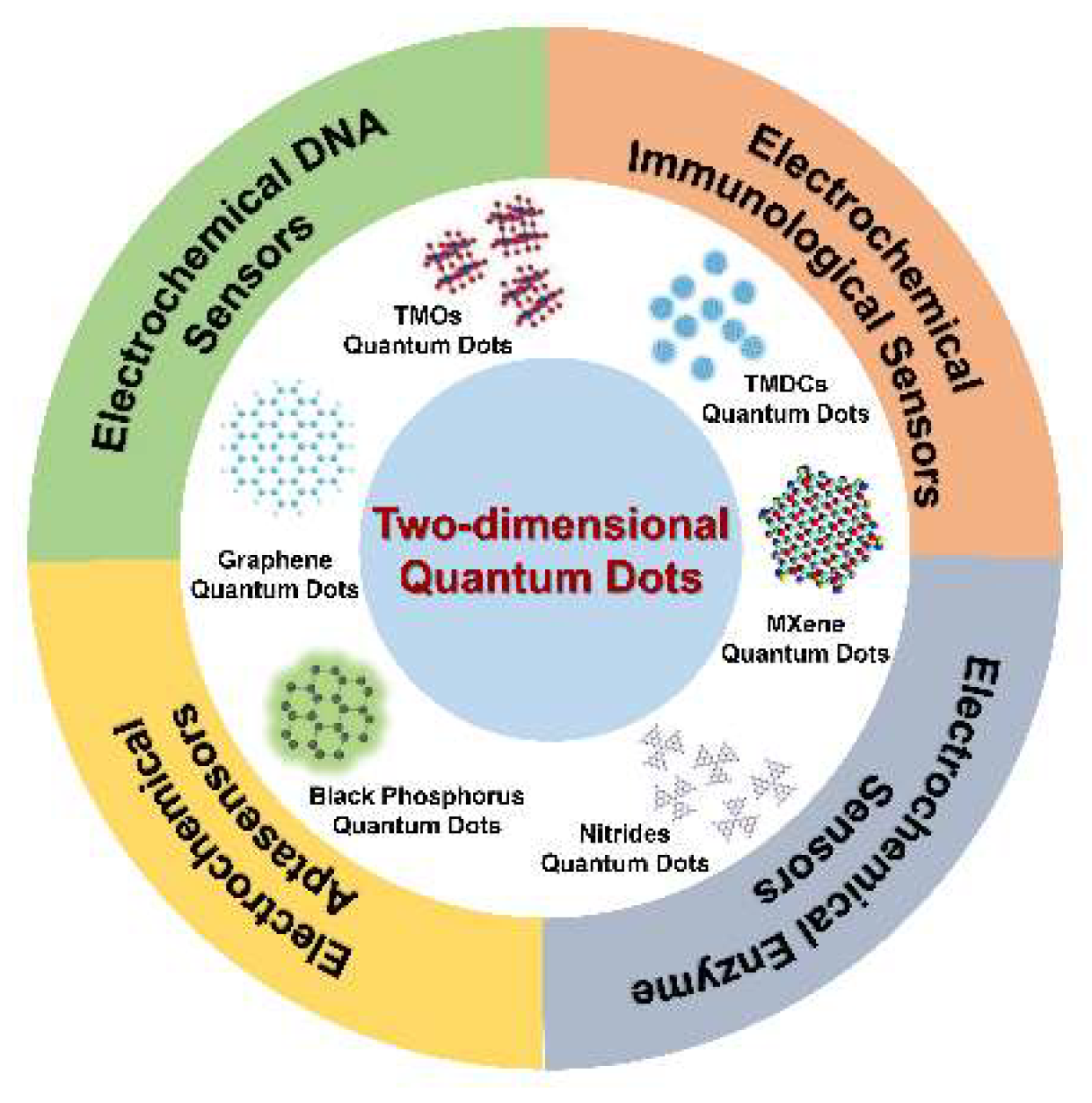
2. Categories of 2D-QDs
2.1. 2D Graphene QDs
2.2. Nitride-Based 2D-QDs
2.3. Black Phosphorus 2D-QDs
2.4. TMDCs-Based 2D-QDs
2.5. TMO-Based 2D-QDs
2.6. MXenes-Based 2D-QDs
3. Synthetic Methods of 2D-QDs
3.1. Top-Down Methods
3.1.1. Ultrasonication-Assisted Methods
3.1.2. Hydro/Solvothermal Methods
3.1.3. Ion Intercalation-Assisted Methods
3.1.4. Microwave-Assisted Methods
3.2. Bottom-Up Methods
4. Applications of 2D-QDs in Electrochemical Biosensors
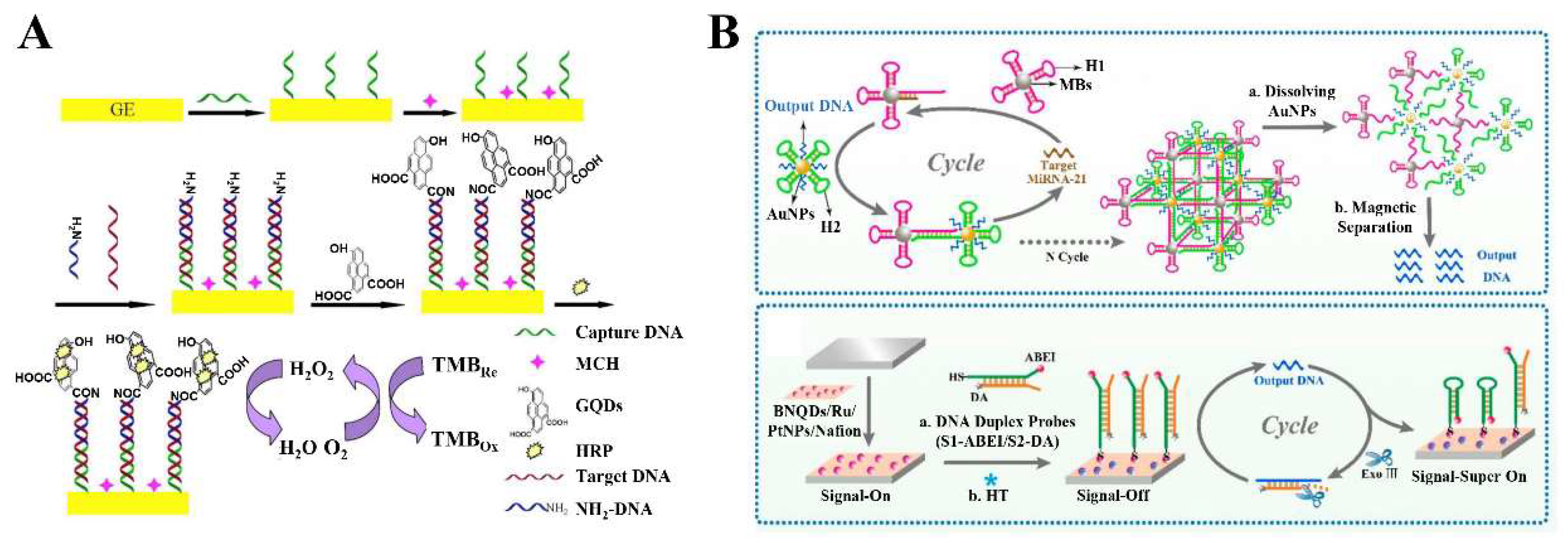
4.1. 2D-QD-Based Electrochemical DNA Sensors
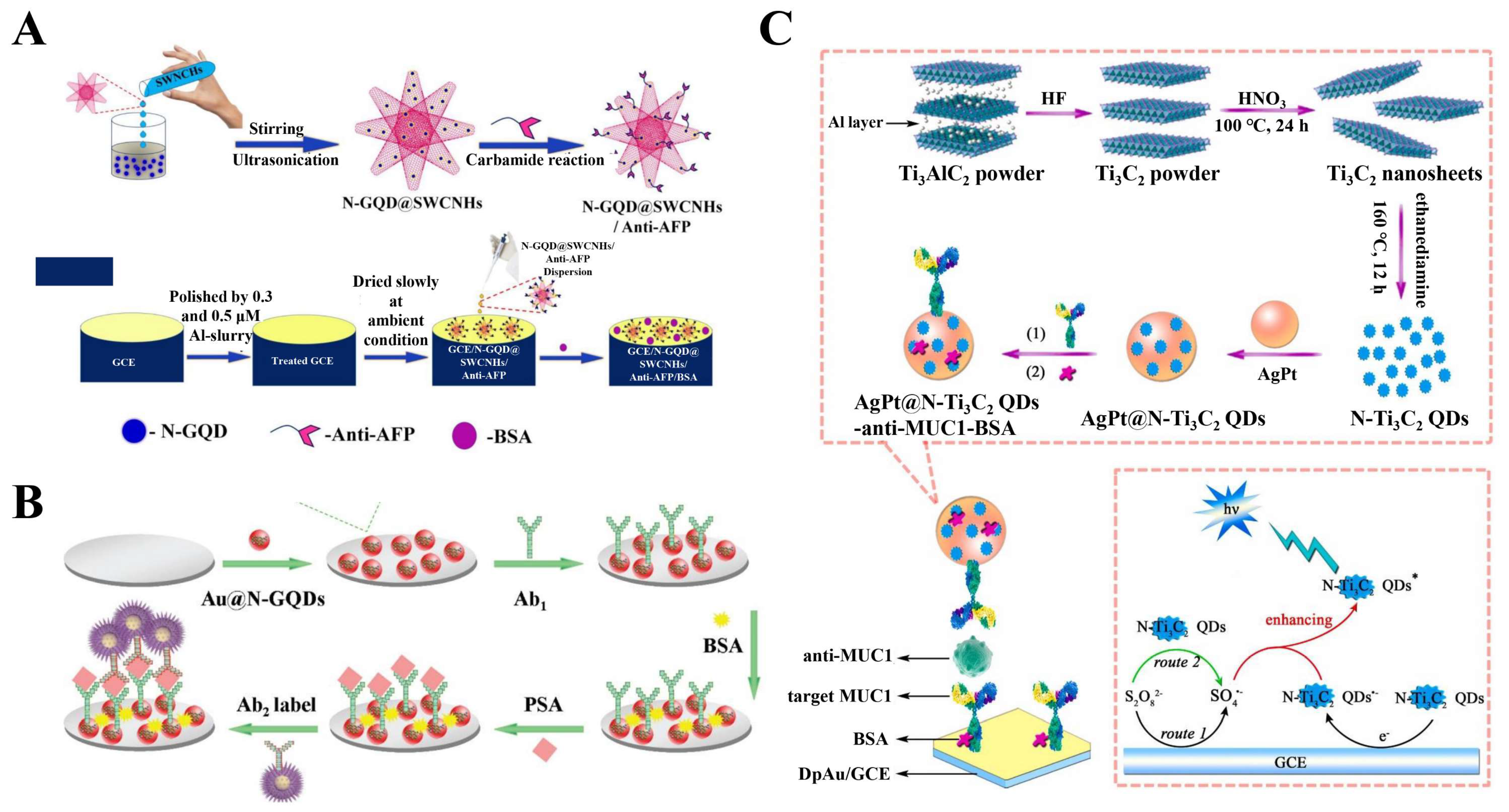
4.2. 2D-QD-Based Electrochemical Immunological Sensors
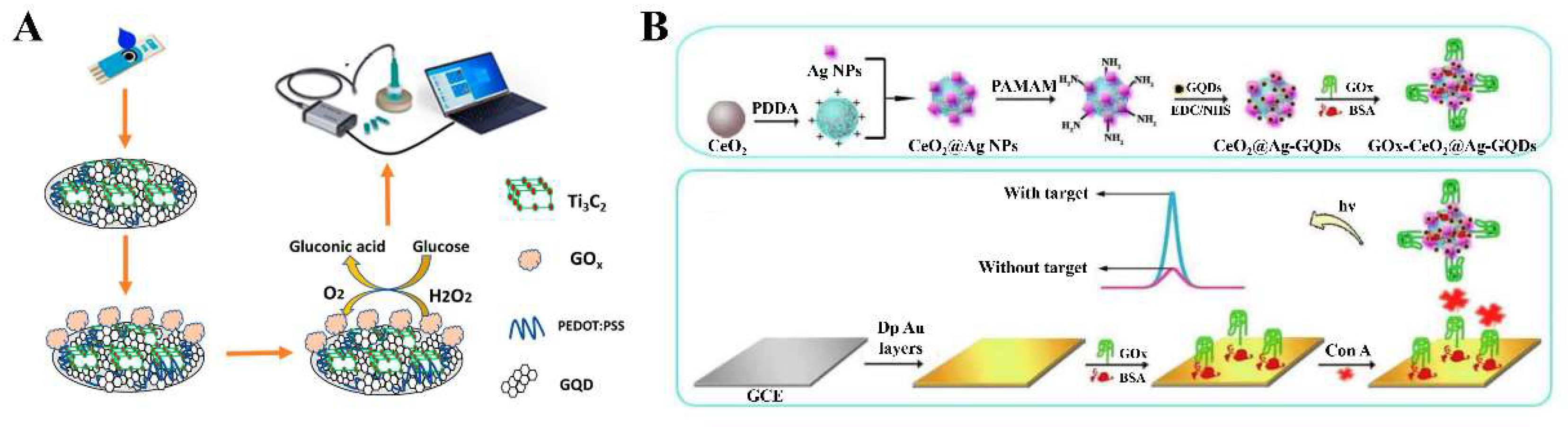
4.3. 2D-QD-Based Electrochemical Enzyme Sensors
4.4. 2D-QD-Based Electrochemical Aptasensors
5. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kim, K.K.; Hsu, A.; Jia, X.; Kim, S.M.; Shi, Y.; Hofmann, M.; Nezich, D.; Rodriguez-Nieva, J.F.; Dresselhaus, M.; Palacios, T.; et al. Synthesis of monolayer hexagonal boron nitride on Cu foil using chemical vapor deposition. Nano Lett. 2012, 12, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Shin, H.J.; Lee, J.; Lee, I.Y.; Kim, G.H.; Choi, J.Y.; Kim, S.W. Large-scale synthesis of high-quality hexagonal boron nitride nanosheets for large-area graphene electronics. Nano Lett. 2012, 12, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, N.; Chen, X.; Ong, W.-J.; Zhao, X. The rising star of 2D black phosphorus beyond graphene: Synthesis, properties and electronic applications. 2D Mater. 2017, 5, 014002. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Deng, Y.; Ye, P.D. Semiconducting black phosphorus: Synthesis, transport properties and electronic applications. Chem. Soc. Rev. 2015, 44, 2732–2743. [Google Scholar] [CrossRef] [Green Version]
- Kumru, B.; Antonietti, M. Colloidal properties of the metal-free semiconductor graphitic carbon nitride. Adv. Colloid Interface Sci. 2020, 283, 102229. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, G.; Wang, X. Synthesis of Carbon Nitride Semiconductors in Sulfur Flux for Water Photoredox Catalysis. ACS Catal. 2012, 2, 940–948. [Google Scholar] [CrossRef]
- Schwinghammer, K.; Mesch, M.B.; Duppel, V.; Ziegler, C.; Senker, J.; Lotsch, B.V. Crystalline Carbon Nitride Nanosheets for Improved Visible-Light Hydrogen Evolution. J. Am. Chem. Soc. 2014, 136, 1730–1733. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Mathis, T.S.; Sarycheva, A.; Hatter, C.B.; Uzun, S.; Levitt, A.; Gogotsi, Y. Selective Etching of Silicon from Ti3 SiC2 (MAX) To Obtain 2D Titanium Carbide (MXene). Angew. Chem. Int. Ed. 2018, 57, 5444–5448. [Google Scholar] [CrossRef]
- Urbankowski, P.; Anasori, B.; Makaryan, T.; Er, D.; Kota, S.; Walsh, P.L.; Zhao, M.; Shenoy, V.B.; Barsoum, M.W.; Gogotsi, Y. Synthesis of two-dimensional titanium nitride Ti4N3 (MXene). Nanoscale. 2016, 8, 11385–11391. [Google Scholar] [CrossRef]
- Fu, Q.; Han, J.; Wang, X.; Xu, P.; Yao, T.; Zhong, J.; Zhong, W.; Liu, S.; Gao, T.; Zhang, Z.; et al. 2D Transition Metal Dichalcogenides: Design, Modulation, and Challenges in Electrocatalysis. Adv. Mater. 2021, 33, 1907818. [Google Scholar] [CrossRef]
- Huang, J.-K.; Pu, J.; Hsu, C.-L.; Chiu, M.-H.; Juang, Z.-Y.; Chang, Y.-H.; Chang, W.-H.; Iwasa, Y.; Takenobu, T.; Li, L.-J. Large-Area Synthesis of Highly Crystalline WSe2 Monolayers and Device Applications. ACS Nano. 2014, 8, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Liao, T.; Dou, Y.; Hwang, S.M.; Park, M.-S.; Jiang, L.; Kim, J.H.; Dou, S.X. Generalized self-assembly of scalable two-dimensional transition metal oxide nanosheets. Nat. Commun. 2014, 5, 3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Song, H.; Lin, S.; Zhou, Y.; Zhan, X.; Hu, Z.; Zhang, Q.; Sun, J.; Yang, B.; Li, T.; et al. Scalable salt-templated synthesis of two-dimensional transition metal oxides. Nat. Commun. 2016, 7, 11296. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jang, M.-H.; Ha, H.D.; Kim, J.-H.; Cho, Y.-H.; Seo, T.S. Facile Synthetic Method for Pristine Graphene Quantum Dots and Graphene Oxide Quantum Dots: Origin of Blue and Green Luminescence. Adv. Mater. 2013, 25, 3657–3662. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Zhang, W.L.; Lv, F.; Guo, S. Recent progress in two-dimensional inorganic quantum dots. Chem. Soc. Rev. 2018, 47, 586–625. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, 1808283. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, L.; Li, F.-F.; Cui, W.-R.; Liang, R.-P.; Qiu, J.-D. Facile and Green Approach to the Synthesis of Boron Nitride Quantum Dots for 2,4,6-Trinitrophenol Sensing. ACS Appl. Mater. Interfaces 2018, 10, 7315–7323. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, C.; Liu, Z.; Han, J.; Fang, Y.; Han, Y.; Niu, Y.; Wu, Y.; Sun, C.; Xu, Y. Rational Design of Hydroxyl-Rich Ti3C2Tx MXene Quantum Dots for High-Performance Electrochemical N2 Reduction. Adv. Energy Mater. 2020, 10, 2000797. [Google Scholar] [CrossRef]
- Li, D.; Liang, L.; Tang, Y.; Fu, L.; Xiao, S.; Yuan, Q. Direct and single-step sensing of primary ovarian cancers related glycosidases. Chin. Chem. Lett. 2019, 30, 1013–1016. [Google Scholar] [CrossRef]
- Faridbod, F.; Sanati, L.A. Graphene Quantum Dots in Electrochemical Sensors/Biosensors. Curr. Analy. Chem. 2019, 15, 103–123. [Google Scholar] [CrossRef]
- Pothipor, C.; Jakmunee, J.; Bamrungsap, S.; Ounnunkad, K. An electrochemical biosensor for simultaneous detection of breast cancer clinically related microRNAs based on a gold nanoparticles/graphene quantum dots/graphene oxide film. Analyst 2021, 146, 4000–4009. [Google Scholar] [CrossRef] [PubMed]
- Akbarnia, A.; Zare, H.R. A voltammetric assay for microRNA-25 based on the use of amino-functionalized graphene quantum dots and ss- and ds-DNAs as gene probes. Mikrochim. Acta 2018, 185, 503. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Meng, H.; Zhang, G.; Song, L.; Han, Q.; Wang, C.; Fu, Y. Ultrasensitive dual-quenching electrochemiluminescence immunosensor for prostate specific antigen detection based on graphitic carbon nitride quantum dots as an emitter. Mikrochim. Acta 2021, 188, 350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, H.; Shen, Y.; Hu, N.; Shi, W. Nitrogen-doped Ti3C2 MXene quantum dots as novel high-efficiency electrochemiluminescent emitters for sensitive mucin 1 detection. Sens. Actuators B Chem. 2022, 350, 130891. [Google Scholar] [CrossRef]
- Li, L.-L.; Ji, J.; Fei, R.; Wang, C.-Z.; Lu, Q.; Zhang, J.-R.; Jiang, L.-P.; Zhu, J.-J. A Facile Microwave Avenue to Electrochemiluminescent Two-Color Graphene Quantum Dots. Adv. Funct. Mater. 2012, 22, 2971–2979. [Google Scholar] [CrossRef]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2021, 33, 1904362. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Wang, C.; Wu, X.; Yang, Y.; Zheng, B.; Wu, H.; Guo, S.; Zhang, J. Photo-Fenton Reaction of Graphene Oxide: A New Strategy to Prepare Graphene Quantum Dots for DNA Cleavage. ACS Nano 2012, 6, 6592–6599. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, L.-s. Synthesis of Large, Stable Colloidal Graphene Quantum Dots with Tunable Size. J. Am. Chem. Soc. 2010, 132, 5944–5945. [Google Scholar] [CrossRef]
- Zhao, H.; Chang, Y.; Liu, M.; Gao, S.; Yu, H.; Quan, X. A universal immunosensing strategy based on regulation of the interaction between graphene and graphene quantum dots. Chem. Commun. 2013, 49, 234–236. [Google Scholar] [CrossRef]
- Patnaik, S.; Martha, S.; Parida, K.M. An overview of the structural, textural and morphological modulations of g-C3N4 towards photocatalytic hydrogen production. RSC Adv. 2016, 6, 46929–46951. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Chen, J.; Wang, A.-J.; Bao, N.; Feng, J.-J.; Wang, W.; Shao, L. Facile synthesis of oxygen and sulfur co-doped graphitic carbon nitride fluorescent quantum dots and their application for mercury(ii) detection and bioimaging. J. Mater. Chem. B 2015, 3, 73–78. [Google Scholar] [CrossRef]
- Song, Z.; Li, Z.; Lin, L.; Zhang, Y.; Lin, T.; Chen, L.; Cai, Z.; Lin, S.; Guo, L.; Fu, F.; et al. Phenyl-doped graphitic carbon nitride: Photoluminescence mechanism and latent fingerprint imaging. Nanoscale 2017, 9, 17737–17742. [Google Scholar] [CrossRef]
- Garg, M.; Rani, R.; Sharma, A.L.; Singh, S. White graphene quantum dots as electrochemical sensing platform for ferritin. Faraday Discuss. 2021, 227, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Angizi, S.; Alem, S.A.A.; Hasanzadeh, M.; Shayeganfar, F.; Manning, M.; Hatamie, A.; Pakdel, A.; Simchi, A. A Comprehensive Review on Planar Boron Nitride Nanomaterials: From 2D Nanosheets Towards 0D Quantum Dots. Prog. Mater Sci. 2021, 124, 100884. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.; Liu, Z.; Tan, C.; Luo, Z.; Li, H.; Lin, J.; Sun, L.; Chen, W.; Xu, Z.; et al. Black Phosphorus Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 3653–3657. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Wang, Z.; Li, J. Black phosphorus quantum dots: Synthesis, properties, functionalized modification and applications. Chem. Soc. Rev. 2018, 47, 6795–6823. [Google Scholar] [CrossRef]
- Gu, W.; Pei, X.; Cheng, Y.; Zhang, C.; Zhang, J.; Yan, Y.; Ding, C.; Xian, Y. Black Phosphorus Quantum Dots as the Ratiometric Fluorescence Probe for Trace Mercury Ion Detection Based on Inner Filter Effect. ACS Sens. 2017, 2, 576–582. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Lin, S.; Onofrio, N.; Zhou, L.; Shi, F.; Lu, W.; Kang, K.; Zhang, Q.; Lau, S.P. Exceptional catalytic effects of black phosphorus quantum dots in shuttling-free lithium sulfur batteries. Nat. Commun. 2018, 9, 4164. [Google Scholar] [CrossRef]
- Guo, T.; Wu, Y.; Lin, Y.; Xu, X.; Lian, H.; Huang, G.; Liu, J.-Z.; Wu, X.; Yang, H.-H. Black Phosphorus Quantum Dots with Renal Clearance Property for Efficient Photodynamic Therapy. Small 2018, 14, 1702815. [Google Scholar] [CrossRef]
- Liu, J.; Yi, K.; Zhang, Q.; Xu, H.; Zhang, X.; He, D.; Wang, F.; Xiao, X. Strong Penetration-Induced Effective Photothermal Therapy by Exosome-Mediated Black Phosphorus Quantum Dots. Small 2021, 17, 2104585. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.-F.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P.K. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Duong, D.L.; Keum, D.H.; Yun, S.J.; Lee, Y.H. van der Waals Metallic Transition Metal Dichalcogenides. Chem. Rev. 2018, 118, 6297–6336. [Google Scholar] [CrossRef] [PubMed]
- Abid; Sehrawat, P.; Julien, C.M.; Islam, S.S. WS2 Quantum Dots on e-Textile as a Wearable UV Photodetector: How Well Reduced Graphene Oxide Can Serve as a Carrier Transport Medium? ACS Appl. Mater. Interfaces 2020, 12, 39730–39744. [Google Scholar] [CrossRef]
- Garg, M.; Chatterjee, M.; Sharma, A.L.; Singh, S. Label-free approach for electrochemical ferritin sensing using biosurfactant stabilized tungsten disulfide quantum dots. Biosens. Bioelectron. 2020, 151, 111979. [Google Scholar] [CrossRef]
- Xiao, S.J.; Zhao, X.J.; Hu, P.P.; Chu, Z.J.; Huang, C.Z.; Zhang, L. Highly Photoluminescent Molybdenum Oxide Quantum Dots: One-Pot Synthesis and Application in 2,4,6-Trinitrotoluene Determination. ACS Appl. Mater. Interfaces 2016, 8, 8184–8191. [Google Scholar] [CrossRef]
- Xiao, S.J.; Zhao, X.J.; Chu, Z.J.; Xu, H.; Liu, G.Q.; Huang, C.Z.; Zhang, L. New Off-On Sensor for Captopril Sensing Based on Photoluminescent MoOx Quantum Dots. ACS Omega 2017, 2, 1666–1671. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, Y.; Jiang, Y.; Liu, K. Improved Current Extraction of Cu/Si Nanowire Heterojunctions for Self-Powered Photodetecting with Insertion of MoOx Quantum Dots Film. ACS Omega 2019, 4, 12418–12424. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, X.; Xu, Y.; Chen, T.; Liu, M.; Niu, F.; Wei, S.; Liu, J. Simultaneous Synthesis of WO3-xQuantum Dots and Bundle-Like Nanowires Using a One-Pot Template-Free Solvothermal Strategy and Their Versatile Applications. Small 2017, 13, 1603689. [Google Scholar] [CrossRef]
- Gao, H.; Xue, C.; Guoxin, H.; Kunxu, Z. Production of graphene quantum dots by ultrasound-assisted exfoliation in supercritical CO2/H2O medium. Ultrason. Sonochem. 2017, 37, 120–127. [Google Scholar] [CrossRef]
- Lee, H.U.; Park, S.Y.; Lee, S.C.; Choi, S.; Seo, S.; Kim, H.; Won, J.; Choi, K.; Kang, K.S.; Park, H.G.; et al. Black Phosphorus (BP) Nanodots for Potential Biomedical Applications. Small 2016, 12, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xu, J.; Wang, X.; Li, L.; Antonietti, M.; Shalom, M. Phenyl-Modified Carbon Nitride Quantum Dots with Distinct Photoluminescence Behavior. Angew. Chem. Int. Ed. 2016, 55, 3672–3676. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, D.; Guo, M.; Yu, Y.; Cao, Y. Facile and efficient fabrication of g-C3N4 quantum dots for fluorescent analysis of trace copper(II) in environmental samples. Chin. Chem. Lett. 2019, 30, 1639–1642. [Google Scholar] [CrossRef]
- Stengl, V.; Henych, J.; Kormunda, M. Self-Assembled BN and BCN Quantum Dots Obtained from High Intensity Ultrasound Exfoliated Nanosheets. Sci. Adv. Mater. 2014, 6, 1106–1116. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, A.Y.; Huang, D.; Chai, Y.Q.; Zhuo, Y.; Yuan, R. MoS2 Quantum Dots as New Electrochemiluminescence Emitters for Ultrasensitive Bioanalysis of Lipopolysaccharide. Anal. Chem. 2017, 89, 8335–8342. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Du, P.; Zhou, Y.; Zhang, L.; Li, D.; Tan, H.; Zhao, Z.; Xie, Z.; Sun, Z. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272–12277. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ding, L.; Wen, Y.; Yang, W.; Zhou, H.; Chen, X.; Street, J.; Zhou, A.; Ong, W.-J.; Li, N. High photoluminescence quantum yield of 18.7% by using nitrogen-doped Ti3C2 MXene quantum dots. J. Phys. Chem. C 2018, 6, 6360–6369. [Google Scholar] [CrossRef]
- Xu, S.; Li, D.; Wu, P. One-Pot, Facile, and Versatile Synthesis of Monolayer MoS2/WS2 Quantum Dots as Bioimaging Probes and Efficient Electrocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, H.; Zhu, M.; Pei, Z.; Li, H.; Wang, Z.; Huang, Y.; Huang, Y.; Deng, Q.; Zhou, J.; et al. Photoluminescent Ti3C2 MXene Quantum Dots for Multicolor Cellular Imaging. Adv. Mater. 2017, 29, 1604847. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, C.; Wang, X. One-pot solvothermal synthesis of water-soluble boron nitride nanosheets and fluorescent boron nitride quantum dots. Mater. Lett. 2019, 234, 306–310. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, S.; Song, X.; Zhang, X.; He, X.; Zhong, W.; Du, Y. Luminescent monolayer MoS2 quantum dots produced by multi-exfoliation based on lithium intercalation. Appl. Surf. Sci. 2015, 359, 130–136. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Y.; Xia, Z.; Wei, W. As-prepared MoS2quantum dot as a facile fluorescent probe for long-term tracing of live cells. Nanotechnology 2016, 27, 275101. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, Y.; Zhang, S.; Ross, I.M.; Ong, A.C.M.; Allwood, D.A. Fabrication and Luminescence of Monolayered Boron Nitride Quantum Dots. Small 2014, 10, 60–65. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Gao, T.; Yao, T.; Han, J.; Han, Z.; Zhang, Z.; Wu, Q.; Song, B. One-pot evaporation–condensation strategy for green synthesis of carbon nitride quantum dots: An efficient fluorescent probe for ion detection and bioimaging. Mater. Chem. Phys. 2017, 194, 293–301. [Google Scholar] [CrossRef]
- Fan, L.; Zhou, Y.; He, M.; Tong, Y.; Zhong, X.; Fang, J.; Bu, X. Facile microwave approach to controllable boron nitride quantum dots. J. Mater. Sci. 2017, 52, 13522–13532. [Google Scholar] [CrossRef]
- Ganganboina, A.; Dutta Chowdhury, A.; Doong, R.-a. N-Doped Graphene Quantum Dots-Decorated V2O5 Nanosheet for Fluorescence Turn Off-On Detection of Cysteine. ACS Appl. Mater. Interfaces 2018, 10, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Ganganboina, A.B.; Doong, R.A. Graphene Quantum Dots Decorated Gold-Polyaniline Nanowire for Impedimetric Detection of Carcinoembryonic Antigen. Sci. Rep. 2019, 9, 7214. [Google Scholar] [CrossRef]
- Tang, Y.; Su, Y.; Yang, N.; Zhang, L.; Lv, Y. Carbon nitride quantum dots: A novel chemiluminescence system for selective detection of free chlorine in water. Anal. Chem. 2014, 86, 4528–4535. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Nie, Y.; Zhang, Q.; Ma, Q. Sulfur Regulated Boron Nitride Quantum Dots Electrochemiluminescence with Amplified Surface Plasmon Coupling Strategy for BRAF Gene Detection. Anal. Chem. 2019, 91, 6250–6258. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Liu, M.; Liu, Y. 2D titanium carbide MXenes as emerging optical biosensing platforms. Biosens. Bioelectron. 2021, 171, 112730. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhang, L.; Wen, W.; Zhang, X.; Wang, S. Enzyme catalytic amplification of miRNA-155 detection with graphene quantum dot-based electrochemical biosensor. Biosens. Bioelectron. 2016, 77, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Akbarnia, A.; Zare, H.R.; Moshtaghioun, S.M.; Benvidi, A. Highly selective sensing and measurement of microRNA-541 based on its sequence-specific digestion by the restriction enzyme Hinf1. Colloids Surf. B Biointerfaces 2019, 182, 110360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chai, Y.; Wang, H.; Yuan, R. Target-Induced 3D DNA Network Structure as a Novel Signal Amplifier for Ultrasensitive Electrochemiluminescence Detection of MicroRNAs. Anal. Chem. 2019, 91, 14368–14374. [Google Scholar] [CrossRef]
- Liang, Z.; Nie, Y.; Zhang, X.; Wang, P.; Ma, Q. Multiplex Electrochemiluminescence Polarization Assay Based on the Surface Plasmon Coupling Effect of Au NPs and Ag@Au NPs. Anal. Chem. 2021, 93, 7491–7498. [Google Scholar] [CrossRef]
- Dutta Chowdhury, A.; Ganganboina, A.B.; Nasrin, F.; Takemura, K.; Doong, R.A.; Utomo, D.I.S.; Lee, J.; Khoris, I.M.; Park, E.Y. Femtomolar Detection of Dengue Virus DNA with Serotype Identification Ability. Anal. Chem. 2018, 90, 12464–12474. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, X.; Zhang, Q.; Liang, Z.; Ma, Q.; Su, X. A novel high efficient electrochemiluminescence sensor based on reductive Cu(I) particles catalyzed Zn-doped MoS2 QDs for HPV 16 DNA determination. Biosens. Bioelectron. 2020, 160, 112217. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, C.X.; Zhu, J.W.; Zong, H.L.; Hu, Y.H.; Wang, Y.Z. Ultrasensitive and Visual Electrochemiluminescence Ratiometry Based on a Constant Resistor-Integrated Bipolar Electrode for MicroRNA Detection. Anal. Chem. 2022, 94, 4303–4310. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Y.; Li, D.; Li, W.; Gou, X.; Gou, Y.; Hu, F. Development of a sensitive electrochemical immunosensor using polyaniline functionalized graphene quantum dots for detecting a depression marker. Mater. Sci. Eng. C 2020, 111, 110797. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, Q.; Liu, Q.; Li, Y.; Liu, H.; Wang, P.; Chen, L.; Zhang, D.; Li, Y.; Dong, Y. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification strategy of echinoidea-shaped Au@Ag-Cu2O nanoparticles for prostate specific antigen detection. Biosens. Bioelectron. 2018, 99, 450–457. [Google Scholar] [CrossRef]
- Nie, G.; Wang, Y.; Tang, Y.; Zhao, D.; Guo, Q. A graphene quantum dots based electrochemiluminescence immunosensor for carcinoembryonic antigen detection using poly(5-formylindole)/reduced graphene oxide nanocomposite. Biosens. Bioelectron. 2018, 101, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhao, Z.; Zheng, M.; Su, B.; Chen, X.; Chen, X. Electrochemical synthesis of phosphorus and sulfur co-doped graphene quantum dots as efficient electrochemiluminescent immunomarkers for monitoring okadaic acid. Sens. Actuators B Chem. 2020, 304, 127383. [Google Scholar] [CrossRef]
- Yan, Q.; Yang, Y.; Tan, Z.; Liu, Q.; Liu, H.; Wang, P.; Chen, L.; Zhang, D.; Li, Y.; Dong, Y. A label-free electrochemical immunosensor based on the novel signal amplification system of AuPdCu ternary nanoparticles functionalized polymer nanospheres. Biosens. Bioelectron. 2018, 103, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, S.; Lv, L.; Hong, Z.; Dai, H.; Lin, Y. Integrated heterojunction and photothermal effect multiple enhanced ratiometric electrochemiluminescence immunosensor based on calcination controlled and tunable TiO2 mesocrystals. Sens. Actuators B Chem. 2021, 346, 130565. [Google Scholar] [CrossRef]
- Baluta, S.; Lesiak, A.; Cabaj, J. Graphene Quantum Dots-based Electrochemical Biosensor for Catecholamine Neurotransmitters Detection. Electroanalysis 2018, 30, 1781–1790. [Google Scholar] [CrossRef]
- Gupta, S.; Smith, T.; Banaszak, A.; Boeckl, J. Graphene Quantum Dots Electrochemistry and Development of Ultrasensitive Enzymatic Glucose Sensor. MRS Adv. 2018, 3, 831–847. [Google Scholar] [CrossRef]
- Nashruddin, S.N.A.; Abdullah, J.; Mohammad Haniff, M.A.S.; Mat Zaid, M.H.; Choon, O.P.; Mohd Razip Wee, M.F. Label Free Glucose Electrochemical Biosensor Based on Poly(3,4-ethylenedioxy thiophene):Polystyrene Sulfonate/Titanium Carbide/Graphene Quantum Dots. Biosensors 2021, 11, 267. [Google Scholar] [CrossRef]
- Erkmen, C.; Demir, Y.; Kurbanoglu, S.; Uslu, B. Multi-Purpose electrochemical tyrosinase nanobiosensor based on poly (3,4 ethylenedioxythiophene) nanoparticles decorated graphene quantum dots: Applications to hormone drugs analyses and inhibition studies. Sens. Actuators B Chem. 2021, 343, 130164. [Google Scholar] [CrossRef]
- Salehnia, F.; Hosseini, M.; Ganjali, M.R. Enhanced electrochemiluminescence of luminol by an in situ silver nanoparticle-decorated graphene dot for glucose analysis. Anal. Methods 2018, 10, 508–514. [Google Scholar] [CrossRef]
- Zuo, F.; Zhang, C.; Zhang, H.; Tan, X.; Chen, S.; Yuan, R. A solid-state electrochemiluminescence biosensor for Con A detection based on CeO2@Ag nanoparticles modified graphene quantum dots as signal probe. Electrochim. Acta 2019, 294, 76–83. [Google Scholar] [CrossRef]
- Ghanbari, K.; Roushani, M. A novel electrochemical aptasensor for highly sensitive and quantitative detection of the streptomycin antibiotic. Bioelectrochemistry 2018, 120, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gogola, J.L.; Martins, G.; Gevaerd, A.; Blanes, L.; Cardoso, J.; Marchini, F.K.; Banks, C.E.; Bergamini, M.F.; Marcolino-Junior, L.H. Label-free aptasensor for p24-HIV protein detection based on graphene quantum dots as an electrochemical signal amplifier. Anal. Chim. Acta 2021, 1166, 338548. [Google Scholar] [CrossRef] [PubMed]
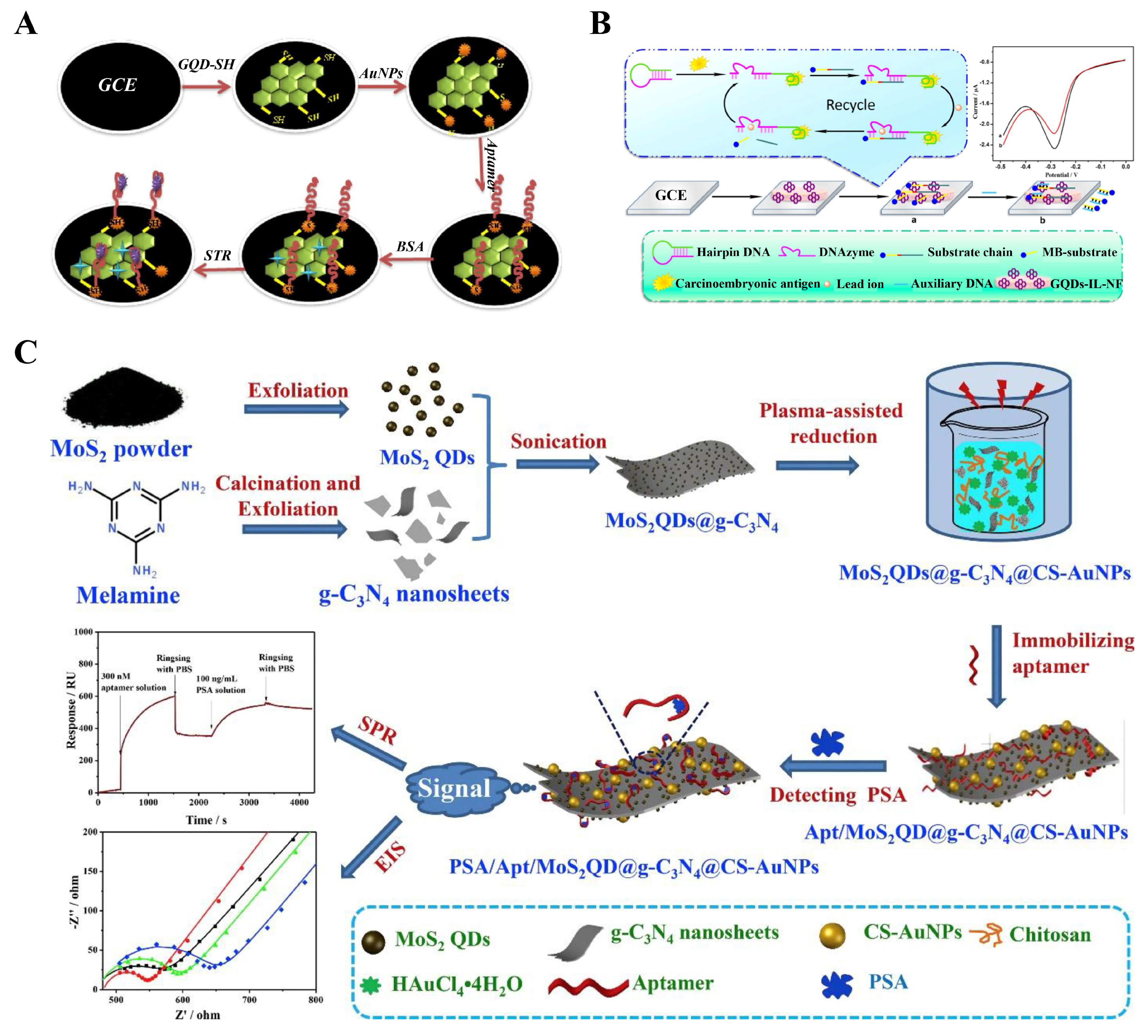
- Huang, J.Y.; Zhao, L.; Lei, W.; Wen, W.; Wang, Y.J.; Bao, T.; Xiong, H.Y.; Zhang, X.H.; Wang, S.F. A high-sensitivity electrochemical aptasensor of carcinoembryonic antigen based on graphene quantum dots-ionic liquid-nafion nanomatrix and DNAzyme-assisted signal amplification strategy. Biosens. Bioelectron. 2018, 99, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Zhang, S.; Yang, L.; Zhang, Z.; He, L.; Wang, M. Bifunctional aptasensor based on novel two-dimensional nanocomposite of MoS2 quantum dots and g-C3N4 nanosheets decorated with chitosan-stabilized Au nanoparticles for selectively detecting prostate specific antigen. Anal. Chim. Acta 2018, 1036, 121–132. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Dong, Y.; Chu, X. Electrogenerated chemiluminescence aptasensor for lysozyme based on copolymer nanospheres encapsulated black phosphorus quantum dots. Talanta 2019, 199, 507–512. [Google Scholar] [CrossRef]
- Yin, H.; Shi, Y.; Liu, H.; Dong, Y.; Chu, X. Dual-potential electrochemiluminescence of single luminophore for detection of biomarker based on black phosphorus quantum dots as co-reactant. Mikrochim. Acta 2021, 188, 181. [Google Scholar] [CrossRef]
- Nxele, S.R.; Nyokong, T. The effects of the composition and structure of quantum dots combined with cobalt phthalocyanine and an aptamer on the electrochemical detection of prostate specific antigen. Dyes Pigment. 2021, 192, 109407. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, C.; Zhang, S.; He, L.; Wang, M.; Peng, D.; Tian, J.; Fang, S. Carbon-based nanocomposites with aptamer-templated silver nanoclusters for the highly sensitive and selective detection of platelet-derived growth factor. Biosens. Bioelectron. 2017, 89, 735–742. [Google Scholar] [CrossRef]
- Khosropour, H.; Rezaei, B.; Rezaei, P.; Ensafi, A.A. Ultrasensitive voltammetric and impedimetric aptasensor for diazinon pesticide detection by VS2 quantum dots-graphene nanoplatelets/carboxylated multiwalled carbon nanotubes as a new group nanocomposite for signal enrichment. Anal. Chim. Acta 2020, 1111, 92–102. [Google Scholar] [CrossRef]
- Jiang, D.; Qin, M.; Zhang, L.; Shan, X.; Chen, Z. Ultrasensitive all-solid-state electrochemiluminescence platform for kanamycin detection based on the pore confinement effect of 0D g-C3N4 quantum dots/3D graphene hydrogel. Sens. Actuators B Chem. 2021, 345, 130343. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Huang, K.-J.; Wu, X. Recent advances in transition-metal dichalcogenides based electrochemical biosensors: A review. Biosens. Bioelectron. 2017, 97, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Luo, Y.; Zhu, C.; Li, H.; Du, D.; Lin, Y. Recent Advances in Electrochemical Biosensors based on Graphene Two-Dimensional Nanomaterials. Biosens. Bioelectron. 2015, 76, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Hai, X.; Li, Y.; Zhu, C.; Song, W.; Cao, J.; Bi, S. DNA-based label-free electrochemical biosensors: From principles to applications. TrAC Trends Anal. Chem. 2020, 133, 116098. [Google Scholar] [CrossRef]
- Wu, L.; Erhu, X.; Zhang, X.; Xiaohua, Z.; Chen, J. Nanomaterials as signal amplification elements in DNA-based electrochemical sensing. Nano Today 2014, 9, 197–211. [Google Scholar]
- Dutta, K.; De, S.; Das, B.; Bera, S.; Guria, B.; Ali, M.S.; Chattopadhyay, D. Development of an Efficient Immunosensing Platform by Exploring Single-Walled Carbon Nanohorns (SWCNHs) and Nitrogen Doped Graphene Quantum Dot (N-GQD) Nanocomposite for Early Detection of Cancer Biomarker. ACS Biomater. Sci. Eng. 2021, 7, 5541–5554. [Google Scholar] [CrossRef]
- Gupta, S.; Smith, T.; Banaszak, A.; Boeckl, J. Graphene Quantum Dots Electrochemistry and Sensitive Electrocatalytic Glucose Sensor Development. Nanomaterials 2017, 7, 301. [Google Scholar] [CrossRef]
| Method | Product Type | Precursors | Size [nm] | Ref. |
|---|---|---|---|---|
| Ultrasonication assisted method | PGQDs | Natural graphite powder | 2–4 | [50] |
| EGQDs | Expanded graphite powder | |||
| GOQDs | Graphite oxide powder | |||
| BPQDs | Black phosphorus | 4.9 ± 1.6 | [36] | |
| BPQDs | Black phosphorus | <20 | [51] | |
| g-C3N4 QDs | Cyanuric acid 2,4-diamino-6-phenyl-1,3,5-triazine | <100 | [52] | |
| g-C3N4 QDs | Recrystallized dicyandiamide | 5–200 | [53] | |
| BNQDs | h-BN | 7.71–13.2 | [54] | |
| MoS2 QDs | Molybdenum disulfide powder | 4.2 ± 0.1 | [55] | |
| Hydro/ Solvothermal method | WO3−x QDs | WCl6 | 3.25 ± 0.25 | [49] |
| N, S-GQDs | Citric acid, thiourea | 3.10 ± 0.54 | [56] | |
| N-MXene QDs | Layered Ti3C2 nanosheet | 3.4 | [57] | |
| MoS2/WS2 dots | MoS2/WS2 powder | 3 | [58] | |
| Ti3C2 QDs | Ti3C2 MXene | 2.9/3.7/6.2 | [59] | |
| BNQDs | h-BN powder | 1.7–10.9 | [60] | |
| Ion intercalation-assisted method | MoS2 QDs | MoS2 powder | 3 | [61] |
| MoS2 QDs | MoS2 bulk crystal | 3.5 | [62] | |
| BN QDs | h-BN flakes | 10 | [63] | |
| Microwave-assisted method | g-CNQDs | g-C3N4 | 3.5 ± 0.5 | [64] |
| BNQDs | h-BN powder | 1.98–7.05 | [65] |
| Method | Product Type | Precursors | Size (nm) | Ref. |
|---|---|---|---|---|
| Hydro/Solvothermal Method | N-GQDs | Citric acid, urea | 4.7±0.5 | [66] |
| N, S-GQDs | Citric acid, thiourea | 4.8 ± 0.5 | [67] | |
| External Microwave and Laser Assisted Method | g-CNQDs | Guanidine hydrochloride, EDTA | 5 | [68] |
| S-BN QDsT | Boric acid, melamine, thiourea | 9.8 | [69] | |
| S-BN QDsL | Boric acid, melamine, L-cysteine | 9.2 |
| Type | Sensors | Analyte | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|
| DNA Sensors | AuNPs/GQDs/GO/SPCE | microRNA-21 | 0.04 fM | 10−15–10−9 M | [21] |
| microRNA-155 | 0.33 fM | 10−15–10−9 M | |||
| microRNA-210 | 0.28 fM | 10−15–10−9 M | |||
| H2N-GQD/GCE | microRNA-25 | 0.95 pM | 0.3 nM–1.0 μM | [22] | |
| S-BNQDs/GCE | BRAF | 0.3 pM | 1 pM–1.5 nM | [69] | |
| NH2-DNA/GQDs/HRP/GE | microRNA-155 | 0.14 fM | 10−15–10−10 M | [72] | |
| GQDs/PGE | microRNA-541 | 0.7 fM | 1 fM–1 nM | [73] | |
| BNQDs/Ru/PtNPs/Nafion/GCE | microRNA-21 | 0.33 aM | 10−18–10−10 M | [74] | |
| BNQDs/GCE | BRCA | 0.33 fM | 10−16–10−9 M | [75] | |
| N,S-GQDs@AuNP/GE | DNA | 9.4 fM | 10−14–10−6 M | [76] | |
| Zn-doped MoS2 QDs/GCE | HPV 16 DNA | 0.03 nM | 0.1 nM–0.2 μM | [77] | |
| BNQDs/BPE | microRNA-141 | 0.1 aM | 10−17–10−7 M | [78] | |
| Immunological Sensors | Ab1/g-CNQDs/Ag@TCM/GCE | PSA | 6.9 fg/mL | 10 fg/mL–0.1 pg/mL | [23] |
| N-Ti3C2 QDs/GCE | MUC1 | 0.31 fg/mL | 1 fg/mL–1 ng/mL | [24] | |
| WS2-B QDs/SPE | Ferritin | 3.8 ng/mL | 10 ng/mL–1.5 μg/mL | [45] | |
| N,S-GQDs@Au/PANI/Pt | CEA | 10 pg/mL | 0.5 ng/mL–1 μg/mL | [67] | |
| HRP-Strept-Biotin-Ab-HSP70/ PAGD/GCE | HSP70 | 0.05 ng/mL | 0.0976–100 ng/mL | [79] | |
| Au@N-GQDs/GCE | PSA | 3 fg/mL | 10 pg/mL–0.1 μg/mL | [80] | |
| GQDs@AuNP-Ab2/CEA/BSA/Ab1/ AuNP/P5FIn/erGO/GE | CEA | 3.78 fg/mL | 0.1 pg/mL–10 ng/mL | [81] | |
| CMCNT-PDDA-AuNC/ GCE | Okadaic acid | 0.25 ng/mL | 0.01–20 ng/mL | [82] | |
| AuPdCu/N-GQDs@PS/GCE | HBsAg | 3.3 fg/mL | 10 fg/mL–50 ng/mL | [83] | |
| C-TiO2@g-CNQDs-Ab2/SFN/ Ab1/AuNPs/PVPTiO2@PFBT/GCE | SFN | 0.33 fg/mL | 1 fg/mL–100 pg/mL | [84] | |
| Enzyme Sensors | GCE/GQDs/Laccase | Epinephrine | 83 nM | 1–120 µM | [85] |
| GOx-GQD/GCE | Glucose | 1.35 µM | 10–250 µM | [86] | |
| PEDOT:PSS/Ti3C2/GQD/GOx/SPCE | Glucose | 65.0 µM | 0−500 µM | [87] | |
| Tyr/GQDs@PEDOT NPs/SPE | Catechol | 0.002 μM | 0.005–11 μM | [88] | |
| Epinephrine | 0.065 μM | 0.2–12 μM | |||
| norepinephrine | 0.035 μM | 0.1–2.5 μM | |||
| Nafion/GOx/GQD–luminol–AgNP/GCE | Glucose | 8 μM | 25–250 μM | [89] | |
| GOx-CeO2@Ag-GQDs/GCE | Concanavalin A | 0.16 pg/mL | 0.0005–1.0 ng/mL | [90] | |
| Aptasensors | AuNPs/GQD-SH/GCE | STR | 33 fg/mL | 0.1 pg/mL–0.7 ng/mL | [91] |
| GQDs/SPEs | HIV | 51.7 pg/mL | 0.93 ng/mL–93 mg/mL | [92] | |
| GQDs -IL-NF/GCE | CEA | 0.34 fg/mL | 0.5 fg/mL–0.5 ng mL | [93] | |
| MoS2QDs@g-C3N4@CS-AuNPs/AE | PSA | 0.72 ng/mL | 1.0 ng/mL–0.25 ng/mL | [94] | |
| BSAN/DNA/probe/GE | Lysozyme | 29 fg/mL | 0.1 pg/mL–0.1 ng/mL | [95] | |
| Fc-aptamer/BPQDs/RuNDs/GCE | MUC1 | 6.2 pg/mL | 20 pg/mL–10 ng/mL | [96] | |
| Aptamer/CoPc/NGQDs/GCE | PSA | 1.54 pM | 34 pg/mL–57 pg/mL | [97] | |
| GODs@AgNCs@Apt/GE | PGDF-BB | 0.82 pg/mL | 32.3 fM–1.61 pM | [98] | |
| VS2 QDs-GNP/CMWCNTs/GCE | Diazinon | 2.0 fM | 10−14–1.0–10−8 M | [99] | |
| g-C3N4 QDs-graphene hydrogel/GCE | Kanamycin | 0.33 pM | 1 pM–50 nM | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, X.; Bi, S. Two-Dimensional Quantum Dot-Based Electrochemical Biosensors. Biosensors 2022, 12, 254. https://doi.org/10.3390/bios12040254
Zhang J, Zhang X, Bi S. Two-Dimensional Quantum Dot-Based Electrochemical Biosensors. Biosensors. 2022; 12(4):254. https://doi.org/10.3390/bios12040254
Chicago/Turabian StyleZhang, Jian, Xiaoyue Zhang, and Sai Bi. 2022. "Two-Dimensional Quantum Dot-Based Electrochemical Biosensors" Biosensors 12, no. 4: 254. https://doi.org/10.3390/bios12040254
APA StyleZhang, J., Zhang, X., & Bi, S. (2022). Two-Dimensional Quantum Dot-Based Electrochemical Biosensors. Biosensors, 12(4), 254. https://doi.org/10.3390/bios12040254





