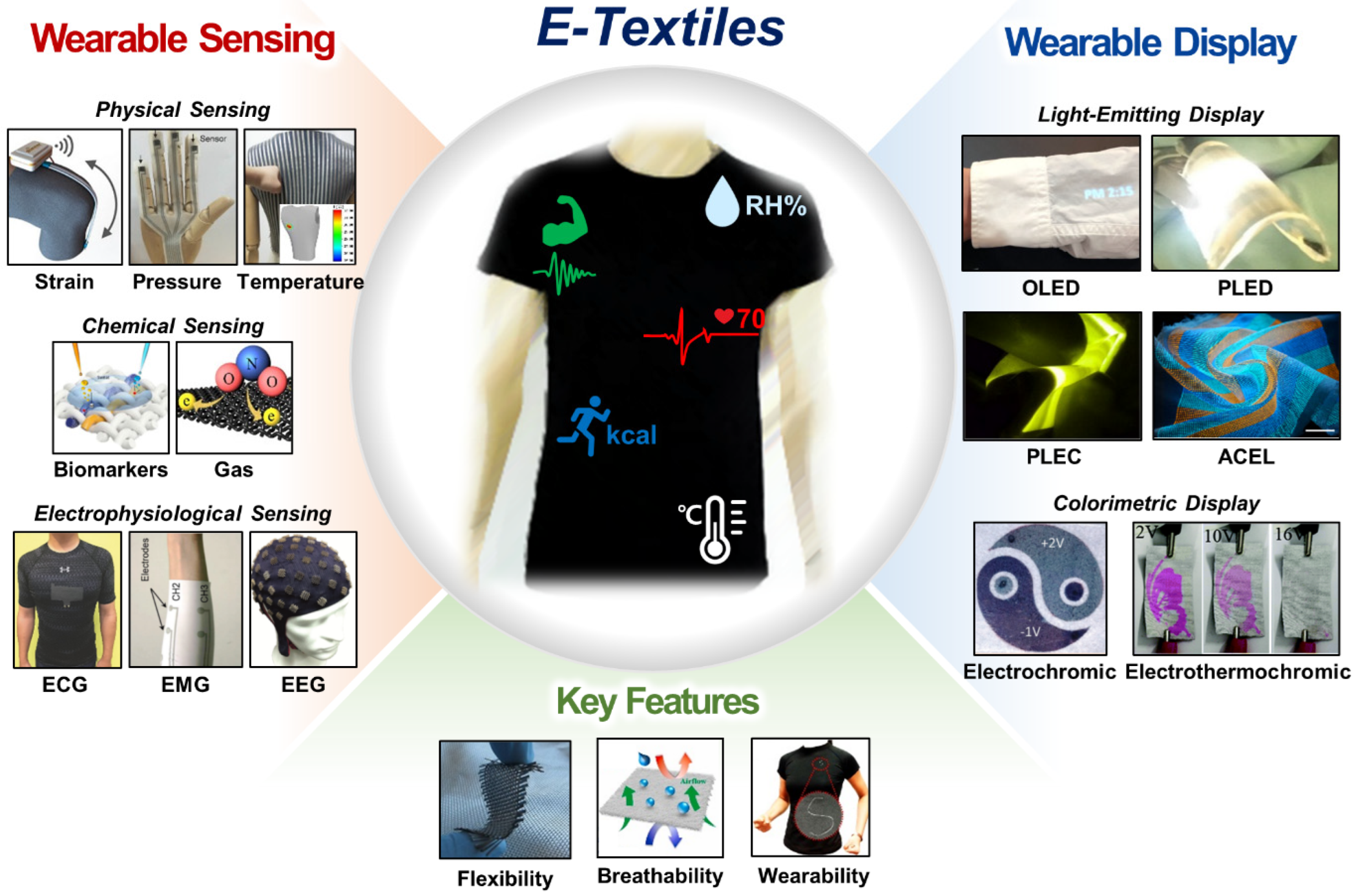
Smart Electronic Textiles for Wearable Sensing and Display
Abstract
:1. Introduction
2. Physical Sensing (Temperature, Strain, and Pressure)
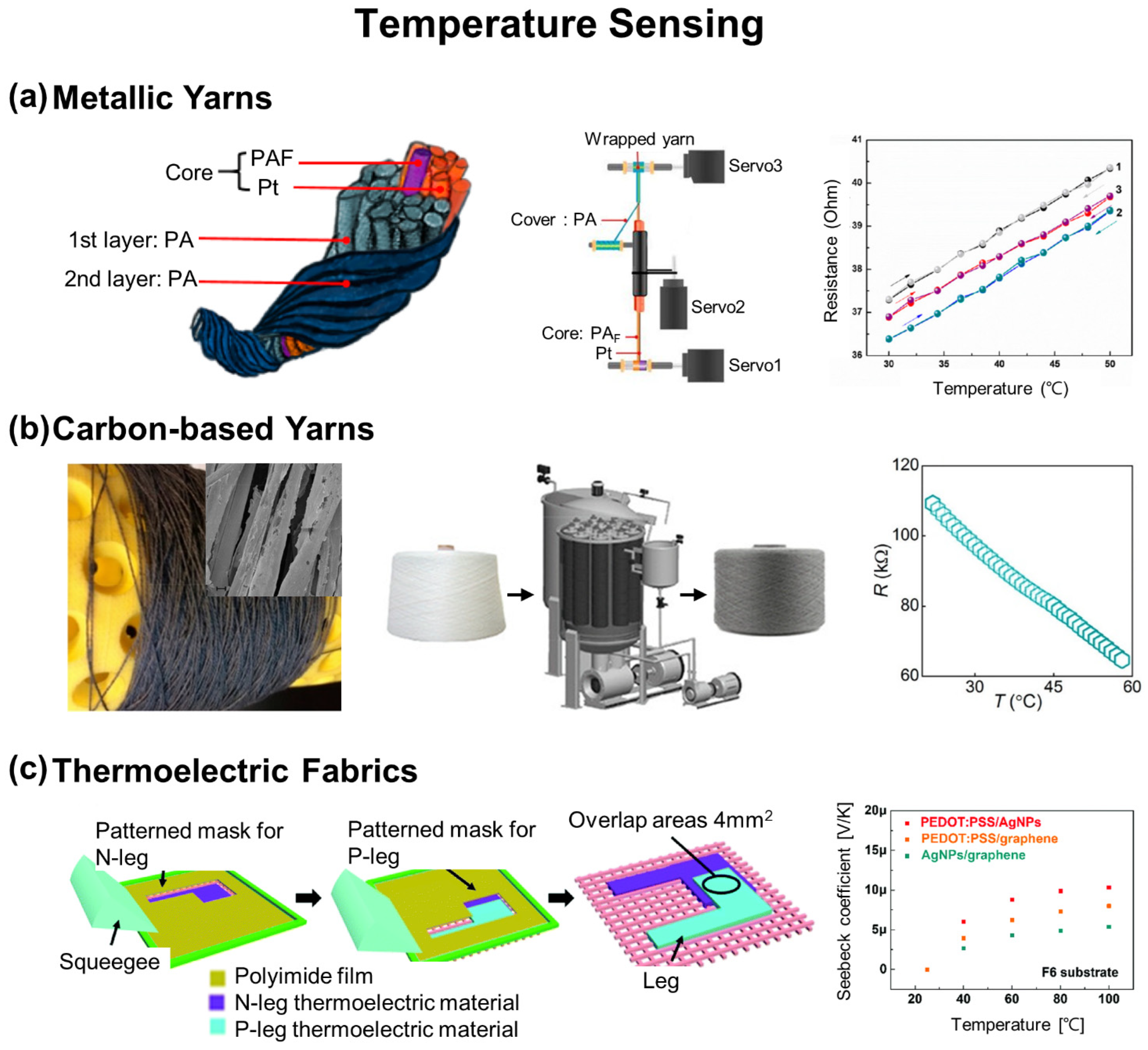
2.1. Temperature Sensing
2.2. Strain and Pressure Sensing
3. Chemical Sensing
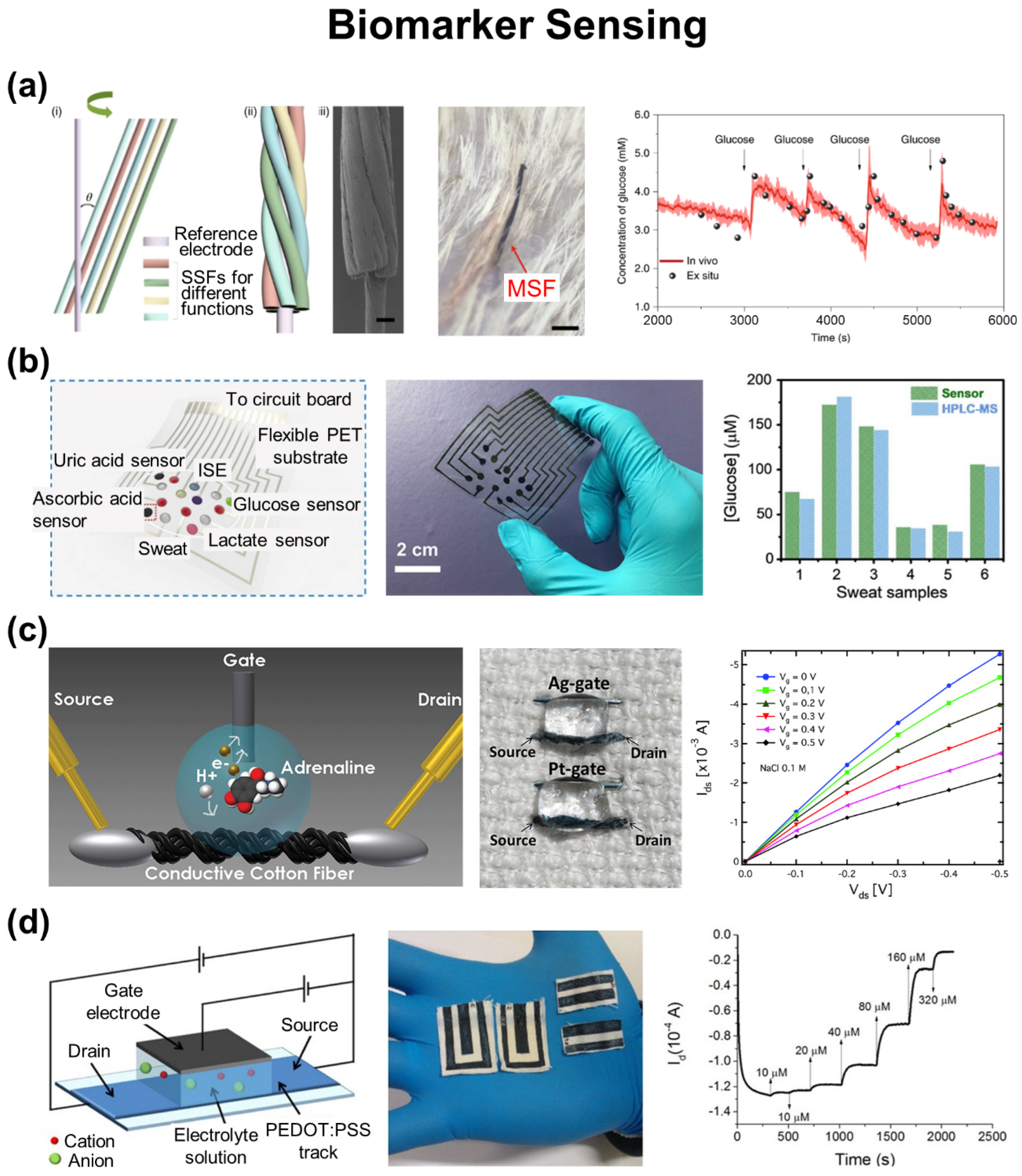
3.1. Biomarker Sensing
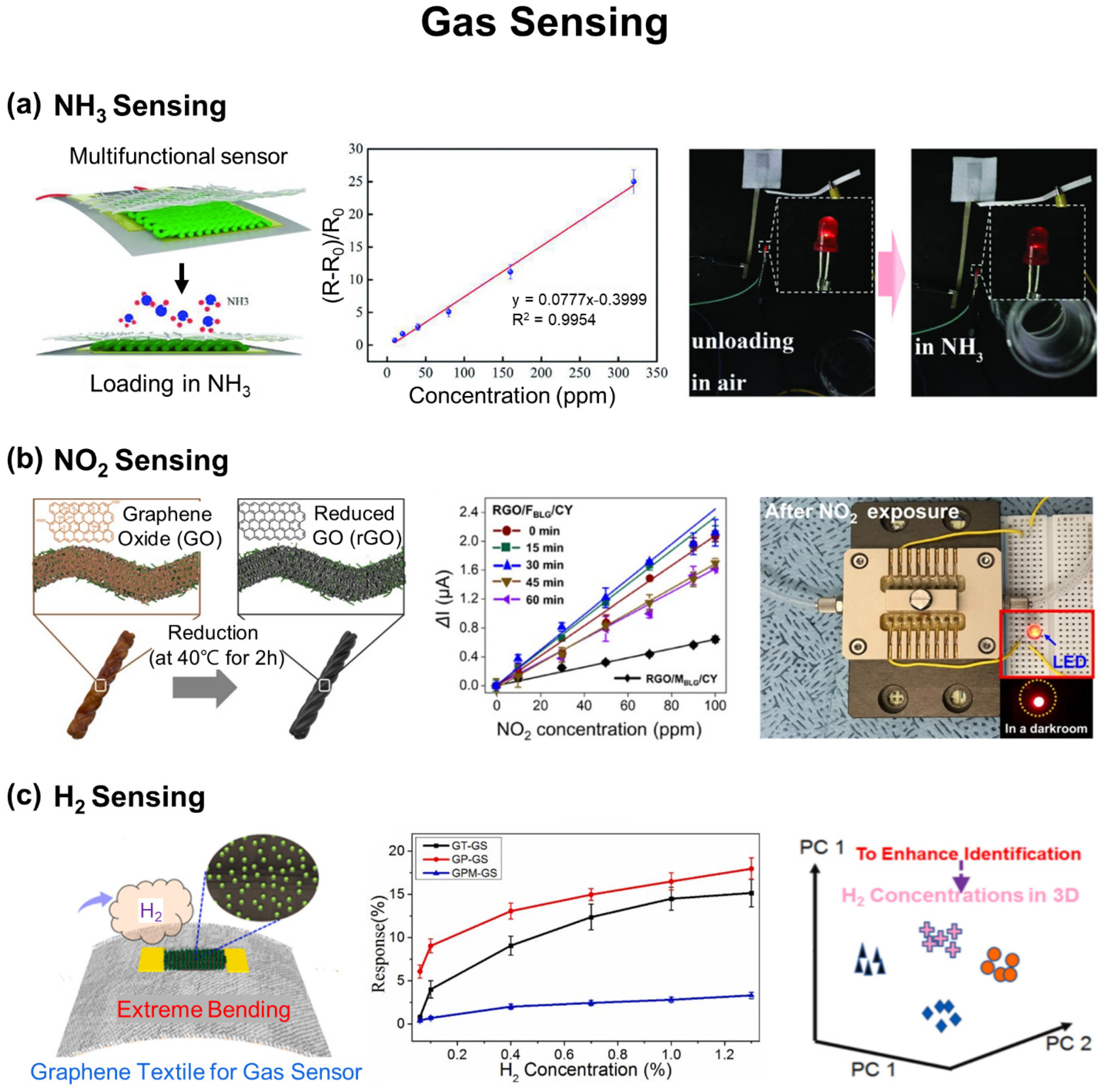
3.2. Gas Sensing
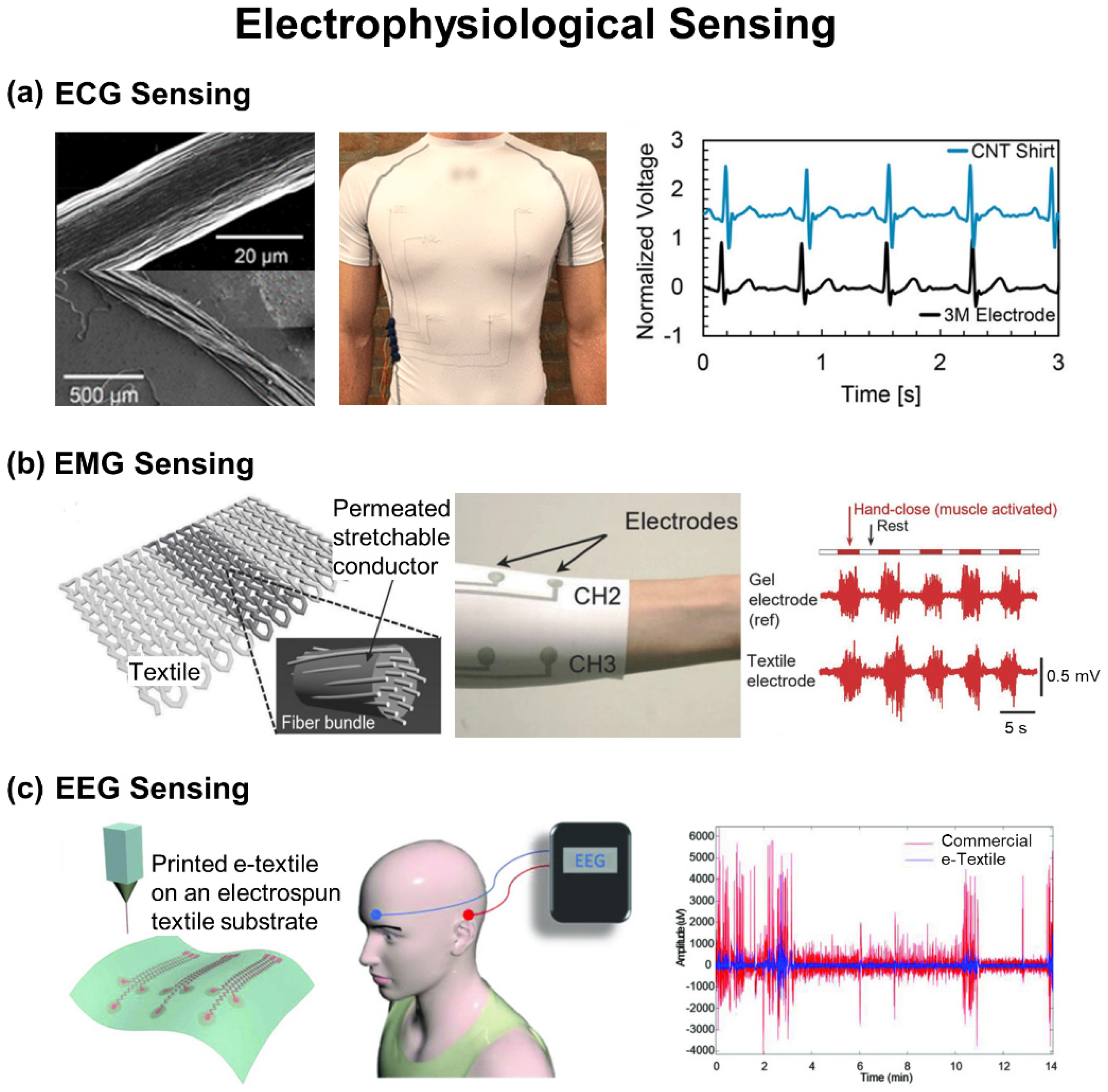
4. Electrophysiological Sensing
4.1. ECG Sensing
4.2. EMG Sensing
4.3. EEG Sensing
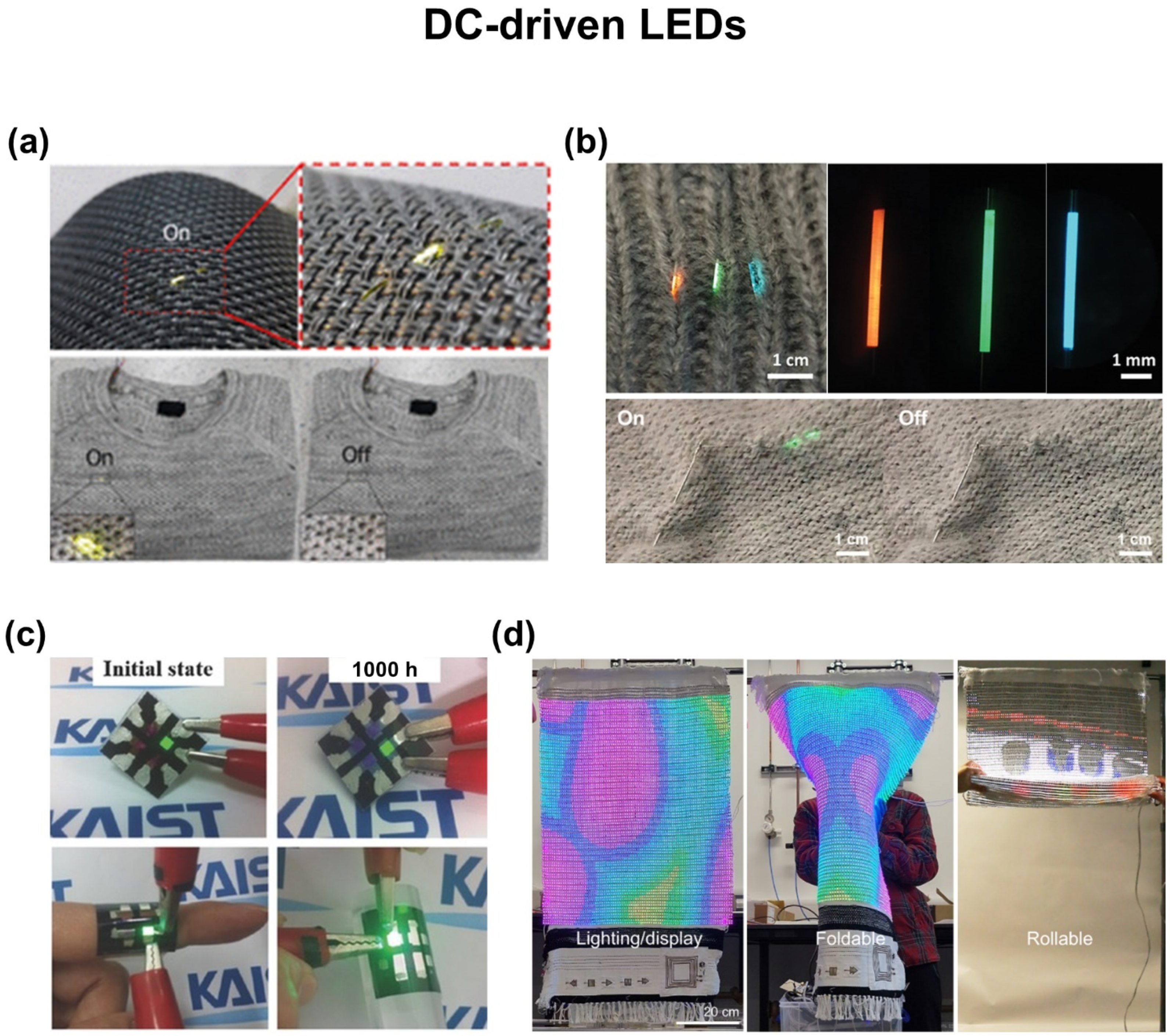
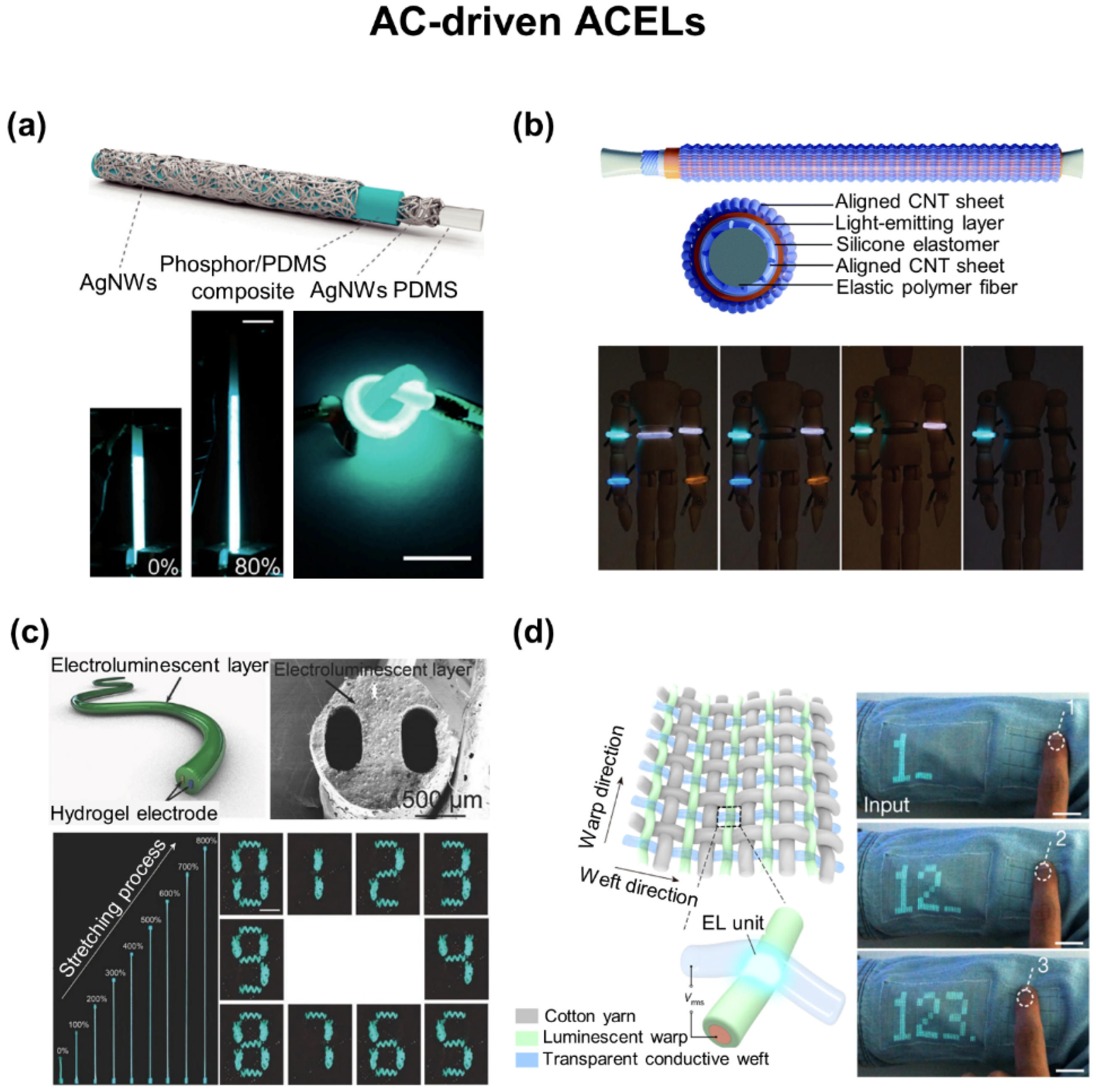
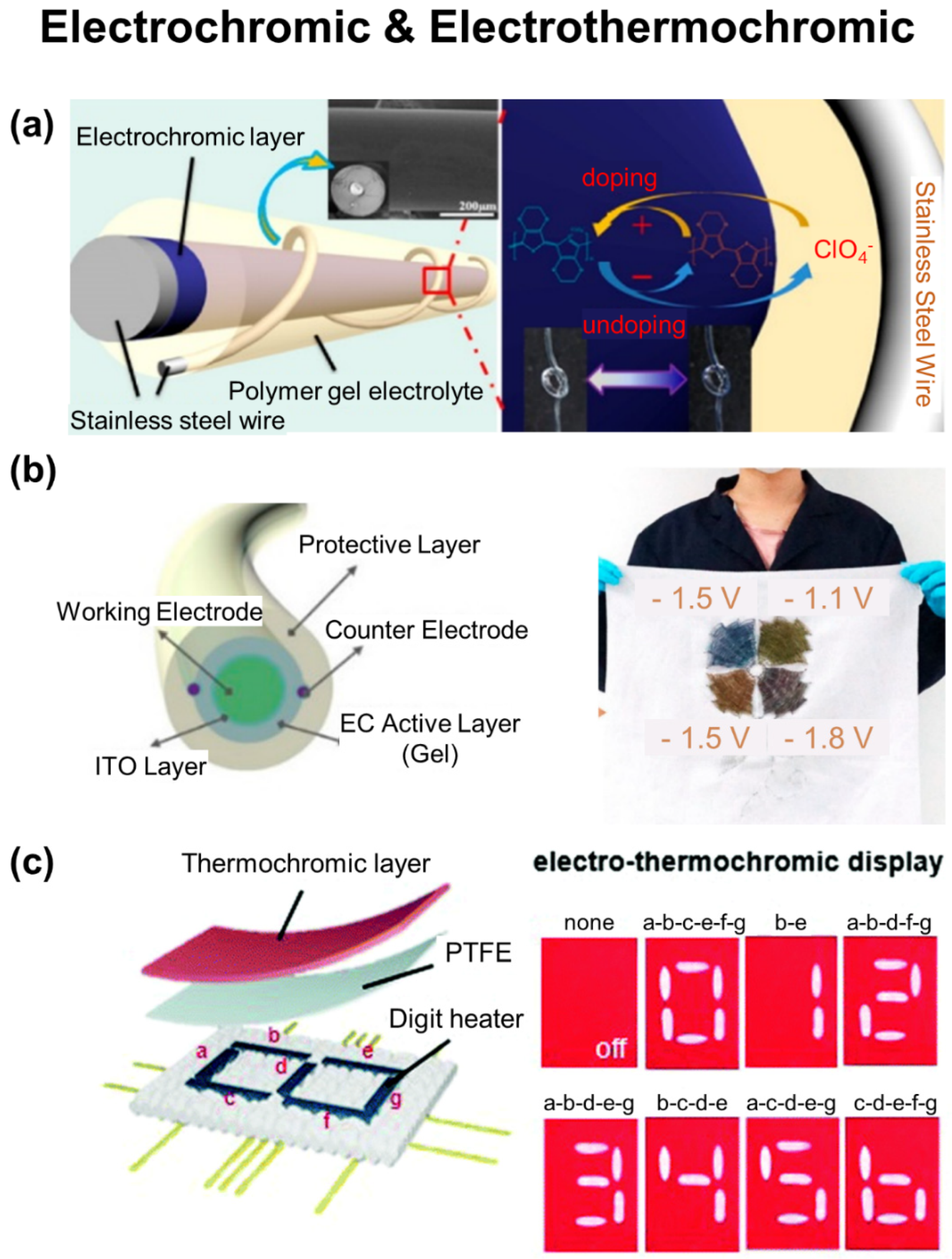
5. Wearable Displays
5.1. DC-Driven LEDs
5.2. AC-Driven ACELs
5.3. Colorimetric Display
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wearable Technology Market Size, Share & Trends Analysis Report by Product (Wrist-Wear, Eye-Wear & Head-Wear, Foot-Wear, Neck-Wear, Body-Wear), by Application, by Region, and Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/wearable-technology-market (accessed on 27 February 2022).
- Shi, Q.; Dong, B.; He, T.; Sun, Z.; Zhu, J.; Zhang, Z.; Lee, C. Progress in Wearable Electronics/Photonics-Moving Toward the Era of Artificial Intelligence and Internet of Things. InfoMat 2020, 2, 1131–1162. [Google Scholar] [CrossRef]
- Mamdiwar, S.D.; Akshith, R.; Shakruwala, Z.; Chadha, U.; Srinivasan, K.; Chang, C.-Y. Recent Advances on IoT-Assisted Wearable Sensor Systems for Healthcare Monitoring. Biosensors 2021, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, J.; Lim, J.; Nolan, J.K.; Lee, H.; Lee, C.H. Wearable Glucose Monitoring and Implantable Drug Delivery Systems for Diabetes Management. Adv. Healthcare Mater. 2021, 10, 2100194. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, W.; Lee, C.H. Electrochemically Active Materials and Wearable Biosensors for the in Situ Analysis of Body Fluids for Human Healthcare. NPG Asia Mater. 2021, 13, 23. [Google Scholar] [CrossRef]
- Kim, K.; Kim, B.; Lee, C.H. Printing Flexible and Hybrid Electronics for Human Skin and Eye-Interfaced Health Monitoring Systems. Adv. Mater. 2020, 32, 1902051. [Google Scholar] [CrossRef]
- Wang, J.; Lin, M.-F.; Park, S.; Lee, P.S. Deformable Conductors for Human–Machine Interface. Mater. Today 2018, 21, 508–526. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, 1801072. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Heo, J.S.; Eom, J.; Kim, Y.-H.; Park, S.K. Recent Progress of Textile-Based Wearable Electronics: A Comprehensive Review of Materials, Devices, and Applications. Small 2017, 14, 1703034. [Google Scholar] [CrossRef]
- Weng, W.; Chen, P.; He, S.; Sun, X.; Peng, H. Smart Electronic Textiles. Angew. Chem. Int. Ed. 2016, 55, 6140–6169. [Google Scholar] [CrossRef]
- Lee, J.; Zambrano, B.L.; Woo, J.; Yoon, K.; Lee, T. Recent Advances in 1D Stretchable Electrodes and Devices for Textile and Wearable Electronics: Materials, Fabrications, and Applications. Adv. Mater. 2019, 32, 1902532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhry, N.A.; Arnold, L.; Rasheed, A.; Khan, I.A.; Wang, L. Textronics—A Review of Textile-Based Wearable Electronics. Adv. Eng. Mater. 2021, 23, 2100469. [Google Scholar] [CrossRef]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.-M. Fiber-Based Wearable Electronics: A Review of Materials, Fabrication, Devices, and Applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in Textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiang, Z.; Ouyang, X.; Zhang, J.; Lau, N.; Zhou, J.; Chan, C.C. Wearable Fiber Optic Technology Based on Smart Textile: A Review. Materials 2019, 12, 3311. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Xiao, X.; Zhao, X.; Tat, T.; Bick, M.; Chen, J. Electronic Textiles for Wearable Point-of-Care Systems. Chem. Rev. 2021, 122, 3259–3291. [Google Scholar] [CrossRef]
- Kim, J.-E.; Kim, Y.-H.; Oh, J.-H.; Kim, K.-D. Interactive Smart Fashion Using User-Oriented Visible Light Communication: The Case of Modular Strapped Cuffs and Zipper Slider Types. Wirel. Commun. Mob. Comput. 2017, 2017, 5203053. [Google Scholar] [CrossRef] [Green Version]
- Cinquino, M.; Prontera, C.T.; Pugliese, M.; Giannuzzi, R.; Taurino, D.; Gigli, G.; Maiorano, V. Light-Emitting Textiles: Device Architectures, Working Principles, and Applications. Micromachines 2021, 12, 652. [Google Scholar] [CrossRef]
- Iakovlev, D.; Hu, S.; Hassan, H.; Dwyer, V.; Ashayer-Soltani, R.; Hunt, C.; Shen, J. Smart Garment Fabrics to Enable Non-Contact Opto-Physiological Monitoring. Biosensors 2018, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Fu, X.; He, J.; Shi, X.; Chen, T.; Chen, P.; Wang, B.; Peng, H. Application Challenges in Fiber and Textile Electronics. Adv. Mater. 2019, 32, 1901971. [Google Scholar] [CrossRef]
- Ismar, E.; Tao, X.; Rault, F.; Dassonville, F.; Cochrane, C. Towards Embroidered Circuit Board from Conductive Yarns for E-Textiles. IEEE Access 2020, 8, 155329–155336. [Google Scholar] [CrossRef]
- Lund, A.; Darabi, S.; Hultmark, S.; Ryan, J.D.; Andersson, B.; Ström, A.; Müller, C. Roll-to-Roll Dyed Conducting Silk Yarns: A Versatile Material for E-Textile Devices. Adv. Mater. Technol. 2018, 3, 1800251. [Google Scholar] [CrossRef] [Green Version]
- Atalay, O.; Kalaoglu, F.; Bahadir, S.K. Development of Textile-Based Transmission Lines Using Conductive Yarns and Ultrasonic Welding Technology for E-Textile Applications. J. Eng. Fibers Fabr. 2019, 14, 1558925019856603. [Google Scholar] [CrossRef]
- Seyedin, S.; Razal, J.M.; Innis, P.C.; Jeiranikhameneh, A.; Beirne, S.; Wallace, G.G. Knitted Strain Sensor Textiles of Highly Conductive All-Polymeric Fibers. ACS Appl. Mater. Interfaces 2015, 7, 21150–21158. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pu, X.; Jiang, C.; Liu, T.; Huang, X.; Chen, L.; Du, C.; Sun, J.; Hu, W.; Wang, Z.L. Large-Area All-Textile Pressure Sensors for Monitoring Human Motion and Physiological Signals. Adv. Mater. 2017, 29, 1703700. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, J.; Zhong, W.; Luo, M.; Wang, W.; Qing, X.; Lu, Y.; Liu, Q.; Liu, K.; Wang, Y.; et al. Large-Area, Wearable, Self-Powered Pressure-Temperature Sensor Based on 3D Thermoelectric Spacer Fabric. ACS Sens. 2020, 5, 2545–2554. [Google Scholar] [CrossRef]
- Possanzini, L.; Decataldo, F.; Mariani, F.; Gualandi, I.; Tessarolo, M.; Scavetta, E.; Fraboni, B. Textile Sensors Platform for the Selective and Simultaneous Detection of Chloride Ion and pH in Sweat. Sci. Rep. 2020, 10, 17180. [Google Scholar] [CrossRef]
- Jung, W.T.; Jang, H.-S.; Jeon, J.W.; Kim, B.H. Effect of Oxygen Functional Groups in Reduced Graphene Oxide-Coated Silk Electronic Textiles for Enhancement of NO2 Gas-Sensing Performance. ACS Omega 2021, 6, 27080–27088. [Google Scholar] [CrossRef]
- Tao, X.; Huang, T.-H.; Shen, C.-L.; Ko, Y.-C.; Jou, G.-T.; Koncar, V. Bluetooth Low Energy-Based Washable Wearable Activity Motion and Electrocardiogram Textronic Monitoring and Communicating System. Adv. Mater. Technol. 2018, 3, 1700309. [Google Scholar] [CrossRef]
- Jin, H.; Matsuhisa, N.; Lee, S.; Abbas, M.; Yokota, T.; Someya, T. Enhancing the Performance of Stretchable Conductors for E-Textiles by Controlled Ink Permeation. Adv. Mater. 2017, 29, 1605848. [Google Scholar] [CrossRef]
- Fiedler, P.; Pedrosa, P.; Griebel, S.; Fonseca, C.; Vaz, F.; Supriyanto, E.; Zanow, F.; Haueisen, J. Novel Multipin Electrode Cap System for Dry Electroencephalography. Brain Topogr. 2015, 28, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Jo, W.; Jeon, Y.; Kwon, S.; Kwon, J.H.; Son, Y.H.; Kim, J.; Park, J.H.; Kim, H.; Lee, H.S.; et al. Multi-Directionally Wrinkle-Able Textile OLEDs for Clothing-Type Displays. NPJ Flex. Electron. 2020, 4, 33. [Google Scholar] [CrossRef]
- Park, M.; Lee, K.S.; Shim, J.; Liu, Y.; Lee, C.; Cho, H.; Kim, M.J.; Park, S.-J.; Yun, Y.J.; Kim, H.Y.; et al. Environment Friendly, Transparent Nanofiber Textiles Consolidated with High Efficiency PLEDs for Wearable Electronics. Org. Electron. 2016, 36, 89–96. [Google Scholar] [CrossRef]
- Lanz, T.; Sandström, A.; Tang, S.; Chabrecek, P.; Sonderegger, U.; Edman, L. A Light–Emission Textile Device: Conformal Spray-Sintering of a Woven Fabric Electrode. Flex. Print. Electron. 2016, 1, 025004. [Google Scholar] [CrossRef]
- Shi, X.; Zuo, Y.; Zhai, P.; Shen, J.; Yang, Y.; Gao, Z.; Liao, M.; Wu, J.; Wang, J.; Xu, X.; et al. Large-Area Display Textiles Integrated with Functional Systems. Nature 2021, 591, 240–245. [Google Scholar] [CrossRef]
- Sinha, S.; Daniels, R.; Yassin, O.; Baczkowski, M.; Tefferi, M.; Deshmukh, A.; Cao, Y.; Sotzing, G. Electrochromic Fabric Displays from a Robust, Open-Air Fabrication Technique. Adv. Mater. Technol. 2022, 7, 2100548. [Google Scholar] [CrossRef]
- Ji, X.; Liu, W.; Yin, Y.; Wang, C.; Torrisi, F. A Graphene-Based Electro-Thermochromic Textile Display. J. Mater. Chem. C 2020, 8, 15788–15794. [Google Scholar] [CrossRef]
- Jur, J.S.; Sweet, W.J.; Oldham, C.J.; Parsons, G.N. Atomic Layer Deposition of Conductive Coatings on Cotton, Paper, and Synthetic Fibers: Conductivity Analysis and Functional Chemical Sensing Using “All-Fiber” Capacitors. Adv. Funct. Mater. 2011, 21, 1993–2002. [Google Scholar] [CrossRef]
- Yang, S.; Liu, S.; Ding, X.; Zhu, B.; Shi, J.; Yang, B.; Liu, S.; Chen, W.; Tao, X. Permeable and Washable Electronics Based on Polyamide Fibrous Membrane for Wearable Applications. Compos. Sci. Technol. 2021, 207, 108729. [Google Scholar] [CrossRef]
- Yin, Z.; Jian, M.; Wang, C.; Xia, K.; Liu, Z.; Wang, Q.; Zhang, M.; Wang, H.; Liang, X.; Liang, X.; et al. Splash-Resistant and Light-Weight Silk-Sheathed Wires for Textile Electronics. Nano Lett. 2018, 18, 7085–7091. [Google Scholar] [CrossRef]
- Schmied, H.; Reiter, A.; Kurz, A.; Sessler, D.I.; Kozek, S. Mild Hypothermia Increases Blood Loss and Transfusion Requirements During Total Hip Arthroplasty. Lancet 1996, 347, 289–292. [Google Scholar] [CrossRef]
- Tiainen, M.; Meretoja, A.; Strbian, D.; Suvanto, J.; Curtze, S.; Lindsberg, P.J.; Soinne, L.; Tatlisumak, T. Body Temperature, Blood Infection Parameters, and Outcome of Thrombolysis-Treated Ischemic Stroke Patients. Int. J. Stroke 2013, 8, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Epstein, Y.; Yanovich, R. Heatstroke. N. Engl. J. Med. 2019, 380, 2449–2459. [Google Scholar] [CrossRef] [PubMed]
- Marcy, S.M.; Kohl, K.S.; Dagan, R.; Nalin, D.; Blum, M.; Jones, M.C.; Hansen, J.; Labadie, J.; Lee, L.; Martin, B.L.; et al. Fever as an Adverse Event Following Immunization: Case Definition and Guidelines of Data Collection, Analysis, and Presentation. Vaccine 2004, 22, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Yamazaki, S.; Shimizu, T.; Takeshima, T.; Fukuma, S.; Yamamoto, Y.; Tochitani, K.; Tsuchido, Y.; Shinohara, K.; Fukuhara, S. Body Temperature at the Emergency Department as a Predictor of Mortality in Patients with Bacterial Infection. Medicine 2016, 95, e3628. [Google Scholar] [CrossRef]
- Husain, M.D.; Kennon, R.; Dias, T. Design and Fabrication of Temperature Sensing Fabric. J. Ind. Text. 2014, 44, 398–417. [Google Scholar] [CrossRef]
- Jung, M.; Jeon, S.; Bae, J. Scalable and Facile Synthesis of Stretchable Thermoelectric Fabric for Wearable Self-Powered Temperature Sensors. RSC Adv. 2018, 8, 39992–39999. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lu, C.; Zhang, K. Textile-Based Strain Sensor for Human Motion Detection. Energy Environ. Mater. 2020, 3, 80–100. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Ma, L.; Hou, C.; Meng, Z.; Guo, W.; Yu, W.; Yu, R.; Hu, F.; Liu, X.Y. Silk Composite Electronic Textile Sensor for High Space Precision 2D Combo Temperature-Pressure Sensing. Small 2019, 15, 1901558. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.; Hua, T.; Xu, B.; Jiang, S. Wearable Strain Sensing Textile Based on One-Dimensional Stretchable and Weavable Yarn Sensors. Nano Res. 2018, 11, 5799–5811. [Google Scholar] [CrossRef]
- Eom, J.; Jaisutti, R.; Lee, H.; Lee, W.; Heo, J.-S.; Lee, J.-Y.; Park, S.K.; Kim, Y.-H. Highly Sensitive Textile Strain Sensors and Wireless User-Interface Devices Using All-Polymeric Conducting Fibers. ACS Appl. Mater. Interfaces 2017, 9, 10190–10197. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Xu, B.; Cai, Z. Polypyrrole Coated Knitted Fabric for Robust Wearable Sensor and Heater. J. Mater. Sci. Mater. Electron. 2018, 29, 9218–9226. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, S.; Shi, J.; Zhang, H.; Zhu, F.; Yang, X. Determinants of Electrical Resistance Change of in Situ PPy-Polymerized Stretch Plain Woven Fabric under Uniaxial Tensile Strain. J. Text. Inst. 2017, 108, 1545–1551. [Google Scholar] [CrossRef]
- Jiyong, H.; Xiaofeng, Z.; Guohao, L.; Xudong, Y.; Xin, D. Electrical Properties of Ppy-coated Conductive Fabrics for Human Joint Motion Monitoring. Autex Res. J. 2016, 16, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Bai, Y.; Li, L.; Wang, S.; Zhang, T. A Superhydrophobic Smart Coating for Flexible and Wearable Sensing Electronics. Adv. Mater. 2017, 29, 1702517. [Google Scholar] [CrossRef]
- Zhang, L.; He, J.; Liao, Y.; Zeng, X.; Qiu, N.; Liang, Y.; Xiao, P.; Chen, T. A Self-Protective, Reproducible Textile Sensor with High Performance Towards Human–Machine Interactions. J. Mater. Chem. A 2019, 7, 26631–26640. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.W.; Hong, S.Y.; Lee, G.; Lee, H.; Song, C.; Keum, K.; Jeong, Y.R.; Jin, S.W.; Kim, D.S.; et al. Dynamically Stretchable Supercapacitor for Powering an Integrated Biosensor in an All-in-One Textile System. ACS Nano 2019, 13, 10469–10480. [Google Scholar] [CrossRef]
- Du, D.; Li, P.; Ouyang, J. Graphene Coated Nonwoven Fabrics as Wearable Sensors. J. Mater. Chem. C 2016, 4, 3224–3230. [Google Scholar] [CrossRef]
- Yang, Z.; Pang, Y.; Han, X.-L.; Yang, Y.; Ling, J.; Jian, M.; Zhang, Y.; Yang, Y.; Ren, T.-L. Graphene Textile Strain Sensor with Negative Resistance Variation for Human Motion Detection. ACS Nano 2018, 12, 9134–9141. [Google Scholar] [CrossRef]
- Yang, S.; Li, C.; Wen, N.; Xu, S.; Huang, H.; Cong, T.; Zhao, Y.; Fan, Z.; Liu, K.; Pan, L. All-Fabric-Based Multifunctional Textile Sensor for Detection and Discrimination of Humidity, Temperature, and Strain Stimuli. J. Mater. Chem. C 2021, 9, 13789–13798. [Google Scholar] [CrossRef]
- Biccai, S.; Boland, C.S.; O’driscoll, D.P.; Harvey, A.; Gabbett, C.; O’suilleabhain, D.R.; Griffin, A.J.; Li, Z.; Young, R.J.; Coleman, J.N. Negative Gauge Factor Piezoresistive Composites Based on Polymers Filled with MoS2 Nanosheets. ACS Nano 2019, 13, 6845–6855. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwon, H.; Seo, J.; Shin, S.; Koo, J.H.; Pang, C.; Son, S.; Kim, J.H.; Jang, Y.H.; Kim, D.E.; et al. Conductive Fiber-Based Ultrasensitive Textile Pressure Sensor for Wearable Electronics. Adv. Mater. 2015, 27, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Feng, Y.; Selven, D.S.M.T.; Yao, L.; Soon, R.H.; Yeo, J.C.; Lim, C.T. Dual-Core Capacitive Microfiber Sensor for Smart Textile Applications. ACS Appl. Mater. Interfaces 2019, 11, 33347–33355. [Google Scholar] [CrossRef] [PubMed]
- Keum, K.; Eom, J.; Lee, J.H.; Heo, J.S.; Park, S.K.; Kim, Y.-H. Fully-Integrated Wearable Pressure Sensor Array Enabled by Highly Sensitive Textile-Based Capacitive Ionotronic Devices. Nano Energy 2021, 79, 105479. [Google Scholar] [CrossRef]
- Uzun, S.; Seyedin, S.; Stoltzfus, A.L.; Levitt, A.S.; Alhabeb, M.; Anayee, M.; Strobel, C.J.; Razal, J.M.; Dion, G.; Gogotsi, Y. Knittable and Washable Multifunctional MXene-Coated Cellulose Yarns. Adv. Funct. Mater. 2019, 29, 1905015. [Google Scholar] [CrossRef]
- Meng, K.; Zhao, S.; Zhou, Y.; Wu, Y.; Zhang, S.; He, Q.; Wang, X.; Zhou, Z.; Fan, W.; Tan, X.; et al. A Wireless Textile-Based Sensor System for Self-Powered Personalized Health Care. Matter 2020, 2, 896–907. [Google Scholar] [CrossRef]
- Fan, W.; He, Q.; Meng, K.; Tan, X.; Zhou, Z.; Zhang, G.; Yang, J.; Wang, Z.L. Machine-Knitted Washable Sensor Array Textile for Precise Epidermal Physiological Signal Monitoring. Sci. Adv. 2020, 6, eaay2840. [Google Scholar] [CrossRef] [Green Version]
- Lei, H.; Chen, Y.; Gao, Z.; Wen, Z.; Sun, X. Advances in Self-Powered Triboelectric Pressure Sensors. J. Mater. Chem. A 2021, 9, 20100–20130. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Ding, X.; Li, Q. Fabrication and Characterization of Wrapped Metal Yarns-based Fabric Temperature Sensors. Polymers 2019, 11, 1549. [Google Scholar] [CrossRef] [Green Version]
- Polanský, R.; Soukup, R.; Řeboun, J.; Kalčík, J.; Moravcová, D.; Kupka, L.; Švantner, M.; Honnerová, P.; Hamáček, A. A Novel Large-Area Embroidered Temperature Sensor Based on an Innovative Hybrid Resistive Thread. Sens. Actuators A Phys. 2017, 265, 111–119. [Google Scholar] [CrossRef]
- Afroj, S.; Karim, N.; Wang, Z.; Tan, S.; He, P.; Holwill, M.; Ghazaryan, D.; Fernando, A.; Novoselov, K.S. Engineering Graphene Flakes for Wearable Textile Sensors via Highly Scalable and Ultrafast Yarn Dyeing Technique. ACS Nano 2019, 13, 3847–3857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon Nanotubes: Sensor Properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Hasanpour, S.; Rashidi, A.; Walsh, T.; Pagan, E.; Milani, A.S.; Akbari, M.; Djilali, N. Electrode-Integrated Textile-Based Sensors for in Situ Temperature and Relative Humidity Monitoring in Electrochemical Cells. ACS Omega 2021, 6, 9509–9519. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, S.; Lee, S.; Song, J.; Kang, S.; Han, H.; Kim, S.; Kim, S.; Seo, J.; Kim, D.; et al. Highly Sensitive Multifilament Fiber Strain Sensors with Ultrabroad Sensing Range for Textile Electronics. ACS Nano 2018, 12, 4259–4268. [Google Scholar] [CrossRef] [PubMed]
- Tabor, J.; Agcayazi, T.; Fleming, A.; Thompson, B.; Kapoor, A.; Liu, M.; Lee, M.Y.; Huang, H.; Bozkurt, A.; Ghosh, T.K. Textile-Based Pressure Sensors for Monitoring Prosthetic-Socket Interfaces. IEEE Sens. J. 2021, 21, 9413–9422. [Google Scholar] [CrossRef]
- Tessarolo, M.; Gualandi, I.; Fraboni, B. Recent Progress in Wearable Fully Textile Chemical Sensors. Adv. Mater. Technol. 2018, 3, 1700310. [Google Scholar] [CrossRef]
- Piper, A.; Månsson, I.Ö.; Khaliliazar, S.; Landin, R.; Hamedi, M.M. A Disposable, Wearable, Flexible, Stitched Textile Electrochemical Biosensing Platform. Biosens. Bioelectron. 2021, 194, 113604. [Google Scholar] [CrossRef]
- Choudhary, T.; Rajamanickam, G.P.; Dendukuri, D. Woven Electrochemical Fabric-Based Test Sensors (WEFTS): A New Class of Multiplexed Electrochemical Sensors. Lab Chip 2015, 15, 2064–2072. [Google Scholar] [CrossRef]
- Liu, X.; Lillehoj, P.B. Embroidered Electrochemical Sensors for Biomolecular Detection. Lab Chip 2016, 16, 2093–2098. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, Q.; Dong, D.; An, T.; Gong, S.; Shi, Q.; Cheng, W. Highly Stretchable and Strain-Insensitive Fiber-Based Wearable Electrochemical Biosensor to Monitor Glucose in the Sweat. Anal. Chem. 2019, 91, 6569–6576. [Google Scholar] [CrossRef]
- Xu, W.; Lu, J.; Huo, W.; Li, J.; Wang, X.; Zhang, C.; Gu, X.; Hu, C. Direct Growth of CuCo2S4 Nanosheets on Carbon Fiber Textile with Enhanced Electrochemical Pseudocapacitive Properties and Electrocatalytic Properties Towards Glucose Oxidation. Nanoscale 2018, 10, 14304–14313. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhai, Q.; An, T.; Gong, S.; Cheng, W. Stretchable Gold Fiber-Based Wearable Textile Electrochemical Biosensor for Lactate Monitoring in Sweat. Talanta 2021, 222, 121484. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Kim, K.N.; Lv, J.; Tehrani, F.; Lin, M.; Lin, Z.; Moon, J.-M.; Ma, J.; Yu, J.; Xu, S.; et al. A Self-Sustainable Wearable Multi-Modular E-Textile Bioenergy Microgrid System. Nat. Commun. 2021, 12, 1542. [Google Scholar] [CrossRef] [PubMed]
- Manjakkal, L.; Dang, W.; Yogeswaran, N.; Dahiya, R. Textile-Based Potentiometric Electrochemical pH Sensor for Wearable Applications. Biosensors 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppedè, N.; Giannetto, M.; Villani, M.; Lucchini, V.; Battista, E.; Careri, M.; Zappettini, A. Ion Selective Textile Organic Electrochemical Transistor for Wearable Sweat Monitoring. Org. Electron. 2020, 78, 105579. [Google Scholar] [CrossRef]
- Battista, E.; Lettera, V.; Villani, M.; Calestani, D.; Gentile, F.; Netti, P.A.; Iannotta, S.; Zappettini, A.; Coppedè, N. Enzymatic Sensing with Laccase-Functionalized Textile Organic Biosensors. Org. Electron. 2017, 40, 51–57. [Google Scholar] [CrossRef]
- Tarabella, G.; Villani, M.; Calestani, D.; Mosca, R.; Iannotta, S.; Zappettini, A.; Coppedè, N. A Single Cotton Fiber Organic Electrochemical Transistor for Liquid Electrolyte Saline Sensing. J. Mater. Chem. 2012, 22, 23830–23834. [Google Scholar] [CrossRef]
- Dutkiewicz, E.P.; Lin, J.-D.; Tseng, T.-W.; Wang, Y.-S.; Urban, P.L. Hydrogel Micropatches for Sampling and Profiling Skin Metabolites. Anal. Chem. 2014, 86, 2337–2344. [Google Scholar] [CrossRef]
- Xing, S.; Jiang, J.; Pan, T. Interfacial Microfluidic Transport on Micropatterned Superhydrophobic Textile. Lab Chip 2013, 13, 1937–1947. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, C. Low-Cost, High-Throughput Fabrication of Cloth-Based Microfluidic Devices Using a Photolithographical Patterning Technique. Lab Chip 2015, 15, 1598–1608. [Google Scholar] [CrossRef]
- He, X.; Yang, S.; Pei, Q.; Song, Y.; Liu, C.; Xu, T.; Zhang, X. Integrated Smart Janus Textiles Bands for Self-Pumping Sweat Sampling and Analysis. ACS Sens. 2020, 5, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Jung, H.G.; Jang, J.W.; Park, D.; Lee, D.; Kim, I.; Kim, Y.; Cheong, D.Y.; Hwang, K.S.; Lee, G.; et al. Graphene-Based Electronic Textile Sheet for Highly Sensitive Detection of NO2 and NH3. Sens. Actuators B Chem. 2021, 345, 130361. [Google Scholar] [CrossRef]
- Zhu, J.; Cho, M.; Li, Y.; He, T.; Ahn, J.; Park, J.; Ren, T.-L.; Lee, C.; Park, I. Machine Learning-Enabled Textile-Based Graphene Gas Sensing with Energy Harvesting-Assisted IoT Application. Nano Energy 2021, 86, 106035. [Google Scholar] [CrossRef]
- Yun, Y.J.; Hong, W.G.; Choi, N.-J.; Kim, B.H.; Jun, Y.; Lee, H.-K. Ultrasensitive and Highly Selective Graphene-Based Single Yarn for Use in Wearable Gas Sensor. Sci. Rep. 2015, 5, 10904. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, R.; Qi, W.; Cai, L.; Sun, Y.; Sun, M.; Li, C.; Yang, X.; Xiang, L.; Xie, D.; et al. Reduced Graphene Oxide/Mesoporous ZnO NSs Hybrid Fibers for Flexible, Stretchable, Twisted, and Wearable NO2 E-Textile Gas Sensor. ACS Sens. 2019, 4, 2809–2818. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wang, Y.; Su, X.; Wang, C.; Zhang, M.; Jian, M.; Xia, K.; Liang, X.; Lu, H.; et al. Laser Writing of Janus Graphene/Kevlar Textile for Intelligent Protective Clothing. ACS Nano 2020, 14, 3219–3226. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Jung, H.G.; Kim, I.; Lee, D.; Kim, W.; Kim, S.H.; Lee, J.-H.; Park, J.; Lee, J.H.; Lee, G.; et al. Highly Conductive and Flexible Dopamine-Graphene Hybrid Electronic Textile Yarn for Sensitive and Selective NO2 Detection. ACS Appl. Mater. Interfaces 2020, 12, 46629–46638. [Google Scholar] [CrossRef]
- Yun, Y.J.; Hong, W.G.; Kim, D.Y.; Kim, H.J.; Jun, Y.; Lee, H.-K. E-Textile Gas Sensors Composed of Molybdenum Disulfide and Reduced Graphene Oxide for High Response and Reliability. Sens. Actuators B Chem. 2017, 248, 829–835. [Google Scholar] [CrossRef]
- Jung, W.T.; Jeon, J.W.; Jang, H.-S.; Kim, D.Y.; Lee, H.-K.; Kim, B.H. Commercial Silk-Based Electronic Textiles for NO2 Sensing. Sens. Actuators B Chem. 2020, 307, 127596. [Google Scholar] [CrossRef]
- Kang, M.-A.; Ji, S.; Kim, S.; Park, C.-Y.; Myung, S.; Song, W.; Lee, S.S.; Lim, J.; An, K.-S. Highly Sensitive and Wearable Gas Sensors Consisting of Chemically Functionalized Graphene Oxide Assembled on Cotton Yarn. RSC Adv. 2018, 8, 11991–11996. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Keum, K.; Lee, G.; Kim, D.; Lee, S.-S.; Ha, J.S. Flexible, Water-Proof, Wire-Type Supercapacitors Integrated with Wire-Type UV/NO2 Sensors on Textiles. Nano Energy 2017, 35, 199–206. [Google Scholar] [CrossRef]
- Han, J.-W.; Kim, B.; Li, J.; Meyyappan, M. A Carbon Nanotube Based Ammonia Sensor on Cotton Textile. Appl. Phys. Lett. 2013, 102, 193104. [Google Scholar] [CrossRef]
- Tang, X.; Wu, C.; Zhang, T.; Zhou, T.; Wang, H.; Xie, C.; Zeng, D. A Low-Cost Polyaniline@Textile-Based Multifunctional Sensor for Simultaneously Detecting Tactile and Olfactory Stimuli. Macromol. Mater. Eng. 2018, 303, 1800340. [Google Scholar] [CrossRef]
- Indarit, N.; Kim, Y.-H.; Petchsang, N.; Jaisutti, R. Highly Sensitive Polyaniline-Coated Fiber Gas Sensors for Real-Time Monitoring of Ammonia Gas. RSC Adv. 2019, 9, 26773–26779. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, R.; Walia, S.; Kandjani, A.E.; Balendran, S.; Mohammadtaheri, M.; Bhargava, S.K.; Kalantar-Zadeh, K.; Bansal, V. Low-Temperature Fabrication of Alkali Metal-Organic Charge Transfer Complexes on Cotton Textile for Optoelectronics and Gas Sensing. Langmuir 2015, 31, 1581–1587. [Google Scholar] [CrossRef]
- Pauksch, L.; Hartmann, S.; Rohnke, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of Silver Nanoparticles and Silver Ions in Primary Human Mesenchymal Stem Cells and Osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef]
- Lugoda, P.; Costa, J.C.; Garcia-Garcia, L.A.; Pouryazdan, A.; Jocys, Z.; Spina, F.; Salvage, J.; Roggen, D.; Münzenrieder, N. Coco Stretch: Strain Sensors Based on Natural Coconut Oil and Carbon Black Filled Elastomers. Adv. Mater. Technol. 2021, 6, 2000780. [Google Scholar] [CrossRef]
- Buaki-Sogó, M.; García-Carmona, L.; Gil-Agustí, M.; García-Pellicer, M.; Quijano-López, A. Flexible and Conductive Bioelectrodes Based on Chitosan-Carbon Black Membranes: Towards the Development of Wearable Bioelectrodes. Nanomaterials 2021, 11, 2052. [Google Scholar] [CrossRef]
- Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Wu, X.; Liu, P.; Li, Y.; Saiyin, H.; et al. Functionalized Helical Fibre Bundles of Carbon Nanotubes as Electrochemical Sensors for Long-Term in Vivo Monitoring of Multiple Disease Biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171. [Google Scholar] [CrossRef]
- Wang, R.; Zhai, Q.; Zhao, Y.; An, T.; Gong, S.; Guo, Z.; Shi, Q.; Yong, Z.; Cheng, W. Stretchable Gold Fiber-Based Wearable Electrochemical Sensor Toward pH Monitoring. J. Mater. Chem. B 2020, 8, 3655–3660. [Google Scholar] [CrossRef]
- He, W.; Wang, C.; Wang, H.; Jian, M.; Lu, W.; Liang, X.; Zhang, X.; Yang, F.; Zhang, Y. Integrated Textile Sensor Patch for Real-Time and Multiplex Sweat Analysis. Sci. Adv. 2019, 5, eaax0649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppedè, N.; Tarabella, G.; Villani, M.; Calestani, D.; Iannotta, S.; Zappettini, A. Human Stress Monitoring Through an Organic Cotton-Fiber Biosensor. J. Mater. Chem. B 2014, 2, 5620–5626. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, I.; Marzocchi, M.; Achilli, A.; Cavedale, D.; Bonfiglio, A.; Fraboni, B. Textile Organic Electrochemical Transistors as a Platform for Wearable Biosensors. Sci. Rep. 2016, 6, 35419. [Google Scholar] [CrossRef]
- Buaki-Sogó, M.; García-Carmona, L.; Gil-Agustí, M.; Zubizarreta, L.; García-Pellicer, M.; Quijano-López, A. Enzymatic Glucose-Based Bio-batteries: Bioenergy to Fuel Next-Generation Devices. Top. Curr. Chem. 2020, 378, 49. [Google Scholar] [CrossRef] [PubMed]
- Jeerapan, I.; Sempionatto, J.R.; Pavinatto, A.; You, J.-M.; Wang, J. Stretchable Biofuel Cells as Wearable Textile-Based Self-Powered Sensors. J. Mater. Chem. A 2016, 4, 18342–18353. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, W.; Kim, I.; Lee, D.; Park, D.; Kim, W.; Park, J.; Lee, J.H.; Lee, G.; Yoon, D.S. Bio-Inspired Electronic Textile Yarn-Based NO2 Sensor Using Amyloid-Graphene Composite. ACS Sens. 2021, 6, 777–785. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, G.; Ehrmann, A. Textile-Based Sensors for Biosignal Detection and Monitoring. Sensors 2021, 21, 6042. [Google Scholar] [CrossRef]
- Nigusse, A.B.; Mengistie, D.A.; Malengier, B.; Tseghai, G.B.; Langenhove, L.V. Wearable Smart Textiles for Long-Term Electrocardiography Monitoring—A Review. Sensors 2021, 21, 4174. [Google Scholar] [CrossRef]
- Yeh, L.-R.; Chen, W.-C.; Chan, H.-Y.; Lu, N.-H.; Wang, C.-Y.; Twan, W.-H.; Du, W.-C.; Huang, Y.-H.; Hsu, S.-Y.; Chen, T.-B. Integrating ECG Monitoring and Classification via IoT and Deep Neural Networks. Biosensors 2021, 11, 188. [Google Scholar] [CrossRef]
- Huang, Y.; Song, Y.; Gou, L.; Zou, Y. A Novel Wearable Flexible Dry Electrode Based on Cowhide for ECG Measurement. Biosensors 2021, 11, 101. [Google Scholar] [CrossRef]
- Takamatsu, S.; Lonjaret, T.; Crisp, D.; Badier, J.-M.; Malliaras, G.G.; Ismailova, E. Direct Patterning of Organic Conductors on Knitted Textiles for Long-Term Electrocardiography. Sci. Rep. 2015, 5, 15003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankhili, A.; Tao, X.; Cochrane, C.; Coulon, D.; Koncar, V. Washable and Reliable Textile Electrodes Embedded into Underwear Fabric for Electrocardiography (ECG) Monitoring. Materials 2018, 11, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankhili, A.; Tao, X.; Cochrane, C.; Koncar, V.; Coulon, D.; Tarlet, J.-M. Ambulatory Evaluation of ECG Signals Obtained Using Washable Textile-Based Electrodes Made with Chemically Modified PEDOT:PSS. Sensors 2019, 19, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankhili, A.; Tao, X.; Cochrane, C.; Koncar, V.; Coulon, D.; Tarlet, J.-M. Comparative Study on Conductive Knitted Fabric Electrodes for Long-Term Electrocardiography Monitoring: Silver-Plated and PEDOT:PSS Coated Fabrics. Sensors 2018, 18, 3890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lidón-Roger, J.V.; Prats-Boluda, G.; Ye-Lin, Y.; Garcia-Casado, J.; Garcia-Breijo, E. Textile Concentric Ring Electrodes for ECG Recording Based on Screen-Printing Technology. Sensors 2018, 18, 300. [Google Scholar] [CrossRef] [Green Version]
- Taylor, L.W.; Williams, S.M.; Yan, J.S.; Dewey, O.S.; Vitale, F.; Pasquali, M. Washable, Sewable, All-Carbon Electrodes and Signal Wires for Electronic Clothing. Nano Lett. 2021, 21, 7093–7099. [Google Scholar] [CrossRef]
- Yapici, M.K.; Alkhidir, T.E. Intelligent Medical Garments with Graphene-Functionalized Smart-Cloth ECG Sensors. Sensors 2017, 17, 875. [Google Scholar] [CrossRef] [Green Version]
- Nigusse, A.B.; Malengier, B.; Mengistie, D.A.; Tseghai, G.B.; Langenhove, L. Van Development of Washable Silver Printed Textile Electrodes for Long-Term ECG Monitoring. Sensors 2020, 20, 6233. [Google Scholar] [CrossRef]
- Arquilla, K.; Webb, A.K.; Anderson, A.P. Textile Electrocardiogram (ECG) Electrodes for Wearable Health Monitoring. Sensors 2020, 20, 1013. [Google Scholar] [CrossRef] [Green Version]
- Arquilla, K.; Devendorf, L.; Webb, A.K.; Anderson, A.P. Detection of the Complete ECG Waveform with Woven Textile Electrodes. Biosensors 2021, 11, 331. [Google Scholar] [CrossRef]
- Dore, H.; Aviles-Espinosa, R.; Luo, Z.; Anton, O.; Rabe, H.; Rendon-Morales, E. Characterisation of Textile Embedded Electrodes for Use in a Neonatal Smart Mattress Electrocardiography System. Sensors 2021, 21, 999. [Google Scholar] [CrossRef] [PubMed]
- Piuzzi, E.; Pisa, S.; Pittella, E.; Podestà, L.; Sangiovanni, S. Wearable Belt with Built-in Textile Electrodes for Cardio—Respiratory Monitoring. Sensors 2020, 20, 4500. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Nayeem, M.O.G.; Lee, S.; Matsuhisa, N.; Inoue, D.; Yokota, T.; Hashizume, D.; Someya, T. Highly Durable Nanofiber-Reinforced Elastic Conductors for Skin-Tight Electronic Textiles Article. ACS Nano 2019, 13, 7905–7912. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Yang, J.; Poblete, F.R.; Hu, X.; Zhu, Y. Multifunctional Electronic Textiles Using Silver Nanowire Composites. ACS Appl. Mater. Interfaces 2019, 11, 31028–31037. [Google Scholar] [CrossRef]
- Fang, C.; He, B.; Wang, Y.; Cao, J.; Gao, S. EMG-Centered Multisensory Based Technologies for Pattern Recognition in Regabilitation: State of the Art and Challenges. Biosensors 2020, 10, 85. [Google Scholar] [CrossRef]
- Chihi, I.; Sidhom, L.; Kamavuako, E.N. Hammerstein-Wiener Multimodel Approach for Fast and Efficient Muscle Force Estimation from EMG Signals. Biosensors 2022, 12, 117. [Google Scholar] [CrossRef]
- Jang, K.-I.; Han, S.Y.; Xu, S.; Mathewson, K.E.; Zhang, Y.; Jeong, J.-W.; Kim, G.-T.; Webb, R.C.; Lee, J.W.; Dawidczyk, T.J.; et al. Rugged and Breathable Forms of Stretchable Electronics with Adherent Composite Substrates for Transcutaneous Monitoring. Nat. Commun. 2014, 5, 4779. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Kaltenbrunner, M.; Yokota, T.; Jinno, H.; Kuribara, K.; Sekitani, T.; Someya, T. Printable elastic conductors with a high conductivity for electronic textile applications. Nat. Commun. 2015, 6, 7461. [Google Scholar] [CrossRef]
- Fira, M.; Costin, H.-N.; Goras, L. On the Classification of ECG and EEG Signals with Various Degrees of Dimensionality Reduction. Biosensors 2021, 11, 161. [Google Scholar] [CrossRef]
- Wu, C.-T.; Huang, H.-C.; Huang, S.; Chen, I.-M.; Liao, S.-C.; Chen, C.-K.; Lin, C.; Lee, S.-H.; Chen, M.-H.; Tsai, C.-F.; et al. Resting-State EEG Signal for Major Depressive Disorder Detection: A Systematic Validation on a Large and Diverse Dataset. Biosensors 2021, 11, 499. [Google Scholar] [CrossRef]
- Löfhede, J.; Seoane, F.; Thordstein, M. Soft Textile Electrodes for EEG Monitoring. In Proceedings of the 10th IEEE International Conference on Information Technology and Applications in Biomedicine (ITAB), Corfu, Greece, 3–5 November 2010; pp. 1–4. [Google Scholar]
- La, T.-G.; Qiu, S.; Scott, D.K.; Bakhtiari, R.; Kuziek, J.W.P.; Mathewson, K.E.; Rieger, J.; Chung, H.-J. Two-Layered and Stretchable e-Textile Patches for Wearable Healthcare Electronics. Adv. Healthcare Mater. 2018, 7, 1801033. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Xu, T.; Xu, X. Multilayer Sweat-Absorbable Textile Electrode for EEG Measurement in Forehead Site. IEEE Sens. J. 2019, 19, 5995–6005. [Google Scholar] [CrossRef]
- Muthukumar, N.; Thilagavathi, G.; Kannaian, T. Polyaniline-Coated Foam Electrodes for Electroencephalography (EEG) Measurement. J. Text. Inst. 2015, 107, 283–290. [Google Scholar] [CrossRef]
- Golparvar, A.; Ozturk, O.; Yapici, M.K. Gel-Free Wearable Electroencephalography (EEG) with Soft Graphene Textiles. In Proceedings of the 2021 IEEE Sensors, Virtual Format, 31 October–3 November 2021; pp. 1–4. [Google Scholar]
- Xu, P.J.; Zhang, H.; Tao, X.M. Textile-Structured Electrodes for Electrocardiogram. Text. Prog. 2008, 40, 183–213. [Google Scholar] [CrossRef]
- Yapici, M.K.; Alkhidir, T.; Samad, Y.A.; Liao, K. Graphene-Clad Textile Electrodes for Electrocardiogram Monitoring. Sens. Actuators B Chem. 2015, 221, 1469–1474. [Google Scholar] [CrossRef]
- Das, P.S.; Kim, J.W.; Park, J.Y. Fashionable Wrist Band Using Highly Conductive Fabric for Electrocardiogram Signal Monitoring. J. Ind. Text. 2019, 49, 243–261. [Google Scholar] [CrossRef]
- Lim, T.; Zhang, H.; Lee, S. Gold and Silver Nanocomposite-Based Biostable and Biocompatible Electronic Textile for Wearable Electromyographic Biosensors. APL Mater. 2021, 9, 091113. [Google Scholar] [CrossRef]
- Chang, T.; Akin, S.; Kim, M.K.; Murray, L.; Kim, B.; Cho, S.; Huh, S.; Teke, S.; Couetil, L.; Jun, M.B.-G.; et al. A Programmable Dual-Regime Spray for Large-Scale and Custom-Designed Electronic Textiles. Adv. Mater. 2022, 34, 2108021. [Google Scholar] [CrossRef]
- Zhang, H.; Rogers, J.A. Recent Advances in Flexible Inorganic Light Emitting Diodes: From Materials Design to Integrated Optoelectronic Platforms. Adv. Opt. Mater. 2019, 7, 1800936. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Guo, K.; Li, Y.; Li, X.; Guan, G.; Li, H.; Luo, Y.; Zhao, F.; Zhang, Q.; Wei, B.; et al. A Colour-Tunable, Weavable Fibre-Shaped Polymer Light-Emitting Electrochemical Cell. Nat. Photon. 2015, 9, 233–238. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Guo, K.; Li, Y.; Li, X.; Wang, L.; Luo, Y.; Li, H.; Zhang, Y.; Guan, G.; et al. Flexible Electroluminescent Fiber Fabricated from Coaxially Wound Carbon Nanotube Sheets. J. Mater. Chem. C 2015, 3, 5621–5624. [Google Scholar] [CrossRef]
- O’Connor, B.; An, K.H.; Zhao, Y.; Pipe, K.P.; Shtein, M. Fiber Shaped Light Emitting Device. Adv. Mater. 2007, 19, 3897–3900. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Lightner, C.R.; Dong, L. Light-Emitting Coaxial Nanofibers. ACS Nano 2012, 6, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.J.; Lee, H.B.; Kim, H.M.; Lee, G.J.; Shin, S.-R.; Kumar, N.; Song, Y.M.; Kang, J.-W. High-Performance, Color-Tunable Fiber Shaped Organic Light-Emitting Diodes. Nanoscale 2018, 10, 16184–16192. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.-J.; Lee, B.; Kang, J.-W. Flexible, Wearable Organic Light-Emitting Fibers Based on PEDOT:PSS/Ag-Fiber Embedded Hybrid Electrodes for Large-Area Textile Lighting. Adv. Mater. Technol. 2020, 5, 2000168. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, S.; Kim, H.; Kim, W.; Kwon, J.H.; Lim, M.S.; Lee, H.S.; Choi, K.C. Highly Flexible and Efficient Fabric-Based Organic Light-Emitting Devices for Clothing-Shaped Wearable Displays. Sci. Rep. 2017, 7, 6424. [Google Scholar] [CrossRef]
- Yin, D.; Chen, Z.-Y.; Jiang, N.-R.; Liu, Y.-F.; Bi, Y.-G.; Zhang, X.-L.; Han, W.; Feng, J.; Sun, H.-B. Highly Transparent and Flexible Fabric-Based Organic Light Emitting Devices for Unnoticeable Wearable Displays. Org. Electron. 2020, 76, 105494. [Google Scholar] [CrossRef]
- Sohn, S.; Kim, S.; Shim, J.W.; Jung, S.K.; Jung, S. Printed Organic Light-Emitting Diodes on Fabric with Roll-to-Roll Sputtered ITO Anode and Poly(vinyl alcohol) Planarization Layer. ACS Appl. Mater. Interfaces 2021, 13, 28521–28528. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kwon, S.; Shin, J.B.; Kim, H.; Son, Y.H.; Lee, H.S.; Noh, B.; Nam, M.; Choi, K.C. Bright-Multicolor, Highly Efficient, and Addressable Phosphorescent Organic Light-Emitting Fibers: Toward Wearable Textile Information Displays. Adv. Funct. Mater. 2021, 31, 2009336. [Google Scholar] [CrossRef]
- Song, Y.J.; Kim, J.-W.; Cho, H.-E.; Son, Y.H.; Lee, M.H.; Lee, J.; Choi, K.C.; Lee, S.-M. Fibertronic Organic Light-Emitting Diodes toward Fully Addressable, Environmentally Robust, Wearable Displays. ACS Nano 2020, 14, 1133–1140. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, W.; Kim, H.; Choi, S.; Park, B.-C.; Kang, S.-H.; Choi, K.C. High Luminance Fiber-Based Polymer Light-Emitting Devices by a Dip-Coating Method. Adv. Electron. Mater. 2015, 1, 1500103. [Google Scholar] [CrossRef]
- Kim, H.; Kwon, S.; Choi, S.; Choi, K.C. Solution-Processed Bottom-Emitting Polymer Light-Emitting Diodes on a Textile Substrate Towards a Wearable Display. J. Inf. Disp. 2015, 16, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Dias, T.; Monaragala, R. Development and Analysis of Novel Electroluminescent Yarns and Fabrics for Localized Automotive Interior Illumination. Text. Res. J. 2012, 82, 1164–1176. [Google Scholar] [CrossRef]
- Liang, G.; Yi, M.; Hu, H.; Ding, K.; Wang, L.; Zeng, H.; Tang, J.; Liao, L.; Nan, C.; He, Y.; et al. Coaxial-Structured Weavable and Wearable Electroluminescent Fibers. Adv. Electron. Mater. 2017, 3, 1700401. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, X.; Zuo, Y.; Liao, M.; Shi, X.; Chen, C.; Xie, S.; Zhou, P.; Sun, X.; Peng, H. A Fiber-Shaped Light-Emitting Pressure Sensor for Visualized Dynamic Monitoring. J. Mater. Chem. C 2020, 8, 935–942. [Google Scholar] [CrossRef]
- Mi, H.; Zhong, L.; Tang, X.; Xu, P.; Liu, X.; Luo, T.; Jiang, X. Electroluminescent Fabric Woven by Ultrastretchable Fibers for Arbitrarily Controllable Pattern Display. ACS Appl. Mater. Interfaces 2021, 13, 11260–11267. [Google Scholar] [CrossRef]
- Hu, B.; Li, D.; Manandharm, P.; Fan, Q.; Kasilingam, D.; Calvert, P. CNT/Conducting Polymer Composite Conductors Impart High Flexibility to Textile Electroluminescent Devices. J. Mater. Chem. 2012, 22, 1598–1605. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, X.; Lou, H.; Xu, Y.; Zhang, J.; Li, Y.; Cheng, X.; Peng, H. A Stretchable and Sensitive Light-Emitting Fabric. J. Mater. Chem. C 2017, 5, 4139–4144. [Google Scholar] [CrossRef]
- Wu, Y.; Mechael, S.S.; Chen, Y.; Carmichael, T.B. Solution Deposition of Conformal Gold Coatings on Knitted Fabric for E-Textiles and Electroluminescent Clothing. Adv. Mater. Technol. 2018, 3, 1700292. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Lin, Y.; Yuan, W.; Ding, C.; Su, W.; Meng, X.; Cui, Z. Fully Printed, Large-Size Alternating Current Electroluminescent Device on Fabric for Wearable Textile Display. ACS Appl. Electron. Mater. 2021, 3, 1747–1757. [Google Scholar] [CrossRef]
- Cho, S.; Kang, D.-h.; Lee, H.; Kim, M.P.; Kang, S.; Shanker, R.; Ko, H. Highly Stretchable Sound-in-Display Electronics Based on Strain-Insensitive Metallic Nanonetworks. Adv. Sci. 2021, 8, 2001647. [Google Scholar] [CrossRef] [PubMed]
- Shanker, R.; Cho, S.; Choe, A.; Kim, M.P.; Khan, Z.; Kang, S.; Ko, H. Solution-Processable, High-Performance Flexible Electroluminescent Devices Based on High-k Nanodielectrics. Adv. Funct. Mater. 2019, 29, 1904377. [Google Scholar] [CrossRef]
- Invernale, M.A.; Ding, Y.; Sotzing, G.A. All-Organic electrochromic spandex. ACS Appl. Mater. Interfaces 2010, 2, 296–300. [Google Scholar] [CrossRef]
- Chen, X.; Lin, H.; Deng, J.; Zhang, Y.; Sun, X.; Chen, P.; Fang, X.; Zhang, Z.; Guan, G.; Peng, H. Electrochromic Fiber-Shaped Supercapacitors. Adv. Mater. 2014, 26, 8126–8132. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Xiong, J.; Chen, J.; Zhou, X.; Cai, G.; Lai, Y.; Lee, P.S. Flexible Electrochromic Fiber with Rapid Color Switching and High Optical Modulation. Nano Res. 2021, 1–7. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Q.; Wang, H.; Li, Y. Red, Green, Blue (RGB) Electrochromic Fibers for the New Smart Color Change Fabrics. ACS Appl. Mater. Interfaces 2014, 6, 13043–13050. [Google Scholar] [CrossRef]
- Fan, H.; Li, K.; Liu, X.; Xu, K.; Su, Y.; Hou, C.; Zhang, Q.; Li, Y.; Wang, H. Continuously Processed, Long Electrochromic Fibers with Multi-Environmental Stability. ACS Appl. Mater. Interfaces 2020, 12, 28451–28460. [Google Scholar] [CrossRef]
- Kim, S.H.; Ko, H.C. Double-Sided Printed Circuit Textiles Based on Stencil-Type Layer-by-Layer Coating with PEDOT:PSS:Ag Nanowires and Chitosan for Electrothermochromic Displays. J. Mater. Chem. C 2019, 7, 14525–14534. [Google Scholar] [CrossRef]
- Huang, G.; Liu, L.; Wang, R.; Zhang, J.; Sun, X.; Peng, H. Smart Color-Changing Textile with High Contrast Based on a Single-Sided Conductive Fabric. J. Mater. Chem. C 2016, 4, 7589–7594. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, H.; Choi, S.; Jeong, E.G.; Kim, D.; Lee, S.; Lee, H.S.; Seo, Y.C.; Choi, K.C. Weavable and Highly Efficient Organic Light-Emitting Fibers for Wearable Electronics: A Scalable, Low-Temperature Process. Nano Lett. 2018, 18, 347–356. [Google Scholar] [CrossRef]
- Kim, W.; Kwon, S.; Han, Y.C.; Kim, E.; Choi, K.C.; Kang, S.-H.; Park, B.-C. Reliable Actual Fabric-Based Organic Light-Emitting Diodes: Toward a Wearable Display. Adv. Electron. Mater. 2016, 2, 1600220. [Google Scholar] [CrossRef]
- Choi, H.W.; Shin, D.-W.; Yang, J.; Lee, S.; Figueiredo, C.; Sinopoli, S.; Ullrich, K.; Jovančić, P.; Marrani, A.; Momentè, R.; et al. Smart Textile Lighting/Display System with Multifunctional Fibre Devices for Large Scale Smart Home and IoT Applications. Nat. Commun. 2022, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Xu, X.; Miao, J.; Gidron, O.; Meng, H. A Stretchable Alternating Current Electroluminescent Fiber. Materials 2018, 11, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Shi, X.; Lou, H.; Cheng, X.; Xu, Y.; Zhang, J.; Li, Y.; Wang, L.; Peng, H. A One-Dimensional Soft and Color-Programmable Light-Emitting Device. J. Mater. Chem. C 2018, 6, 1328–1333. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, L.; Shi, X.; Tian, X.; Wang, D.; Gu, C.; Chen, E.; Cheng, X.; Xu, Y.; Hu, Y.; et al. Textile Display for Electronic and Brain-Interfaced Communications. Adv. Mater. 2018, 30, 1800323. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Chang, T.; Yu, T.; Lee, C.H. Smart Electronic Textiles for Wearable Sensing and Display. Biosensors 2022, 12, 222. https://doi.org/10.3390/bios12040222
Cho S, Chang T, Yu T, Lee CH. Smart Electronic Textiles for Wearable Sensing and Display. Biosensors. 2022; 12(4):222. https://doi.org/10.3390/bios12040222
Chicago/Turabian StyleCho, Seungse, Taehoo Chang, Tianhao Yu, and Chi Hwan Lee. 2022. "Smart Electronic Textiles for Wearable Sensing and Display" Biosensors 12, no. 4: 222. https://doi.org/10.3390/bios12040222
APA StyleCho, S., Chang, T., Yu, T., & Lee, C. H. (2022). Smart Electronic Textiles for Wearable Sensing and Display. Biosensors, 12(4), 222. https://doi.org/10.3390/bios12040222






