Interdigitated Organic Sensor in Multimodal Facemask’s Barrier Integrity and Wearer’s Respiration Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. E-Mask Fabrication
2.3. E-Mask Characterization
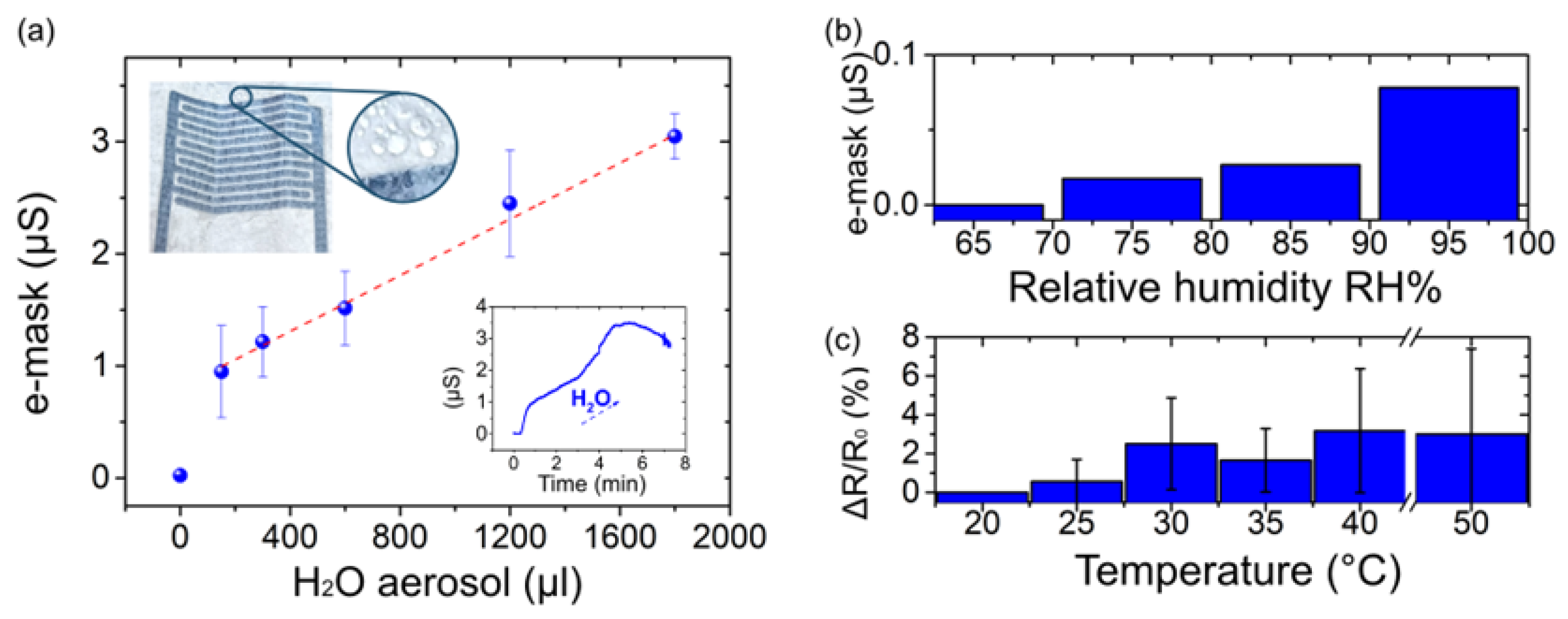
2.3.1. E-Mask Response to an Aerosol Flow
2.3.2. E-Mask Response to Relative Humidity Variations
2.3.3. IDEs Response to the Temperature Variation
2.3.4. Contact Angle Measurement
2.4. E-Mask Evaluation
2.5. E-Mask Data Processing
3. Results and Discussion
3.1. IDEs Printing on Facemask
3.2. E-Mask Design and Working Principle
3.2.1. Dampness Sensing Mechanism
3.2.2. Respiration Sensing Mechanism
3.3. E-Mask Characteristics
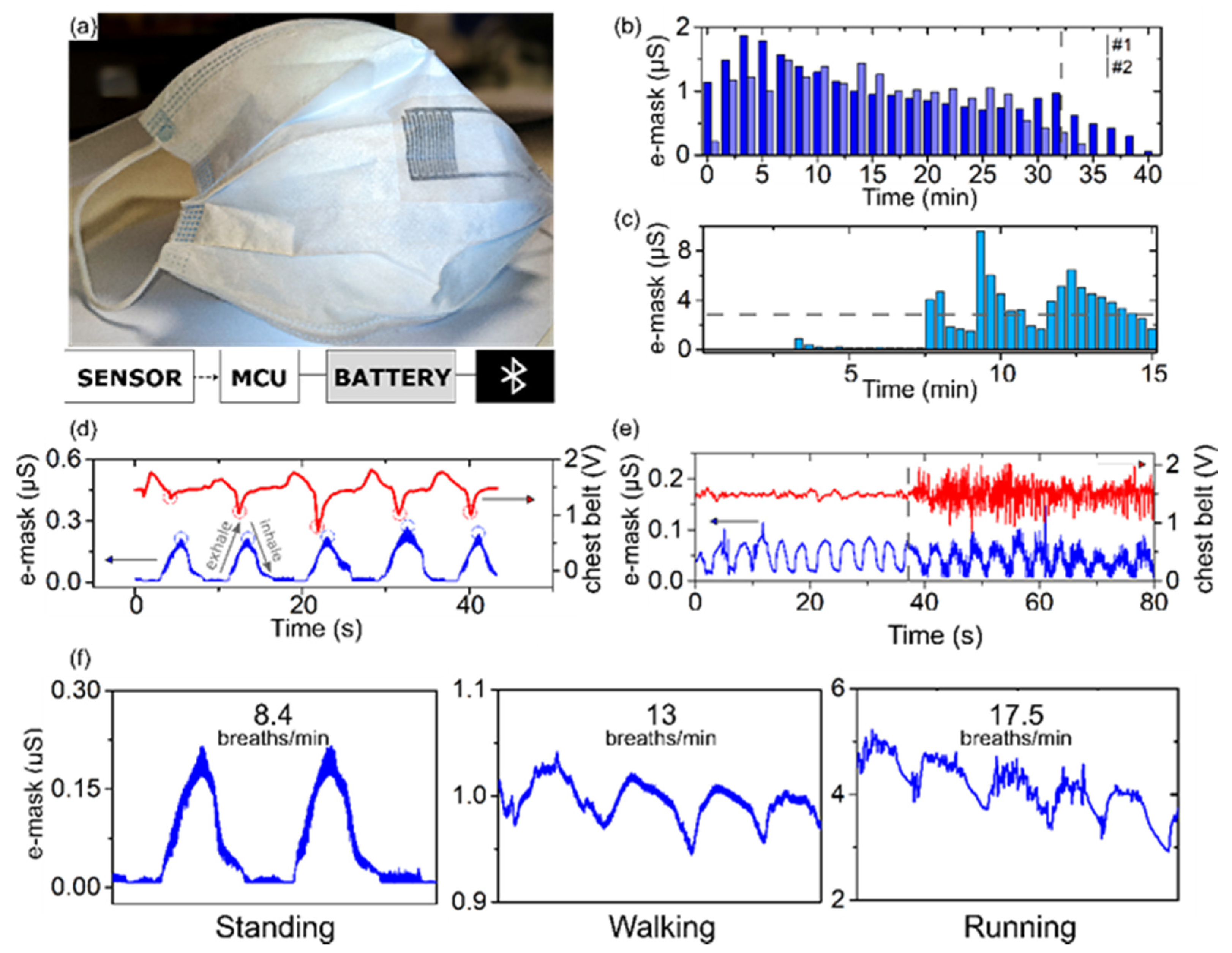
3.4. Multimodal E-Mask System Validation
3.4.1. E-Mask Dampness Monitoring
3.4.2. Respiration Rate Monitoring
3.4.3. Motion Artifacts Tolerance
3.4.4. Multimodal Monitoring
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marijon, E.; Karam, N.; Jost, D.; Perrot, D.; Frattini, B.; Derkenne, C.; Sharifzadehgan, A.; Waldmann, V.; Beganton, F.; Narayanan, K.; et al. Out-of-Hospital Cardiac Arrest during the COVID-19 Pandemic in Paris, France: A Population-Based, Observational Study. Lancet Public Health 2020, 5, e437–e443. [Google Scholar] [CrossRef]
- Arumugam, S.; Colburn, D.A.M.; Sia, S.K. Biosensors for Personal Mobile Health: A System Architecture Perspective. Adv. Mater. Technol. 2020, 5, 1900720. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Advice on the Use of Masks in the Context of COVID-19: Interim Guidance, 6 April 2020; World Health Organization: Geneva, Switzerland, 2020; p. 5. [Google Scholar]
- Jeong, H.; John, A.R.; Xu, S. Continuous On-Body Sensing for the COVID-19 Pandemic: Gaps and Opportunities. Sci. Adv. 2020, 6, eabd4794. [Google Scholar] [CrossRef] [PubMed]
- Channa, A.; Popescu, N.; Skibinska, J.; Burget, R. The Rise of Wearable Devices during the COVID-19 Pandemic: A Systematic Review. Sensors 2021, 21, 5787. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhang, Z.; Sun, P.; Chen, H. High-Sensitivity Wearable and Flexible Humidity Sensor Based on Graphene Oxide/Non-Woven Fabric for Respiration Monitoring. Langmuir 2020, 36, 9443–9448. [Google Scholar] [CrossRef]
- Pang, Y.; Jian, J.; Tu, T.; Yang, Z.; Ling, J.; Li, Y.; Wang, X.; Qiao, Y.; Tian, H.; Yang, Y.; et al. Wearable Humidity Sensor Based on Porous Graphene Network for Respiration Monitoring. Biosens. Bioelectron. 2018, 116, 123–129. [Google Scholar] [CrossRef]
- Bianco, G.M.; Marrocco, G. Sensorized Facemask With Moisture-Sensitive RFID Antenna. IEEE Sens. Lett. 2021, 5, 6000604. [Google Scholar] [CrossRef]
- Wang, W.; Ouaras, K.; Rutz, A.L.; Li, X.; Gerigk, M.; Naegele, T.E.; Malliaras George, G.; Huang, Y.Y.S. Inflight Fiber Printing toward Array and 3D Optoelectronic and Sensing Architectures. Sci. Adv. 2020, 6, eaba0931. [Google Scholar] [CrossRef]
- Escobedo, P.; Fernández-Ramos, M.D.; López-Ruiz, N.; Moyano-Rodríguez, O.; Martínez-Olmos, A.; Pérez de Vargas-Sansalvador, I.M.; Carvajal, M.A.; Capitán-Vallvey, L.F.; Palma, A.J. Smart Facemask for Wireless CO2 Monitoring. Nat. Commun. 2022, 13, 72. [Google Scholar] [CrossRef]
- Rahimzadeh, Z.; Naghib, S.M.; Zare, Y.; Rhee, K.Y. An Overview on the Synthesis and Recent Applications of Conducting Poly(3,4-Ethylenedioxythiophene) (PEDOT) in Industry and Biomedicine. J. Mater. Sci. 2020, 55, 7575–7611. [Google Scholar] [CrossRef]
- Ferrari, L.M.; Rodríguez-Meana, B.; Bonisoli, A.; Cutrone, A.; Micera, S.; Navarro, X.; Greco, F.; del Valle, J. All-Polymer Printed Low-Cost Regenerative Nerve Cuff Electrodes. Front. Bioeng. Biotechnol. 2021, 9, 615218. [Google Scholar] [CrossRef]
- Khodagholy, D.; Doublet, T.; Quilichini, P.; Gurfinkel, M.; Leleux, P.; Ghestem, A.; Ismailova, E.; Hervé, T.; Sanaur, S.; Bernard, C.; et al. In Vivo Recordings of Brain Activity Using Organic Transistors. Nat. Commun. 2013, 4, 1575. [Google Scholar] [CrossRef]
- Papaiordanidou, M.; Takamatsu, S.; Rezaei-Mazinani, S.; Lonjaret, T.; Martin, A.; Ismailova, E. Cutaneous Recording and Stimulation of Muscles Using Organic Electronic Textiles. Adv. Healthc. Mater. 2016, 5, 2001–2006. [Google Scholar] [CrossRef]
- Khau, B.V.; Scholz, A.D.; Reichmanis, E. Advances and Opportunities in Development of Deformable Organic Electrochemical Transistors. J. Mater. Chem. C 2020, 8, 15067–15078. [Google Scholar] [CrossRef]
- Xu, K.; Chen, Y.; Okhai, T.A.; Snyman, L.W. Micro Optical Sensors Based on Avalanching Silicon Light-Emitting Devices Monolithically Integrated on Chips. Opt. Mater. Express 2019, 9, 3985–3997. [Google Scholar] [CrossRef]
- Bihar, E.; Roberts, T.; Ismailova, E.; Saadaoui, M.; Isik, M.; Sanchez-Sanchez, A.; Mecerreyes, D.; Hervé, T.; De Graaf, J.B.; Malliaras, G.G. Fully Printed Electrodes on Stretchable Textiles for Long-Term Electrophysiology. Adv. Mater. Technol. 2017, 2, 1600251. [Google Scholar] [CrossRef]
- Takamatsu, S.; Lonjaret, T.; Ismailova, E.; Masuda, A.; Itoh, T.; Malliaras, G.G. Wearable Keyboard Using Conducting Polymer Electrodes on Textiles. Adv. Mater. 2016, 28, 4485–4488. [Google Scholar] [CrossRef]
- Ferrari, L.M.; Ismailov, U.; Badier, J.-M.; Greco, F.; Ismailova, E. Conducting Polymer Tattoo Electrodes in Clinical Electro- and Magneto-Encephalography. Npj Flex. Electron. 2020, 4, 4. [Google Scholar] [CrossRef]
- Ferrari, L.M.; Ismailov, U.; Greco, F.; Ismailova, E. Capacitive Coupling of Conducting Polymer Tattoo Electrodes with the Skin. Adv. Mater. Interfaces 2021, 8, 2100352. [Google Scholar] [CrossRef]
- Lo, L.-W.; Zhao, J.; Wan, H.; Wang, Y.; Chakrabartty, S.; Wang, C. An Inkjet-Printed PEDOT:PSS-Based Stretchable Conductor for Wearable Health Monitoring Device Applications. ACS Appl. Mater. Interfaces 2021, 13, 21693–21702. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Silva, A.F.; Tavakoli, M. Domiciliary Hospitalization through Wearable Biomonitoring Patches: Recent Advances, Technical Challenges, and the Relation to COVID-19. Sensors 2020, 20, 6835. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Davies, E.V.; Harlow, E.R.; Hsu, J.J.; Knighton, S.C.; Walker, T.A.; Voos, J.E.; Drummond, C.K. Wearable Sensors for COVID-19: A Call to Action to Harness Our Digital Infrastructure for Remote Patient Monitoring and Virtual Assessments. Front. Digit. Health 2020, 2, 8. [Google Scholar] [CrossRef]
- Cherrie, J.W.; Wang, S.; Mueller, W.; Wendelboe-Nelson, C.; Loh, M. In-Mask Temperature and Humidity Can Validate Respirator Wear-Time and Indicate Lung Health Status. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 578–583. [Google Scholar] [CrossRef]
- Minh, Q.N.; Tong, H.D.; Kuijk, A.; van de Bent, F.; Beekman, P.; van Rijn, C.J.M. Gas Sensing Performance at Room Temperature of Nanogap Interdigitated Electrodes for Detection of Acetone at Low Concentration. RSC Adv. 2017, 7, 50279–50286. [Google Scholar] [CrossRef] [Green Version]
- Igreja, R.; Dias, C.J. Analytical Evaluation of the Interdigital Electrodes Capacitance for a Multi-Layered Structure. Sens. Actuators A Phys. 2004, 112, 291–301. [Google Scholar] [CrossRef]
- Xu, K. Silicon Electro-Optic Micro-Modulator Fabricated in Standard CMOS Technology as Components for All Silicon Monolithic Integrated Optoelectronic Systems. J. Micromechanics Microengineering 2021, 31, 054001. [Google Scholar] [CrossRef]
- Molina-Lopez, F.; Briand, D.; de Rooij, N.F. All Additive Inkjet Printed Humidity Sensors on Plastic Substrate. Sens. Actuators B Chem. 2012, 166–167, 212–222. [Google Scholar] [CrossRef]
- Song, S.-H.; Yang, H.-H.; Han, C.-H.; Ko, S.-D.; Lee, S.-H.; Yoon, J.-B. Metal-Oxide-Semiconductor Field Effect Transistor Humidity Sensor Using Surface Conductance. Appl. Phys. Lett. 2012, 100, 101603. [Google Scholar] [CrossRef] [Green Version]
- Tcharkhtchi, A.; Abbasnezhad, N.; Zarbini Seydani, M.; Zirak, N.; Farzaneh, S.; Shirinbayan, M. An Overview of Filtration Efficiency through the Masks: Mechanisms of the Aerosols Penetration. Bioact. Mater. 2021, 6, 106–122. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galliani, M.; Ferrari, L.M.; Ismailova, E. Interdigitated Organic Sensor in Multimodal Facemask’s Barrier Integrity and Wearer’s Respiration Monitoring. Biosensors 2022, 12, 305. https://doi.org/10.3390/bios12050305
Galliani M, Ferrari LM, Ismailova E. Interdigitated Organic Sensor in Multimodal Facemask’s Barrier Integrity and Wearer’s Respiration Monitoring. Biosensors. 2022; 12(5):305. https://doi.org/10.3390/bios12050305
Chicago/Turabian StyleGalliani, Marina, Laura M. Ferrari, and Esma Ismailova. 2022. "Interdigitated Organic Sensor in Multimodal Facemask’s Barrier Integrity and Wearer’s Respiration Monitoring" Biosensors 12, no. 5: 305. https://doi.org/10.3390/bios12050305
APA StyleGalliani, M., Ferrari, L. M., & Ismailova, E. (2022). Interdigitated Organic Sensor in Multimodal Facemask’s Barrier Integrity and Wearer’s Respiration Monitoring. Biosensors, 12(5), 305. https://doi.org/10.3390/bios12050305




