Recent Advances in Microfluidic Platform for Physical and Immunological Detection and Capture of Circulating Tumor Cells
Abstract
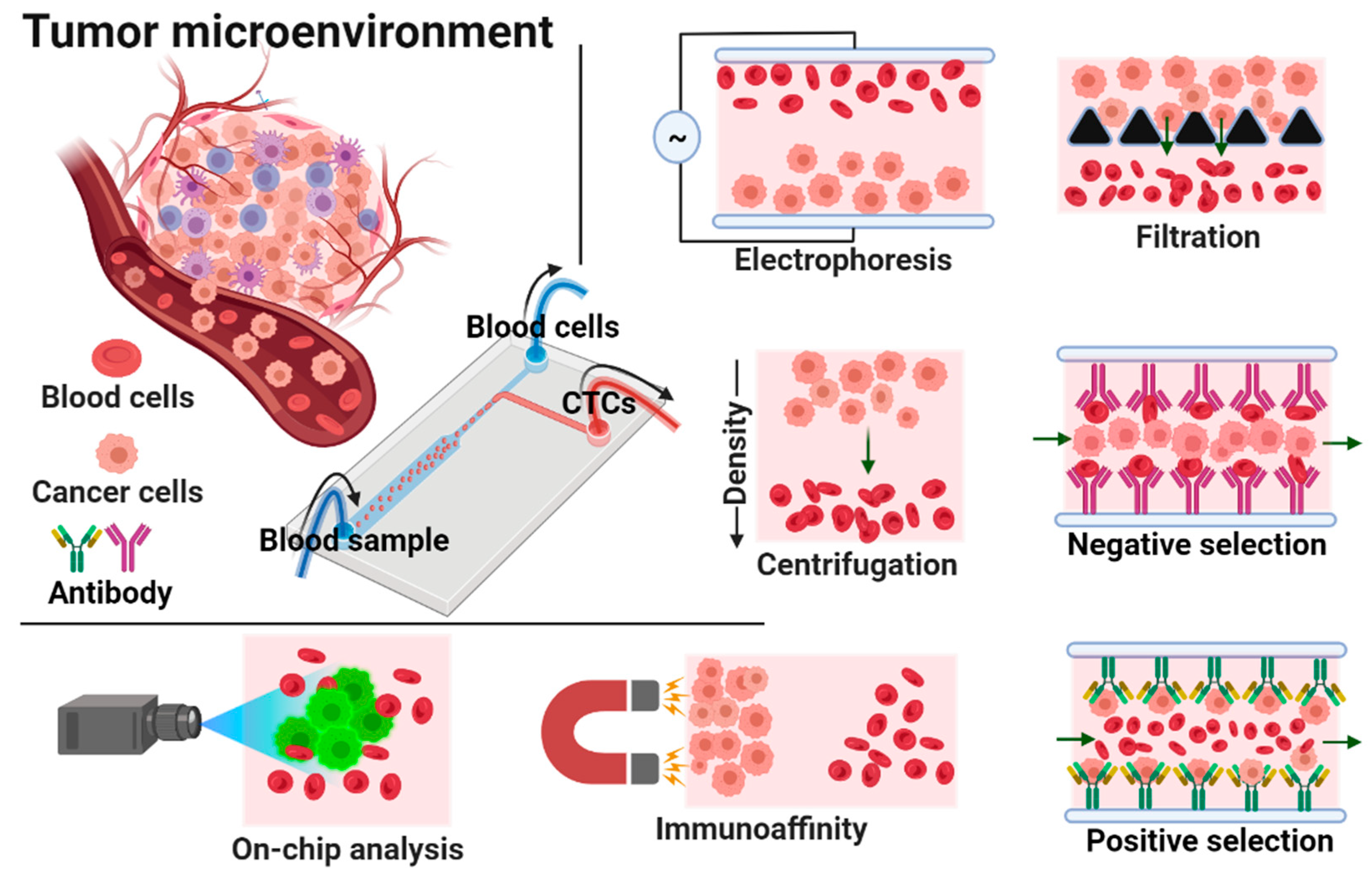
:1. Introduction
2. Fabrication of Microfluidic Devices for the Isolation of CTCs
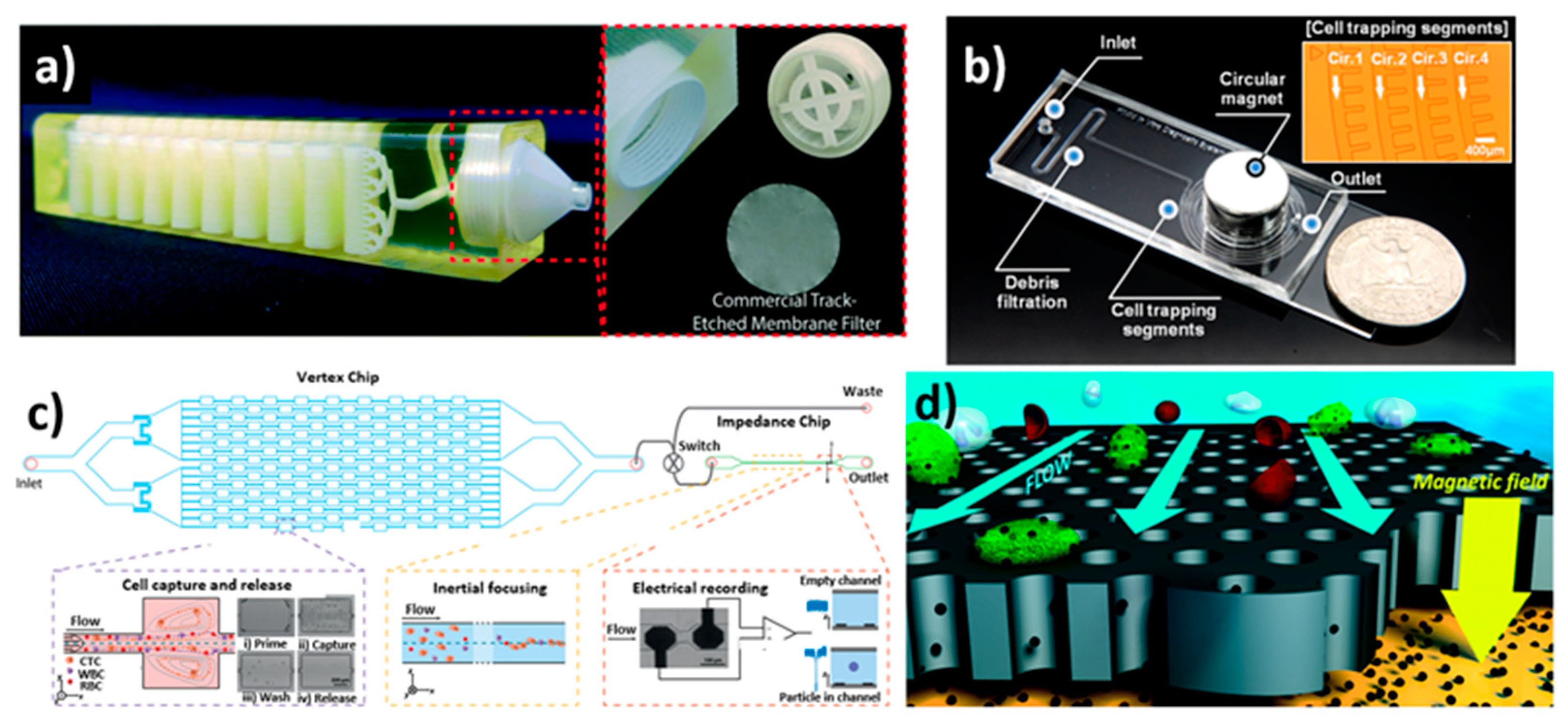
2.1. Additive Manufacturing
2.2. Etching Technique
2.3. Mold Punching Technique
2.4. Photolithography Technique
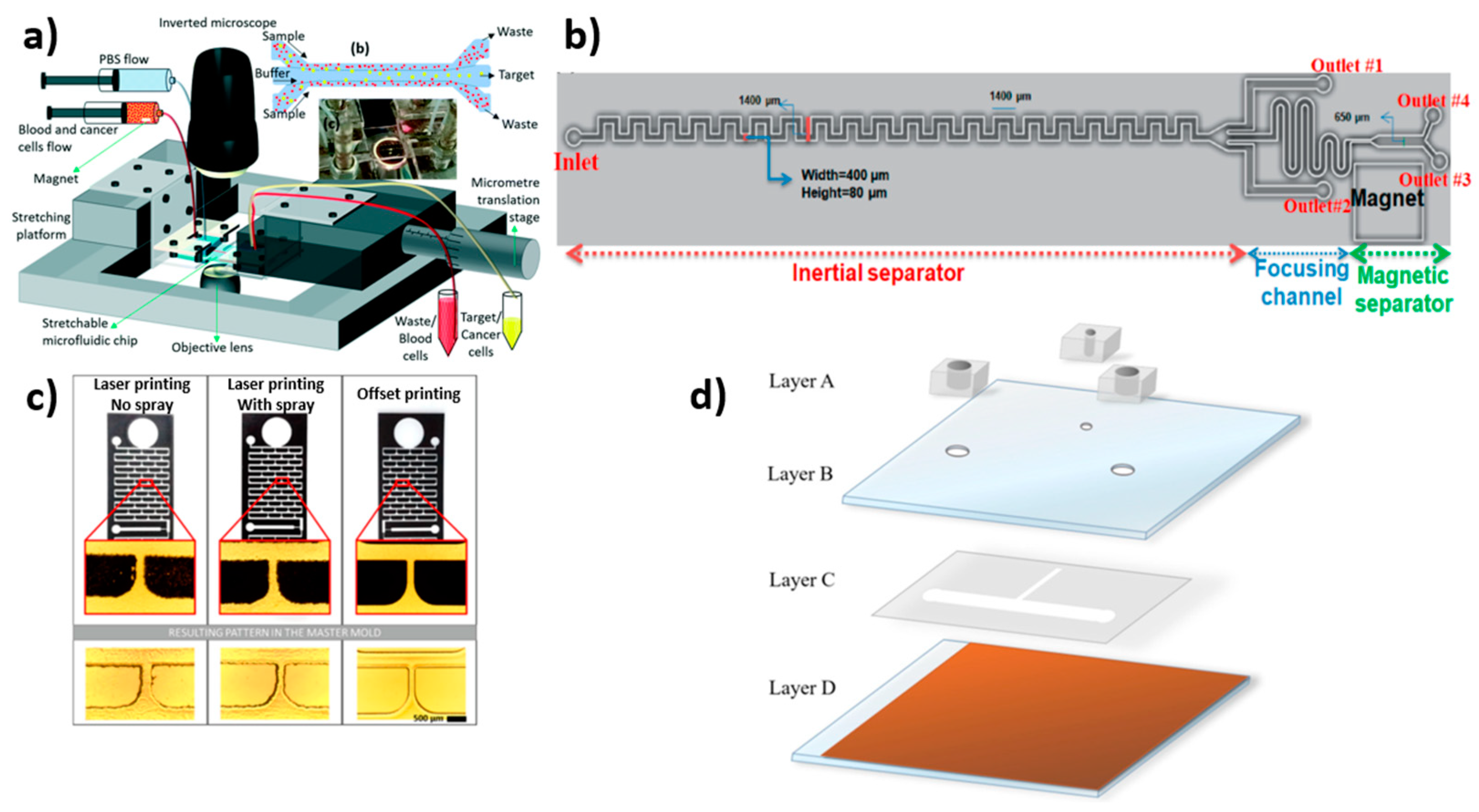
2.5. Printing Technique
2.6. Overall Summary of the Fabrication Process
3. Isolation of Circulating Tumor Cells (CTCs) by Microfluidic Devices
3.1. Size-Based Isolation
3.2. Inertial Focusing Microchannel-Based Isolation
3.3. Dielectrophoresis-Based Isolation
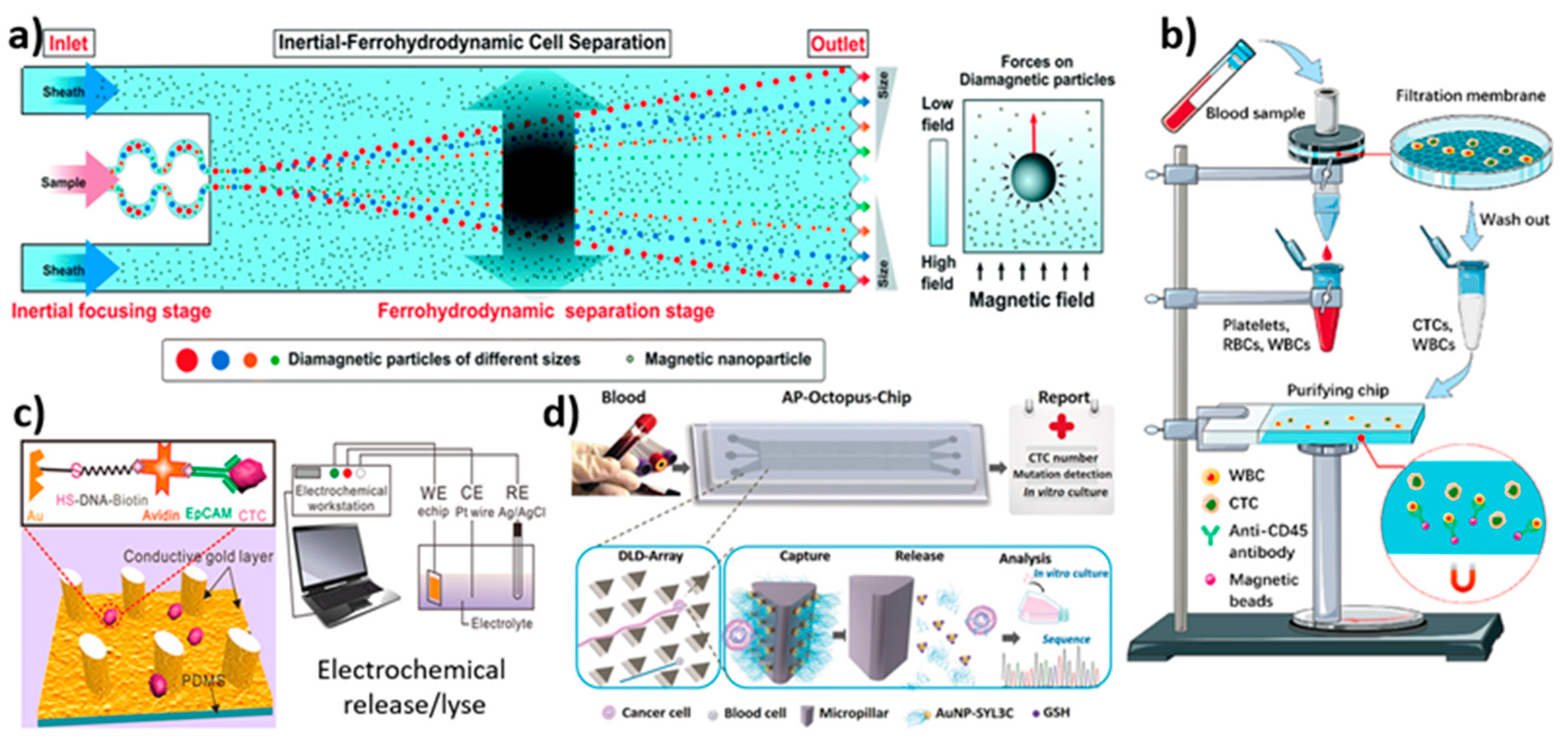
3.4. Magnetic Field-Based Isolation
3.4.1. Immunomagnetic (Label)-Based Isolation
3.4.2. Label-Free-Based Magnetic Isolation
3.5. Acoustic-Based Isolation

3.6. Combined Method-Based Isolation
3.7. Electrochemical-Based Isolation
3.8. Biological Interaction-Based Isolation
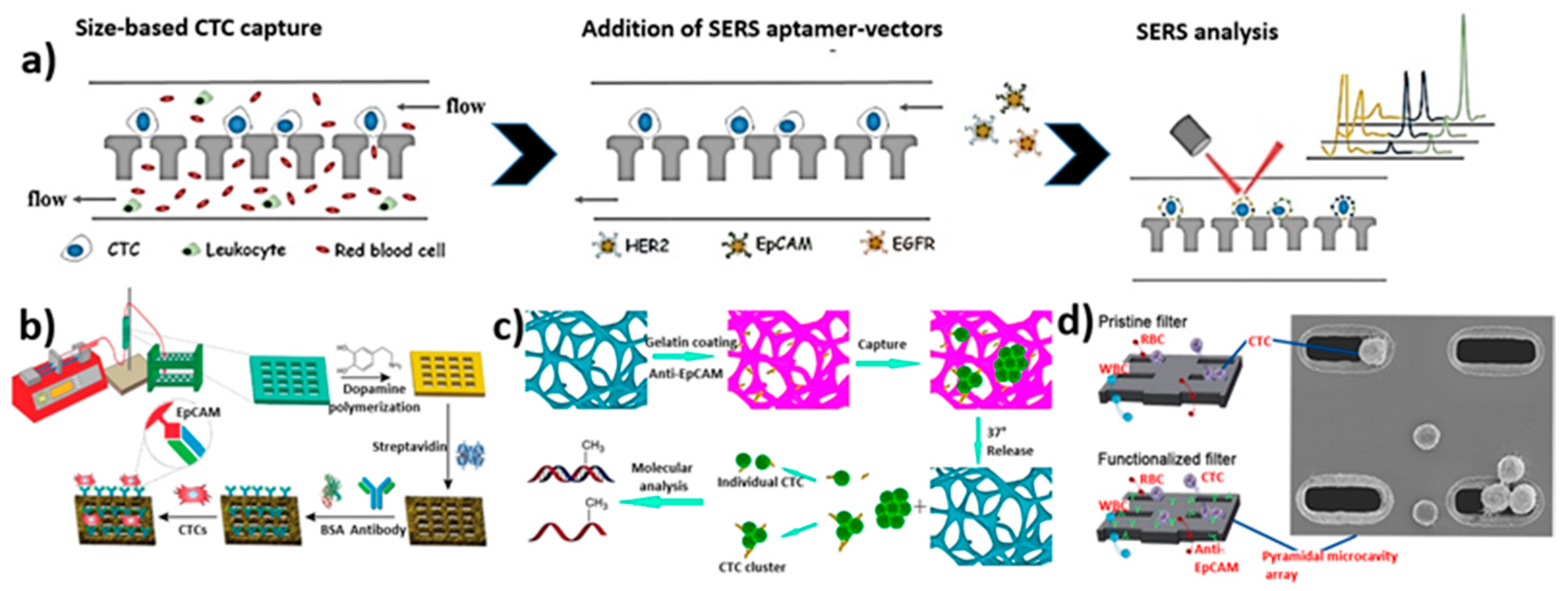
3.9. Overview of Microfluidic Device Performance for the Isolation of Circulating Tumor Cells
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.; Liu, S.; Liu, Z.; Huang, J.; Pu, X.; Li, J.; Yang, D.; Deng, H.; Yang, N.; Xu, J. Classification of Circulating Tumor Cells by Epithelial-Mesenchymal Transition Markers. PLoS ONE 2015, 10, e0123976. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, J.; Song, H.; Sohn, K.Y.; Jeon, M.; Han, K.-H. Microfluidic technologies for circulating tumor cell isolation. Analyst 2018, 143, 2936–2970. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.M.; Witek, M.A.; Kamande, J.W.; Soper, S.A. Materials and microfluidics: Enabling the efficient isolation and analysis of circulating tumour cells. Chem. Soc. Rev. 2017, 46, 4254–4280. [Google Scholar] [CrossRef] [PubMed]
- Potdar, P.D.; Lotey, N.K. Role of circulating tumor cells in future diagnosis and therapy of cancer. J. Cancer Metastasis Treat. 2015, 1, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Navas, C.; de Miguel-Pérez, D.; Exposito-Hernandez, J.; Bayarri, C.; Amezcua, V.; Ortigosa, A.; Valdivia, J.; Guerrero, R.; Garcia Puche, J.L.; Lorente, J.A. Cooperative and escaping mechanisms between circulating tumor cells and blood constituents. Cells 2019, 8, 1382. [Google Scholar] [CrossRef] [Green Version]
- Kurkuri, M.D.; Al-Ejeh, F.; Shi, J.Y.; Palms, D.; Prestidge, C.; Griesser, H.J.; Brown, M.P.; Thierry, B. Plasma functionalized PDMS microfluidic chips: Towards point-of-care capture of circulating tumor cells. J. Mater. Chem. 2011, 21, 8841–8848. [Google Scholar] [CrossRef]
- Liu, H.Y.; Hille, C.; Haller, A.; Kumar, R.; Pantel, K.; Hirtz, M. Highly efficient capture of circulating tumor cells by microarray in a microfluidic device. FASEB J. 2019, 33, lb230. [Google Scholar] [CrossRef]
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef]
- Jie, X.-X.; Zhang, X.-Y.; Xu, C.-J. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: Mechanisms and clinical applications. Oncotarget 2017, 8, 81558. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.-B.; Chen, M.-M.; Wang, Y.-K.; Sun, Z.-H.; Xie, M.; Huang, W.-H. Current techniques and future advance of microfluidic devices for circulating tumor cells. TrAC Trends Anal. Chem. 2019, 117, 116–127. [Google Scholar] [CrossRef]
- Madhuprasad; Bhat, M.P.; Jung, H.-Y.; Losic, D.; Kurkuri, M.D. Anion Sensors as Logic Gates: A Close Encounter? Chem. Eur. J. 2016, 22, 6148–6178. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.P.; Patil, P.; Nataraj, S.K.; Altalhi, T.; Jung, H.-Y.; Losic, D.; Kurkuri, M.D. Turmeric, naturally available colorimetric receptor for quantitative detection of fluoride and iron. Chem. Eng. J. 2016, 303, 14–21. [Google Scholar] [CrossRef]
- Patil, P.; Bhat, M.P.; Gatti, M.G.; Kabiri, S.; Altalhi, T.; Jung, H.-Y.; Losic, D.; Kurkuri, M. Chemodosimeter functionalized diatomaceous earth particles for visual detection and removal of trace mercury ions from water. Chem. Eng. J 2017, 327, 725–733. [Google Scholar] [CrossRef]
- Patil, P.; Ajeya, K.V.; Bhat, M.P.; Sriram, G.; Yu, J.; Jung, H.-Y.; Altalhi, T.; Kigga, M.; Kurkuri, M.D. Real-Time Probe for the Efficient Sensing of Inorganic Fluoride and Copper Ions in Aqueous Media. ChemistrySelect 2018, 3, 11593–11600. [Google Scholar] [CrossRef]
- Bhat, M.P.; Kigga, M.; Govindappa, H.; Patil, P.; Jung, H.-Y.; Yu, J.; Kurkuri, M. A reversible fluoride chemosensor for the development of multi-input molecular logic gates. New J. Chem. 2019, 43, 12734–12743. [Google Scholar] [CrossRef]
- Bhat, M.P.; Vinayak, S.; Yu, J.; Jung, H.-Y.; Kurkuri, M. Colorimetric Receptors for the Detection of Biologically Important Anions and Their Application in Designing Molecular Logic Gate. ChemistrySelect 2020, 5, 13135–13143. [Google Scholar] [CrossRef]
- Pirzada, M.; Altintas, Z. Nanomaterials for healthcare biosensing applications. Sensors 2019, 19, 5311. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.; Nedosekin, D.A.; Galanzha, E.I.; Zharov, V.P. Detection of apoptotic circulating tumor cells using in vivo fluorescence flow cytometry. Cytom. Part A 2019, 95, 664–671. [Google Scholar] [CrossRef]
- Safaei, T.S.; Mohamadi, R.M.; Sargent, E.H.; Kelley, S.O. In situ electrochemical ELISA for specific identification of captured cancer cells. ACS Appl. Mater. Interfaces 2015, 7, 14165–14169. [Google Scholar] [CrossRef]
- Huaman, J.; Naidoo, M.; Zang, X.; Ogunwobi, O.O. Fibronectin regulation of integrin B1 and SLUG in circulating tumor cells. Cells 2019, 8, 618. [Google Scholar] [CrossRef] [Green Version]
- Andergassen, U.; Zebisch, M.; Kölbl, A.C.; König, A.; Heublein, S.; Schröder, L.; Hutter, S.; Friese, K.; Jeschke, U. Real-time qPCR-based detection of circulating tumor cells from blood samples of adjuvant breast cancer patients: A preliminary study. Breast Care 2016, 11, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Sun, L.; Zhang, H.; Wei, L.; Qu, W.; Zeng, Z.; Liu, Y.; Zhu, Z. Microfluidic chip combined with magnetic-activated cell sorting technology for tumor antigen-independent sorting of circulating hepatocellular carcinoma cells. PeerJ 2019, 7, e6681. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Weaver, W.M.; Mach, A.J.; Hur, S.C.; Tse, H.T.K.; Lee, W.; Amini, H.; Di Carlo, D. Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 2010, 397, 3249–3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Shen, M.; Shi, X. Design of functional electrospun nanofibers for cancer cell capture applications. J. Mater. Chem. B 2018, 6, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Nieto, D.; Couceiro, R.; Aymerich, M.; Lopez-Lopez, R.; Abal, M.; Flores-Arias, M.T. A laser-based technology for fabricating a soda-lime glass based microfluidic device for circulating tumour cell capture. Colloids Surf. B Biointerfaces 2015, 134, 363–369. [Google Scholar] [CrossRef]
- Bhat, M.P.; Kurkuri, M.; Losic, D.; Kigga, M.; Altalhi, T. New optofluidic based lab-on-a-chip device for the real-time fluoride analysis. Anal. Chim. Acta 2021, 1159, 338439. [Google Scholar] [CrossRef]
- Leung, C.-H.; Wu, K.-J.; Li, G.; Wu, C.; Ko, C.-N.; Ma, D.-L. Application of label-free techniques in microfluidic for biomolecules detection and circulating tumor cells analysis. TrAC Trends Anal. Chem. 2019, 117, 78–83. [Google Scholar] [CrossRef]
- Edd, J.F.; Mishra, A.; Dubash, T.D.; Herrera, S.; Mohammad, R.; Williams, E.K.; Hong, X.; Mutlu, B.R.; Walsh, J.R.; de Carvalho, F.M. Microfluidic concentration and separation of circulating tumor cell clusters from large blood volumes. Lab Chip 2020, 20, 558–567. [Google Scholar] [CrossRef]
- Gharaghani, F.M.; Akhond, M.; Hemmateenejad, B. A three-dimensional origami microfluidic device for paper chromatography: Application to quantification of Tartrazine and Indigo carmine in food samples. J. Chromatogr. A 2020, 1621, 461049. [Google Scholar] [CrossRef]
- He, G.; Yang, C.; Feng, J.; Wu, J.; Zhou, L.; Wen, R.; Huang, S.; Wu, Q.; Liu, F.; Chen, H.J. Hierarchical spiky microstraws-integrated microfluidic device for efficient capture and in situ manipulation of cancer cells. Adv. Funct. Mater. 2019, 29, 1806484. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Xie, Y.; Yuan, T.; Chen, X. Progress of microfluidics for biology and medicine. Nano-Micro Lett. 2013, 5, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Mo Jung, S.; Lee, K.H.; Jun Kang, Y.; Yang, S. A microfluidic device for continuous white blood cell separation and lysis from whole blood. Artif. Organs 2010, 34, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yang, X.; Zhang, J.; Cao, X. Developing a non-fouling hybrid microfluidic device for applications in circulating tumour cell detections. Colloids Surf. B Biointerfaces 2017, 151, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R.M. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Panesar, S.; Neethirajan, S. Microfluidics: Rapid diagnosis for breast cancer. Nano-Micro Lett. 2016, 8, 204–220. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Bhat, M.P.; Kim, C.S.; Kim, J.; Lee, K.-H. Improved 3D-Printability of Cellulose Acetate to Mimic Water Absorption in Plant Roots through Nanoporous Networks. Macromolecules 2022, 55, 1855–1865. [Google Scholar] [CrossRef]
- Castillo-León, J. Microfluidics and lab-on-a-chip devices: History and challenges. In Lab-on-a-Chip Devices and Micro-Total Analysis Systems; Springer: Cham, Switzerland, 2015; pp. 1–15. [Google Scholar]
- Muhsin, S.A.; Al-Amidie, M.; Shen, Z.; Mlaji, Z.; Liu, J.; Abdullah, A.; El-Dweik, M.; Zhang, S.; Almasri, M. A microfluidic biosensor for rapid simultaneous detection of waterborne pathogens. Biosens. Bioelectron. 2022, 203, 113993. [Google Scholar] [CrossRef]
- Leong, S.Y.; Ong, H.B.; Tay, H.M.; Kong, F.; Upadya, M.; Gong, L.; Dao, M.; Dalan, R.; Hou, H.W. Microfluidic Size Exclusion Chromatography (μSEC) for Extracellular Vesicles and Plasma Protein Separation. Small 2022, 18, 2104470. [Google Scholar] [CrossRef]
- Livak-Dahl, E.; Sinn, I.; Burns, M. Microfluidic Chemical Analysis Systems. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 325–353. [Google Scholar] [CrossRef]
- Sanjay, S.T.; Zhou, W.; Dou, M.; Tavakoli, H.; Ma, L.; Xu, F.; Li, X. Recent advances of controlled drug delivery using microfluidic platforms. Adv. Drug Deliv. Rev. 2018, 128, 3–28. [Google Scholar] [CrossRef]
- Riahi, R.; Tamayol, A.; Shaegh, S.A.M.; Ghaemmaghami, A.M.; Dokmeci, M.R.; Khademhosseini, A. Microfluidics for advanced drug delivery systems. Curr. Opin. Chem. Eng. 2015, 7, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Borecki, M.; Korwin-Pawlowski, M.L.; Beblowska, M.; Szmidt, J.; Jakubowski, A. Optoelectronic Capillary Sensors in Microfluidic and Point-of-Care Instrumentation. Sensors 2010, 10, 3771–3797. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Fan, L.; Zhu, R.; Sun, D. Microfluidic single-cell manipulation and analysis: Methods and applications. Micromachines 2019, 10, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendre, A.; Bhat, M.P.; Lee, K.-H.; Altalhi, T.; Alruqi, M.A.; Kurkuri, M. Recent developments in microfluidic technology for synthesis and toxicity-efficiency studies of biomedical nanomaterials. Mater. Today Adv. 2022, 13, 100205. [Google Scholar] [CrossRef]
- Elvira, K.S.; Wootton, R.C.; de Mello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, Z.; Andarge, H.; Li, W.; Pappas, D. Nanoparticle modification of microfluidic cell separation for cancer cell detection and isolation. Analyst 2020, 145, 257–267. [Google Scholar] [CrossRef]
- Qu, L.; Xu, J.; Tan, X.; Liu, Z.; Xu, L.; Peng, R. Dual-aptamer modification generates a unique interface for highly sensitive and specific electrochemical detection of tumor cells. ACS Appl. Mater. Interfaces 2014, 6, 7309–7315. [Google Scholar] [CrossRef]
- Safdar, M.; Jänis, J.; Sanchez, S. Microfluidic fuel cells for energy generation. Lab Chip 2016, 16, 2754–2758. [Google Scholar] [CrossRef]
- Aykar, S.S.; Reynolds, D.E.; McNamara, M.C.; Hashemi, N.N. Manufacturing of poly(ethylene glycol diacrylate)-based hollow microvessels using microfluidics. RSC Adv. 2020, 10, 4095–4102. [Google Scholar] [CrossRef]
- Jung, B.-J.; Kim, J.; Kim, J.-a.; Jang, H.; Seo, S.; Lee, W. PDMS-parylene hybrid, flexible microfluidics for real-time modulation of 3D helical inertial microfluidics. Micromachines 2018, 9, 255. [Google Scholar] [CrossRef] [Green Version]
- Friend, J.; Yeo, L. Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 2010, 4, 026502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddings, M.A.; Johnson, M.A.; Gale, B.K. Determining the optimal PDMS–PDMS bonding technique for microfluidic devices. J. Micromech. Microeng. 2008, 18, 067001. [Google Scholar] [CrossRef]
- Yin, J.; Deng, J.; Du, C.; Zhang, W.; Jiang, X. Microfluidics-based approaches for separation and analysis of circulating tumor cells. TrAC Trends Anal. Chem. 2019, 117, 84–100. [Google Scholar] [CrossRef]
- Murlidhar, V.; Zeinali, M.; Grabauskiene, S.; Ghannad-Rezaie, M.; Wicha, M.S.; Simeone, D.M.; Ramnath, N.; Reddy, R.M.; Nagrath, S. A Radial Flow Microfluidic Device for Ultra-High-Throughput Affinity-Based Isolation of Circulating Tumor Cells. Small 2014, 10, 4895–4904. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Park, J.; Lim, M.; Sunkara, V.; Kim, S.Y.; Kim, G.H.; Kim, M.-H.; Cho, Y.-K. All-in-one centrifugal microfluidic device for size-selective circulating tumor cell isolation with high purity. Anal. Chem. 2014, 86, 11349–11356. [Google Scholar] [CrossRef]
- Sato, K. Microdevice in Cellular Pathology: Microfluidic Platforms for Fluorescence in situ Hybridization and Analysis of Circulating Tumor Cells. Anal. Sci. 2015, 31, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Park, E.S.; Jin, C.; Guo, Q.; Ang, R.R.; Duffy, S.P.; Matthews, K.; Azad, A.; Abdi, H.; Todenhöfer, T.; Bazov, J.; et al. Continuous Flow Deformability-Based Separation of Circulating Tumor Cells Using Microfluidic Ratchets. Small 2016, 12, 1909–1919. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Ren, Y.; Yan, Y.; Wu, A. Numerical simulation of circulating tumor cell separation in a dielectrophoresis based Y-Y shaped microfluidic device. Sep. Purif. Technol. 2021, 255, 117343. [Google Scholar] [CrossRef]
- Shi, W.; Wang, S.; Maarouf, A.; Uhl, C.G.; He, R.; Yunus, D.; Liu, Y. Magnetic particles assisted capture and release of rare circulating tumor cells using wavy-herringbone structured microfluidic devices. Lab Chip 2017, 17, 3291–3299. [Google Scholar] [CrossRef]
- Rafeie, M.; Zhang, J.; Asadnia, M.; Li, W.; Warkiani, M.E. Multiplexing slanted spiral microchannels for ultra-fast blood plasma separation. Lab Chip 2016, 16, 2791–2802. [Google Scholar] [CrossRef]
- Zhou, J.; Kulasinghe, A.; Bogseth, A.; O’Byrne, K.; Punyadeera, C.; Papautsky, I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst. Nanoeng. 2019, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, F.; Liu, C.; Lin, L.; Chen, Q.; Sun, J. Microfluidic analysis of circulating tumor cells and tumor-derived extracellular vesicles. TrAC Trends Anal. Chem. 2019, 117, 128–145. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.-T.; Ebrahimi Warkiani, M.; Li, W. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, D.; Wang, X.; Jin, Y.; Huo, B.; Wang, Y.; He, C.; Fu, X.; Lu, N. Size-matching hierarchical micropillar arrays for detecting circulating tumor cells in breast cancer patients’ whole blood. Nanoscale 2019, 11, 6677–6684. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Yu, V.; Garon, E.B.; Goldman, J.W.; Di Carlo, D. Biophysical isolation and identification of circulating tumor cells. Lab Chip 2017, 17, 1452–1461. [Google Scholar] [CrossRef] [Green Version]
- Brimmo, A.T.; Menachery, A.; Qasaimeh, M.A. Microelectrofluidic probe for sequential cell separation and patterning. Lab Chip 2019, 19, 4052–4063. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Jiang, H.; Zhang, L.; Yi, K.; Cui, H.; Wang, F.; Liu, W.; Zhao, X.; Zhou, F.; Guo, S. The acoustofluidic focusing and separation of rare tumor cells using transparent lithium niobate transducers. Lab Chip 2019, 19, 3922–3930. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, D.; Chen, M.; Chen, K.; Liu, H.; Liu, W.; Yang, Y. Precise and non-invasive circulating tumor cell isolation based on optical force using homologous erythrocyte binding. Lab Chip 2019, 19, 2549–2556. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.; Jenkins, B.D.; Cheng, R.; Harris, B.N.; Zhang, W.; Xie, J.; Murrow, J.R.; Hodgson, J.; Egan, M.; et al. Tumor antigen-independent and cell size variation-inclusive enrichment of viable circulating tumor cells. Lab Chip 2019, 19, 1860–1876. [Google Scholar] [CrossRef]
- Ahmed, M.G.; Abate, M.F.; Song, Y.; Zhu, Z.; Yan, F.; Xu, Y.; Wang, X.; Li, Q.; Yang, C. Isolation, Detection, and Antigen-Based Profiling of Circulating Tumor Cells Using a Size-Dictated Immunocapture Chip. Angew. Chem. Int. Ed. 2017, 56, 10681–10685. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, Y.; Zhao, Y.; Zhang, L.; Zhang, L.; Mao, H.; Huang, C. Nanotechnology-assisted isolation and analysis of circulating tumor cells on microfluidic devices. Micromachines 2020, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Cui, D. Advances in isolation and detection of circulating tumor cells based on microfluidics. Cancer Biol. Med. 2018, 15, 335–353. [Google Scholar] [PubMed] [Green Version]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Lee, W.; Folch, A. Mail-order microfluidics: Evaluation of stereolithography for the production of microfluidic devices. Lab Chip 2014, 14, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Tseng, P.; Murray, C.; Kim, D.; Di Carlo, D. Research highlights: Printing the future of microfabrication. Lab Chip 2014, 14, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Sochol, R.; Sweet, E.; Glick, C.; Venkatesh, S.; Avetisyan, A.; Ekman, K.; Raulinaitis, A.; Tsai, A.; Wienkers, A.; Korner, K. 3D printed microfluidic circuitry via multijet-based additive manufacturing. Lab Chip 2016, 16, 668–678. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.-H.; Liu, R.; Ozkaya-Ahmadov, T.; Boya, M.; Swain, B.E.; Owens, J.M.; Burentugs, E.; Bilen, M.A.; McDonald, J.F.; Sarioglu, A.F. Hybrid negative enrichment of circulating tumor cells from whole blood in a 3D-printed monolithic device. Lab Chip 2019, 19, 3427–3437. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Woolley, A.T.; Nordin, G.P. 3D printed high density, reversible, chip-to-chip microfluidic interconnects. Lab Chip 2018, 18, 639–647. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.-Y.; Wang, X.; Sweet, E.; Liu, N.; Gong, X.; Lin, L. 3D printed microfluidic devices for circulating tumor cells (CTCs) isolation. Biosens. Bioelectron. 2020, 150, 111900. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Lu, Z.; Sun, Y.; Yang, C.; Zhou, Q.; Hong, S.; Wang, S.; Xiong, B.; Liu, K.; et al. A simple pyramid-shaped microchamber towards highly efficient isolation of circulating tumor cells from breast cancer patients. Biomed. Microdevices 2018, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, N.; Wang, S.; Shi, D.; Zhang, C.; Liu, K.; Xiong, B. Wedge-shaped microfluidic chip for circulating tumor cells isolation and its clinical significance in gastric cancer. J. Transl. Med. 2018, 16, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, S.M.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-J.; Hsieh, C.-H.; Chiu, T.-K.; Zhu, Y.-X.; Wang, H.-M.; Hung, F.-C.; Chou, W.-P.; Wu, M.-H. An Optically Induced Dielectrophoresis (ODEP)-Based Microfluidic System for the Isolation of High-Purity CD45(neg)/EpCAM(neg) Cells from the Blood Samples of Cancer Patients-Demonstration and Initial Exploration of the Clinical Significance of These Cells. Micromachines 2018, 9, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.T.; Low, H.Y.; Ng, J.K.; Liu, W.-T.; Zhang, Y. Fabrication of three-dimensional hemispherical structures using photolithography. Microfluid. Nanofluid. 2009, 7, 721. [Google Scholar] [CrossRef]
- Tian, W.-C.; Finehout, E. Microfluidics for Biological Applications; Springer Science & Business Media: Berlin, Germany, 2009; Volume 16. [Google Scholar]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef] [Green Version]
- Kwak, B.; Lee, J.; Lee, J.; Kim, H.S.; Kang, S.; Lee, Y. Spiral shape microfluidic channel for selective isolating of heterogenic circulating tumor cells. Biosens. Bioelectron. 2018, 101, 311–316. [Google Scholar] [CrossRef]
- Fan, X.; Jia, C.; Yang, J.; Li, G.; Mao, H.; Jin, Q.; Zhao, J. A microfluidic chip integrated with a high-density PDMS-based microfiltration membrane for rapid isolation and detection of circulating tumor cells. Biosens. Bioelectron. 2015, 71, 380–386. [Google Scholar] [CrossRef]
- Yan, S.; Chen, P.; Zeng, X.; Zhang, X.; Li, Y.; Xia, Y.; Wang, J.; Dai, X.; Feng, X.; Du, W.; et al. Integrated Multifunctional Electrochemistry Microchip for Highly Efficient Capture, Release, Lysis, and Analysis of Circulating Tumor Cells. Anal. Chem. 2017, 89, 12039–12044. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Zhou, J.; Kenny, L.; Papautsky, I.; Punyadeera, C. Capture of Circulating Tumour Cell Clusters Using Straight Microfluidic Chips. Cancers 2019, 11, 89. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.; Lee, J.; Ra, M.; Gwon, H.; Lee, S.; Kim, M.Y.; Yoo, K.-C.; Sul, O.; Kim, C.G.; Kim, W.-Y.; et al. Continuous Separation of Circulating Tumor Cells from Whole Blood Using a Slanted Weir Microfluidic Device. Cancers 2019, 11, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-H.; Pulikkathodi, A.K.; Ma, Y.-D.; Wang, Y.-L.; Lee, G.-B. A microfluidic platform integrated with field-effect transistors for enumeration of circulating tumor cells. Lab Chip 2019, 19, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Raillon, C.; Che, J.; Thill, S.; Duchamp, M.; Desbiolles, B.X.E.; Millet, A.; Sollier, E.; Renaud, P. Toward Microfluidic Label-Free Isolation and Enumeration of Circulating Tumor Cells from Blood Samples. Cytom. Part A 2019, 95, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-L.; Huang, W.; Jalal, S.I.; Chan, B.-D.; Mahmood, A.; Shahda, S.; O’Neil, B.H.; Matei, D.E.; Savran, C.A. Circulating tumor cell detection using a parallel flow micro-aperture chip system. Lab Chip 2015, 15, 1677–1688. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Liu, H.; Zhang, Z.; Lin, C.; Wang, B. Hybrid magnetic and deformability based isolation of circulating tumor cells using microfluidics. AIP Adv. 2019, 9, 025023. [Google Scholar] [CrossRef] [Green Version]
- Varillas, J.I.; Zhang, J.; Chen, K.; Barnes, I.I.; Liu, C.; George, T.J.; Fan, Z.H. Microfluidic Isolation of Circulating Tumor Cells and Cancer Stem-Like Cells from Patients with Pancreatic Ductal Adenocarcinoma. Theranostics 2019, 9, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Ahmad, S.; Momeni, M. Design and Parameter Study of Integrated Microfluidic Platform for CTC Isolation and Enquiry; A Numerical Approach. Biosensors 2018, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wu, J.; Zhang, Y.; Lin, Z.; Lin, J.-M. Targeted isolation and analysis of single tumor cells with aptamer-encoded microwell array on microfluidic device. Lab Chip 2012, 12, 5180–5185. [Google Scholar] [CrossRef]
- Hoshino, K.; Huang, Y.-Y.; Lane, N.; Huebschman, M.; Uhr, J.W.; Frenkel, E.P.; Zhang, X. Microchip-based immunomagnetic detection of circulating tumor cells. Lab Chip 2011, 11, 3449–3457. [Google Scholar] [CrossRef]
- Fallahi, H.; Yadav, S.; Phan, H.-P.; Ta, H.; Zhang, J.; Nguyen, N.-T. Size-tuneable isolation of cancer cells using stretchable inertial microfluidics. Lab Chip 2021, 21, 2008–2018. [Google Scholar] [CrossRef]
- Jiang, R.; Agrawal, S.; Aghaamoo, M.; Parajuli, R.; Agrawal, A.; Lee, A.P. Rapid isolation of circulating cancer associated fibroblasts by acoustic microstreaming for assessing metastatic propensity of breast cancer patients. Lab Chip 2021, 21, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Jou, H.-J.; Chou, L.-Y.; Chang, W.-C.; Ho, H.-C.; Zhang, W.-T.; Ling, P.-Y.; Tsai, K.-H.; Chen, S.-H.; Chen, T.-H.; Lo, P.-H.; et al. An Automatic Platform Based on Nanostructured Microfluidic Chip for Isolating and Identification of Circulating Tumor Cells. Micromachines 2021, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, X.; Gao, W.; Wang, Y.; Jia, C.; Cong, H. A label-free microfluidic chip for the highly selective isolation of single and cluster CTCs from breast cancer patients. Transl. Oncol. 2021, 14, 100959. [Google Scholar] [CrossRef] [PubMed]
- Reinholt, S.J.; Craighead, H.G. Microfluidic Device for Aptamer-Based Cancer Cell Capture and Genetic Mutation Detection. Anal. Chem. 2018, 90, 2601–2608. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, R.; Shamloo, A.; Akbari, J. Design of a Hybrid Inertial and Magnetophoretic Microfluidic Device for CTCs Separation from Blood. Micromachines 2021, 12, 877. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-T.; Thach, H.; Roy, E.; Huynh, K.; Perrault, C.M.-T. Low-Cost, Accessible Fabrication Methods for Microfluidics Research in Low-Resource Settings. Micromachines 2018, 9, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldbaur, A.; Rapp, H.; Länge, K.; Rapp, B.E. Let there be chip—Towards rapid prototyping of microfluidic devices: One-step manufacturing processes. Anal. Methods 2011, 3, 2681–2716. [Google Scholar] [CrossRef]
- Kamei, K.-i.; Mashimo, Y.; Koyama, Y.; Fockenberg, C.; Nakashima, M.; Nakajima, M.; Li, J.; Chen, Y. 3D printing of soft lithography mold for rapid production of polydimethylsiloxane-based microfluidic devices for cell stimulation with concentration gradients. Biomed. Microdevices 2015, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, P.; Luo, Z.; Ren, Y.; Liao, M.; Feng, L.; Li, Y.; He, L. Rapid fabrication of microfluidic chips based on the simplest LED lithography. J. Micromech. Microeng. 2015, 25, 055020. [Google Scholar] [CrossRef]
- Isiksacan, Z.; Guler, M.T.; Aydogdu, B.; Bilican, I.; Elbuken, C. Rapid fabrication of microfluidic PDMS devices from reusable PDMS molds using laser ablation. J. Micromech. Microeng. 2016, 26, 035008. [Google Scholar] [CrossRef]
- Thaweskulchai, T.; Schulte, A. A Low-Cost 3-in-1 3D Printer as a Tool for the Fabrication of Flow-Through Channels of Microfluidic Systems. Micromachines 2021, 12, 947. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, W.; Zou, K.; Wei, S.; Zhang, X.; Li, E.; Wang, Q. Design and Clinical Application of an Integrated Microfluidic Device for Circulating Tumor Cells Isolation and Single-Cell Analysis. Micromachines 2021, 12, 49. [Google Scholar] [CrossRef]
- Gurudatt, N.G.; Chung, S.; Kim, J.-M.; Kim, M.-H.; Jung, D.-K.; Han, J.-Y.; Shim, Y.-B. Separation detection of different circulating tumor cells in the blood using an electrochemical microfluidic channel modified with a lipid-bonded conducting polymer. Biosens. Bioelectron. 2019, 146, 111746. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, Z.; Li, G.; Lin, F.; Shao, K.; Cao, B.; Hou, Y. Characterization of circulating tumor cells in breast cancer patients by spiral microfluidics. Cell Biol. Toxicol. 2019, 35, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Autebert, J.; Coudert, B.; Bidard, F.-C.; Pierga, J.-Y.; Descroix, S.; Malaquin, L.; Viovy, J.-L. Microfluidic: An innovative tool for efficient cell sorting. Methods 2012, 57, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.S.; Hu, M.; Huang, M.C.; Cheong, W.C.; Gan, A.T.L.; Looi, X.L.; Leong, S.M.; Koay, E.S.-C.; Li, M.-H. Microsieve lab-chip device for rapid enumeration and fluorescence in situ hybridization of circulating tumor cells. Lab Chip 2012, 12, 4388–4396. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Tran, T.H.P.; Blick, T.; O’Byrne, K.; Thompson, E.W.; Warkiani, M.E.; Nelson, C.; Kenny, L.; Punyadeera, C. Enrichment of circulating head and neck tumour cells using spiral microfluidic technology. Sci. Rep. 2017, 7, 42517. [Google Scholar] [CrossRef] [Green Version]
- Abdulla, A.; Zhang, T.; Ahmad, K.Z.; Li, S.; Lou, J.; Ding, X. Label-free Separation of Circulating Tumor Cells Using a Self-Amplified Inertial Focusing (SAIF) Microfluidic Chip. Anal. Chem. 2020, 92, 16170–16179. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Z.; Ai, Y. Sheathless inertial cell focusing and sorting with serial reverse wavy channel structures. Microsyst. Nanoeng. 2018, 4, 5. [Google Scholar] [CrossRef]
- Martel, J.M.; Toner, M. Inertial Focusing in Microfluidics. Annu. Rev. Biomed. Eng. 2014, 16, 371–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Sun, S.; Chen, Y.; Cheng, Z.; Li, Y.; Jia, L.; Lin, P.; Yang, Z.; Shu, R. Inertial particle focusing and spacing control in microfluidic devices. Microfluid. Nanofluidics 2018, 22, 25. [Google Scholar] [CrossRef]
- Ying, Y.; Lin, Y. Inertial Focusing and Separation of Particles in Similar Curved Channels. Sci. Rep. 2019, 9, 16575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.; Cheng, L.; Wang, S.; Bi, X.; Wang, X.; Wang, R.; Chen, X.; Zha, Z.; Wang, F.; Xu, X.; et al. Efficient separation of tumor cells from untreated whole blood using a novel multistage hydrodynamic focusing microfluidics. Talanta 2020, 207, 120261. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Khoo, B.L.; Wu, L.; Tay, A.K.P.; Bhagat, A.A.S.; Han, J.; Lim, C.T. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat. Protoc. 2016, 11, 134–148. [Google Scholar] [CrossRef]
- Ozbey, A.; Karimzadehkhouei, M.; Kocaturk, N.M.; Bilir, S.E.; Kutlu, O.; Gozuacik, D.; Kosar, A. Inertial focusing of cancer cell lines in curvilinear microchannels. Micro Nano Eng. 2019, 2, 53–63. [Google Scholar] [CrossRef]
- Nam, J.; Tan, J.K.S.; Khoo, B.L.; Namgung, B.; Leo, H.L.; Lim, C.T.; Kim, S. Hybrid capillary-inserted microfluidic device for sheathless particle focusing and separation in viscoelastic flow. Biomicrofluidics 2015, 9, 064117. [Google Scholar] [CrossRef] [Green Version]
- Che, J.; Yu, V.; Dhar, M.; Renier, C.; Matsumoto, M.; Heirich, K.; Garon, E.B.; Goldman, J.; Rao, J.; Sledge, G.W.; et al. Classification of large circulating tumor cells isolated with ultra-high throughput microfluidic Vortex technology. Oncotarget 2016, 7, 12748. [Google Scholar] [CrossRef] [Green Version]
- Thanormsridetchai, A.; Ketpun, D.; Srituravanich, W.; Piyaviriyakul, P.; Sailasuta, A.; Jeamsaksiri, W.; Sripumkhai, W.; Pimpin, A. Focusing and sorting of multiple-sized beads and cells using low-aspect-ratio spiral microchannels. J. Mech. Sci. Technol. 2017, 31, 5397–5405. [Google Scholar] [CrossRef]
- Khoshmanesh, K.; Nahavandi, S.; Baratchi, S.; Mitchell, A.; Kalantar-zadeh, K. Dielectrophoretic platforms for bio-microfluidic systems. Biosens. Bioelectron. 2011, 26, 1800–1814. [Google Scholar] [CrossRef]
- Abd Rahman, N.; Ibrahim, F.; Yafouz, B. Dielectrophoresis for Biomedical Sciences Applications: A Review. Sensors 2017, 17, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.Y.; Kayani, A.B.A.; Ali, M.A.M.; Kok, C.K.; Majlis, B.Y.; Hoe, S.L.L.; Marzuki, M.; Khoo, A.S.-B.; Ostrikov, K.; Rahman, M.A.; et al. Dielectrophoresis-based microfluidic platforms for cancer diagnostics. Biomicrofluidics 2018, 12, 011503. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.-K.; Chou, W.-P.; Huang, S.-B.; Wang, H.-M.; Lin, Y.-C.; Hsieh, C.-H.; Wu, M.-H. Application of optically-induced-dielectrophoresis in microfluidic system for purification of circulating tumour cells for gene expression analysis—Cancer cell line model. Sci. Rep. 2016, 6, 32851. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Anand, R.K. High-Throughput Selective Capture of Single Circulating Tumor Cells by Dielectrophoresis at a Wireless Electrode Array. J. Am. Chem. Soc. 2017, 139, 8950–8959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Ito, H.; Kozuka, M.; Hirai, M.; Fujii, T. Localization of low-abundant cancer cells in a sharply expanded microfluidic step-channel using dielectrophoresis. Biomicrofluidics 2017, 11, 054114. [Google Scholar] [CrossRef]
- Chikaishi, Y.; Yoneda, K.; Ohnaga, T.; Tanaka, F. EpCAM-independent capture of circulating tumor cells with a ‘universal CTC-chip’. Oncol. Rep. 2017, 37, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Fusi, A.; Klopocki, E.; Schmittel, A.; Tinhofer, I.; Nonnenmacher, A.; Keilholz, U. Negative enrichment by immunomagnetic nanobeads for unbiased characterization of circulating tumor cells from peripheral blood of cancer patients. J. Med. 2011, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Kim, J.; Cho, H.; Han, K.-H. Evaluation of Positive and Negative Methods for Isolation of Circulating Tumor Cells by Lateral Magnetophoresis. Micromachines 2019, 10, 386. [Google Scholar] [CrossRef] [Green Version]
- Poudineh, M.; Aldridge, P.M.; Ahmed, S.; Green, B.J.; Kermanshah, L.; Nguyen, V.; Tu, C.; Mohamadi, R.M.; Nam, R.K.; Hansen, A.; et al. Tracking the dynamics of circulating tumour cell phenotypes using nanoparticle-mediated magnetic ranking. Nat. Nanotechnol. 2017, 12, 274–281. [Google Scholar] [CrossRef]
- Poudineh, M.; Labib, M.; Ahmed, S.; Nguyen, L.N.M.; Kermanshah, L.; Mohamadi, R.M.; Sargent, E.H.; Kelley, S.O. Profiling Functional and Biochemical Phenotypes of Circulating Tumor Cells Using a Two-Dimensional Sorting Device. Angew. Chem. Int. Ed. 2017, 56, 163–168. [Google Scholar] [CrossRef]
- Yin, C.; Wang, Y.; Ji, J.; Cai, B.; Chen, H.; Yang, Z.; Wang, K.; Luo, C.; Zhang, W.; Yuan, C.; et al. Molecular Profiling of Pooled Circulating Tumor Cells from Prostate Cancer Patients Using a Dual-Antibody-Functionalized Microfluidic Device. Anal. Chem. 2018, 90, 3744–3751. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhu, T.; Cheng, R.; Liu, Y.; He, J.; Qiu, H.; Wang, L.; Nagy, T.; Querec, T.D.; Unger, E.R.; et al. Label-Free and Continuous-Flow Ferrohydrodynamic Separation of HeLa Cells and Blood Cells in Biocompatible Ferrofluids. Adv. Funct. Mater. 2016, 26, 3990–3998. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Cheng, R.; Jenkins, B.D.; Zhu, T.; Okonkwo, N.E.; Jones, C.E.; Davis, M.B.; Kavuri, S.K.; Hao, Z.; Schroeder, C.; et al. Label-free ferrohydrodynamic cell separation of circulating tumor cells. Lab Chip 2017, 17, 3097–3111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cheng, R.; Lim, S.H.; Miller, J.R.; Zhang, W.; Tang, W.; Xie, J.; Mao, L. Biocompatible and label-free separation of cancer cells from cell culture lines from white blood cells in ferrofluids. Lab Chip 2017, 17, 2243–2255. [Google Scholar] [CrossRef]
- Lenshof, A.; Evander, M.; Laurell, T.; Nilsson, J. Acoustofluidics 5: Building microfluidic acoustic resonators. Lab Chip 2012, 12, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, M.; Lin, Y.; Xu, J. Acoustic Microfluidic Separation Techniques and Bioapplications: A Review. Micromachines 2020, 11, 921. [Google Scholar] [CrossRef]
- Ding, X.; Li, P.; Lin, S.-C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F.; et al. Surface acoustic wave microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef]
- Wu, M.; Huang, P.-H.; Zhang, R.; Mao, Z.; Chen, C.; Kemeny, G.; Li, P.; Lee, A.V.; Gyanchandani, R.; Armstrong, A.J.; et al. Circulating Tumor Cell Phenotyping via High-Throughput Acoustic Separation. Small 2018, 14, 1801131. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, W.; Lin, Z.; Cai, F.; Li, F.; Wu, J.; Meng, L.; Niu, L.; Zheng, H. Sorting of tumour cells in a microfluidic device by multi-stage surface acoustic waves. Sens. Actuators B Chem. 2018, 258, 1174–1183. [Google Scholar] [CrossRef]
- Karthick, S.; Pradeep, P.N.; Kanchana, P.; Sen, A.K. Acoustic impedance-based size-independent isolation of circulating tumour cells from blood using acoustophoresis. Lab Chip 2018, 18, 3802–3813. [Google Scholar] [CrossRef]
- Bai, X.; Bin, S.; Yuguo, D.; Wei, Z.; Yanmin, F.; Yuanyuan, C.; Deyuan, Z.; Fumihito, A.; Lin, F. Parallel trapping, patterning, separating and rotating of micro-objects with various sizes and shapes using acoustic microstreaming. Sens. Actuators A Phys. 2020, 315, 112340. [Google Scholar] [CrossRef]
- Cushing, K.; Undvall, E.; Ceder, Y.; Lilja, H.; Laurell, T. Reducing WBC background in cancer cell separation products by negative acoustic contrast particle immuno-acoustophoresis. Anal. Chim. Acta 2018, 1000, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Naghdloo, A.; Besanjideh, M. Cancer cell enrichment on a centrifugal microfluidic platform using hydrodynamic and magnetophoretic techniques. Sci. Rep. 2021, 11, 1939. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. A Triplet Parallelizing Spiral Microfluidic Chip for Continuous Separation of Tumor Cells. Sci. Rep. 2018, 8, 4042. [Google Scholar] [CrossRef] [PubMed]
- Antfolk, M.; Kim, S.H.; Koizumi, S.; Fujii, T.; Laurell, T. Label-free single-cell separation and imaging of cancer cells using an integrated microfluidic system. Sci. Rep. 2017, 7, 46507. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhao, W.; Cheng, R.; Puig, A.; Hodgson, J.; Egan, M.; Cooper Pope, C.N.; Nikolinakos, P.G.; Mao, L. Label-free inertial-ferrohydrodynamic cell separation with high throughput and resolution. Lab Chip 2021, 21, 2738–2750. [Google Scholar] [CrossRef]
- Garg, N.; Westerhof, T.M.; Liu, V.; Liu, R.; Nelson, E.L.; Lee, A.P. Whole-blood sorting, enrichment and in situ immunolabeling of cellular subsets using acoustic microstreaming. Microsyst. Nanoeng. 2018, 4, 17085. [Google Scholar] [CrossRef]
- Li, X.-R.; Zhou, Y.-G. Electrochemical detection of circulating tumor cells: A mini review. Electrochem. Commun. 2021, 124, 106949. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Y.; Huang, M.; Wang, W.; Wang, Y.; Cheng, J.; Lei, Z.; Zhu, Z.; Yang, C. Bioinspired Engineering of a Multivalent Aptamer-Functionalized Nanointerface to Enhance the Capture and Release of Circulating Tumor Cells. Angew. Chem. Int. Ed. 2019, 58, 2236–2240. [Google Scholar] [CrossRef]
- Stott, S.L.; Hsu, C.-H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef] [Green Version]
- Sheng, W.; Ogunwobi, O.O.; Chen, T.; Zhang, J.; George, T.J.; Liu, C.; Fan, Z.H. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip 2014, 14, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
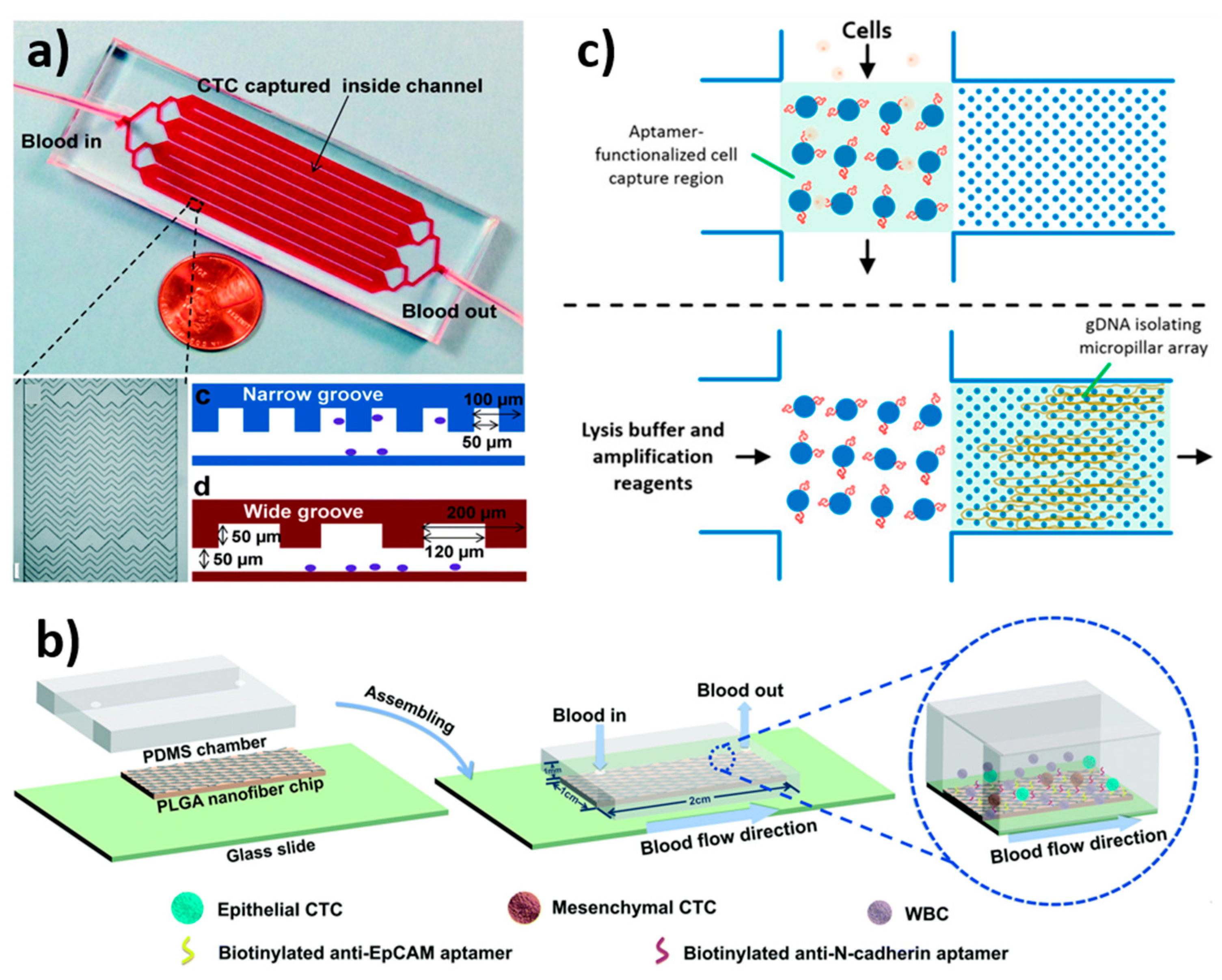
- Wu, Z.; Pan, Y.; Wang, Z.; Ding, P.; Gao, T.; Li, Q.; Hu, M.; Zhu, W.; Pei, R. A PLGA nanofiber microfluidic device for highly efficient isolation and release of different phenotypic circulating tumor cells based on dual aptamers. J. Mater. Chem. B 2021, 9, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Pulikkathodi, A.K.; Sarangadharan, I.; Hsu, C.-P.; Chen, Y.-H.; Hung, L.-Y.; Lee, G.-Y.; Chyi, J.-I.; Lee, G.-B.; Wang, Y.-L. Enumeration of circulating tumor cells and investigation of cellular responses using aptamer-immobilized AlGaN/GaN high electron mobility transistor sensor array. Sens. Actuators B Chem. 2018, 257, 96–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Wu, L.; Zong, S.; Yun, B.; Cui, Y. Combining Multiplex SERS Nanovectors and Multivariate Analysis for In Situ Profiling of Circulating Tumor Cell Phenotype Using a Microfluidic Chip. Small 2018, 14, 1704433. [Google Scholar] [CrossRef]
- Cheng, S.-B.; Xie, M.; Chen, Y.; Xiong, J.; Liu, Y.; Chen, Z.; Guo, S.; Shu, Y.; Wang, M.; Yuan, B.-F.; et al. Three-Dimensional Scaffold Chip with Thermosensitive Coating for Capture and Reversible Release of Individual and Cluster of Circulating Tumor Cells. Anal. Chem. 2017, 89, 7924–7932. [Google Scholar] [CrossRef]
- Jiang, X.; Wong, K.H.K.; Khankhel, A.H.; Zeinali, M.; Reategui, E.; Phillips, M.J.; Luo, X.; Aceto, N.; Fachin, F.; Hoang, A.N.; et al. Microfluidic isolation of platelet-covered circulating tumor cells. Lab Chip 2017, 17, 3498–3503. [Google Scholar] [CrossRef]
- Zeinali, M.; Murlidhar, V.; Fouladdel, S.; Shao, S.; Zhao, L.; Cameron, H.; Bankhead, A., III; Shi, J.; Cuneo, K.C.; Sahai, V.; et al. Profiling Heterogeneous Circulating Tumor Cells (CTC) Populations in Pancreatic Cancer Using a Serial Microfluidic CTC Carpet Chip. Adv. Biosyst. 2018, 2, 1800228. [Google Scholar] [CrossRef]
- Yin, J.; Mou, L.; Yang, M.; Zou, W.; Du, C.; Zhang, W.; Jiang, X. Highly efficient capture of circulating tumor cells with low background signals by using pyramidal microcavity array. Anal. Chim. Acta 2019, 1060, 133–141. [Google Scholar] [CrossRef]
- Kermanshah, L.; Poudineh, M.; Ahmed, S.; Nguyen, L.N.M.; Srikant, S.; Makonnen, R.; Pena Cantu, F.; Corrigan, M.; Kelley, S.O. Dynamic CTC phenotypes in metastatic prostate cancer models visualized using magnetic ranking cytometry. Lab Chip 2018, 18, 2055–2064. [Google Scholar] [CrossRef]
- Sun, N.; Li, X.; Wang, Z.; Li, Y.; Pei, R. High-purity capture of CTCs based on micro-beads enhanced isolation by size of epithelial tumor cells (ISET) method. Biosens. Bioelectron. 2018, 102, 157–163. [Google Scholar] [CrossRef]

| Isolation Method | Device Fabrication | Device Dimension | Flow Rate | Efficiency | Cancer Cell Lines | Ref. |
|---|---|---|---|---|---|---|
| Size-based isolation | ||||||
| Size and deformability | Double-layer photolithography | L = 500 μm T = 23 μm | 2.5 mL/h | ~97% | LM2 MDA-MB-231 | [93] |
| Size | Wet etching technique and thermal bonding technique | L = 22 mm H = 40 μm | 200 μL/min | 85% | BGC823, H1975, PC-3, SKBR3 | [82] |
| Size-based PDMS microflitration membrane | Photolithography | T = 60 μm | 10 mL/h | >90% | A549, SK-MES-1, H446 | [90] |
| Size | Photolithography | Main channel L = 80 µm; Main channel L = 50 µm H = 50 µm | 10 mL/h | 82% | SKBR3, MCF-7, MDAMB231 | [105] |
| Inertial focusing microchannel-based isolation | ||||||
| Label-free, inertial migration of cells | Photolithography | L = 20 mm W = 150 µm H = 50 µm | 300 µL/min | >99% | H460, HCC827 | [62] |
| Rotation-induced inertial lift force | photolithography | W = 100, 200, 400 µm D = 30 µm | 9 µL/min | 90% | U87 | [126] |
| Dean vortex flow, inertial lift force | Photolithography | - | 1.7 mL/min | 54% | FaDu, CAL27, RPMI2650, UD-SCC9 HNC cells, MDA-MB-468 | [120] |
| Inertial and Dean drag forces | Photolithography | W = 500 μm H = 170 μm | 100 μL/min | ≥85% | MDA-MB-231, MCF-7, T24 | [127] |
| Inertial microfluidics and Dean flow physics | Photolithography | L = 9.75 mm W = 350 µm | 400–2700 μL/min | >94% | MDA-MB-231, Jurkat, K562, HeLa | [128] |
| Size-dependent lateral migration | Photolithography | Capillary inner and outer diameter = 50 and 360 μm; H = 200 μm L = 5 and 1 cm | 200 μL/min | 94% | MCF-7 | [129] |
| Self-amplified inertial-focused (SAIF) separation | Photolithography | Zigzag channel W = 40 μm; First expansion region W = 0.84 mm; Second expansion region W = 1.64 mm; H = 50 μm | 0.4 mL/min | ~80% | A549, MCF-7, HeLa | [121] |
| Vortex and inertial cell focusing lift force | Photolithography | L = 1000 μm W = 40 μm H = 70 μm; Trapping zone L, W = 720, 230 μm | 8 mL/min | 83% | MCF-7 | [130] |
| Inertial lift force and Dean drag force | Photolithography | L = 5.5 mm W = 130 μm H = 500 μm | 1 mL/min | 90% | MCTC | [131] |
| Dielectrophoresis-based isolation | ||||||
| Optically induced dielectrophoretic (ODEP) force | Metal mould-punching | Main channel, L = 25 mm, W = 1000 μm, H = 100 μm; Side channel, L = 15 mm, W = 400 μm, H = 100 μm | 2.5 μL/min | 41.5% | PC-3 | [135] |
| Dielectrophoresis at wireless bipolar electrode (BPE) array | Photolithography | L = 2.95 mm W = 200 µm H = 25 µm | 20 μm/s | 96% | MDA-MB-231, Jurkat E6-1 T | [136] |
| Dielectrophoresis (DEP) force | Photolithography and wet etching | L = 7 mm H = 50 µm | 100 µL/min | 92 ± 9% | NCI-H1975 | [137] |
| Optically induced dielectrophoresis (ODEP) | Metal mould-punching | Main channel, L = 2500 µm, W = 1000 μm, H = 60 μm; Side channel, L = 2500 μm, W = 400 μm, H = 60 μm | - | 81.0 ± 0.7% | PC-3, SW620 | [85] |
| Magnetic field-based isolation | ||||||
| Immunomagnetics and size-based filtration | Photolithography | T = 50 μm | 2 mL/min | ~89% | MCF-7 | [96] |
| EpCAM-specific conjugation of MNPs | Photolithography | Microchannel W = 250 μm; Trapping site H = 400 μm, W = 100 μm | 150 µL/min | ~81.2–96.3% | MDA-MB-231, MCF-7 | [89] |
| EpCAM-based positive method and CD45/CD66b-based negative method by lateral magnetophoresis | Photolithography | Free-bead capture microchannel, L = 42.5 mm, W = 1 mm, H = 50 µm; Lateral magnetophoretic microchannel, L = 42.5 mm, W = 2.8 mm, H = 100 µm | 2 mL/h and 3.2 mL/h | 83.1% | MDA-MB-231, PC-3, SKBR3, MCF-7 | [140] |
| Magnet deformability | Photolithography | L = 49,000 µm W = 10,000 µm | 3 mL/h | 90% | HCT116, SW480, MCF-7 | [97] |
| Immunomagnetic technique | Photolithography | L = 9 mm W = 1 mm | - | 97–107% | SKBR3, PC-3, Colo205 | [99] |
| Magnetic-ranking cytometry and phenotypic profiling of CTCs | Photolithography | L = 8.75 cm H = 50 µm | 500 µL/h | >90% | SKBR3, PC-3, MDA-MB-231 | [141] |
| MNP-labeled aptamers | Photolithography | - | 25 mL/h | ~79% | PC-3, SKBR3 | [142] |
| Magnetic-bead-mediated dual-antibody functionalised microfluidics | Photolithography | - | 0.8 mL/h | >85% | LnCAP and LnCAP-EMP | [143] |
| Cell size difference in ferrofluids under permanent magnetic influence | Photolithography | L = 2.54 mm W, H = 635 µm | 8 µL/min | >99% | HeLa | [144] |
| Ferrodynamic cell separation | Photolithography | L = 4.94 cm W = 900 µm | 6 mL/h | ~92.9% | H1299, A549, H3122, PC-3, MCF-7, HCC1806 | [145] |
| Acoustic-based isolation | ||||||
| Cell size difference in ferrofluids | Photolithography | L = 5.81 cm W = 900 µm | 20 µL/min | 82.2% | A549, H1299, MCF-7, MDA-MB-231 | [146] |
| Lateral cavity acoustic transducers | Photolithography | W = 750 µm H = 100 µm | 25 µL/min | 94% | Breast, bone, lung cancer cells | [103] |
| Hydrodynamic and SAW focusing separation | Photolithography | - | 7.5 mL/h | >86% | MCF-7, HeLa, PC-3, LNCaP | [150] |
| Interdigital transducers (IDTs) and focused interdigital transducers (FIDTs) generating standing SAWs and travelling pulsed SAWs | Photolithography | W = 65 µm H = 50 µm | 0.3 µL/min | ~90% | U87 | [151] |
| Acoustic impedance contrast | Photolithography and deep reactive ion etching (DRIE) | L = 20 mm W = 380 µm H = 200 µm | 20–60 µL/min | >86% | HeLa, MDA-MA-231 | [152] |
| Microvortices generated by acoustic vibration | Photolithography | L = 50 mm W = 40 mm H = 200 µm | 10 µL/min | >90% | DU145 | [153] |
| Continuous flow acoustophoretic negative selection | Photolithography | Maun channel, L = 20 mm, W = 375 µm, H = 150 µm; Sub channel, L = 10 mm, W = 300 µm, H = 150 µm | 100, 400 µL/min | >98% | MCF-7, DU145 | [154] |
| Combined method-based isolation | ||||||
| Inertial and magnetic method | Photolithography | W = 400 µm H = 80 µm | 1000 µL/min | ~95% | MCF-7 | [107] |
| Vortex trapping and impedance cytometry | - | L = 1 cm H = 70 µm | 100 µL/min | ~ 98% | MCF-7, LoVo, HT-29 human colon cells, | [95] |
| Inertial hydrodynamic forces and bifurcation law | CNC micromachining | W = 0.26 mm H = 0.2 mm | - | 85% | MCF-7 | [155] |
| Inertial and deformability-based principle | Photolithography | L = 1–1.5 cm W = 400, 300, 200 µm | 80 mL/h | >90% | MCF-7 | [156] |
| Integrated device with acoustofluidic label-free separation and direct dielectrophoretic cell trapping | Photolithography | L = 2.3 cm W = 375 µm H = 150 µm | 80, 160 µL/min | ~76% | DU145 | [157] |
| Inertial-ferrohydrodynamic cell separation | Photolithography | H = 60 µm | ~60 mL/h | 94.8% | H1299, MDA-MB-231, MCF-7, H3122 | [158] |
| Micropore-arrayed filtration and magnetic bead-functionalised antibody-mediated detection | Molding technique | Micropore L, W = 20 mm, diameter = 10 µm | - | ~85% | PC-9 | [115] |
| Lateral cavity acoustic transducers (LCAT) and biomarker-based immuno-labelling | Photolithography | Main, side channel W = 500, 100 µm H = 100 µm | 25 µL/min | ~100% | MCF-7, SKBR3 | [159] |
| Electrochemical isolation | ||||||
| Antibody-mediated electrochemical release and lysis | Photolithography | L = 40 mm W = 20 mm | 1 mL/h | 85–100% | PC-3, MCF-7, NCl-H1650 | [91] |
| Electrochemical detection and electric-filed influenced hydrodynamic flow | Screen printing | W = 95 ± 2.5 µm H = 15 ± 1.5 µm | 5 µL/min | 92 ± 0.5% | HEK-293, HeLa | [116] |
| Biological interaction-based isolation | ||||||
| EpCAM-expressing cells using antibody-coated microposts | Photolithography | L = 20 mm H = 50–100 µm | 1.5–2.5 mL/h | 93% | PC-3 | [162] |
| Aptamer-functionalized micropillars | Photolithography | - | 1 mL/h | 80% | W480 colorectal, LNCap prostate, KATO III gastric cancer cells, K-562 chronic myelogenous leukemia cells | [161] |
| Anti-EpCAM-coated channel surface with herringbone grooves | Photolithography | L = 50 mm W = 2.1 mm H = 50 µm | 1 µL/s | >90% | L3.6pl, BxPC-3, MIAPaCa-2 | [163] |
| EpCAM antibody-functionalised pillars | Laser direct-write technique | Micropost diameter = 420 µm; Pitch = 245 µm | 90 µL/min | ~76% | HEC-1A | [25] |
| Combination of anti-EpCAM antibody and anti-N-cadherin antibody | Photolithography | L = 32 mm W = 34 mm H = 0.7 mm | 0.6 mL/h | 89.6% | SKOV-3 ovarian tumor cells | [104] |
| Dual aptamer (EpCAM-5-1 and NC3S)-modified poly(lactic-co-glycolic acid) (PLGA) nanofiber | Electrospinning | L = 2 cm W = 1 cm H = 1 mm | 300 µL/min | 89–91% | A2780, OVCAR-3 | [164] |
| Aptamer-immobilized microchannel | Photolithography | Cell channel W = 1 mm; DNA channel W = 0.5–1 mm H = ~25 µm | 5 µL/min | - | HeLa, CAOV-3 | [106] |
| AlGaN/GaN HEMT biosensor array | Photolithography | L = 22 mm W = 13 mm | - | - | HCT-8 | [165] |
| Size-based and multiplex SERS nanovectors | - | Filter gap = 12 µm, H = 40 µm | 1 µL/min | ~87–93% | SKBR3, MCF7, and MDA-MB-231 | [166] |
| Microchannel functionalised with anti-EpCAM | 3D printing | L = 2 cm | 1 mL/h | ~87–92% | MCF-7, SW480, PC-3, 293T | [81] |
| Gelatin-coated Ni foam functionalised with anti-WpCAM | Ni foam surface modification | L = 20 mm W = 4 mm H = 1 mm | 50 µL/min | ~88% | MCF-7 | [167] |
| Lateral displacement (DLD) and herringbone CTC chip functionalised with EpCAM and CD41 antibodies | Deep reactive ion etching | H = 150 µm | 1.14 ± 0.24 mL/h | 60–83% | Lung, breast, melanoma cancer cells | [168] |
| EpCAM and CD133 antibodies functionalised hexagonal array of posts | Photolithography | L = 44.6 mm W = 16.9 mm H = 100 µm | 1 mL/h | 13.6–97.5% | HT-29, Panc-1, PC-3, Hs-578T, Capan-1 | [169] |
| Microcavity array functionalised with anti-EpCAM | Photolithography | H = 200 ± 10 µm Microcavity L, W = 30, 8 µm | 0.1 mL/min | ~76–83% | MCF-7, SW620 | [170] |
| Magnetic ranking cytometry and CTC surface marker expression | Photolithography | L = 5.4 cm W = 4.3 cm H = 50 µm, Radii of Ni magnet = 145–235 µm | 400 µL/h | >90% | LNCaP, PC-3, PC-3M | [171] |
| Isolation by size of epithelial tumor cell (ISET) and microbeads assisting ISET | - | L = 4 mm W = 17 mm H = 300 µm | 1 mL/min | ~72–93% | MCF-7, KATO III, PC-3 | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, M.P.; Thendral, V.; Uthappa, U.T.; Lee, K.-H.; Kigga, M.; Altalhi, T.; Kurkuri, M.D.; Kant, K. Recent Advances in Microfluidic Platform for Physical and Immunological Detection and Capture of Circulating Tumor Cells. Biosensors 2022, 12, 220. https://doi.org/10.3390/bios12040220
Bhat MP, Thendral V, Uthappa UT, Lee K-H, Kigga M, Altalhi T, Kurkuri MD, Kant K. Recent Advances in Microfluidic Platform for Physical and Immunological Detection and Capture of Circulating Tumor Cells. Biosensors. 2022; 12(4):220. https://doi.org/10.3390/bios12040220
Chicago/Turabian StyleBhat, Mahesh Padmalaya, Venkatachalam Thendral, Uluvangada Thammaiah Uthappa, Kyeong-Hwan Lee, Madhuprasad Kigga, Tariq Altalhi, Mahaveer D. Kurkuri, and Krishna Kant. 2022. "Recent Advances in Microfluidic Platform for Physical and Immunological Detection and Capture of Circulating Tumor Cells" Biosensors 12, no. 4: 220. https://doi.org/10.3390/bios12040220
APA StyleBhat, M. P., Thendral, V., Uthappa, U. T., Lee, K.-H., Kigga, M., Altalhi, T., Kurkuri, M. D., & Kant, K. (2022). Recent Advances in Microfluidic Platform for Physical and Immunological Detection and Capture of Circulating Tumor Cells. Biosensors, 12(4), 220. https://doi.org/10.3390/bios12040220








