Remote Healthcare for Elderly People Using Wearables: A Review
Abstract
1. Introduction
- Cardiovascular diseases (30.3 percent);
- Cancer (15.1 percent);
- Chronic lung diseases (9.5 percent);
- Musculoskeletal diseases (7.5 percent);
- Mental disorders and diseases of the nervous system (6.6 percent).
2. Physiological Variables of Prevalent Diseases in Older Adults
2.1. Heart Rate (HR)
2.2. Heart Rate Variability (HRV)
2.3. Pulse Rate Variability (PRV)
2.4. Respiratory Rate (RR)/Breathing Rate (BR)
2.5. Oxygen Saturation of the Blood (SpO2)
2.6. Blood Pressure (BP)
2.7. Blood Glucose (BC)
2.8. Other Physiological Variables
3. Methods
- The main global deadly, chronic, or degenerative diseases for older people
- The physiological variables used in diagnosed diseases
- The sensors and biosensors that measure those physiological variables
- The consumer wearable devices available in the market that use those sensors
- The wearable devices that were available or not in the market
- The FDA-approved commercial wearable devices available
- The remote healthcare monitoring devices.
‘main global disease’ AND (‘deadly disease’ OR ‘chronic disease’ OR ‘degenerative disease’) AND (‘older people’ OR ‘elderly people’ OR ‘aged population’)
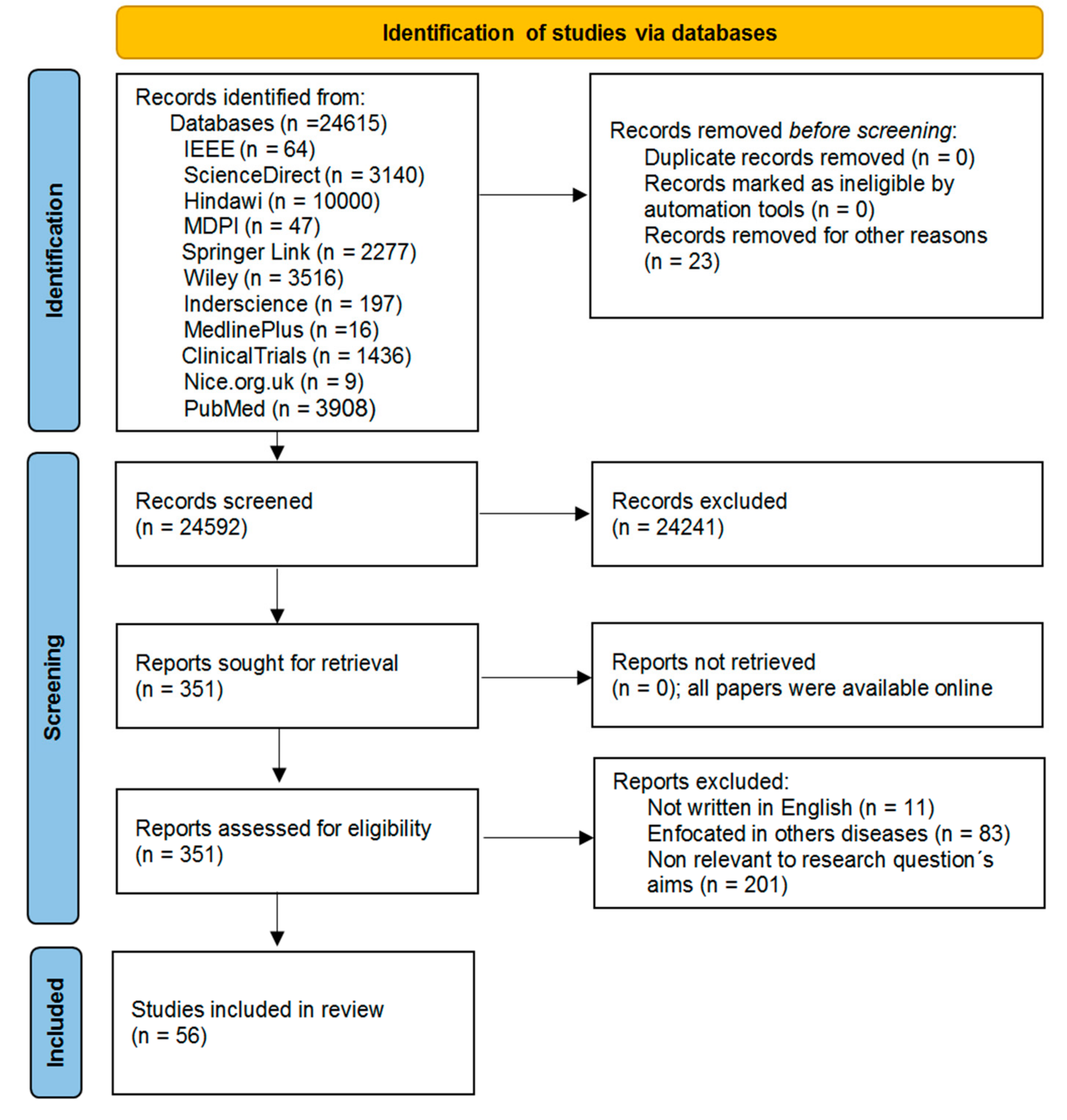
4. Results
4.1. Study Selection
4.2. Study Characteristics
4.2.1. Commercial Wearable Devices
4.2.2. Non-Commercial Wearable Devices
- Target refers to the physiological parameter that the described device can measure.
- Device Type describes the device’s category (i.e., watch and bracelet) and the year of publication.
- Functioning is a brief description of how the device works.
- Sensors Used shows the sensors found as part of the device.
- Real-Time Monitoring indicates if the device can monitor the physiological parameter in real-time.
- Elderly User Ready indicates if the device in its proposed version has the optimal characteristics and ease of use for elderly users.
5. Discussion
5.1. Challenges and Trends
5.2. Emerging Solutions
5.3. Limitations
6. Conclusions
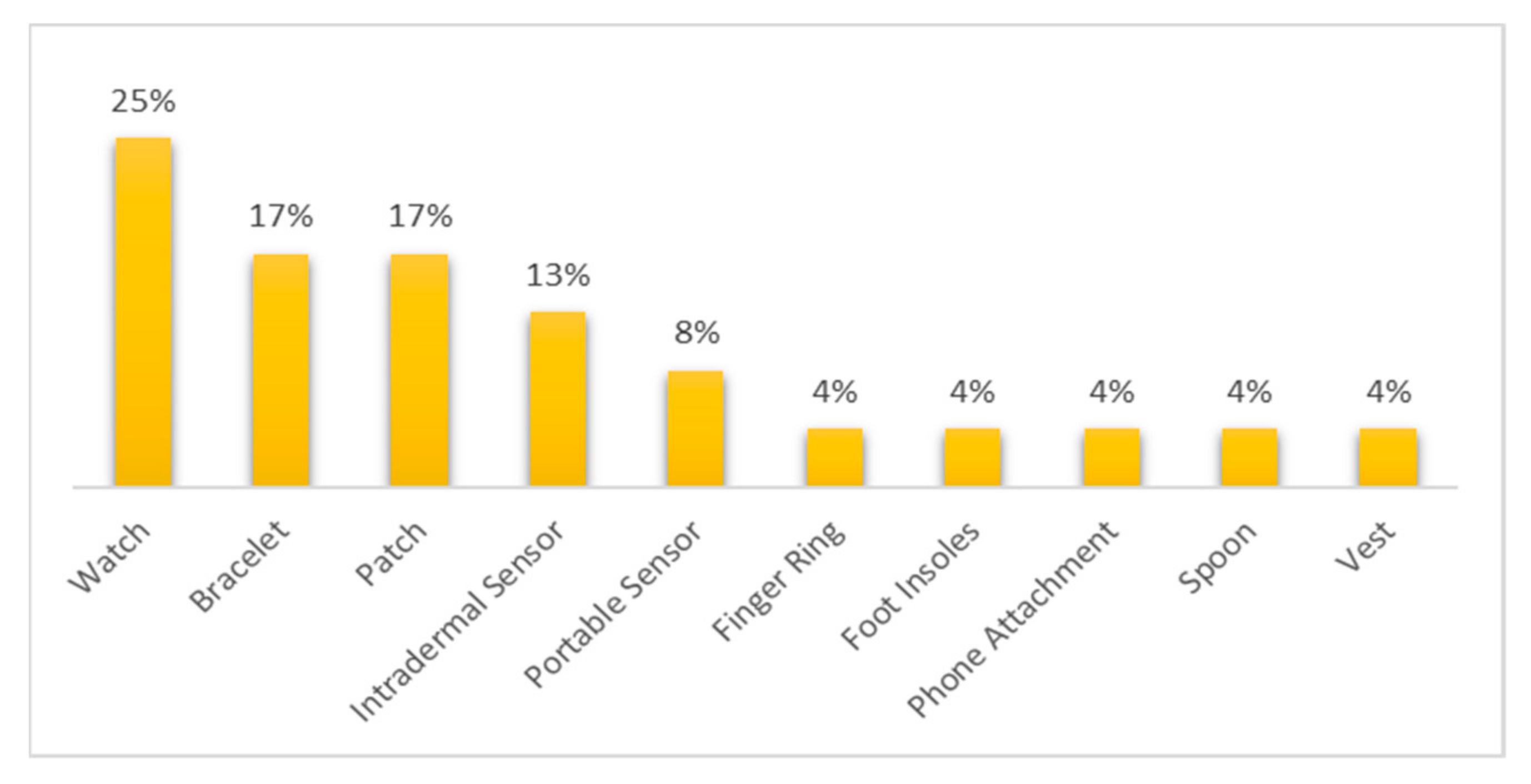
- Among the commercial devices reviewed, 25% belonged to the smartwatch category.
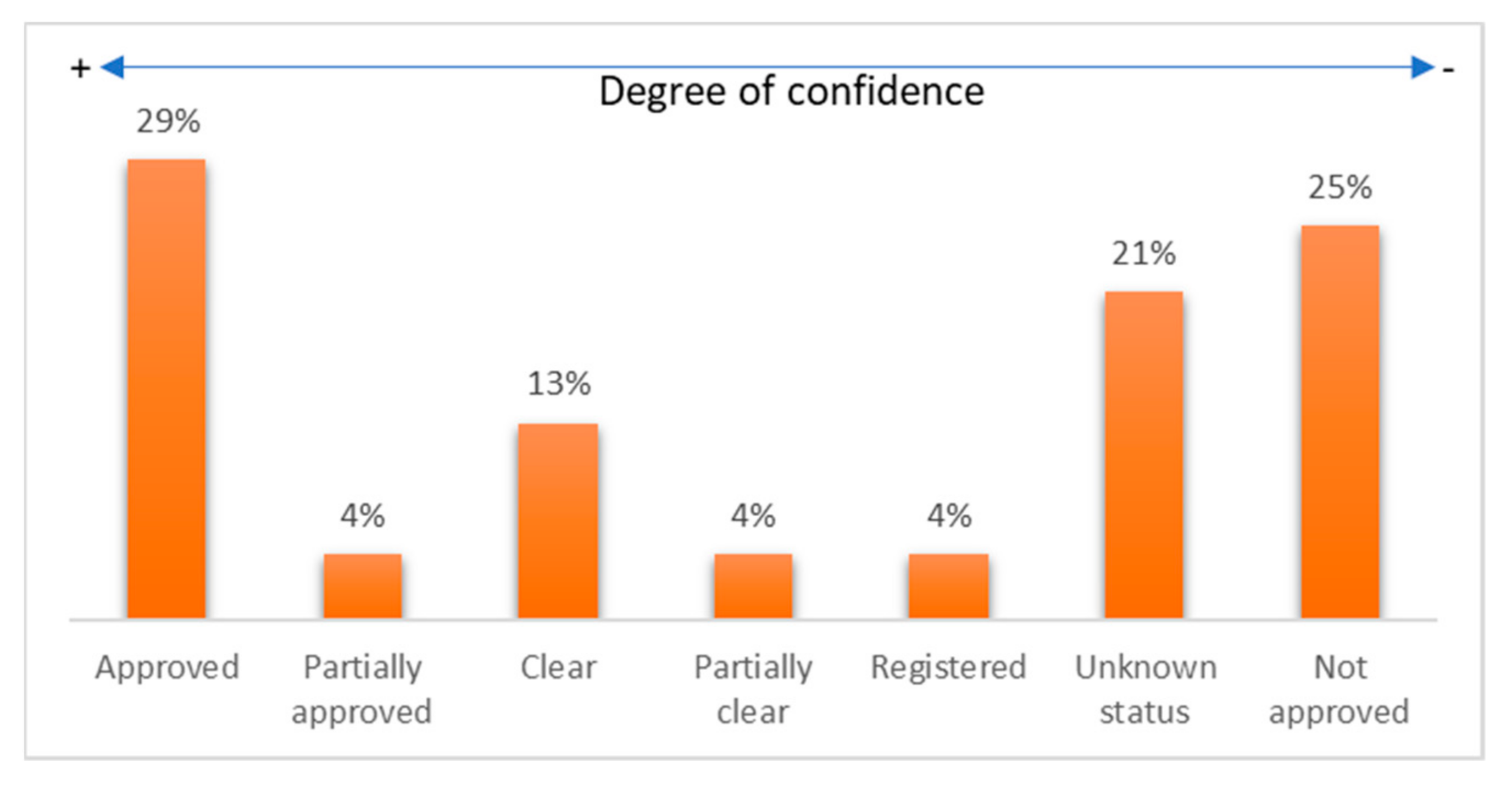
- Among the commercial devices, 54% had some FDA evaluation (approved, partially approved, cleared, partially cleared, or registered).
- The diagnosed diseases that an FDA-approved wearable device can monitor were cardiovascular diseases, diabetes, general body tracking, sleep disorders, and alcoholism.
- Most of the commercial devices reviewed were devoted to cardiovascular diseases and general body tracking.
- Among the non-commercial wearable devices, those in the band, bracelet/watch, ear wear, and patch category were the most used.
- The physiological parameters that non-commercial wearable devices could monitor were glucose, heart rate, oxygen saturation of blood, blood pressure, pulse rate variability, heart rate variability, and respiratory rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs. World Population Ageing 2020: Highlights—Living Arrangements of Older Persons; United Nations: New York, NY, USA, 2021. [Google Scholar]
- Prince, M.J.; Wu, F.; Guo, Y.; Gutierrez Robledo, L.M.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef]
- Rizzuto, D.; Melis, R.J.F.; Angleman, S.; Qiu, C.; Marengoni, A. Effect of Chronic Diseases and Multimorbidity on Survival and Functioning in Elderly Adults. J. Am. Geriatr. Soc. 2017, 6, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Geriatría. Available online: http://www.geriatria.salud.gob.mx (accessed on 13 June 2021).
- Rizzuto, D.; Bellocco, R.; Kivipelto, M.; Clerici, F.; Wimo, A.; Fratiglioni, L. Dementia After Age 75: Survival in Different Severity Stages and Years of Life Lost. Curr. Alzheimer Res. 2012, 9, 795–800. [Google Scholar] [CrossRef]
- Jagger, C.; Matthews, R.; Matthews, F.; Robinson, T.; Robine, J.M.; Brayne, C. The burden of diseases on disability-free life expectancy in later life. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, Z.; Dong, T. A Review of Wearable Technologies for Elderly Care that Can Accurately Track Indoor Position, Recognize Physical Activities and Monitor Vital Signs in Real Time. Sensors 2017, 17, 341. [Google Scholar] [CrossRef]
- Leirós-Rodríguez, R.; García-Soidán, J.L.; Romo-Pérez, V. Analyzing the use of accelerometers as a method of early diagnosis of alterations in balance in elderly people: A systematic review. Sensors 2019, 19, 3883. [Google Scholar] [CrossRef]
- Rucco, R.; Sorriso, A.; Liparoti, M.; Ferraioli, G.; Sorrentino, P.; Ambrosanio, M.; Baselice, F. Type and location of wearable sensors for monitoring falls during static and dynamic tasks in healthy elderly: A review. Sensors 2018, 18, 1613. [Google Scholar] [CrossRef]
- Stavropoulos, T.G.; Papastergiou, A.; Mpaltadoros, L.; Nikolopoulos, S.; Kompatsiaris, I. Iot wearable sensors and devices in elderly care: A literature review. Sensors 2020, 20, 2826. [Google Scholar] [CrossRef]
- Tun, S.Y.Y.; Madanian, S.; Mirza, F. Internet of things (IoT) applications for elderly care: A reflective review. Aging Clin. Exp. Res. 2020, 33, 855–867. [Google Scholar] [CrossRef]
- Allet, L.; Knols, R.H.; Shirato, K.; de Bruin, E.D. Wearable systems for monitoring mobility-related activities in chronic disease: A systematic review. Sensors 2010, 10, 9026–9052. [Google Scholar] [CrossRef]
- Yang, C.C.; Hsu, Y.L. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors 2010, 10, 7772–7788. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.M.; GholamHosseini, H.; Moqeem, A.A.; Mirza, F.; Lindén, M. A Systematic Review of Wearable Patient Monitoring Systems—Current Challenges and Opportunities for Clinical Adoption. J. Med. Syst. 2017, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, X.; Peng, S.; Jiang, X.; Xu, K.; Chen, C.; Wang, Z.; Dai, C.; Chen, W. A review of wearable and unobtrusive sensing technologies for chronic disease management. Comput. Biol. Med. 2021, 129, 104163. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Aghayi, E.; Noferesti, M.; Memarzadeh-Tehran, H.; Mondal, T.; Pang, Z.; Deen, M.J. Smart homes for elderly healthcare—Recent advances and research challenges. Sensors 2017, 17, 2496. [Google Scholar] [CrossRef] [PubMed]
- Gal, R.; May, A.M.; van Overmeeren, E.J.; Simons, M.; Monninkhof, E.M. The Effect of Physical Activity Interventions Comprising Wearables and Smartphone Applications on Physical Activity: A Systematic Review and Meta-analysis. Sport. Med. Open 2018, 4, 1–15. [Google Scholar] [CrossRef]
- Yen, H.Y.; Chiu, H.L. The effectiveness of wearable technologies as physical activity interventions in weight control: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2019, 20, 1485–1493. [Google Scholar] [CrossRef]
- Kirk, M.A.; Amiri, M.; Pirbaglou, M.; Ritvo, P. Wearable Technology and Physical Activity Behavior Change in Adults with Chronic Cardiometabolic Disease: A Systematic Review and Meta-Analysis. Am. J. Health Promot. 2019, 33, 778–791. [Google Scholar] [CrossRef]
- Jalloul, N. Wearable sensors for the monitoring of movement disorders. Biomed. J. 2018, 41, 249–253. [Google Scholar] [CrossRef]
- Tucker, C.S.; Behoora, I.; Nembhard, H.B.; Lewis, M.; Sterling, N.W.; Huang, X. Machine learning classification of medication adherence in patients with movement disorders using non-wearable sensors. Comput. Biol. Med. 2015, 66, 120–134. [Google Scholar] [CrossRef]
- Warmerdam, E.; Hausdorff, J.; Atrsaei, A.; Zhou, Y.; Mirelman, A.; Aminian, K.; Espay, A.J.; Hansen, C.; Evers, L.J.W.; Keller, A.; et al. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol. 2020, 19, 462–470. [Google Scholar] [CrossRef]
- Srinivasan, R.; Ben-Pazi, H.; Dekker, M.; Cubo, E.; Bloem, B. Telemedicine for Hyperkinetic Movement Disorders. Tremor and Other Hyperkinetic Movements. Tremor. Other. Hyperkinetic. Mov. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Espay, A.J.; Bonato, P.; Nahab, F.B.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Technology in Parkinson’s disease: Challenges and opportunities. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef]
- Maetzler, W.; Domingos, J.; Srulijes, K.; Ferreira, J.J.; Bloem, B.R. Quantitative Wearable Sensors for Objective Assessment of Parkinson’s Disease. Mov. Disord. 2013, 28, 1628–1637. [Google Scholar] [CrossRef]
- De Lima, A.L.S.; Evers, L.J.; Hahn, T.; Bataille, L.; Hamilton, J.L.; Little, M.A.; Okuma, Y.; Bloem, B.R.; Faber, M.J. Freezing of gait and fall detection in Parkinson’s disease using wearable sensors: A systematic review. J. Neurol. 2017, 264, 1642–1654. [Google Scholar] [CrossRef]
- Sweeney, D.; Quinlan, L.R.; Browne, P.; Richardson, M.; Meskell, P.; ÓLaighin, G. A Technological Review of Wearable Cueing Devices Addressing Freezing of Gait in Parkinson’s Disease. Sensors 2019, 19, 1277. [Google Scholar] [CrossRef]
- Mazzetta, I.; Zampogna, A.; Suppa, A.; Gumiero, A.; Pessione, M.; Irrera, F. Wearable Sensors System for an Improved Analysis of Freezing of Gait in Parkinson’s Disease Using Electromyography and Inertial Signals. Sensors 2019, 19, 948. [Google Scholar] [CrossRef]
- Pardoel, S.; Kofman, J.; Nantel, J.; Lemaire, E.D. Wearable-Sensor-Based Detection and Prediction of Freezing of Gait in Parkinson’s Disease: A Review. Sensors 2019, 19, 5141. [Google Scholar] [CrossRef]
- Demrozi, F.; Bacchin, R.; Tamburin, S.; Cristani, M.; Pravadelli, G. Toward a Wearable System for Predicting Freezing of Gait in People Affected by Parkinson’s Disease. IEEE J. Biomed. Health Inform. 2020, 24, 2444–2451. [Google Scholar] [CrossRef]
- Deb, R.; Bhat, G.; An, S.; Shill, H.; Ogras, U.Y. Trends in Technology Usage for Parkinson’s Disease Assessment: A Systematic Review. medRxiv 2021. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 1–17. [Google Scholar] [CrossRef]
- Martín-Vaquero, J.; Encinas, A.H.; Queiruga-Dios, A.; Bullón, J.J.; Martínez-Nova, A.; González, J.T.; Bullón-Carbajo, C. Review on wearables to monitor foot temperature in diabetic patients. Sensors 2019, 19, 776. [Google Scholar] [CrossRef]
- Wu, M.; Luo, J. Wearable Technology Applications in Healthcare: A Literature Review. Online J. Nurs. Inform. Contrib. 2019, 23, 3. [Google Scholar]
- Anastasova, S.; Crewther, B.; Bembnowicz, P.; Curto, V.; Ip, H.M.; Rosa, B.; Yang, G.-Z. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 2017, 93, 139–145. [Google Scholar] [CrossRef]
- Dang, W.; Manjakkal, L.; Navaraj, W.T.; Lorenzelli, L.; Vinciguerra, V.; Dahiya, R. Stretchable wireless system for sweat pH monitoring. Biosens Bioelectron 2018, 107, 192–202. [Google Scholar] [CrossRef]
- Godfrey, A. Wearables for independent living in older adults: Gait and falls. Maturitas 2017, 100, 16–26. [Google Scholar] [CrossRef]
- Tedesco, S.; Barton, J.; O’Flynn, B. A review of activity trackers for senior citizens: Research perspectives, commercial landscape and the role of the insurance industry. Sensors 2017, 17, 1277. [Google Scholar] [CrossRef]
- Kekade, S.; Hseieh, C.H.; Islam, M.M.; Atique, S.; Mohammed Khalfan, A.; Li, Y.C.; Abdul, S.S. The usefulness and actual use of wearable devices among the elderly population. Comput. Methods Programs Biomed. 2018, 153, 137–159. [Google Scholar] [CrossRef]
- Alharbi, M.; Straiton, N.; Smith, S.; Neubeck, L.; Gallagher, R. Data management and wearables in older adults: A systematic review. Maturitas 2019, 124, 100–110. [Google Scholar] [CrossRef]
- Teixeira, E.; Fonseca, H.; Diniz-Sousa, F.; Veras, L.; Boppre, G.; Oliveira, J.; Pinto, D.; Alves, A.J.; Barbosa, A.; Mendes, R.; et al. Wearable devices for physical activity and healthcare monitoring in elderly people: A critical review. Geriatrics 2021, 6, 38. [Google Scholar] [CrossRef]
- Uddin, M.Z.; Khaksar, W.; Torresen, J. Ambient sensors for elderly care and independent living: A survey. Sensors 2018, 18, 2027. [Google Scholar] [CrossRef]
- Isravel, D.P.; Arulkumar, D.; Raimond, K.; Issac, B. A novel framework for quality care in assisting chronically impaired patients with ubiquitous computing and ambient intelligence technologies. In Systems Simulation and Modeling for Cloud Computing and Big Data Applications; Peter, J.D., Fernandes, D.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–79. [Google Scholar]
- Rodbard, D. Continuous Glucose Monitoring: A Review of Successes, Challenges, and Opportunities. Diabetes Technol. Ther. 2016, 18, S23–S213. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Ahn, D.; Drincic, A. Continuous glucose monitoring: A review of the technology and clinical use. Diabetes Res. Clin. Pract. 2017, 133, 178–192. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Taj-Eldin, M.; Ryan, C.; O’flynn, B.; Galvin, P. A review of wearable solutions for physiological and emotional monitoring for use by people with autism spectrum disorder and their caregivers. Sensors 2018, 18, 4271. [Google Scholar] [CrossRef]
- Dias, D.; Cunha, J.P.S. Wearable health devices—Vital sign monitoring, systems and technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef]
- Hunkin, H.; King, D.L.; Zajac, I.T. Wearable devices as adjuncts in the treatment of anxiety-related symptoms: A narrative review of five device modalities and implications for clinical practice. Clin. Psychol. Sci. Pract. 2019, 26, e12290. [Google Scholar] [CrossRef]
- Dinh, T.; Nguyen, T.; Phan, H.P.; Nguyen, N.T.; Dao, D.V.; Bell, J. Stretchable respiration sensors: Advanced designs and multifunctional platforms for wearable physiological monitoring. Biosens. Bioelectron. 2020, 166, 112460. [Google Scholar] [CrossRef]
- Vanegas, E.; Igual, R.; Plaza, I. Sensing systems for respiration monitoring: A technical systematic review. Sensors 2020, 20, 1–84. [Google Scholar] [CrossRef]
- Hickey, B.A.; Chalmers, T.; Newton, P.; Lin, C.T.; Sibbritt, D.; McLachlan, C.S.; Clifton-Bligh, R.; Morley, J.; Lal, S. Smart devices and wearable technologies to detect and monitor mental health conditions and stress: A systematic review. Sensors 2021, 21, 3461. [Google Scholar] [CrossRef]
- Temko, A. Accurate Heart Rate Monitoring during Physical Exercises Using PPG. IEEE Trans. Biomed. Eng. 2017, 64, 2016–2024. [Google Scholar] [CrossRef]
- Achten, J.; Jeukendrup, A.E. Heart rate monitoring: Applications and limitations. Sport. Med. 2003, 33, 517–538. [Google Scholar] [CrossRef]
- Schäfer, A.; Vagedes, J. How accurate is pulse rate variability as an estimate of heart rate variability?: A review on studies comparing photoplethysmographic technology with an electrocardiogram. Int. J. Cardiol. 2013, 166, 15–29. [Google Scholar] [CrossRef]
- Birrenkott, D.A.; Pimentel, M.A.F.; Watkinson, P.J.; Clifton, D.A. A Robust Fusion Model for Estimating Respiratory Rate from Photoplethysmography and Electrocardiography. IEEE Trans. Biomed. Eng. 2018, 65, 2033–2041. [Google Scholar] [CrossRef]
- Tereshchenko, L.G.; Josephson, M.E. Frequency content and characteristics of ventricular conduction. J. Electrocardiol. 2015, 48, 933–937. [Google Scholar] [CrossRef]
- Collins, J.A.; Rudenski, A.; Gibson, J.; Howard, L.; O’Driscoll, R. Relating oxygen partial pressure, saturation and content: The haemoglobin–oxygen dissociation curve. Breathe 2015, 11, 194–201. [Google Scholar] [CrossRef]
- Chan, E.D.; Chan, M.M.; Chan, M.M. Pulse oximetry: Understanding its basic principles facilitates appreciation of its limitations. Respir. Med. 2013, 107, 789–799. [Google Scholar] [CrossRef]
- Sinex, J.E. Pulse oximetry: Principles and limitations. Am. J. Emerg. Med. 1999, 17, 59–66. [Google Scholar] [CrossRef]
- Harvey, J.; Salehizadeh, S.M.; Mendelson, Y.; Chon, K.H. Oxima: A frequency-domain approach to address motion artifacts in photoplethysmograms for improved estimation of arterial oxygen saturation and pulse rate. IEEE Trans. Biomed. Eng. 2018, 66, 311–318. [Google Scholar] [CrossRef]
- American Heart Association. What Is Blood Pressure? Available online: https://dc.statelibrary.sc.gov/bitstream/handle/10827/25131/DHEC_What_is_High_Blood_Pressure_2017-07.pdf?sequence=1 (accessed on 23 June 2021).
- Güemes, M.; Rahman, S.A.; Hussain, K. What is a normal blood glucose? Arch. Dis. Child. 2016, 101, 569–574. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Sistema Flash de Monitorización de Glucosa. Available online: https://www.freestylelibre.es/libre/ (accessed on 28 July 2021).
- KardiaMobile. Available online: https://www.alivecor.es/kardiamobile (accessed on 28 July 2021).
- Apple Watch Series 6—Apple (MX). Available online: https://www.apple.com/mx/apple-watch-series-6/ (accessed on 28 July 2021).
- BACtrack SkynTM—The World’s 1st Wearable Alcohol Monitor. Available online: https://skyn.bactrack.com/ (accessed on 28 July 2021).
- G5 mobile Spain. Available online: https://www.dexcom.com/es-ES/g5-mobile-spain (accessed on 28 July 2021).
- Halford, J.J.; Sperling, M.R.; Nair, D.R.; Dlugos, D.J.; Tatum, W.O.; Harvey, J.; French, J.A.; Pollard, J.R.; Faught, E.; Noe, K.H.; et al. Detection of generalized tonic–clonic seizures using surface electromyographic monitoring. Epilepsia 2017, 58, 1861–1869. [Google Scholar] [CrossRef]
- E4 Wristband|Real-Time Physiological Signals|Wearable PPG, EDA, Temperature, Motion Sensors. Available online: https://www.empatica.com/research/e4/ (accessed on 28 July 2021).
- Smartwatch Fitbit Versa 2. Available online: https://www.fitbit.com/global/es/products/smartwatches/versa (accessed on 28 July 2021).
- Fitbit Charge 4 | Pulsera Avanzada de Salud y Actividad Física. Available online: https://www.fitbit.com/global/es/products/trackers/charge4 (accessed on 28 July 2021).
- Health Care Originals—Breathe Easy, Always. Available online: https://healthcareoriginals.com/ (accessed on 28 July 2021).
- Uninterrupted Ambulatory Cardiac Monitoring. Available online: https://www.irhythmtech.com/ (accessed on 28 July 2021).
- Sensor Enlite® | Medtronic. Available online: https://www.medtronicdiabeteslatino.com/productos/monitoreo-de-glucosa/sensor-enliter (accessed on 28 July 2021).
- Diabetes—GuardianTM Sensor 3|Medtronic. Available online: https://www.medtronic.com/us-en/healthcare-professionals/products/diabetes/continuous-glucose-monitoring-systems/guardian-sensor-3.html (accessed on 28 July 2021).
- About Orpyx SI Sensory Insoles—Orpyx Medical Technologies. Available online: https://www.orpyx.com/about-orpyx-si (accessed on 28 July 2021).
- Ōura Ring: Accurate Health Information Accessible to Everyone. Available online: https://ouraring.com/ (accessed on 28 July 2021).
- LISTENS TO THE BEAT—Preventice Solutions. Available online: https://www.preventicesolutions.com/patients/body-guardian-heart (accessed on 28 July 2021).
- Biomarkers & Digital Therapeutics for Mental Health. Available online: https://www.myfeel.co/ (accessed on 28 July 2021).
- ZOLL LifeVest Wearable Defibrillator|ZOLL Medical Corporation. Available online: https://lifevest.zoll.com/ (accessed on 28 July 2021).
- Mi Mexico. Available online: https://www.mi.com/mx/mi-smart-band-5/ (accessed on 28 July 2021).
- ECG Monitor & Activity Watch—Move ECG|Withings. Available online: https://www.withings.com/ca/en/move-ecg (accessed on 28 July 2021).
- HUAWEI Band 6—HUAWEI México. Available online: https://consumer.huawei.com/mx/wearables/band6/ (accessed on 28 July 2021).
- Akintola, A.A.; van de Pol, V.; Bimmel, D.; Maan, A.C.; van Heemst, D. Comparative Analysis of the Equivital EQ02 Lifemonitor with Holter Ambulatory ECG Device for Continuous Measurement of ECG, Heart Rate, and Heart Rate Variability: A Validation Study for Precision and Accuracy. Front. Physiol. 2016, 7, 391. [Google Scholar] [CrossRef]
- GYENNO SPOON. Available online: https://www.gyenno.com/spoon-en.html (accessed on 28 July 2021).
- Muvone, el Wearable Que Cuida de Tus Huesos. Available online: https://secmotic.com/muvone/ (accessed on 28 July 2021).
- Chen, Y.; Lu, S.; Zhang, S.; Li, Y.; Qu, Z.; Chen, Y.; Lu, B.; Wang, X.; Feng, X. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 2017, 3, e1701629. [Google Scholar] [CrossRef]
- Rachim, V.P.; Chung, W.Y. Wearable-band type visible-near infrared optical biosensor for non-invasive blood glucose monitoring. Sens. Actuators B Chem. 2019, 286, 173–180. [Google Scholar] [CrossRef]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Wearable Contact Lens Biosensors for Continuous Glucose Monitoring Using Smartphones. ACS Nano 2018, 12, 5452–5462. [Google Scholar] [CrossRef]
- Hanna, J.; Bteich, M.; Tawk, Y.; Ramadan, A.H.; Dia, B.; Asadallah, F.A.; Eid, A.; Kanj, R.; Costantine, J.; Eid, A.A. Noninvasive, wearable, and tunable electromagnetic multisensing system for continuous glucose monitoring, mimicking vasculature anatomy. Sci. Adv. 2020, 6, eaba5320. [Google Scholar] [CrossRef]
- Wang, G.; Poscente, M.D.; Park, S.S.; Andrews, C.N.; Yadid-Pecht, O.; Mintchev, M.P. Wearable Microsystem for Minimally Invasive, Pseudo-Continuous Blood Glucose Monitoring: The e-Mosquito. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 979–987. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef] [PubMed]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Lee, H.; Kim, J.; Lee, M.; Choi, H.J.; Hyeon, T.; Kim, D.-H. Multifunctional Wearable System that Integrates Sweat-Based Sensing and Vital-Sign Monitoring to Estimate Pre-/Post-Exercise Glucose Levels. Adv. Funct. Mater. 2018, 28, 1805754. [Google Scholar] [CrossRef]
- Xiao, N.; Yu, W.; Han, X. Wearable heart rate monitoring intelligent sports bracelet based on Internet of things. Measurement 2020, 164, 108102. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, D.; Bardill, A.; De Gelidi, S.; Bayford, R.; Demosthenous, A. A high frame rate wearable EIT system using active electrode ASICs for lung respiration and heart rate monitoring. IEEE Trans. Circuits Syst. I Regul. Pap. 2018, 65, 3810–3820. [Google Scholar] [CrossRef]
- Hussein, A.F.; Hashim, S.J.; Aziz, A.F.A.; Rokhani, F.Z.; Adnan, W.A.W. A real time ECG data compression scheme for enhanced bluetooth low energy ECG system power consumption. J. Ambient Intell. Humaniz. Comput. 2017, 1–14. [Google Scholar] [CrossRef]
- Sani, M.I.; Mutiara, G.A.; Putra, R.S.D.W. Fit-NES: Wearable bracelet for heart rate monitoring. Telkomnika 2019, 17, 392–399. [Google Scholar] [CrossRef]
- Raluca Maria, A.; Pasca, S.; Strungaru, R. Heart rate monitoring by using non-invasive wearable sensor. In Proceedings of the 2017 E-Health and Bioengineering Conference, Sinaia, Romania, 22–24 June 2017. [Google Scholar]
- Irawan, H.C.; Juhana, T. Heart rate monitoring using IoT wearable for ambulatory patient. In Proceedings of the 11th International Conference on Telecommunication Systems Services and Applications, Lombok, Indonesia, 26–27 October 2017. [Google Scholar]
- Lázaro, J.; Reljin, N.; Noh, Y.; Laguna, P.; Chon, K.H. Heart Rate Variability Monitoring Using a Wearable Armband. In Proceedings of the Computing in Cardiology, Singapore, 8–11 September 2019. [Google Scholar]
- Ahn, J.W.; Ku, Y.; Kim, H.C. A novel wearable EEG and ECG recording system for stress assessment. Sensors 2019, 19, 1991. [Google Scholar] [CrossRef]
- Raj, A.; Karthik, A.K.; Sachin, S.; Sanchana, M.; Ganesan, M. A Wearable Device to Detect Blood Volume Change. In Proceedings of the 5th International Conference on Advanced Computing and Communication Systems, Coimbatore, India, 15–16 March 2019. [Google Scholar]
- Kos, M.; Kramberger, I. A Wearable Device and System for Movement and Biometric Data Acquisition for Sports Applications. IEEE Access 2017, 5, 6411–6420. [Google Scholar] [CrossRef]
- Schneider, J.; Schroth, M.; Ottenbacher, J.; Stork, W. A novel wearable sensor device for continuous monitoring of cardiac activity during sleep. In Proceedings of the 2018 IEEE Sensors Applications Symposium, Seoul, Korea, 12–14 March 2018. [Google Scholar]
- Yeh, K.Y.; Lin, T.H.; Hsieh, Y.Y.; Chang, C.M.; Yang, Y.J.; Lu, S.S. A cuffless wearable system for real-time cutaneous pressure monitoring with cloud computing assistance. In Proceedings of the 2018 International Symposium on VLSI Design, Automation and Test, Hsinchu, Taiwan, 16–19 April 2018. [Google Scholar]
- Molinaro, N.; Massaroni, C.; Lo Presti, D.; Saccomandi, P.; Di Tomaso, G.; Zollo, L.; Perego, P.; Andreoni, G.; Schena, E. Wearable textile based on silver plated knitted sensor for respiratory rate monitoring. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Honolulu, HI, USA, 18–21 July 2018. [Google Scholar]
- Al-Halhouli, A.; Al-Ghussain, L.; El Bouri, S.; Liu, H.; Zheng, D. Fabrication and evaluation of a novel non-invasive stretchable and wearable respiratory rate sensor based on silver nanoparticles using inkjet printing technology. Polymers 2019, 11, 1518. [Google Scholar] [CrossRef]
- Lázaro, J.; Bailón, R.; Gil, E.; Noh, Y.; Laguna, P.; Chon, K.H. Pilot Study on Electrocardiogram Derived Respiratory Rate Using a Wearable Armband. In Proceedings of the Computers in Cardiology (CinC), Maastricht, The Netherlands; 2018; Volume 45, pp. 1–4. [Google Scholar]
- Adiputra, R.R.; Hadiyoso, S.; Hariyani, Y.S. Internet of Things: Low Cost and Wearable SpO2 Device for Health Monitoring. Int. J. Electr. Comput. Eng. 2018, 8, 939–945. [Google Scholar] [CrossRef]
- Davies, H.J.; Williams, I.; Peters, N.S.; Mandic, D.P. In-Ear SpO2: A Tool for Wearable, Unobtrusive Monitoring of Core Blood Oxygen Saturation. Sensors 2020, 20, 4879. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, E.; Lee, Y.; Kim, H.; Lee, J.; Kim, M.; Yoo, H.-J.; Yoo, S. Toward all-day wearable health monitoring: An ultralow-power, reflective organic pulse oximetry sensing patch. Sci. Adv. 2018, 4, eaas9530. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kozlowski, M.; Garcia-Lopez, I.; Jiang, Z.; Rodriguez-Villegas, E. Proof-of-concept of a novel neck-situated wearable PPG system for continuous physiological monitoring. IEEE Trans. Instrum. Meas. 2021, 70, 9509609. [Google Scholar] [CrossRef]
- Chacon, P.J.; Pu, L.; Da Costa, T.H.; Shin, Y.H.; Ghomian, T.; Shamkhalichenar, H.; Wu, H.C.; Irving, B.A.; Choi, J.W. A Wearable Pulse Oximeter with Wireless Communication and Motion Artifact Tailoring for Continuous Use. IEEE Trans. Biomed. Eng. 2019, 66, 1505–1513. [Google Scholar] [CrossRef]
- Carek, A.M.; Conant, J.; Joshi, A.; Kang, H.; Inan, O.T. SeismoWatch: Wearable cuffless blood pressure monitoring using pulse transit time. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2017, 1, 1–16. [Google Scholar] [CrossRef]
- Bui, N.; Pham, N.; Barnitz, J.J.; Zou, Z.; Nguyen, P.; Truong, H.; Kim, T.; Farrow, N.; Nguyen, A.; Xiao, J.; et al. eBP: A wearable system for frequent and comfortable blood pressure monitoring from user’s ear. In Proceedings of the 25th Annual International Conference on Mobile Computing and Networking, Los Cabos, Mexico, 21–25 October 2019. [Google Scholar]
- Zhang, Q.; Zeng, X.; Hu, W.; Zhou, D. A Machine Learning-Empowered System for Long-Term Motion-Tolerant Wearable Monitoring of Blood Pressure and Heart Rate with Ear-ECG/PPG. IEEE Access 2017, 5, 10547–10561. [Google Scholar] [CrossRef]
- Holz, C.; Wang, E.J. Glabella. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies; ACM PUB27: New York, NY, USA, 2017; Volume 1, pp. 1–23. [Google Scholar]
- Xing, N.; Rincon-Mora, G. A Self-Synchronized Maximum-Power-Point Inductively Coupled Wireless Battery Charger for Embedded Microsensors. IEEE J. Emerg. Sel. Top. Ind. Electron. 2021, 2, 297–304. [Google Scholar] [CrossRef]
- Amutha, J.; Sharma, S.; Sharma, S.K. Strategies based on various aspects of clustering in wireless sensor networks using classical, optimization and machine learning techniques: Review, taxonomy, research findings, challenges and future directions. Comput. Sci. Rev. 2021, 40, 100376. [Google Scholar] [CrossRef]
- Heifler, O.; Borberg, E.; Harpak, N.; Zverzhinetsky, M.; Krivitsky, V.; Gabriel, I.; Fourman, V.; Sherman, D.; Patolsky, F. Clinic-on-a-Needle Array toward Future Minimally Invasive Wearable Artificial Pancreas Applications. ACS Nano 2021, 15, 12019–12033. [Google Scholar] [CrossRef]
- O’Connell, P.J.; Guilbault, G.G. Future Trends in Biosensor Research. Anal. Lett. 2001, 34, 1063–1078. [Google Scholar] [CrossRef]
- Martínez-Pérez, B.; de la Torre-Díez, I.; López-Coronado, M.; Herreros-González, J. Mobile Apps in Cardiology: Review. JMIR Mhealth Uhealth 2013, 1, e15. [Google Scholar] [CrossRef] [PubMed]
- Neubeck, L.; Lowres, N.; Benjamin, E.J.; Freedman, S.B.; Coorey, G.; Redfern, J. The mobile revolution—Using smartphone apps to prevent cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 350–360. [Google Scholar] [CrossRef]
- Xie, B.; Su, Z.; Zhang, W.; Cai, R. Chinese Cardiovascular Disease Mobile Apps’ Information Types, Information Quality, and Interactive Functions for Self-Management: Systematic Review. JMIR Mhealth Uhealth 2017, 5, e195. [Google Scholar] [CrossRef] [PubMed]
- Al-Arkee, S.; Mason, J.; Lane, D.A.; Fabritz, L.; Chua, W.; Haque, M.S.; Jalal, Z. Mobile Apps to Improve Medication Adherence in Cardiovascular Disease: Systematic Review and Meta-analysis. J. Med. Internet Res. 2021, 23, e24190. [Google Scholar] [CrossRef] [PubMed]
- Cucciniello, M.; Petracca, F.; Ciani, O.; Tarricone, R. Development features and study characteristics of mobile health apps in the management of chronic conditions: A systematic review of randomised trials. NPJ Digit. Med. 2021, 4, 144. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, E.M.; Marvel, F.A.; Piasecki, R.J.; Martin, S.S.; Allen, J.K. User Engagement with Smartphone Apps and Cardiovascular Disease Risk Factor Outcomes: Systematic Review. JMIR Cardio 2021, 5, e18834. [Google Scholar] [CrossRef]
- Holmen, H.; Wahl, A.K.; Småstuen, M.C.; Ribu, L. Tailored communication within mobile apps for diabetes self-management: A systematic review. J. Med. Internet Res. 2017, 19, e227. [Google Scholar] [CrossRef]
- Wu, X.; Guo, X.; Zhang, Z. The efficacy of mobile phone apps for lifestyle modification in diabetes: Systematic review and meta-analysis. JMIR Mhealth Uhealth 2019, 7, e12297. [Google Scholar] [CrossRef]
- Bonoto, B.C.; Piassi Godói, I.; Lovato Pires de Lemos, L.; Godman, B.; Bennie, M.; Diniz, L.M.; Afonso, A.; Junior, G. Efficacy of Mobile Apps to Support the Care of Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. JMIR Mhealth Uhealth 2017, 5, e4. [Google Scholar] [CrossRef]
- Rossi, M.G.; Bigi, S. mHealth for diabetes support: A systematic review of apps available on the italian market. Mhealth 2017, 3, 16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adu, M.D.; Malabu, U.H.; Callander, E.J.; Malau-Aduli, A.E.; Malau-Aduli, B.S. Considerations for the development of mobile phone apps to support diabetes self-management: Systematic review. JMIR Mhealth Uhealth 2018, 6, e10115. [Google Scholar] [CrossRef] [PubMed]
- Larbi, D.; Randine, P.; Årsand, E.; Antypas, K.; Bradway, M.; Gabarron, E. Methods and Evaluation Criteria for Apps and Digital Interventions for Diabetes Self-Management: Systematic Review. J. Med. Internet Res. 2020, 22, e18480. [Google Scholar] [CrossRef]
- Brzan, P.P.; Rotman, E.; Pajnkihar, M.; Klanjsek, P. Mobile applications for control and self management of diabetes: A systematic review. J. Med. Syst. 2016, 40, 210. [Google Scholar] [CrossRef] [PubMed]
- Martos, M.B.; Velando, A.; Pradas, L.; Suleiman, N.; Cañadas, G.A.; Albendín, L.; Gómez, J.L. Smartphones and Apps to Control Glycosylated Hemoglobin (HbA1c) Level in Diabetes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 693. [Google Scholar] [CrossRef] [PubMed]



| Brand | Model | Target | Device Type | Functioning | Sensors Used | FDA Status |
|---|---|---|---|---|---|---|
| Abbot | Libre 2 [68] | Diabetes | Patch | Reading of blood glucose levels. | Intradermal Glucose Sensor | Approved (2020) |
| AliveCor® | KardiaMobile [69] | Cardiology | Phone attachment | Reading the heart rate by positioning the fingers on the sensors | Electrodes | Clear (2014) |
| Apple | Watch 6 [70] | General Purposes | Smart Watch | Reading the heart rate by positioning the fingers on the sensors. | Oximeter, Electrical Heart Rate Sensor, Optical Heart Rate Sensor, Accelerometer, Gyroscope | ECG Approved (2018)/Oximeter not Approved |
| BACtrack® | Skyn™ [71] | Alcoholism | Bracelet | Measurement of alcohol levels. | - | Not Approved |
| Dexcom | G5 Mobile [72] | Diabetes | Intradermal sensor | A sensor under the skin measures glucose levels. A transmitter attaches to the top of the sensor and sends the data wirelessly to a smart device. | Intradermal Glucose Sensor | Approved (2015) |
| Empatica | Embrace 2 [73] | Seizures | Smart Watch | Use machine learning (ML) to detect unusual patterns that are possibly associated with seizures. | EDA Sensor, Peripheral Temperature Sensor, 3-Axis accelerometer, Gyroscope | Approved (2018) |
| Empatica | E4 [74] | General Purposes | Bracelet | It enables researchers to record physiological signals at home or in the laboratory. After recording, they can access the data for deep analysis. | PPG Sensor, 3-axis Accelerometer, EDA Sensor (GSR Sensor), Infrared Thermopile | Not Approved |
| Fitbit | Versa 2™ [75] | General Purposes | Smart Watch | It monitors the heart rate, physical activity, sleep quality, oxygen saturation, and body temperature. | 3-axis accelerometer, optical heart rate monitor, altimeter, ambient light sensor, relative SpO2 sensor, built-in microphone | ECG app cleared (2020) |
| Fitbit | Charge 4 [76] | Cardiology | Smart Watch | It monitors the heart rate, physical activity, sleep quality, oxygen saturation, and body temperature. | 3-axis accelerometer, optical heart rate monitor, altimeter | Not Approved |
| Health Care Originals | ADAMM [77] | Asthma | Patch | It is worn discreetly under clothing. Follow-up of cough, breathing patterns, wheezing, heart rate, skin temperature, and activity level. | Acoustic, HR, temperature | - |
| iRhythm | Zio® [78] | Cardiology | Patch | The physiological data collected for a predefined time interval is sent by mail to the provider, who generates reports for the patient and the doctor. | ECG | Clear (2021) |
| Medtronic | Sensor Enlite™ [79] | Diabetes | Intradermal sensor | The sensor is inserted under the skin and captures glucose readings every 5 min, which it communicates wirelessly to the MiniMed pump or its Guardian system so that glucose levels can be observed in real-time. After 6 days, it is removed, discarded, and replaced with a new sensor. | Intradermal glucose sensing electrode | Approved (2016) |
| Medtronic | Guardian™ Sensor 3 [80] | Diabetes | Intradermal sensor | Once inserted, it remains under the skin, capturing glucose readings every 5 min, sending them wirelessly to the MiniMed pump or its Guardian system so that glucose levels can be seen in real-time. After 6 days, it is removed, discarded, and replaced with a new sensor. | Intradermal glucose sensing electrode | Approved (2018) |
| Orpyx® | Orpyx SI [81] | Diabetic foot | Foot Insoles | Custom insoles incorporate sensors to monitor pressure, step count, hours of wear, and temperature. Provides real-time audiovisual alerts and flushing instructions when sustained high-pressure levels occur. | Pressure sensors | Registered |
| Oura | Oura Ring [82] | General Purposes | Finger ring | It uses a monitoring technology that collects the heart rate, heart rate variability, temperature, activity, and sleep quality from a non-invasive ring. | Body temperature sensor, optical, infrared sensors, and a 3D accelerometer and gyroscope | Not Approved |
| Preventice | BodyGuardian® Heart [83] | Cardiology | Patch | Small wireless monitor that adheres to the chest via a disposable strip. The strip can be repositioned as needed due to its medical-grade adhesive and electrode gel and should be replaced periodically during the monitoring period. The monitor is returned to the service provider. | Accelerometer, ECG | Clear (2012) |
| Sentio Solutions | Feel [84] | Emotional/mental health | Bracelet | A bracelet that monitors physiological signals throughout the day and learns to recognize emotional patterns. | EDA, PPG HR, skin sensor | - |
| Zoll® | LifeVest® [85] | Cardiology | Vest | It is a portable cardioverter-defibrillator used by patients at risk of sudden cardiac death (SCD). It controls dangerously fast heart rhythms by applying an electric shock to the heart. LifeVest WCD is used directly against the patient’s skin. | Temperature sensor | Approved (2018) |
| Xiaomi | Mi Band 5 [86] | General Purposes | Bracelet | It monitors heart rate, physical activity, sleep quality, oxygen saturation, body temperature, menstrual cycle. | ECG | Not Approved |
| Withings | Move ECG [87] | Cardiology | Analog watch | In 30 s, a medical-grade ECG is ready by simply pressing the side button and placing a finger on the bezel. It can record an ECG with or without a phone nearby, as the data can be stored on the watch until the next sync. | Heart rate sensor, 3-axis accelerometer, 3-axis gyroscope | Not Approved |
| Huawei | Band 6 [88] | General Purposes | Smart Watch | Measurement of oxygen levels in the blood through the use of LED clusters and photodiodes. Heart rate measurement. Sleep quality monitoring. | Accelerometer, three electrodes, ECG, barometric altimeter | Not Approved |
| Holter | Stat-On™ [89] | Parkinson’s | Portable sensor | It is a non-invasive device worn on a belt that records the user’s motor status at all times of the day. | - | - |
| Gyenno | Gyenno Spoon [90] | Parkinson’s | Spoon | By detecting involuntary hand movements, sensors activate internal motors that keep the spoon stable, helping the person eat normally. | Accelerometer | - |
| Secmotic | Muvone [91] | Osteoporosis | Portable sensor | A device that checks if the activity carried out is appropriate to help strengthen bones or how much sun is needed to assimilate adequate amounts of Vitamin D. | - | - |
| Disease for Which It Can Be Used | FDA Devices | Non-FDA Devices | Total |
|---|---|---|---|
| Cardiovascular Diseases | 6 | 7 | 13 |
| General Body Tracking | 2 | 5 | 7 |
| Diabetes | 5 | 0 | 5 |
| Sleep Disorders | 1 | 4 | 5 |
| Parkinson’s | 0 | 2 | 2 |
| Alcoholism | 1 | 0 | 1 |
| Seizures | 1 | 0 | 1 |
| Osteoporosis | 0 | 1 | 1 |
| Respiratory Diseases | 0 | 1 | 1 |
| Target | Device Type (Year of Publication) | Functioning | Sensors Used | Real-Time Monitoring | Elderly User Ready |
|---|---|---|---|---|---|
| Glucose Monitoring | Non-invasive intravascular glucose measuring sensor (2017) | It consists of ultra-thin skin-like biosensors on a flexible biocompatible paper battery. The battery generates subcutaneous electrochemical channels (ETC) by binding to the skin; the sensors act through the penetration of hyaluronic acid into the anode channel, the refiltration of intravascular blood glucose from the vessels, and the reverse iontophoresis of glucose to the skin surface [92]. | Ultrathin skin-like biosensors | No | Yes |
| Glucose Monitoring | Wearable-band type visible-near infrared optical biosensor (2019) | It is a highly portable blood glucose sensor with a data acquisition time window that enables long-term, non-invasive continuous blood glucose monitoring (CGM). The biosensor exploits information from the pulsatile components that continuously measure the arterial blood volume in the wrist tissue during the change in blood glucose concentration [93]. | Multi-chip sensor package of SFH7060 (OSRAM Semiconductor Inc., Regensburg, Germany) | Yes | Yes |
| Glucose Monitoring | Contact Lens (2018) | The human eye is read using a photon microstructure with a periodicity of 1.6 µm on a selective glucose hydrogel film functionalized with phenylboronic acid [94]. | A photonic structure glucose sensor | Yes | No |
| Glucose Monitoring | Patch (2020) | It is a non-invasive, continuous, portable system, inspired by the anatomy of the vasculature, based on electro-magnetism (EM) for glycemic measurements. The structure of the sensor mimics the vasculature anatomy. The multiple detection system, depending on the patient’s characteristics, provides personalized monitoring [95]. | EM sensors | Yes | No |
| Glucose Monitoring | Wearable-band type (2017) | It is an autonomous and minimally invasive portable microsystem for pseudo-continuous monitoring of blood glucose. With a shape memory alloy (SMA) microactuator, the microsystem pierces a slight wound in the skin and draws a whole blood sample from the skin [96]. | Shape memory alloy (SMA)-based microactuator | Pseudo | Yes |
| Glucose Monitoring | Patch (2017) | It is a disposable patch-type device that measures glucose levels in sweat and automatically applies metformin, thanks to a transdermal drug delivery device [97]. | Extendable sensors (humidity, glucose, pH, and temperature) are integrated in a monolithic way. | Yes | Yes |
| Glucose Monitoring | Band (2017) | The system induces sweat with different excretion rates at periodic intervals employing wirelessly programmable iontophoresis. The induced sweat can be immediately analyzed for glucose monitoring by integrating sensor iontophoresis electrodes on the same substrate [98]. | Iontophoresis and sweat sensing electrodes for detection of Na + and Cl− | Yes | yes |
| Glucose Monitoring | Patch and Smart Band (2018) | It is a multifunctional wearable health management system that analyzes sweat glucose levels using a disposable sweat-based glucose detector strip and a wearable smart band [99]. It also continuously monitors vital signs (i.e., heart rate, blood oxygen saturation level, and activity). | Sensors for light-based photoplethysmography, accelerometer-based activity monitoring, and sweat-based electrochemical analysis | Yes | No |
| HR Monitoring | Bracelet (2020) | It is an IoT-based wearable HR monitoring smart sports bracelet. IoT technology enables real-time monitoring, storage, and analysis of data transmitted to a PC or mobile phone. After data processing and analysis, abnormal data will receive an alarm in time to track the health status [100]. | heart rate sensor son7015 and step acceleration sensor mma9555lr1 | Yes | Yes |
| HR Monitoring | Belt (2018) | It is a multifunctional portable electrical impedance tomography (EIT) system based on a high-performance application-specific integrated circuit (ASIC) active electrode that can record heart rate signals and measure humidity and ambient temperature [101]. | ECG, accelerometers | Yes | No |
| HRV | Leg belt (2017) | It is a portable ECG sensor system that captures vital patient skin data from amplified signals detected by patched electrodes. These modules are capable of collecting 6 ECG lead signals [102]. | ECG, accelerometers | Yes | yes |
| HR Monitoring | Bracelet (2019) | It integrates an HR measurement device using an optics-based pulse sensor and a Bluetooth-based communication module. In addition, an Android-based smartphone application receives and processes the sensor data [103]. | Optical based pulse sensor | Yes | Yes |
| HR Monitoring | Finger case (2017) | A portable heart rate monitoring system that uses photoplethysmography (PPG). Based on the detection of the cardiovascular pulse, this method presents the analysis of light variations in biological tissues [104]. | Pulse sensors | Yes | Yes |
| HR Monitoring | Smartwatch (2018) | It is a prototype that allows monitoring of the heart rate and the intervals between beats for some subjects. This prototype was made using the Samsung Gear S3 Smartwatch, with WebSocket library, nodejs, and JavaScript [105]. | Samsung Gear S3 sensors | Yes | No |
| HRV | Armband (2019) | The device consists of a cuff designed to fit on the upper left arm that provides 3 ECG channels based on three pairs of dry electrodes (without hydrogel) [106]. | ECG | Yes | Yes |
| HRV | Ear wear (2019) | It is a lightweight, portable device that continuously monitors stress in daily life by measuring electrocardiograms (ECG) and EEG. The system can be easily worn by hanging it from both ears [107]. | ECG | No | No |
| PRV | (2019) | It is a portable device that collects PRV values in real-time. The device includes an amplifier and filter for signal accuracy. An accelerometer is used to eliminate noise due to motion. This device can transmit the acquired PPG signal wirelessly with the use of Wi-Fi technology [108]. | Pulse sensors | Yes | |
| PRV | Wristband (2017) | A small portable device worn on the wrist detects and records gestures, arm movements, and biometric information such as skin temperature and pulse rate during sports activities using an inertial measurement unit [109]. | 6DOF motion sensor, temperature sensor, pulse rate sensor | Yes | No |
| PRV | Wristband (2018) | A portable sensing device capable of continuously monitoring cardiac movements and parameters on the wrist by using impedance plethysmography (IPG) technology. The sensor’s design consistently allows getting high-resolution measurements for up to 48 h [110]. | - | Yes | Yes |
| PRV | Wristband (2018) | A handheld cuffless integrated system utilizes a piezoresistive tunneling sensor, achieving ultra-high sensitivity to detect slight wrist artery pressure. After the read, a circuit amplifies and converts the pulse pressure-induced signal to be wirelessly transmitted to the cloud for its storage [111]. | Tunneling piezoresistive sensor | Yes | No |
| Respiratory Rate | Fabric (2018) | It is a smart textile based on a piezoresistive sensor element for respiratory monitoring [112]. | Silver-plated nylon knitted fabric | Yes | No |
| Respiratory Rate | Stretchable sensor (2019) | It is an easy-to-use, low-cost, stretchable, and portable RR sensor that measures respiratory volumetric changes. The sensor is manufactured using polydimethylsiloxane substrates (PDMS) and a soft lithography technique for the stretchable sensor body. An inkjet printing technology creates the conductive circuit by depositing silver nanoparticles on top of PDMS substrates that detect inductance fluctuations [113]. | RR sensor | Yes | No |
| Respiratory Rate | Armband (2018) | Respiratory rate is estimated from a cuff ECG using a method based on variations in the slopes of the QRS and the angle of the R wave. The estimates are compared with those obtained from the respiration signal. The cuff includes a pair of dry electrodes that record the ECG and is designed for long-term monitoring. [114]. | ECG | Yes | No |
| Oxygen saturation of blood | Finger case (2018) | The device connects to a cloud gateway to support IoT applications using an MCU node as a data processor. The data sent to the cloud can be later accessed online for detailed analysis [115]. | Photodetector | Yes | No |
| Oxygen saturation of blood | In-ear device (2020) | It is a device entered into the ear canal for real-time oxygen saturation measurement in the blood using a photoplethysmography sensor. It consists of green (537 nm), red (660 nm), and infrared (880 nm) emitting diodes, as well as a photodiode to measure reflected light [116]. | Photoplethysmography sensor | Yes | No |
| Oxygen saturation of blood | Patch (2018) | It is a patch-type device that uses green light emitters to calculate oxygen saturation levels in the blood [117]. | Photoplethysmography sensor | Yes | No |
| Oxygen saturation of blood | Neck device (2021) | An integrated PPG sensor (MAX30102 by MAXIM integrated) housed in a PCB emits red light (650–670 nm) and IR (870–900 nm). Then, the PPG sensor coupled to a photodiode quantifies light absorption. A three-axis linear accelerometer (LIS2DH12 by ST Electronics) assesses activity and eliminates motion artifacts as necessary [118]. | PPG sensor, accelerometers | Yes | No |
| Oxygen saturation of blood | Finger case (2019) | It is a portable optical biosensor system that continuously measures pulse oximetry and heart rate using a reflectance-based probe [119]. | Photodetector | Yes | No |
| Blood pressure | Wrist-watch (2017) | It is a wristwatch blood pressure monitor to measure blood pressure by holding the watch against the sternum wall to detect micro-vibrations of the chest related to the heartbeat. As the pulse wave travels from the heart to the wrist, an optical sensor and an accelerometer in the watch allow estimating the travel time (pulse transit time (PTT) to estimate BP [120]. | Optical based pulse sensor | Yes | Yes |
| Blood pressure | In-ear device (2019) | A device called eBP measures BP from inside the ear, minimizing interference with the user’s everyday activities while maximizing their comfort level. Three key components provide this functionality: (1) a light-based pulse sensor connected to an inflatable tube placed into the ear, (2) a digital air pump with a controller, and (3) a BP calculation algorithm [121]. | Optical based pulse sensor | Yes | No |
| Blood pressure and HR | Ear wear (2017) | ECG and PPG-based HR and BP monitor attachable to the ear for greater usability. It is suggested to place the ECG and PPG sensors at the back of the ears with the possibility of integrating them into glasses or headphones [122]. | ECG and PPG | Yes | No |
| Blood pressure and HR | Glasses (2017) | It is a portable device that monitors the HR at three points on the user’s head. The lens prototype incorporates optical sensors, processing, storage, and communication components. The device continuously records the flow of reflected light intensities from the bloodstream and the inertial measurements of the wearer’s head [123]. | Optical based pulse sensor | Yes | Yes |
| Real-Time Monitoring | No. of Devices | % |
|---|---|---|
| Yes | 29 | 91% |
| No | 3 | 9% |
| Parameter Target | No. of Devices | % |
|---|---|---|
| Glucose | 8 | 24% |
| Heart Rate | 7 | 21% |
| Oxygen Saturation of Blood | 5 | 15% |
| Blood Pressure | 4 | 12% |
| Pulse Rate Variability | 4 | 12% |
| Heart Rate Variability | 3 | 9% |
| Respiratory Rate | 3 | 9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedo-Aguirre, J.O.; Reyes-Campos, J.; Alor-Hernández, G.; Machorro-Cano, I.; Rodríguez-Mazahua, L.; Sánchez-Cervantes, J.L. Remote Healthcare for Elderly People Using Wearables: A Review. Biosensors 2022, 12, 73. https://doi.org/10.3390/bios12020073
Olmedo-Aguirre JO, Reyes-Campos J, Alor-Hernández G, Machorro-Cano I, Rodríguez-Mazahua L, Sánchez-Cervantes JL. Remote Healthcare for Elderly People Using Wearables: A Review. Biosensors. 2022; 12(2):73. https://doi.org/10.3390/bios12020073
Chicago/Turabian StyleOlmedo-Aguirre, José Oscar, Josimar Reyes-Campos, Giner Alor-Hernández, Isaac Machorro-Cano, Lisbeth Rodríguez-Mazahua, and José Luis Sánchez-Cervantes. 2022. "Remote Healthcare for Elderly People Using Wearables: A Review" Biosensors 12, no. 2: 73. https://doi.org/10.3390/bios12020073
APA StyleOlmedo-Aguirre, J. O., Reyes-Campos, J., Alor-Hernández, G., Machorro-Cano, I., Rodríguez-Mazahua, L., & Sánchez-Cervantes, J. L. (2022). Remote Healthcare for Elderly People Using Wearables: A Review. Biosensors, 12(2), 73. https://doi.org/10.3390/bios12020073







