Electrical Impedance-Based Characterization of Hepatic Tissue with Early-Stage Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Hepatic Fibrotic Model, Biopsies Preparation, and Histology
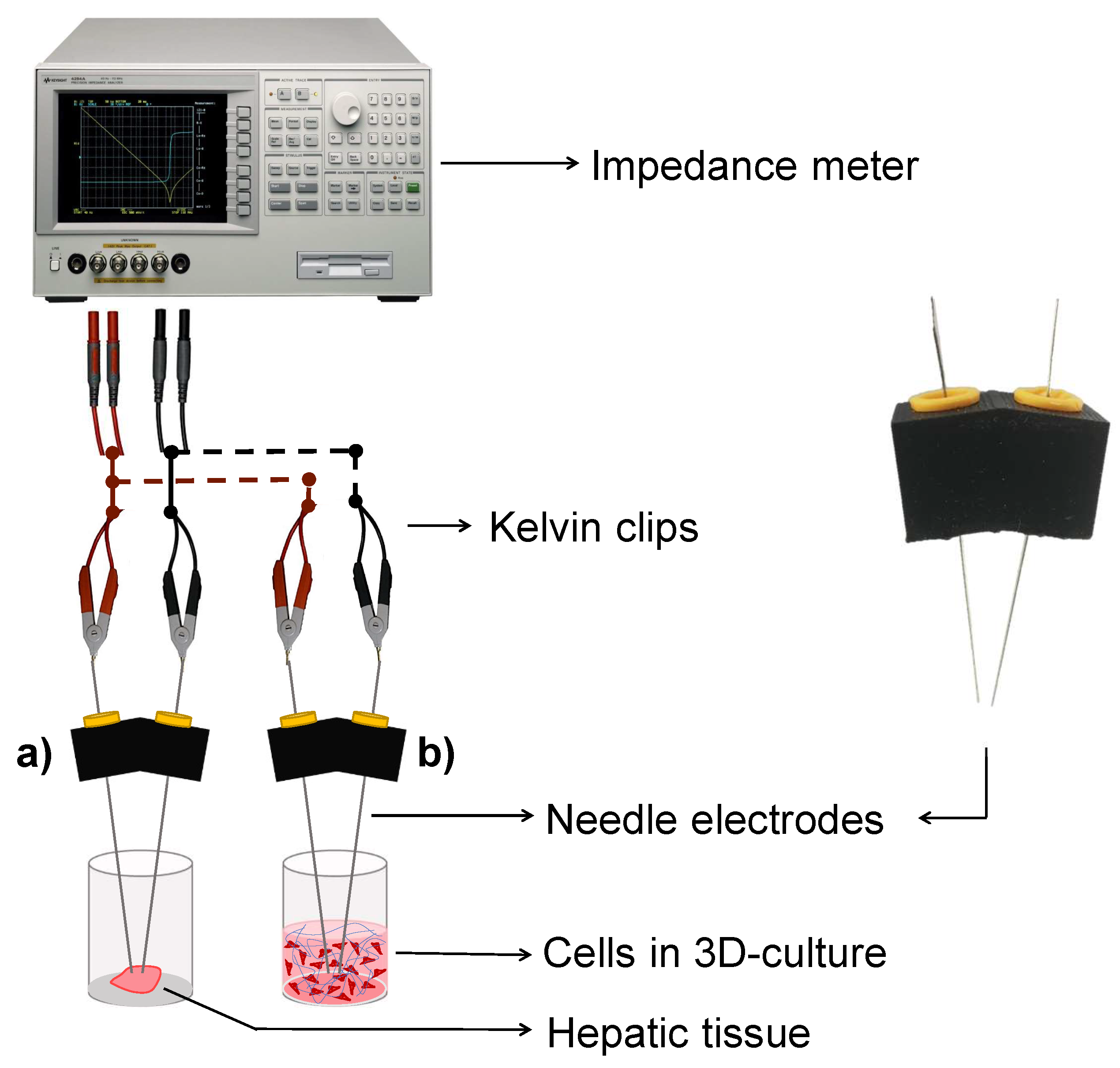
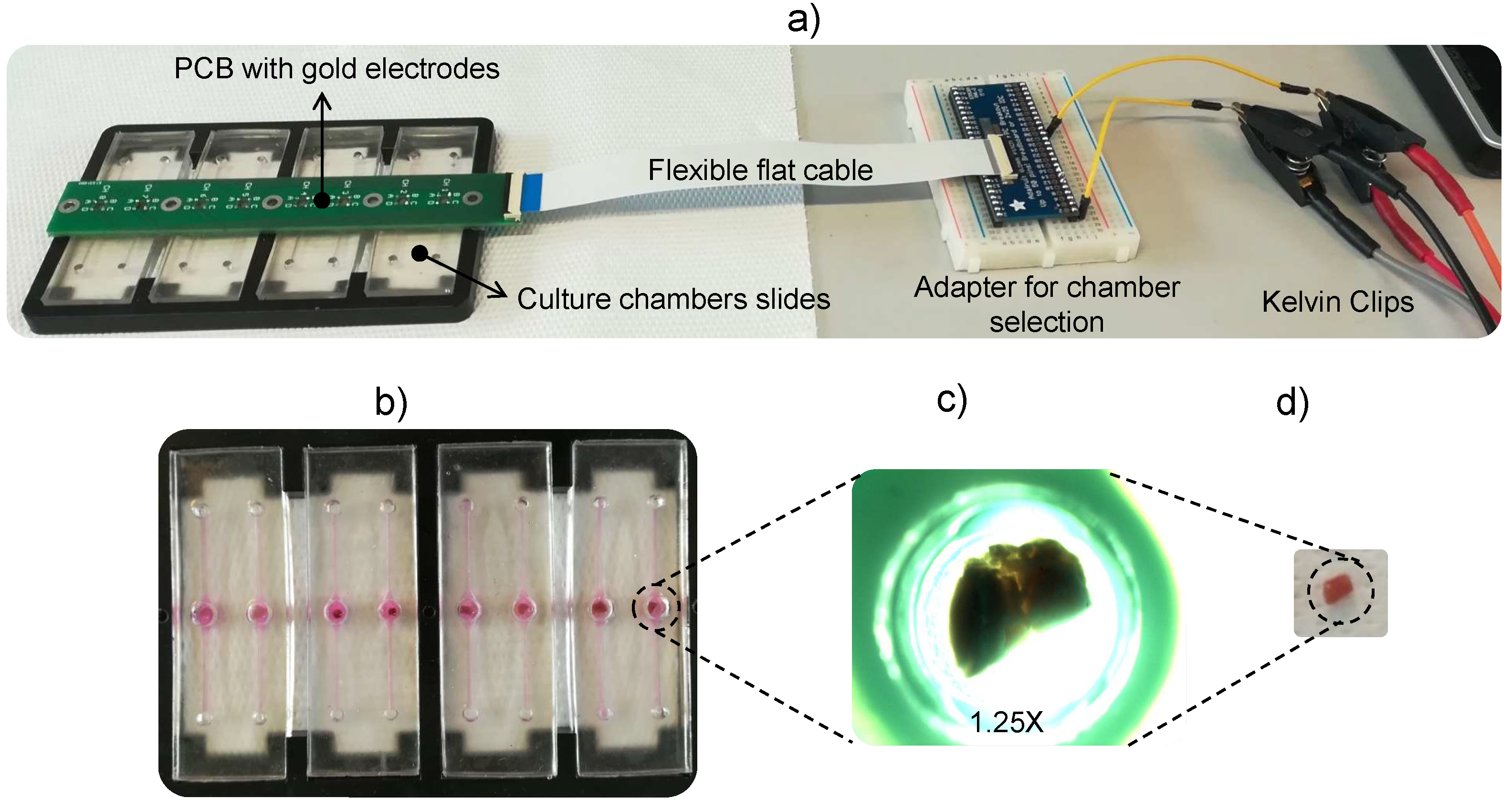
2.2. EIS Measurement Setup and Data Acquisition
2.3. 3D Cell Culture Model
2.4. Data Processing and Statistical Analysis
- The impedance module of each chamber was normalized to the culture medium measurement and the sample mass (m). The normalized impedance (NI) magnitude was .
- Mean curves were obtained from computed NI triplicate biopsies from the same mouse liver (k = 3, number of biopsy replicates from the same mouse).
- Mean curves were obtained from mice triplicates (n = 3, number of mice).
3. Results
3.1. Dielectric Response of Healthy and Fibrotic Tissue Biopsies
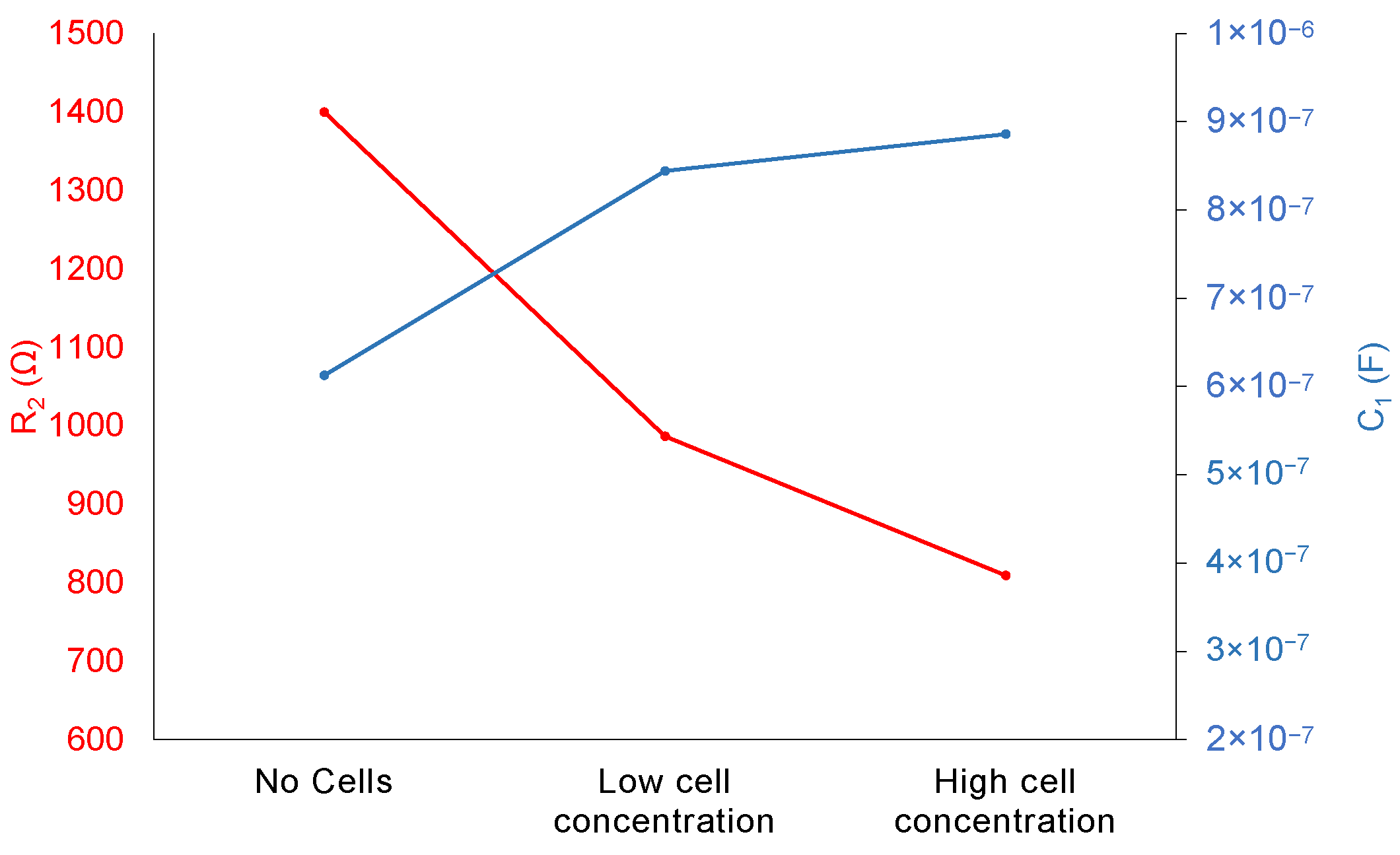
3.2. Equivalent Circuit Model and BDL Evolution Analysis Through Impedance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Long-Axis | Short-Axis | |
|---|---|---|
| Mean | 3.23 | 2.22 |
| Std. Dev | 0.44 | 0.12 |
| Std. Error | 0.22 | 0.06 |
| Long-Axis | Short-Axis | |
|---|---|---|
| Mean | 3.09 | 2.25 |
| Std. Dev | 0.23 | 0.44 |
| Std. Error | 0.22 | 0.06 |
| Mean Mass Values (n = 30) | |
|---|---|
| Mean | 13.40 mg |
| Std. Dev | 3.89 mg |
| Std. Error | 0.71 |
Appendix B
Appendix C

Appendix D

References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, A.; Tsipouras, M.G.; Tzallas, A.T.; Forlano, R.; Manousou, P.; Giannakeas, N. Quantification of liver fibrosis-a comparative study. Appl. Sci. 2020, 10, 447. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Rosso, C.; Fagoonee, S.; Saracco, G.M.; Pellicano, R. Liver fibrosis: The 2017 state of art. Panminerva Med. 2017, 59, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.; Desai, V.; Janardhan, S. Con: Liver Biopsy Remains the Gold Standard to Evaluate Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2019, 13, 114–116. [Google Scholar] [CrossRef]
- You, S.; Sun, Y.; Yang, L.; Park, J.; Tu, H.; Marjanovic, M.; Sinha, S.; Boppart, S.A. Real-time intraoperative diagnosis by deep neural network driven multiphoton virtual histology. Npj Precis. Oncol. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Ianni Filho, D.; Boin, I.d.F.S.F.; Yamanaka, A. Bioimpedance: New Approach To Non-Invasive Detection of Liver Fibrosis—A Pilot Study. Arq. Gastroenterol. 2018, 55, 2–6. [Google Scholar] [CrossRef][Green Version]
- Morgan, K.; Gamal, W.; Samuel, K.; Morley, S.D.; Hayes, P.C.; Bagnaninchi, P.; Plevris, J.N. Application of Impedance-Based Techniques in Hepatology Research. J. Clin. Med. 2019, 9, 50. [Google Scholar] [CrossRef]
- Laufer, S.; Ivorra, A.; Reuter, V.E.; Rubinsky, B.; Solomon, S.B. Electrical impedance characterization of normal and cancerous human hepatic tissue. Physiol. Meas. 2010, 31, 995–1009. [Google Scholar] [CrossRef]
- Bhati, C.S.; Silva, M.A.; Wigmore, S.J.; Bramhall, S.R.; Mayer, D.A.; Buckels, J.A.; Neil, D.A.; Murphy, N.; Mirza, D.F. Use of Bioelectrical Impedance Analysis to Assess Liver Steatosis. Transplant. Proc. 2009, 41, 1677–1681. [Google Scholar] [CrossRef]
- Parramon, D.; Erill, I.; Guimerà, A.; Ivorra, A.; Muñoz, Á.; Sola, A.; Fondevila, C.; García-Valdecasas, J.C.; Villa, R. In vivo detection of liver steatosis in rats based on impedance spectroscopy. Physiol. Meas. 2007, 28, 813–828. [Google Scholar] [CrossRef]
- Fuentes Vélez, S.; Fagoonee, S.; Sanginario, A.; Gallo, V.; Riganti, C.; Pizzi, M.; Altruda, F.; Demarchi, D. Impedance-based drug-resistance characterization of colon cancer cells through real-time cell culture monitoring. Talanta 2021, 222, 121441. [Google Scholar] [CrossRef] [PubMed]
- Abshagen, K.; König, M.; Hoppe, A.; Müller, I.; Ebert, M.; Weng, H.; Holzhütter, H.G.; Zanger, U.M.; Bode, J.; Vollmar, B.; et al. Pathobiochemical signatures of cholestatic liver disease in bile duct ligated mice. BMC Syst. Biol. 2015, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Fagoonee, S.; Arigoni, M.; Manco, M.; Olivero, M.; Bizzaro, F.; Magagnotti, C.; Andolfo, A.; Miniscalco, B.; Forni, M.; Todeschi, S.; et al. Circulating extracellular vesicles contain liver-derived RNA species as indicators of severe cholestasis-induced early liver fibrosis in mice. Antioxid. Redox Signal. 2021, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Rosso, C.; Caviglia, G.P.; Younes, R.; Ribaldone, D.G.; Fagoonee, S.; Pellicano, R.; Bugianesi, E. Molecular mechanisms of hepatic fibrosis in chronic liver diseases. Minerva Biotecnol. 2020, 32, 121–127. [Google Scholar] [CrossRef]
- Georgiev, P.; Jochum, W.; Heinrich, S.; Jang, J.H.; Nocito, A.; Dahm, F.; Clavien, P.A. Characterization of time-related changes after experimental bile duct ligation. Br. J. Surg. 2008, 95, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, W.M.; Kim, K.; Jeong, W.I.; Seo, J.B.; Park, I. Biopsy Needle Integrated with Electrical Impedance Sensing Microelectrode Array towards Real-time Needle Guidance and Tissue Discrimination. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Korelova, K.; Jirouskova, M.; Sarnova, L.; Gregor, M. Isolation and 3D collagen sandwich culture of primary mouse hepatocytes to study the role of cytoskeleton in bile canalicular formation in vitro. J. Vis. Exp. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Min, M. Fundamentals, recent advances, and future challenges in bioimpedance devices for healthcare applications. J. Sens. 2019, 42, 9210258. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Liaw, B.Y.; Zhang, J. Graphical analysis of electrochemical impedance spectroscopy data in Bode and Nyquist representations. J. Power Sources 2016, 309, 82–98. [Google Scholar] [CrossRef]
- Mahdavi, R.; Hosseinpour, P.; Abbasvandi, F.; Mehrvarz, S.; Yousefpour, N.; Ataee, H.; Parniani, M.; Mamdouh, A.; Ghafari, H.; Abdolahad, M. Bioelectrical pathology of the breast; real-time diagnosis of malignancy by clinically calibrated impedance spectroscopy of freshly dissected tissue. Biosens. Bioelectron. 2020, 165, 112421. [Google Scholar] [CrossRef]
- Yun, J.; Kang, G.; Park, Y.; Kim, H.W.; Cha, J.J.; Lee, J.H. Electrochemical impedance spectroscopy with interdigitated electrodes at the end of hypodermic needle for depth profiling of biotissues. Sens. Actuators B Chem. 2016, 237, 984–991. [Google Scholar] [CrossRef]
- Balasubramani, L.; Brown, B.H.; Healey, J.; Tidy, J.A. The detection of cervical intraepithelial neoplasia by electrical impedance spectroscopy: The effects of acetic acid and tissue homogeneity. Gynecol. Oncol. 2009, 115, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H.; Chui, C.K.; Teoh, S.H.; Chang, S.K. A multiscale model for bioimpedance dispersion of liver tissue. IEEE Trans. Biomed. Eng. 2012, 59, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Han, N.; Lee, R.; Choi, I.H.; Park, Y.B.; Shin, J.S.; Yoo, K.H. Real-time monitoring of 3D cell culture using a 3D capacitance biosensor. Biosens. Bioelectron. 2016, 77, 56–61. [Google Scholar] [CrossRef]
- Yin, M.; Glaser, K.J.; Manduca, A.; Mounajjed, T.; Malhi, H.; Simonetto, D.A.; Wang, R.; Yang, L.; Mao, S.A.; Glorioso, J.M.; et al. Distinguishing between hepatic inflammation and fibrosis with MR elastography. Radiology 2017, 284, 694–705. [Google Scholar] [CrossRef]
- Matko, V.; Milanovič, M. Sensitivity and accuracy of dielectric measurements of liquids significantly improved by coupled capacitive-dependent quartz crystals. Sensors 2021, 21, 3565. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-Vélez, S.; Fagoonee, S.; Sanginario, A.; Pizzi, M.; Altruda, F.; Demarchi, D. Electrical Impedance-Based Characterization of Hepatic Tissue with Early-Stage Fibrosis. Biosensors 2022, 12, 116. https://doi.org/10.3390/bios12020116
Fuentes-Vélez S, Fagoonee S, Sanginario A, Pizzi M, Altruda F, Demarchi D. Electrical Impedance-Based Characterization of Hepatic Tissue with Early-Stage Fibrosis. Biosensors. 2022; 12(2):116. https://doi.org/10.3390/bios12020116
Chicago/Turabian StyleFuentes-Vélez, Susana, Sharmila Fagoonee, Alessandro Sanginario, Marco Pizzi, Fiorella Altruda, and Danilo Demarchi. 2022. "Electrical Impedance-Based Characterization of Hepatic Tissue with Early-Stage Fibrosis" Biosensors 12, no. 2: 116. https://doi.org/10.3390/bios12020116
APA StyleFuentes-Vélez, S., Fagoonee, S., Sanginario, A., Pizzi, M., Altruda, F., & Demarchi, D. (2022). Electrical Impedance-Based Characterization of Hepatic Tissue with Early-Stage Fibrosis. Biosensors, 12(2), 116. https://doi.org/10.3390/bios12020116






