Optical Light Sources and Wavelengths within the Visible and Near-Infrared Range Using Photoacoustic Effects for Biomedical Applications
Abstract
:1. Introduction
2. Laser Wavelengths and Applications for Photoacoustic Effects
2.1. Applications at 400–700 nm Visible Wavelength
2.1.1. The 450 nm Wavelength Laser Diode
2.1.2. The 532 nm Wavelength Pulsed Laser
2.1.3. The 680 nm Wavelength Pulsed Laser
2.1.4. The 700 nm Wavelength Pulsed Laser
2.2. Applications at 800–1600 nm NIR Wavelength
2.2.1. The 800 nm Wavelength Pulsed Laser
2.2.2. The 820 nm Wavelength Pulsed Laser
2.2.3. The 905 nm Wavelength Pulsed Laser
2.2.4. The 1064 nm Wavelength Pulsed Laser
2.2.5. The 1600 nm Wavelength Pulsed Laser
2.3. Multi-Wavelength Laser Applications
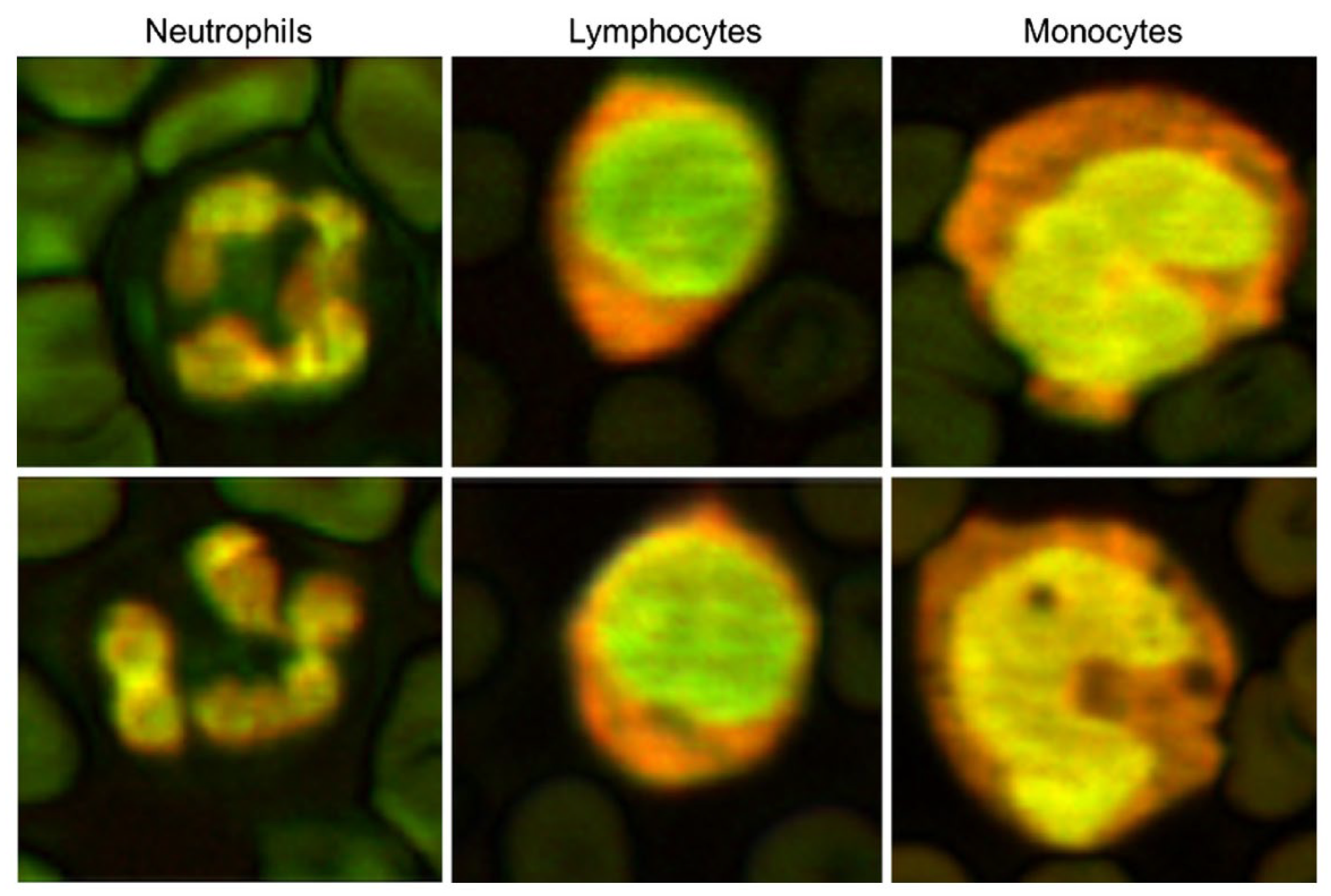
2.3.1. The 532 and 600 nm Wavelength Pulsed Lasers for Leukocyte Imaging
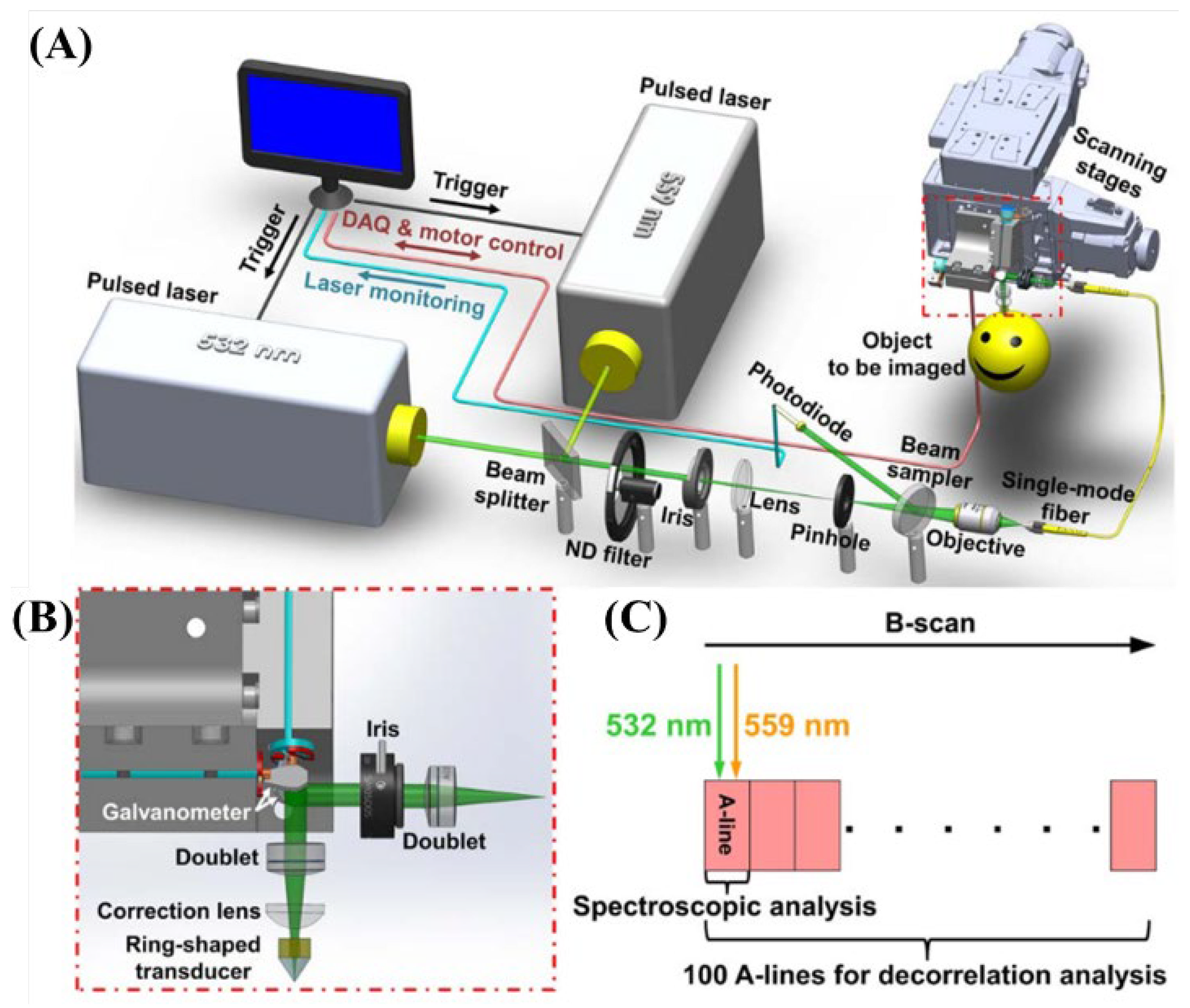
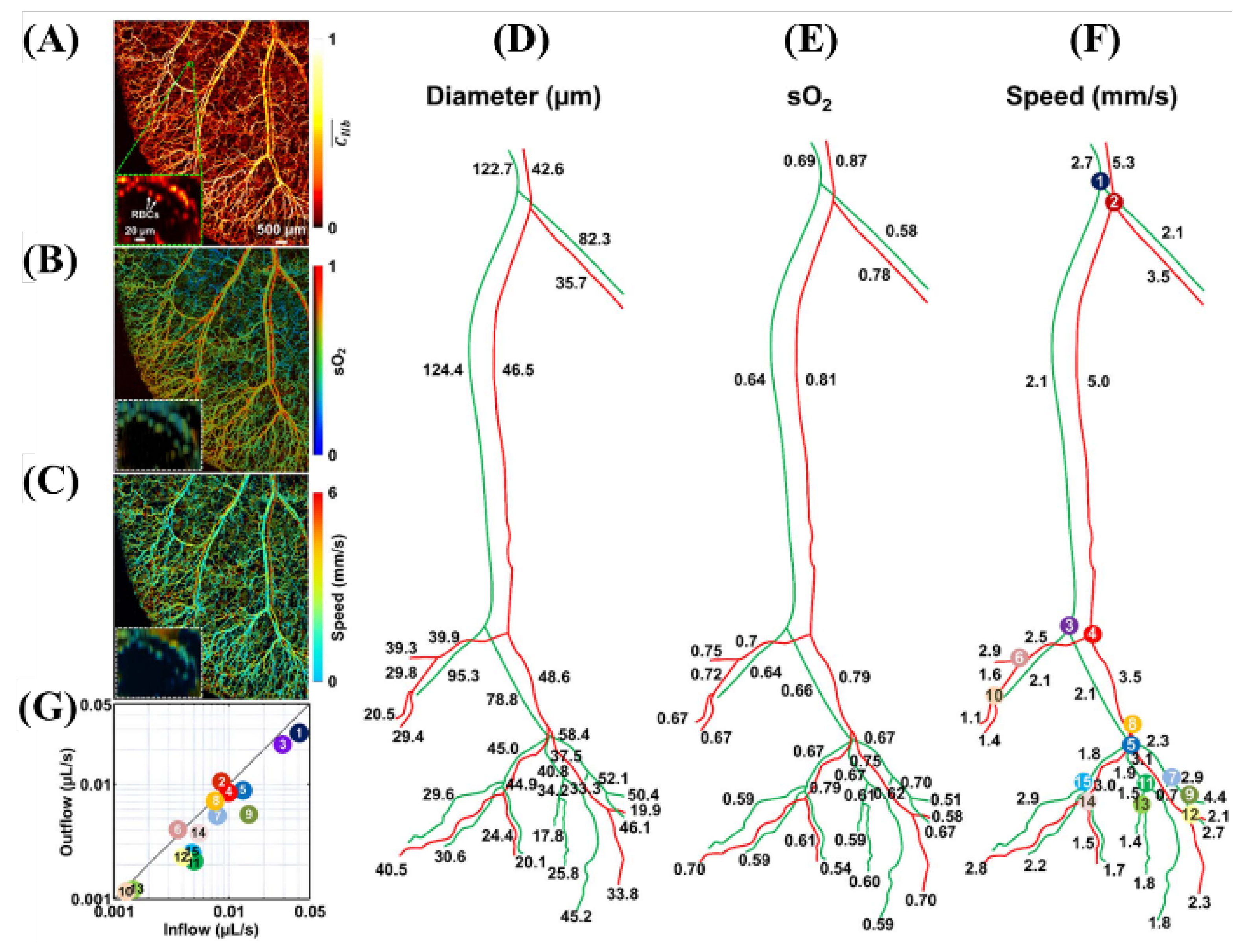
2.3.2. The 532 and 559 nm Wavelength Pulsed Lasers for Oxy- and Deoxy-Hemoglobin Imaging
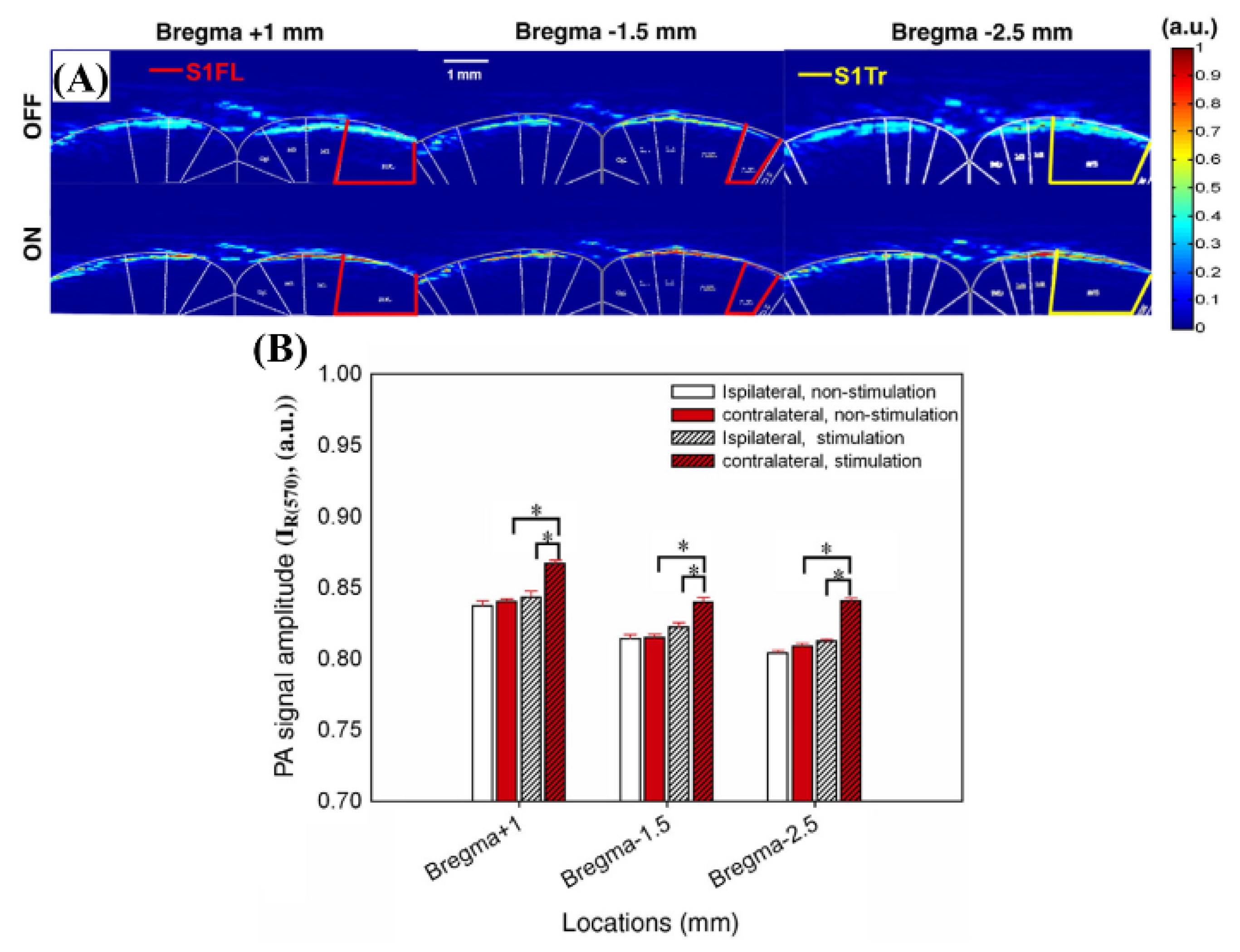
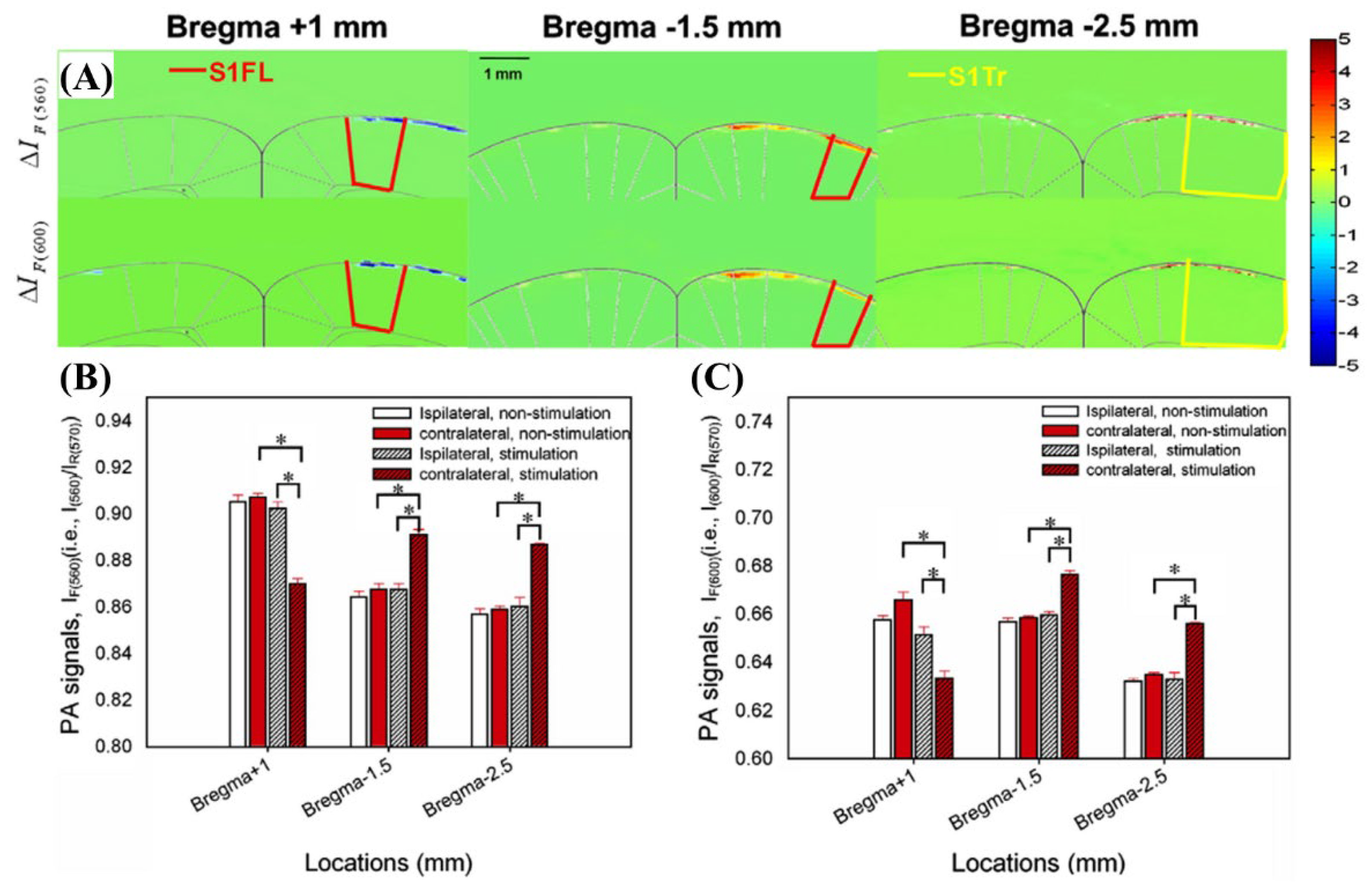
2.3.3. The 560, 570, and 600 nm Wavelength Pulsed Lasers for Imaging of Rat Brain Hemodynamic Changes
2.3.4. The 562 and 584 nm Wavelength Pulsed Lasers for In Vivo Internal Organ Imaging Integrated with Ultrasound Imaging
2.3.5. Beyond 650 nm Wavelength Pulsed Laser for Cells and Tissues Using Tyrosinase (Tyr)
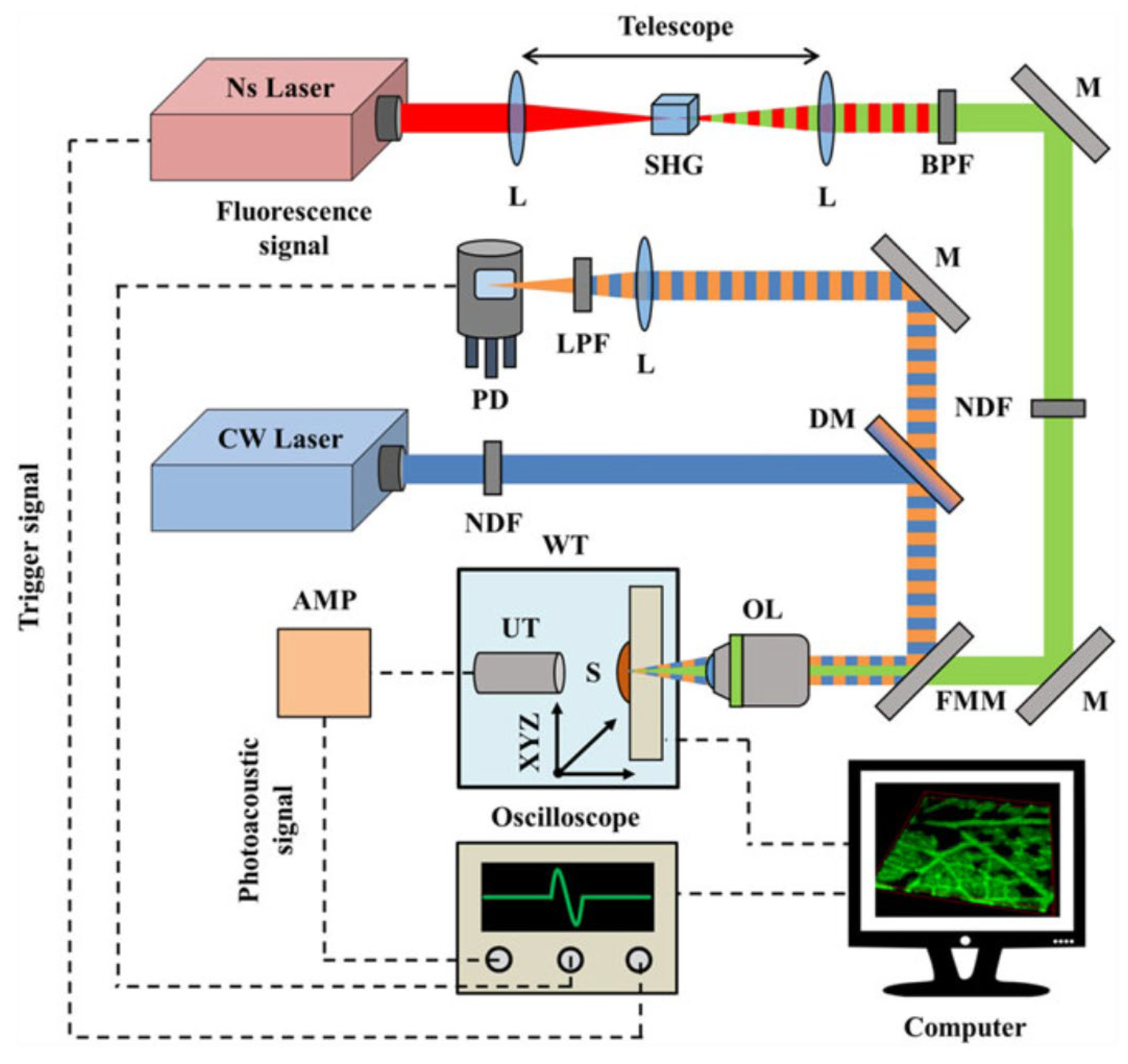
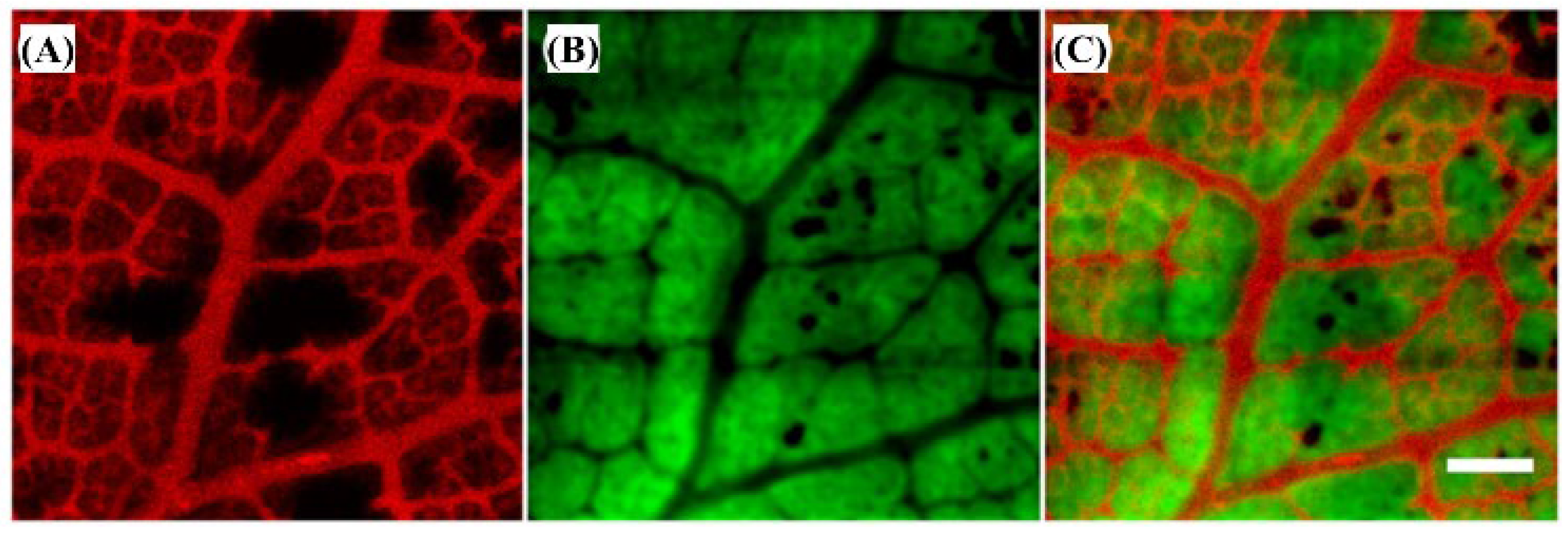
2.3.6. The 532 nm Wavelength Pulsed Laser for Vegetative Tissue Imaging Integrated with 473 nm CW Laser Fluorescence Imaging
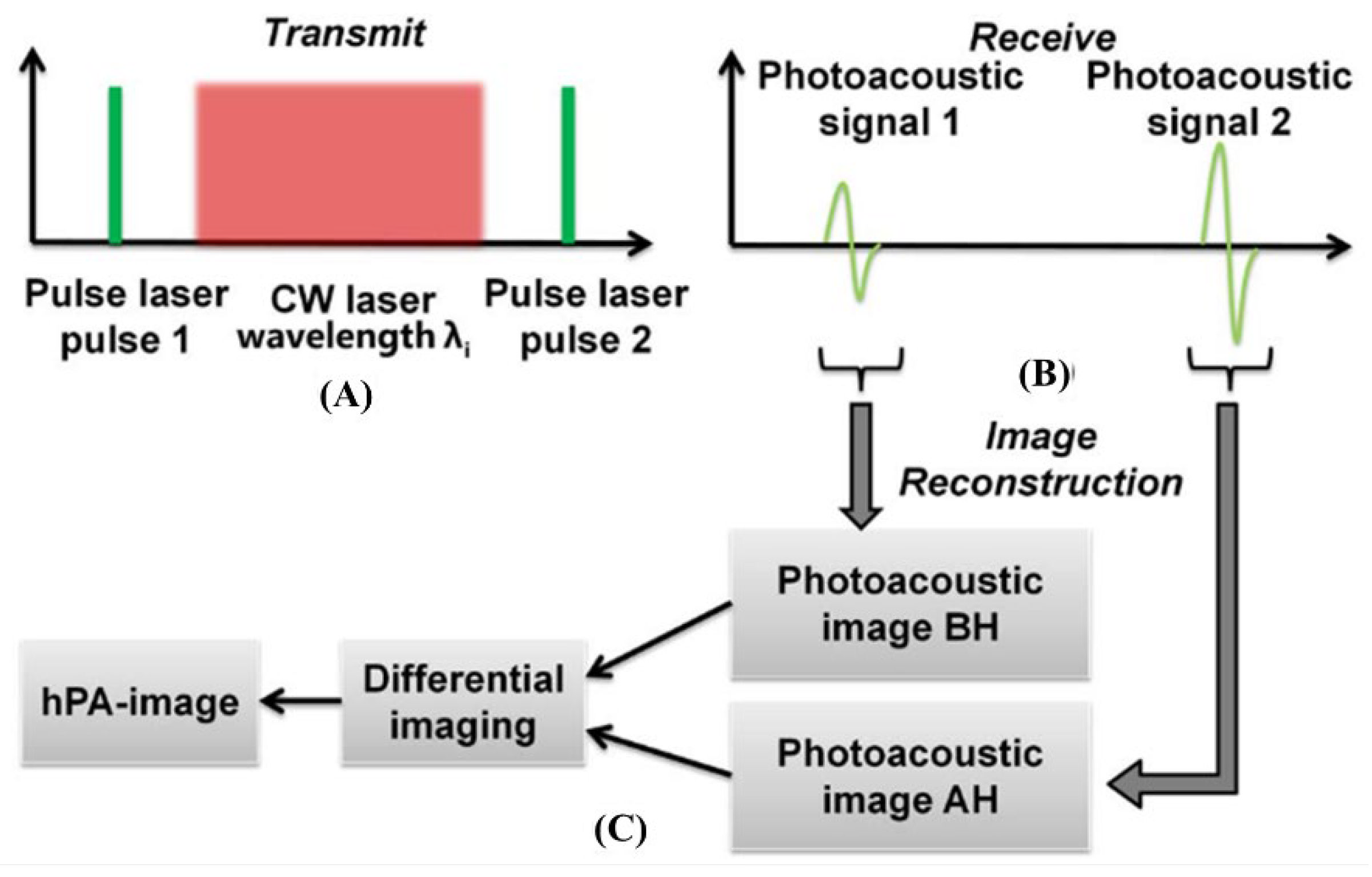
2.3.7. Single Wavelength Pulsed Laser with Multiple CW Laser Heating for Photoacoustic Imaging
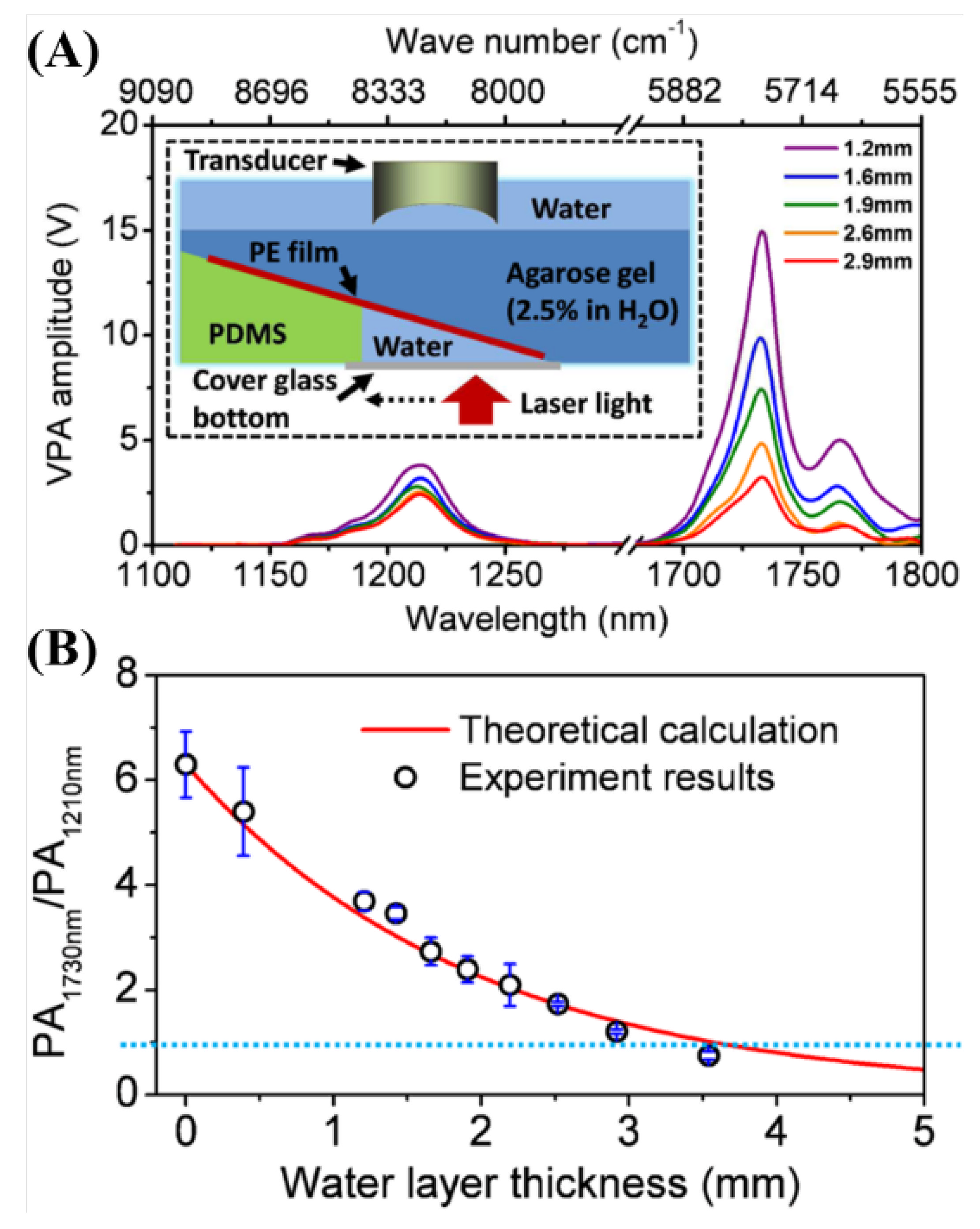
2.3.8. Spectroscopic Photoacoustic Imaging
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, A.G. Upon the Production and Reproduction of Sound by Light. J. Soc. Telegr. Eng. 1880, 9, 404–426. [Google Scholar] [CrossRef]
- Maslov, K.; Wang, L.V. Photoacoustic Imaging of Biological Tissue with Intensity-Modulated Continuous-Wave Laser. J. Biomed. Opt. 2008, 13, 024006. [Google Scholar] [CrossRef]
- Murray, T.W.; Balogun, O. High-Sensitivity Laser-Based Acoustic Microscopy Using a Modulated Excitation Source. Appl. Phys. Lett. 2004, 85, 2974–2976. [Google Scholar] [CrossRef]
- Erfanzadeh, M.; Zhu, Q. Photoacoustic Imaging with Low-Cost Sources; A Review. Photoacoustics 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Zhong, H.; Duan, T.; Lan, H.; Zhou, M.; Gao, F. Review of Low-Cost Photoacoustic Sensing and Imaging Based on Laser Diode and Light-Emitting Diode. Sensors 2018, 18, 2264. [Google Scholar] [CrossRef] [Green Version]
- Manohar, S.; Razansky, D. Photoacoustics: A Historical Review. Adv. Opt. Photon. 2016, 8, 586. [Google Scholar] [CrossRef] [Green Version]
- Koushki, E.; Ghasedi, A.; Tayebee, R. Origins of Photoacoustic Effect in Solutions with a Single Non-Pulsed Continue Wave Laser Beam; Study on the CrTPP Solutions. J. Photochem. Photobiol. A Chem. 2022, 427, 113811. [Google Scholar] [CrossRef]
- Beard, P. Biomedical Photoacoustic Imaging. Interface Focus. 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed]
- Hale, G.M.; Querry, M.R. Optical Constants of Water in the 200-Nm to 200-Μm Wavelength Region. Appl. Opt. AO 1973, 12, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Olvera Cano, L.I.; Villanueva Lopez, G.C.; Mateos, E.R.; Orea, A.C. Photoacoustic Spectroscopy and Hyperglycemia in Experimental Type 1 Diabetes. Appl. Spectrosc. 2021, 75, 1465–1474. [Google Scholar] [CrossRef]
- Lister, T.; Wright, P.A.; Chappell, P.H. Optical Properties of Human Skin. J. Biomed. Opt. 2012, 17, 0909011. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yao, J.; Wang, L.V. Tutorial on Photoacoustic Tomography. J. Biomed. Opt. 2016, 21, 061007. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, R.; Sahin, O.; Emelianov, S. Ultrasound-Guided Photoacoustic Imaging: Current State and Future Development. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2014, 61, 450–466. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Photoacoustic Microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H. Photoacoustic Tomography; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-315-21390-3. [Google Scholar]
- Cho, S.-W.; Park, S.M.; Park, B.; Kim, D.Y.; Lee, T.G.; Kim, B.-M.; Kim, C.; Kim, J.; Lee, S.-W.; Kim, C.-S. High-Speed Photoacoustic Microscopy: A Review Dedicated on Light Sources. Photoacoustics 2021, 24, 100291. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.F. Photoacoustic Imaging of the Eye: A Mini Review. Photoacoustics 2016, 4, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Wang, L.V. Photoacoustic Tomography: Fundamentals, Advances and Prospects: Photoacoustic Tomography: Review. Contrast Media Mol. Imaging 2011, 6, 332–345. [Google Scholar] [CrossRef] [Green Version]
- Kratkiewicz, K.; Pattyn, A.; Alijabbari, N.; Mehrmohammadi, M. Ultrasound and Photoacoustic Imaging of Breast Cancer: Clinical Systems, Challenges, and Future Outlook. JCM 2022, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- Piras, D.; Xia, W.; Steenbergen, W.; van Leeuwen, T.G.; Manohar, S.G. Photoacoustic Imaging of the Breast Using the Twente Photoacoustic Mammoscope: Present Status and Future Perspectives. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 730–739. [Google Scholar] [CrossRef]
- Li, S.; Lu, J.; Shang, Z.; Zeng, X.; Yuan, Y.; Wu, H.; Pan, Y.; Sampaolo, A.; Patimisco, P.; Spagnolo, V.; et al. Compact Quartz-Enhanced Photoacoustic Sensor for Ppb-Level Ambient NO2 Detection by Use of a High-Power Laser Diode and a Grooved Tuning Fork. Photoacoustics 2022, 25, 100325. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Chen, N.; Zhao, H.; Yin, T.; Zhang, J.; Zheng, W.; Song, L.; Liu, C.; Zheng, R. Optical-Resolution Photoacoustic Microscopy for Monitoring Vascular Normalization during Anti-Angiogenic Therapy. Photoacoustics 2019, 15, 100143. [Google Scholar] [CrossRef]
- Brunker, J.; Beard, P. Acoustic Resolution Photoacoustic Doppler Velocimetry in Blood-Mimicking Fluids. Sci. Rep. 2016, 6, 20902. [Google Scholar] [CrossRef]
- Ahn, J.; Baik, J.W.; Kim, Y.; Choi, K.; Park, J.; Kim, H.; Kim, J.Y.; Kim, H.H.; Nam, S.H.; Kim, C. Fully Integrated Photoacoustic Microscopy and Photoplethysmography of Human In Vivo. Photoacoustics 2022, 27, 100374. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.A.; Fernandes, D.D.; Li, Y.; Wang, Y.; Zhang, Z.; Rousseau, D.; Gradinaru, C.C.; Kolios, M.C. Synthesis of Stable Multifunctional Perfluorocarbon Nanoemulsions for Cancer Therapy and Imaging. Langmuir 2016, 32, 10870–10880. [Google Scholar] [CrossRef] [PubMed]
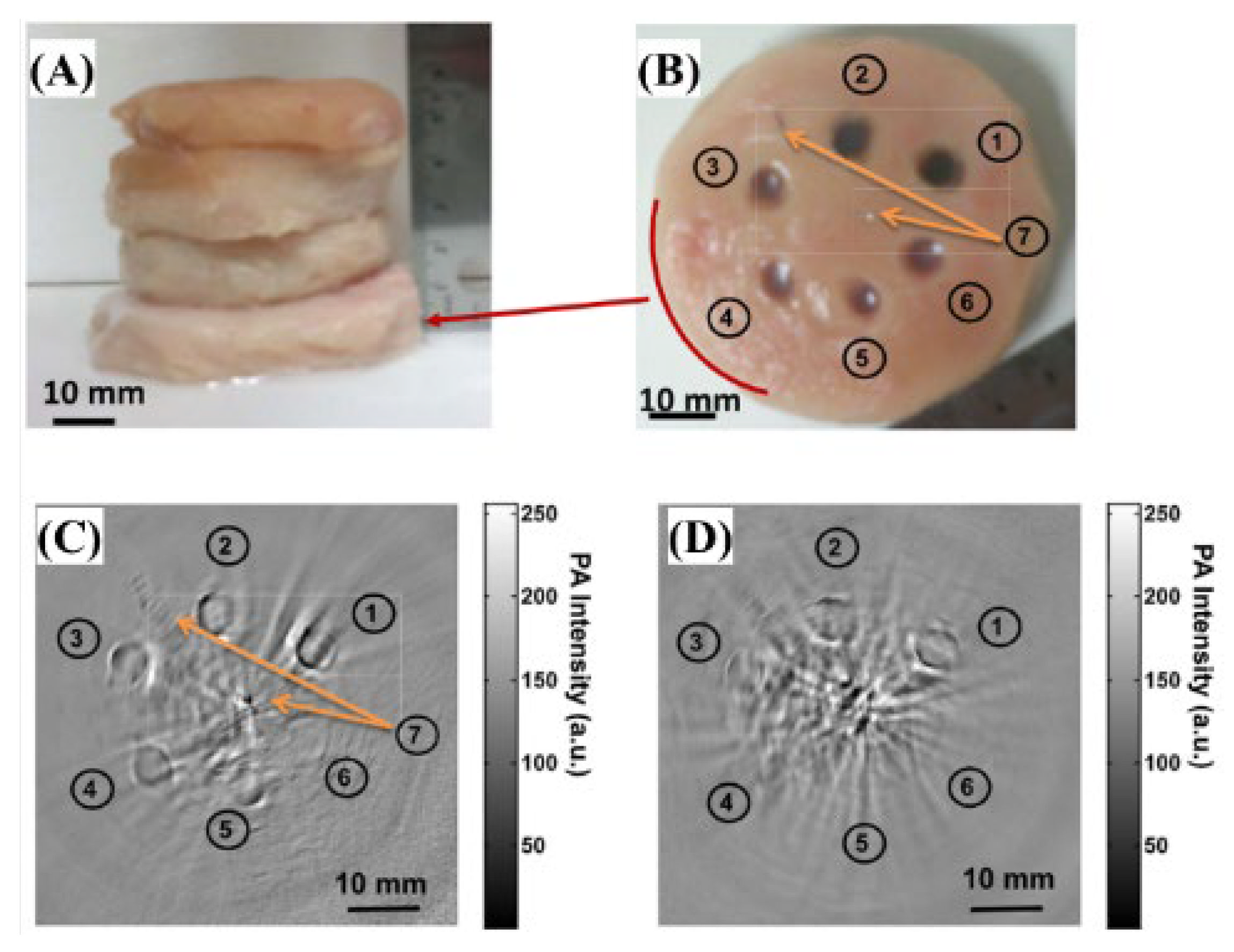
- Bui, N.Q.; Cho, S.-W.; Moorthy, M.S.; Park, S.M.; Piao, Z.; Nam, S.Y.; Kang, H.W.; Kim, C.-S.; Oh, J. In Vivo Photoacoustic Monitoring Using 700-Nm Region Raman Source for Targeting Prussian Blue Nanoparticles in Mouse Tumor Model. Sci. Rep. 2018, 8, 2000. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-L.; Wang, J.C.; Schwartz, J.A.; Gill-Sharp, K.L.; Stoica, G.; Wang, L.V. In-Vivo Photoacoustic Microscopy of Nanoshell Extravasation from Solid Tumor Vasculature. J. Biomed. Opt. 2009, 14, 010507. [Google Scholar] [CrossRef] [Green Version]
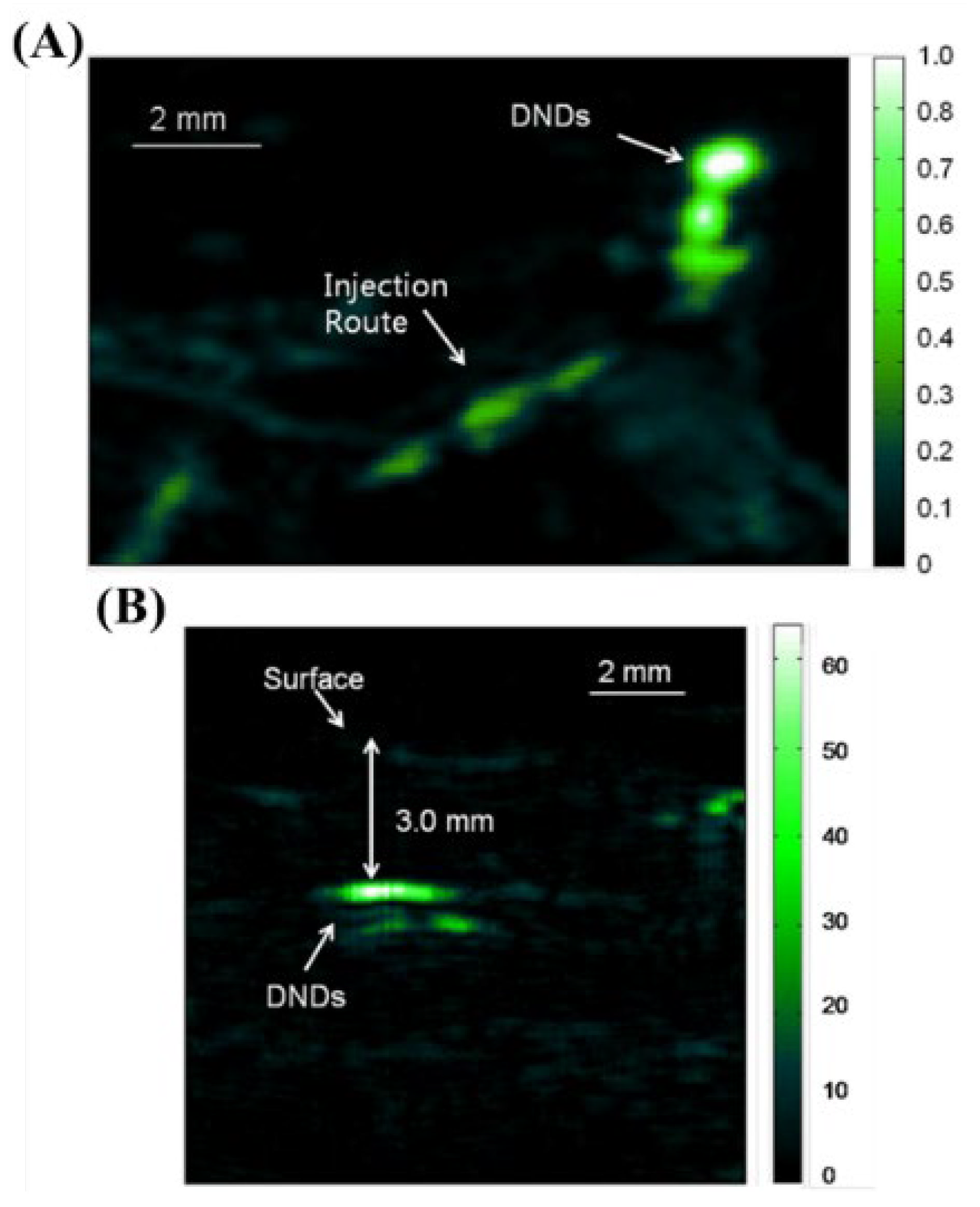
- Zhang, T.; Cui, H.; Fang, C.-Y.; Su, L.-J.; Ren, S.; Chang, H.-C.; Yang, X.; Forrest, M.L. Photoacoustic Contrast Imaging of Biological Tissues with Nanodiamonds Fabricated for High Near-Infrared Absorbance. J. Biomed. Opt. 2013, 18, 026018. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-S.; Hung, Y.-C.; Liau, I.; Huang, G.S. Assessment of the In Vivo Toxicity of Gold Nanoparticles. Nanoscale Res. Lett. 2009, 4, 858. [Google Scholar] [CrossRef] [Green Version]
- Nune, S.K.; Gunda, P.; Majeti, B.K.; Thallapally, P.K.; Forrest, M.L. Advances in Lymphatic Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 876–885. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Liu, G.; Yang, D.; Ji, X. 3D-Visual Laser-Diode-Based Photoacoustic Imaging. Opt. Express 2012, 20, 1237. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, G.; Yang, D.; Ji, X. Cost-Efficient Laser-Diode-Induced Optical-Resolution Photoacoustic Microscopy for Two-Dimensional/Three-Dimensional Biomedical Imaging. J. Biomed. Opt. 2014, 19, 076017. [Google Scholar] [CrossRef]
- Homan, K.; Kim, S.; Chen, Y.-S.; Wang, B.; Mallidi, S.; Emelianov, S. Prospects of Molecular Photoacoustic Imaging at 1064 Nm Wavelength. Opt. Lett. 2010, 35, 2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitcham, T.; Dextraze, K.; Taghavi, H.; Melancon, M.; Bouchard, R. Photoacoustic Imaging Driven by an Interstitial Irradiation Source. Photoacoustics 2015, 3, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Ku, G.; Zhou, M.; Song, S.; Huang, Q.; Hazle, J.; Li, C. Copper Sulfide Nanoparticles As a New Class of Photoacoustic Contrast Agent for Deep Tissue Imaging at 1064 Nm. ACS Nano 2012, 6, 7489–7496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, L.; Cai, X.; Maslov, K.; Garcia-Uribe, A.; Anastasio, M.A.; Wang, L.V. Photoacoustic Tomography through a Whole Adult Human Skull with a Photon Recycler. J. Biomed. Opt. 2012, 17, 110506. [Google Scholar] [CrossRef] [Green Version]
- Piao, Z.; Zeng, L.; Chen, Z.; Kim, C.-S. Q-Switched Erbium-Doped Fiber Laser at 1600 Nm for Photoacoustic Imaging Application. Appl. Phys. Lett. 2016, 108, 143701. [Google Scholar] [CrossRef] [Green Version]
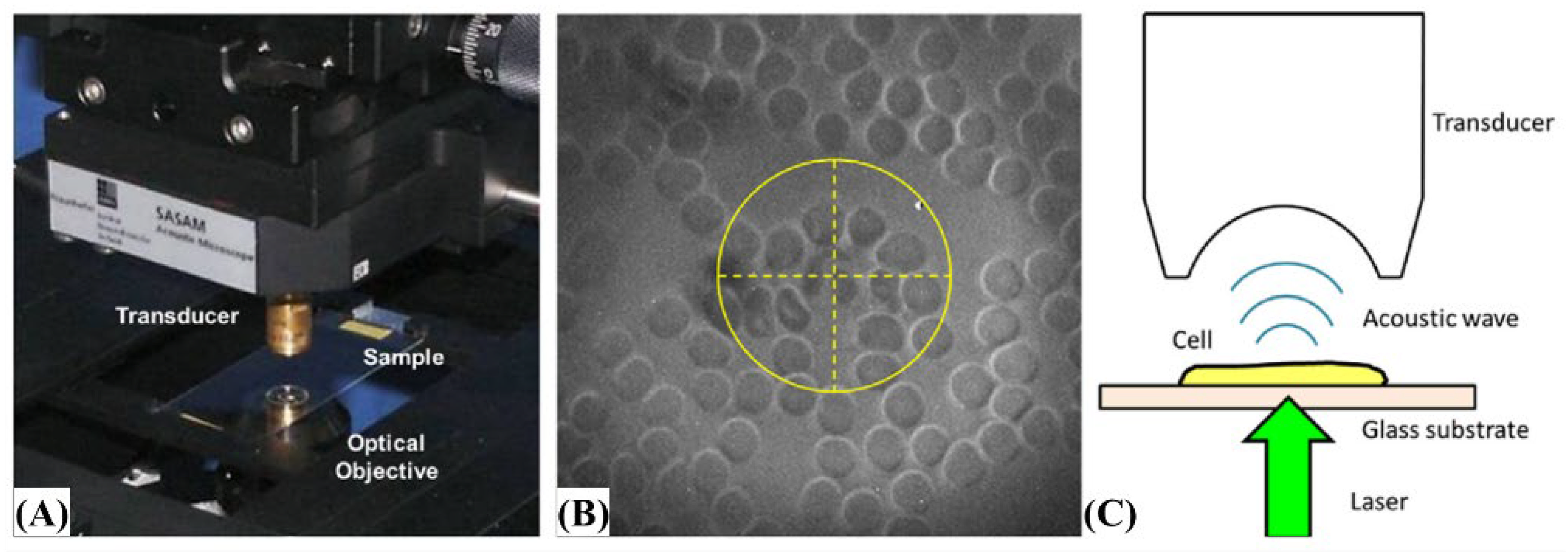
- Strohm, E.M.; Moore, M.J.; Kolios, M.C. High Resolution Ultrasound and Photoacoustic Imaging of Single Cells. Photoacoustics 2016, 4, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, B.; Kennedy, M.J.; Dixon, A.J.; Sun, N.; Cao, R.; Soetikno, B.T.; Chen, R.; Zhou, Q.; Kirk Shung, K.; Hossack, J.A.; et al. Simultaneous Photoacoustic Microscopy of Microvascular Anatomy, Oxygen Saturation, and Blood Flow. Opt. Lett. 2015, 40, 910. [Google Scholar] [CrossRef]
- Liao, L.-D.; Li, M.-L.; Lai, H.-Y.; Shih, Y.-Y.I.; Lo, Y.-C.; Tsang, S.; Chao, P.C.-P.; Lin, C.-T.; Jaw, F.-S.; Chen, Y.-Y. Imaging Brain Hemodynamic Changes during Rat Forepaw Electrical Stimulation Using Functional Photoacoustic Microscopy. NeuroImage 2010, 52, 562–570. [Google Scholar] [CrossRef]
- Yang, J.-M.; Favazza, C.; Chen, R.; Yao, J.; Cai, X.; Maslov, K.; Zhou, Q.; Shung, K.K.; Wang, L.V. Simultaneous Functional Photoacoustic and Ultrasonic Endoscopy of Internal Organs In Vivo. Nat. Med. 2012, 18, 1297–1302. [Google Scholar] [CrossRef]
- Yao, J.; Maslov, K.I.; Zhang, Y.; Xia, Y.; Wang, L.V. Label-Free Oxygen-Metabolic Photoacoustic Microscopy In Vivo. J. Biomed. Opt. 2011, 16, 076003. [Google Scholar] [CrossRef] [PubMed]
- Jathoul, A.P.; Laufer, J.; Ogunlade, O.; Treeby, B.; Cox, B.; Zhang, E.; Johnson, P.; Pizzey, A.R.; Philip, B.; Marafioti, T.; et al. Deep In Vivo Photoacoustic Imaging of Mammalian Tissues Using a Tyrosinase-Based Genetic Reporter. Nat. Photon 2015, 9, 239–246. [Google Scholar] [CrossRef]
- Tserevelakis, G.J.; Tsagkaraki, M.; Zacharakis, G. Hybrid Photoacoustic and Optical Imaging of Pigments in Vegetative Tissues: Hybrid Photoacoustic And Optical Imaging Of Pigments In Vegetative Tissues. J. Microsc. 2016, 263, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Lan, H.; Zhong, H.; Zhou, M.; Zhang, R.; Gao, F. Hybrid Multi-Wavelength Nonlinear Photoacoustic Sensing and Imaging. Opt. Lett. 2018, 43, 5611. [Google Scholar] [CrossRef]
- Wang, P.; Rajian, J.R.; Cheng, J.-X. Spectroscopic Imaging of Deep Tissue through Photoacoustic Detection of Molecular Vibration. J. Phys. Chem. Lett. 2013, 4, 2177–2185. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.-X. New “HOPE” Laser for Photoacoustic Imaging of Water. Light Sci. Appl. 2022, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Aloraynan, A.; Rassel, S.; Xu, C.; Ban, D. A Single Wavelength Mid-Infrared Photoacoustic Spectroscopy for Noninvasive Glucose Detection Using Machine Learning. Biosensors 2022, 12, 166. [Google Scholar] [CrossRef] [PubMed]
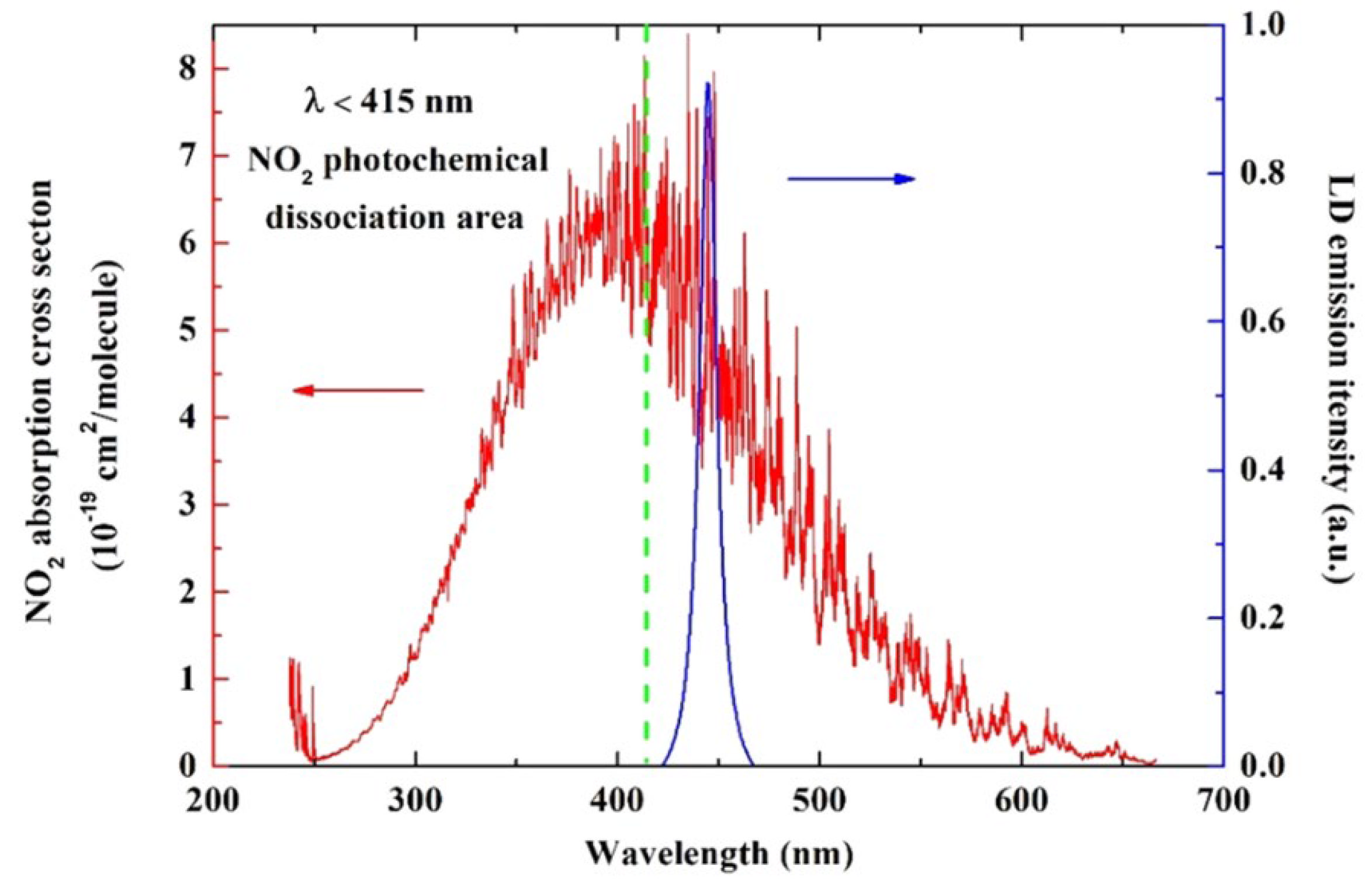
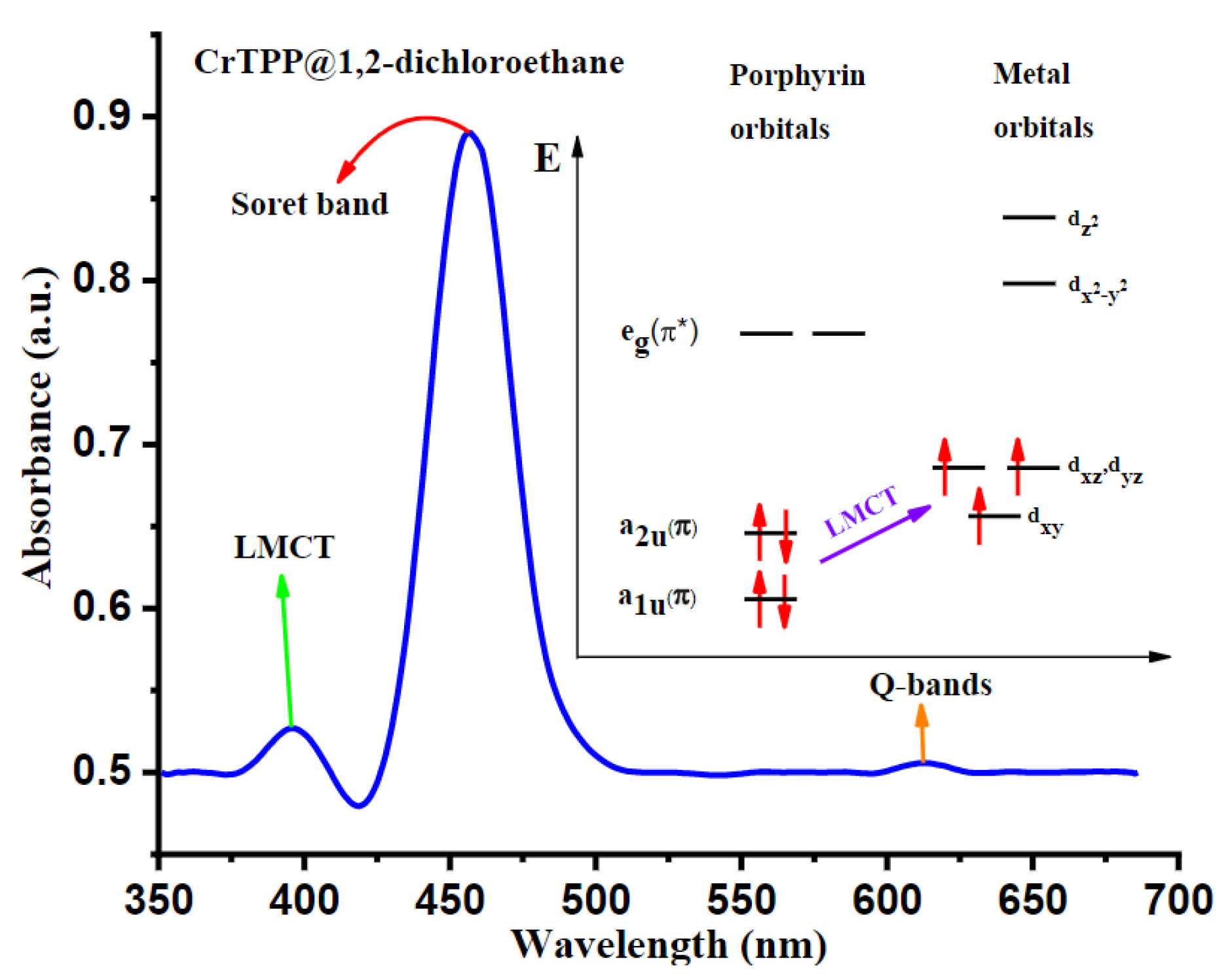

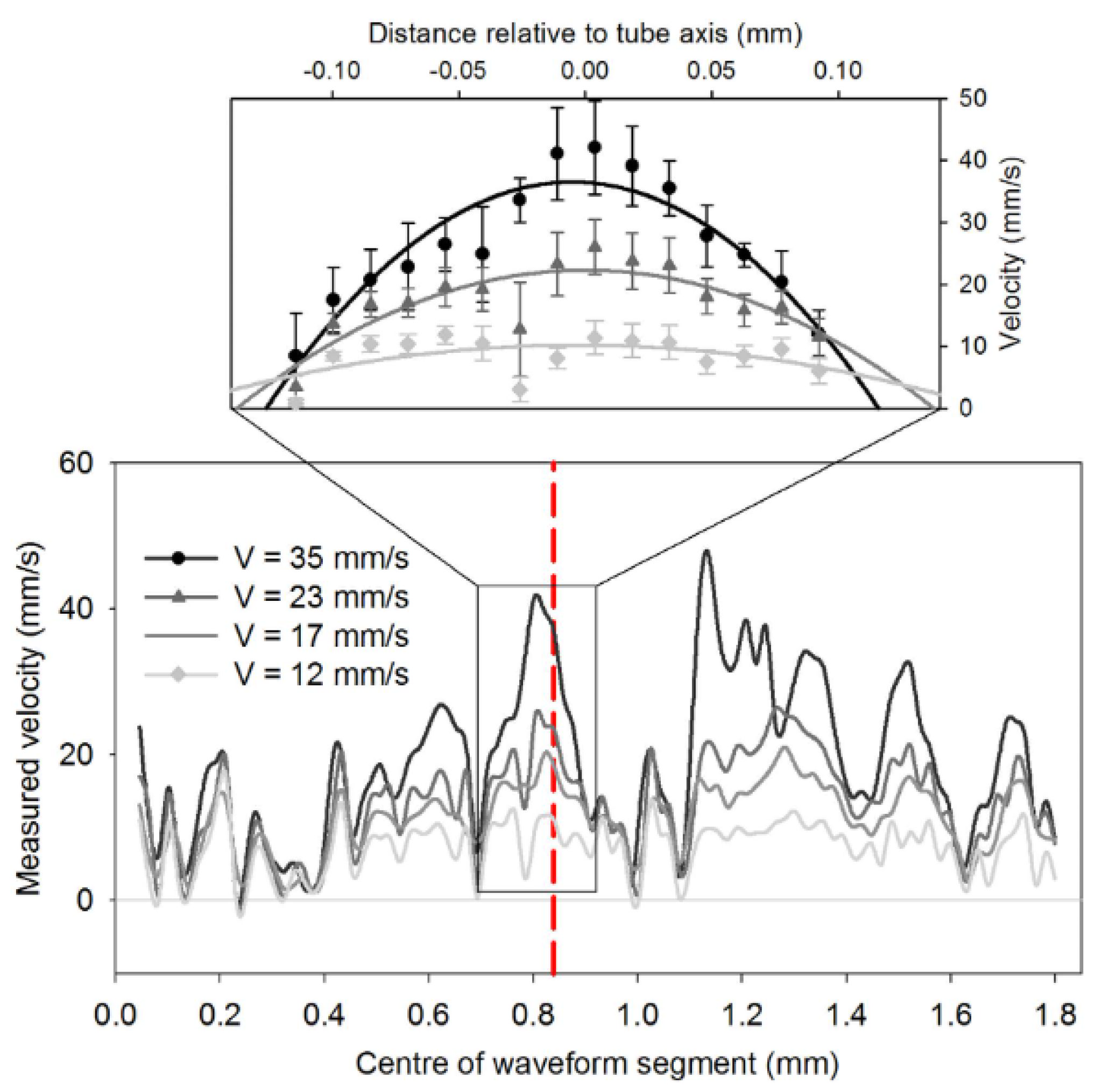

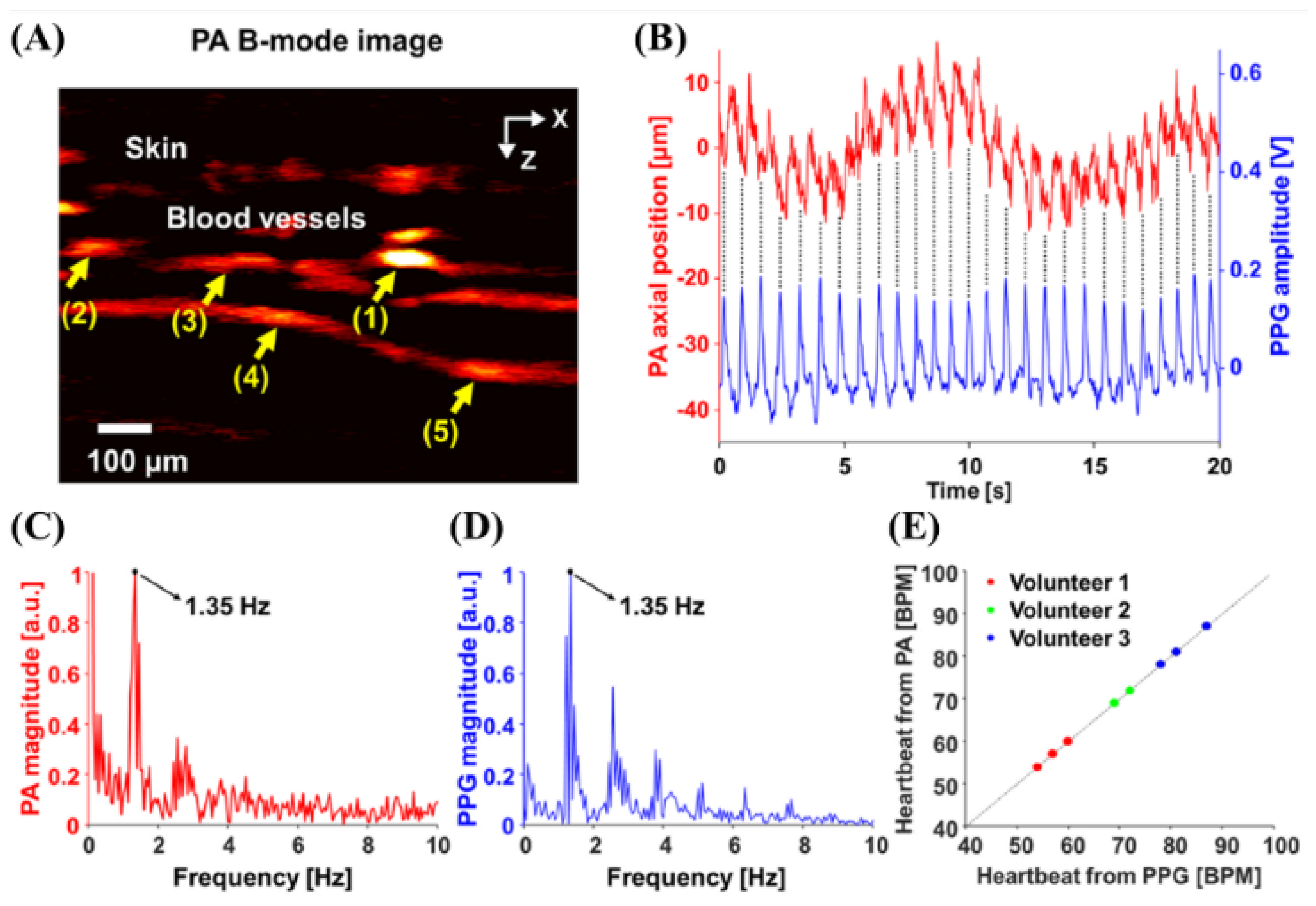
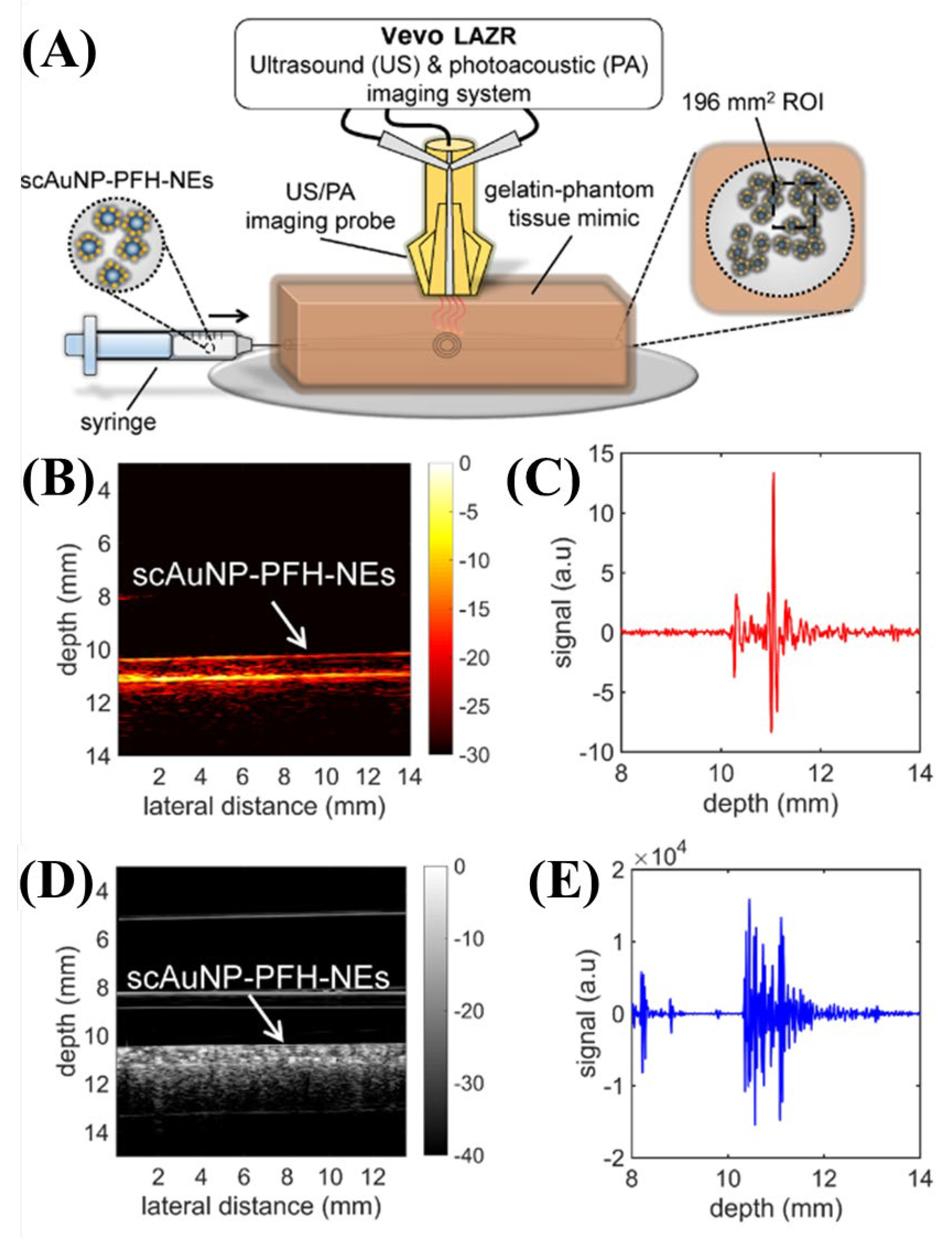
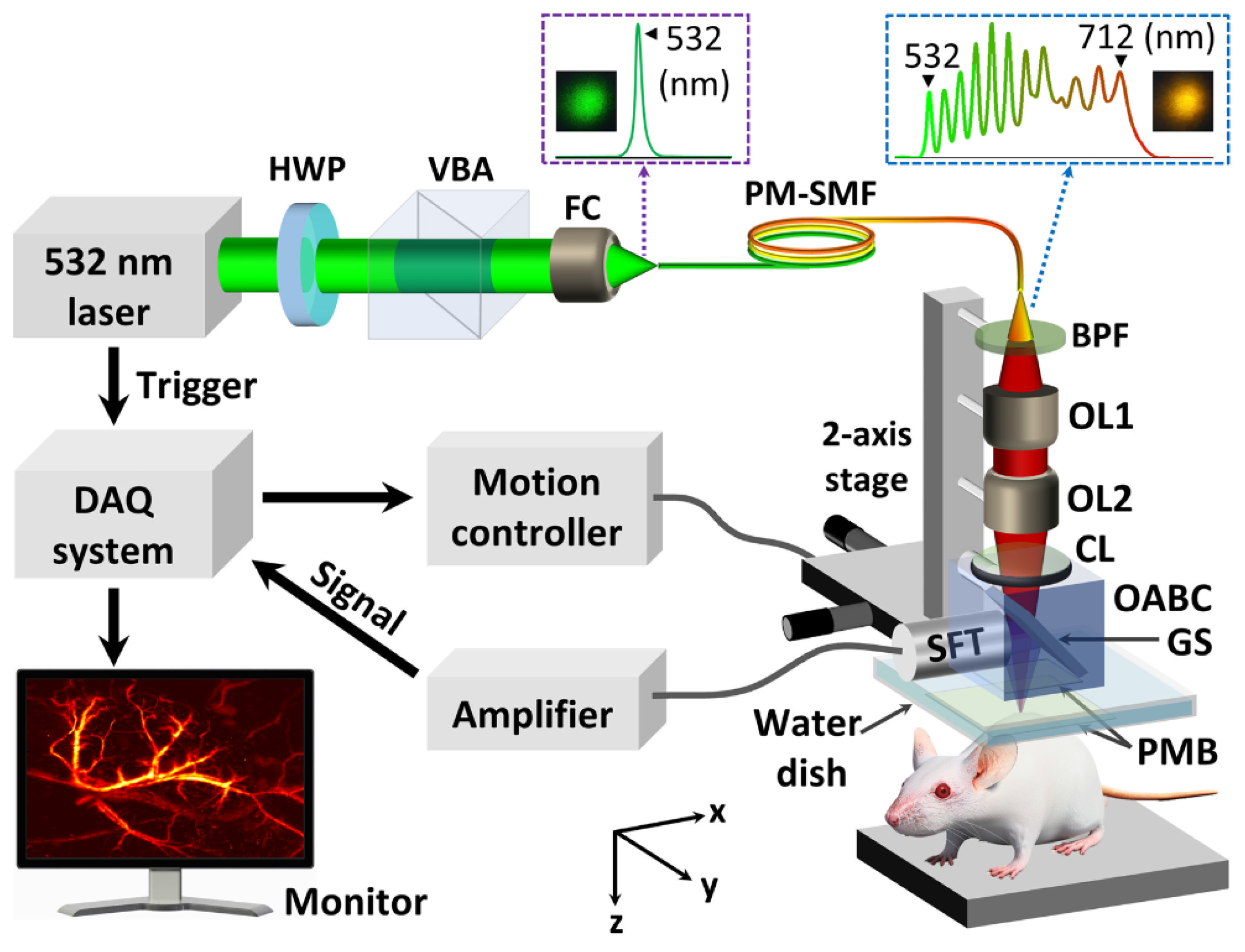
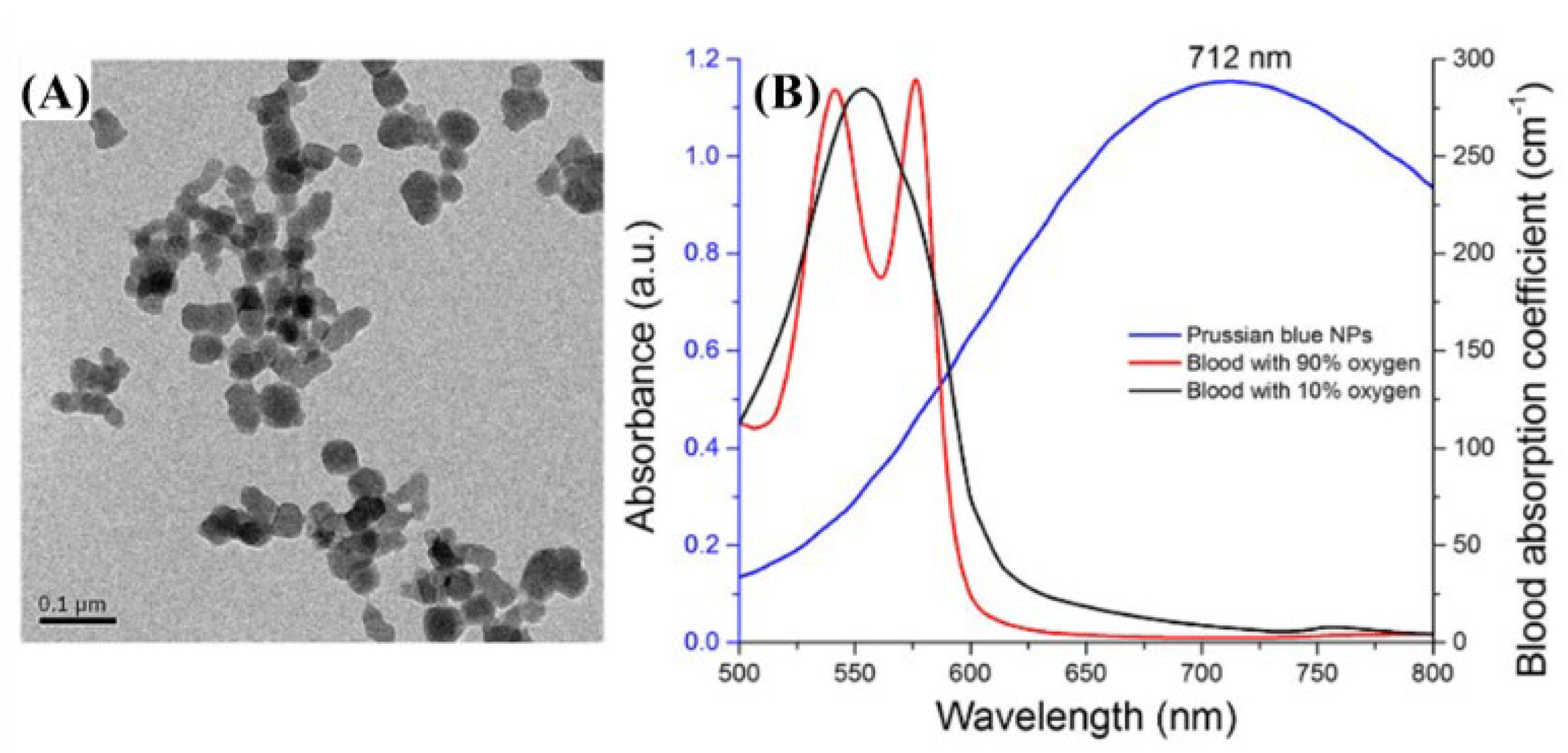


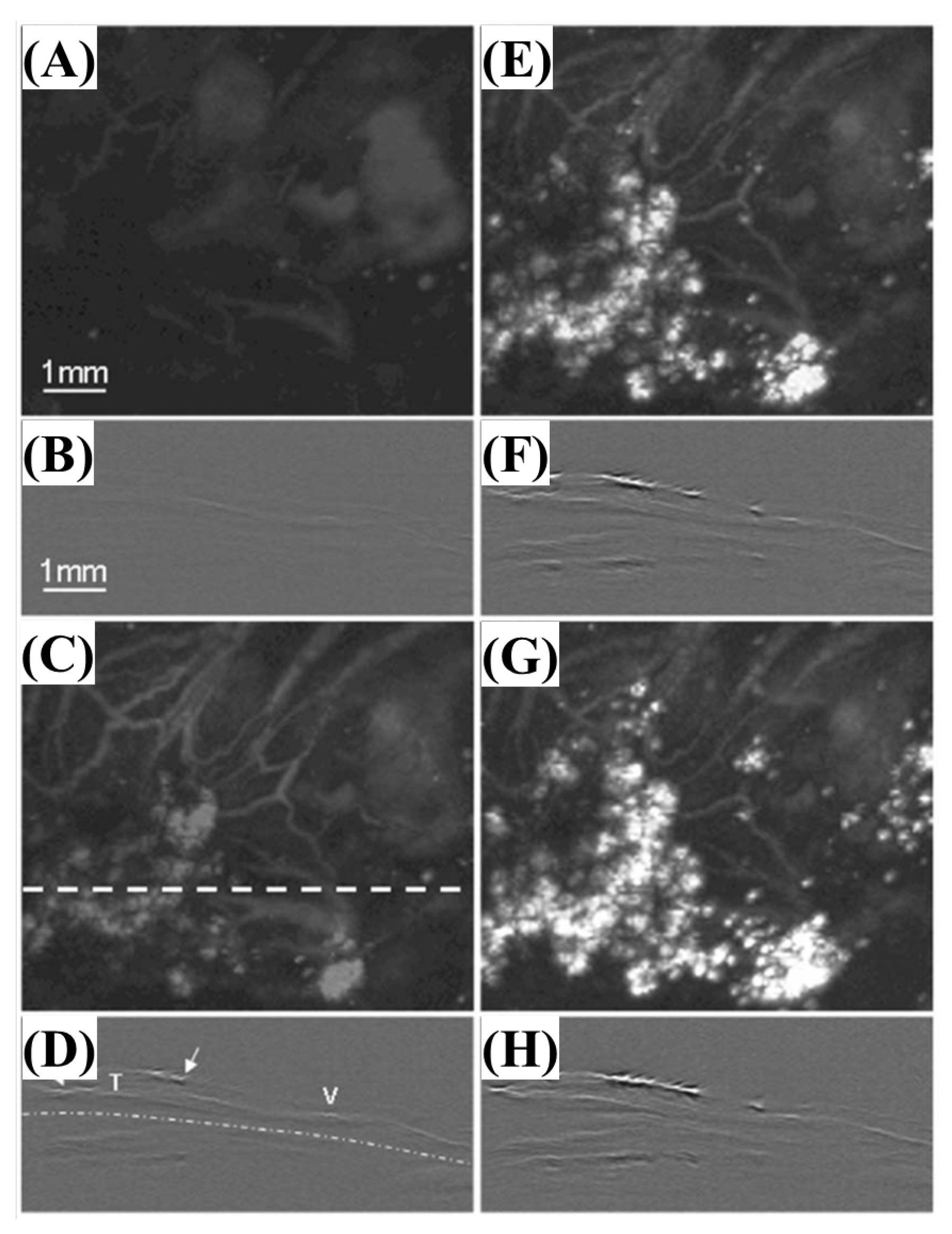





| Wavelength | Pulsed/CW | Model Type | Light Source | Pump Source | Duration | Repetition Rate | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| 450 nm | CW | Laser diode | CW | NO2 detection (3.5 W), CrIIITPP PA(300 mW) | [7,21] | |||
| 532 nm | Pulsed | 532 nm (Nano L 200-15, Litron) | Nd:YAG | 6–9 ns | 0–15 Hz | Blood flowmetry | [23] | |
| 532 nm | Pulsed | 532 nm (GKNQL-532, China) | Nd:YAG | - | 2 kHz | Tumor DC101 therapy monitoring | [22] | |
| 680 nm | Pulsed | 680–970 nm commercial | Nd:YAG | - | 4–6 ns | 20 Hz | PFH-NEs detection | [25] |
| 700 nm | Pulsed | 532 nm (SPOT-10-100-532, Elforlight) | Q-Switched Diode | SRS (stimulated Raman Scattering) | 1.8 ns | ~50 kHz | Prussian blue detection | [26] |
| 800 nm | Pulsed | 800 nm (LT-2211A, Lotis TII) | Ti:sapphire | Nd-YAG | 15 ns | 10 Hz | Polyethylene glycol (PEGylated) gold nanoshell (tumor target) | [27] |
| 820 nm | Pulsed | OPO (Surlite OPO Plus, Continuum, USA) | Nd:YAG | Surelite III, Continuum, USA | 6 | 10 Hz | Radiation-damaged nanodiamond detection | [28] |
| 905 nm | Pulsed | 905 ± 15 nm | Laser diode | 100 ns | 0.8 kHz | Blood vessel phantom | [31] | |
| 1064 nm | Pulsed | 1064 nm (Brilliant-B, Quantel, France) | Nd:YAG | Brilliant-B, Quantel, France | 5 ns | 10 Hz | Mammoscope (Breast cancer detection) | [20] |
| 1064 nm | Pulsed | 1064 nm, 53 2 nm (LS-2137, Symphotic Tii, USA) | Nd:YAG | 15 ns | 10 Hz | CuS NPs detection (tumors contrast agent) | [35] | |
| 1600 nm | Pulsed | 1600 nm (customizable) | EDF | Laser Diode | 12 ns | 100 kHz–1 MHz | Hair | [37] |
| 532 nm, 559 nm | Pulsed | 532 nm (BX40-2-G, Edgewave) 559 nm (BX40-2-GR, Edgewave) | 5 ns | 30 kHz | sO2, flow speed, vessels diameter | [39] | ||
| 532 nm, 600 nm | Pulsed | 532 nm (Teem Photonics, France) | Fiber-coupled wavelength broadening & bandpass filter selection | Leukocytes imaging (1–5 nJ/pulse, 30–160 mJ/cm2) | [38] | |||
| 560 nm, 570 nm, 600 nm | Pulsed | OPO (Surlite OPO Plus, Continuum, USA) | Nd:YAG | Frequency-tripled Nd:YAG Q-switched laser (Surlite II-10, Continuum, USA) | ~4 ns | 10 Hz | Imaging brain hemodynamic changes | [40] |
| 562 nm, 584 nm | Pulsed | Tunable dye laser (Cobra HRR, Sirah) | Solid-state, diode-pumped, neodymium-doped yttrium lithium fluoride laser (INNOSLAB IS811-E, Edgewave) | Internal organs in vivo | [41] | |||
| 532 nm(pulsed)473 nm(CW) | Pulsed CW | Variable repetition rate | Nd:YAG LD | 1064 nm second harmonic generation | 10 ns | 6.8 kHz | Chlorophylls, Anthocyanins 29.4 μJ (at 1064 nm) | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, U.; Ryu, J.; Choi, H. Optical Light Sources and Wavelengths within the Visible and Near-Infrared Range Using Photoacoustic Effects for Biomedical Applications. Biosensors 2022, 12, 1154. https://doi.org/10.3390/bios12121154
Jung U, Ryu J, Choi H. Optical Light Sources and Wavelengths within the Visible and Near-Infrared Range Using Photoacoustic Effects for Biomedical Applications. Biosensors. 2022; 12(12):1154. https://doi.org/10.3390/bios12121154
Chicago/Turabian StyleJung, Unsang, Jaemyung Ryu, and Hojong Choi. 2022. "Optical Light Sources and Wavelengths within the Visible and Near-Infrared Range Using Photoacoustic Effects for Biomedical Applications" Biosensors 12, no. 12: 1154. https://doi.org/10.3390/bios12121154





