Abstract
Klebsiella pneumoniae is an important human pathogen that causes diseases such as urinary tract infections, pneumonia, bloodstream infections, bacteremia, and sepsis. The rise of multidrug-resistant strains has severely limited the available treatments for K. pneumoniae infections. On the other hand, K. pneumoniae activity (and related infections) urgently requires improved management strategies. A growing number of medical applications are using nanotechnology, which uses materials with atomic or molecular dimensions, to diagnose, eliminate, or reduce the activity of different infections. In this review, we start with the traditional treatment and detection method for K. pneumoniae and then concentrate on selected studies (2015–2022) that investigated the application of nanoparticles separately and in combination with other techniques against K. pneumoniae.
1. Introductions
Infectious diseases such as pneumonia, diarrhea, tuberculosis, and malaria, are leading causes of death, responsible for 2.2 million, 1.8 million, 1.5 million, and 1.2 million deaths, respectively [1]. More than 95% of the deaths happen in low-income and middle-income countries [2]. The notable increase in drug resistance of infectious agents inhibits efficient therapy for diseases [3]. Klebsiella species are Gram-negative, nonmotile, encapsulated bacteria found in nature, including surface waters, soil, animals, medical devices, and healthcare environments [4]. They were first identified in the late nineteenth century and became known as Friedlander’s bacterium [5]. K. pneumoniae virulence factors play various roles in different K. pneumoniae infections. These virulence agents include fimbriae, siderophores, lipopolysaccharide (LPS), porins, outer membrane proteins (OMPs), iron transport systems, efflux pumps, and genes related to allantoin metabolism [6]. This bacterium can produce biofilms on different levels in the body to escape the host defense. In addition, due to the presence of a polysaccharide capsule that acts as an outer shell, it is significantly protected from phagocytosis by polymorphonuclear granulocytes [7]. In general, K. pneumoniae has long been known as an organism that causes serious primary infections in immunocompromised people. Still, the number of individuals at risk has increased due to the emergence of excessive virulence strains. Even healthy people and persons with adequate immunity are at risk of becoming infected with the bacterium [8]. Klebsiella pneumoniae as an opportunistic pathogen can be easily colonized in human mucous membranes such as the oropharynx and gastrointestinal tract; this colonization appears to be benign [4]. Nevertheless, if this bacterium spreads from the mucosa to other tissues in the body, it can lead to serious and threatening infections such as pneumonia, sepsis, and urinary tract infections (UTIs) [9]. Klebsiella infections cause serious problems in immunocompromised patients, infants, and the elderly. This organism is also one of the bacteria that cause nosocomial infections and community-acquired infections [10]. K. pneumoniae can be divided into hospital-acquired pneumonias (HAPs) and community-acquired pneumonias (CAPs). HAPs are much more common than CAPs, and the underlying cause of 11.8% of HAPs is K. pneumoniae [11]. One of the most serious and dangerous consequences of K. pneumoniae pneumonia and urinary tract infections is bacteremia caused by bacteria entering the bloodstream. K. pneumoniae is the second most deadly bacteria among Gram-negative bacteria as a cause of both community- and hospital-based bacteremia [12]. A K. pneumoniae pathotype known as hypervirulent K. pneumoniae (hvKp) is spreading worldwide. Unlike infections caused by the classical K. pneumoniae (cKp), hvKp causes invasive tissue infections in healthy people in the community and often involves multiple sites [13]. In recent years, the resistance to a wide range of antibiotics of K. pneumoniae has increased significantly. As a result of this increase in antibiotic resistance, infections such as pneumonia and bacteremia are increasingly threatening human health. Simple infections such as urinary tract infections often do not respond well to treatment [14,15]. The emergence of multidrug-resistant (MDR) bacterial strains is a major threat to patients because, with the spread of this type of antibiotic resistance, most existing treatments fail [16,17]. Several mechanisms of resistance to antibiotic agents, including the product of ion extended-spectrum β-lactamases (ESBLs) and carbapenemases such as Klebsiella pneumoniae carbapenemases (KPC), over-activation of efflux pumps, and porin modification, have been reported in K. pneumoniae. This is important as the bacterium becomes resistant to all β-lactams, including carbapenems [18]. The emergence of KPC-producing strains has become a significant medical problem. β-lactamases can hydrolyze carbapenem and make this bacterium resistant to a wide range of β-lactam antibiotics. Therefore, treating infections caused by this pathogen has become a considerable challenge [19]. KPCs are frequent in K. pneumoniae but may also be seen in other Enterobacteriaceae types, including Salmonella enterica, Enterobacter species, Escherichia coli, Citrobacter freundii, and Proteus mirabilis [20,21]. Because of the presence of KPC genes, for example, blaKPC-2, on the plasmid, has a high ability to spread [20,22]. Due to the lack of appropriate alternative therapies, K. pneumoniae infections created by ESBL-producing strains and carbapenem-resistant strains have a higher mortality rate than non-resistant bacteria [23]. The emergence of MDR K. pneumoniae strains, including colistin-resistant strains, has caused great concern. This type of resistance occurs due to mutations in the mgrB gene, which is stably conserved in Klebsiella populations and by the plasmids carrying mcr-1 and mcr-2 genes [24]. Furthermore, an extensive drug-resistant (XDR) cKp strain, which obtained a segment of a virulence plasmid carrying hvKp, led to a lethal nosocomial outbreak [25]. Today, the search for alternative therapies for antibiotic-resistant K. pneumoniae and other bacteria is vital and essential worldwide [26].
Due to rising antibiotic resistance, K. pneumoniae infection has become a significant health hazard, restricting effective treatments. Therapies reprogramming lung defenses and improving immune response to clear bacteria may help prevent K. pneumoniae infection [27]. Accordingly, in recent years, there has been increased demand for new strategies, pharmaceuticals, and devices to diagnose and treat diseases precisely and competently [28,29]. Many types of nanoparticles, such as graphene, polymers, vesicles, and green synthesized NPs, have been developed as drug delivery systems in cancer and infectious diseases [30,31,32]. The ability of nanomaterial-based therapeutics and diagnostics to overcome established processes linked to acquired drug resistance makes them intriguing tools for treating difficult-to-treat bacterial infections. Additionally, nanoparticles’ distinctive size and physical characteristics enable them to target biofilms and treat resistant illnesses [33]. Many kinds of nanomaterials have been considered to control infectious diseases effectively [34,35,36]. Nanomedicine has recently been used to increase immune responses to antigens for efficient vaccination, targeted delivery and sustained release of medications, and rapid and reliable disease detection and diagnosis at low cost [37,38].
In this review, we will discuss the structure and health problems of K. pneumoniae and treatment options and diagnostic techniques for K. pneumoniae–induced infectious diseases [39].
2. Traditional Detection and Treatment Methods
Clinical criteria for the identification of hvKp strains and related infections are often problematic; because the clinical definition for hvKp strains, which includes the occurrence of community-acquired infection and tissue invasion in a healthy host, prevents the detection of hvKp infection in immunocompromised patients or individuals that stay in a health care center, it is difficult to identify strains that lack clinical information [13]. For this reason, detection of iucA, iroB, peg-344, and prmpA2 genes and string testing are done to identify hvKp strains [13]. Because the delay of appropriate treatment for Klebsiella spp., infections is associated with adverse prognosis and increased mortality, rapid diagnosis is essential to manage these infections [40]. Many molecular methods are used for the detection of antibiotic-resistant K. pneumoniae, such as multiplex PCR assay [41], DNA microarray [42], Real-time PCR assay [43], single-colony whole-genome sequencing [44], Raman spectroscopic analysis [45], loop-mediated isothermal amplification (LAMP) [46], matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS) [47], and chromogenic media [48]. The gold standard for confirming the presence of KPC is the detection of hydrolysis of a carbapenem by spectrophotometry testing and detection of blaKPC by the PCR technique [49]. There are several phenotypic tests to identify carbapenem-resistant K. pneumoniae strains. These methods, which have also been approved by the Clinical & Laboratory Standards Institute (CLSI), are the CarbaNP test, mCIM, and eCIM; these have good sensitivity and specificity for detecting different carbapenemase enzymes [50]. Another phenotypic method is boronic acid-based compounds used as reversible inhibitors of class C β-lactamases [51]. Table 1 summarizes the conventional detection methods of K. pneumoniae and compares their properties.

Table 1.
Conventional detection methods of K. pneumoniae.
Routine antibiotic treatments used for the resistant Klebsiella spp. show different percentages of effectiveness. In a study reviewing the reports, the significance of each of the antibiotics was stated as follows: polymyxin (14%), carbapenem (40%), tigecycline (71%), polymyxin combinations (73%), and aminoglycosides (75%) [49]. To overcome bacterial resistance and maximize bacterial killing, combination therapies are sometimes suggested, which include a combination of colistin, tigecycline, and meropenem, which can be effective against KPC-producing isolates and colistin-producing strains [57]. New β-lactamase inhibitors have been developed to resist hydrolysis by class A carbapenemases and ESBLs, including LK-157, NXL104, and BLI-489 [58]. Today, various methods are being developed to treat drug-resistant K. pneumoniae infections, including probiotics, phytochemicals, metal salts, and bacteriophage therapy [59]. One of the treatment methods considered an alternative to antibiotic treatments is bacteriophages. An animal model study has shown that bacteriophages provide reasonable protection against respiratory infections and other infections caused by K. pneumoniae, such as bacteremia and liver abscesses [60]. Probiotic refers to living microorganisms that benefit the host if present in sufficient quantities. Studies have shown that the administration of probiotics has been associated with a reduction in pneumonia incidence, a decrease in the duration of cold infection, and protection against respiratory pathogens [61]. Several studies have examined the effect of probiotic Bifidobacterium longum 51A and lactic acid bacteria (LAB) on K. pneumoniae [62]. Despite this, there are still many challenges in treating antibiotic-resistant K. pneumoniae infections, and research is ongoing to investigate other factors and treatment approaches [63,64].
3. Nanotechnology-Assisted Approaches for Effective Detection of K. pneumoniae
3.1. Nanoparticle-Assisted Multiple Cross-Displacement Amplification
Numerous isothermal amplifying approaches using nanostructures to amplify nucleic acids in water baths or a simple heating block have been widely reported. The multiple cross displacement amplification (MCDA) test, developed by Wang et al., is a quick and easy method for amplifying nucleic acids at a fixed temperature in under 40 min [65]. For example, the detection of K. pneumoniae by label-free MCDA coupled with nanoparticle-based biosensors was developed by Wang et al. [66]. The MCDA reaction was carried out for only 30 min at a constant temperature (65 °C), and the amplification findings were directly presented utilizing a lateral flow biosensor (LFB). The results demonstrated that reaction products might be detected in clinical samples with as few as 100 fg and 4.8 CFU of pure K. pneumoniae templates and as few as 480 CFU in 1 mL of spiked clinical specimens. All K. pneumoniae strains tested were positive for label-free MCDA-LFB analysis, while all non–K. pneumoniae strains tested were negative for label-free MCDA-LFB analysis, showing the label-free MCDA-LFB assay’s excellent specificity. The label-free MCDA-LFB test was further enhanced with Antarctic heat-sensitive uracil-DNA-glycosylase (AUDG) to reduce carryover contamination and remove misleading data. As a result, the label-free MCDA-LFB assay combined with the AUDG enzyme proved to be a quick, easy, sensitive, and reliable method for detecting the target pathogen. It also could efficiently avoid carryover contamination, making it a valuable tool for clinical diagnosis, “on-site” identification, and primary quarantine [66].
A similar study reported that an MCDA assay for identifying K. pneumoniae could produce positive results after 40 min of isothermal amplification [67]. For the quick readouts of MCDA amplification, colorimetric indicators and an Au NP LFB were used. The LOD of this assay was 100 fg per reaction at 65 °C, which was validated by real-time turbidimeters to be the best amplification temperature. For the MCDA assay’s specificity, all 30 clinical-source K. pneumoniae strains were positive, whereas all non–K. pneumoniae strains from 31 different species were negative. The approach’s practicability was used to identify K. pneumoniae strains in sputum samples (24 CFU per reaction) and DNA templates from 100 sputum samples using the MCDA-LFB technique. The MCDA test correctly recognized all of the sputum samples that were positive for K. pneumoniae (30/100) utilizing the culture technique, and its identification sensitivity was greater than that of the polymerase chain reaction (PCR) (25/100). As a result, the MCDA test for K. pneumoniae and the gold nanoparticle LFB is a straightforward, specific, and accurate approach for detecting K. pneumoniae in clinical specimens [67].
In addition, in another study, using MCDA and Au NPs LFB, a simple approach for detecting Pseudomonas aeruginosa (P. aeruginosa) was developed [68]. This approach performed the reaction in 40 min at an optimal constant temperature (67 °C). An LFB could visually detect the reaction product, obviating the need for specialized equipment. The P. aeruginosa-MCDA-LFB technique was highly specialized for P. aeruginosa and accurately separated it from other infections. It was possible to identify only 10 fg of the genomic DNA template (from pure culture). The assay had the same specificity and sensitivity as the reference (culture-biochemical) approach for detecting P. aeruginosa in clinical sputum samples [68].
To discover the mcr−1 gene, Gong et al. employed a similar approach and developed an MCDA linked with the detection of amplified products using an Au NP LFB system [69]. The MCDA-LFB test was done at an isothermal temperature (63 °C) for only 30 min during the amplification phase. The reaction products were directly recognized using LFB, which produced results in less than 2 min. The whole test process took about 60 min, from template extraction to the results from evaluation. All of the 16 mcr-1-producing strains were positive, while all of the non-mcr-1 isolates yielded negative findings, demonstrating the analytical sensitivity of this approach. In pure culture, the specificity of the mcr-1-MCDA-LFB test was as low as 600 fg of plasmid DNA per response, while in spiked fecal samples, it was around 4.5 × 103 CFU/mL [69].
Because of its low cost, good effectiveness, and real-time identification, the nanopore test has recently been employed for screening biomarkers of diseases [70]. For example, Niu et al. separated CRKP from carbapenem-sensitive K. pneumoniae (CSKP) by detecting increased amounts of extracted 16S ribosomal RNA (16S rRNA) from bacterial culture using imipenem, showing that CRKP’s growth was unaffected by the antibiotic [70]. The quick and ultra-sensitive identification of 16S rRNA was enabled by specific signals from the single-channel recording of 16S rRNA bound with probes by MspA nanopores. This study demonstrated that only 4 h of CRKP growth time was required for the nanopore analysis to discriminate between the two proteins. The test takes about 5% of the time of the disk diffusion approach while achieving equivalent precision (See Figure 1) [70].
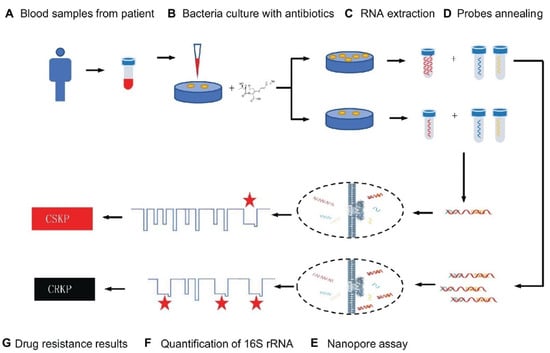
Figure 1.
Schematic representation for carbapenem-resistant K. pneumoniae nanopore assay adapted from [70], Frontiers, 2019.
3.2. Nanoparticle-Assisted Loop-Mediated Isothermal Amplification
There have been numerous investigations on the LAMP (loop-mediated isothermal amplification) assay for pneumoniae identification. However, this diagnostic system often produces false positive results due to a high rate of non-specific reactions caused by the formation of hairpin structures, self-dimers, and mismatched hybridization [71]. Recent advances have proven that nanomaterials can effectively inhibit undesired amplification and reduce non-specific signals effectively [72]. For example, a LAMP-coupled nanoparticle-based LFB assay (LAMP-LFB) was developed for the specific detection of pneumoniae (P-LAMP-LFB) [73]. The optimal temperature for this experiment was proven to be 65 °C, and six primers corresponding to the P1 gene of pneumoniae were prepared. Within 2 min, LFB was able to evaluate the amplified outputs accurately. The P-LAMP-LFB test recognized DNA templates of pneumoniae exclusively, with no cross-reactivity with other infections. The LOD of this approach in pure cultures was about 600 fg of the DNA templates, which was in perfect agreement with agarose gel electrophoresis assay and colorimetric indicator identification. This technique was compared to real-time PCR analysis on 209 oropharyngeal swab specimens obtained from children suffering respiratory tract infections. Positive rates of pneumoniae were 47.8% and 31.6%, respectively, using the LAMP-LFB and real-time PCR assays. Compared to the real-time PCR approach, the LAMP-LFB assay exhibited greater selectivity [73].
3.3. Optical Nanosensors
Colorimetric and fluorescence sensor arrays have generated great interest in recent years because of their capacity to differentiate a wide range of bacteria with a high recognition rate [74]. These biosensors rely on the optical characteristics of the sensor surface being altered by the bound analyte, and these changes are subsequently communicated to a detector [75,76,77]. The development of nanotechnology and the application of nanomaterials’ advantages on a biosensor platform allow for an improvement in the biosensors’ sensing parameters. Nanomaterials are employed to great advantage in nanosensors as a label-free detection approach because of their capacity to induce SPR [78,79]. In this light, a colorimetric nanosensor was developed for selectively identifying bacteria like K. pneumoniae. Four gold nanoparticles (AuNPs) with different surface charges were employed as sensing components. The interactions of AuNPs with microbes resulted in visible hue shifts that could be seen with bare eyes. A total of 15 bacteria exhibited distinct reactions that were effectively distinguished using linear discriminant analysis (LDA). Microorganism combinations may also be determined with ease. This approach is simple, quick (less than 5 s), efficient, and visual, indicating that it could be used for pathogen detection and environmental sensing (See Figure 2) [80].
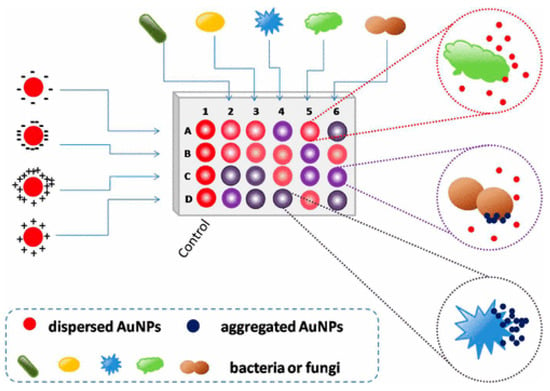
Figure 2.
Schematic representation of the colorimetric nanosensor with four types of coated AuNPs. The interactions of microorganisms and AuNPs result in color changes. In the diagram, column 1 represents the blank control, and other columns show various organisms. Rows A to D represent four coated AuNPs, adapted from [80], ACS, 2017.
Surface plasmon resonance (SPR), a label-free optical biosensor, has been used to detect a variety of compounds [81]. Additionally, surface-enhanced Raman scattering (SERS) boosts a Raman spectrum manifold’s amplitude and has been used in conjunction with other techniques to find bacterial cells in blood medium [81,82]. The nanophotonic interferometric biosensor is another cutting-edge biosensing device that offers a quick way to identify nosocomial pathogens for the diagnosis of illnesses [83]. For example, a recent study established a label-free approach for quickly detecting clinically relevant multilocus sequencing typing (MLST)-verified quinolone-resistant K. pneumoniae strains. This approach was also used to recognize three quinolone-resistant K. pneumoniae strains from colony samples, ST15, ST11, and ATCC70063 (control), which are the most common quinolone-resistant K. pneumoniae strains in East Asia. A multivariate statistical method combined with a drop-coating deposition surface-enhanced Raman scattering (DCD-SERS) approach was used to identify the colonies. The process had a correlation coefficient of 0.98 LOD of 100 pM rhodamine 6G, strong repeatability (relative standard deviation of 7.4%), and a Raman enhancement factor of 11.3 × 106. Compared to Escherichia coli (E. coli), all quinolone-resistant K. pneumoniae strains displayed similar spectral Raman shifts (high correlations) and distinct Raman vibrational modes. The suggested DCD-SERS strategy, in combination with a multivariate statistics-based identification method, performed exceptionally well in subtyping K. pneumoniae strains and distinguishing identical microorganisms. As a result, the label-free DCD-SERS approach combined with the computational decision-supporting method could be a valuable technique for accurately identifying clinically relevant K. pneumoniae strains [84].
3.4. Cantilevers-Based Nanosensors
As a result of their high responsivity and ease of integration, cantilevers are gaining increasing popularity as new-generation biosensors. Microcantilever-based nanosensors are highly appealing for biomedical purposes because of their fast and real-time analysis, ultrasensitive features, and label-free potential [85]. For example, a new array biosensor made up of a microcantilever and gold nanoparticle could detect ultralow quantities of pathogenic bacteria, such as Listeria monocytogenes, Shigella spp., Vibrio parahaemolyticus, Staphylococcus aureus, E. coli O157:H7, Salmonella spp., Listeria monocytogenes, Shigella spp., and many others [86]. The process was significantly quicker than traditional methods that need PCR amplification or germi-culturing. Six pairs of ssDNA probes (ssDNA1 + ssDNA2) were designed and verified based on sequence examination of the bacteria’s particular gene. The -S-(CH2)6 parts were attached at the 5′ end of ssDNA1 probes and immobilized as a self-assembled monolayer (SAM) on the surface of cantilevers and finally integrated with Au NPs. In addition, the 6-mercapto-1-hexanol SAM were attached to reference cantilevers to remove interferences from non-specific interactions (Figure 3). Microcantilever array sensors and Au NP platforms can detect quickly and precisely various bacteria with working ranges of 3 to 4 orders of magnitude and LOD of 1–9 cells/mL. There was negligible cross-reaction between the probing of various organisms. This has a promise for quick, combinatorial, and extremely sensitive identification for environmental, clinical, and food items [86].
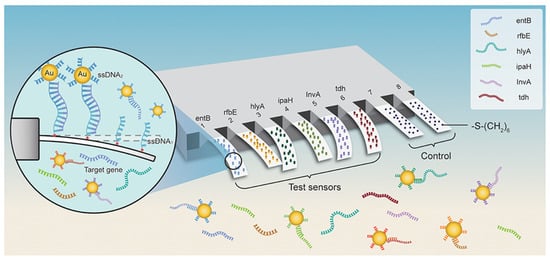
Figure 3.
Schematic representation for microcantilever array biosensor modified with gold nanoparticles for foodborne bacteria detection, adapted from [86], Frontiers, 2019.
3.5. Electrochemical Nanosensors
Electrochemical nanosensors have potential as a diagnostic tool for people and animals. These sensors can detect pathogens and biomarkers in bodily fluids such as urine and blood [87]. For example, the development of a label-free DNA biosensor for the identification of K. pneumoniae that can also diagnose other types of bacterial infections was reported by Zhang et al. [88]. In this light, on a glassy carbon electrode, graphene oxide (GO) and indole-5-carboxylic acid (ICA) were successfully deposited, and the resultant ICA/GO (rGO) hybrid film was used to immobilize oligonucleotides on an ssDNA sequence (single-stranded DNA). In optimized conditions, the electrochemical nanosensor showed outstanding performance. A considerable change was seen after hybridizing the target probe with ssDNA under optimal conditions. Differential pulse voltammetry was used to investigate hybridization with three-base mismatched, one-base mismatched, noncomplementary, and complementary DNA targets. With a linear range of 1 × 10−6 to 1 × 10−10 M, the suggested method could identify target DNA as low as 3 × 10−11 M, demonstrating great sensitivity of the nanosensor. The nanosensor showed fast analysis time, devoid of indicators, and had a high degree of specificity. As a result, the proposed nanoplatform can help diagnose K. pneumoniae and other pathogen-related infections [88].
Another work presented a glassy carbon electrode (GCE) enhanced with graphene and Au-NPs as a new electrochemical nanosensor for DNA identification. After that, electrochemical impedance spectroscopy, cyclic voltammetry (CV), and scanning electron microscopy (SEM) was used to analyze Au-NPs/Gr/GCE (EIS). In addition, differential pulse voltammetry (DPV) using methylene blue (MB) as the hybridization marker was performed to identify hybridization processes. The sensor’s LOD and dynamic range for the target DNA sequences were 2 × 10−13 mol/L and 1 × 10−12 to 1 × 10−7 mol/L, respectively. In the presence of mismatched and non-complementary DNA sequences, the DNA nanosensor demonstrated remarkable selectivity for recognizing complementary DNA sequences. The findings showed that the Au-NP/Gr nanostructure is a potential substrate for developing high-performance catalytic systems for KPC measurement [89].
3.6. Biomimetic Nanosensors
Nature is a source of inspiration for solving biological problems. Potentiometric, voltammetric, and impedance spectrum sensors are among the biomimetic nanosensors that mimic the behavior and operation of living organisms by being modified with nanomaterials and specially designed biomimetic materials [90]. A new biosensor was designed using graphene and two-photon polymerization to provide an improved biosensor for detecting motile bacteria. Around graphene-based sensing electronics, a cage with a directed micro-architecture was covered that was inspired by venous valves. The designed 3D-mucro architecture enables motile cells to move from the outside of the cage to the center area, leading to an accumulation of bacteria around the core sensing zone, which improves the received signal. The concentrating effect has been shown in cell cultures, ranging from 101 to 109 CFU/mL. Fluorescence evaluation indicated a signal enhancement of 3.38–3.5 fold. Identifying cellar metabolites improves the pH sensor by 2.14–3.08 fold. Electrical tests showed an increase in current of 8.8–26.7 fold. The suggested architecture enables the construction of smart biomedical sensors using bio-inspired 2D materials and 3D printing in a novel manner [91]. Table 2 summarizes the nano-based detection method of K. pneumoniae.

Table 2.
Nano-based detection method of K. pneumoniae.
4. The Application of Nanomaterials for Treatment of Klebsiella pneumoniae
Antimicrobial resistance (AMR) threatens human well-being globally. The mortality rate due to AMR is expanding and is anticipated to reach 50 million by 2050 [96]. Microorganisms such as Klebsiella spp. have increased resistance to accessible antimicrobial specialists. Currently, there is a pressing need for alternative medicine to address the AMR risk [97]. One of these is nanotechnology-based pharmaceuticals [98]. Nanoscience allows us to synthesize a variety of nanoparticles (NPs) from bulk materials [99,100]. Utilizing nanoparticles to transport drugs, heat, light, or other chemicals to particular types of cells is currently under development [101,102]. The use of nanomaterial-based therapeutics, which have the ability to circumvent current defenses linked to acquired drug resistance, is a potential strategy for treating bacterial infections that are challenging to cure. Additionally, nanomaterials’ distinct dimensions and physical characteristics enable them to target biofilms and treat resistant illnesses [103]. Ag nanoparticles (AgNPs) have a large surface area and small size, and they are one of the most effective antimicrobials [104]. This encourages contact between them and their targets, such as bacterial cells [105]. Tsung-Ying Yang et al. fabricated antibacterial silver nanoparticles (Ag NPs) that were restricted to a mesostructured material and used as an Ag/80S bioactive nanocomposite against K. pneumoniae. Ag/80S had a 7.5 nm mesoporous size and 307.6 m/g surface area, according to textural analysis. The Ag/80S UV–Vis spectrum and TEM pictures exhibited a homogeneous Ag NP size distribution with a mean size of ~3.5 nm. The minimum inhibitory concentration (MIC) for KP isolates was 0.25 to 0.5% (2.5 to 5.0 mg/mL). The inspired Ag/80S adhered to and distorted bacterial cells, causing a time-dependent amassing of reactive oxygen species (ROS), which resulted in bacterial death, according to the results of the mechanistic investigation [106]. In a separate investigation, Aghigh Dolatabadi and colleagues examined the inhibitory effects of commercial and green Ag NPs on OxqAB efflux pump genes in ciprofloxacin-resistant K. pneumoniae strains. The effectiveness of the synthetic nanoparticles was determined by comparing the activity of the antiefflux pump with that of commercial Ag NPs. The oxqAB gene expression levels were lowered in ciprofloxacin-resistant isolates in the sub-minimum inhibitory concentration (MIC) of both Ag NPs, suggesting that efflux pumps could be a promising target for biosynthesized AgNPs. The expression of the oxqAB gene was lowered in both Ag NPs’ subMIC, although biosynthesized Ag NPs showed better bactericidal effects than commercial Ag NPs [107]. In addition, Sanjay Chhibber et al. investigated the therapeutic efficiency of histidine-capped silver nanoparticles delivered through microemulsions in the treatment of K. pneumoniae–induced infection. Thixotropy, texturing, differential scanning calorimetry, and release kinetics were used to construct and analyze the emulgel. The treatment’s effectiveness was assessed using bacterial load, histology, tissue repair, and other infectious indicators. When compared to untreated animals, the developed emulgel showed a remarkable in vivo antimicrobial effect of the histidine-capped silver nanoparticle preparations when applied topically, associated with decreased microbial infection, tissue regeneration, and improved skin healing, as well as reduced systemic inflammation like malondialdehyde, myeloperoxidase, and reactive oxygen and nitrogen intermediates [108].
Researchers in earlier trials combined two therapeutic approaches to treat bacterium-induced infectious disease. For example, Mai I. El-kaliuoby and colleagues investigated the synergistic effects of combining exposure to a low frequency pulsed magnetic field (LF-PMF) with the administration of AgNPs. KP bacterium, which is Gram-negative, was tested under varied AgNP concentrations and contacted LF-PMF at various frequencies. The optimal synergistic impact is obtained by achieving the highest inhibitory concentration of AgNPs and the lowest ELF-PMF resonance frequency that inhibits bacterial growth the most. Exposure to 20 Hz PMF for 30 min with a supplement of 150 ppm Ag NPs resulted in a highly synergistic impact, with a 90 percent increase in growth inhibition, according to growth kinetics [109]. In a separate study, Kate Kotlhao and colleagues used various chemical procedures to produce three different types of nanoparticles, silver (Ag), zinc oxide (ZnO), and titanium dioxide (TiO2), and compared their antibacterial efficacy. The nanoparticles were in the nanoscale range, according to TEM (1100 nm). The various absorption bands of the fabricated nanoparticles were revealed using FTIR and UV-Vis spectroscopy, respectively. Ag NPs displayed an antibacterial effect more than TiO2 and ZnO NPs against a variety of bacteria. K. pneumoniae showed the greatest inhibition. The findings shown that the antibacterial effect of NPs increases as the concentration of NPs increases [110].
Ciobanu et al. fabricated Ag-doped crystalline hydroxyapatite NPs (Ag:HAp-NPs) (Ca-xAgx(PO4)6(OH)2, xAg = 0.3, 0.2, and 0.05) via a coprecipitation method and investigated their antibacterial capabilities. The antibacterial effect of Ag:HAp NPs against Citrobacter freundii, Providencia stuartii, S. aureus, K. pneumoniae, and Serratia marcescens was studied. The results revealed that S. aureus, K. pneumoniae, P. stuartii, and C. freundii had the highest antimicrobial effect, regardless of sample type. This illustrated the antibacterial activity of different values of xAg on K. pneumoniae. The antibacterial effect of samples with xAg = 0.2 and 0.3 was unaffected by the Ag:Hap NPs concentration, whereas samples with xAg = 0.05 showed little antibacterial effect up to 31.25 g/mL Ag:Hap NPs concentration (Figure 4) [111].
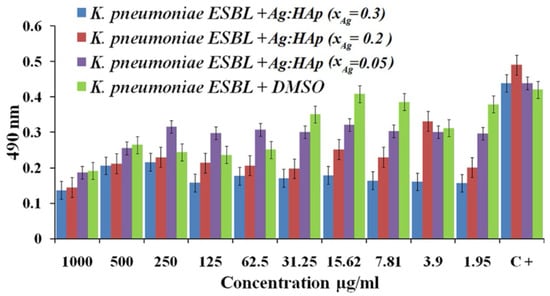
Figure 4.
Antibacterial effect of Ag:Hap-NPs (xAg = 0.3, 0.2, and 0.05) on KP, adapted from [111], Springer, 2012.
Syed Zeeshan Haider Naqvi and colleagues studied the synergistic antibacterial effect of five conventional antibiotics and Ag NPs in order to determine an effective therapy for K. pneumoniae using the Kirby–Bauer disk-diffusion technique. They tested eight distinct multidrug-resistant bacterial species (K. pneumoniae is among them). According to the findings, the antibiotics and nanoparticles worked together to improve the antibacterial effect by 2.8-fold, demonstrating that NPs can be employed in conjunction with antibiotics to enhance their effectiveness against numerous pathogenic germs (Figure 5) [112].
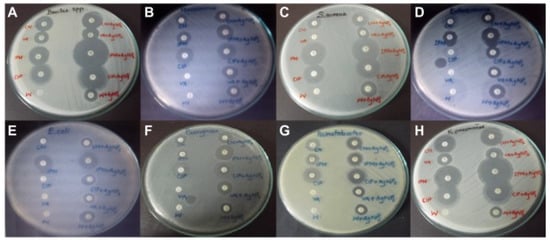
Figure 5.
Zones of inhibition of multidrug-resistant bacteria by conventional antibiotics and AgNPs. (A) Bacillus; (B) Micrococcus luteus; (C) Staphylococcus aureus; (D) Enterococcus faecalis; (E) E. coli; (F) Pseudomonas aeruginosa; (G) Acinetobacter baumannii; (H) K. pneumoniae. Adapted from [112], Dove Medical Press Ltd., Macclesfield, United Kingdom, 2013.
Au NPs are an antibacterial nanoparticle that has been used as a treatment agent for K. pneumoniae in recent decades. Ayaz Ahmed and colleagues tested the antibiofilm efficiency of gold nanoparticles linked with chlorhexidine (Au CHX) towards K. pneumoniae. MTT assay was used to determine biofilm inhibition and eradication, and florescence and AFM microscopy were used to confirm these findings. A surface plasmon resonance (SPR) band maxima at 535 nm, spherical shape, and polydispersity with sizes ranging from 20 to 100 nm were observed for Au-CHX nanoparticles. The biofilm construction and metabolic effect inside biofilms of KP reference and three tested clinical isolates were fully suppressed at micro molar concentrations (i.e., 25 and 100 M) of Au-CHX, respectively. The concentrations of 70 and 100 µM eradicated the K. pneumoniae established biofilms [113].
Antimicrobial substances such as ZnO and ZnO NPs are becoming more widely used as antibacterial agents. Jehad M. Yousef et al. investigated the minimum inhibitory concentration (MIC) or minimum bactericidal concentration (MBC) of ZnO NPs against a variety of pathogens, including K. pneumoniae. According to the findings, nano-Zno has an excellent bacteriostatic impact but a weak bactericidal activity against the whole pathogens studied. ZnO NPs could be a viable antibacterial agent due to the inexpensive cost of manufacture and great antimicrobial effectiveness and could be used in a variety of industries to address safety concerns [114].
Doping of some ions in the ZnO NPs can improve their antibacterial activity. Accordingly, Hameed et al. used the coprecipitation approach to fabricate pure ZnO and Nd-ZnO NPs with nanorod and nanoflower shapes, respectively. Antibacterial experiments on E. coli and K. pneumoniae ESBLs revealed that the Nd-ZnO NPs had a higher antibacterial activity than the pure ZnO NPs [115].
Antibacterial properties of titanium oxide (TiO2) NPs have been investigated against K. pneumoniae. Many studies have used TiO2 nanoparticles. Venkatasubbu et al. reported TiO2 and ZnO’s ability to preserve food. A wet chemical process was used to synthesis TiO2 and ZnO NPs. Both materials’ antibacterial activity was examined to ensure that they were effective as food preservatives toward Salmonella typhi, Shigella flexneri, and K. pneumoniae. The findings shows that ZnO and TiO2 NPs hinder growth of Salmonella, Klebsiella and Shigella spp. [116]. B.K. Thakur and colleagues published a paper in which they described a method for synthesizing TiO2 NPs using Azadirachta indica leaves. E. coli, B. subtilis, S. typhi, and K. pneumoniae were used to study the antibacterial efficacy of the produced ZnO and TiO2 NPs compound. The findings of this investigation show that TiO2 NPs prevented the full growth of the examined bacteria. The lowermost MBC value, i.e., 83.3 μg/mL was detected toward K. pneumoniae [117]. One-dimensional CuO/TiO2 nanofibers with excellent antibacterial effect were synthesized and studied by Ayman Yousef and colleagues. The antibacterial effect was determined by calculating the MIC against K. pneumoniae as a model organism, and the manufactured NPs exhibited high antibacterial activity against K. pneumoniae based on MIC measurements compared to when they were utilized separately [118].
Cadmium nanoparticles are among the most promising and new treatment agents for infectious diseases. Ashwani Kumar and coworkers tested the antibacterial susceptibility of CdS NPs (pure and 1% Cu doped) toward bacteria S. aureus, Salmonella typhimurium, P. aeruginosa, E. coli, and K. pneumoniae. Using the well diffusion technique, the antimicrobial effect of the synthesized NPs was investigated by measuring the zone of inhibition in the concentration range of 1 mg/mL to 100 mg/mL of CdS NPs. The MBC and MIC were determined after the initial antibacterial qualitative test. Cu-doped CdS NPs were more effective than pure CdS nanoparticles, with MICs ranging from 0.078 to 0.52 mg/mL, as compared to pure CdS NPs, which had MICs ranging from 0.15 to 0.83 mg/mL [119].
In recent years, silica (SiO2) NPs have shown antibacterial properties. Na Xu and colleagues synthesized and then characterized SiO2 NPs by TEM and DLS techniques. They then tested their antibacterial effect toward K. pneumoniae using the minimum inhibitory concentration (MIC) procedure. The findings exhibited the influence of NP size on antibacterial ability; when compared to Sulfamethoxazole as control, the produced NPs with a size of 40 nm demonstrated a good inhibitory efficacy against K. pneumoniae [120].
Rahman et al. described the influence of flexible and multimodal hybrid membranes (BC-SiO2-TiO2/Ag) that contain bacterial cellulose. This nanoplatform had Ag and TiO2 that awarded antibacterial properties, UV guarding, and photocatalytic and self-cleaning characteristics. The BC-SiO2-TiO2/Ag membranes showed good photoactivity and significant antibacterial efficacy towards five different bacterial strains [121].
Bushra Al Edhari and colleagues studied the synergistic effect of Ag NPs, Ni NPs, and Al2O3 NPs against all K. pneumoniae strains separately and in combination. Ag/Ni NPs and Ag/Al2O3 NPs exhibited much more antibacterial and antibiofilm activity against K. pneumoniae [122]. Table 3 gives a set of nanostructures that have been used to treat K. pneumoniae–related infections.
The problem of hospital-acquired infection is now compounded by the rise of antimicrobial resistance to antibiotics. Hospitals are loci of the intensive deployment of antibiotics. This, in turn, imposes intense selection pressure on the microorganisms, which generally have the capacity to deploy or evolve resistance mechanisms. One of practical techniques to combat hospital-acquired infections is photocatalytic nanomaterials. Photocatalytic antimicrobial coatings, especially those based on titanium dioxide, which is the most extensively investigated material, appear to be capable of keeping the environmental microbial burden of hospital surfaces close to zero [123,124]. Reid et al. sprayed titanium dioxide–based photo-catalytic coating onto six surfaces in a four-bed bay in a ward and compared them under normal lighting to the same surfaces in an untreated ward in order to assess the impact of a photocatalytic antimicrobial coating at close-to-patient, high-touch sites. These surfaces included right and left bed rails, bed controls, bedside lockers, overbed tables, and bed footboards. Significantly lower levels of bacteria were present on treated surfaces than on control sites, and this difference between treated and untreated surfaces grew over the course of the investigation [124].

Table 3.
Nanostructures that have been used to treat K. pneumoniae–related infectious.
Table 3.
Nanostructures that have been used to treat K. pneumoniae–related infectious.
| Substrates | NP Size (nm) | Key Features | Ref. |
|---|---|---|---|
| Ag-rifampicin | 15–18 | Greater antimicrobial effect than free drug | [125] |
| Ag | 29–50 | High antibacterial effect | [126] |
| Au-imipenem | 12–27 | Strong synergistic antibacterial effect | [127] |
| Ag | 34–90 | Low minimal inhibitory concentration | [128,129] |
| Ag | 3 | 100% bactericidal effect at 0.05 g/mL | [130] |
| TiO2 and Ag | 20 and 90 | Antibiotics and nanoparticles are combined; they have a synergistic impact | [131] |
| TiO2 | - | High antibacterial activity | [132] |
| TiO2 | 50 and 100 | Good antibacterial nature | [133] |
| TiO2 + L. lactis | - | Reliable and operative inorganic antimicrobial agents | [134] |
| CML@Ag-NPs and CML@Au-NPs | 40–60 | Obtained MIC values for Ag and Au NPs were 0.5 and 370 ppm | [135] |
| Ag/AgCl-imipenem (IPM) | 55-89 | Synergetic effect between the IPM antibiotic and Ag/AgCl NPs | [136] |
| ZnO | 6–18 | The antibacterial effectiveness of ZnO NPs-E was 40 g/mL, which was higher than previously reported values | [137] |
| ZnO | 94 | Low MIC and MBC in comparison with those obtained for imipenem and meropenem antibiotics | [138] |
| ZnO | 11–25 | During a lunar eclipse, ZnO NPs have a better antimicrobial activity than on a regular day | [139] |
| Cy-da/ZnO | 29–35 | The highest antibacterial activity for Cy-da/ ZnO NPs in comparison with ZnO NPs | [140] |
| ZnO | 45–50 | Green synthesis with proper size | [141] |
| ZnO | 88 | At dosages of 0.50 to 0.75 mM, the NPs-treated K. pneumoniae was five times less infectious | [142] |
| ZnO/bovine serum albumin(BSA) | 11 | BSA improved antibacterial effect of ZnO NPs | [143] |
| ZnO/Zeolite | - | In sublethal levels, it has strong antibiofilm efficacy against K. pneumoniae | [144] |
| Ag/ZnO | 143 and 154 | The toxicity of nanoparticles strongly depend on surface charge effect | [145] |
| ZnO | 90–110 | Eco-friendly and simple method for synthesis of ZnO NPs | [146] |
| Fe/Co-ZnO | - | 6% Fe and 4% doped ZnO show the maximum antibacterial effect | [147] |
| Carbon cloth/Ag/ZnO | - | Cloth carbons improved antibacterial activity of metal or metal oxide NPs | [148] |
| Ga-ZnO | - | The bioactivity of undoped and Ga-ZnO nanocrystals is significantly improved | [149] |
| Ag@SiO2 | 118 | Antibacterial activity greater than than Ag and SiO2 NPs separately | [150] |
| Mn-TiO2 | 150 | 100% reduction of Klebsiella pneumoniae within 120 min under sunlight | [151] |
| Ag-TiO2 | 163 | Superior antimicrobial activities to Ag and TiO2 | [152] |
| N-TiO2, C-TiO2, N-T-TiO2, and Pd-C-TiO2 | 5–60 | Under visible-light irradiation, Pd-C-TiO2 had the maximum capacity for bacterial inactivation | [153] |
| Au-Ag | 12–67 | The synthesis procedure is environmentally benign because it does not use any solvents or harmful chemicals | [154] |
| Au | 5.5–10 | Remarkable bactericidal activities against polymyxin-resistant Klebsiella pneumoniae, which are superior to clinical antibiotics | [155] |
| Fe3O4 | 24 | Good additional antimicrobial | [156] |
5. Conclusions, Challenges and Future Perspective
K. pneumoniae has been described as an immediate threat to human health. The grade of pathogenicity and virulence of K. pneumoniae and the emergence of new MDR strains has led scientists to seek new antibacterial compounds. Alternative methods include the use of multiple antibiotics, phage therapy, probiotics, and phytochemicals; however, each has its limitations. The delivery of antibacterial agents, the clinical acceptance of several nanotechnology-based devices for the diagnosis of pathogen infection, and the advancements in medical equipment with antimicrobial coatings have highlighted the promising effect of nanoscience on microbial infectious diseases. Nanostructures with specific physicochemical characteristics have allowed for great specificity and sensitivity in the detection of K. pneumoniae, as well as quick readout. Furthermore, covering medical equipment with antimicrobial nanoparticles, particularly Ag, has significantly decreased device-related bacterial infection and biofilm growth, as well as improved wound repair.
While nanomedicine is promoting a range of developments in the antimicrobial area, practical design of antimicrobial nanoscience still faces significant obstacles. The fast appearance of novel nanomaterials that have been clinically disproved will need a more thorough evaluation of their long-term safety and biocompatibility. The design and marketing of sophisticated nanostructured materials (e.g., targeted multimodal nanostructures) is hampered by the inability to mass produce them with low batch-to-batch variance. Novel monitoring approaches are also critical for fast in vitro and in vivo testing of nanoparticles and their biophysicochemical properties, such as composition, size, structure, zeta potential, targeting ligand, and surface area. Additionally, other properties such as loading performance, stability, matrices, release kinetics, immune response, pharmacokinetics, and biodistribution can be different for each approach. Moreover, designing more clinically useful animal studies, knowing the microenvironment of bacterial infection sites, recognizing pathways of new biomarkers and microbial pathogenesis, and lowering burdensome regulations can all help with the successful translation of antimicrobial nanodevices. We should anticipate many more nanotechnologies to be introduced into the clinic to address every element of microbial illness as antimicrobial nanomedicine advances.
Despite these remarkable advances, nanotechnology’s full promise in the management of microbial illness, notably in the fields of antimicrobial treatment and vaccinations, is still far from being exploited. Some suggestions for improving the efficiency of nanoparticles to address K. pneumoniae are (i) taking full advantage of the EPR effect in infection sites; (ii) development of theranostic nanoparticles by combining diagnostic and therapeutic agents; and (iii) the use of nanotechnology along with gene silencing technologies such as the antisense strategy and RNA interference (RNAi).
Author Contributions
Conceptualization, M.B. and D.K.-N.; methodology, M.B. and H.F.; software, R.A.-S.; validation, D.K.-N., A.T.J. and H.A.; formal analysis, H.F.; investigation, M.R.A.; resources, A.T.J.; data curation, M.B. and D.K.-N.; writing—original draft preparation, M.B., D.K.-N., H.F., H.A. and R.A.-S.; writing—review and editing, M.B.; visualization, M.B.; supervision, M.B. and D.K.-N.; project administration, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This article is the result of the research project approved by the student research committee of Kerman University of Medical Sciences under the number 400001085, which was carried out with the financial support of the Research and Technology Vice-Chancellor of this university.
Institutional Review Board Statement
This review study with Reg. No. 400001085 was approved by the Ethical Committee of Kerman University of Medical Sciences. The ethic approval code is IR.KMU.REC.1401.006.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The challenge of emerging and re-emerging infectious diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef]
- Millet, J.-P.; Moreno, A.; Fina, L.; Del Baño, L.; Orcau, A.; De Olalla, P.G.; Cayla, J.A. Factors that influence current tuberculosis epidemiology. Eur. Spine J. 2013, 22, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Rafael, D.; Videira, M.; Ferreira, D.; Sosnik, A.; Sarmento, B. Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv. Drug Deliv. Rev. 2013, 65, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.; Thom, K.A.; Masnick, M.; Johnson, J.K.; Harris, A.D.; Morgan, D.J. Frequency of Klebsiella pneumoniae Carbapenemase (KPC)–producing and Non-KPC-producing klebsiella species contamination of healthcare workers and the environment. Infect. Control Hosp. Epidemiol. 2014, 35, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, C. Ueber die Schizomyceten bei der acuten fibrösen Pneumonie. Arch. Für Pathol. Anat. Und Physiol. Und Für Klin. Med. 1882, 87, 319–324. [Google Scholar]
- Clegg, S.; Murphy, C.N. Epidemiology and virulence of Klebsiella pneumoniae. Urin. Tract Infect. Mol. Pathog. Clin. Manag. 2017, 4, 435–457. [Google Scholar]
- Vuotto, C.; Longo, F.; Balice, M.P.; Donelli, G.; Varaldo, P.E. Antibiotic resistance related to biofilm formation in Klebsiella pneumoniae. Pathogens 2014, 3, 743–758. [Google Scholar] [CrossRef]
- Sargazi, G.; Afzali, D.; Ghafainazari, A.; Saravani, H. Rapid synthesis of cobalt metal organic framework. J. Inorg. Organomet. Polym. Mater. 2014, 24, 786–790. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Ko, W.-C.; Paterson, D.L.; Sagnimeni, A.J.; Hansen, D.S.; Von Gottberg, A.; Mohapatra, S.; Casellas, J.M.; Goossens, H.; Mulazimoglu, L.; Trenholme, G. Community-acquired Klebsiella pneumoniae bacteremia: Global differences in clinical patterns. Emerg. Infect. Dis. 2002, 8, 160. [Google Scholar] [CrossRef]
- Gross, A.E.; Van Schooneveld, T.C.; Olsen, K.M.; Rupp, M.E.; Bui, T.H.; Forsung, E.; Kalil, A.C. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob. Agents Chemother. 2014, 58, 5262–5268. [Google Scholar] [CrossRef] [PubMed]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Olson, R.; Fang, C.-T.; Stoesser, N.; Miller, M.; MacDonald, U.; Hutson, A.; Barker, J.H.; La Hoz, R.M.; Johnson, J.R. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J. Clin. Microbiol. 2018, 56, e00776-18. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. “Nightmare” bacteria on the rise in US hospitals, long-term care facilities. Jama 2013, 309, 1573–1574. [Google Scholar] [PubMed]
- Pidot, S.J.; Coyne, S.; Kloss, F.; Hertweck, C. Antibiotics from neglected bacterial sources. Int. J. Med. Microbiol. 2014, 304, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S.; Guidos, R.; Baragona, S.; Bartlett, J.G.; Rubinstein, E.; Zhanel, G.G.; Tino, M.D.; Pompliano, D.L.; Tally, F.; Tipirneni, P. Anti-infective research and development—problems, challenges, and solutions. Lancet Infect. Dis. 2007, 7, 68–78. [Google Scholar] [CrossRef]
- Yigit, H.; Queenan, A.M.; Anderson, G.J.; Domenech-Sanchez, A.; Biddle, J.W.; Steward, C.D.; Alberti, S.; Bush, K.; Tenover, F.C. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1151–1161. [Google Scholar] [CrossRef]
- Jeffs, M.A.; Lohans, C.T. Inhibiting the metallo-β-lactamases: Challenges and strategies to overcome bacterial β-lactam resistance. Future Med Chem. 2022, 14, 1021–1025. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile β-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Paterson, D.L. Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Infect. Control 2006, 34, S20–S28. [Google Scholar] [CrossRef]
- Rasheed, J.K.; Biddle, J.W.; Anderson, K.F.; Washer, L.; Chenoweth, C.; Perrin, J.; Newton, D.W.; Patel, J.B. Detection of the Klebsiella pneumoniae carbapenemase type 2 carbapenem-hydrolyzing enzyme in clinical isolates of Citrobacter freundii and K. oxytoca carrying a common plasmid. J. Clin. Microbiol. 2008, 46, 2066–2069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, X.; Zhang, W.; Zhao, Z.; Ye, C.; Zhou, S.; Wu, S.; Han, L.; Han, Z.; Ye, H. Molecular characterization of carbapenem-resistant Klebsiella pneumoniae isolates with focus on antimicrobial resistance. BMC Genom. 2019, 20, 822. [Google Scholar] [CrossRef]
- Cannatelli, A.; Santos-Lopez, A.; Giani, T.; Gonzalez-Zorn, B.; Rossolini, G.M. Polymyxin resistance caused by mgrB inactivation is not associated with significant biological cost in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2015, 59, 2898–2900. [Google Scholar] [CrossRef]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Nagel, T.E.; Chan, B.K.; De Vos, D.; El-Shibiny, A.; Kang’ethe, E.K.; Makumi, A.; Pirnay, J.-P. The developing world urgently needs phages to combat pathogenic bacteria. Front. Microbiol. 2016, 7, 882. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Jass, J.; Sebulsky, M.T.; McCormick, J.K. Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 2003, 16, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Hosseinikhah, S.M.; Barani, M.; Rahdar, A.; Madry, H.; Arshad, R.; Mohammadzadeh, V.; Cucchiarini, M. Nanomaterials for the diagnosis and treatment of inflammatory arthritis. Int. J. Mol. Sci. 2021, 22, 3092. [Google Scholar] [CrossRef]
- Qindeel, M.; Barani, M.; Rahdar, A.; Arshad, R.; Cucchiarini, M. Nanomaterials for the diagnosis and treatment of urinary tract infections. Nanomaterials 2021, 11, 546. [Google Scholar] [CrossRef]
- Lai, W.-F. Non-conjugated polymers with intrinsic luminescence for drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101916. [Google Scholar] [CrossRef]
- Lai, W.-F.; Wong, W.-T. Use of graphene-based materials as carriers of bioactive agents. Asian J. Pharm. Sci. 2021, 16, 577–588. [Google Scholar] [CrossRef]
- Almansob, A.; Bahkali, A.H.; Albarrag, A.; Alshomrani, M.; Binjomah, A.; Hailan, W.A.; Ameen, F. Effective treatment of resistant opportunistic fungi associated with immuno-compromised individuals using silver biosynthesized nanoparticles. Appl. Nanosci. 2022, 12, 3871–3882. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Abbasi, M.; Alijani, H.Q.; Akbarizadeh, M.R.; Iravani, S.; Barani, M.; Najafi, K.; Khatami, S.; Khatami, M. Ceramic magnetic ferrite nanoribbons: Eco-friendly synthesis and their antifungal and parasiticidal activity. Ceram. Int. 2022, 48, 3448–3454. [Google Scholar] [CrossRef]
- Barani, M.; Rahdar, A.; Sargazi, S.; Amiri, M.S.; Sharma, P.K.; Bhalla, N. Nanotechnology for inflammatory bowel disease management: Detection, imaging and treatment. Sens. Bio-Sens. Res. 2021, 32, 100417. [Google Scholar] [CrossRef]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic acid nanoplatforms: Antimicrobial, tissue engineering, and cancer theranostic applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef]
- Moghadam, N.C.Z.; Jasim, S.A.; Ameen, F.; Alotaibi, D.H.; Nobre, M.A.; Sellami, H.; Khatami, M. Nickel oxide nanoparticles synthesis using plant extract and evaluation of their antibacterial effects on Streptococcus mutans. Bioprocess Biosyst. Eng. 2022, 45, 1201–1210. [Google Scholar] [CrossRef]
- Satarzadeh, N.; Shakibaie, M.; Adeli-Sardou, M.; Jabari-Morouei, F.; Forootanfar, H.; Sadeghi-Dousari, A. Facile Microwave-Assisted Biosynthesis of Arsenic Nanoparticles and Evaluation their Antioxidant Properties and Cytotoxic Effects: A Preliminary in Vitro Study. J. Clust. Sci. 2022, 1–9. [Google Scholar] [CrossRef]
- Jadoun, S.; Chauhan, N.P.S.; Zarrintaj, P.; Barani, M.; Varma, R.S.; Chinnam, S.; Rahdar, A. Synthesis of nanoparticles using microorganisms and their applications: A review. Environ. Chem. Lett. 2022, 20, 3153–3197. [Google Scholar] [CrossRef]
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef]
- Rastegar, S.; Moradi, M.; Kalantar-Neyestanaki, D.; Hosseini-Nave, H. Virulence factors, capsular serotypes and antimicrobial resistance of hypervirulent Klebsiella pneumoniae and classical Klebsiella pneumoniae in Southeast Iran. Infect. Chemother. 2019, 51, e39. [Google Scholar] [CrossRef]
- Spanu, T.; Fiori, B.; D’Inzeo, T.; Canu, G.; Campoli, S.; Giani, T.; Palucci, I.; Tumbarello, M.; Sanguinetti, M.; Rossolini, G.M. Evaluation of the new NucliSENS EasyQ KPC test for rapid detection of Klebsiella pneumoniae carbapenemase genes (bla KPC). J. Clin. Microbiol. 2012, 50, 2783–2785. [Google Scholar] [CrossRef] [PubMed]
- Peter, H.; Berggrav, K.; Thomas, P.; Pfeifer, Y.; Witte, W.; Templeton, K.; Bachmann, T.T. Direct detection and genotyping of Klebsiella pneumoniae carbapenemases from urine by use of a new DNA microarray test. J. Clin. Microbiol. 2012, 50, 3990–3998. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.D.; Adie, K.; McNabb, A.; Purych, D.; Mannan, K.; Azana, R.; Ng, C.; Tang, P.; Hoang, L.M. Rapid detection of KPC, NDM, and OXA-48-like carbapenemases by real-time PCR from rectal swab surveillance samples. J. Clin. Microbiol. 2015, 53, 2731–2733. [Google Scholar] [CrossRef] [PubMed]
- Köser, C.U.; Fraser, L.J.; Ioannou, A.; Becq, J.; Ellington, M.J.; Holden, M.T.; Reuter, S.; Török, M.E.; Bentley, S.D.; Parkhill, J. Rapid single-colony whole-genome sequencing of bacterial pathogens. J. Antimicrob. Chemother. 2014, 69, 1275–1281. [Google Scholar] [CrossRef]
- Willemse-Erix, D.; Bakker-Schut, T.; Slagboom-Bax, F.; Jachtenberg, J.-W.; Lemmens-den Toom, N.; Papagiannitsis, C.C.; Kuntaman, K.; Puppels, G.; van Belkum, A.; Severin, J.A. Rapid typing of extended-spectrum β-lactamase-and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae isolates by use of SpectraCell RA. J. Clin. Microbiol. 2012, 50, 1370–1375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakano, R.; Nakano, A.; Ishii, Y.; Ubagai, T.; Kikuchi-Ueda, T.; Kikuchi, H.; Tansho-Nagakawa, S.; Kamoshida, G.; Mu, X.; Ono, Y. Rapid detection of the Klebsiella pneumoniae carbapenemase (KPC) gene by loop-mediated isothermal amplification (LAMP). J. Infect. Chemother. 2015, 21, 202–206. [Google Scholar] [CrossRef]
- Carvalhaes, C.G.; Cayô, R.; Visconde, M.F.; Barone, T.; Frigatto, E.A.; Okamoto, D.; Assis, D.M.; Juliano, L.; Machado, A.M.; Gales, A.C. Detection of carbapenemase activity directly from blood culture vials using MALDI-TOF MS: A quick answer for the right decision. J. Antimicrob. Chemother. 2014, 69, 2132–2136. [Google Scholar] [CrossRef]
- Girlich, D.; Anglade, C.; Zambardi, G.; Nordmann, P. Comparative evaluation of a novel chromogenic medium (chromID OXA-48) for detection of OXA-48 producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2013, 77, 296–300. [Google Scholar] [CrossRef]
- Hirsch, E.B.; Tam, V.H. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): An emerging cause of multidrug-resistant infection. J. Antimicrob. Chemother. 2010, 65, 1119–1125. [Google Scholar] [CrossRef]
- Humphries, R.; Bobenchik, A.M.; Hindler, J.A.; Schuetz, A.N. Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100. J. Clin. Microbiol. 2021, 59, e00213–e00221. [Google Scholar] [CrossRef]
- Yagi, T.; Wachino, J.-i.; Kurokawa, H.; Suzuki, S.; Yamane, K.; Doi, Y.; Shibata, N.; Kato, H.; Shibayama, K.; Arakawa, Y. Practical methods using boronic acid compounds for identification of class C β-lactamase-producing Klebsiella pneumoniae and Escherichia coli. J. Clin. Microbiol. 2005, 43, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Shang, Y. The Detection Techniques of Genetically Modified Organisms. In Genetically Modified Organisms in Food; Elsevier Academic Press: Cambridge, MA, USA, 2015; pp. 343–351. [Google Scholar]
- Ma, X.; Li, Y.; Liang, Y.; Liu, Y.; Yu, L.; Li, C.; Liu, Q.; Chen, L. Development of a DNA microarray assay for rapid detection of fifteen bacterial pathogens in pneumonia. BMC Microbiol. 2020, 20, 177. [Google Scholar] [CrossRef] [PubMed]
- Rebrosova, K.; Samek, O.; Kizovsky, M.; Bernatova, S.; Hola, V.; Ruzicka, F. Raman Spectroscopy—A Novel Method for Identification and Characterization of Microbes on a Single-Cell Level in Clinical Settings. Front. Cell. Infect. Microbiol. 2022, 367, 866463. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.P.; Othman, S.; Lau, Y.L.; Radu, S.; Chee, H.Y. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018, 124, 626–643. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Freydiere, A. The application of chromogenic media in clinical microbiology. J. Appl. Microbiol. 2007, 103, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Clancy, C.J.; Cheng, S.; Shields, R.K.; Chen, L.; Doi, Y.; Zhao, Y.; Perlin, D.S.; Kreiswirth, B.N.; Nguyen, M.H. Characterization of porin expression in Klebsiella pneumoniae Carbapenemase (KPC)-producing K. pneumoniae identifies isolates most susceptible to the combination of colistin and carbapenems. Antimicrob Agents Chemother 2013, 57, 2147–2153. [Google Scholar] [CrossRef]
- Stachyra, T.; Levasseur, P.; Péchereau, M.-C.; Girard, A.-M.; Claudon, M.; Miossec, C.; Black, M.T. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J. Antimicrob. Chemother. 2009, 64, 326–329. [Google Scholar] [CrossRef]
- Khan, A.A.; Manzoor, K.N.; Sultan, A.; Saeed, M.; Rafique, M.; Noushad, S.; Talib, A.; Rentschler, S.; Deigner, H.-P. Pulling the brakes on fast and furious multiple drug-resistant (MDR) bacteria. Int. J. Mol. Sci. 2021, 22, 859. [Google Scholar] [CrossRef]
- Cao, F.; Wang, X.; Wang, L.; Li, Z.; Che, J.; Wang, L.; Li, X.; Cao, Z.; Zhang, J.; Jin, L. Evaluation of the efficacy of a bacteriophage in the treatment of pneumonia induced by multidrug resistance Klebsiella pneumoniae in mice. BioMed Res. Int. 2015, 2015, 752930. [Google Scholar] [CrossRef]
- Gondil, V.S.; Chhibber, S. Alternative Therapies: Toolbox to Combat Antibiotic-Resistant Bugs. In Strategies to Overcome Superbug Invasions: Emerging Research and Opportunities; IGI Global: Hershey, PA, USA, 2021; pp. 160–182. [Google Scholar]
- Vieira, A.T.; Rocha, V.M.; Tavares, L.; Garcia, C.C.; Teixeira, M.M.; Oliveira, S.C.; Cassali, G.D.; Gamba, C.; Martins, F.S.; Nicoli, J.R. Control of Klebsiella pneumoniae pulmonary infection and immunomodulation by oral treatment with the commensal probiotic Bifidobacterium longum 51A. Microbes Infect. 2016, 18, 180–189. [Google Scholar] [CrossRef]
- Barani, M.; Zeeshan, M.; Kalantar-Neyestanaki, D.; Farooq, M.A.; Rahdar, A.; Jha, N.K.; Sargazi, S.; Gupta, P.K.; Thakur, V.K. Nanomaterials in the management of gram-negative bacterial infections. Nanomaterials 2021, 11, 2535. [Google Scholar] [CrossRef]
- Alijani, H.Q.; Iravani, S.; Pourseyedi, S.; Torkzadeh-Mahani, M.; Barani, M.; Khatami, M. Biosynthesis of spinel nickel ferrite nanowhiskers and their biomedical applications. Sci. Rep. 2021, 11, 17431. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Ma, A.-J.; Li, D.-X.; Luo, L.-J.; Liu, D.-X.; Jin, D.; Liu, K.; Ye, C.-Y. Rapid and sensitive isothermal detection of nucleic-acid sequence by multiple cross displacement amplification. Sci. Rep. 2015, 5, srep11902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, W.; Wang, Y.; Xu, J.; Ye, C. Rapid, sensitive and reliable detection of Klebsiella pneumoniae by label-free multiple cross displacement amplification coupled with nanoparticles-based biosensor. J. Microbiol. Methods 2018, 149, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhao, F.; Chen, J.; Nong, J.; Wang, C.; Wang, J.; Gao, N.; Zhu, X.; Wu, L.; Hu, S. Isothermal amplification and rapid detection of Klebsiella pneumoniae based on the multiple cross displacement amplification (MCDA) and gold nanoparticle lateral flow biosensor (LFB). PLoS ONE 2018, 13, e0204332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Niu, L.; Nong, J.; Wang, C.; Wang, J.; Liu, Y.; Gao, N.; Zhu, X.; Wu, L.; Hu, S. Rapid and sensitive detection of Pseudomonas aeruginosa using multiple cross displacement amplification and gold nanoparticle-based lateral flow biosensor visualization. FEMS Microbiol. Lett. 2018, 365, fny147. [Google Scholar] [CrossRef]
- Gong, L.; Liu, E.; Che, J.; Li, J.; Liu, X.; Xu, H.; Liang, J. Multiple cross displacement amplification coupled with gold nanoparticles-based lateral flow biosensor for detection of the mobilized colistin resistance gene mcr-1. Front. Cell. Infect. Microbiol. 2019, 9, 226. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, W.; Wei, L.; Liu, M.; Liu, H.; Zhao, C.; Zhang, P.; Liao, Q.; Liu, Y.; Yuan, Q. Rapid nanopore assay for carbapenem-resistant Klebsiella pneumoniae. Front. Microbiol. 2019, 10, 1672. [Google Scholar] [CrossRef]
- Kim, J.-W.; Park, K.-W.; Kim, M.; Lee, K.K.; Lee, C.-S. Highly Specific Loop-Mediated Isothermal Amplification Using Graphene Oxide–Gold Nanoparticles Nanocomposite for Foot-and-Mouth Disease Virus Detection. Nanomaterials 2022, 12, 264. [Google Scholar] [CrossRef]
- Li, J.; Jiang, J.; Zhao, D.; Xu, Z.; Liu, M.; Liu, X.; Tong, H.; Qian, D. Novel hierarchical sea urchin-like Prussian blue@ palladium core–shell heterostructures supported on nitrogen-doped reduced graphene oxide: Facile synthesis and excellent guanine sensing performance. Electrochim. Acta 2020, 330, 135196. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Jiao, W.; Li, J.; Quan, S.; Sun, L.; Wang, Y.; Qi, X.; Wang, X.; Shen, A. Development of loop-mediated isothermal amplification coupled with nanoparticle-based lateral flow biosensor assay for Mycoplasma pneumoniae detection. AMB Express 2019, 9, 196. [Google Scholar] [CrossRef]
- Tao, Y.; Ran, X.; Ren, J.; Qu, X. Array-Based Sensing of Proteins and Bacteria By Using Multiple Luminescent Nanodots as Fluorescent Probes. Small 2014, 10, 3667–3671. [Google Scholar] [CrossRef] [PubMed]
- Péter, B.; Farkas, E.; Kurunczi, S.; Szittner, Z.; Bősze, S.; Ramsden, J.J.; Szekacs, I.; Horvath, R. Review of Label-Free Monitoring of Bacteria: From Challenging Practical Applications to Basic Research Perspectives. Biosensors 2022, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Roostaee, M.; Sheikhshoaie, I. Fabrication of a sensitive sensor for determination of xanthine in the presence of uric acid and ascorbic acid by modifying a carbon paste sensor with Fe3O4@ Au core–shell and an ionic liquid. J. Food Meas. Charact. 2022, 16, 731–739. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Roostaee, M. Coupling of NiO nanoparticles and room temperature ionic liquid for fabrication of highly sensitive voltammetric sensor in tryptophan analysis. Anal. Bioanal. Electrochem 2016, 8, 578–588. [Google Scholar]
- Nirgund, J.; Purana, K.; Selvakumar, D.; Kumar, N.; Sil, S. Nanobiosensors for detection of bacteria: An overview of fiber-optics and Raman spectroscopy based biosensors. In Handbook of Microbial Nanotechnology; Academic Press: Cambridge, MA, USA, 2022; pp. 91–132. [Google Scholar]
- Sargazi, S.; Mukhtar, M.; Rahdar, A.; Bilal, M.; Barani, M.; Díez-Pascual, A.M.; Behzadmehr, R.; Pandey, S. Opportunities and challenges of using high-sensitivity nanobiosensors to detect long noncoding RNAs: A preliminary review. Int. J. Biol. Macromol. 2022, 205, 304–315. [Google Scholar] [CrossRef]
- Li, B.; Li, X.; Dong, Y.; Wang, B.; Li, D.; Shi, Y.; Wu, Y. Colorimetric sensor array based on gold nanoparticles with diverse surface charges for microorganisms identification. Anal. Chem. 2017, 89, 10639–10643. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for whole-cell bacterial detection. Clin. Microbiol. Rev. 2014, 27, 631. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Tsai, K.-T.; Wang, H.-H.; Chen, Y.; Chen, Y.-H.; Chao, Y.-C.; Chang, H.-H.; Lin, C.-H.; Wang, J.-K.; Wang, Y.-L. Functionalized arrays of Raman-enhancing nanoparticles for capture and culture-free analysis of bacteria in human blood. Nat. Commun. 2011, 2, 538. [Google Scholar] [CrossRef]
- Maldonado, J.; Estévez, M.-C.; Fernández-Gavela, A.; González-López, J.J.; González-Guerrero, A.B.; Lechuga, L.M. Label-free detection of nosocomial bacteria using a nanophotonic interferometric biosensor. Analyst 2020, 145, 497–506. [Google Scholar] [CrossRef]
- Cheong, Y.; Kim, Y.J.; Kang, H.; Choi, S.; Lee, H.J. Rapid label-free identification of Klebsiella pneumoniae antibiotic resistant strains by the drop-coating deposition surface-enhanced Raman scattering method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-S.; Pheanpanitporn, Y.; Yen, Y.-K.; Chang, K.-F.; Lin, L.-Y.; Lai, D.-M. Detection of the antiepileptic drug phenytoin using a single free-standing piezoresistive microcantilever for therapeutic drug monitoring. Biosens. Bioelectron. 2014, 59, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wang, P.; Du, Q.; Chen, Y.; Liu, N. Simultaneous and ultrasensitive detection of foodborne bacteria by gold nanoparticles-amplified microcantilever array biosensor. Front. Chem. 2019, 7, 232. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.; Barani, M.; Rahdar, A.; Sargazi, S.; Cucchiarini, M.; Pandey, S.; Kang, M. Multi-functionalized nanomaterials and nanoparticles for diagnosis and treatment of retinoblastoma. Biosensors 2021, 11, 97. [Google Scholar] [CrossRef]
- Marinesco, S. Microelectrode Biosensors for In Vivo Functional Monitoring of Biological Molecules. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Zhang, Z.; Yu, H.-W.; Wan, G.-C.; Jiang, J.-H.; Wang, N.; Liu, Z.-Y.; Chang, D.; Pan, H.-Z. A Label-Free Electrochemical Biosensor Based on a Reduced Graphene Oxide and Indole-5-Carboxylic Acid Nanocomposite for the Detection of Klebsiella pneumoniae. J. AOAC Int. 2017, 100, 548–552. [Google Scholar] [CrossRef]
- Pan, H.-z.; Yu, H.-w.; Wang, N.; Zhang, Z.; Wan, G.-c.; Liu, H.; Guan, X.; Chang, D. Electrochemical DNA biosensor based on a glassy carbon electrode modified with gold nanoparticles and graphene for sensitive determination of Klebsiella pneumoniae carbapenemase. J. Biotechnol. 2015, 214, 133–138. [Google Scholar] [CrossRef]
- Lu, L.; Hu, X.; Zhu, Z. Biomimetic sensors and biosensors for qualitative and quantitative analyses of five basic tastes. TRAC Trends Anal. Chem. 2017, 87, 58–70. [Google Scholar] [CrossRef]
- Li, B.; Tan, H.; Anastasova, S.; Power, M.; Seichepine, F.; Yang, G.-Z. A bio-inspired 3D micro-structure for graphene-based bacteria sensing. Biosens. Bioelectron. 2019, 123, 77–84. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Q.; Dong, S.; Wang, S.; Liu, R.; Wu, X.; Li, S. Multiple cross displacement amplification linked with nanoparticles-based lateral flow biosensor in screening of hepatitis B virus in clinical application. Infect. Drug Resist. 2021, 14, 1219. [Google Scholar] [CrossRef]
- Aylott, J.W. Optical nanosensors—an enabling technology for intracellular measurements. Analyst 2003, 128, 309–312. [Google Scholar] [CrossRef]
- Fritz, J. Cantilever biosensors. Analyst 2008, 133, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Riu, J.; Giussani, B. Electrochemical biosensors for the detection of pathogenic bacteria in food. TrAC Trends Anal. Chem. 2020, 126, 115863. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 1 December 2022).
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, D.K.; Binjawhar, D.N.; Ameen, F.; Veerappan, A. Lectin-Fortified Cationic Copper Sulfide Nanoparticles Gain Dual Targeting Capabilities to Treat Carbapenem-Resistant Acinetobacter baumannii Infection. ACS Omega 2022, 7, 43934–43944. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Dong, H.; Gao, Y.; Sinko, P.J.; Wu, Z.; Xu, J.; Jia, L. The nanotechnology race between China and the United States. Nano Today 2016, 11, 7–12. [Google Scholar] [CrossRef]
- Lai, W.-F.; Tang, R.; Wong, W.-T. Ionically crosslinked complex gels loaded with oleic acid-containing vesicles for transdermal drug delivery. Pharmaceutics 2020, 12, 725. [Google Scholar] [CrossRef]
- Megarajan, S.; Ameen, F.; Singaravelu, D.; Islam, M.A.; Veerappan, A. Synthesis of N-myristoyltaurine stabilized gold and silver nanoparticles: Assessment of their catalytic activity, antimicrobial effectiveness and toxicity in zebrafish. Environ. Res. 2022, 212, 113159. [Google Scholar] [CrossRef]
- Begum, I.; Ameen, F.; Soomro, Z.; Shamim, S.; AlNadhari, S.; Almansob, A.; Al-Sabri, A.; Arif, A. Facile fabrication of malonic acid capped silver nanoparticles and their antibacterial activity. J. King Saud. Univ. -Sci. 2021, 33, 101231. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel biogenic silver nanoparticle-induced reactive oxygen species inhibit the biofilm formation and virulence activities of Methicillin-Resistant Staphylococcus aureus (MRSA) strain. Front. Bioeng. Biotechnol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Yang, T.-Y.; Hsieh, Y.-J.; Lu, P.-L.; Lin, L.; Wang, L.-C.; Wang, H.-Y.; Tsai, T.-H.; Shih, C.-J.; Tseng, S.-P. In vitro and in vivo assessments of inspired ag/80S bioactive nanocomposites against carbapenem-resistant Klebsiella pneumoniae. Mater. Sci. Eng. C 2021, 125, 112093. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, A.; Noorbazargan, H.; Khayam, N.; Moulavi, P.; Zamani, N.; Asghari Lalami, Z.; Ashrafi, F. Ecofriendly Biomolecule-Capped Bifidobacterium bifidum-Manufactured Silver Nanoparticles and Efflux Pump Genes Expression Alteration in Klebsiella pneumoniae. Microb. Drug Resist. 2021, 27, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Chhibber, S.; Gondil, V.S.; Singla, L.; Kumar, M.; Chhibber, T.; Sharma, G.; Sharma, R.K.; Wangoo, N.; Katare, O.P. Effective topical delivery of H-AgNPs for eradication of Klebsiella pneumoniae–induced burn wound infection. AAPS Pharm. Sci. Tech. 2019, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- El-kaliuoby, M.I.; Khalil, A.M.; El-Khatib, A.M.; Shalaby, T.I. Synergistic Antibacterial Effect of Silver Nanoparticles and Extremely Low-Frequency Pulsed Magnetic Fields on Klebsiella pneumoniae. Communications 2018, 15, 23. [Google Scholar]
- Kotlhao, K.; Madiseng, M.D.; Mtunzi, F.M.; Pakade, V.E.; Modise, S.J.; Laloo, N.; Klink, M.J. The synthesis of silver, zinc oxide and titanium dioxide nanoparticles and their antimicrobial activity. In Proceedings of the Advanced Materials Proceedings, Stockholm, Sweden, 1 August 2017; pp. 479–484. [Google Scholar]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Constantin, L.V.; Predoi, D. Antibacterial activity of silver-doped hydroxyapatite nanoparticles against gram-positive and gram-negative bacteria. Nanoscale Res. Lett. 2012, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.Z.H.; Kiran, U.; Ali, M.I.; Jamal, A.; Hameed, A.; Ahmed, S.; Ali, N. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed. 2013, 8, 3187. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Khan, A.K.; Anwar, A.; Ali, S.A.; Shah, M.R. Biofilm inhibitory effect of chlorhexidine conjugated gold nanoparticles against Klebsiella pneumoniae. Microb. Pathog. 2016, 98, 50–56. [Google Scholar] [CrossRef]
- Yousef, J.M.; Danial, E.N. In vitro antibacterial activity and minimum inhibitory concentration of zinc oxide and nano-particle zinc oxide against pathogenic strains. J. Health Sci. 2012, 2, 38–42. [Google Scholar] [CrossRef]
- Hameed, A.S.H.; Karthikeyan, C.; Ahamed, A.P.; Thajuddin, N.; Alharbi, N.S.; Alharbi, S.A.; Ravi, G. In vitro antibacterial activity of ZnO and Nd doped ZnO nanoparticles against ESBL producing Escherichia coli and Klebsiella pneumoniae. Sci. Rep. 2016, 6, 24312. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Baskar, R.; Anusuya, T.; Seshan, C.A.; Chelliah, R. Toxicity mechanism of titanium dioxide and zinc oxide nanoparticles against food pathogens. Colloids Surf. B Biointerfaces 2016, 148, 600–606. [Google Scholar] [CrossRef]
- Thakur, B.; Kumar, A.; Kumar, D. Green synthesis of titanium dioxide nanoparticles using Azadirachta indica leaf extract and evaluation of their antibacterial activity. South Afr. J. Bot. 2019, 124, 223–227. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Amna, T.; Al-Deyab, S.S.; Hassan, M.S.; Abdel-Hay, A.; Kim, H.Y. Inactivation of pathogenic Klebsiella pneumoniae by CuO/TiO2 nanofibers: A multifunctional nanomaterial via one-step electrospinning. Ceram. Int. 2012, 38, 4525–4532. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Kumar, D. Evaluation of antimicrobial potential of cadmium sulphide nanoparticles against bacterial pathogens. Int. J. Pharm. Sci. Rev. Res 2014, 24, n32. [Google Scholar]
- Xu, N.; Lu, W.; Meng, L.; Feng, X.; Xuan, J.; Liu, F.; Feng, Z. Carbonic anhydrase inhibition, antioxidant activity against alveolar epithelial cells and antibacterial effect against Klebsiella pneumoniae enabled by synthesized silica nanoparticles through laser ablation technique. Life Sci. 2021, 278, 119032. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.U.; Ferreira-Neto, E.P.; Rahman, G.U.; Parveen, R.; Monteiro, A.S.; Rahman, G.; Van Le, Q.; Domeneguetti, R.R.; Ribeiro, S.J.; Ullah, S. Flexible bacterial cellulose-based BC-SiO2-TiO2-Ag membranes with self-cleaning, photocatalytic, antibacterial and UV-shielding properties as a potential multifunctional material for combating infections and environmental applications. J. Environ. Chem. Eng. 2021, 9, 104708. [Google Scholar] [CrossRef]
- Al Edhari, B.; Mashreghi, M.; Makhdoumi, A.; Darroudi, M. Antibacterial and antibiofilm efficacy of Ag NPs, Ni NPs and Al2O3 NPs singly and in combination against multidrug-resistant Klebsiella pneumoniae isolates. J. Trace Elem. Med. Biol. 2021, 68, 126840. [Google Scholar] [CrossRef]
- Ramsden, J.J. Photocatalytic antimicrobial coatings. Nanotechnol. Percept. 2015, 11, 146–168. [Google Scholar] [CrossRef]
- Reid, M.; Whatley, V.; Spooner, E.; Nevill, A.M.; Cooper, M.; Ramsden, J.J.; Dancer, S.J. How does a photocatalytic antimicrobial coating affect environmental bioburden in hospitals? Infect. Control. Hosp. Epidemiol. 2018, 39, 398–404. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmad, T.; Khan, A.; Sarwar, R.; Shafiq, J.; Raza, Y.; Ahmed, A.; Ullah, S.; Rehman, N.U.; Al-Harrasi, A. Rifampicin conjugated silver nanoparticles: A new arena for development of antibiofilm potential against methicillin resistant Staphylococcus aureus and Klebsiella pneumoniae. Int. J. Nanomed. 2019, 14, 3983. [Google Scholar] [CrossRef]
- Chhibber, S.; Gondil, V.S.; Sharma, S.; Kumar, M.; Wangoo, N.; Sharma, R.K. A novel approach for combating Klebsiella pneumoniae biofilm using histidine functionalized silver nanoparticles. Front. Microbiol. 2017, 8, 1104. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Yosri, M.; Amin, B.H. Control of imipenem resistant-Klebsiella pneumoniae pulmonary infection by oral treatment using a combination of mycosynthesized Ag-nanoparticles and imipenem. J. Radiat. Res. Appl. Sci. 2017, 10, 353–360. [Google Scholar] [CrossRef][Green Version]
- Shanmugaiah, V.; Harikrishnan, H.; Al-Harbi, N.; Shine, K.; Khaled, J.; Balasubramanian, N.; Kumar, R.S. Facile synthesis of silver nanoparticles using Streptomyces sp. VSMGT1014 and their antimicrobial efficiency. Dig. J. Nanomater. Biostruct. 2015, 10, 179–187. [Google Scholar]
- Saravanan, M.; Arokiyaraj, S.; Lakshmi, T.; Pugazhendhi, A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018, 117, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wu, X.; Zhao, J.; Jia, X. Clinical Study of the Treatment of Klebsiella pneumoniae with Comprehensive Nursing Intervention Combined with New Nano Silver. J. Nanosci. Nanotechnol. 2020, 20, 6063–6069. [Google Scholar] [CrossRef] [PubMed]
- Kareem, P.A.; Alsammak, E.G. The effect of silver and titanium dioxide nanoparticles on Klebsiella pneumoniae isolates multi resistant to antibiotics and observed by scanning electron microscopy. Cihan Univ. Sci. J. 2017, 2017, 284–297. [Google Scholar] [CrossRef][Green Version]
- Kőrösi, L.; Dömötör, D.; Beke, S.; Prato, M.; Scarpellini, A.; Meczker, K.; Schneider, G.; Kovács, T.; Kovács, Á.; Papp, S. Antibacterial activity of nanocrystalline TiO2 (B) on multiresistant Klebsiella pneumoniae strains. Sci. Adv. Mater. 2013, 5, 1184–1192. [Google Scholar] [CrossRef]
- Maheswari, P.; Harish, S.; Navaneethan, M.; Muthamizhchelvan, C.; Ponnusamy, S.; Hayakawa, Y. Bio-modified TiO2 nanoparticles with Withania somnifera, Eclipta prostrata and Glycyrrhiza glabra for anticancer and antibacterial applications. Mater. Sci. Eng. C 2020, 108, 110457. [Google Scholar] [CrossRef]
- Shafiei, M.; Amini, K.; Jafari, P. Impact of Probiotic Lactococcus lactis and Titanium Dioxide Nanoparticles on the Expression of Klebsiella pneumoniae Biofilm Genes; a Comparative Study. Mol. Genet. Microbiol. Virol. 2021, 36, 46–52. [Google Scholar] [CrossRef]
- Shirzadi-Ahodashti, M.; Mortazavi-Derazkola, S.; Ebrahimzadeh, M.A. Biosynthesis of noble metal nanoparticles using crataegus monogyna leaf extract (CML@ X-NPs, X= Ag, Au): Antibacterial and cytotoxic activities against breast and gastric cancer cell lines. Surf. Interfaces 2020, 21, 100697. [Google Scholar] [CrossRef]
- Picoli, S.U.; Durán, M.; Andrade, P.F.; Duran, N. Silver nanoparticles/silver chloride (Ag/AgCl) synthesized from Fusarium oxysporum acting against Klebsiella pneumouniae carbapenemase (KPC) and extended spectrum beta-lactamase (ESBL). Front. Nanosci. Nanotech. 2016, 2, 107–110. [Google Scholar] [CrossRef]
- El-Rab, S.M.G.; Abo-Amer, A.E.; Asiri, A.M. Biogenic synthesis of ZnO nanoparticles and its potential use as antimicrobial agent against multidrug-resistant pathogens. Curr. Microbiol. 2020, 77, 1767–1779. [Google Scholar] [CrossRef] [PubMed]
- Rasha, E.; Monerah, A.; Manal, A.; Rehab, A.; Mohammed, D.; Doaa, E. Biosynthesis of Zinc Oxide Nanoparticles from Acacia nilotica (L.) Extract to Overcome Carbapenem-Resistant Klebsiella Pneumoniae. Molecules 2021, 26, 1919. [Google Scholar] [CrossRef] [PubMed]
- Chouke, P.; Potbhare, A.; Dadure, K.; Mungole, A.; Meshram, N.; Chaudhary, R.; Rai, A.; Chaudhary, R. An antibacterial activity of Bauhinia racemosa assisted ZnO nanoparticles during lunar eclipse and docking assay. Mater. Today Proc. 2020, 29, 815–821. [Google Scholar] [CrossRef]
- Meenatchi, T.; Palanimurugan, A.; Dhanalakshmi, A.; Maheshkumar, V.; Natarajan, B. Green synthesis of Cynodon Dactylon capped concentrations on ZnO nanoparticles for antibacterial activity, ROS/ML-DNA treatment and compilation of best controlling microbes by mathematical comparisons. Chem. Phys. Lett. 2020, 749, 137429. [Google Scholar] [CrossRef]
- Vidhya, E.; Vijayakumar, S.; Prathipkumar, S.; Praseetha, P. Green way biosynthesis: Characterization, antimicrobial and anticancer activity of ZnO nanoparticles. Gene Rep. 2020, 20, 100688. [Google Scholar] [CrossRef]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P. Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharm. Biol. 2014, 52, 1388–1397. [Google Scholar] [CrossRef]
- Prasad, A.R.; Basheer, S.M.; Gupta, I.R.; Elyas, K.; Joseph, A. Investigation on bovine serum albumin (BSA) binding efficiency and antibacterial activity of ZnO nanoparticles. Mater. Chem. Phys. 2020, 240, 122115. [Google Scholar] [CrossRef]
- Partoazar, A.; Bideskan, F.R.; Takzaree, N.; Dallal, M. Antibiofilm Activity of ZnO/Zeolite Nanocomposite (ZnO/ZeoNC) Against Klebsiella pneumoniae and its Biocompatibility in an Animal Model. Anti-Infect. Agents 2021, 19, 174–181. [Google Scholar] [CrossRef]
- Dhas, S.P.; Shiny, P.J.; Khan, S.; Mukherjee, A.; Chandrasekaran, N. Toxic behavior of silver and zinc oxide nanoparticles on environmental microorganisms. J. Basic Microbiol. 2014, 54, 916–927. [Google Scholar] [CrossRef]
- Chandra, H.; Patel, D.; Kumari, P.; Jangwan, J.; Yadav, S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C 2019, 102, 212–220. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Munawar, T.; Mukhtar, F.; Ur Rahman, M.N.; Riaz, M.; Iqbal, F. Enhancement in the photocatalytic and antimicrobial properties of ZnO nanoparticles by structural variations and energy bandgap tuning through Fe and Co co-doping. Ceram. Int. 2021, 47, 11109–11121. [Google Scholar] [CrossRef]
- Sharifuddin, S.A.B.; Ismail, S.B.; Abdullah, I.; Mohamad, I.; Mohammed, J.S. Antibacterial evaluation of activated carbon cloth with Ag+ impregnated with ZnO nanoparticles. Res. J. Text. Appar. 2019, 23, 232–243. [Google Scholar] [CrossRef]
- Sathish, P.; Dineshbabu, N.; Ravichandran, K.; Arun, T.; Karuppasamy, P.; SenthilPandian, M.; Ramasamy, P. Combustion synthesis, characterization and antibacterial properties of pristine ZnO and Ga doped ZnO nanoparticles. Ceram. Int. 2021, 47, 27934–27941. [Google Scholar] [CrossRef]
- Zheng, L.P.; Zhang, Z.; Zhang, B.; Wang, J.W. Antifungal properties of Ag-SiO2 core-shell nanoparticles against phytopathogenic fungi. Adv. Mat. Res. 2012, 476, 814–818. [Google Scholar]
- Zahid, M.; Papadopoulou, E.L.; Suarato, G.; Binas, V.D.; Kiriakidis, G.; Gounaki, I.; Moira, O.; Venieri, D.; Bayer, I.S.; Athanassiou, A. Fabrication of visible light-induced antibacterial and self-cleaning cotton fabrics using manganese doped TiO2 nanoparticles. ACS Appl. Bio Mater. 2018, 1, 1154–1164. [Google Scholar] [CrossRef]
- Ryu, S.-Y.; Chung, J.W.; Kwak, S.-Y. Dependence of photocatalytic and antimicrobial activity of electrospun polymeric nanofiber composites on the positioning of Ag–TiO2 nanoparticles. Compos. Sci. Technol. 2015, 117, 9–17. [Google Scholar] [CrossRef]
- Huang, S.-M.; Weng, C.-H.; Tzeng, J.-H.; Huang, Y.-Z.; Anotai, J.; Yen, L.-T.; Chang, C.-J.; Lin, Y.-T. Photocatalytic inactivation of Klebsiella pneumoniae by visible-light-responsive N/C-doped and N-tourmaline/palladium-C-codoped TiO2. Chem. Eng. J. 2020, 379, 122345. [Google Scholar] [CrossRef]
- Ramakritinan, C.; Kaarunya, E.; Shankar, S.; Kumaraguru, A. Antibacterial effects of Ag, Au and bimetallic (Ag-Au) nanoparticles synthesized from red algae. Solid State Phenom. 2013, 201, 211–230. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, Y.; Li, J.; Dong, R.; Zhang, J.; Ma, C.; Wang, H.; Rui, Y.; Jiang, X. Indole derivative-capped gold nanoparticles as an effective bactericide in vivo. ACS Appl. Mater. Interfaces 2018, 10, 29398–29406. [Google Scholar] [CrossRef]
- Ansari, S.A.; Oves, M.; Satar, R.; Khan, A.; Ahmad, S.I.; Jafri, M.A.; Zaidi, S.K.; Algahtani, M. Antibacterial activity of iron oxide nanoparticles synthesized by co-precipitation technology against Bacillus cereus and Klebsiella pneumoniae. Pol. J. Chem. Technol. 2017, 19, 110–115. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).