Fast and Ultrasensitive Electrochemical Detection for Antiviral Drug Tenofovir Disoproxil Fumarate in Biological Matrices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Preparation of ZrO2
2.4. Preparation of ZrO2-CS-MWCNTs Composite Dispersion
2.5. Fabrication of Modified Electrode
2.6. Electrochemical Detection of TDF
2.7. Sample Preparation
3. Results and Discussion
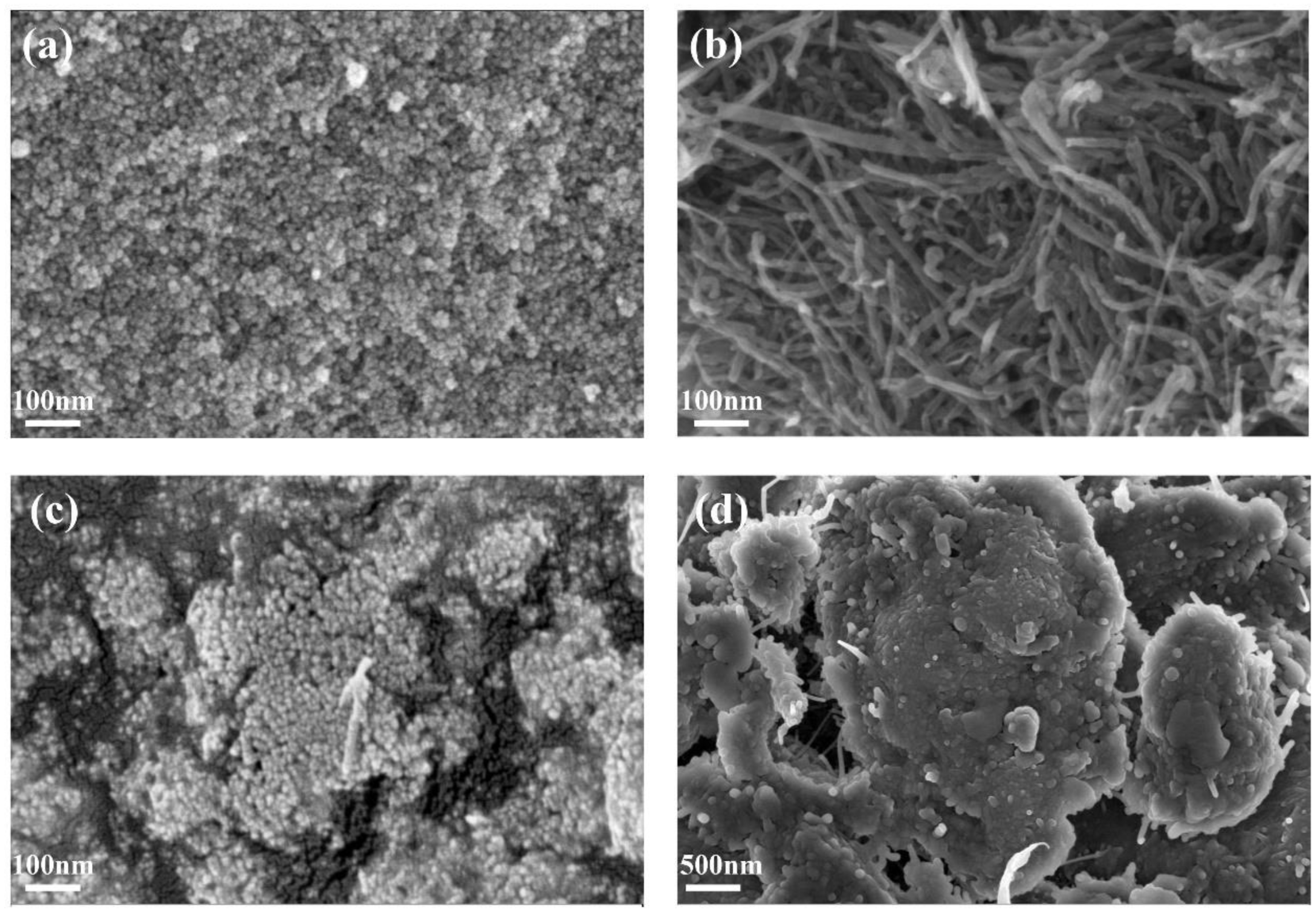
3.1. Crystal Phase and Morphology Characterization
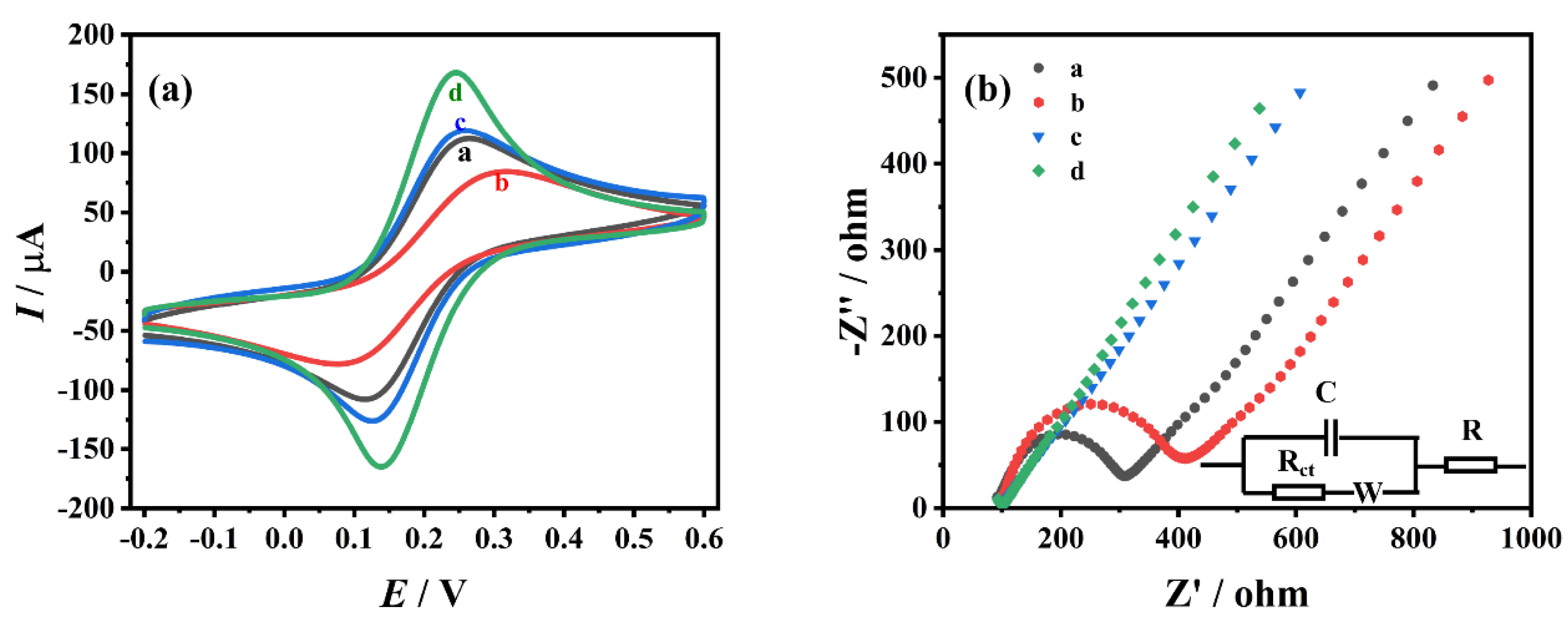
3.2. Electrochemical Characterization
3.3. Electrochemical Behavior of TDF on Different Electrodes
3.4. Effect of pH
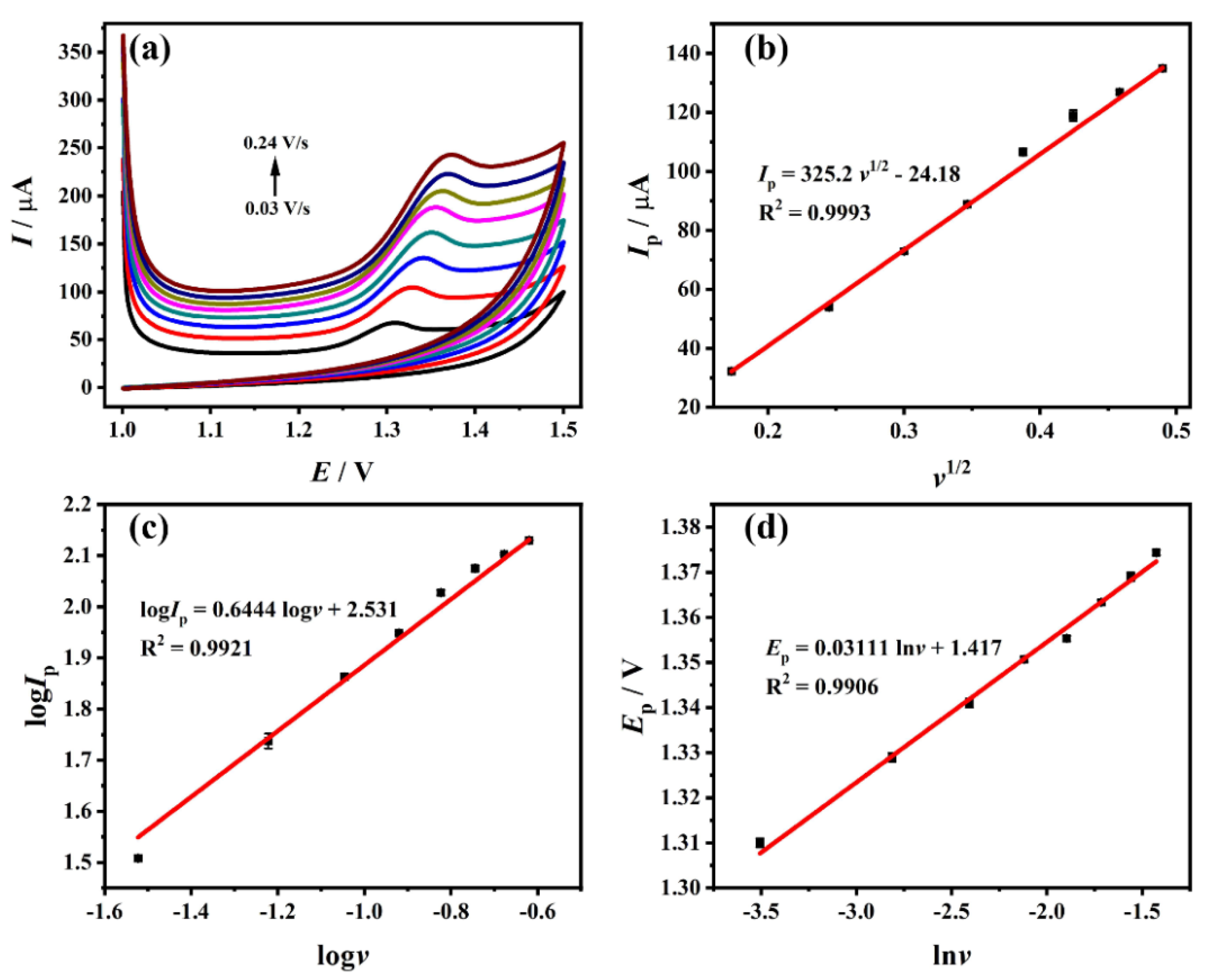
3.5. Effect of Scan Rate
3.6. Effects of Deposition Conditions and Dropping Amount of ZrO2-CS-MWCNTs Composites
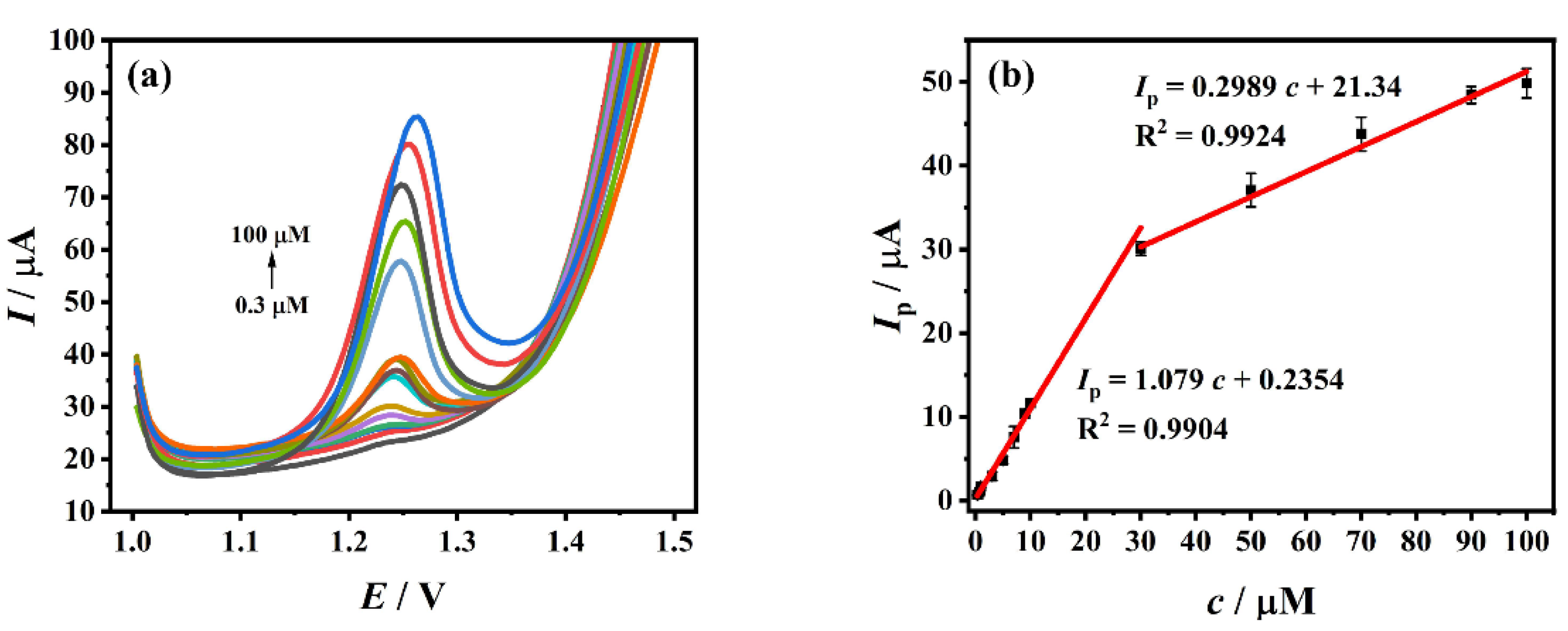
3.7. Standard Curve and Detection Limit
3.8. Selectivity
3.9. Repeatability, Reproducibility and Stability
3.10. Sample Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Douek, D.C.; Roederer, M.; Koup, R.A. Emerging concepts in the immunopathogenesis of AIDS. Annu. Rev. Med. 2009, 60, 471–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorraji, P.S.; Noori, M.; Fotouhi, L. Voltammetric determination of Adefovir dipivoxil by using a nanocomposite prepared from molecularly imprinted poly(o-phenylenediamine), multi-walled carbon nanotubes and carbon nitride. Microchim. Acta 2019, 186, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Arts, E.J.; Hazuda, D.J. HIV-1 antiretroviral drug therapy. Cold Spring Harb. Perspect. Med. 2012, 2, a007161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.J.; You, J.Z.; Wang, X.J. Thermal decomposition mechanism of tenofovir disoproxil fumarate. J. Therm. Anal. Calorim. 2018, 132, 471–482. [Google Scholar] [CrossRef]
- Watkins, M.E.; Wring, S.; Randolph, R.; Park, S.; Powell, K.; Lutz, L.; Nowakowski, M.; Ramabhadran, R.; Domanico, P.L. Development of a novel formulation that improves preclinical bioavailability of Tenofovir disoproxil fumarate. J. Pharm. Sci. 2017, 106, 906–919. [Google Scholar] [CrossRef] [Green Version]
- Riley, D.L.; Walwyn, D.R.; Edlin, C.D. An improved process for the preparation of Tenofovir disoproxil fumarate. Org. Process Res. Dev. 2016, 20, 742–750. [Google Scholar] [CrossRef] [Green Version]
- Yuen, M.F.; Lai, C.L. Treatment of chronic hepatitis B: Evolution over two decades. J. Gastroenterol. Hepatol. 2011, 26, 138–143. [Google Scholar] [CrossRef]
- Chapman, T.M.; McGavin, J.K.; Noble, S. Tenofovir disoproxil fumarate. Drugs 2003, 63, 1597–1608. [Google Scholar] [CrossRef]
- Monteiro, N.; Branco, M.; Peres, S.; Borges, F.; Mansinho, K. The impact of Tenofovir disoproxil fumarate on kidney function: Four-year data from the HIV-infected outpatient cohort. J. Int. AIDS Soc. 2014, 17, 19565. [Google Scholar] [CrossRef]
- Grant, P.M.; Cotter, A.G. Tenofovir and bone health. Curr. Opin. HIV AIDS 2016, 11, 326–332. [Google Scholar] [CrossRef]
- Susmitha, K.; Thirumalachary, M.; Singh, T.C.; Venkateshwarlu, G. Extractive spectrophotometric determination of Tenofovir disoproxil fumarate using acidic triphenylmethane dyes. ISRN Spectrosc. 2014, 2014, 856760. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, S.; Kannappan, N. Simultaneous spectrophotometric method for determination of Emtricitabine and Tenofovir disoproxil fumarate in three-component tablet formulation containing Rilpivirine Hydrochloride. Int. Sch. Res. Not. 2014, 2014, 541727. [Google Scholar] [CrossRef] [PubMed]
- Onah, J.O.; Ajima, U. Spectrophotometric determination of Tenofovir disoproxil fumarate after complexation with ammonium molybdate and picric acid. Int. J. Drug Dev. Res. 2011, 3, 199–204. [Google Scholar]
- Choudhari, V.P.; Parekar, S.R.; Chate, S.G.; Bharande, P.D.; Singh, R.R.; Kuchekar, B.S. Development and validation of UV-visible spectrophotometric baseline manipulation method for simultaneous quantitation of Tenofovir disoproxil fumarate and Emtricitabine in pharmaceutical dosage form. J. Spectrosc. 2013, 1, 146580. [Google Scholar] [CrossRef]
- Shirkhedkar Atul, A.; Bhirud Charushila, H.; Surana Sanjay, J. Application of UV-spectrophotometric methods for estimation of Tenofovir disoproxil fumarate in tablets. Pak. J. Pharm. Sci. 2009, 22, 27–29. [Google Scholar]
- Nye, L.C.; Gray, N.; McClure, M.; Boffit, M.; Holmes, E.; Plumb, R.S.; Wilson, I.D. Identification of a novel human circulating metabolite of Tenofovir disoproxil fumarate with LC-MS/MS. Bioanalysis 2015, 7, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, D.S.; Patel, B.N.; Patel, C.N. RP-HPLC method for simultaneous estimation of Tenofovir disoproxil fumarate, Lamivudine, and Efavirenz in combined tablet dosage form. Pharm. Methods 2012, 3, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurmi, M.; Jayaraman, K.; Natarajan, S.; Kumar, G.S.; Bhutani, H.; Bajpai, L. Rapid and efficient chiral method development for Lamivudine and Tenofovir disoproxil fumarate fixed dose combination using ultra-high performance supercritical fluid chromatography: A design of experiment approach. J. Chromatogr. A 2020, 1625, 461257. [Google Scholar] [CrossRef]
- Bhirud, C.H.; Hiremath, S.N. Indicating high performance thin layer chromatography determination of Tenofovir disoproxil fumarate in bulk and tablet dosage form. Int. J. Chem. Anal. Sci. 2014, 5, 1–5. [Google Scholar]
- Ozkan, S.A.; Uslu, B. From mercury to nanosensors: Past, present and the future perspective of electrochemistry in pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2016, 130, 126–140. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Feng, J.; Xiao, J.; Li, J.; Liu, J.; Li, G.; He, Q. Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J. Nanobiotechnol. 2020, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, B.; Escosura-Muñiz, A. Electrochemical biosensors based on nanomaterials for aflatoxins detection: A review (2015–2021). Anal. Chim. Acta 2022, 1212, 339658. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhong, P.; Ye, Y.; Wan, X.; Cai, Z.; Yang, S.; Xia, Y.; Li, Q.; Liu, J.; He, Q. A highly sensitive and stable dopamine sensor using shuttle-like α-Fe2O3 nanoparticles/electro-reduced graphene oxide composites. J. Electrochem. Soc. 2019, 166, B1552–B1561. [Google Scholar] [CrossRef]
- Wang, T.-T.; Huang, X.-F.; Huang, H.; Luo, P.; Qing, L.-S. Nanomaterial-based optical- and electrochemical-biosensors for urine glucose detection: A comprehensive review. Adv. Sens. Energy Mater. 2022, 1, 100016. [Google Scholar] [CrossRef]
- Muthumariappan, A.; Sakthivel, K.; Chen, S.M.; Chen, T.W.; Mani, G.; Lou, B.S. Effects of annealing temperature on crystal structure and glucose sensing properties of cuprous oxide. Sensors Actuators B Chem. 2018, 266, 655–663. [Google Scholar] [CrossRef]
- Arul, P.; Huang, S.T.; Mani, V.; Huang, C.H. Gold-silver bimetallic alloy nanoparticles in a covalent organic framework for real-time monitoring of hydrogen peroxide from live cells. ACS Appl. Nano Mater. 2022, 5, 6340–6351. [Google Scholar] [CrossRef]
- Liu, J.; Ma, N.; Wu, W.; He, Q. Recent progress on photocatalytic heterostructures with full solar spectral responses. Chem. Eng. J. 2020, 393, 124719. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Xia, Y.; Wu, Y.; Tian, Y.; Liu, J.; Chen, D.; He, Q. Towards emerging EEG applications: A novel printable flexible Ag/AgCl dry electrode array for robust recording of EEG signals at forehead sites. J. Neural Eng. 2020, 17, 026001. [Google Scholar] [CrossRef]
- Ozcelikay, G.; Dogan-Topal, B.; Ozkan, S.A. Electrochemical characteristics of Tenofovir and its determination in dosage form by electroanalytical methods. Rev. Roum. Chim 2017, 62, 569–578. [Google Scholar]
- Sakthinathan, S.; Kubendhiran, S.; Chen, S.M.; Govindasamy, M.; Al-Hemaid, F.M.A.; Ajmal Ali, M.; Tamizhdurai, P.; Sivasanker, S. Metallated porphyrin noncovalent interaction with reduced graphene oxide-modified electrode for amperometric detection of environmental pollutant hydrazine. Appl. Organomet. Chem. 2017, 31, e3703. [Google Scholar] [CrossRef]
- Rajaji, U.; Ms, R.; Al-Kahtani, A.A.; Chen, C.P.; Juang, R.S.; Liu, T.Y. Electrocatalytic oxidation and amperometric determination of sulfasalazine using bimetal oxide nanoparticles-decorated graphene oxide composite modified glassy carbon electrode at neutral pH. Mikrochim. Acta 2022, 189, 409. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Guo, Z.; Liu, J.H.; Huang, X.J. The new age of carbon nanotubes: An updated review of functionalized carbon nanotubes in electrochemical sensors. Nanoscale 2012, 4, 1940–1948. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Xia, Y.; Li, G.; Deng, P.; Chen, D. Novel electrochemical sensors based on cuprous oxide-electrochemically reduced graphene oxide nanocomposites modified electrode toward sensitive detection of sunset yellow. Molecules 2018, 23, 2130. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Deng, Z.; Wu, Z.; Xie, M.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; He, Q. Ta2O5/rGO nanocomposite modified electrodes for detection of tryptophan through electrochemical route. Nanomaterials 2019, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Xia, Y.; Tuo, D.; Deng, P.; Tian, Y.; Wu, Y.; Li, G.; Chen, D. Rapid and sensitive voltammetric detection of Rhodamine B in chili-containing foodstuffs using MnO2 nanorods/electro-reduced graphene oxide composite. J. Electrochem. Soc. 2019, 166, B805–B813. [Google Scholar] [CrossRef]
- Irfan, M.F.; Qurashi, A.; Alam, M.W. Metal oxide nanostructures and nanocomposites for selective catalytic reduction of NOx: A review. Arab. J. Sci. Eng. 2010, 35, 79–92. [Google Scholar]
- Alam, M.W.; Al Qahtani, H.S.; Souayeh, B.; Ahmed, W.; Albalawi, H.; Farhan, M.; Abuzir, A.; Naeem, S. Novel copper-zinc-manganese ternary metal oxide nanocomposite as heterogeneous catalyst for glucose sensor and antibacterial activity. Antioxidants 2022, 11, 1064. [Google Scholar] [CrossRef]
- Arul, P.; Huang, S.T.; Mani, V.; Huang, C.H. Graphene quantum dots-based nanocomposite for electrocatalytic application of L-cysteine in whole blood and live cells. Electrochim. Acta 2022, 428, 140937. [Google Scholar] [CrossRef]
- Wang, M.; Chen, K.; Liu, J.; He, Q.; Li, G.; Li, F. Efficiently enhancing electrocatalytic activity of α-MnO2 nanorods/N-doped ketjenblack carbon for oxygen reduction reaction and oxygen evolution reaction using facile regulated hydrothermal treatment. Catalysts 2018, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Wang, M.; Li, G.; He, Q.; Liu, J.; Li, F. Spherical α-MnO2 supported on N-KB as efficient electrocatalyst for oxygen reduction in Al-air battery. Materials. 2018, 11, 601. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Deng, P.; Wu, Y.; Tian, Y.; Li, G.; Liu, J.; He, Q. A novel modified electrode for detection of the food colorant sunset yellow based on nanohybrid of MnO2 nanorods-decorated electrochemically reduced graphene oxide. Molecules 2019, 24, 1178. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Li, G.; Liu, X.; Liu, J.; Deng, P.; Chen, D. Morphologically tunable MnO2 nanoparticles fabrication, modelling and their influences on electrochemical sensing performance toward dopamine. Catalysts 2018, 8, 323. [Google Scholar] [CrossRef] [Green Version]
- Ning, J.; He, Q.; Luo, X.; Wang, M.; Liu, D.; Wang, J.; Liu, J.; Li, G. Rapid and sensitive determination of vanillin based on a glassy carbon electrode modified with Cu2O-electrochemically reduced graphene oxide nanocomposite film. Sensors 2018, 18, 2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Deng, P.; Wu, Y.; Liu, J.; Li, J.; Li, G.; He, Q. High sensitive voltammetric sensor for nanomolarity vanillin detection in food samples via manganese dioxide nanowires hybridized electrode. Microchem. J. 2020, 157, 104885. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, P.; Wu, Y.; Li, J.; Liu, J.; Li, G.; He, Q. MnO2 nanowires-decorated reduced graphene oxide modified glassy carbon electrode for sensitive determination of Bisphenol A. J. Electrochem. Soc. 2020, 167, 046514. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Magesa, F.; Liu, J.; Li, G.; He, Q. Construction of effective electrochemical sensor for the determination of quinoline yellow based on different morphologies of manganese dioxide functionalized graphene. J. Food Compos. Anal. 2019, 84, 103280. [Google Scholar] [CrossRef]
- Wei, Y.; Yao, L.; Wu, Y.; Liu, X.; Feng, J.; Ding, J.; Li, K.; He, Q. Ultrasensitive electrochemical detection for nanomolarity Acyclovir at ferrous molybdate nanorods and graphene oxide composited glassy carbon electrode. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128601. [Google Scholar] [CrossRef]
- Govindasamy, M.; Sakthinathan, S.; Chen, S.M.; Chiu, T.W.; Sathiyan, A.; Merlin, J.P. Reduced graphene oxide supported cobalt bipyridyl complex for sensitive detection of methyl parathion in fruits and vegetables. Electroanalysis 2017, 29, 1950–1960. [Google Scholar] [CrossRef]
- ReddyPrasad, P.; Naidoo, E.B.; Sreedhar, N.Y. Electrochemical preparation of a novel type of C-dots/ZrO2 nanocomposite onto glassy carbon electrode for detection of organophosphorus pesticide. Arab. J. Chem. 2019, 12, 2300–2309. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Morris, M.A.; Holmes, J.D.; Yu, J.; Xu, R. Thermally stable nanocrystallised mesoporous zirconia thin films. Microporous Mesoporous Mater. 2009, 117, 161–164. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Z.; Chen, Z.; Rimoldi, M.; Howarth, A.J.; Chen, H.; Alayoglu, S.; Snurr, R.Q.; Farha, O.K.; Hupp, J.T. Ammonia capture within zirconium metal−organic frameworks: Reversible and irreversible uptake. ACS Appl. Mater. Interfaces. 2021, 13, 20081–20093. [Google Scholar] [CrossRef] [PubMed]
- Avila-Orta, C.; Cabello-Alvarado, C.; Andrade, M. Zirconium dioxide: Synthesis alternatives and biomedical applications. CienciaUAT 2019, 14, 18–30. [Google Scholar] [CrossRef]
- Dercz, G.; Prusik, K.; Pająk, L. X-ray and SEM studies on zirconia powders. J. Achiev. Mater. Manuf. Eng. 2008, 31, 408–414. [Google Scholar]
- Teymourian, H.; Salimi, A.; Firoozi, S.; Korani, A.; Soltanian, S. One-pot hydrothermal synthesis of zirconium dioxide nanoparticles decorated reduced graphene oxide composite as high performance electrochemical sensing and biosensing platform. Electrochim. Acta 2014, 143, 196–206. [Google Scholar] [CrossRef]
- Chen, T.W.; Sivasamy Vasantha, A.; Chen, S.M.; Al Farraj, D.A.; Soliman Elshikh, M.; Alkufeidy, R.M.; Al Khulaifi, M.M. Sonochemical synthesis and fabrication of honeycomb like zirconium dioxide with chitosan modified electrode for sensitive electrochemical determination of anti-tuberculosis (TB) drug. Ultrason. Sonochem. 2019, 59, 104718. [Google Scholar] [CrossRef]
- Tan, G.L.; Wu, X.J. Electronic conductivity of a ZrO2 thin film as an oxygen sensor. Thin Solid Films 1998, 330, 59–61. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Ding, Z.; Li, G.; Liu, J.; Zuberi, Z.; He, Q. Rapid recognition and determination of tryptophan by carbon nanotubes and molecularly imprinted polymer-modified glassy carbon electrode. Bioelectrochemistry 2020, 131, 107393. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Deng, P.; Fei, J.; Feng, Y. Sensitive voltammetric determination of tryptophan using an acetylene black paste electrode modified with a Schiff’s base derivative of chitosan. Analyst 2011, 136, 5211–5217. [Google Scholar] [CrossRef]
- Suh, J.H.; Lee, Y.Y.; Lee, H.J.; Kang, M.; Hur, Y.; Lee, S.N.; Yang, D.H.; Han, S.B. Dispersive liquid-liquid microextraction based on solidification of floating organic droplets followed by high performance liquid chromatography for the determination of duloxetine in human plasma. J. Pharm. Biomed. Anal. 2013, 75, 214–219. [Google Scholar] [CrossRef]
- Singh, D.K.; Iyer, P.K.; Giri, P.K. Diameter dependence of interwall separation and strain in multiwalled carbon nanotubes probed by X-ray diffraction and Raman scattering studies. Diam. Relat. Mater. 2010, 19, 1281–1288. [Google Scholar] [CrossRef]
- Feng, J.; Deng, P.; Xiao, J.; Li, J.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; He, Q. New voltammetric method for determination of tyrosine in foodstuffs using an oxygen-functionalized multi-walled carbon nanotubes modified acetylene black paste electrode. J. Food Compos. Anal. 2020, 96, 103708. [Google Scholar] [CrossRef]
- Wu, Y.; Li, G.; Tian, Y.; Feng, J.; Xiao, J.; Liu, J.; Liu, X.; He, Q. Electropolymerization of molecularly imprinted polypyrrole film on multiwalled carbon nanotube surface for highly selective and stable determination of carcinogenic amaranth. J. Electroanal. Chem. 2021, 895, 115494. [Google Scholar] [CrossRef]
- Deng, P.; Xiao, J.; Feng, J.; Tian, Y.; Wu, Y.; Li, J.; He, Q. Highly sensitive electrochemical sensor for tyrosine detection using a sub-millimeter electrode. Microchem. J. 2021, 165, 106106. [Google Scholar] [CrossRef]
- Ozcelikay, G.; Dogan-Topal, B.; Ozkan, S.A. An electrochemical sensor based on silver nanoparticles-benzalkonium chloride for the voltammetric determination of antiviral drug Tenofovir. Electroanalysis 2018, 30, 943–954. [Google Scholar] [CrossRef]
- Festinger, N.; Spilarewicz-Stanek, K.; Borowczyk, K.; Guziejewski, D.; Smarzewska, S. Highly sensitive determination of Tenofovir in pharmaceutical formulations and patients urine—Comparative electroanalytical studies using different sensing methods. Molecules 2022, 27, 1992. [Google Scholar] [CrossRef]
- Godasu, S.K.; Sreenivas, S.A. Determination of simultaneous estimation HPLC method for Elvitegravir, Tenofovir disoproxil fumarate, Emtricitabine and cobicistat it’s pure and tablet form. Der Pharma Chem. 2018, 10, 158–165. [Google Scholar]
- Donnell, D.; Baeten, J.M.; Bumpus, N.N.; Brantley, J.; Bangsberg, D.R.; Haberer, J.E.; Mujugira, A.; Mugo, N.; Ndase, P.; Hendrix, C.; et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J. Acquir. Immune Defic. Syndr. 2014, 66, 340–348. [Google Scholar] [CrossRef] [Green Version]
- Boglione, L.; Benedetto, I.D.; Dodaro, V.; Chiecchio, M.; Nicolò, A.D.; Perri, G.D.; D’Avolio, A. Role of plasmatic and urinary concentration of Tenofovir disoproxil fumarate in a cohort of patients affected by chronic hepatitis B. Arch. Virol. 2021, 167, 1669–1674. [Google Scholar] [CrossRef]
- Chavakula, R.; Mutyala, N.; Chennupati, S. Industrially viable synthesis of Tenofovir a key intermediate of Tenofovir disoproxil fumarate and related impurities. OCAIJ 2012, 8, 432–436. [Google Scholar]
- Morawska, K.; Popławski, T.; Ciesielski, W.; Smarzewska, S. Electrochemical and spectroscopic studies of the interaction of antiviral drug Tenofovir with single and double stranded DNA. Bioelectrochemistry 2018, 123, 227–232. [Google Scholar] [CrossRef] [PubMed]


| Method | Material | Linear Range (μM) | Detection Limit (μM) | Reference |
|---|---|---|---|---|
| Spectrophotometry | Acid triphenylmethane dye | 6.29~62.9 | 0.692 | [11] |
| Ultraviolet spectrophotometry | / | 7.87~62.9 | 0.804 | [15] |
| a RP-HPLC | / | 236~708 | 4.75 | [67] |
| First order derivative spectrophotometry | / | 7.87~62.9 | 1.03 | [15] |
| b AdSDPV | GCE | 0.6~60 | 0.102 | [29] |
| c AdSSWV | GCE | 0.6~60 | 0.0839 | [29] |
| d DPV | ZrO2-CS-MWCNTs/GCE | 0.3~30; 30~100 | 0.0625 | This work |
| Sample | Method | Measured/μM | Added/μM | Detected/μM | RSD/% | Recovery/% | Reference |
|---|---|---|---|---|---|---|---|
| TDF tablets | a RP-HPLC | 3.147 | 1.574 | 4.743 | 0.17 | 101.42 ± 0.08 | [17] |
| 3.147 | 3.147 | 6.323 | 0.25 | 100.89 ± 0.07 | |||
| 3.147 | 4.721 | 7.849 | 0.31 | 99.57 ± 0.09 | |||
| TDF tablets | b UV-Vis | 9.441 | 4.721 | / | 0.54 | 100.21 | [14] |
| 9.441 | 9.441 | / | 0.94 | 100.4 | |||
| 9.441 | 14.16 | / | 0.54 | 99.45 | |||
| TDF tables | c AdSDPV | / | / | / | 1.69 | 100.35 | [29] |
| d AdSSWV | / | / | / | 1.50 | 100.50 | ||
| TFV in pharmaceutical dosage form | AdSSWV | / | / | / | 1.36 | 101.33 | [65] |
| TFV in Viread | e SWV | / | / | / | / | 95.8 | [71] |
| TFV in Tenofovir disoproxil Teva | / | / | / | / | 101.9 | ||
| serum 1 | f DPV | g ND | 5 | 4.881 | 2.91 | 97.62 | This work |
| ND | 10 | 9.689 | 2.38 | 96.89 | |||
| ND | 15 | 14.42 | 4.84 | 96.13 | |||
| serum 2 | ND | 5 | 4.985 | 2.01 | 99.70 | ||
| ND | 10 | 9.810 | 4.58 | 98.10 | |||
| ND | 15 | 14.68 | 2.42 | 97.87 | |||
| urine 1 | ND | 5 | 4.862 | 1.32 | 97.24 | ||
| ND | 10 | 9.587 | 2.59 | 95.87 | |||
| ND | 15 | 14.55 | 4.96 | 97.00 | |||
| urine 2 | ND | 5 | 4.851 | 0.196 | 97.02 | ||
| ND | 10 | 9.791 | 2.11 | 97.91 | |||
| ND | 15 | 15.15 | 1.21 | 101.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Shi, S.; Yao, L.; Feng, J.; Zuo, J.; He, Q. Fast and Ultrasensitive Electrochemical Detection for Antiviral Drug Tenofovir Disoproxil Fumarate in Biological Matrices. Biosensors 2022, 12, 1123. https://doi.org/10.3390/bios12121123
Xiao J, Shi S, Yao L, Feng J, Zuo J, He Q. Fast and Ultrasensitive Electrochemical Detection for Antiviral Drug Tenofovir Disoproxil Fumarate in Biological Matrices. Biosensors. 2022; 12(12):1123. https://doi.org/10.3390/bios12121123
Chicago/Turabian StyleXiao, Jingyun, Shuting Shi, Liangyuan Yao, Jinxia Feng, Jinsong Zuo, and Quanguo He. 2022. "Fast and Ultrasensitive Electrochemical Detection for Antiviral Drug Tenofovir Disoproxil Fumarate in Biological Matrices" Biosensors 12, no. 12: 1123. https://doi.org/10.3390/bios12121123
APA StyleXiao, J., Shi, S., Yao, L., Feng, J., Zuo, J., & He, Q. (2022). Fast and Ultrasensitive Electrochemical Detection for Antiviral Drug Tenofovir Disoproxil Fumarate in Biological Matrices. Biosensors, 12(12), 1123. https://doi.org/10.3390/bios12121123





