Plasmonic Refractive Index Sensor Enhanced with Chitosan/Au Bilayer Thin Film for Dopamine Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Preparation
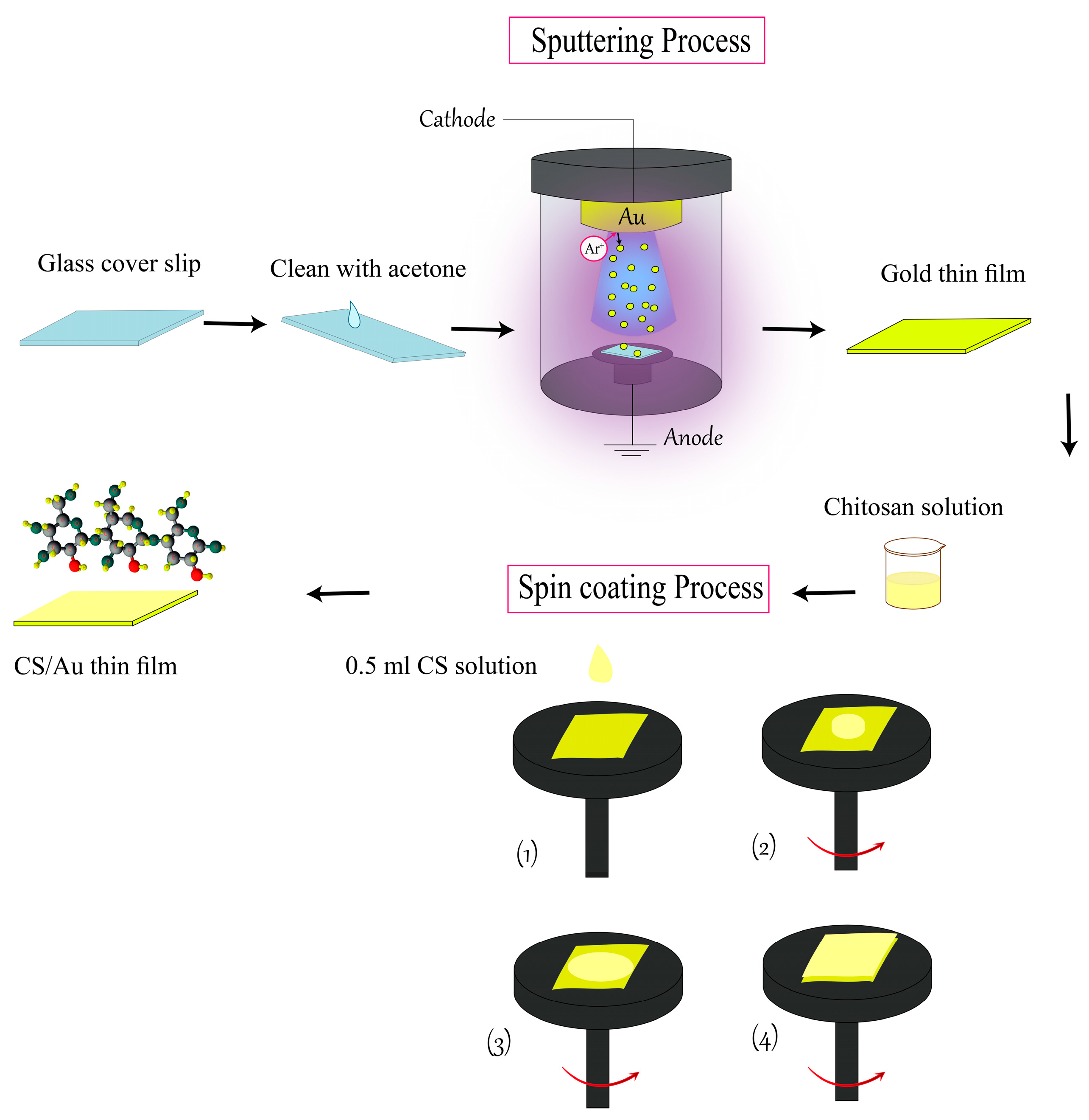
2.2. Preparation Process of Sensor Chip
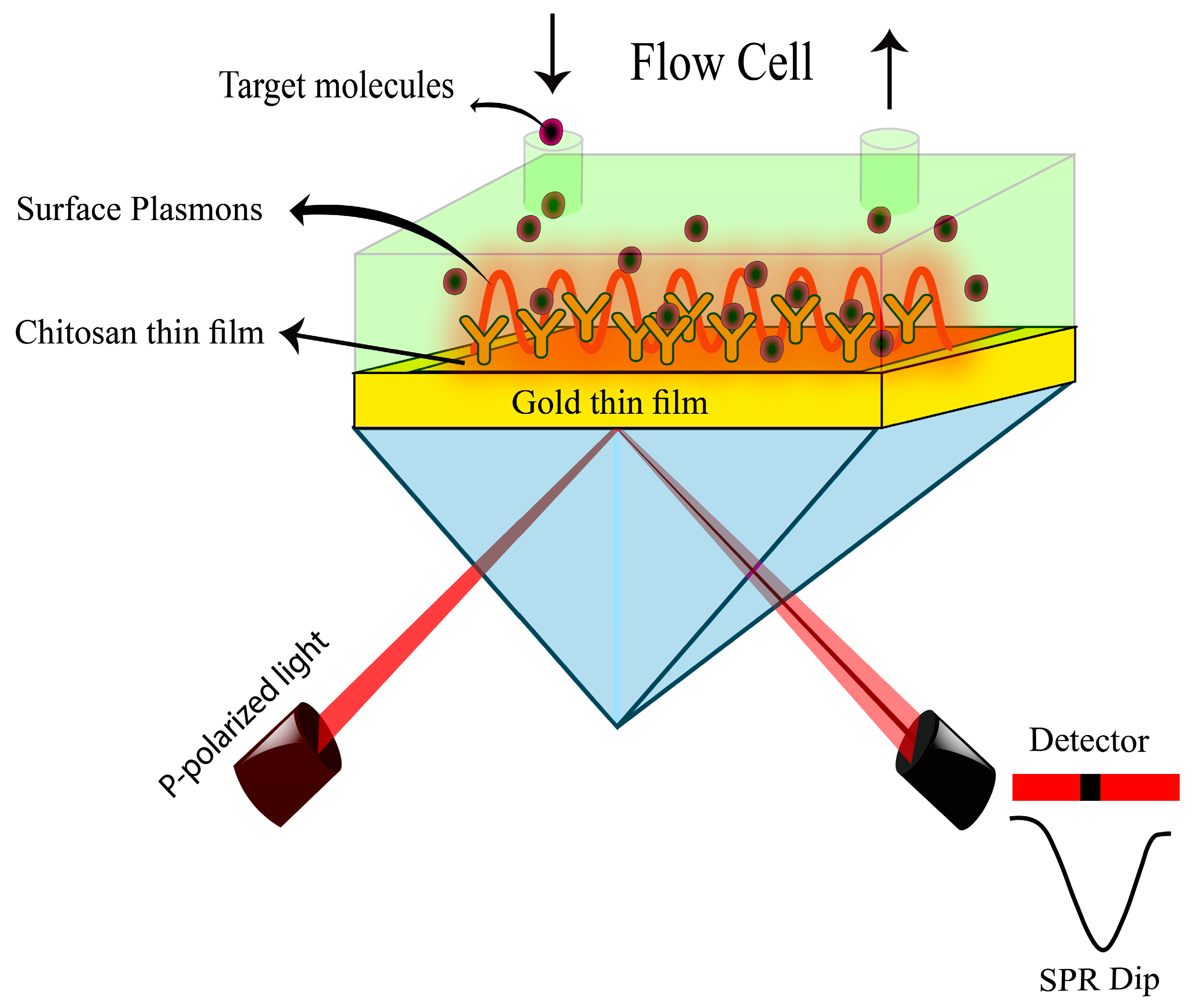
2.3. Sensing System Configuration
2.4. Structural Characterization Techniques
3. Results and Discussion
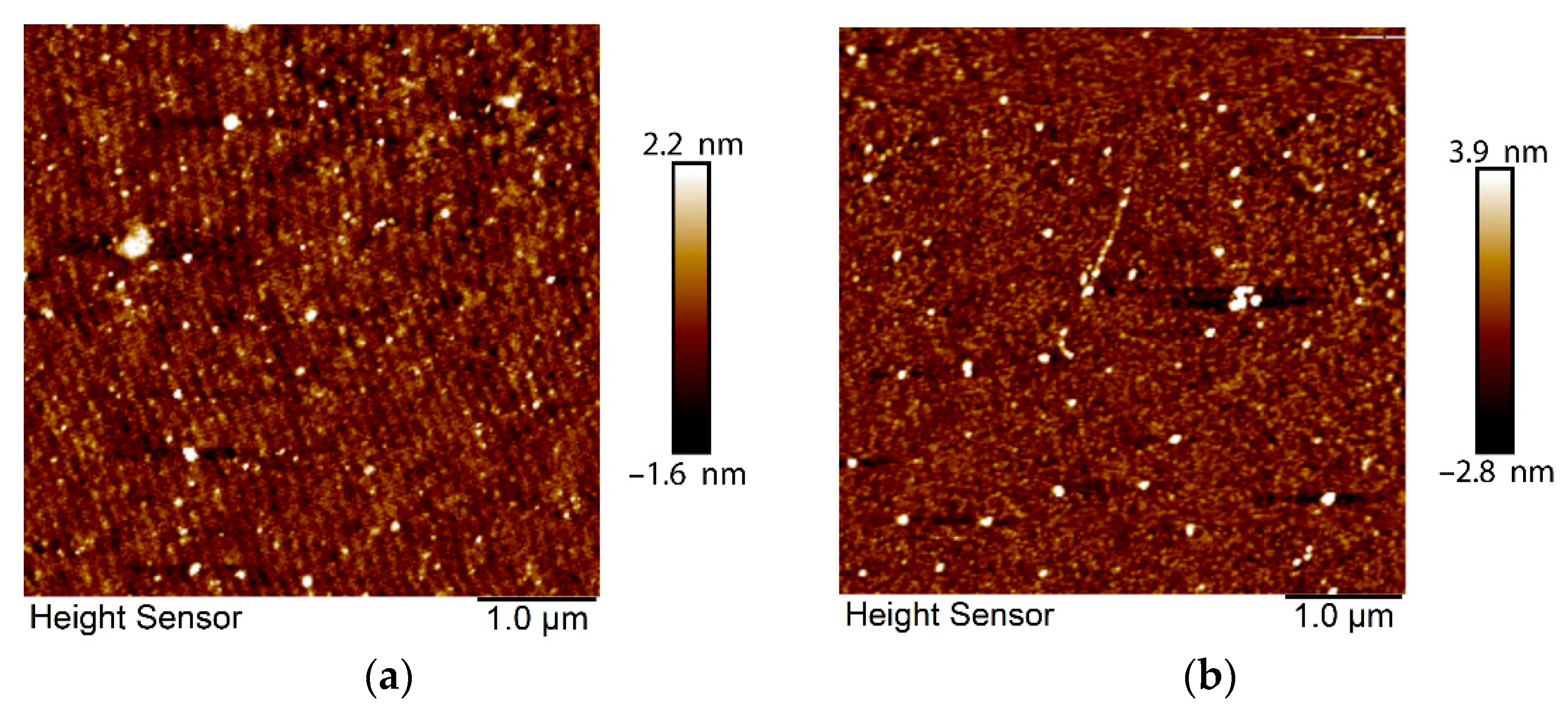
3.1. Structural Characterization
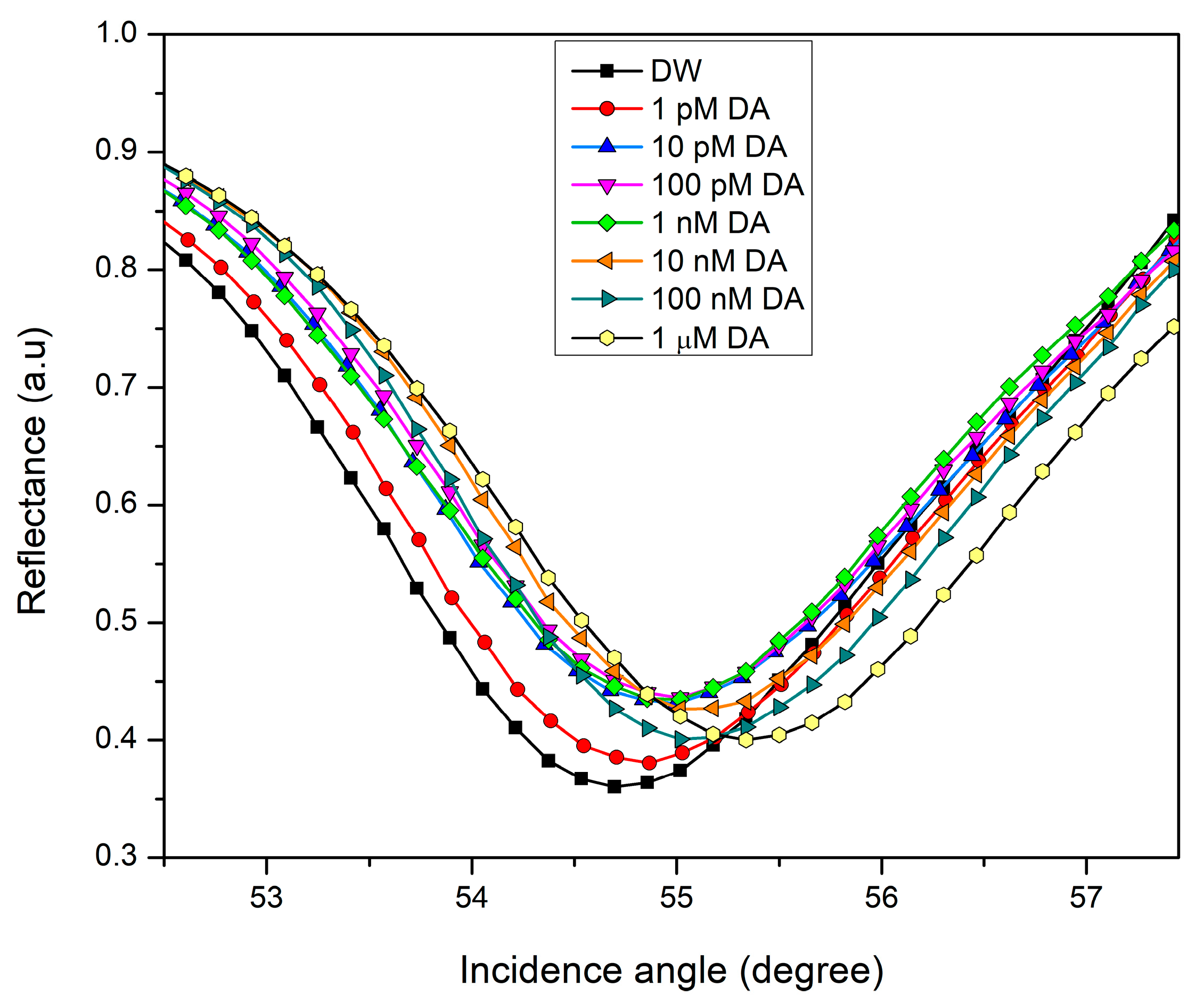
3.2. SPR Reflectivity Curves for DA on CS/Au Bilayer Film
3.3. Sensing Characteristics Analysis
3.4. Optical Characterization and Refractive Index Sensitivity of CS/Au-Based Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yusoff, N.; Pandikumar, A.; Ramaraj, R.; Lim, H.N.; Huang, N.M. Gold nanoparticle based optical and electrochemical sensing of dopamine. Microchim. Acta 2015, 182, 2091–2114. [Google Scholar] [CrossRef]
- Leng, Y.; Xie, K.; Ye, L.; Li, G.; Lu, Z.; He, J. Gold-nanoparticle-based colorimetric array for detection of dopamine in urine and serum yumin. Talanta 2015, 139, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Elayappan, V.; Muthusamy, S.; Mayakrishnan, G.; Balasubramaniam, R.; Lee, Y.S.; Noh, H.S.; Kwon, D.; Mussa, M.M.; Lee, H. Ultrasonication–dry-based synthesis of gold nanoparticle-supported CuFe on RGO nanosheets for competent detection of biological molecules. Appl. Surf. Sci. 2020, 531, 147415. [Google Scholar] [CrossRef]
- Cao, Y.; McDermott, M.T. Femtomolar and selective dopamine detection by a gold nanoparticle enhanced surface plasmon resonance aptasensor. BioRxiv 2018, 2018, 273078. [Google Scholar] [CrossRef]
- Hsu, M.; Chen, Y.; Lee, C.; Chiu, H. Gold nanostructures on flexible substrates as electrochemical dopamine sensors. ACS Appl. Mater. Interfaces 2012, 4, 5570–5575. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, G.; Song, Q.; Tang, T.; Wang, X.; Wei, F.; Hu, Q. Highly sensitive determination of dopamine by a turn-on fluorescent biosensor based on aptamer labeled carbon dots and nano-graphite. Sens. Actuators B Chem. 2016, 231, 506–512. [Google Scholar] [CrossRef]
- Kesby, J.P.; Eyles, D.; McGrath, J.; Scott, J. Dopamine, psychosis and schizophrenia: The widening gap between basic and clinical neuroscience. Transl. Psychiatry 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Fan, L.; Xin, Y.; Xu, Y.; Zhang, X.; Cheng, X.; Liu, L.; Song, H.; Gao, S.; Huo, L. Carbon nanospheres modified with WO2-NaxWO3 nanoparticles for highly sensitive electrochemical detection of dopamine. Microchem. J. 2021, 170, 106770. [Google Scholar] [CrossRef]
- Muzzi, C.; Bertocci, E.; Terzuoli, L.; Porcelli, B.; Ciari, I.; Pagani, R.; Guerranti, R. Simultaneous determination of serum concentrations of levodopa, dopamine, 3-O-methyldopa and α-methyldopa by HPLC. Biomed. Pharmacother. 2008, 62, 253–258. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Yao, X.; Yin, M. Determination of dopamine in rat less differentiated pheochromocytoma cells by capillary electrophoresis with a palladium nanoparticles microdisk electrode. RSC Adv. 2013, 3, 24605–24611. [Google Scholar] [CrossRef]
- Syslová, K.; Rambousek, L.; Kuzma, M.; Najmanová, V.; Bubeníková-Valešová, V.; Šlamberová, R.; Kačer, P. Monitoring of dopamine and its metabolites in brain microdialysates: Method combining freeze-drying with liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3382–3391. [Google Scholar] [CrossRef]
- Jamal, M.; Ameno, K.; Miki, T.; Tanaka, N.; Ito, A.; Ono, J.; Takakura, A.; Kumihashi, M.; Kinoshita, H. Ethanol and acetaldehyde differentially alter extracellular dopamine and serotonin in Aldh2-Knockout mouse dorsal striatum: A reverse microdialysis study. Neurotoxicology 2016, 52, 204–209. [Google Scholar] [CrossRef]
- Wang, X.; Baokang, J.I.N.; Xiangqin, L.I.N. In-situ FTIR spectroelectrochemical study of dopamine at a glassy carbon electrode in a neutral solution. Anal. Sci. 2002, 18, 931–933. [Google Scholar] [CrossRef]
- Wabaidur, S.M.; Alothman, Z.A.; Alam, S.M.; Lee, S.H. Flow injection-chemiluminescence determination of dopamine using potassium permanganate and formaldehyde system. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2012, 96, 221–225. [Google Scholar] [CrossRef]
- Fritzen-Garcia, M.B.; Monteiro, F.F.; Cristofolini, T.; Acuña, J.J.S.; Zanetti-Ramos, B.G.; Oliveira, I.R.W.Z.; Soldi, V.; Pasa, A.A.; Creczynski-Pasa, T.B. Characterization of horseradish peroxidase immobilized on PEGylated polyurethane nanoparticles and its application for dopamine detection. Sens. Actuators B Chem. 2013, 182, 264–272. [Google Scholar] [CrossRef]
- Shin, J.-W.; Kim, K.-J.; Yoon, J.; Jo, J.; El-Said, W.A.; Choi, J.-W. Silver nanoparticle modified electrode covered by graphene oxide for the enhanced electrochemical detection of dopamine. Sensors 2017, 17, 2771. [Google Scholar] [CrossRef]
- Kan, X.; Li, S.F.Y. Rapid detection of bacteria in food by surface plasmon resonance sensors. Int. J. Adv. Sci. Eng. Technol. 2016, 4, 26–29. [Google Scholar]
- Kamal Eddin, F.B.; Fen, Y.W. The principle of nanomaterials based surface plasmon resonance biosensors and its potential for dopamine detection. Molecules 2020, 25, 2769. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Mustapha Kamil, Y.; Daniyal, W.M.E.M.M.; Sadrolhosseini, A.R.; Mahdi, M.A. Sensitive detection of dengue virus type 2 E-Proteins signals using self-assembled monolayers/reduced graphene oxide-PAMAM dendrimer thin film-SPR optical sensor. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Zainuddin, N.H.; Fen, Y.W.; Alwahib, A.A.; Yaacob, M.H.; Bidin, N.; Omar, N.A.S.; Mahdi, M.A. Detection of adulterated honey by surface plasmon resonance optical sensor. Optik 2018, 168, 134–139. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Surface plasmon resonance optical sensor for detection of Pb2+ based on immobilized P-Tert-Butylcalix[4]Arene-Tetrakis in chitosan thin film as an active layer. Sens. Actuators B Chem. 2012, 171–172, 287–293. [Google Scholar] [CrossRef]
- Chen, Z.; Han, K.; Zhang, Y. Reflective fiber surface plasmon resonance sensor for high-sensitive mercury ion detection. Appl. Sci. 2019, 9, 1480. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical properties of chitosan/hydroxyl-functionalized graphene quantum dots thin film for potential optical detection of ferric(III) ion. Opt. Laser Technol. 2019, 120, 105724. [Google Scholar] [CrossRef]
- Yao, G.; Liang, R.; Huang, C.; Wang, Y.; Qiu, J. Surface Plasmon Resonance sensor based on magnetic molecularly imprinted polymers amplification for pesticide recognition. Anal. Chem. 2013, 85, 11944–11951. [Google Scholar] [CrossRef] [PubMed]
- Brasili, F.; Capocefalo, A.; Palmieri, D.; Capitani, F.; Chiessi, E.; Paradossi, G.; Bordi, F.; Domenici, F. Assembling patchy plasmonic nanoparticles with aggregation-dependent antibacterial activity. J. Colloid Interface Sci. 2020, 580, 419–428. [Google Scholar] [CrossRef]
- Hassan, M.M.; Sium, F.S.; Islam, F.; Choudhury, S.M. A review on plasmonic and metamaterial based biosensing platforms for virus detection. Sens. Bio-Sens. Res. 2021, 33, 100429. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nanosci. Technol. A Collect. Rev. Nat. J. 2009, 7, 308–319. [Google Scholar] [CrossRef]
- Pryce, I.M.; Kelaita, Y.A.; Aydin, K.; Atwater, H.A. Compliant metamaterials for resonantly enhanced infrared absorption spectroscopy and refractive index sensing. ACS Nano 2011, 5, 8167–8174. [Google Scholar] [CrossRef]
- Yue, Y.; Ding, H.; Chen, C. Label-free optical antibody testing kit based on a self-assembled whispering-gallery-mode microsphere. J. Biophotonics 2021, 14, 1–8. [Google Scholar] [CrossRef]
- Capocefalo, A.; Quintiero, E.; Conti, C.; Ghofraniha, N.; Viola, I. Droplet lasers for smart photonic labels. ACS Appl. Mater. Interfaces 2021, 13, 51485–51494. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Mehaney, A. Ultra-high sensitive 1D porous silicon photonic crystal sensor based on the coupling of tamm/fano resonances in the mid-infrared region. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Sellami, K.; Khelif, A.; Christensen, R.; Achaoui, Y.; Mehaney, A. Experimental and numerical evidence of using a phononic membrane with the coupling of Fano resonant modes as a highly sensitive temperature sensor. Mech. Syst. Signal Process. 2023, 185, 109763. [Google Scholar] [CrossRef]
- Elsayed, H.A.; Taha, T.A.; Algarni, S.A.; Ahmed, A.M.; Mehaney, A. Evolution of optical Tamm states in a 1D photonic crystal comprising a nanocomposite layer for optical filtering and reflecting purposes. Opt. Quantum Electron. 2022, 54, 15398. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic crystals for chemical sensing and biosensing. Angew. Chem.-Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef]
- Soelberg, S.D.; Chinowsky, T.; Geiss, G.; Spinelli, C.B.; Stevens, R.; Near, S.; Kauffman, P.; Yee, S.; Furlong, C.E. A portable surface plasmon resonance sensor system for real-time monitoring of small to large analytes. J. Ind. Microbiol. Biotechnol. 2005, 32, 669–674. [Google Scholar] [CrossRef]
- Çalışır, M.; Bakhshpour, M.; Yavuz, H.; Denizli, A. HbA1c detection via high-sensitive boronate based surface plasmon resonance sensor. Sens. Actuators B Chem. 2020, 306, 127561. [Google Scholar] [CrossRef]
- Pal, S.; Verma, A.; Raikwar, S.; Prajapati, Y.K.; Saini, J.P. Detection of DNA hybridization using graphene-coated black phosphorus surface plasmon resonance sensor. Appl. Phys. A Mater. Sci. Process. 2018, 124, 1–11. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Moksin, M.M.; Talib, Z.A.; Yusof, N.A. Surface plasmon resonance optical sensor for mercury iondetection by crosslinked chitosan thin film. J. Optoelectron. Adv. Mater. 2011, 13, 279–285. [Google Scholar]
- Matsui, J.; Akamatsu, K.; Hara, N.; Miyoshi, D.; Nawafune, H.; Tamaki, K.; Sugimoto, N. SPR sensor chip for detection of small molecules using molecularly imprinted polymer with embedded gold nanoparticles. Anal. Chem. 2005, 77, 4282–4285. [Google Scholar] [CrossRef]
- Kumbhat, S.; Shankaran, D.R.; Kim, S.J.; Gobi, K.V.; Joshi, V.; Miura, N. Surface plasmon resonance biosensor for dopamine using D3 dopamine receptor as a biorecognition molecule. Biosens. Bioelectron. 2007, 23, 421–427. [Google Scholar] [CrossRef]
- Sebok, D.; Csapó, E.; Preocanin, T.; Bohus, G.; Kallay, N.; Dékány, I. Adsorption of ibuprofen and dopamine on functionalized gold using surface plasmon resonance spectroscopy at solid-liquid interface. Croat. Chem. Acta 2013, 86, 287–295. [Google Scholar] [CrossRef]
- Zangeneh Kamali, K.; Pandikumar, A.; Sivaraman, G.; Lim, H.N.; Wren, S.P.; Sun, T.; Huang, N.M. Silver@graphene oxide nanocomposite-based optical sensor platform for biomolecules. RSC Adv. 2015, 5, 17809–17816. [Google Scholar] [CrossRef]
- Raj, D.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic sensor for mercury detection using gold nanoparticles PVA hybrid. Opt. Commun. 2016, 367, 102–107. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Thakur, G.; Kotsuchibashi, Y.; Naicker, S.; Narain, R.; Thundat, T. Rapid and highly sensitive detection of dopamine using conjugated oxaborole-based polymer and glycopolymer systems. ACS Appl. Mater. Interfaces 2017, 9, 15225–15231. [Google Scholar] [CrossRef] [PubMed]
- Manaf, A.A.; Ghadiry, M.; Soltanian, R.; Ahmad, H.; Lai, C.K. Picomole dopamine detection using optical chips. Plasmonics 2017, 12, 1505–1510. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, B.D. Surface plasmon resonance based highly selective fiber optic dopamine sensor fabricated using molecular imprinted GNP/SnO2 nanocomposite. J. Light. Technol. 2018, 36, 5956–5962. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, S.; Xu, J.; Li, Z.; Li, C.; Jing, Y.; Zhao, X.; Pan, J.; Zhang, C.; Man, B. Sensitive and selective surface plasmon resonance sensor employing a gold-supported graphene composite film/D-shaped fiber for dopamine detection. J. Phys. D. Appl. Phys. 2019, 52, 195402. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Xu, Z.; Chen, Y. Investigation of dopamine immobilized on gold by surface plasmon resonance. AIP Adv. 2019, 9, 035028. [Google Scholar] [CrossRef]
- Kamal Eddin, F.B.; Fen, Y.W.; Omar, N.A.S.; Liew, J.Y.C.; Daniyal, W.M.E.M.M. Femtomolar detection of dopamine using surface plasmon resonance sensor based on chitosan/graphene quantum dots thin film. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2021, 263, 120202. [Google Scholar] [CrossRef]
- Kamal Eddin, F.B.; Fen, Y.W.; Fauzi, N.I.M.; Daniyal, W.M.E.M.M.; Omar, N.A.S.; Anuar, M.F.; Hashim, H.S.; Sadrolhosseini, A.R.; Abdullah, H. Direct and sensitive detection of dopamine using carbon quantum dots based refractive index surface plasmon resonance sensor. Nanomaterials 2022, 12, 1799. [Google Scholar] [CrossRef]
- Kamal Eddin, F.B.; Fen, Y.W.; Sadrolhosseini, A.R.; Liew, J.Y.C.; Mohd Daniyal, W.M.E.M. Optical property analysis of chitosan-graphene quantum dots thin film and dopamine using surface plasmon resonance spectroscopy. Plasmonics 2022, 17, 1985–1997. [Google Scholar] [CrossRef]
- Sonsin, A.F.; Nascimento, S.M.S.; Albuquerque, I.M.B.; Silva, E.C.O.; Rocha, J.C.A.; Oliveira, R.S.; Barbosa, C.D.A.E.S.; Souza, S.T.; Fonseca, E.J.S. Temperature-dependence on the optical properties of chitosan carbon dots in the solid state. RSC Adv. 2021, 11, 2767–2773. [Google Scholar] [CrossRef]
- Marpu, S.B.; Benton, E.N. Shining light on chitosan: A review on the usage of chitosan for photonics and nanomaterials research. Int. J. Mol. Sci. 2018, 19, 1795. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A. Analysis of Pb(II) ion sensing by crosslinked chitosan thin film using surface plasmon resonance spectroscopy. Optik 2013, 124, 126–133. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Seong, K.C.; Ahmed, A.Y.; Ferrell, T.L.; Fen, Y.W.; Sadrolhosseini, A.R.; Ayodele, O.B.; Meriaudeau, F.; Saidu, A. Enhanced sensitivity of surface plasmon resonance biosensor functionalized with doped polyaniline composites for the detection of low-concentration acetone vapour. J. Sens. 2019, 2019, 5786105. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Irmawati, R.; Fen, Y.W.; Noryana Muhamad, E.; Kamal Eddin, F.B.; Anas, N.A.A.; Ramdzan, N.S.M.; Fauzi, N.I.M.; Adzir Mahdi, M. Surface refractive index sensor based on titanium dioxide composite thin film for detection of cadmium ions. Measurement 2021, 187, 110287. [Google Scholar] [CrossRef]
- Samavati, Z.; Samavati, A.; Ismail, A.F.; Rahman, M.A.; Othman, M.H.D.; Yeganeh, F.N. Optical fiber sensor for glycoprotein detection based on localized surface plasmon resonance of discontinuous Ag-deposited nanostructure. Opt. Fiber Technol. 2021, 62, 102476. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Kamal Eddin, F.B.; Abdullah, J.; Mahdi, M.A. Surface plasmon resonance assisted optical characterization of nickel ion solution and nanocrystalline cellulose-graphene oxide thin film for sensitivity enhancement analysis. Phys. B Condens. Matter 2022, 646, 414292. [Google Scholar] [CrossRef]
- Essel, T.Y.A.; Koomson, A.; Seniagya, M.P.O.; Cobbold, G.P.; Kwofie, S.K.; Asimeng, B.O.; Arthur, P.K.; Awandare, G.; Tiburu, E.K. Chitosan composites synthesized using acetic acid and tetraethylorthosilicate respond differently to methylene blue adsorption. Polymers 2018, 10, 466. [Google Scholar] [CrossRef]
- Zam, Z.Z.; Muin, F.; Fataruba, A. Identification of chitosan beads from coconut crab patani variety using Fourier transform infrared spectroscopy (FTIR). J. Phys. Conf. Ser. 2021, 1832, 012014. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Devi, B.M.; Sudha, P.N.; Venkatesan, J.; Anil, S. Synthesis, characterization and applications of nanochitosan/sodium alginate/microcrystalline cellulose film. J. Nanomed. Nanotechnol. 2016, 7, 419. [Google Scholar] [CrossRef]
- Abazar, F.; Noorbakhsh, A. Chitosan-carbon quantum dots as a new platform for highly sensitive insulin impedimetric aptasensor. Sens. Actuators B Chem. 2020, 304, 127281. [Google Scholar] [CrossRef]
- Ramos, J.V.H.; Morawski, F.D.M.; Costa, T.M.H.; Dias, S.L.P.; Benvenutti, E.V.; De Menezes, E.W.; Arenas, L.T. Mesoporous chitosan/silica hybrid material applied for development of electrochemical sensor for paracetamol in presence of dopamine. Microporous Mesoporous Mater. 2015, 217, 109–118. [Google Scholar] [CrossRef]
- Kandra, R.; Bajpai, S. Synthesis, mechanical properties of fluorescent carbon dots loaded nanocomposites chitosan film for wound healing and drug delivery. Arab. J. Chem. 2020, 13, 4882–4894. [Google Scholar] [CrossRef]
- Roshidi, M.D.A.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Omar, N.A.S.; Zulholinda, M. Structural and optical properties of chitosan–Poly (amidoamine) dendrimer composite thin film for potential sensing Pb2+ using an optical spectroscopy. Opt.-Int. J. Light Electron Opt. 2019, 185, 351–358. [Google Scholar] [CrossRef]
- Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M.; Hashim, H.S.; Nasrullah, M. Nanostructured chitosan/maghemite composites thin film for potential optical detection of mercury ion by surface plasmon resonance investigation. Polymers 2020, 12, 1497. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Piliarik, M. Surface plasmon resonance (SPR) sensors. In Surface Plasmon Resonance Based Sensors; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Kashif, M.; Bakar, A.; Arsad, N.; Shaari, S. Development of phase detection schemes based on surface plasmon resonance using interferometry. Sensors 2014, 14, 15914–15938. [Google Scholar] [CrossRef]
- Thomsen, V.; Schatzlein, D.; Mercuro, D. Limits of Detection in Spectroscopy. Spectroscopy 2003, 18, 112–114. [Google Scholar]
- Lim, J.; Kang, I. Chitosan-gold nano composite for dopamine analysis using raman scattering. Bull. Korean Chem. Soc. 2013, 34, 237–242. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, H.; Hu, S.; Li, F.; Weng, W.; Chen, J.; Wang, Q.; He, Y.; Zhang, W.; Bao, X. A sensitive and reliable dopamine biosensor was developed based on the Au@carbon dots-chitosan composite film. Biosens. Bioelectron. 2014, 52, 277–280. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Tang, L.; Lu, J.; Li, J. Application of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of nickel(II) ions onto a Sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Roy, D. Optical characterization of multi-layer thin films using the surface plasmon resonance method: A six-phase model based on the Kretschmann formalism. Opt. Commun. 2001, 200, 119–130. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Sheh Omar, N.A.; Abdullah, J.; Daniyal, W.M.E.M.M.; Saleviter, S. Detection of phenol by incorporation of gold modified-enzyme based graphene oxide thin film with surface plasmon resonance technique. Opt. Express 2020, 28, 9738. [Google Scholar] [CrossRef]
- Hossain, M.M.; Talukder, M.A. Gate-controlled graphene surface plasmon resonance glucose sensor. Opt. Commun. 2021, 493, 126994. [Google Scholar] [CrossRef]
- Szunerits, S.; Shalabney, A.; Boukherroub, R.; Abdulhalim, I. Dielectric coated plasmonic interfaces: Their interest for sensitive sensing of analyte-ligand interactions. Rev. Anal. Chem. 2012, 31, 15–28. [Google Scholar] [CrossRef]
- Mostufa, S.; Akib, T.B.A.; Rana, M.M.; Islam, M.R. Highly sensitive TiO2/Au/graphene layer-based surface plasmon resonance biosensor for cancer detection. Biosensors 2022, 12, 603. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Utilization of chitosan-based sensor thin films for the detection of lead ion by surface plasmon resonance optical sensor. IEEE Sens. J. 2013, 13, 1413–1418. [Google Scholar] [CrossRef]
- Fouad, S.; Sabri, N.; Jamal, Z.A.Z.; Poopalan, P. Surface plasmon resonance sensor sensitivity enhancement using gold-dielectric material. Exp. Theo. Nanotechnol. 2017, 10, 149–158. [Google Scholar] [CrossRef]
- Mudgal, N.; Saharia, A.; Choure, K.K.; Agarwal, A.; Singh, G. Sensitivity enhancement with anti-reflection coating of silicon nitride (Si3N4) layer in silver-based surface plasmon resonance (SPR) sensor for sensing of DNA hybridization. Appl. Phys. A Mater. Sci. Process. 2020, 126, 1–8. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Surface plasmon resonance optical sensor for detection of essential heavy metal ions with potential for toxicity: Copper, zinc and manganese ions. Sens. Lett. 2011, 9, 1704–1711. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Abdullah, J.; Sadrolhosseini, A.R.; Omar, N.A.S. Design and analysis of surface plasmon resonance optical sensor for determining cobalt ion based on chitosan-graphene oxide decorated quantum dots-modified gold active layer. Opt. Express 2019, 27, 32294–32307. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Hu, S.; Zhang, H.; Chen, J.; He, Y.; Li, F.; Weng, W.; Ni, J.; Bao, X.; Lin, Y. Carbon dots and chitosan composite film based biosensor for the sensitive and selective determination of dopamine. Analyst 2013, 138, 5417–5423. [Google Scholar] [CrossRef]
- Aoun, S.B. Nanostructured carbon electrode modified with N-doped graphene quantum dots–chitosan nanocomposite: A sensitive electrochemical dopamine sensor. R. Soc. Open Sci. 2017, 4, 1–12. [Google Scholar]
- Liu, X.; Xie, L.; Li, H. Electrochemical biosensor based on reduced graphene oxide and au nanoparticles entrapped in chitosan/silica sol–Gel hybrid membranes for determination of dopamine and uric acid. J. Electroanal. Chem. 2012, 682, 158–163. [Google Scholar] [CrossRef]


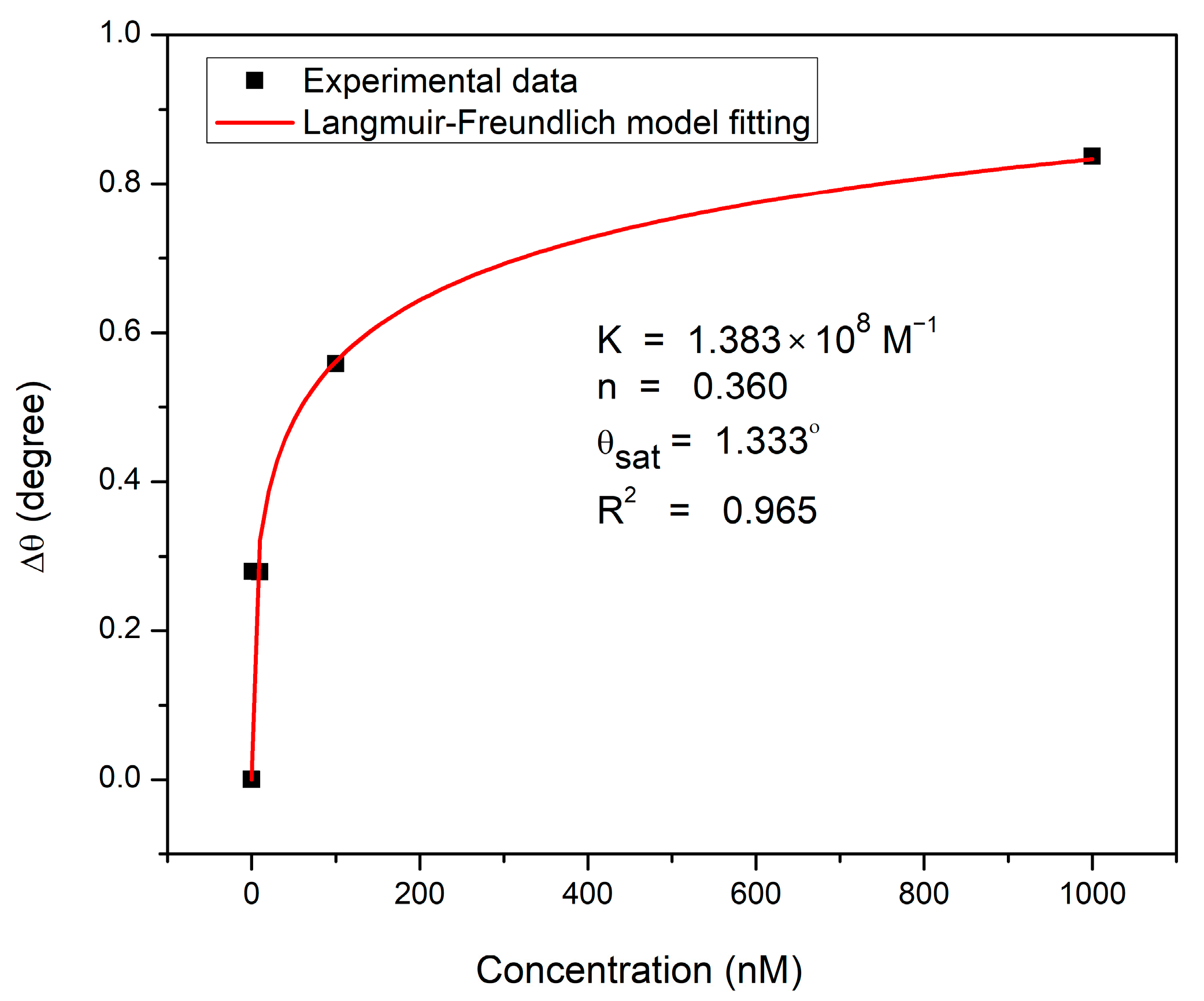
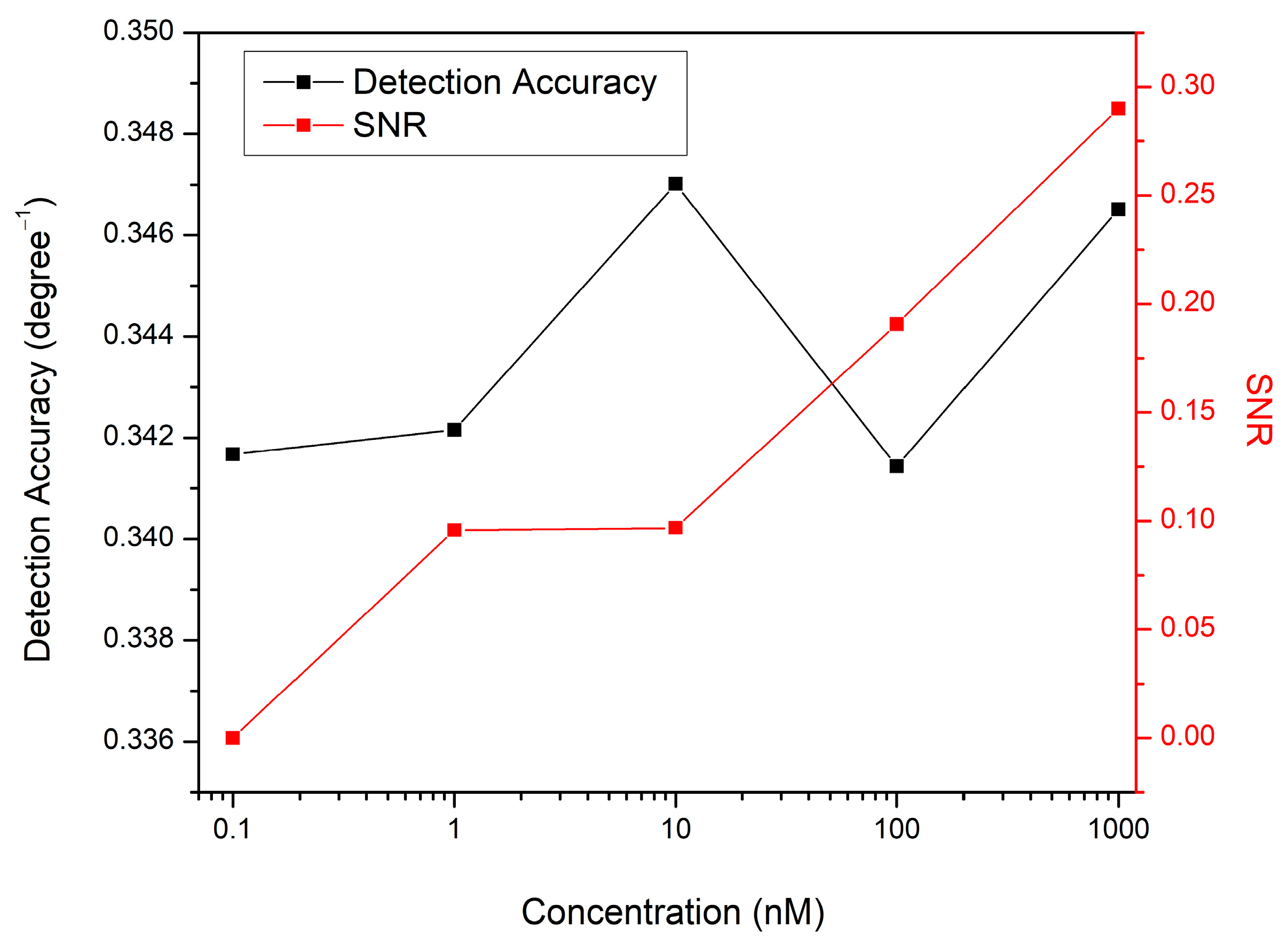
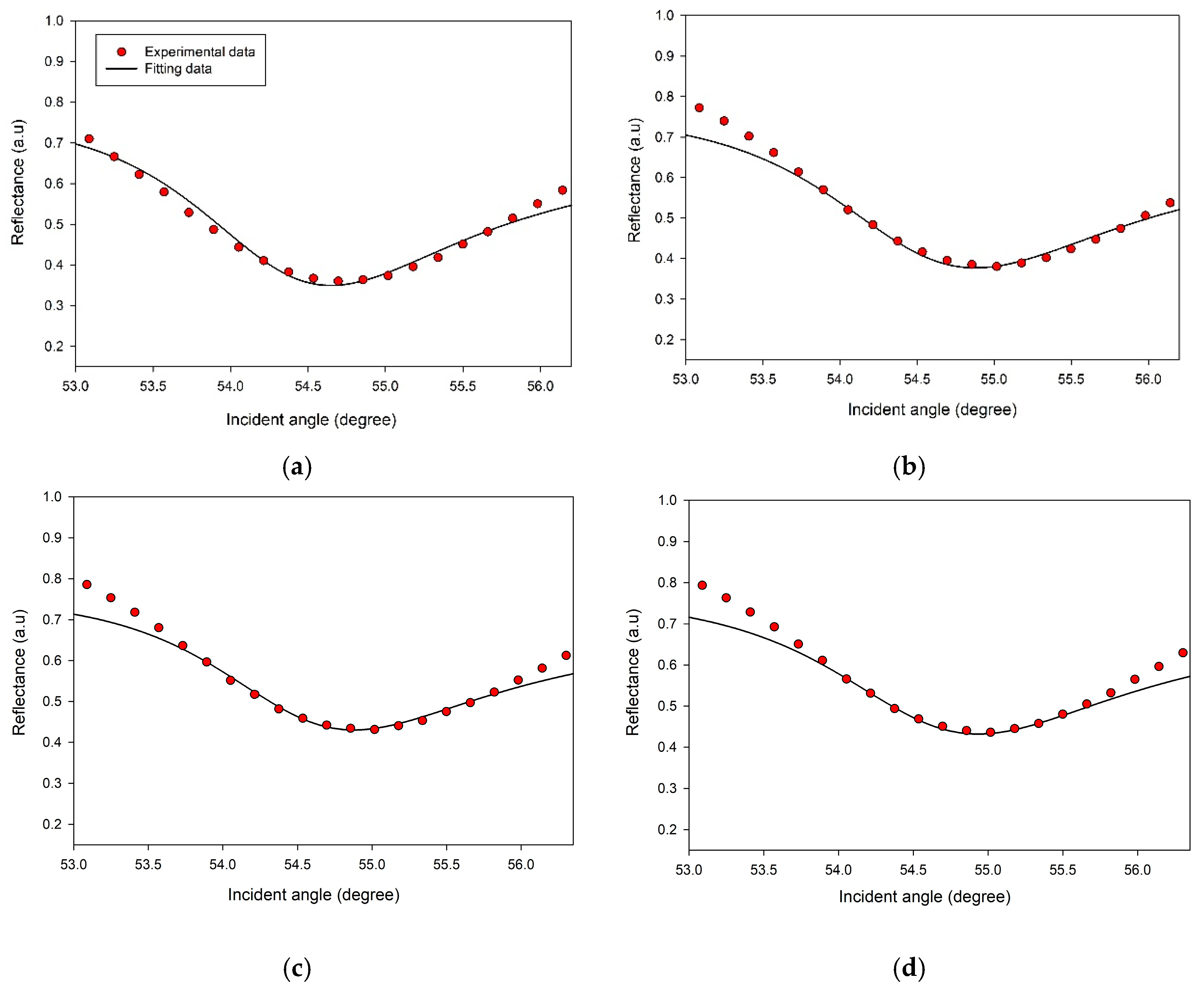

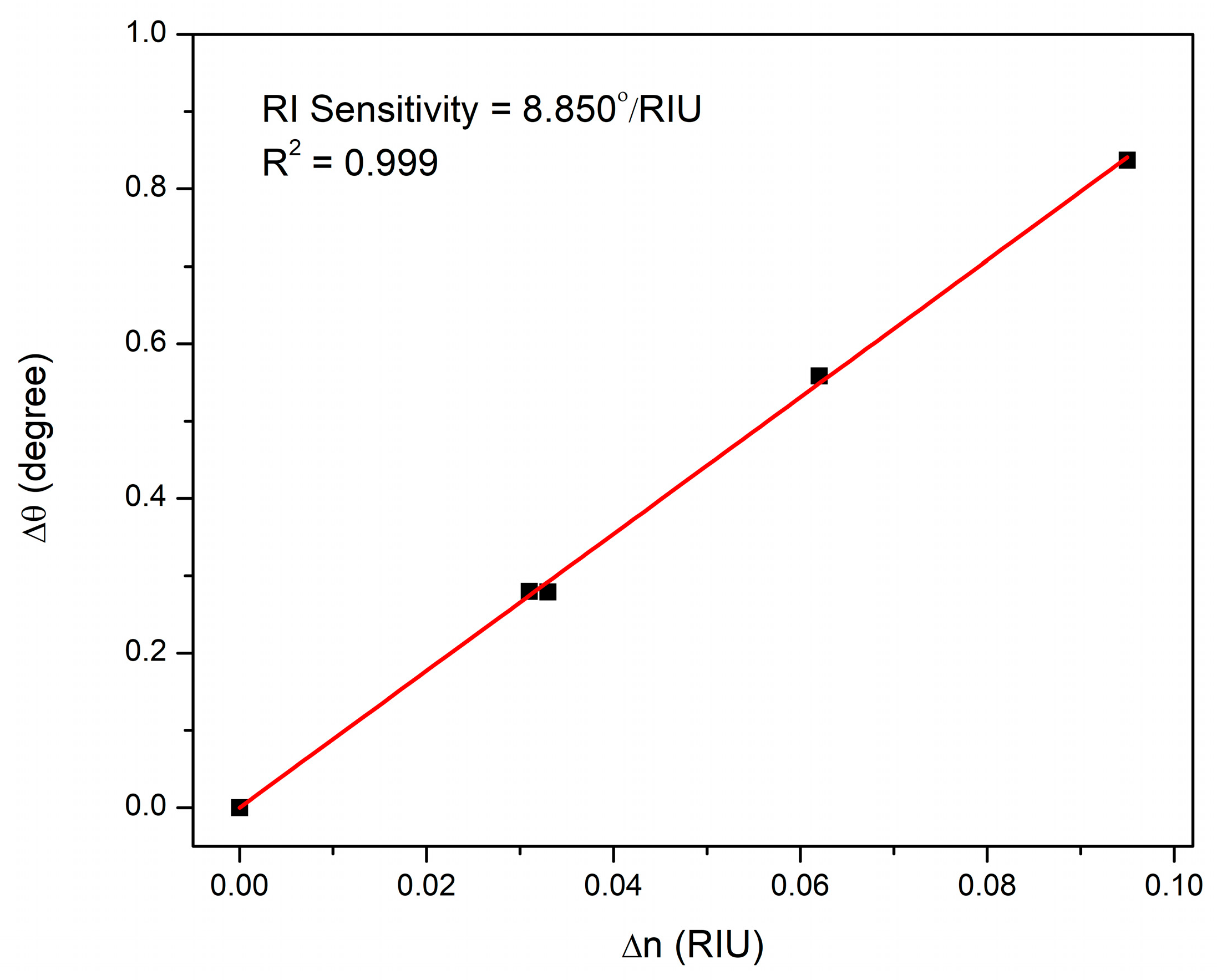
| DA Concentration (nM) | Resonance Angle (deg) | Δθ (deg) |
|---|---|---|
| 0.000 | 54.675 | 0.000 |
| 0.001 | 54.675 | 0.000 |
| 0.01 | 54.675 | 0.000 |
| 0.1 | 54.675 | 0.000 |
| 1 | 54.955 | 0.280 |
| 10 | 54.954 | 0.279 |
| 100 | 55.234 | 0.559 |
| 1000 | 55.512 | 0.837 |
| DA Concentration (nM) | Refractive Index of DA Solution | Refractive Index of CS Layer Exposed to DA | Thickness of CS Layer d (nm) (±0.01) | Δn (±0.0001) | Δθ | ||
|---|---|---|---|---|---|---|---|
| Real Part, n (±0.0001) | Imaginary Part, k (±0.0001) | Real Part, n (±0.0001) | Imaginary Part, k (±0.0001) | ||||
| 0 | 1.3333 | 0.0000 | 1.5350 | 0.0150 | 15.00 | 0.0000 | 0.000 ± 0.001 |
| 0.001 | 1.3333 | 0.0000 | 1.5350 | 0.0150 | 15.00 | 0.0000 | 0.000 ± 0.001 |
| 0.01 | 1.3333 | 0.0030 | 1.5350 | 0.0150 | 15.00 | 0.0000 | 0.000 ± 0.001 |
| 0.1 | 1.3333 | 0.0030 | 1.5350 | 0.0150 | 15.00 | 0.0000 | 0.000 ± 0.001 |
| 1 | 1.3333 | 0.0030 | 1.5660 | 0.0130 | 15.50 | 0.0310 | 0.280 ± 0.018 |
| 10 | 1.3333 | 0.0030 | 1.5680 | 0.0130 | 15.50 | 0.0330 | 0.279 ± 0.017 |
| 100 | 1.3333 | 0.0030 | 1.5970 | 0.0110 | 15.90 | 0.0620 | 0.559 ± 0.022 |
| 1000 | 1.3333 | 0.0050 | 1.6300 | 0.0100 | 16.25 | 0.0950 | 0.837 ± 0.021 |
| Materials | Technique | LOD | Reference |
|---|---|---|---|
| Au/CS | SERS | 1 mM | [70] |
| Au@CDs/CS | Electrochemical | 0.001 µM | [71] |
| Graphene/CS | Electrochemical | 5 µM | [72] |
| CS-CDs/GCE | Electrochemical | 11.2 nM | [84] |
| Nitrogen-doped graphene quantum dots-CS | Electrochemical | 0.145 µM | [85] |
| Reduced graphene oxide-Au nanoparticles-CS/silica | Electrochemical | 0.3 µM | [86] |
| CS /Au | SPR | 0.007 nM | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eddin, F.B.K.; Fen, Y.W.; Liew, J.Y.C.; Daniyal, W.M.E.M.M. Plasmonic Refractive Index Sensor Enhanced with Chitosan/Au Bilayer Thin Film for Dopamine Detection. Biosensors 2022, 12, 1124. https://doi.org/10.3390/bios12121124
Eddin FBK, Fen YW, Liew JYC, Daniyal WMEMM. Plasmonic Refractive Index Sensor Enhanced with Chitosan/Au Bilayer Thin Film for Dopamine Detection. Biosensors. 2022; 12(12):1124. https://doi.org/10.3390/bios12121124
Chicago/Turabian StyleEddin, Faten Bashar Kamal, Yap Wing Fen, Josephine Ying Chyi Liew, and Wan Mohd Ebtisyam Mustaqim Mohd Daniyal. 2022. "Plasmonic Refractive Index Sensor Enhanced with Chitosan/Au Bilayer Thin Film for Dopamine Detection" Biosensors 12, no. 12: 1124. https://doi.org/10.3390/bios12121124
APA StyleEddin, F. B. K., Fen, Y. W., Liew, J. Y. C., & Daniyal, W. M. E. M. M. (2022). Plasmonic Refractive Index Sensor Enhanced with Chitosan/Au Bilayer Thin Film for Dopamine Detection. Biosensors, 12(12), 1124. https://doi.org/10.3390/bios12121124







