Paper-Based Electrochemical Biosensors for Food Safety Analysis
Abstract
1. Introduction
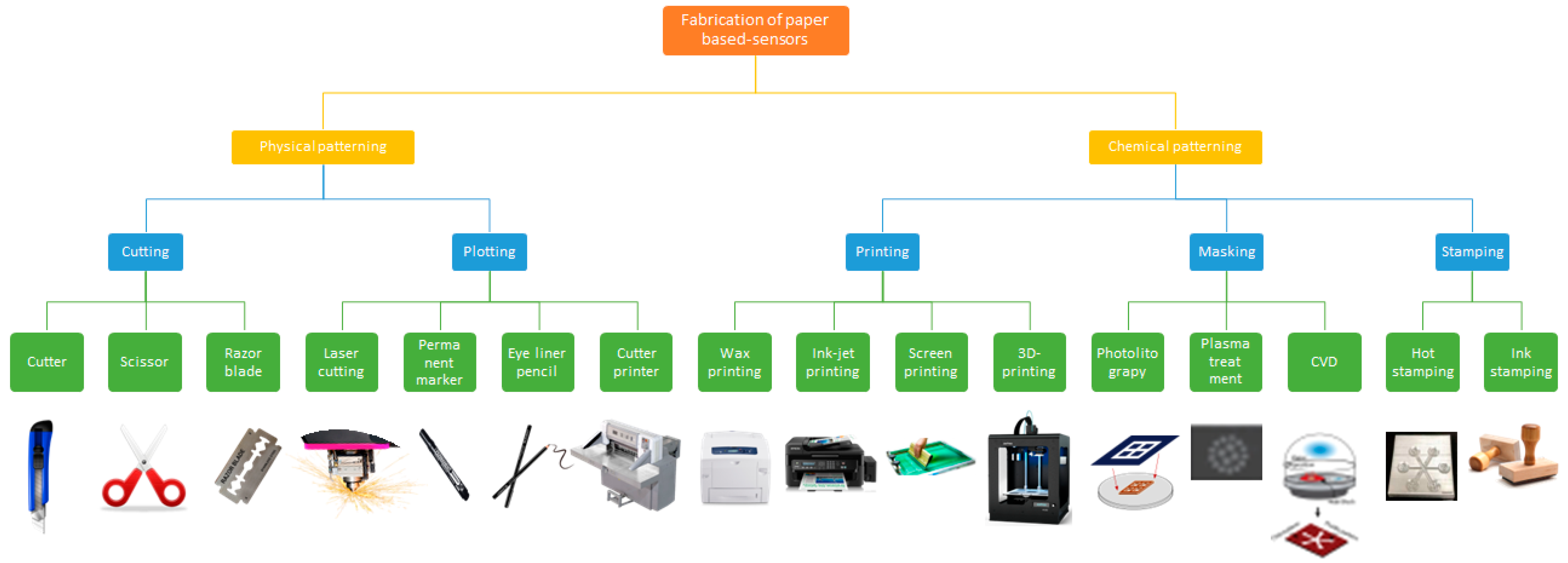
2. Paper Design and Fabrication
2.1. Paper Types
| No. | High-Adsorption | Electrochemical Technique | References | Low-Adsorption | Electrochemical Technique | References |
|---|---|---|---|---|---|---|
| 1 | Whatman No.1 filter paper | Differential pulse voltammetry | [22] | Cellulose acetate filter paper | Cyclic voltammetry & amperometry | [26] |
| 2 | Whatman chromatography paper (3 mm) | Differential pulse voltammetry | [27] | Mixed cellulose ester (MCE) | Cyclic voltammetry | [28] |
| 3 | Whatman RC60 regenerated membrane filter | Cyclic voltammetry | [29] | Office paper | Electrochemical impedance spectroscopy | [30] |
| 4 | Filter papers (102, 15 mm) | Cyclic voltammetry and chronoamperometry | [31] | Art paper | Linear sweep voltammetry | [32] |
| 5 | Labor filter paper (67 g/m2) | Cyclic voltammetry & chronoamperometric | [33] | PVDF filter membrane | Cyclic voltammetry & differential pulse voltammetry | [34] |
| 6 | Nitrocellulose membrane | Cyclic voltammetry & differential pulse voltammetry | [35] | Copy paper (80 g/m2) | chronoamperometry | [19] |
2.2. Device Fabrication
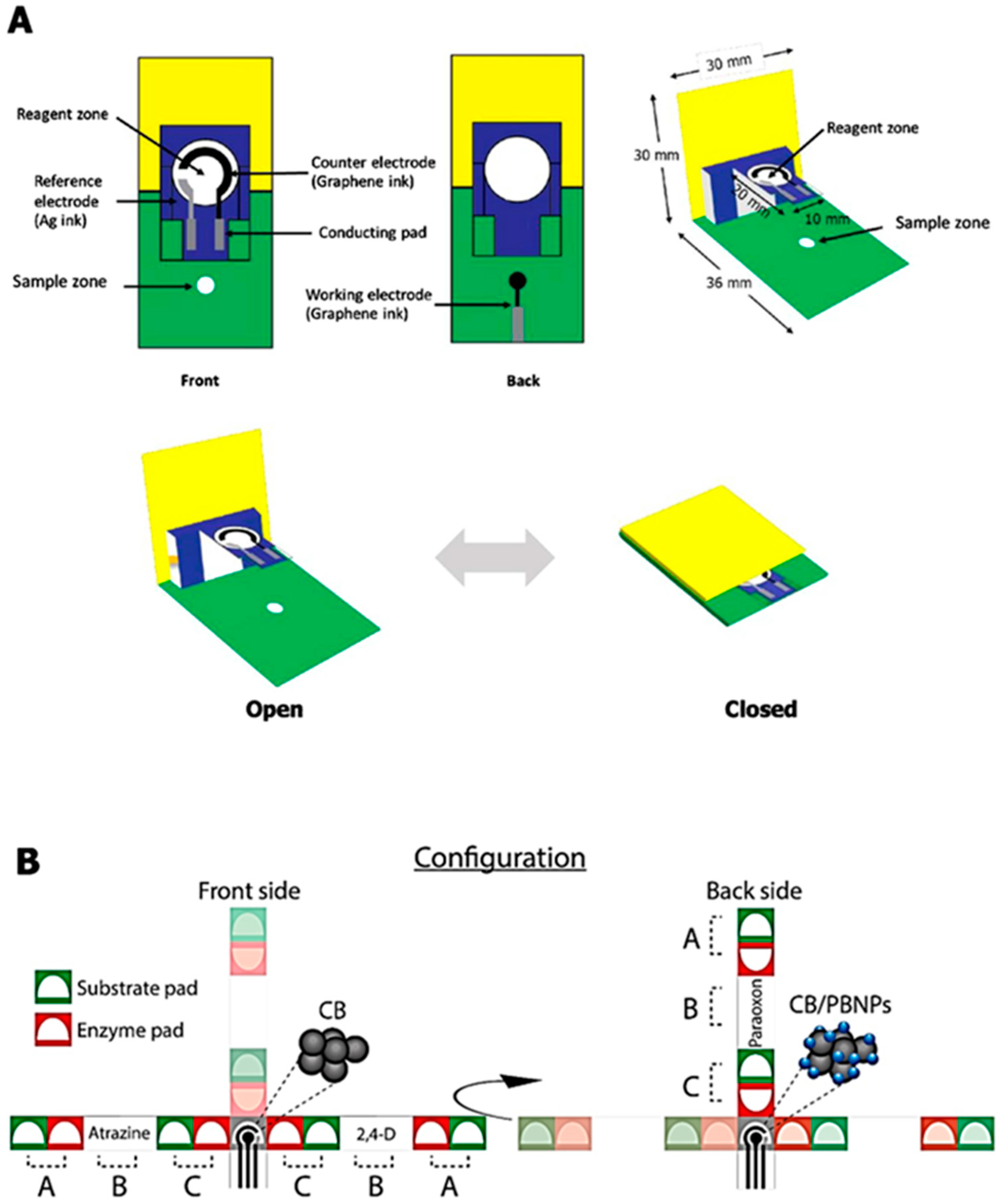
2.3. 2D and 3D Designs
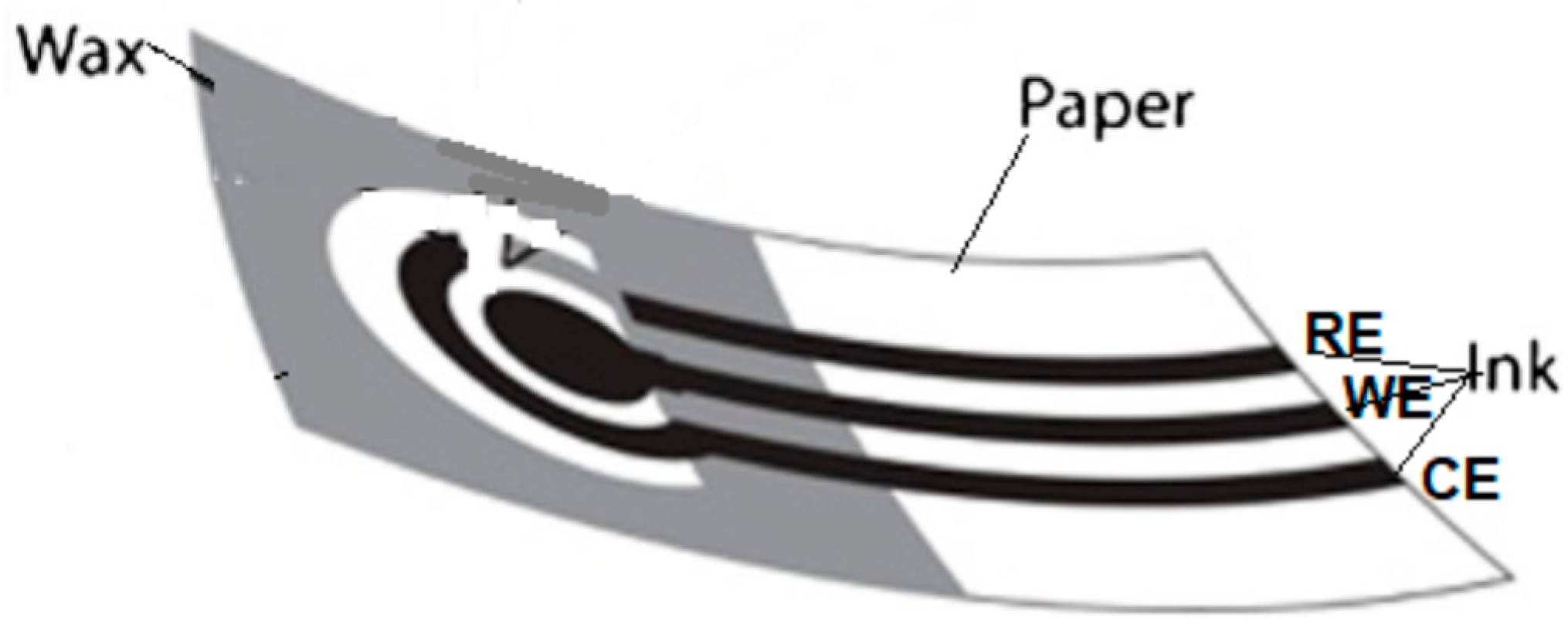
2.4. Patterning Hydrophobic Barriers
2.5. Electrode Fabrication
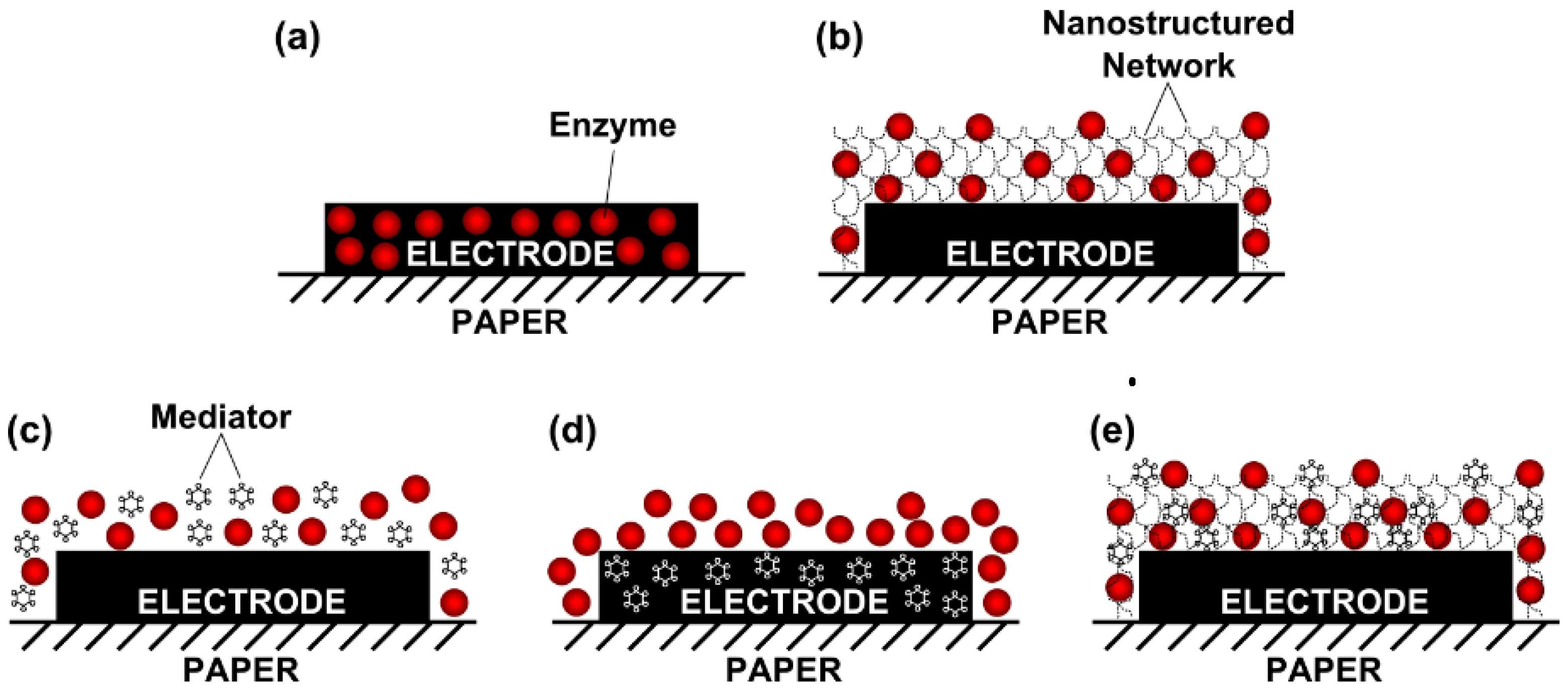
2.6. Surface Modification
3. Applications for Food Safety
3.1. Foodborne Pathogens
| Analyte | Detection Principle | Electrochemical Technique | Sample Matrix | Sample Volume/Size | Detection Limit | RSD | Assay Time | References |
|---|---|---|---|---|---|---|---|---|
| Botulinum toxin (C. botulinum) | Catalytic activity of toxin toward a synthetic peptide | SWV | Orange juice | 100 mL | 10 pM | <10% | 4 h | [75] |
| E. coli O157:H7 | Immunoassay | EIS | Ground beef and cucumber | 10 g | 150 CFU/mL | <15% | NS | [78] |
| Aptamer-based assay | EIS | Standard solution | NS | 4 CFU/mL | <5% | 12 min | [80] | |
| Enzymatic assay | SWV | Alfalfa sprout | 10 g | 10 CFU/mL (after enrichment) | NS | 8 h | [79] | |
| E. faecalis, E. faecium | Enzymatic assay | SWV | Alfalfa sprout | 10 g | 10 CFU/mL (after enrichment) | NS | 12 h | [79] |
| L. monocytogenes | Aptamer-based assay | EIS | Cheese and milk | NS | 10 CFU/mL | <6% | NS | [81] |
| S. aureus | Immunoassay | DPV | Milk | NS | 13 CFU/mL | <11% | ~30 min | [83] |
| DNA hybridization | DPV | Fruit juice | NS | 0.1 nM | <5% | 10 s (response time) | [84] | |
| S. typhimurium | Immunoassay | Potentiometry | Apple juice | NS | 5 cells/mL | <15% | <1 h | [85] |
| Methylene blue-mediated detection of LAMP-amplified DNA | DPV | Drinking water and milk | 10 mL | 2 CFU/mL (water), 5 CFU/mL (milk) | <10% | NS, 45 min for LAMP | [86] | |
| Norovirus | DNA hybridization | DPV | Standard solution | 5 mL | 100 fM | <5% | 5 s (response time) | [76] |
3.2. Pesticides
3.3. Veterinary Drugs
3.4. Allergens
3.5. Heavy Metals
| Analyte | Detection Principle | Electrochemical Technique | Sample Matrix | Sample Volume/Size | Detection Limit | RSD | Assay Time | References |
|---|---|---|---|---|---|---|---|---|
| Cd(II) | Direct detection | DPV | Rice | 0.2 g | 0.1 ng/mL | 20–40% | ~1 h | [127] |
| Cd(II), Pb(II) | Direct detection | SWASV | Soda water | 100 mL | 2.3 ng/mL (Cd) 2.0 ng/mL (Pb) | <5% | 4 min | [125] |
| ASV | Drinking water | 500 mL | 2.33 ng/mL (Cd) 0.97 ng/mL (Pb) | 5–10% | ~20 min | [124] | ||
| DPASV | Fish food | 1 g | 3.1 ng/mL (Cd) 4.5 ng/mL (Pb) | <15% | ~5 min * | [123] | ||
| DPASV/SWASV | Tap water | 160 mL | 2.4 ng/mL (Cd) 4.2 ng/mL (Pb) | <15% | ~8 min | [128] | ||
| Aptamer-based assay | SWV | Vegetable and fruit | 60 mL | 23.3 pM (Cd) 46.2 pM (Pb) | <10% | 15 min * | [123] | |
| Cd(II), Pb(II), Zn(II) | Direct detection | SWASV | River water | 100 mL | 1.3 ng/mL (Cd) 0.9 ng/mL (Pb) 10.5 ng/mL (Zn) | <15% | ~5 min | [121] |
| Hg(II) | Direct detection | ASV | River water | 40 mL | 30 nM | <10% | ~10 min | [129] |
| Ni(II) | Direct detection | AdCSV | Water | 20 mL | 6.27 ng/mL | <5% | ~ 3 min | [130] |
| Pb(II), Sn(II) | Direct detection | SWASV | Canned food | 500 mL/1 g | 0.26 ng/mL (Pb) 0.44 ng/mL (Sn) | <5% | 2 min * | [89] |
| Zn(II) | DNAzyme-based assay | DPV | Tap water | 5 mL | 0.03 nM | <15% | ~40 min | [131] |
4. Conclusions, Challenges, and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Popa, A.; Hnatiuc, M.; Paun, M.; Geman, O.; Hemanth, D.J.; Dorcea, D.; Son, L.H.; Ghita, S. An Intelligent IoT-Based Food Quality Monitoring Approach Using Low-Cost Sensors. Symmetry 2019, 11, 374. [Google Scholar] [CrossRef]
- Wu, M.Y.C.; Hsu, M.Y.; Chen, S.J.; Hwang, D.K.; Yen, T.H.; Cheng, C.M. Point-of-Care Detection Devices for Food Safety Monitoring: Proactive Disease Prevention. Trends Biotechnol. 2017, 35, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Emerging Point-of-Care Technologies for Food Safety Analysis. Sensors 2019, 19, 817. [Google Scholar] [CrossRef]
- Vidic, J.; Vizzini, P.; Manzano, M.; Kavanaugh, D.; Ramarao, N.; Zivkovic, M.; Radonic, V.; Knezevic, N.; Giouroudi, I.; Gadjanski, I. Point-of-Need DNA Testing for Detection of Foodborne Pathogenic Bacteria. Sensors 2019, 19, 1100. [Google Scholar] [CrossRef] [PubMed]
- Rovina, K.; Siddiquee, S. A Review of Recent Advances in Melamine Detection Techniques. J. Food Compos. Anal. 2015, 43, 25–38. [Google Scholar] [CrossRef]
- Wojnowski, W.; Kalinowska, K.; Majchrzak, T.; Płotka-Wasylka, J.; Namieśnik, J. Prediction of the Biogenic Amines Index of Poultry Meat Using an Electronic Nose. Sensors 2019, 19, 1580. [Google Scholar] [CrossRef] [PubMed]
- Scordo, G.; Moscone, D.; Palleschi, G.; Arduini, F. A Reagent-Free Paper-Based Sensor Embedded in a 3D Printing Device for Cholinesterase Activity Measurement in Serum. Sens. Actuators B Chem. 2018, 258, 1015–1021. [Google Scholar] [CrossRef]
- Cinti, S.; Fiore, L.; Massoud, R.; Cortese, C.; Moscone, D.; Palleschi, G.; Arduini, F. Low-Cost and Reagent-Free Paper-Based Device to Detect Chloride Ions in Serum and Sweat. Talanta 2018, 179, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Moscone, D.; Arduini, F. Preparation of Paper-Based Devices for Reagentless Electrochemical (Bio)Sensor Strips. Nat. Protoc. 2019, 14, 2437–2451. [Google Scholar] [CrossRef]
- Jemmeli, D.; Marcoccio, E.; Moscone, D.; Dridi, C.; Arduini, F. Highly Sensitive Paper-Based Electrochemical Sensor for Reagent Free Detection of Bisphenol A. Talanta 2020, 216, 120924. [Google Scholar] [CrossRef] [PubMed]
- Caratelli, V.; Ciampaglia, A.; Guiducci, J.; Sancesario, G.; Moscone, D.; Arduini, F. Precision Medicine in Alzheimer’s Disease: An Origami Paper-Based Electrochemical Device for Cholinesterase Inhibitors. Biosens. Bioelectron. 2020, 165, 112411. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Mazzaracchio, V.; Cinti, S.; Colozza, N.; Di Natale, C.; Netti, P.A.; Saraji, M.; Roggero, S.; Moscone, D.; Arduini, F. Electroanalytical Sensor Based on Gold-Nanoparticle-Decorated Paper for Sensitive Detection of Copper Ions in Sweat and Serum. Anal. Chem. 2021, 93, 5225–5233. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Cusenza, R.; Moscone, D.; Arduini, F. Paper-Based Synthesis of Prussian Blue Nanoparticles for the Development of Whole Blood Glucose Electrochemical Biosensor. Talanta 2018, 187, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Cinti, S.; Caratelli, V.; Amendola, L.; Palleschi, G.; Moscone, D. Origami Multiple Paper-Based Electrochemical Biosensors for Pesticide Detection. Biosens. Bioelectron. 2019, 126, 346–354. [Google Scholar] [CrossRef]
- Colozza, N.; Tazzioli, S.; Sassolini, A.; Agosta, L.; di Monte, M.G.; Hermansson, K.; Arduini, F. Multiparametric Analysis by Paper-Assisted Potentiometric Sensors for Diagnostic and Monitoring of Reinforced Concrete Structures. Sens. Actuators B Chem. 2021, 345, 130352. [Google Scholar] [CrossRef]
- Colozza, N.; Kehe, K.; Dionisi, G.; Popp, T.; Tsoutsoulopoulos, A.; Steinritz, D.; Moscone, D.; Arduini, F. A Wearable Origami-like Paper-Based Electrochemical Biosensor for Sulfur Mustard Detection. Biosens. Bioelectron. 2019, 129, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem.-Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef]
- Ellerbee, A.K.; Phillips, S.T.; Siegel, A.C.; Mirica, K.A.; Martinez, A.W.; Striehl, P.; Jain, N.; Prentiss, M.; Whitesides, G.M. Quantifying Colorimetric Assays in Paper-Based Microfluidic Devices by Measuring the Transmission of Light through Paper. Anal. Chem. 2009, 81, 8447–8452. [Google Scholar] [CrossRef] [PubMed]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical Detection for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Du, D.; Hua, X.; Yu, X.Y.; Lin, Y. Paper-Based Electrochemical Biosensors: From Test Strips to Paper-Based Microfluidics. Electroanalysis 2014, 26, 1214–1223. [Google Scholar] [CrossRef]
- Colozza, N.; Caratelli, V.; Moscone, D.; Arduini, F. Origami Paper-Based Electrochemical (Bio)Sensors: State of the Art and Perspective. Biosensors 2021, 11, 328. [Google Scholar] [CrossRef]
- Ma, C.; Li, W.; Kong, Q.; Yang, H.; Bian, Z.; Song, X.; Yu, J.; Yan, M. 3D Origami Electrochemical Immunodevice for Sensitive Point-of-Care Testing Based on Dual-Signal Amplification Strategy. Biosens. Bioelectron. 2015, 63, 7–13. [Google Scholar] [CrossRef]
- Lee, V.B.C.; Mohd-Naim, N.F.; Tamiya, E.; Ahmed, M.U. Trends in Paper-Based Electrochemical Biosensors: From Design to Application. Anal. Sci. 2018, 34, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Novel Paper-Based Electroanalytical Tools for Food Surveillance. Anal. Bioanal. Chem. 2019, 411, 4303–4311. [Google Scholar] [CrossRef]
- Silveira, C.; Monteiro, T.; Almeida, M. Biosensing with Paper-Based Miniaturized Printed Electrodes–A Modern Trend. Biosensors 2016, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Basso, M.; Moscone, D.; Arduini, F. A Paper-Based Nanomodified Electrochemical Biosensor for Ethanol Detection in Beers. Anal. Chim. Acta 2017, 960, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.; Devarakonda, S.; Kumar, S.; Jang, J. Development of a Paper-Based Electrochemical Immunosensor Using an Antibody-Single Walled Carbon Nanotubes Bio-Conjugate Modified Electrode for Label-Free Detection of Foodborne Pathogens. Sens. Actuators B Chem. 2017, 253, 115–123. [Google Scholar] [CrossRef]
- Wang, P.; Wang, M.; Zhou, F.; Yang, G.; Qu, L.; Miao, X. Development of a Paper-Based, Inexpensive, and Disposable Electrochemical Sensing Platform for Nitrite Detection. Electrochem. commun. 2017, 81, 74–78. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A.; Bakker, E. Paper-Based Thin-Layer Coulometric Sensor for Halide Determination. Anal. Chem. 2015, 87, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Boonyasit, Y.; Chailapakul, O.; Laiwattanapaisal, W. A Multiplexed Three-Dimensional Paper-Based Electrochemical Impedance Device for Simultaneous Label-Free Affinity Sensing of Total and Glycated Haemoglobin: The Potential of Using a Specific Single-Frequency Value for Analysis. Analytica Chimica Acta. 2016, 936, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, F.; Xing, Y.; Liu, Z.; You, M.; Li, Y.; Wen, T.; Qu, Z.; Li, X.L.; Xu, F. Pen-on-Paper Strategy for Point-of-Care Testing: Rapid Prototyping of Fully Written Microfluidic Biosensor. Biosens. Bioelectron. 2017, 98, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, W.; Lv, Q.; Xi, G.; Bai, H.; Zhang, Q. Disposable Paper-Based Electrochemical Sensor Based on Stacked Gold Nanoparticles Supported Carbon Nanotubes for the Determination of Bisphenol A. Electrochem. Commun. 2016, 68, 104–107. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. Nanomaterials in Electrochemical Biosensors for Pesticide Detection: Advances and Challenges in Food Analysis. Mikrochim. Acta 2016, 183, 2063–2083. [Google Scholar] [CrossRef]
- Wang, X.; Lin, G.; Cui, G.; Zhou, X.; Liu, G.L. White Blood Cell Counting on Smartphone Paper Electrochemical Sensor. Biosens. Bioelectron. 2017, 90, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.H.; Tran, V.K.; Ko, E.; Park, C.H.; Chung, W.S.; Seong, G.H. Determination of Acetaminophen Using Functional Paper-Based Electrochemical Devices. Sens. Actuators B Chem. 2016, 232, 514–522. [Google Scholar] [CrossRef]
- Lim, S.A.; Ahmed, M.U. A Carbon Nanofiber-Based Label Free Immunosensor for High Sensitive Detection of Recombinant Bovine Somatotropin. Biosens. Bioelectron. 2015, 70, 48–53. [Google Scholar] [CrossRef]
- Narang, J.; Malhotra, N.; Singhal, C.; Mathur, A.; Chakraborty, D.; Anil, A.; Ingle, A.; Pundir, C.S. Point of Care with Micro Fluidic Paper Based Device Integrated with Nano Zeolite–Graphene Oxide Nanoflakes for Electrochemical Sensing of Ketamine. Biosens. Bioelectron. 2017, 88, 249–257. [Google Scholar] [CrossRef]
- Caratelli, V.; Fegatelli, G.; Moscone, D.; Arduini, F. A Paper-Based Electrochemical Device for the Detection of Pesticides in Aerosol Phase Inspired by Nature: A Flower-like Origami Biosensor for Precision Agriculture. Biosens. Bioelectron. 2022, 205, 114119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ge, P.; Wang, L.; Jiang, H.; Yang, M.; Yuan, L.; Ge, Q.; Fang, W.; Ju, X. A Novel Electrochemical Mast Cell-Based Paper Biosensor for the Rapid Detection of Milk Allergen Casein. Biosens. Bioelectron. 2019, 130, 299–306. [Google Scholar] [CrossRef]
- Sun, X.; Jian, Y.; Wang, H.; Ge, S.; Yan, M.; Yu, J. Ultrasensitive Microfluidic Paper-Based Electrochemical Biosensor Based on Molecularly Imprinted Film and Boronate Affinity Sandwich Assay for Glycoprotein Detection. ACS Appl. Mater. Interfaces 2019, 11, 16198–16206. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, Q.; Zhang, C.; Sun, Z.; Weng, X. Microfluidic Origami Nano-Aptasensor for Peanut Allergen Ara H1 Detection. Food Chem. 2021, 365, 130511. [Google Scholar] [CrossRef] [PubMed]
- Terzi, F.; Zanfrognini, B.; Ruggeri, S.; Dossi, N.; Casagrande, G.M.; Piccin, E. Amperometric Paper Sensor Based on Cu Nanoparticles for the Determination of Carbohydrates. Sens. Actuators B Chem. 2017, 245, 352–358. [Google Scholar] [CrossRef]
- Lamas-Ardisana, P.J.; Casuso, P.; Fernandez-Gauna, I.; Martínez-Paredes, G.; Jubete, E.; Añorga, L.; Cabañero, G.; Grande, H.J. Disposable Electrochemical Paper-Based Devices Fully Fabricated by Screen-Printing Technique; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Cinti, S.; Colozza, N.; Cacciotti, I.; Moscone, D.; Polomoshnov, M.; Sowade, E.; Baumann, R.R.; Arduini, F. Electroanalysis Moves towards Paper-Based Printed Electronics: Carbon Black Nanomodified Inkjet-Printed Sensor for Ascorbic Acid Detection as a Case Study. Sens. Actuators B Chem. 2018, 265, 155–160. [Google Scholar] [CrossRef]
- Li, W.; Qian, D.; Wang, Q.; Li, Y.; Bao, N.; Gu, H.; Yu, C. Fully-Drawn Origami Paper Analytical Device for Electrochemical Detection of Glucose. Sens. Actuators, B Chem. 2016, 231, 230–238. [Google Scholar] [CrossRef]
- Nie, Z.; Nijhuis, C.A.; Gong, J.; Chen, X.; Kumachev, A.; Martinez, A.W.; Narovlyansky, M.; Whitesides, G.M. Electrochemical Sensing in Paper-Based Microfluidic Devices. Lab Chip 2010, 10, 477–483. [Google Scholar] [CrossRef]
- Kong, F.Y.; Gu, S.X.; Li, W.W.; Chen, T.T.; Xu, Q.; Wang, W. A Paper Disk Equipped with Graphene/Polyaniline/Au Nanoparticles/Glucose Oxidase Biocomposite Modified Screen-Printed Electrode: Toward Whole Blood Glucose Determination. Biosens. Bioelectron. 2014, 56, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Srisomwat, C.; Yakoh, A.; Chuaypen, N.; Tangkijvanich, P.; Vilaivan, T.; Chailapakul, O. Amplification-Free DNA Sensor for the One-Step Detection of the Hepatitis B Virus Using an Automated Paper-Based Lateral Flow Electrochemical Device. Anal. Chem. 2021, 93, 2879–2887. [Google Scholar] [CrossRef]
- Li, X.; Scida, K.; Crooks, R.M. Detection of Hepatitis B Virus DNA with a Paper Electrochemical Sensor. Anal. Chem. 2015, 87, 9009–9015. [Google Scholar] [CrossRef]
- Srisomwat, C.; Teengam, P.; Chuaypen, N.; Tangkijvanich, P.; Vilaivan, T.; Chailapakul, O. Pop-up Paper Electrochemical Device for Label-Free Hepatitis B Virus DNA Detection. Sens. Actuators B Chem. 2020, 316, 128077. [Google Scholar] [CrossRef]
- Fischer, C.; Fraiwan, A.; Choi, S. A 3D Paper-Based Enzymatic Fuel Cell for Self-Powered, Low-Cost Glucose Monitoring. Biosens. Bioelectron. 2016, 79, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Su, W.; Ding, X. A Review on Microfluidic Paper-Based Analytical Devices for Glucose Detection. Sensors 2016, 16, 2086. [Google Scholar] [CrossRef]
- Tenjimbayashi, M.; Higashi, M.; Yamazaki, T.; Takenaka, I.; Matsubayashi, T.; Moriya, T.; Komine, M.; Yoshikawa, R.; Manabe, K.; Shiratori, S. Droplet Motion Control on Dynamically Hydrophobic Patterned Surfaces as Multifunctional Liquid Manipulators. ACS Appl. Mater. Interfaces 2017, 9, 10371–10377. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Hidayat, M.A.; Noviana, E. Paper-Based Sensors for Rapid Important Biomarkers Detection. Biosens. Bioelectron. X 2022, 12, 100246. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, C.; Sciences, Y.L.-A. Screen Printed Paper-Based Diagnostic Devices with Polymeric Inks. Anal. Sci. 2015, 31, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. A Low-Cost, Simple, and Rapid Fabrication Method for Paper-Based Microfluidics Using Wax Screen-Printing. Analyst 2011, 136, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Cook, B.S.; Fang, Y.; Tentzeris, M.M. Fully Inkjet-Printed Microfluidics: A Solution to Low-Cost Rapid Three-Dimensional Microfluidics Fabrication with Numerous Electrical and Sensing Applications. Sci. Rep. 2016, 6, 35111. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Fang, C.; Zeng, R.; Zhao, X.; Jiang, Y.; Chen, Z. Paper-Based Microfluidic Devices for Electrochemical Immunofiltration Analysis of Human Chorionic Gonadotropin. Biosens. Bioelectron. 2017, 92, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.; Blondeel, E.; Analyst, M.K. Creating Compact and Microscale Features in Paper-Based Devices by Laser Cutting. Analyst 2016, 141, 6449–6454. [Google Scholar] [CrossRef] [PubMed]
- Liana, D.; Raguse, B.; Gooding, J.; Chow, E. Recent Advances in Paper-Based Sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent Developments in Paper-Based Microfluidic Devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Xiao, F.; Wu, Z.; Talanta, R.Y. Undefined Sensitive Inkjet Printing Paper-Based Colormetric Strips for Acetylcholinesterase Inhibitors with Indoxyl Acetate Substrate; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Adkins, J.; Boehle, K.; Henry, C. Electrochemical Paper-Based Microfluidic Devices. Electrophoresis 2015, 36, 1811–1824. [Google Scholar] [CrossRef]
- Cunningham, J.C.; DeGregory, P.R.; Crooks, R.M. New Functionalities for Paper-Based Sensors Lead to Simplified User Operation, Lower Limits of Detection, and New Applications. Annu. Rev. Anal. Chem. 2016, 9, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Ruecha, N.; Rangkupan, R.; Rodthongkum, N.; Chailapakul, O. Novel Paper-Based Cholesterol Biosensor Using Graphene/Polyvinylpyrrolidone/Polyaniline Nanocomposite. Biosens. Bioelectron. 2014, 52, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zaman, M.H. Amperometric Measurements of Ethanol on Paper with a Glucometer. Talanta 2015, 134, 194–199. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N. Electrochemical and Photoelectrochemical Nano-Immunesensing Using Origami Paper Based Method. Mater. Sci. Eng. C 2016, 61, 979–1001. [Google Scholar] [CrossRef] [PubMed]
- Scida, K.; Cunningham, J.C.; Renault, C.; Richards, I.; Crooks, R.M. Simple, Sensitive, and Quantitative Electrochemical Detection Method for Paper Analytical Devices. Anal. Chem. 2014, 86, 6501–6507. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, L.; Yu, J. Paper-Based Microfluidic Devices in Bioanalysis: How Far Have We Come? Bioanalysis 2015, 7, 633–636. [Google Scholar] [CrossRef]
- Wu, L.; Ma, C.; Zheng, X.; Liu, H.; Yu, J. Paper-Based Electrochemiluminescence Origami Device for Protein Detection Using Assembled Cascade DNA–Carbon Dots Nanotags Based on Rolling Circle Amplification. Biosens. Bioelectron. 2015, 68, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Vergara, A.; Rossi, M.; Gravagnuolo, A.M.; Valadan, M.; Corrado, F.; Conte, M.; Gesuele, F.; Giardina, P.; Altucci, C. Electrostatically Driven Scalable Synthesis of MoS2-Graphene Hybrid Films Assisted by Hydrophobins. RSC Adv. 2017, 7, 50166–50175. [Google Scholar] [CrossRef]
- Khan, S.; Verma, N.C.; Chethana; Nandi, C.K. Carbon Dots for Single-Molecule Imaging of the Nucleolus. ACS Appl. Nano Mater. 2018, 1, 483–487. [Google Scholar] [CrossRef]
- Lettieri, S.; Avitabile, A.; Della Ventura, B.; Funari, R.; Ambrosio, A.; Maddalena, P.; Valadan, M.; Velotta, R.; Altucci, C. Nano- and Femtosecond UV Laser Pulses to Immobilize Biomolecules onto Surfaces with Preferential Orientation. Appl. Phys. A Mater. Sci. Process. 2014, 117, 185–190. [Google Scholar] [CrossRef][Green Version]
- Caratelli, V.; Fillo, S.; D’Amore, N.; Rossetto, O.; Pirazzini, M.; Moccia, M.; Avitabile, C.; Moscone, D.; Lista, F.; Arduini, F. Paper-Based Electrochemical Peptide Sensor for on-Site Detection of Botulinum Neurotoxin Serotype A and C. Biosens. Bioelectron. 2021, 183, 113210. [Google Scholar] [CrossRef]
- Rana, A.; Killa, M.; Yadav, N.; Mishra, A.; Mathur, A.; Kumar, A.; Khanuja, M.; Narang, J.; Pilloton, R. Graphitic Carbon Nitride as an Amplification Platform on an Electrochemical Paper-Based Device for the Detection of Norovirus-Specific DNA. Sensors 2020, 20, 2070. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Yoon, J.W.; Hovde, C.J. A Brief Overview of Escherichia Coli O157: H7 and Its Plasmid O157. J. Microbiol. Biotechnol. 2010, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ping, J.; Ye, Z.; Wu, J.; Ying, Y. Impedimetric Immunosensor Based on Gold Nanoparticles Modified Graphene Paper for Label-Free Detection of Escherichia Coli O157:H7. Biosens. Bioelectron. 2013, 49, 492–498. [Google Scholar] [CrossRef]
- Adkins, J.A.; Boehle, K.; Friend, C.; Chamberlain, B.; Bisha, B.; Henry, C.S. Colorimetric and Electrochemical Bacteria Detection Using Printed Paper- and Transparency-Based Analytic Devices. Anal. Chem. 2017, 89, 3613–3621. [Google Scholar] [CrossRef]
- Burrs, S.L.; Bhargava, M.; Sidhu, R.; Kiernan-Lewis, J.; Gomes, C.; Claussen, J.C.; McLamore, E.S. A Paper Based Graphene-Nanocauliflower Hybrid Composite for Point of Care Biosensing. Biosens. Bioelectron. 2016, 85, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Pilloton, R.; Jain, S.; Roy, S.; Khanuja, M.; Mathur, A.; Narang, J. Paper-Based Electrodes Conjugated with Tungsten Disulfide Nanostructure and Aptamer for Impedimetric Detection of Listeria Monocytogenes. Biosensors 2022, 12, 88. [Google Scholar] [CrossRef]
- Hamon, M.; Bierne, H.; Cossart, P. Listeria Monocytogenes: A Multifaceted Model. Nat. Rev. Microbiol. 2006, 4, 423–434. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.C.; Alves, G.F.; Lisboa, T.P.; Matos, M.A.C.; Matos, R.C. Low-Cost Paper-Based Electrochemical Sensor for the Detection of Ciprofloxacin in Honey and Milk Samples. J. Food Compos. Anal. 2022, 112, 104700. [Google Scholar] [CrossRef]
- Mathur, A.; Gupta, R.; Kondal, S.; Wadhwa, S.; Pudake, R.N.; Kansal, R.; Pundir, C.S.; Narang, J. A New Tactics for the Detection of S. Aureus via Paper Based Geno-Interface Incorporated with Graphene Nano Dots and Zeolites. Int. J. Biol. Macromol. 2018, 112, 364–370. [Google Scholar] [CrossRef]
- Silva, N.F.D.; Almeida, C.M.R.; Magalhães, J.M.C.S.; Gonçalves, M.P.; Freire, C.; Delerue-Matos, C. Development of a Disposable Paper-Based Potentiometric Immunosensor for Real-Time Detection of a Foodborne Pathogen. Biosens. Bioelectron. 2019, 141, 111317. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Li, J.; Liu, L.; Ge, S.; Yan, M.; Liu, H.; Yu, J. Origami-Based “Book” Shaped Three-Dimensional Electrochemical Paper Microdevice for Sample-to-Answer Detection of Pathogens. RSC Adv. 2020, 10, 25808–25816. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Monis, P.T.; Giglio, S. Nucleic Acid Amplification-Based Techniques for Pathogen Detection and Identification. Infect. Genet. Evol. 2006, 6, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Pungjunun, K.; Nantaphol, S.; Praphairaksit, N.; Siangproh, W.; Chaiyo, S.; Chailapakul, O. Enhanced Sensitivity and Separation for Simultaneous Determination of Tin and Lead Using Paper-Based Sensors Combined with a Portable Potentiostat. Sens. Actuators B Chem. 2020, 318, 128241. [Google Scholar] [CrossRef]
- WHO Pesticide Residues in Food. Available online: https://www.who.int/news-room/fact-sheets/detail/pesticide-residues-in-food (accessed on 15 September 2022).
- Vodova, M.; Nejdl, L.; Pavelicova, K.; Zemankova, K.; Rrypar, T.; Sterbova, D.S.; Bezdekova, J.; Nuchtavorn, N.; Macka, M.; Adam, V. Detection of Pesticides in Food Products Using Paper-Based Devices by UV-Induced Fluorescence Spectroscopy Combined with Molecularly Imprinted Polymers. Food Chem. 2022, 380, 132141. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Patel, S.; Thakur, S.S.; Shankar, R. Food Safety Monitoring of the Pesticide Phenthoate Using a Smartphone-Assisted Paper-Based Sensor with Bimetallic Cu@ Ag Core–Shell Nanoparticles. Lab Chip 2020, 20, 3996–4006. [Google Scholar] [CrossRef]
- Jin, L.; Hao, Z.; Zheng, Q.; Chen, H.; Zhu, L.; Wang, C.; Liu, X.; Lu, C. A Facile Microfluidic Paper-Based Analytical Device for Acetylcholinesterase Inhibition Assay Utilizing Organic Solvent Extraction in Rapid Detection of Pesticide Residues in Food. Anal. Chim. Acta 2020, 1100, 215–224. [Google Scholar] [CrossRef]
- Chen, H.; Hu, O.; Fan, Y.; Xu, L.; Zhang, L.; Lan, W.; Hu, Y.; Xie, X.; Ma, L.; She, Y.; et al. Fluorescence Paper-Based Sensor for Visual Detection of Carbamate Pesticides in Food Based on CdTe Quantum Dot and Nano ZnTPyP. Food Chem. 2020, 327, 127075. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, A.; Mancini, M.; Gioia, V.; Cinti, S. Office Paper-Based Electrochemical Strips for Organophosphorus Pesticide Monitoring in Agricultural Soil. Environ. Sci. Technol. 2021, 55, 8859–8865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, S.; Luo, J.; Xiong, Y.; Ming, T.; Liu, J.; Ma, Y.; Yan, S.; Yang, Y.; Yang, Z. Low Sample Volume Origami-Paper-Based Graphene-Modified Aptasensors for Label-Free Electrochemical Detection of Cancer Biomarker-EGFR. Microsyst. Nanoeng. 2020, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Reid, R.C.; Minteer, S.D. A Paper-Based Mitochondrial Electrochemical Biosensor for Pesticide Detection. Electroanalysis 2016, 28, 854–859. [Google Scholar] [CrossRef]
- Ding, J.; Li, B.; Chen, L.; Qin, W. A Three-Dimensional Origami Paper-Based Device for Potentiometric Biosensing. Angew. Chem.-Int. Ed. 2016, 55, 13033–13037. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Shaffo, F.C.; Grodzki, A.C.; Fryer, A.D.; Lein, P.J. Mechanisms of Organophosphorus Pesticide Toxicity in the Context of Airway Hyperreactivity and Asthma. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2018, 315, L485–L501. [Google Scholar] [CrossRef]
- Yang, N.; Zhou, X.; Yu, D.; Jiao, S.; Han, X.; Zhang, S.; Yin, H.; Mao, H. Pesticide Residues Identification by Impedance Time-Sequence Spectrum of Enzyme Inhibition on Multilayer Paper-Based Microfluidic Chip. J. Food Process Eng. 2020, 43, e13544. [Google Scholar] [CrossRef]
- Joint FAO/WHO Codex Alimentarius Commission General Standard for Contaminants and Toxins in Food and Feed; Codex Alimentarius International Food Standards; CXS 193-19; Food and Agriculture Organization of the United Nations/World Health Organization: Rome, Italy, 1995.
- Chen, C.; Wu, J. A Fast and Sensitive Quantitative Lateral Flow Immunoassay for Cry1AB Based on a Novel Signal Amplification Conjugate. Sensors 2012, 12, 11684–11696. [Google Scholar] [CrossRef]
- Nilghaz, A.; Liu, X.; Ma, L.; Huang, Q.; Lu, X. Development of Fabric-Based Microfluidic Devices by Wax Printing. Cellulose 2019, 26, 3589–3599. [Google Scholar] [CrossRef]
- Sousa, A.C.R.; Makara, C.N.; Brazaca, L.C.; Carrilho, E. A Colorimetric Microfluidic Paper-Based Analytical Device for Sulfonamides in Cow Milk Using Enzymatic Inhibition. Food Chem. 2021, 356, 129692. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Liu, L.; Wu, X.; Kuang, H.; Xu, C.; Xu, L. A Colorimetric Paper-Based Sensor for Toltrazuril and Its Metabolites in Feed, Chicken, and Egg Samples. Food Chem. 2019, 276, 707–713. [Google Scholar] [CrossRef]
- Trofimchuk, E.; Nilghaz, A.; Sun, S.; Lu, X. Determination of Norfloxacin Residues in Foods by Exploiting the Coffee-ring Effect and Paper-based Microfluidics Device Coupling with Smartphone-based Detection. J. Food Sci. 2020, 85, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Liu, J.-J.; Xu, Y.; Wu, Z.-Y. Fast and Sensitive Screening Detection of Tetracyclines with a Paper-Based Analytical Device. Microchem. J. 2019, 145, 703–707. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Ban, L.; Du, W.; Feng, X.; Liu, B.-F. A Paper-Based Device with an Adjustable Time Controller for the Rapid Determination of Tumor Biomarkers. Sens. Actuators B Chem. 2018, 254, 855–862. [Google Scholar] [CrossRef]
- Mo, M.; Wang, X.; Ye, L.; Su, Y.; Zhong, Y.; Zhao, L.; Zhou, Y.; Peng, J. A Simple Paper-Based Ratiometric Luminescent Sensor for Tetracyclines Using Copper Nanocluster-Europium Hybrid Nanoprobes. Anal. Chim. Acta 2022, 1190, 339257. [Google Scholar] [CrossRef]
- Kuo, Z.K.; Chang, T.H.; Chen, Y.S.; Cheng, C.M.; Tsai, C.Y. Two Potential Clinical Applications of Origami-Based Paper Devices. Diagnostics 2019, 9, 203. [Google Scholar] [CrossRef]
- De Oliveira, T.R.; Fonseca, W.T.; de Oliveira Setti, G.; Faria, R.C. Fast and Flexible Strategy to Produce Electrochemical Paper-Based Analytical Devices Using a Craft Cutter Printer to Create Wax Barrier and Screen-Printed Electrodes. Talanta 2019, 195, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Primpray, V.; Chailapakul, O.; Tokeshi, M.; Rojanarata, T.; Laiwattanapaisal, W. A Paper-Based Analytical Device Coupled with Electrochemical Detection for the Determination of Dexamethasone and Prednisolone in Adulterated Traditional Medicines. Anal. Chim. Acta 2019, 1078, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, T.P.; de Faria, L.V.; Alves, G.F.; Matos, M.A.C.; Matos, R.C. Development of Paper Devices with Conductive Inks for Sulfanilamide Electrochemical Determination in Milk, Synthetic Urine, and Environmental and Pharmaceutical Samples. J. Solid State Electrochem. 2021, 25, 2301–2308. [Google Scholar] [CrossRef]
- Wu, X.; Kuang, H.; Hao, C.; Xing, C.; Wang, L.; Xu, C. Paper Supported Immunosensor for Detection of Antibiotics. Biosens. Bioelectron. 2012, 33, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food Allergy. Nat. Rev. Dis. Prim. 2018, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.L.K.; Mullins, R.J. Food Allergy: Is Prevalence Increasing? Intern. Med. J. 2017, 47, 256–261. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA). In Public Law; US Food and Drug Administration: Silver Spring, MD, USA, 2004; Volume 108. [Google Scholar]
- Jiang, D.; Jiang, H.; Wang, L. A Novel Paper-Based Capacitance Mast Cell Sensor for Evaluating Peanut Allergen Protein Ara h 2. Food Anal. Methods 2020, 13, 1993–2001. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy Metals in Food Crops: Health Risks, Fate, Mechanisms, and Management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Silva-Neto, H.A.; Cardoso, T.M.G.; McMahon, C.J.; Sgobbi, L.F.; Henry, C.S.; Coltro, W.K.T. Plug-and-Play Assembly of Paper-Based Colorimetric and Electrochemical Devices for Multiplexed Detection of Metals. Analyst 2021, 146, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- FDA Lead in Food, Foodwares, and Dietary Supplements 2022. Available online: https://www.fda.gov/food/metals-and-your-food/lead-food-foodwares-and-dietary-supplements (accessed on 26 October 2022).
- Soulis, D.; Pagkali, V.; Kokkinos, C.; Economou, A. Plot-on-Demand Integrated Paper-Based Sensors for Drop-Volume Voltammetric Monitoring of Pb (II) and Cd (II) Using a Bismuth Nanoparticle-Modified Electrode. Microchim. Acta 2022, 189, 240. [Google Scholar] [CrossRef]
- Ninwong, B.; Ratnarathorn, N.; Henry, C.S.; Mace, C.R.; Dungchai, W. Dual Sample Preconcentration for Simultaneous Quantification of Metal Ions Using Electrochemical and Colorimetric Assays. ACS Sens. 2020, 5, 3999–4008. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tang, F.; Xing, H.; Zheng, H.; Lianhua, B.; Wei, W. Electrochemical Detection of Pb and Cd in Paper-Based Microfluidic Devices. J. Braz. Chem. Soc. 2012, 23, 1124–1130. [Google Scholar] [CrossRef]
- Qian, S.; Han, Y.; Xu, F.; Feng, D.; Yang, X.; Wu, X.; Hao, L.; Yuan, M. A Fast, Sensitive, Low-Cost Electrochemical Paper-Based Chip for Real-Time Simultaneous Detection of Cadmium (Ⅱ) and Lead (Ⅱ) via Aptamer. Talanta 2022, 247, 123548. [Google Scholar] [CrossRef]
- Bi, X.-M.; Wang, H.-R.; Ge, L.-Q.; Zhou, D.-M.; Xu, J.-Z.; Gu, H.-Y.; Bao, N. Gold-Coated Nanostructured Carbon Tape for Rapid Electrochemical Detection of Cadmium in Rice with in Situ Electrodeposition of Bismuth in Paper-Based Analytical Devices. Sens. Actuators B Chem. 2018, 260, 475–479. [Google Scholar] [CrossRef]
- Soulis, D.; Trachioti, M.; Kokkinos, C.; Economou, A.; Prodromidis, M. Single-Use Fluidic Electrochemical Paper-Based Analytical Devices Fabricated by Pen Plotting and Screen-Printing for on-Site Rapid Voltammetric Monitoring of Pb (II) and Cd (II). Sensors 2021, 21, 6908. [Google Scholar] [CrossRef]
- Sánchez-Calvo, A.; Fernández-Abedul, M.T.; Blanco-López, M.d.C.; Costa-García, A. Paper-Based Electrochemical Transducer Modified with Nanomaterials for Mercury Determination in Environmental Waters. Sens. Actuators B Chem. 2019, 290, 87–92. [Google Scholar] [CrossRef]
- Pokpas, K.; Jahed, N.; Iwuoha, E. Tuneable, Pre-Stored Paper-Based Electrochemical Cells ($μ$PECs): An Adsorptive Stripping Voltammetric Approach to Metal Analysis. Electrocatalysis 2019, 10, 352–364. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhang, L.; Ge, S.; Yan, M.; Yu, J. Steric Paper Based Ratio-Type Electrochemical Biosensor with Hollow-Channel for Sensitive Detection of Zn2+. Sci. Bull. 2017, 62, 1114–1121. [Google Scholar] [CrossRef]
- Miniature Bluetooth Low Energy Module for IoT Application. Available online: https://www.electronicsforu.com/tech-zone/electronics-components/miniature-bluetooth-low-energy-module-for-iot-applications (accessed on 12 November 2022).
- Ainla, A.; Mousavi, M.P.S.; Tsaloglou, M.N.; Redston, J.; Bell, J.G.; Fernández-Abedul, M.T.; Whitesides, G.M. Open-Source Potentiostat for Wireless Electrochemical Detection with Smartphones. Anal. Chem. 2018, 90, 6240–6246. [Google Scholar] [CrossRef]
- Dryden, M.D.M.; Wheeler, A.R. DStat: A Versatile, Open-Source Potentiostat for Electroanalysis and Integration. PLoS ONE 2015, 10, e0140349. [Google Scholar] [CrossRef]
- Rowe, A.A.; Bonham, A.J.; White, R.J.; Zimmer, M.P.; Yadgar, R.J.; Hobza, T.M.; Honea, J.W.; Ben-Yaacov, I.; Plaxco, K.W. CheapStat: An Open-Source, “Do-It-Yourself” Potentiostat for Analytical and Educational Applications. PLoS ONE 2011, 6, e23783. [Google Scholar] [CrossRef]

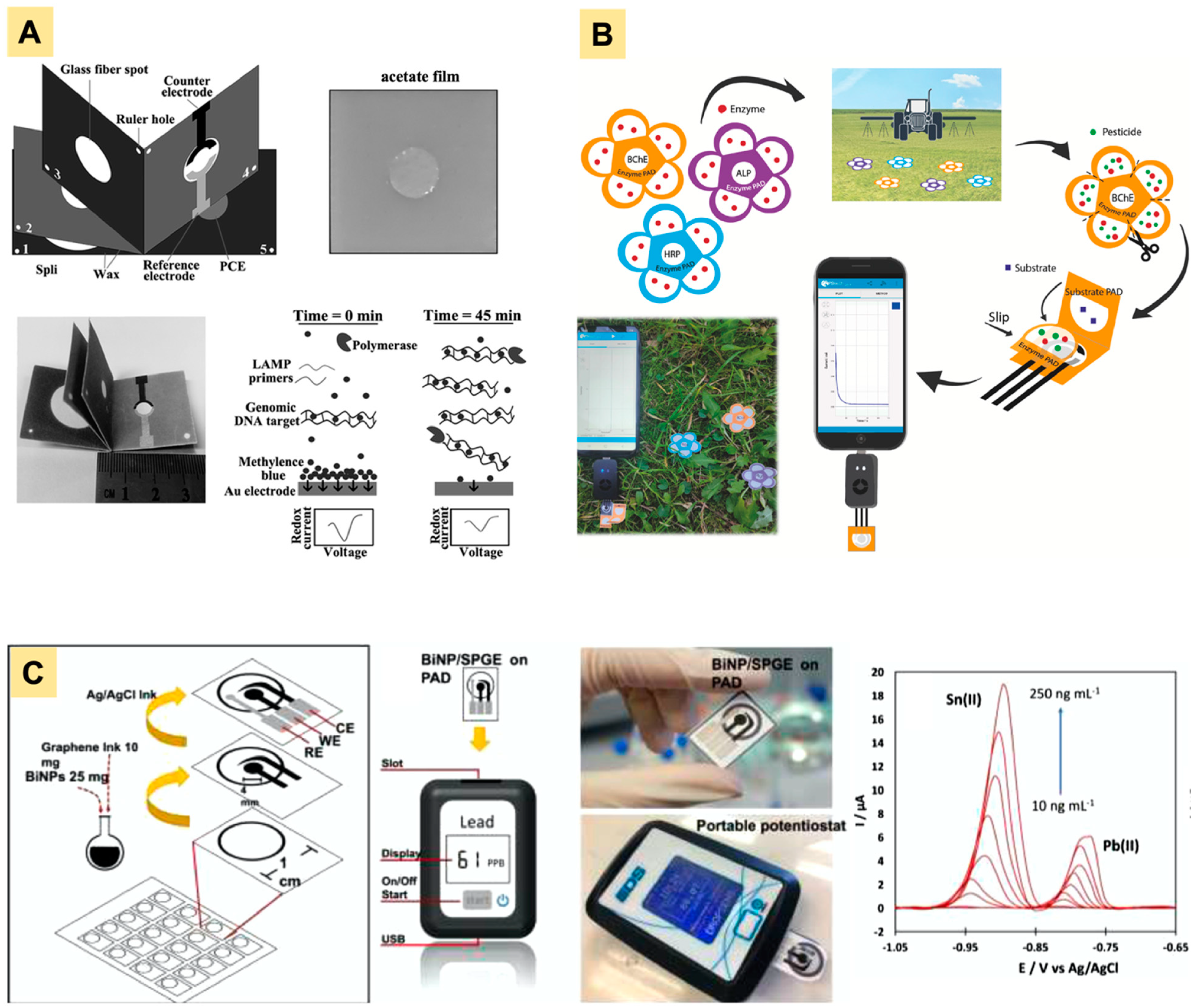
| No. | Fabrication Technique | Analyte | Sample | References |
|---|---|---|---|---|
| 1 | Wax printing | Ethanol | Beer | [26] |
| 2 | Wax printing | Ketamine | Alcoholic and non-alcoholic beer | [37] |
| 3 | Wax printing | Pesticides (insecticides and herbicides) | River water | [14] |
| 4 | Wax printing | Pesticides | Aerosol | [38] |
| 5 | Wax printing | Casein allergen | Milk | [39] |
| 6 | Wax printing | Glycoproteins | Eggs white | [40] |
| 7 | Wax Printing | Peanut allergen Ara h1 | Cookie dough | [41] |
| 8 | Wax Printing | Glucose and total carbohydrate | Food stuff | [42] |
| 8 | Screen Printing | Ferricyanide | Standard solution | [43] |
| 9 | Inkjet Printing | Ascorbic acid | Dietary supplement | [44] |
| 10 | Laser Printing | Glucose | Blood | [45] |
| 11 | Photolithography | Heavy-metal ions and glucose | Aqueous solutions | [46] |
| Analyte | Detection Principle | Electrochemical Technique | Sample Matrix | Sample Volume/Size | Detection Limit | RSD | Assay Time | References |
|---|---|---|---|---|---|---|---|---|
| 2,4-dichlorophenoxy-acetic acid | Enzymatic assay | Chrono-amperometry | River water | 5 mL | 50 ng/mL | <5% | ~10 min | [14] |
| Standard solution | NS | 30 ng/mL | 6% | <10 min | [38] | |||
| Avermectin, dimethoate, and phoxim | Enzymatic assay, combined with multivariate analysis | Electrochemical impedance spectroscopy | Vegetable | 30 mL | NS (tested conc.: 0.1–0.3 mg/kg) | NS | 15 min | [96] |
| Glyphosate | Enzymatic assay | Chrono-amperometry | Standard solution | NS | 10 ng/mL | 7% | <10 min | [38] |
| Malathion | Mitochondria-based assay | Cyclic voltammetry | Standard solution | NS | 20 nM | ~20% | NS | [97] |
| Paraoxon | Enzymatic assay | Chrono-amperometry | Soil and vegetable | 1 g | 1.3 ng/mL | <15% | <1 h | [95] |
| River water | 5 mL | 2 ng/mL | <5% | ~10 min | [14] | |||
| Standard solution | NS | 2 ng/mL | 3% | <10 min | [38] | |||
| River water and wastewater | 5 mL | 3 ng/mL | <15% | ~5 min | [26] | |||
| Parathion | Enzymatic assay | Potentiometry | Standard solution | 10 mL | 0.06 nM | <10% | ~10 min | [98] |
| Triazine | Enzymatic assay | Chrono-amperometry | River water | 5 mL | NS | <5% | ~10 min | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuswandi, B.; Hidayat, M.A.; Noviana, E. Paper-Based Electrochemical Biosensors for Food Safety Analysis. Biosensors 2022, 12, 1088. https://doi.org/10.3390/bios12121088
Kuswandi B, Hidayat MA, Noviana E. Paper-Based Electrochemical Biosensors for Food Safety Analysis. Biosensors. 2022; 12(12):1088. https://doi.org/10.3390/bios12121088
Chicago/Turabian StyleKuswandi, Bambang, Mochammad Amrun Hidayat, and Eka Noviana. 2022. "Paper-Based Electrochemical Biosensors for Food Safety Analysis" Biosensors 12, no. 12: 1088. https://doi.org/10.3390/bios12121088
APA StyleKuswandi, B., Hidayat, M. A., & Noviana, E. (2022). Paper-Based Electrochemical Biosensors for Food Safety Analysis. Biosensors, 12(12), 1088. https://doi.org/10.3390/bios12121088






