Development of Fluorescent Turn-On Probes for CAG-RNA Repeats
Abstract
1. Introduction
2. Materials and Methods
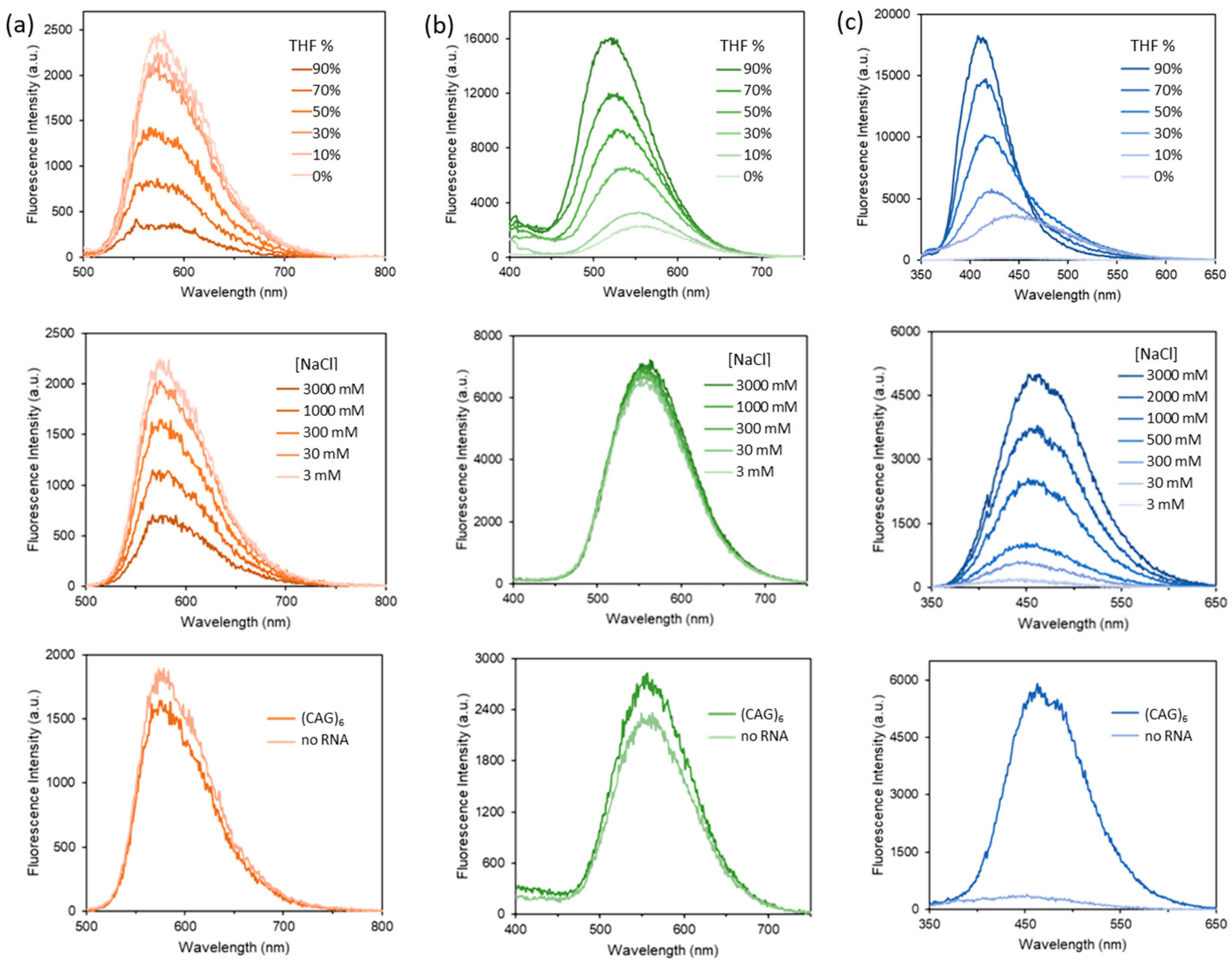
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ross, C.A. Polyglutamine pathogenesis: Emergence of unifying mechanisms for Huntington’s disease and related disorders. Neuron 2002, 35, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Orr, H.T.; Zoghbi, H.Y. Trinucleotide Repeat Disorders. Annu. Rev. Neurosci. 2007, 30, 575–621. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, A.P.; Shakkottai, V.G.; Albin, R.L. Polyglutamine Repeats in Neurodegenerative Diseases. Annu. Rev. Pathol. 2019, 14, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Diamond, M.I. Polyglutamine Diseases: Emerging Concepts in Pathogenesis and Therapy. Hum. Mol. Genet. 2007, 16, R115–R123. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, B.; Robberecht, W.; Van Den Bosch, L. RNA Toxicity in Non-Coding Repeat Expansion Disorders. EMBO J. 2020, 39, e101112–e101114. [Google Scholar] [CrossRef]
- Kouroku, Y.; Fujita, E.; Jimbo, A.; Kikuchi, T.; Yamagata, T.; Momoi, M.Y.; Kominami, E.; Kuida, K.; Sakamaki, K.; Yonehara, S.; et al. Polyglutamine aggregates stimulate ER stress signals and caspase-12 activation. Hum. Mol. Genet. 2002, 11, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.S.; Li, L.; Peng, S.; Chen, F.M.; Zhang, Q.; An, Y.; Lin, X.; Li, W.; Koon, A.C.; Chan, T.F.; et al. Planar cell polarity gene Fuz triggers apoptosis in neurodegenerative disease models. EMBO Rep. 2018, 19, e45409. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chadwick, S.R.; Lajoie, P. Endoplasmic reticulum stress: The cause and solution to Huntington’s disease? Brain Res. 2016, 1648, 650–657. [Google Scholar] [CrossRef]
- Rusmini, P.; Crippa, V.; Cristofani, R.; Rinaldi, C.; Cicardi, M.E.; Galbiati, M.; Carra, S.; Malik, B.; Greensmith, L.; Poletti, A. The role of the protein quality control system in SBMA. J. Mol. Neurosci. 2016, 58, 348–364. [Google Scholar] [CrossRef]
- Fardghassemi, Y.; Tauffenberger, A.; Gosselin, S.; Parker, J.A. Rescue of ATXN3 neuronal toxicity in Caenorhabditis elegans by chemical modification of endoplasmic reticulum stress. Dis. Model. Mech. 2017, 10, 1465–1480. [Google Scholar]
- Bañez-Coronel, M.; Porta, S.; Kagerbauer, B.; Mateu-Huertas, E.; Pantano, L.; Ferrer, I.; Guzmán, M.; Estivill, X.; Martí, E. A Pathogenic Mechanism in Huntington’s Disease Involves Small CAG-Repeated RNAs with Neurotoxic Activity. PLoS Genet. 2012, 8, e1002481–e1002495. [Google Scholar] [CrossRef] [PubMed]
- Nalavade, R.; Griesche, N.; Ryan, D.P.; Hildebrand, S.; Krauss, S. Mechanisms of RNA-Induced Toxicity in CAG Repeat Disorders. Cell Death Dis. 2013, 4, e752–e762. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.Y.E. RNA-Mediated Pathogenic Mechanisms in Polyglutamine Diseases and Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2014, 8, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Martí, E. RNA Toxicity Induced by Expanded CAG Repeats in Huntington’s Disease. Brain Pathol. 2016, 26, 779–786. [Google Scholar] [CrossRef]
- Peng, S.; Guo, P.; Lin, X.; An, Y.; Sze, K.H.; Lau, M.H.Y.; Chen, Z.S.; Wang, Q.; Li, W.; Sun, J.K.-L.; et al. CAG RNAs induce DNA damage and apoptosis by silencing NUDT16 expression in polyglutamine degeneration. Proc. Natl. Acad. Sci. USA 2021, 118, e2022940118. [Google Scholar] [CrossRef]
- Murata, A.; Sato, S.-I.; Kawazoe, Y.; Uesugi, M. Small-Molecule Fluorescent Probes for Specific RNA Targets. Chem. Commun. 2011, 47, 4712–4714. [Google Scholar] [CrossRef]
- Cao, C.; Wei, P.; Li, R.; Zhong, Y.; Li, X.; Xue, F.; Shi, Y.; Yi, T. Ribosomal RNA-Selective Light-up Fluorescent Probe for Rapidly Imaging the Nucleolus in Live Cells. ACS Sens. 2019, 4, 1409–1416. [Google Scholar] [CrossRef]
- Das, B.; Murata, A.; Nakatani, K. A Small-Molecule Fluorescence Probe ANP77 for Sensing RNA Internal Loop of C, U and A/CC Motifs and Their Binding Molecules. Nucleic Acids Res. 2021, 49, 8462–8470. [Google Scholar] [CrossRef]
- Wong, C.-H.; Nguyen, L.; Peh, J.; Luu, L.M.; Sanchez, J.S.; Richardson, S.L.; Tuccinardi, T.; Tsoi, H.; Chan, W.Y.; Chan, E.H.Y.; et al. Targeting toxic RNAs that cause myotonic dystrophy type 1 (DM1) with a bisamidinium inhibitor. J. Am. Chem. Soc. 2014, 136, 6355–6361. [Google Scholar] [CrossRef]
- Nguyen, L.; Luu, L.M.; Peng, S.; Serrano, J.F.; Chan, H.Y.E.; Zimmerman, S.C. Rationally Designed Small Molecules That Target Both the DNA and RNA Causing Myotonic Dystrophy Type 1. J. Am. Chem. Soc. 2015, 137, 14180–14189. [Google Scholar] [CrossRef]
- Luu, L.M.; Nguyen, L.; Peng, S.; Lee, J.Y.; Lee, H.Y.; Wong, C.-H.; Hergenrother, P.J.; Chan, H.Y.E.; Zimmerman, S.C. A Potent Inhibitor of Protein Sequestration by Expanded Triplet (CUG) Repeats that Shows Phenotypic Improvements in a Drosophila Model of Myotonic Dystrophy. ChemMedChem 2016, 11, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Rhee, W.J.; Tsourkas, A. Fluorescent Probes for Live-Cell RNA Detection. Annu. Rev. Biomed. Eng. 2009, 11, 25–47. [Google Scholar] [CrossRef]
- Juskowiak, B. Nucleic Acid-Based Fluorescent Probes and Their Analytical Potential. Anal. Bioanal. Chem. 2011, 399, 3157–3176. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Frei, M.S.; Salim, A.; Johnsson, K. Small-Molecule Fluorescent Probes for Live-Cell Super-Resolution Microscopy. J. Am. Chem. Soc. 2019, 141, 2770–2781. [Google Scholar] [CrossRef] [PubMed]
- Terai, T.; Nagano, T. Small-Molecule Fluorophores and Fluorescent Probes for Bioimaging. Pflugers Arch. 2013, 465, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Loving, G.S.; Sainlos, M.; Imperiali, B. Monitoring Protein Interactions and Dynamics with Solvatochromic Fluorophores. Trends Biotechnol. 2010, 28, 73–83. [Google Scholar] [CrossRef]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef]
- Liese, D.; Haberhauer, G. Rotations in Excited ICT States—Fluorescence and Its Microenvironmental Sensitivity. Isr. J. Chem. 2018, 58, 813–826. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Solvent and Environmental Effects. In Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; pp. 205–235. [Google Scholar]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Mocanu, S.; Ionita, G.; Ionescu, S.; Tecuceanu, V.; Enache, M.; Leonties, A.R.; Stavarache, C.; Matei, I. New Environment-Sensitive Bis-Dansyl Molecular Probes Bearing Alkyl Diamine Linkers: Emissive Features and Interaction with Cyclodextrins. Chem. Phys. Lett. 2018, 713, 226–234. [Google Scholar] [CrossRef]
- Demeter, A.; Kovalenko, S.A. Photoinduced Intramolecular Charge Transfer and Relaxation Dynamics of 4-Dimethylaminopyridine in Water, Alcohols, and Aprotic Solvents. J. Photochem. Photobiol. A Chem. 2021, 412, 113246–113254. [Google Scholar] [CrossRef]
- Leung, N.L.C.; Xie, N.; Yuan, W.; Liu, Y.; Wu, Q.; Peng, Q.; Miao, Q.; Lam, J.W.Y.; Tang, B.Z. Restriction of Intramolecular Motions: The General Mechanism behind Aggregation-Induced Emission. Chemistry 2014, 20, 15349–15353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-F.; Chen, Z.-Q.; Aldred, M.P.; Hu, Z.; Chen, T.; Huang, Z.; Meng, X.; Zhu, M.-Q. Direct Validation of the Restriction of Intramolecular Rotation Hypothesis via the Synthesis of Novel Ortho-Methyl Substituted Tetraphenylethenes and Their Application in Cell Imaging. Chem. Commun. 2014, 50, 12058–12060. [Google Scholar] [CrossRef] [PubMed]
- Marcheschi, R.J.; Tonelli, M.; Kumar, A.; Butcher, S.E. Structure of the HIV-1 frameshift site RNA bound to a small molecule inhibitor of viral replication. ACS Chem. Biol. 2011, 6, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kwok, R.T.K.; Liu, J.; Xing, B.; Tang, B.Z.; Liu, B. Real-Time Monitoring of Cell Apoptosis and Drug Screening Using Fluorescent Light-up Probe with Aggregation-Induced Emission Characteristics. J. Am. Chem. Soc. 2012, 134, 17972–17981. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Mohy Ei Dine, T.; Vincent, S.P. Synthesis of Functionalized Copillar[4+1]Arenes and Rotaxane as Heteromultivalent Scaffolds. Chem. Commun. 2021, 57, 492–495. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, M.H.Y.; Wong, C.-H.; Chan, H.Y.E.; Au-Yeung, H.Y. Development of Fluorescent Turn-On Probes for CAG-RNA Repeats. Biosensors 2022, 12, 1080. https://doi.org/10.3390/bios12121080
Lau MHY, Wong C-H, Chan HYE, Au-Yeung HY. Development of Fluorescent Turn-On Probes for CAG-RNA Repeats. Biosensors. 2022; 12(12):1080. https://doi.org/10.3390/bios12121080
Chicago/Turabian StyleLau, Matthew Ho Yan, Chun-Ho Wong, Ho Yin Edwin Chan, and Ho Yu Au-Yeung. 2022. "Development of Fluorescent Turn-On Probes for CAG-RNA Repeats" Biosensors 12, no. 12: 1080. https://doi.org/10.3390/bios12121080
APA StyleLau, M. H. Y., Wong, C.-H., Chan, H. Y. E., & Au-Yeung, H. Y. (2022). Development of Fluorescent Turn-On Probes for CAG-RNA Repeats. Biosensors, 12(12), 1080. https://doi.org/10.3390/bios12121080




