Nano-Engineered Surface Comprising Metallic Dendrites for Biomolecular Analysis in Clinical Perspective
Abstract
:1. Introduction
2. Properties
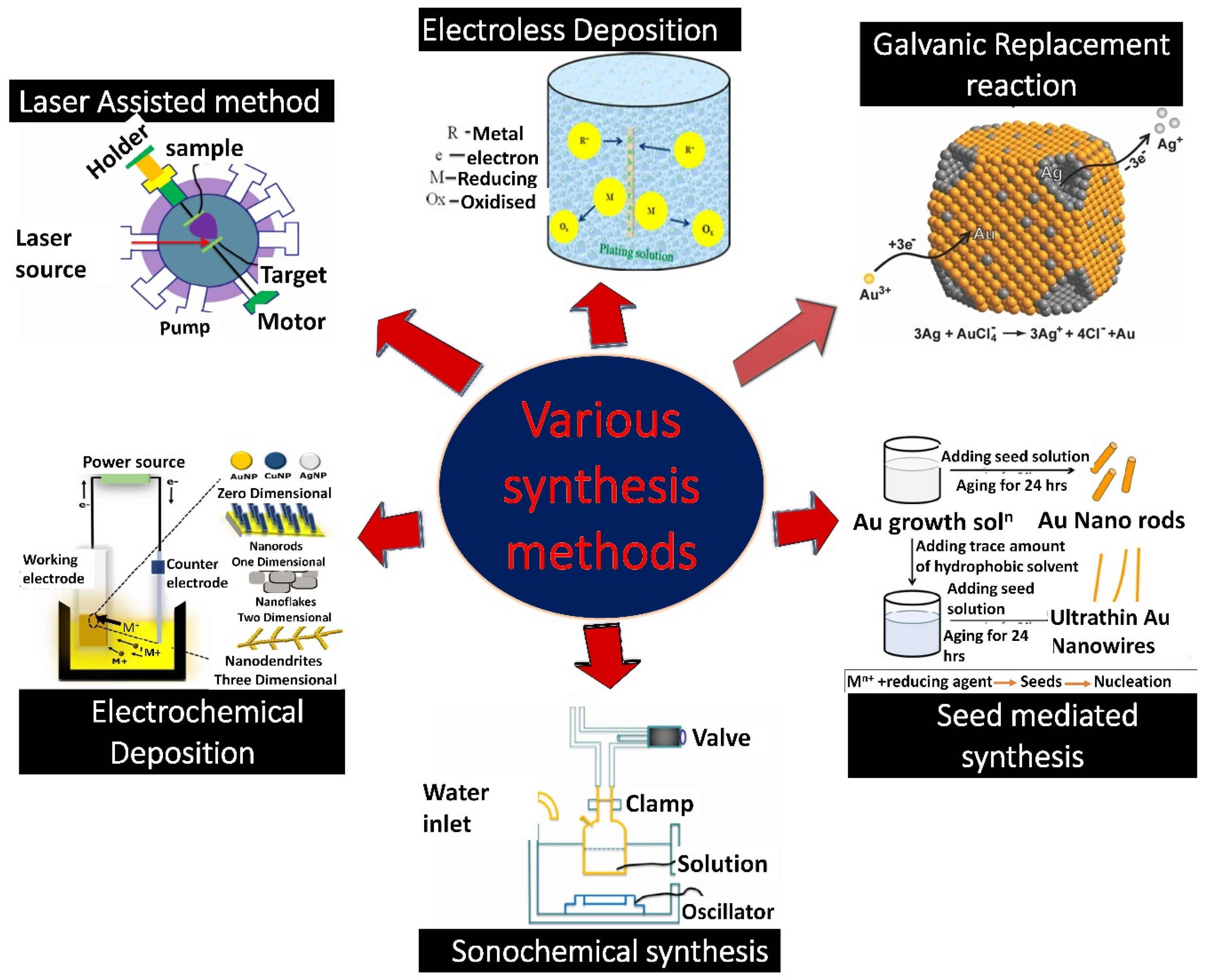
3. Methods of Syntheses
3.1. Galvanic Replacement Reaction
3.2. Seed-Mediated
3.3. Co-Reduction
3.4. Sonochemical Reduction
3.5. Laser-Assisted Method
3.6. Electroless Deposition
3.7. Electrochemical Deposition
4. Models of Nanodendrites Formation
5. Small-Molecule Detection Using Metallic Nanodendrite-Based Sensors
6. Macro-Molecules Detection Using Metallic Nanodendrite-Based Sensors
7. Detection of Cells Using Metallic Nanodendrite-Based Sensors
8. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Sriphathoorat, R.; Wang, K.; Luo, S.; Tang, M.; Du, H.; Du, X.; Shen, P.K. Well-Defined PtNiCo Core-Shell Nanodendrites with Enhanced Catalytic Performance for Methanol Oxidation. J. Mater. Chem. A 2016, 4, 18015–18021. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, X.; Han, L.; Peng, Z.; Yang, J. A Universal Approach to the Synthesis of Nanodendrites of Noble Metals. Nanoscale 2014, 6, 6173–6179. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Wu, F.; Han, Q.; Qi, J.; Gao, W.; Wang, Y.; Li, T.; Yang, Y.; Sun, M. Electrochemical Synthesis of Tin Plasmonic Dendritic Nanostructures with SEF Capability through: In Situ Replacement. RSC Adv. 2020, 10, 36042–36050. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Lin, Z.; Cao, Y.; Zheng, Z.; Zeng, Z.; Hu, Z. Shape-Controllable Pulse Electrodeposition of Ultrafine Platinum Nanodendrites for Methanol Catalytic Combustion and the Investigation of Their Local Electric Field Intensification by Electrostatic Force Microscope and Finite Element Method. Electrochim. Acta 2014, 136, 66–74. [Google Scholar] [CrossRef]
- Sui, R.; Charpentier, P.A.; Marriott, R.A. Metal Oxide-Related Dendritic Structures: Self-Assembly and Applications for Sensor, Catalysis, Energy Conversion and Beyond. Nanomaterials 2021, 11, 1686. [Google Scholar] [CrossRef]
- Gentile, A.; Ruffino, F.; Grimaldi, M.G. Complex-Morphology Metal-Based Nanostructures: Fabrication, Characterization, and Applications. Nanomaterials 2016, 6, 110. [Google Scholar] [CrossRef] [Green Version]
- Purohit, B.; Kumar, A.; Mahato, K.; Chandra, P. Novel Sensing Assembly Comprising Engineered Gold Dendrites and MWCNT-AuNPs Nanohybrid for Acetaminophen Detection in Human Urine. Electroanalysis 2020, 32, 561–570. [Google Scholar] [CrossRef]
- Noh, H.B.; Lee, K.S.; Chandra, P.; Won, M.S.; Shim, Y.B. Application of a Cu-Co Alloy Dendrite on Glucose and Hydrogen Peroxide Sensors. Electrochim. Acta 2012, 61, 36–43. [Google Scholar] [CrossRef]
- Anh, N.H.; Duy, P.K.; Hai Yen, P.T.; Hung, L.Q.; Phong, P.H.; Thu Ha, V.T.; Chung, H. Role of Br- on the Formation of a Bismuth Nanodendrite Structure and Its Use as an Electrochemical Sensor for Heavy Metal Detection. Int. J. Electrochem. Sci. 2020, 15, 5373–5384. [Google Scholar] [CrossRef]
- Huynh, K.H.; Pham, X.H.; Kim, J.; Lee, S.H.; Chang, H.; Rho, W.Y.; Jun, B.H. Synthesis, Properties, and Biological Applications of Metallic Alloy Nanoparticles. Int. J. Mol. Sci. 2020, 21, 5174. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.Q.; Li, Z.W.; Yao, R.; Xiong, K.W.; Cheng, G.L.; Zhou, Y.H.; Luo, X.; Liu, Z.M. Improved SERS Performance and Catalytic Activity of Dendritic Au/Ag Bimetallic Nanostructures Based on Ag Dendrites. Nanoscale Res. Lett. 2020, 15, 117. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Lin, X.X.; Zhang, X.F.; Wang, A.J.; Zhu, X.Y.; Feng, J.J. Bimetallic PtPd Alloyed Core-Shell Nanodendrites Supported on Reduced Graphene Oxide: One-Pot Green Synthesis and Efficient Electrocatalytic Performances for Glycerol Oxidation and Hydrogen Evolution. J. Alloys Compd. 2018, 735, 2123–2132. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Z.; Yang, Y.; Su, D. The Growth and Enhanced Catalytic Performance of Au@Pd Core-Shell Nanodendrites. Nanoscale 2013, 5, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.K.; Joo, J.; Kwon, H.B.; Kim, B.; Kim, H.Y.; Joo, S.H.; Lee, K. Nanodendrites of Platinum-Group Metals for Electrocatalytic Applications. Nano Res. 2018, 11, 6111–6140. [Google Scholar] [CrossRef]
- Guo, S.; Li, J.; Dong, S.; Wang, E. Three-Dimensional Pt-on-Au Bimetallic Dendritic Nanoparticle: One-Step, High-Yield Synthesis and Its Bifunctional Plasmonic and Catalytic Properties. J. Phys. Chem. C 2010, 114, 15337–15342. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [Green Version]
- Oladipo, A.O.; Nkambule, T.T.I.; Mamba, B.B.; Msagati, T.A.M. Therapeutic Nanodendrites: Current Applications and Prospects. Nanoscale Adv. 2020, 2, 5152–5165. [Google Scholar] [CrossRef]
- Huang, T.; Meng, F.; Qi, L. Controlled Synthesis of Dendritic Gold Nanostructures Assisted by Supramolecular Complexes of Surfactant with Cyclodextrin. Langmuir 2010, 26, 7582–7589. [Google Scholar] [CrossRef]
- Mohanty, A.; Garg, N.; Jin, R. A Universal Approach to the Synthesis of Noble Metal Nanodendrites and Their Catalytic Properties. Angew. Chemie Int. Ed. 2010, 49, 4962–4966. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.Q.; Li, Z.W.; Xu, J.H.; Yao, R.; Li, Z.L.; Liang, S.; Cheng, G.L.; Zhou, Y.H.; Luo, X.; Zhong, J. Morphology-Controlled Fabrication of Large-Scale Dendritic Silver Nanostructures for Catalysis and SERS Applications. Nanoscale Res. Lett. 2019, 14, 89. [Google Scholar] [CrossRef]
- Kang, Y.; Li, F.; Li, S.; Ji, P.; Zeng, J.; Jiang, J.; Chen, Y. Unexpected Catalytic Activity of Rhodium Nanodendrites with Nanosheet Subunits for Methanol Electrooxidation in an Alkaline Medium. Nano Res. 2016, 9, 3893–3902. [Google Scholar] [CrossRef]
- Hau, N.Y.; Yang, P.; Liu, C.; Wang, J.; Lee, P.H.; Feng, S.P. Aminosilane-Assisted Electrodeposition of Gold Nanodendrites and Their Catalytic Properties. Sci. Rep. 2017, 7, 39839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Sun, Z.; Tian, D.; Nevirkovets, I.P.; Dou, S.X. Platinum Dendritic Nanoparticles with Magnetic Behavior. J. Appl. Phys. 2014, 116, 033911. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Lin, Y.; Chu, X.; Tang, J.; Xu, S. Facile Synthesis of Supported AuNi and PtNi Bimetallic Nanomaterials and Their Enhanced Catalytic Properties. J. Mater. Res. Technol. 2020, 9, 2237–2246. [Google Scholar] [CrossRef]
- Qingming, S.; Qianhao, M.; Jianjun, S.; Liping, J.; Jian-Rong, Z.; Wenhua, H.; Jun-Jie, Z. Morphology-Controlled Synthesis of Palladium Nanostructures by Sonoelectrochemical Method and Their Application in Direct Alcohol Oxidation. J. Phys. Chem. C 2009, 113, 1267–1273. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, X.; Peng, H.C. Shape-Controlled Synthesis of Colloidal Metal Nanocrystals: Thermodynamic versus Kinetic Products. J. Am. Chem. Soc. 2015, 137, 7947–7966. [Google Scholar] [CrossRef]
- Xiao, C.; Lu, B.A.; Xue, P.; Tian, N.; Zhou, Z.Y.; Lin, X.; Lin, W.F.; Sun, S.G. High-Index-Facet- and High-Surface-Energy Nanocrystals of Metals and Metal Oxides as Highly Efficient Catalysts. Joule 2020, 4, 2562–2598. [Google Scholar] [CrossRef]
- Mohan Mundotiya, B.; Ullah, W. Morphology Controlled Synthesis of the Nanostructured Gold by Electrodeposition Techniques. In Novel Metal Electrodeposition and the Recent Application; IntechOpen: London, UK, 2019. [Google Scholar]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-Dimensional, One-Dimensional, Two-Dimensional and Three-Dimensional Nanostructured Materials for Advanced Electrochemical Energy Devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Purohit, B.; Kumar, A.; Mahato, K.; Chandra, P. Electrodeposition of Metallic Nanostructures for Biosensing Applications in Health Care. J. Sci. Res. 2020, 64, 68–73. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Ruditskiy, A.; Xia, Y. 25th Anniversary Article: Galvanic Replacement: A Simple and Versatile Route to Hollow Nanostructures with Tunable and Well-Controlled Properties. Adv. Mater. 2013, 25, 6313–6333. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jiang, B.; Tang, H.; Lin, Z. Unconventional Seed-Mediated Growth of Ultrathin Au Nanowires in Aqueous Solution. Chem. Sci. 2015, 6, 6349–6354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canepa, S.; Yesibolati, M.N.; Schiøtz, J.; Kadkhodazadeh, S.; Huang, W.; Sun, H.; Mølhave, K. Initiation and Progression of Anisotropic Galvanic Replacement Reactions in a Single Ag Nanowire: Implications for Nanostructure Synthesis. ACS Appl. Nano Mater. 2021, 4, 12346–12355. [Google Scholar] [CrossRef]
- Bhol, P.; Bhavya, M.B.; Swain, S.; Saxena, M.; Samal, A.K. Modern Chemical Routes for the Controlled Synthesis of Anisotropic Bimetallic Nanostructures and Their Application in Catalysis. Front. Chem. 2020, 8, 357. [Google Scholar] [CrossRef]
- Li, G.; Zhang, W.; Luo, N.; Xue, Z.; Hu, Q.; Zeng, W.; Xu, J. Bimetallic Nanocrystals: Structure, Controllable Synthesis and Applications in Catalysis, Energy and Sensing. Nanomaterials 2021, 11, 1926. [Google Scholar] [CrossRef]
- Cai, W.F.; Pu, K.B.; Ma, Q.; Wang, Y.H. Insight into the Fabrication and Perspective of Dendritic Ag Nanostructures. J. Exp. Nanosci. 2017, 12, 319–337. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Jiang, Z. Facile Fabrication of Dendritic Silver Structures and Their Surface Enhanced Raman Spectroscopic Properties. J. Chem. Sci. 2015, 127, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.G.M.; Rodrigues, T.S.; Haigh, S.J.; Camargo, P.H.C. Galvanic Replacement Reaction: Recent Developments for Engineering Metal Nanostructures towards Catalytic Applications. Chem. Commun. 2017, 53, 7135–7148. [Google Scholar] [CrossRef]
- Niu, W.; Zhang, L.; Xu, G. Seed-Mediated Growth of Noble Metal Nanocrystals: Crystal Growth and Shape Control. Nanoscale 2013, 5, 3172–3181. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Evidence for Seed-Mediated Nucleation in the Chemical Reduction of Gold Salts to Gold Nanoparticles. Chem. Mater. 2001, 13, 2313–2322. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Yu, T.; Camargo, P.H.C.; Xia, Y. Nucleation and Growth Mechanisms for Pd-Pt Bimetallic Nanodendrites and Their Electrocatalytic Properties. Nano Res. 2010, 3, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Lim, B.; Wang, J.; Camargo, P.H.C.; Yu, T.; Kim, M.J.; Xia, Y. Seed-Mediated Synthesis of Pd-Rh Bimetallic Nanodendrites. Chem. Phys. Lett. 2010, 494, 249–254. [Google Scholar] [CrossRef]
- Fan, F.R.; Liu, D.Y.; Wu, Y.F.; Duan, S.; Xie, Z.X.; Jiang, Z.Y.; Tian, Z.Q. Epitaxial Growth of Heterogeneous Metal Nanocrystals: From Gold Nano-Octahedra to Palladium and Silver Nanocubes. J. Am. Chem. Soc. 2008, 130, 6949–6951. [Google Scholar] [CrossRef]
- Chao, Y.J.; Lyu, Y.P.; Wu, Z.W.; Lee, C.L. Seed-Mediated Growth of Ag Nanocubes and Their Size-Dependent Activities toward Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2016, 41, 3896–3903. [Google Scholar] [CrossRef]
- Ward, C.J.; Tronndorf, R.; Eustes, A.S.; Auad, M.L.; Davis, E.W. Seed-Mediated Growth of Gold Nanorods: Limits of Length to Diameter Ratio Control. J. Nanomater. 2014, 2014, 765618. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, N.; Weiner, R.G.; Skrabalak, S.E. Ligand-Controlled Co-Reduction versus Electroless Co-Deposition: Synthesis of Nanodendrites with Spatially Defined Bimetallic Distributions. ACS Nano 2014, 8, 12461–12467. [Google Scholar] [CrossRef] [Green Version]
- Niu, Z.; Li, Y. Removal and Utilization of Capping Agents in Nanocatalysis. Chem. Mater. 2014, 26, 72–83. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic Nanocrystals: Syntheses, Properties, and Applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef]
- Fuentes-García, J.A.; Santoyo-Salzar, J.; Rangel-Cortes, E.; Goya, G.F.; Cardozo-Mata, V.; Pescador-Rojas, J.A. Effect of Ultrasonic Irradiation Power on Sonochemical Synthesis of Gold Nanoparticles. Ultrason. Sonochem. 2021, 70, 105274. [Google Scholar] [CrossRef]
- Wang, X.K.; Shao, L.; Guo, W.L.; Wang, J.G.; Zhu, Y.P.; Wang, C. Synthesis of Dendritic Silver Nanostructures by Means of Ultrasonic Irradiation. Ultrason. Sonochem. 2009, 16, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.P.; Xie, Y.; Tang, R.; Chen, M.; Tian, X.B. Novel Ultrasonically Assisted Templated Synthesis of Palladium and Silver Dendritic Nanostructures. Adv. Mater. 2001, 13, 1887–1891. [Google Scholar] [CrossRef]
- Low, S.S.; Yew, M.; Lim, C.N.; Chai, W.S.; Low, L.E.; Manickam, S.; Tey, B.T.; Show, P.L. Sonoproduction of Nanobiomaterials—A Critical Review. Ultrason. Sonochem. 2022, 82, 105887. [Google Scholar] [CrossRef] [PubMed]
- Theerthagiri, J.; Lee, S.J.; Karuppasamy, K.; Park, J.; Yu, Y.; Kumari, M.L.A.; Chandrasekaran, S.; Kim, H.S.; Choi, M.Y. Fabrication Strategies and Surface Tuning of Hierarchical Gold Nanostructures for Electrochemical Detection and Removal of Toxic Pollutants. J. Hazard. Mater. 2021, 420, 126648. [Google Scholar] [CrossRef] [PubMed]
- Gera, T.; Nagy, E.; Smausz, T.; Budai, J.; Ajtai, T.; Kun-Szabó, F.; Homik, Z.; Kopniczky, J.; Bozóki, Z.; Szabó-Révész, P.; et al. Application of Pulsed Laser Ablation (PLA) for the Size Reduction of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Sci. Rep. 2020, 10, 15806. [Google Scholar] [CrossRef] [PubMed]
- Swiatkowska-Warkocka, Z.; Koga, K.; Kawaguchi, K.; Wang, H.; Pyatenko, A.; Koshizaki, N. Pulsed Laser Irradiation of Colloidal Nanoparticles: A New Synthesis Route for the Production of Non-Equilibrium Bimetallic Alloy Submicrometer Spheres. RSC Adv. 2013, 3, 79–83. [Google Scholar] [CrossRef]
- Xu, L.; Li, S.; Zhang, H.; Wang, D.; Chen, M. Laser-Induced Photochemical Synthesis of Branched Ag@Au Bimetallic Nanodendrites as a Prominent Substrate for Surface-Enhanced Raman Scattering Spectroscopy. Opt. Express 2017, 25, 7408–7417. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of Nanoparticles by Laser Ablation: A Review. KONA Powder Part. J. 2017, 34, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Coluccio, M.L.; Gentile, F.; Francardi, M.; Perozziello, G.; Malara, N.; Candeloro, P.; Di Fabrizio, E. Electroless Deposition and Nanolithography Can Control the Formation of Materials at the Nano-Scale for Plasmonic Applications. Sensors 2014, 14, 6056–6083. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Tamanna, T.; Hu, W.J.; Yu, A. Chemical Preparation and Applications of Silver Dendrites. Chem. Pap. 2014, 68, 1283–1297. [Google Scholar] [CrossRef]
- Qiu, T.; Wu, X.L.; Mei, Y.F.; Chu, P.K.; Siu, G.G. Self-Organized Synthesis of Silver Dendritic Nanostructures via an Electroless Metal Deposition Method. Appl. Phys. A Mater. Sci. Process. 2005, 81, 669–671. [Google Scholar] [CrossRef]
- Lahiri, A.; Pulletikurthi, G.; Endres, F. A Review on the Electroless Deposition of Functional Materials in Ionic Liquids for Batteries and Catalysis. Front. Chem. 2019, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of Nanomaterials for Electrochemical Sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurrappa, I.; Binder, L. Electrodeposition of Nanostructured Coatings and Their Characterization—A Review. Sci. Technol. Adv. Mater. 2008, 9, 043001. [Google Scholar] [CrossRef]
- De Campos, A.M.; Silva, R.R.; Calegaro, M.L. Design and Fabrication of Flexible Copper Sensor Decorated with Bismuth Micro/Nanodentrites to Detect Lead and Cadmium in Noninvasive Samples of Sweat. Chemosensors 2022, 10, 446. [Google Scholar] [CrossRef]
- Al-Bat’hi, S.A.M. Electrodeposition of Nanostructure Materials. In Electroplating of Nanostructures; IntechOpen: London, UK, 2015. [Google Scholar]
- Purohit, B.; Kumar, A.; Mahato, K.; Srivastava, A.; Chandra, P. Engineered Three-Dimensional Au-Cu Bimetallic Dendritic Nanosensor for Ultrasensitive Drug Detection in Urine Samples and in Vitro Human Embryonic Kidney Cells Model. Microchem. J. 2022, 176, 107239. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Zheng, Y.; Cai, W.; Fu, Z. Electrodeposition of Ag Dendrites/AgCl Hybrid Film as a Novel Photodetector. Mater. Lett. 2015, 142, 119–121. [Google Scholar] [CrossRef]
- Kaniyankandy, S.; Nuwad, J.; Thinaharan, C.; Dey, G.K.; Pillai, C.G.S. Electrodeposition of Silver Nanodendrites. Nanotechnology 2007, 18, 125610. [Google Scholar] [CrossRef]
- Bakthavatsalam, R.; Ghosh, S.; Biswas, R.K.; Saxena, A.; Raja, A.; Thotiyl, M.O.; Wadhai, S.; Banpurkar, A.G.; Kundu, J. Solution Chemistry-Based Nano-Structuring of Copper Dendrites for Efficient Use in Catalysis and Superhydrophobic Surfaces. RSC Adv. 2016, 6, 8416–8430. [Google Scholar] [CrossRef]
- Meakin, P. The Diffusion-Limited Aggregation Model and Geological Pattern Formation. In Growth, Dissolution and Pattern Formation in Geosystems; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Witten, T.A.; Sander, L.M. Diffusion-Limited Aggregation, a Kinetic Critical Phenomenon. Phys. Rev. Lett. 1981, 47, 1400–1403. [Google Scholar] [CrossRef]
- Santos, N.M.; Santos, D.M.F. A Fractal Dimension Minimum in Electrodeposited Copper Dendritic Patterns. Chaos, Solitons and Fractals 2018, 116, 381–385. [Google Scholar] [CrossRef]
- Tenti, J.M.; Hernández Guiance, S.N.; Irurzun, I.M. Fractal Dimension of Diffusion-Limited Aggregation Clusters Grown on Spherical Surfaces. Phys. Rev. E 2021, 103, 012138. [Google Scholar] [CrossRef] [PubMed]
- Meakin, P.; Majid, I.; Havlin, S.; Eugene Stanley, H. Topological Properties of Diffusion Limited Aggregation and Cluster-Cluster Aggregation. J. Phys. A Gen. Phys. 1984, 17, L975–L981. [Google Scholar] [CrossRef]
- Li, C.; Xiong, H. 3D Simulation of the Cluster-Cluster Aggregation Model. Comput. Phys. Commun. 2014, 185, 3424–3429. [Google Scholar] [CrossRef]
- Jullien, R.; Kolb, M. Hierarchical Model for Chemically Limited Cluster-Cluster Aggregation. J. Phys. A Gen. Phys. 1984, 17, L639–L643. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, X.; Xiong, H. Aggregation Modeling of the Influence of PH on the Aggregation of Variably Charged Nanoparticles. Sci. Rep. 2021, 11, 17386. [Google Scholar] [CrossRef]
- Lu, L.; Kobayashi, A.; Kikkawa, Y.; Tawa, K.; Ozaki, Y. Oriented Attachment-Based Assembly of Dendritic Silver Nanostructures at Room Temperature. J. Phys. Chem. B 2006, 110, 23234–23241. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Xue, X.; Penn, R.L.; Leite, E.R.; Huang, F.; Lin, Z. Crystal Growth by Oriented Attachment: Kinetic Models and Control Factors. CrystEngComm 2014, 16, 1419–1429. [Google Scholar] [CrossRef]
- Salzmann, B.B.V.; Van Der Sluijs, M.M.; Soligno, G.; Vanmaekelbergh, D. Oriented Attachment: From Natural Crystal Growth to a Materials Engineering Tool. Acc. Chem. Res. 2021, 54, 787–797. [Google Scholar] [CrossRef]
- Govardhanagiri, S.; Bethi, S.; Nagaraju, G.P. Small Molecules and Pancreatic Cancer Trials and Troubles. In Breaking Tolerance to Pancreatic Cancer Unresponsiveness to Chemotherapy; Academic Press: Cambridge, MA, USA, 2019; pp. 117–131. [Google Scholar]
- Wang, X.; Cohen, L.; Wang, J.; Walt, D.R. Competitive Immunoassays for the Detection of Small Molecules Using Single Molecule Arrays. J. Am. Chem. Soc. 2018, 140, 18132–18139. [Google Scholar] [CrossRef] [PubMed]
- Stone, J. Sample Preparation Techniques for Mass Spectrometry in the Clinical Laboratory. In Mass Spectrometry for the Clinical Laboratory; Academic Press: Cambridge, MA, USA, 2017; pp. 37–62. ISBN 9780128008713. [Google Scholar]
- Chung, S.; Chandra, P.; Koo, J.P.; Shim, Y.B. Development of a Bifunctional Nanobiosensor for Screening and Detection of Chemokine Ligand in Colorectal Cancer Cell Line. Biosens. Bioelectron. 2018, 100, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Baranwal, A.; Purohit, B.; Roy, S.; Mahto, S.K.; Chandra, P. Advanced Biosensing Methodologies for Ultrasensitive Detection of Human Coronaviruses. In Diagnostic Strategies for COVID-19 and Other Coronaviruses; Springer: Singapore, 2020; pp. 19–36. [Google Scholar]
- Purohit, B.; Vernekar, P.R.; Shetti, N.P.; Chandra, P. Biosensor Nanoengineering: Design, Operation, and Implementation for Biomolecular Analysis. Sensors Int. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Kumar, A.; Purohit, B.; Mahato, K.; Roy, S.; Srivastava, A.; Chandra, P. Design and Development of Ultrafast Sinapic Acid Sensor Based on Electrochemically Nanotuned Gold Nanoparticles and Solvothermally Reduced Graphene Oxide. Electroanalysis 2020, 32, 59–69. [Google Scholar] [CrossRef]
- Nanobiosensors: Next Generation Point-of-Care Biomedical Devices for Personalized Diagnosis. J. Anal. Bioanal. Tech. 2016, 7, 2. [CrossRef] [Green Version]
- Akhtar, M.H.; Hussain, K.K.; Gurudatt, N.G.; Chandra, P.; Shim, Y.B. Ultrasensitive Dual Probe Immunosensor for the Monitoring of Nicotine Induced-Brain Derived Neurotrophic Factor Released from Cancer Cells. Biosens. Bioelectron. 2018, 116, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Divya; Mahapatra, S.; Srivastava, V.R.; Chandra, P. Nanobioengineered Sensing Technologies Based on Cellulose Matrices for Detection of Small Molecules, Macromolecules, and Cells. Biosensors 2021, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
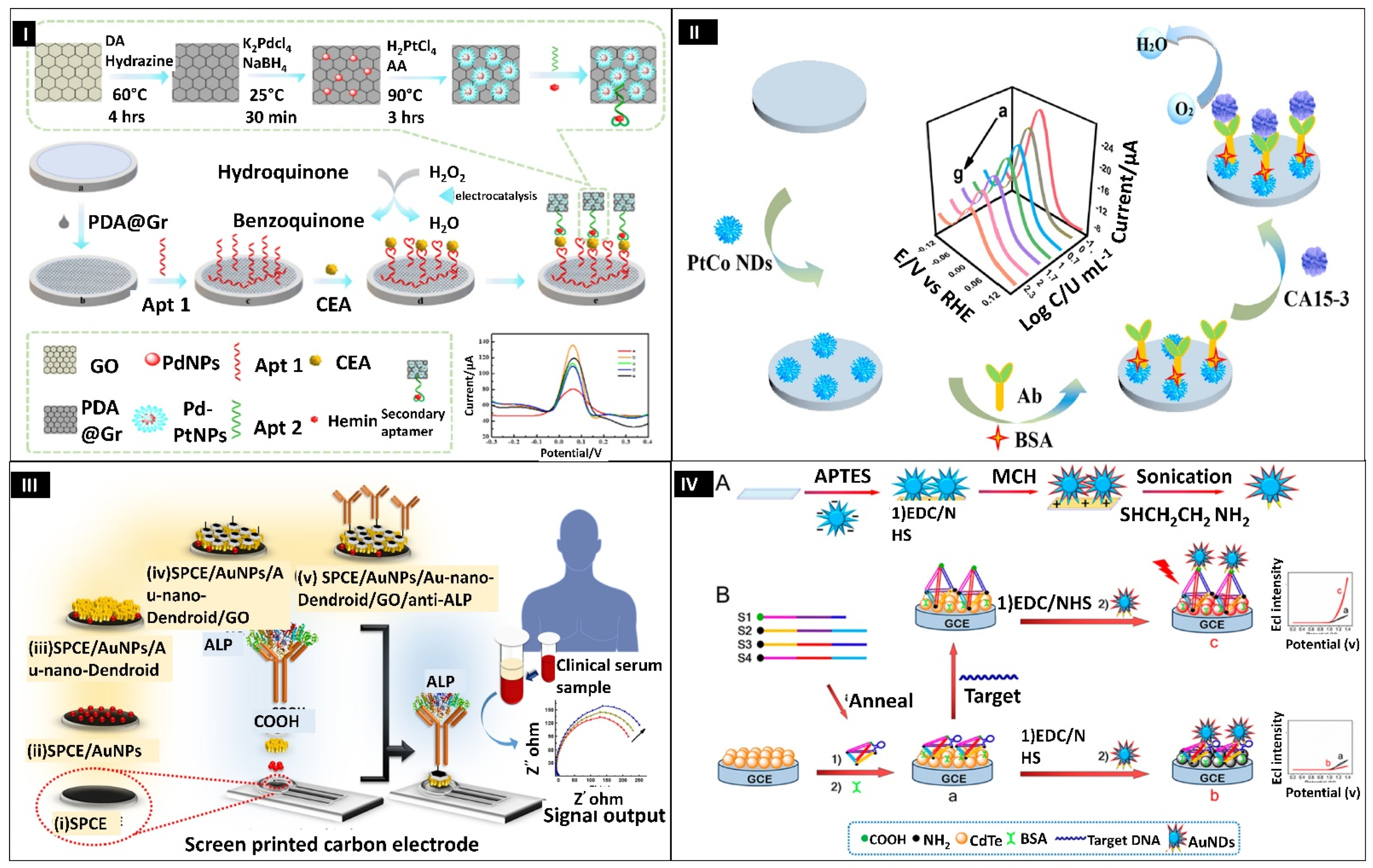
- Mahato, K.; Purohit, B.; Kumar, A.; Chandra, P. Clinically Comparable Impedimetric Immunosensor for Serum Alkaline Phosphatase Detection Based on Electrochemically Engineered Au-Nano-Dendroids and Graphene Oxide Nanocomposite. Biosens. Bioelectron. 2020, 148, 111815. [Google Scholar] [CrossRef]
- Mahato, K.; Kumar, A.; Purohit, B.; Mahapatra, S.; Srivastava, A.; Chandra, P. Nanomaterial Functionalization Strategies in Bio-Interface Development for Modern Diagnostic Devices. In Biointerface Engineering: Prospects in Medical Diagnostics and Drug Delivery; Springer: Singapore, 2020; pp. 195–214. [Google Scholar]
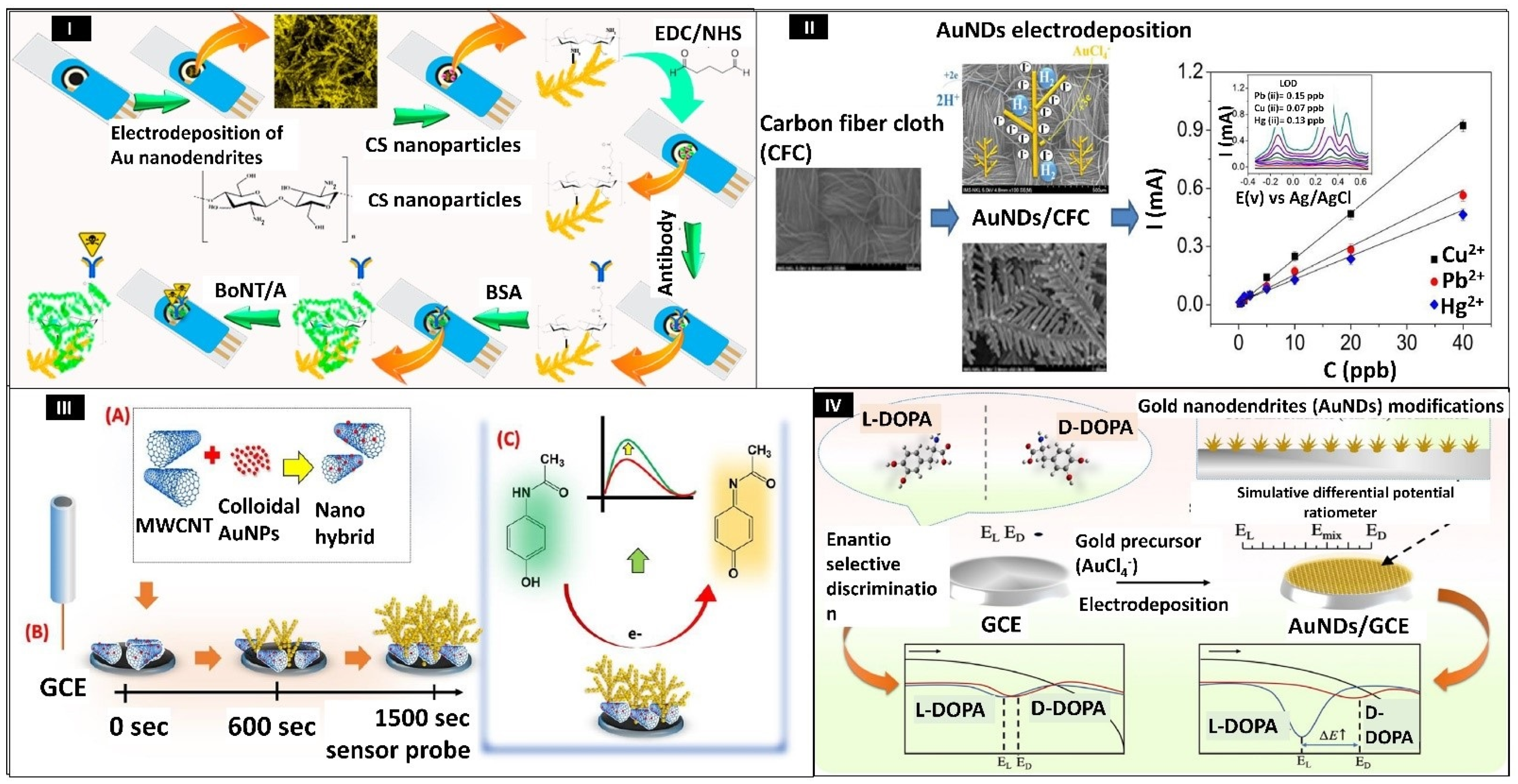
- Sorouri, R.; Bagheri, H.; Afkhami, A.; Salimian, J. Fabrication of a Novel Highly Sensitive and Selective Immunosensor for Botulinum Neurotoxin Serotype a Based on an Effective Platform of Electrosynthesized Gold Nanodendrites/Chitosan Nanoparticles. Sensors 2017, 17, 1074. [Google Scholar] [CrossRef]
- Dang, V.H.; Yen, P.T.H.; Giao, N.Q.; Phong, P.H.; Ha, V.T.T.; Duy, P.K.; Hoeil, C. A Versatile Carbon Fiber Cloth-Supported Au Nanodendrite Sensor for Simultaneous Determination of Cu(II), Pb(II) and Hg(II). Electroanalysis 2018, 30, 2222–2227. [Google Scholar] [CrossRef]
- Lian, H.; Huang, S.; Wei, X.; Guo, J.; Sun, X.; Liu, B. Gold Nanodendrite-Based Differential Potential Ratiometric Sensing Strategy for Enantioselective Recognition of DOPA. Talanta 2020, 210, 120654. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Chen, W.; Zhou, Q. Electrochemical Sensor for Chloramphenicol Based on Copper Nanodendrites and Carbon Nanotubes. Ionics 2022, 28, 451–462. [Google Scholar] [CrossRef]
- Sun, Y.; Zhai, X.; Xu, Y.; Liu, C.; Zou, X.; Li, Z.; Shi, J.; Huang, X. Facile Fabrication of Three-Dimensional Gold Nanodendrites Decorated by Silver Nanoparticles as Hybrid SERS-Active Substrate for the Detection of Food Contaminants. Food Control 2021, 122, 107772. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; German, N.; Kausaite-Minkstimiene, A.; Ramanavicius, A. Glucose Biosensor Based on Dendritic Gold Nanostructures Electrodeposited on Graphite Electrode by Different Electrochemical Methods. Chemosensors 2021, 9, 188. [Google Scholar] [CrossRef]
- Khumngern, S.; Choosang, J.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. Flow Injection Enzyme-Free Amperometric Uric Acid Sensor Consisting of Ordered Mesoporous Carbon Decorated with 3D Pd-Pt Alloy Nanodendrite Modified Screen-Printed Carbon Electrode. Microchem. J. 2020, 157, 104923. [Google Scholar] [CrossRef]
- Gopi, P.K.; Ngo, D.B.; Chen, S.M.; Ravikumar, C.H.; Surareungchai, W. High-Performance Electrochemical Sensing of Hazardous Pesticide Paraoxon Using BiVO4 Nano Dendrites Equipped Catalytic Strips. Chemosphere 2022, 288, 132511. [Google Scholar] [CrossRef]
- Pham, T.B.; Bui, H.; Pham, V.H.; Do, T.C. Surface-Enhanced Raman Spectroscopy Based on Silver Nano-Dendrites on Microsphere End-Shape Optical Fibre for Pesticide Residue Detection. Optik 2020, 219, 165172. [Google Scholar] [CrossRef]
- Pham, T.B.; Hoang, T.H.C.; Pham, V.H.; Nguyen, V.C.; Van Nguyen, T.; Vu, D.C.; Pham, V.H.; Bui, H. Detection of Permethrin Pesticide Using Silver Nano-Dendrites SERS on Optical Fibre Fabricated by Laser-Assisted Photochemical Method. Sci. Rep. 2019, 9, 12590. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Pan, D.; Cui, Y.; Liu, H.; Gao, G.; Xia, J. Anodic Stripping Determination of Selenium in Seawater Using an Electrode Modified with Gold Nanodendrites/Perforated Reduced Graphene Oxide. Int. J. Electrochem. Sci. 2020, 15, 1669–1680. [Google Scholar] [CrossRef]
- Wang, C.; Tang, Q.; Zhao, K.; Deng, A.; Li, J. Peroxydisulfate/Oxygen System-Based Electrochemiluminescent Immunosensing of Hg2+ Using Pt/Pd Nanodendrites-Thiosemicarbazide/Norfloxacin as a Signal Enhancer. Analyst 2019, 144, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Giao, N.Q.; Dang, V.H.; Yen, P.T.H.; Phong, P.H.; Ha, V.T.T.; Duy, P.K.; Chung, H. Au Nanodendrite Incorporated Graphite Pencil Lead as a Sensitive and Simple Electrochemical Sensor for Simultaneous Detection of Pb(II), Cu(II) and Hg(II). J. Appl. Electrochem. 2019, 49, 839–846. [Google Scholar] [CrossRef]
- Chu, G.; Wang, B.; Huang, J.; Zhang, Y.; Guo, Y.; Sun, X.; Li, M. Novel Three-dimensional Senor Based on Nanodendrites for Nitrite Determination. J. Appl. Electrochem. 2021, 51, 1059–1070. [Google Scholar] [CrossRef]
- Lien, N.T.; Quoc Hung, L.; Hoang, N.T.; Thu, V.T.; Ngoc Nga, D.T.; Hai Yen, P.T.; Phong, P.H.; Thu Ha, V.T. An Electrochemical Sensor Based on Gold Nanodendrite/Surfactant Modified Electrode for Bisphenol A Detection. J. Anal. Methods Chem. 2020, 2020, 6693595. [Google Scholar] [CrossRef] [PubMed]
- Juska, V.B.; Walcarius, A.; Pemble, M.E. Cu Nanodendrite Foams on Integrated Band Array Electrodes for the Nonenzymatic Detection of Glucose. ACS Appl. Nano Mater. 2019, 2, 5878–5889. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, R.; Wang, L.; Yu, L.; Wang, J.; Geng, B.; Wang, G. Controllable Synthesis of Silver Nanodendrites on Copper Rod and Its Application to Hydrogen Peroxide and Glucose Detection. CrystEngComm 2013, 15, 1173–1178. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, C.; Yang, Y.; Yang, N.; Lu, S.; You, T.; Yin, P. A High Sensitive Glucose Sensor Based on Ag Nanodendrites/Cu Mesh Substrate via Surface-Enhanced Raman Spectroscopy and Electrochemical Analysis. J. Alloys Compd. 2021, 863, 158758. [Google Scholar] [CrossRef]
- Ridhuan, N.S.; Mohamad Nor, N.; Abdul Razak, K.; Lockman, Z.; Zakaria, N.D. ITO Electrode Modified with Pt Nanodendrites-Decorated ZnO Nanorods for Enzymatic Glucose Sensor. J. Solid State Electrochem. 2021, 25, 1065–1072. [Google Scholar] [CrossRef]
- Valera, A.E.; Nesbitt, N.T.; Archibald, M.M.; Naughton, M.J.; Chiles, T.C. On-Chip Electrochemical Detection of Cholera Using a Polypyrrole-Functionalized Dendritic Gold Sensor. ACS Sens. 2019, 4, 654–659. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Noh, H.B.; Shim, Y.B. Dealloyed AuNi Dendrite Anchored on a Functionalized Conducting Polymer for Improved Catalytic Oxygen Reduction and Hydrogen Peroxide Sensing in Living Cells. Adv. Funct. Mater. 2016, 26, 1590–1601. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Zhang, D.; Ma, M.; Wang, W.; Chen, Q. Nano-Assemblies Consisting of Pd/Pt Nanodendrites and Poly (Diallyldimethylammonium Chloride)-Coated Reduced Graphene Oxide on Glassy Carbon Electrode for Hydrogen Peroxide Sensors. Mater. Sci. Eng. C 2016, 58, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.M.; Zhang, M.R.; Luo, L.Q.; Pan, G.B. Electrosynthesis of Bismuth Nanodendrites/Gallium Nitride Electrode for Non-Enzymatic Hydrogen Peroxide Detection. Talanta 2017, 171, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Amini, R.; Asadpour-Zeynali, K. Layered Double Hydroxide Decorated with Ag Nanodendrites as an Enhanced Sensing Platform for Voltammetric Determination of Pyrazinamide. New J. Chem. 2018, 42, 2140–2148. [Google Scholar] [CrossRef]
- Porifreva, A.V.; Gorbatchuk, V.V.; Evtugyn, V.G.; Stoikov, I.I.; Evtugyn, G.A. Glassy Carbon Electrode Modified with Silver Nanodendrites Implemented in Polylactide-Thiacalix[4]Arene Copolymer for the Electrochemical Determination of Tryptophan. Electroanalysis 2018, 30, 641–649. [Google Scholar] [CrossRef]
- GunaVathana, S.D.; Thivya, P.; Wilson, J.; Peter, A.C. Sensitive Voltammetric Sensor Based on Silver Dendrites Decorated Polythiophene Nanocomposite: Selective Determination of L-Tryptophan. J. Mol. Struct. 2020, 1205, 127649. [Google Scholar] [CrossRef]
- Yu, S.; Li, H.; Li, G.; Niu, L.; Liu, W.; Di, X. Reduced Graphene Oxide-Supported Gold Dendrite for Electrochemical Sensing of Acetaminophen. Talanta 2018, 184, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, R.; Sangaranarayanan, M.V. A Facile Formation of Silver Dendrites on Indium Tin Oxide Surfaces Using Electrodeposition and Amperometric Sensing of Hydrazine. Sens. Actuators B Chem. 2015, 213, 92–101. [Google Scholar] [CrossRef]
- Dhanush, S.; Sreejesh, M.; Bindu, K.; Chowdhury, P.; Nagaraja, H.S. Synthesis and Electrochemical Properties of Silver Dendrites and Silver Dendrites/RGO Composite for Applications in Paracetamol Sensing. Mater. Res. Bull. 2018, 100, 295–301. [Google Scholar] [CrossRef]
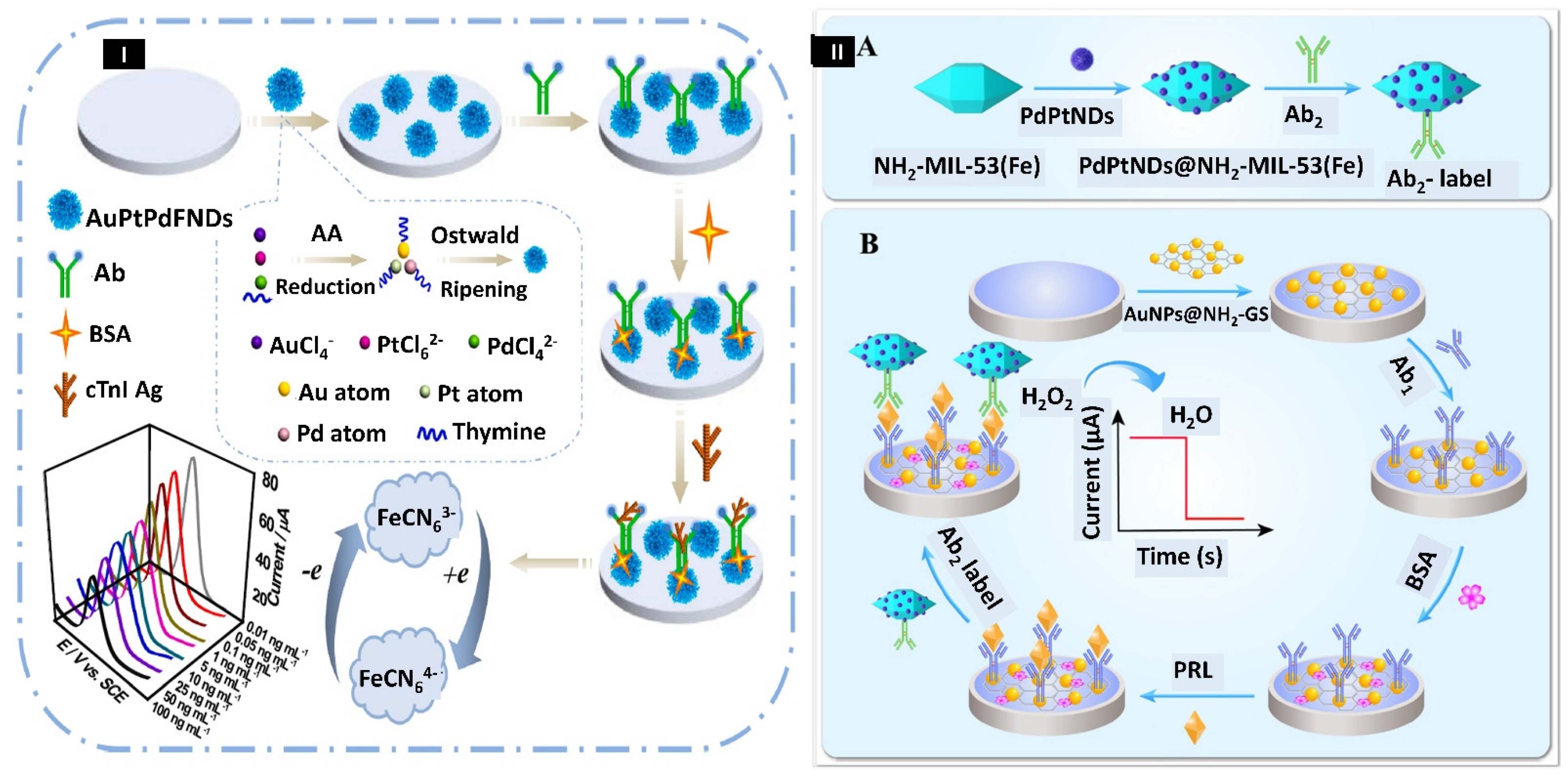
- Cen, S.Y.; Ge, X.Y.; Chen, Y.; Wang, A.J.; Feng, J.J. Label-Free Electrochemical Immunosensor for Ultrasensitive Determination of Cardiac Troponin I Based on Porous Fluffy-like AuPtPd Trimetallic Alloyed Nanodendrites. Microchem. J. 2021, 169, 106568. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, F.; Gong, W.; Tian, F.; Wu, H.; Ding, S.; Li, S.; Luo, R. Multi-Branched PdPt Nanodendrites Decorated Amino-Rich Fe-Based Metal-Organic Framework as Signal Amplifier for Ultrasensitive Electrochemical Detection of Prolactin. J. Electroanal. Chem. 2021, 882, 115032. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; Han, Y.; Fan, L.; Guo, Y. Sandwich Electrochemical Carcinoembryonic Antigen Aptasensor Based on Signal Amplification of Polydopamine Functionalized Graphene Conjugate Pd-Pt Nanodendrites. Bioelectrochemistry 2021, 142, 107947. [Google Scholar] [CrossRef]
- Ge, X.Y.; Feng, Y.G.; Cen, S.Y.; Wang, A.J.; Mei, L.P.; Luo, X.; Feng, J.J. A Label-Free Electrochemical Immnunosensor Based on Signal Magnification of Oxygen Reduction Reaction Catalyzed by Uniform PtCo Nanodendrites for Highly Sensitive Detection of Carbohydrate Antigen 15-3. Anal. Chim. Acta 2021, 1176, 338750. [Google Scholar] [CrossRef]
- Li, M.X.; Feng, Q.M.; Zhou, Z.; Zhao, W.; Xu, J.J.; Chen, H.Y. Plasmon-Enhanced Electrochemiluminescence for Nucleic Acid Detection Based on Gold Nanodendrites. Anal. Chem. 2018, 90, 1340–1347. [Google Scholar] [CrossRef]
- Fan, L.; Yan, Y.; Guo, B.; Zhao, M.; Li, J.; Bian, X.; Wu, H.; Cheng, W.; Ding, S. Trimetallic Hybrid Nanodendrites and Magnetic Nanocomposites-Based Electrochemical Immunosensor for Ultrasensitive Detection of Serum Human Epididymis Protein 4. Sens. Actuators B Chem. 2019, 296, 126697. [Google Scholar] [CrossRef]
- Jiao, L.; Mu, Z.; Zhu, C.; Wei, Q.; Li, H.; Du, D.; Lin, Y. Graphene Loaded Bimetallic Au@Pt Nanodendrites Enhancing Ultrasensitive Electrochemical Immunoassay of AFP. Sens. Actuators B Chem. 2016, 231, 513–519. [Google Scholar] [CrossRef]
- Pei, F.; Wang, P.; Ma, E.; Yang, Q.; Yu, H.; Liu, J.; Yin, H.; Li, Y.; Liu, Q.; Dong, Y. A Sensitive Label-Free Immunosensor for Alpha Fetoprotein Detection Using Platinum Nanodendrites Loaded on Functional MoS 2 Hybridized Polypyrrole Nanotubes as Signal Amplifier. J. Electroanal. Chem. 2019, 835, 197–204. [Google Scholar] [CrossRef]
- He, L.; Rodda, T.; Haynes, C.L.; Deschaines, T.; Strother, T.; Diez-Gonzalez, F.; Labuza, T.P. Detection of a Foreign Protein in Milk Using Surface-Enhanced Raman Spectroscopy Coupled with Antibody-Modified Silver Dendrites. Anal. Chem. 2011, 83, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Feng, X.; Hu, J.; Bo, S.; Zhang, J.; Wang, W.; Li, S.; Yang, Y. Preparation of Hemoglobin (Hb)-Imprinted Poly(Ionic Liquid)s via Hb-Catalyzed EATRP on Gold Nanodendrites. Anal. Bioanal. Chem. 2020, 412, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, C.; Gao, R.; Geng, Y.; Zhao, Y.; Niu, Y.; Zhang, L.; Yu, Y.; He, J. A Palladium-Platinum Bimetal Nanodendritic Melamine Network for Signal Amplification in Voltammetric Sensing of DNA. Microchim. Acta 2018, 185, 138. [Google Scholar] [CrossRef]
- Song, Y.; Xu, T.; Xu, L.P.; Zhang, X. Nanodendritic Gold/Graphene-Based Biosensor for Tri-Mode MiRNA Sensing. Chem. Commun. 2019, 55, 1742–1745. [Google Scholar] [CrossRef]
- Liu, F.; Xiang, G.; Jiang, D.; Zhang, L.; Chen, X.; Liu, L.; Luo, F.; Li, Y.; Liu, C.; Pu, X. Ultrasensitive Strategy Based on PtPd Nanodendrite/Nano-Flower-Like@GO Signal Amplification for the Detection of Long Non-Coding RNA. Biosens. Bioelectron. 2015, 74, 214–221. [Google Scholar] [CrossRef]
- Zhou, X.; Qian, X.; Tan, X.; Ran, X.; Li, Z.; Huang, Z.; Yang, L.; Xie, X. Water-Soluble Pillar[6]Arene Functionalized PdPt Porous Core-Shell Octahedral Nanodendrites to Construct Highly Sensitive and Robust Neuron-Specific Enolase Immunosensor by Host-Guest Chemistry Assisted Catalytic Amplification. Anal. Chim. Acta 2019, 1068, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.J.; Zhu, X.Y.; Chen, Y.; Luo, X.; Xue, Y.; Feng, J.J. Ultrasensitive Label-Free Electrochemical Immunoassay of Carbohydrate Antigen 15-3 Using Dendritic Au@Pt Nanocrystals/Ferrocene-Grafted-Chitosan for Efficient Signal Amplification. Sens. Actuators B Chem. 2019, 292, 164–170. [Google Scholar] [CrossRef]
- Shao, F.; Jiao, L.; Wei, Q.; Li, H. Ternary Pt-Co-Cu Nanodendrites for Ultrasensitive Voltammetric Determination of Insulin at Very Low Working Potential. Microchim. Acta 2017, 184, 2031–2038. [Google Scholar] [CrossRef]
- Ke, H.; Liu, M.; Zhuang, L.; Li, Z.; Fan, L.; Zhao, G. A Fetomolar Level 17β-Estradiol Electrochemical Aptasensor Constructed on Hierachical Dendritic Gold Modified Boron-Doped Diamond Electrode. Electrochim. Acta 2014, 137, 146–153. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Zhang, X.; Gao, Z.; Feng, J.; Wang, P.; Dong, Y. The Label-Free Immunosensor Based on Rhodium@palladium Nanodendrites/Sulfo Group Functionalized Multi-Walled Carbon Nanotubes for the Sensitive Analysis of Carcino Embryonic Antigen. Anal. Chim. Acta 2018, 1007, 61–70. [Google Scholar] [CrossRef]
- Yang, G.; Liu, H.; Liu, X.; Zhang, P.; Huang, C.; Xu, T.; Jiang, L.; Wang, S. Underwater-Transparent Nanodendritic Coatings for Directly Monitoring Cancer Cells. Adv. Healthc. Mater. 2014, 3, 332–337. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, Y.; Zhang, L.; Liang, L.; Liu, H.; Yan, M.; Huang, J.; Yu, J. Ultrasensitive Electrochemical Cancer Cells Sensor Based on Trimetallic Dendritic Au@PtPd Nanoparticles for Signal Amplification on Lab-on-Paper Device. Sens. Actuators B Chem. 2015, 220, 665–672. [Google Scholar] [CrossRef]

| Sr. No. | Sensing Molecule | Detection Techniques | Description | Deposition Potential | Response Time | Real Sample | LDR | LOD | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Uric acid | Amperometry | After being fabricated by ordered mesoporous carbon (OMC), screen-printed carbon electrode (SPCE) was electrodeposited with a three-dimensional (3D) dendritic nanomaterial of the palladium−platinum (Pd−Pt) alloy | 0.14 V | NR | Serum | 0.00025–0.80 mM | 0.25 μM | [103] |
| 2 | Pesticides | ||||||||
| (a) Paraoxon | DPV | First, BiVO4 was hydrothermally synthesized and characterized. It was then decorated on the screen-printed electrode for sensing of Paraoxon, an organophosphorus pesticide | NR | NR | River water | 0.199–1.96 μM | 0.03 μM | [104] | |
| (b) Dimethoate | Optical | Ag nanodendrite structures were developed on the optical fibers’ surface by a cost-effective laser-assisted photochemical method | NR | NR | NR | 0.005–4 ppm | 0.002 ppm | [105] | |
| (c) Permethrin | Optical | The procedure was initiated with the synthesis of SERS-active optical fiber substrates. Then, using a laser-assisted photochemical technique, silver (Ag) nanodendrites were deposited on the tip of the fiber core | NR | NR | NR | 0.1–20 ppm | 0.0035 ppm | [106] | |
| 3 | Metal ions | ||||||||
| (a) Selenium | Anodic stripping voltammetry | Glassy carbon electrode (GCE) modified with reduced graphene oxide (rGO) and further AuNDs was electrodeposited to form GCE/P-rGO/AuNDs | −0.2 V | NR | Seawater | 3–300 nM | 0.9 nM | [107] | |
| (b) Cadmium ion (Cd2+) and Lead ion (Pb2+) | DPASV | Bismuth nanodendrites (BiNDs) were fabricated by one-step electrodeposition of bismuth (Bi) and simultaneously detected Cd2+ and Pb2+ ions. Bromide ion (Br-) was used as a co-reagent to inhibit agglomeration of Bi | −2.8–−2.6 V | NR | Pure water, seawater, lake water | 2–270 ppb | 0.09 ppb (Cd2+) 0.05 ppb (Pb2+) | [11] | |
| (c) Mercury ion (Hg2+) | Electro chemiluminescent immunoassay | GCE was modified with gold nanoparticles (GNP20), and further nanodendritic structure of Pt/Pd was loaded on it. In this experiment, GNP50 was employed as a biocarrier to load more Pt/Pd NDs | NR | NR | Tap water, Lake water | 0.05–1000 ng/mL | 16 pg/mL | [108] | |
| (d) Hg (II), Cu (II), and Pb (II) | DPASV | One-step electrodeposition was used to create AuNDs structures on graphite pencil lead (GPL) | −0.3 V | NR | Lake water | 1–50 ppb | 0.18 ppb for Hg (II), 0.19 ppb for Cu (II), 0.12 ppb for Pb (II) | [109] | |
| (e) Nitrite | Amperometry | GCE modified with poly dimethyl diallyl ammonium chloride-reduced graphene oxide (PDDA-RGO), and further copper nanodendrites (CuNDs) were electrodeposited on it to form PDDA-RGO/ Cu NDs/GCE | −1 V | 3 s | NR | 1–15,000 μM | 0.06 μM | [110] | |
| 4 | Bisphenol A | CV, DPV | GCE modified with cetyl trimethyl ammonium bromide (CTAB), and further AuND were electrostatically deposited | NR | 5 min | Drinking water | 0.025–10 μM | 22 nM | [111] |
| 5 | (a) Glucose | LSV | Cu nanodendrite foams (CuND foams) were electrodeposited on gold array electrodes under acidic conditions at negative overpotentials | −5.0 V | NR | Human serum | 0.01–22.55 mM | NR | [112] |
| (b) Glucose | Amperometry | A simple and easy displacement process, without any surfactants, was used to construct silver nanodendrites on copper rods | NR | <3 s | NR | 0.02–7.4 mM | 0.1 µM | [113] | |
| (c) Glucose | Amperometry | A simple electrochemical deposition approach was used to produce Ag nanodendrites on a Cu mesh substrate, which showed high electrocatalytic activity and SERS sensitivity | 1.7 V | NR | Human urine | 0.5–5 mM | 0.005 mM | [114] | |
| (d) Glucose | CV | A Cu–Co alloy nanodendritic surfaces, with a hierarchical structure, was electrochemically prepared for detection of glucose | −0.80 V | 5 s | Human blood | 0.5 µM–14.0 mM | 0.10µM | [10] | |
| (e) Glucose | Amperometry | Indium tin oxide (ITO) electrode was decorated with zinc oxide nanorods (ZnONRs) and further platinum nanodendrites (PtNDs) were synthesized on it via the chemical reduction method | NR | NR | Human blood | 0.05–1 mM | 0.03 mM | [115] | |
| 6 | Cholera toxin | DPV | Using poly-(2-cyano-ethyl)pyrrole (PCEPy), dendritic gold architecture was functionalized with antibodies. Here, conductive polypyrrole polymer PCEPy and directed electrochemical nanowire assembly (DENA) were combined to facilitate functionalization. | NR | NR | NR | NR | 1 ng/mL | [116] |
| 7 | (a) H2O2 | Chronoamperometry | GCE modified with p-benzoic acid-2,2′:5′,2″-terthiophene (TBA) polymer and further gold nickel (AuNi) dendrites were deposited electrochemically to detect H2O2 | –0.8 V | 3 s | Cancer cell, normal cell | 5–40 nM, 80 nM–30μM, 200 μM–2.5 mM | 5 nM | [117] |
| (b) H2O2 | Amperometry | A simple and easy displacement process, without any surfactants, was used to construct silver nanodendrites on copper rods | NR | <3 s | NR | 0.2–19.2 mM | 0.1 µM | [113] | |
| (c) H2O2 | CV | GCE electrode modified with (Pd/Pt-NDs) and rGO, which was coated with poly (diallyldimethylammonium chloride) (PDDA) | 0.018 V | 5 s | Fetal bovine serum (FBS) | 0.005–0.5 mM | 0.027 μM | [118] | |
| (d) H2O2 | CV | A copper–cobalt (Cu–Co) alloy nanodendritic surface, with a hierarchical structure, was electrochemically prepared for detection of glucose | −0.80 V | 5 s | Human blood | 1.0 μM–11 mM | 0.75 μM | [10] | |
| (e) H2O2 | CV | DPV technique was used to electrodeposit bismuth nanodendrites (BiNDs) on gallium nitride (GaN) electrode | −0.05 V | NR | Fetal bovine serum (FBS), milk, tap water | 10 μM–1 mM, 1–10 mM | 5 μM | [119] | |
| 8 | Pyrazinamide (PZA) drug | DPV | GCE was altered with zinc–aluminum layered double hydroxide (Zn–Al LDH), and further nanodendritic silver (AgNDs) were electrodeposited on the surface | –0.3 V | NR | Human serum and urine | 9.0 × 10−7–5.2 × 10−4 mol/L | 7.2 × 10−7 mol/L | [120] |
| 9 | Amino acids | ||||||||
| (a) Tryptophan | DPV | GCE was modified by new polymeric materials made from oligolactides by cross-linking with tetracarboxylated thiacalix [4] arene in a cone, partial cone and 1,3-alternate configurations and then silver was deposited by potential cycling in the polymer film pores | 0.7 V | NR | Sedative medicine | 0.1–100 µM | 0.03 µM | [121] | |
| (b) Tryptophan | SWV | First, Ag dendrites were synthesized, and then polythiophene (PT)–Ag nanodendrites composite were formed by electrostatic interaction and fabricated on the GCE surface | NR | NR | Soybeans extract | 200 nM–400 μM | 20 nM | [122] | |
| 10 | Acetaminophen | Amperometry | First, PDDA-coated gold dendrite, and poly (sodium 4-styrenesulfonate) (PSS) coated rGO was synthesized. Finally, rGO-gold dendritic surface was constructed by self-assembly of both for acetaminophen detection | NR | NR | Tablets, human urine | 0.07–3000 μM | 0.005 μM | [123] |
| 11 | Hydrazine | Amperometry | ITO electrodes were modified with silver dendritic structures by using an aqueous solution of AgNO3 and KNO3 without any surfactants | −0.80 V | <5 s | Tap water, distilled water, and river water samples | 100–1700 μM | 0.5 μM | [124] |
| 12 | Paracetamol | CV, Chronoamperometry | Silver nanodendrites and its composite with graphene oxide (GO) were constructed by galvanic replacement method and dropcasted on GCE | NR | <3 s | NR | 0.5–10 mM | 0.025 μM | [125] |
| Sr. No | Sensing Molecule | Detection Techniques | Description | Deposition Potential | Response Time | Real Sample | LDR | LOD | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Proteins | ||||||||
| (a) Human epididymis protein 4 | DPV | Trimetallic AgPtCo nanodendrites were synthesized by convenient one-pot method | NR | NR | HE-4-positive ovarian cancer patients | 0.001–50 ng/mL | 0.487 pg/mL | [131] | |
| (b) Alpha-fetoprotein (AFP) | Amperometry | Graphene (NH2-GS) doped mesoporous Au@Pt nanodendrites (NH2-GS/Au@Pt) and poly-dopamine coated N-doped multi-walled carbon nanotube (PDA-N-MWCNT) was used to synthesize sandwich electrochemical immunosensor for AFP sensing | NR | NR | NR | 0.1 pg/mL–10 ng/mL | 0.05 pg/mL | [132] | |
| (c) Alpha-fetoprotein (AFP) | CV | First, poly (diallyldimethylammonium chloride) decorated molybdenum disulfide nanosheet (MoS2) was synthesized and hybridized with polypyrrole nanotubes. Then, platinum nanodendrites were fabricated to form Pt NDs/PDDA/MoS2@PPy NTs | NR | NR | Human serum | 50 fg/mL–50 ng/mL | 17 fg/mL | [133] | |
| (d) Ovalbumin (OVA) | Optical | Antibody-modified silver dendrites were coupled with surface-enhancedRaman scattering (SERS) phenomena for identification of OVA | NR | 30 min | Milk | NR | 5 μg/mL | [134] | |
| 2 | Hemoglobin | DPV | On the Au electrode surface, haemoglobin (Hb)-imprinted poly(ionic liquids) (HIPILs) were built to create Au/AuND/HIPILs. Gold nanodendrites were earlier used to alter the Au electrode surface | −0.9 V | NR | Bovine blood sample | 1.0 × 10−14 –1.0 × 10−4 mg/mL | 5.22 × 10−15 mg/mL | [135] |
| 3 | Nucleic acid | ||||||||
| (a) DNA | Chronoamperometry | The one-pot method was utilized to construct PdPt nanodendrites, which acted as a carrier for the DNA probe. Further, the PdPt NDs were combined with melamine | NR | NR | Human serum | 1 fmol/L–1 nmol/L | 0.33 fmol/L | [136] | |
| (b) miRNA | DPV | Nanodendritic gold structure was electrodeposited on the ITO/Ti/Au, and further graphene was deposited on the surface. | −1.8 V | NR | NR | 0.43 pM–1.13 nM | 0.34 nM | [137] | |
| (c) lncRNAs | CV | Graphene oxide/Au/horseradish peroxidase surface was decorated with Pt–Pd bimetallic nanodendrites to form PtPd/BND/BNF@GO/Au/HRP nanocomposites. Thionine or a detecting probe was coated over Au particles | NR | NR | Serum | 1.00 × 10−3–1.00 × 103 pM/mL | 0.247 fM/mL | [138] | |
| 4 | Enolase | DPV | GCE modified with AuNPs and further forms GCE/Au/Ab1/BSA/NSE surface. Finally, TB/WP6@PdPt-Ab2 were deposited on the surface | NR | NR | Human serum | 0.0003–100.00 ng/mL | 0.095 pg/mL | [139] |
| Carbohydrates | |||||||||
| (a) Carbohydrate antigen 15-3 (CA15-3) | DPV | First, Au@Pt core–shell nanodendritic crystals (Au@Pt NCs) were synthesized by one-pot wet-chemical strategy. Then, it was dispersed homogenously with ferrocene-grafted-chitosan (Fcg-CS) on GCE surface | NR | NR | Serum | 0.5–200 U/mL | 0.17 U/mL | [140] | |
| 5 | (b) Carbohydrate antigen 15-3 (CA15-3) | DPV | Using a one-pot solvothermal technique, and co-structure-directing agent, L-carnosine platinum-cobalt nanodendritic (Pt-Co NDs) surfaces were made | NR | NR | Human serum | 0.1–200 U/mL | 0.0114 U/mL | [129] |
| 6 | Insulin hormone | Amperometry | Antibody 1 was immobilized on glassy carbon electrode (GCE) surface altered with gold nanoparticles (AuNPs). Finally, antibody 2 conjugated Pt-Co-Cu nanodendrites were electrodeposited | −0.2 V | NR | Serum | 0.2–2000 pM | 0.08 pM | [141] |
| 7 | 17 β-estradiol (E2) hormone | EIS | Boron doped diamond (BDD) electrode surface was used to grow dendritic gold by a double template method. Further 17 β estradiol (E2) aptamers were functionalized on the surface of the Au/BDD electrode by covalent bonding (Au-S) to capture E2. | NR | NR | Water | 1.0 × 10−14 to 1.0 × 10−9 mol/L | 5.0 × 10−15 mol/L | [142] |
| 8 | Glycoprotein Carcinoembryonic antigen (CEA) | DPV | Bimetallic core–shell rhodium@palladium nanodendrites (Rh@Pd NDs) synthesized on MWCNT, functionalized with sulfo group (MWCNTs-SO3H) to prepare Rh@PdNDs/MWCNTs-SO3H composite surfaces | NR | NR | Human serum | 25 fg/mL to 100 ng/mL | 8.3 fg/mL | [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, R.; Dkhar, D.S.; Mahapatra, S.; Divya; Singh, S.P.; Chandra, P. Nano-Engineered Surface Comprising Metallic Dendrites for Biomolecular Analysis in Clinical Perspective. Biosensors 2022, 12, 1062. https://doi.org/10.3390/bios12121062
Kumari R, Dkhar DS, Mahapatra S, Divya, Singh SP, Chandra P. Nano-Engineered Surface Comprising Metallic Dendrites for Biomolecular Analysis in Clinical Perspective. Biosensors. 2022; 12(12):1062. https://doi.org/10.3390/bios12121062
Chicago/Turabian StyleKumari, Rohini, Daphika S. Dkhar, Supratim Mahapatra, Divya, Surinder P. Singh, and Pranjal Chandra. 2022. "Nano-Engineered Surface Comprising Metallic Dendrites for Biomolecular Analysis in Clinical Perspective" Biosensors 12, no. 12: 1062. https://doi.org/10.3390/bios12121062
APA StyleKumari, R., Dkhar, D. S., Mahapatra, S., Divya, Singh, S. P., & Chandra, P. (2022). Nano-Engineered Surface Comprising Metallic Dendrites for Biomolecular Analysis in Clinical Perspective. Biosensors, 12(12), 1062. https://doi.org/10.3390/bios12121062






