Flash Characterization of Smartphones Used in Point-of-Care Diagnostics
Abstract
:1. Introduction
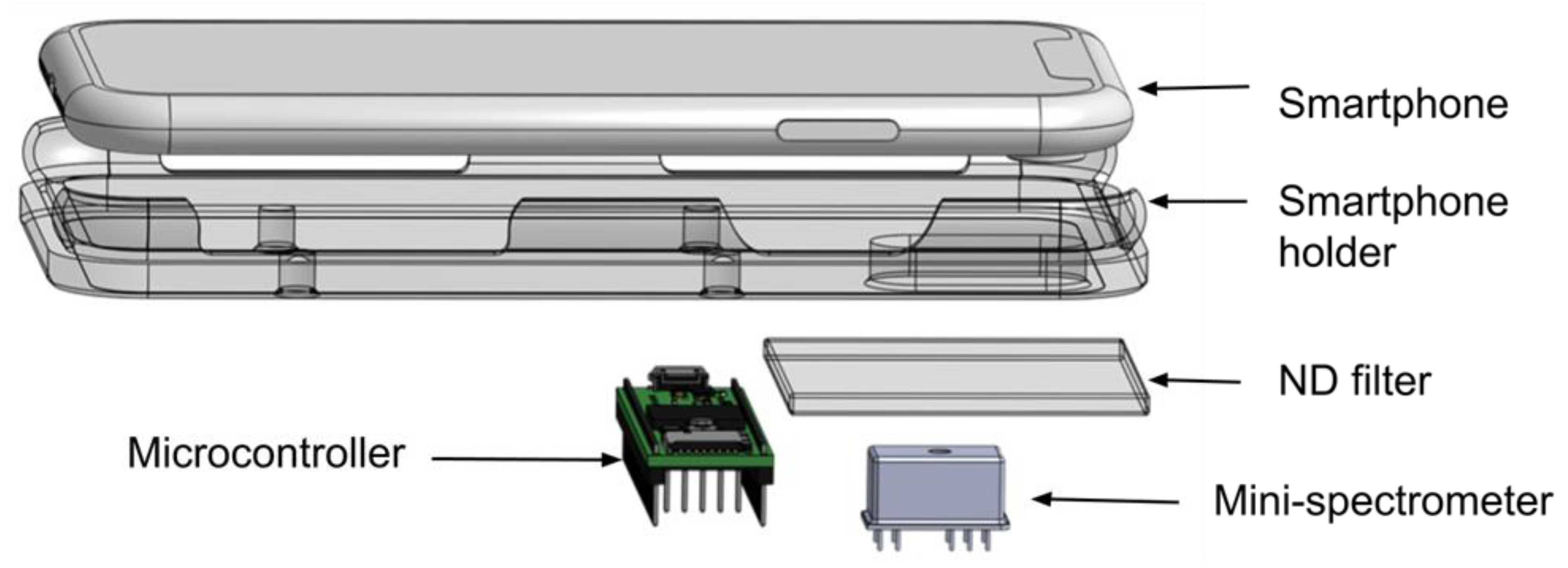
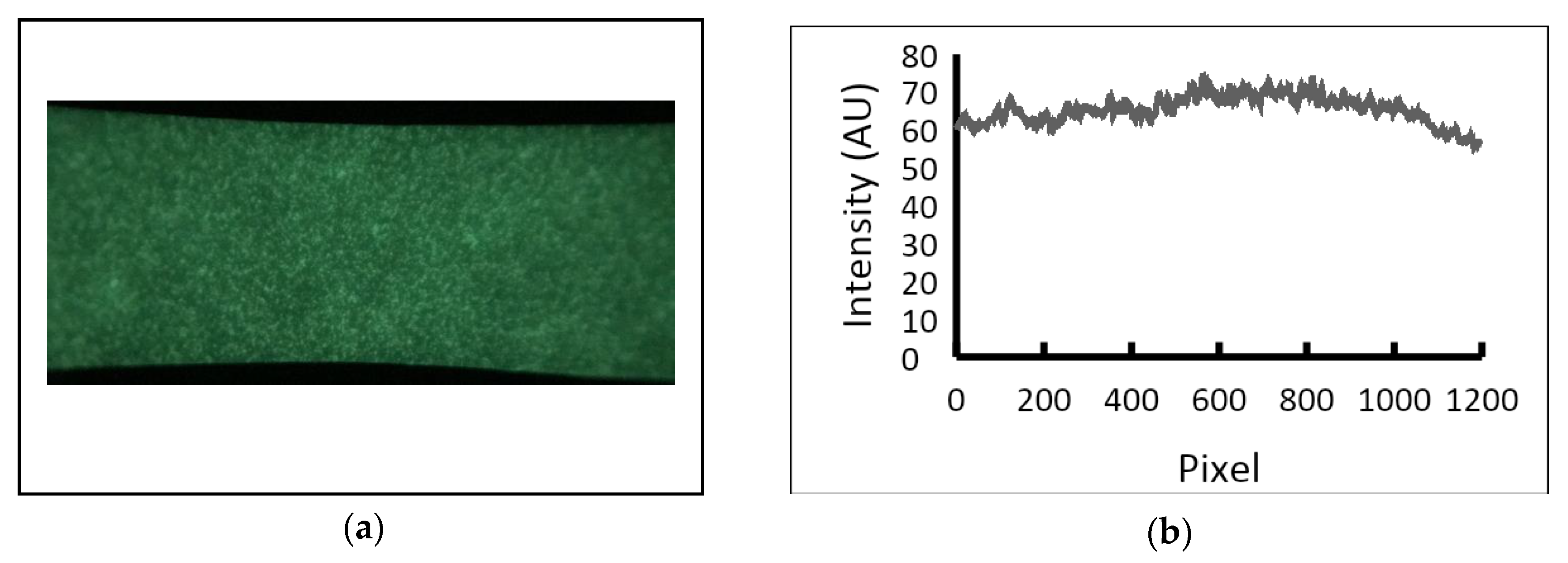
2. Materials and Methods
3. Results and Discussion
3.1. Flash Duration and Variability as a Function of Power State
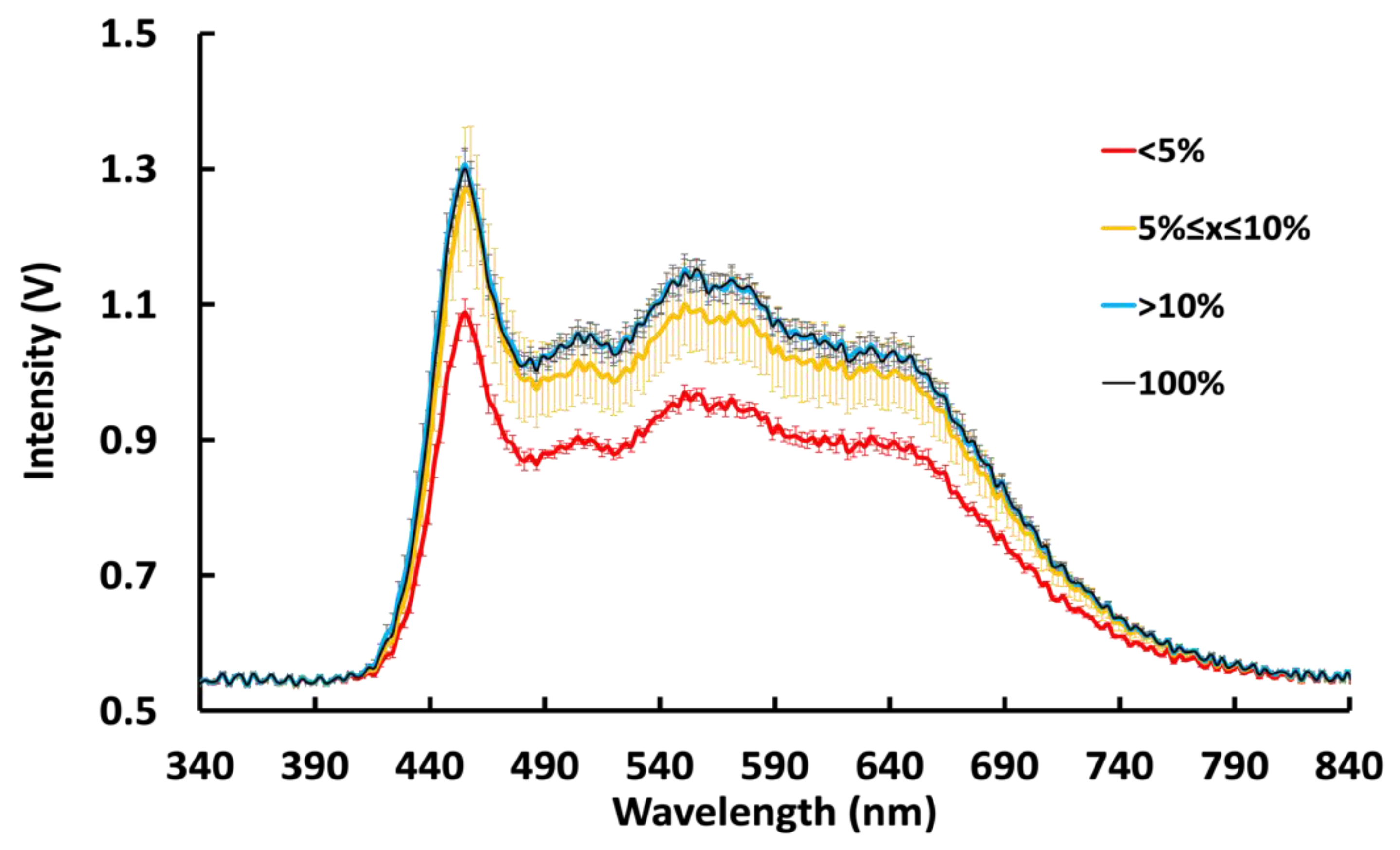
3.2. Spectral Variation as a Function of Power State
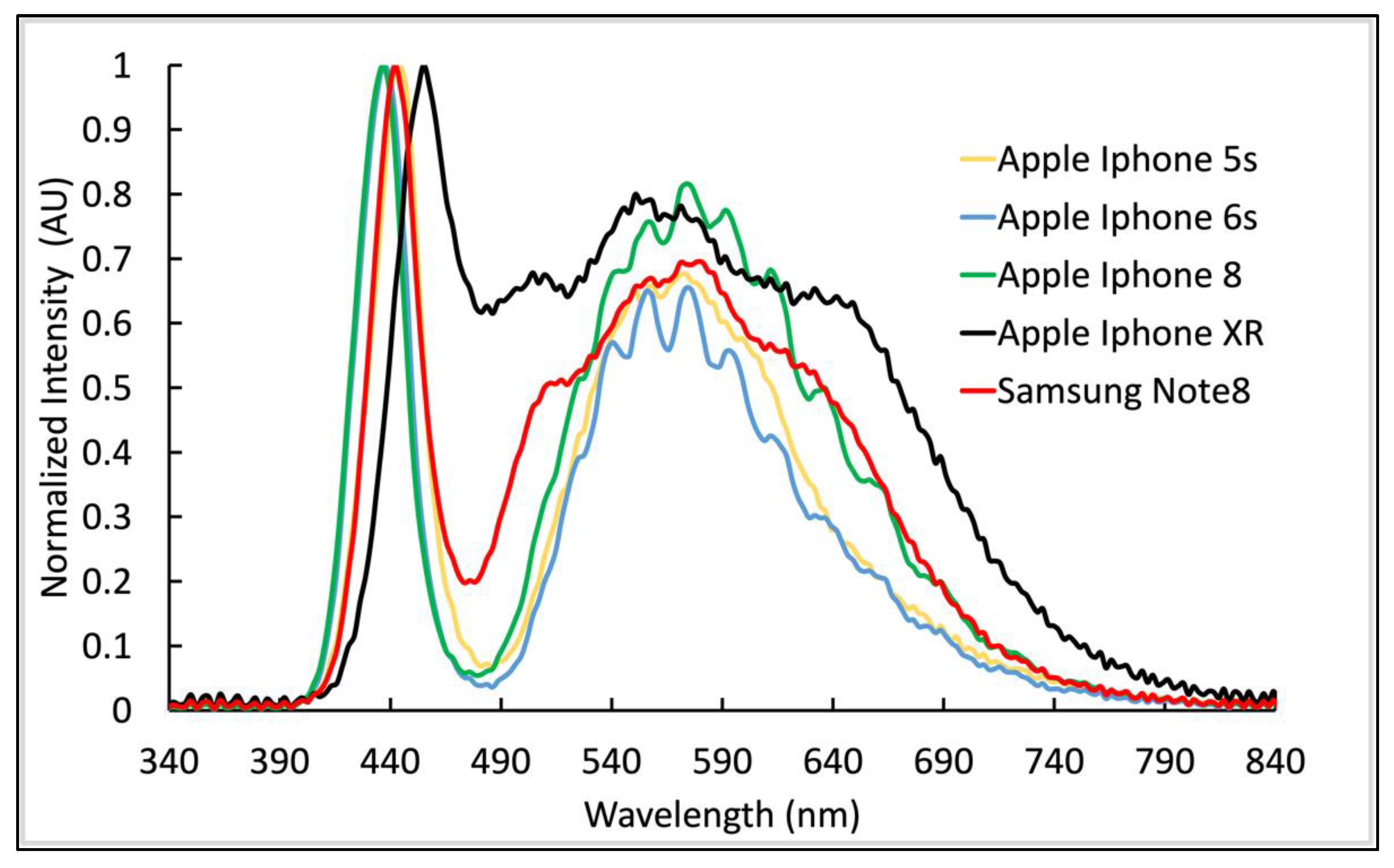
3.3. Intra-Model Flash Variation
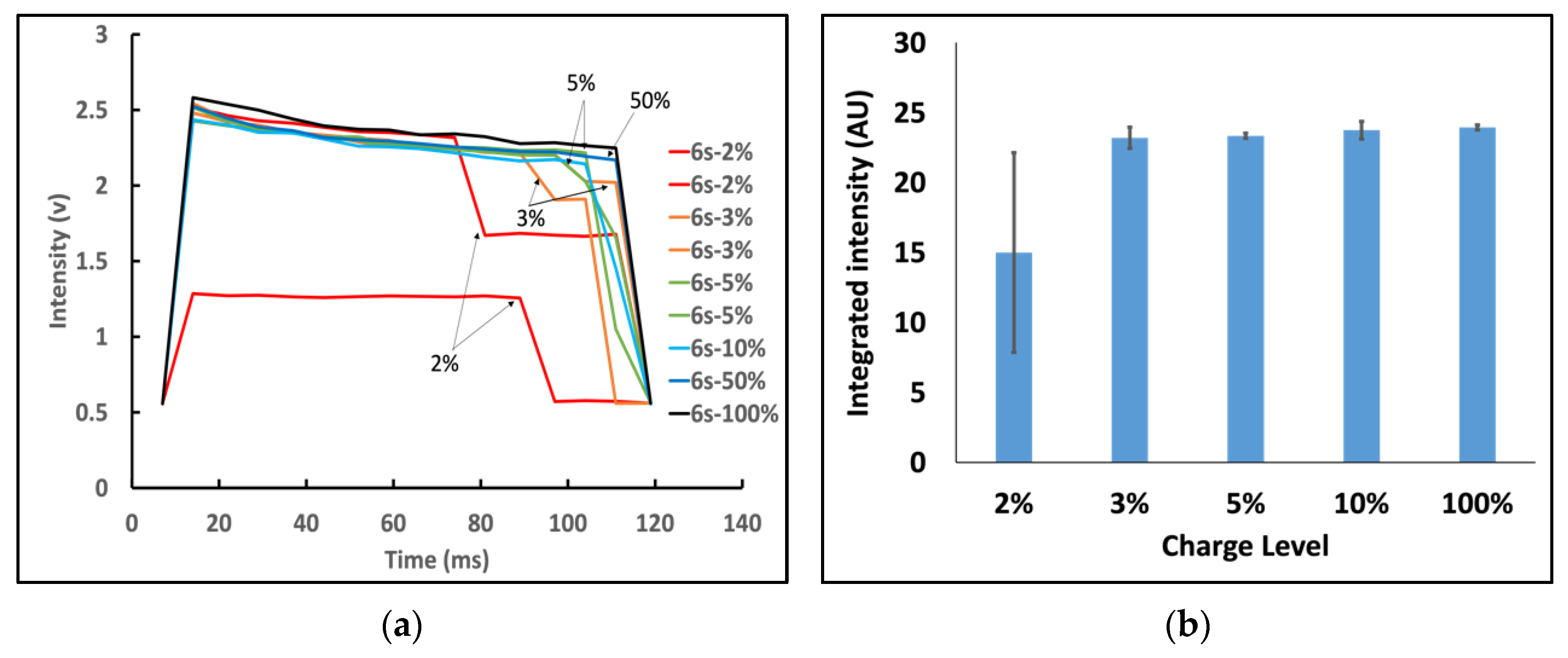
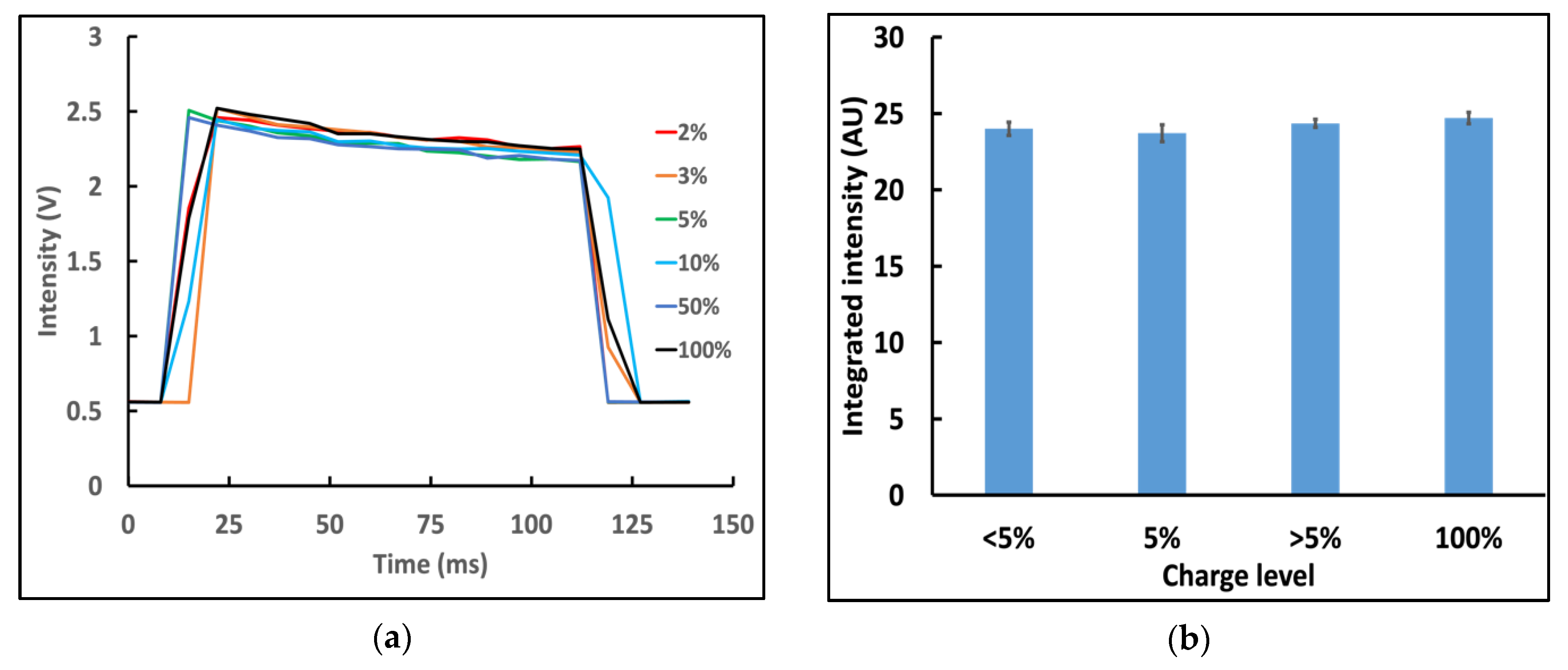
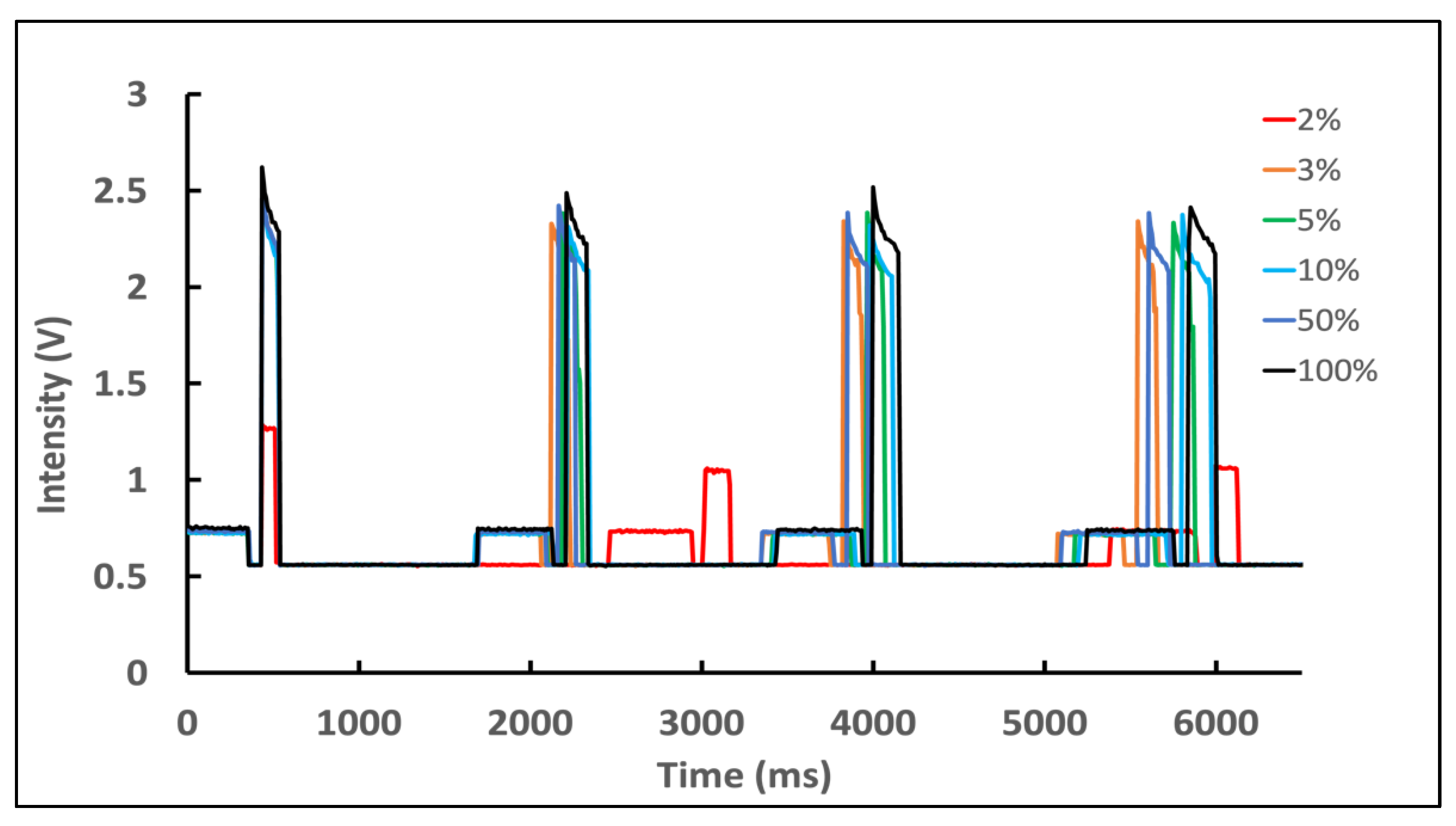
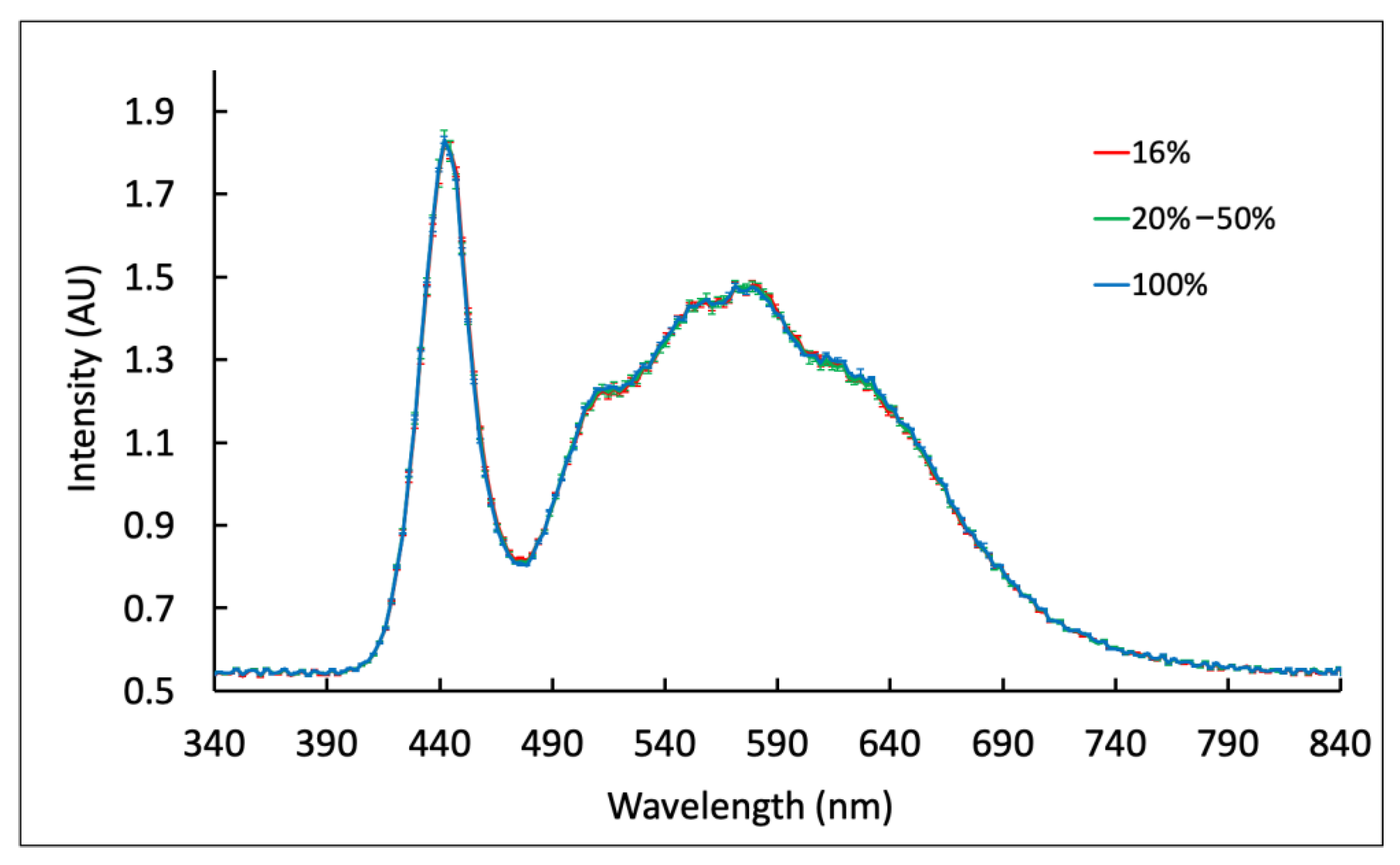
3.4. Effect of Battery Level on Nanophosphor Reporter-Based LFA Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-Neuta, I.; Neumann, F.; Brightmeyer, J.; Tis, T.B.; Madaboosi, N.; Wei, Q.; Ozcan, A.; Nilsson, M. Smartphone-based clinical diagnostics: Towards democratization of evidence-based health care. J. Intern. Med. 2019, 285, 19–39. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, V.K.; Bakthavathsalam, P.; Bergquist, P.L.; Sunna, A. Smartphone technology facilitates point-of-care nucleic acid diagnosis: A beginner’s guide. Crit. Rev. Clin. Lab. Sci. 2021, 58, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Kanchi, S.; Sabela, M.I.; Mdluli, P.S.; Inamuddin; Bisetty, K. Smartphone based bioanalytical and diagnosis applications: A review. Biosens. Bioelectron. 2018, 102, 136–149. [Google Scholar] [CrossRef]
- Hunt, B.; Ruiz, A.J.; Pogue, B.W. Smartphone-based imaging systems for medical applications: A critical review. J. Biomed. Opt. 2021, 26, 040902. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.M.S.; Bremer, M.G.E.G.; Nielen, M.W.F. Consumer-friendly food allergen detection: Moving towards smartphone-based immunoassays. Anal. Bioanal. Chem. 2018, 410, 5353–5371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-H.; Park, E.-K.; Cho, Y.K.; Shin, I.-S.; Lee, H. Normalizing the Optical Signal Enables Robust Assays with Lateral Flow Biosensors. ACS Omega 2022, 7, 17723–17731. [Google Scholar] [CrossRef]
- Kong, T.; You, J.B.; Zhang, B.; Nguyen, B.; Tarlan, F.; Jarvi, K.; Sinton, D. Accessory-free quantitative smartphone imaging of colorimetric paper-based assays. Lab A Chip 2019, 19, 1991–1999. [Google Scholar] [CrossRef]
- McCracken, K.E.; Tat, T.; Paz, V.; Yoon, J.-Y. Smartphone-based fluorescence detection of bisphenol A from water samples. RSC Adv. 2017, 7, 9237–9243. [Google Scholar] [CrossRef] [Green Version]
- Coskun, A.F.; Nagi, R.; Sadeghi, K.; Phillips, S.; Ozcan, A. Albumin testing in urine using a smart-phone. Lab A Chip 2013, 13, 4231–4238. [Google Scholar] [CrossRef]
- Amin, R.; Knowlton, S.; Yenilmez, B.; Hart, A.; Joshi, A.; Tasoglu, S. Smart-phone attachable, flow-assisted magnetic focusing device. RSC Adv. 2016, 6, 93922–93931. [Google Scholar] [CrossRef]
- Ji, X.; Chen, W.; Long, L.; Huang, F.; Sessler, J.L. Double layer 3D codes: Fluorescent supramolecular polymeric gels allowing direct recognition of the chloride anion using a smart phone. Chem. Sci. 2018, 9, 7746–7752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awqatty, B.; Samaddar, S.; Cash, K.J.; Clark, H.A.; Dubach, J.M. Fluorescent sensors for the basic metabolic panel enable measurement with a smart phone device over the physiological range. Anal. 2014, 139, 5230–5238. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging Technologies for Next-Generation Point-of-Care Testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, T.-Y.; Lillehoj, P.B. Smartphones for Cell and Biomolecular Detection. Ann. Biomed. Eng. 2014, 42, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.-J.; Choi, K.; Cuc, B.T.; Hong, N.N.; Bao, D.T.; Ngoc, N.M.; Le, M.Q.; Hang, N.L.K.; Thach, N.C.; Mallik, S.K.; et al. Smartphone-Based Fluorescent Diagnostic System for Highly Pathogenic H5N1 Viruses. Theranostics 2016, 6, 231–242. [Google Scholar] [CrossRef]
- Im, H.; Castro, C.M.; Shao, H.; Liong, M.; Song, J.; Pathania, D.; Fexon, L.; Min, C.; Avila-Wallace, M.; Zurkiya, O.; et al. Digital diffraction analysis enables low-cost molecular diagnostics on a smartphone. Proc. Natl. Acad. Sci. 2015, 112, 5613–5618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vashist, S.K.; Mudanyali, O.; Schneider, E.M.; Zengerle, R.; Ozcan, A. Cellphone-based devices for bioanalytical sciences. Anal. Bioanal. Chem. 2014, 406, 3263–3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Li, Z.; Zhang, L.; Sawaya, P.; Shi, J.; Wang, P. Quantitation of Femtomolar-Level Protein Biomarkers Using a Simple Microbubbling Digital Assay and Bright-Field Smartphone Imaging. Angew. Chem. Int. Ed. 2019, 58, 13922–13928. [Google Scholar] [CrossRef]
- Chung, S.; Breshears, L.E.; Perea, S.; Morrison, C.M.; Betancourt, W.Q.; Reynolds, K.A.; Yoon, J.-Y. Smartphone-Based Paper Microfluidic Particulometry of Norovirus from Environmental Water Samples at the Single Copy Level. ACS Omega 2019, 4, 11180–11188. [Google Scholar] [CrossRef]
- Lee, S.; O’Dell, D.; Hohenstein, J.; Colt, S.; Mehta, S.; Erickson, D. NutriPhone: A mobile platform for low-cost point-of-care quantification of vitamin B12 concentrations. Sci. Rep. 2016, 6, 28237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, B.; O’Dell, D.; Finkelstein, J.L.; Lee, S.; Erickson, D.; Mehta, S. ironPhone: Mobile device-coupled point-of-care diagnostics for assessment of iron status by quantification of serum ferritin. Biosens. Bioelectron. 2018, 99, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.S.; Raja, B.; Mandadi, V.; Townsend, B.; Lee, M.; Buell, A.; Vu, B.; Brgoch, J.; Willson, R.C. A low-cost smartphone-based platform for highly sensitive point-of-care testing with persistent luminescent phosphors. Lab A Chip 2017, 17, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Paterson, A.S.; Raja, B.; Garvey, G.; Kolhatkar, A.; Hagström, A.E.V.; Kourentzi, K.; Lee, T.R.; Willson, R.C. Persistent Luminescence Strontium Aluminate Nanoparticles as Reporters in Lateral Flow Assays. Anal. Chem. 2014, 86, 9481–9488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goux, H.J.; Raja, B.; Kourentzi, K.; Trabuco, J.R.C.; Vu, B.V.; Paterson, A.S.; Kirkpatrick, A.; Townsend, B.; Lee, M.; Truong, V.T.T.; et al. Evaluation of a nanophosphor lateral-flow assay for self-testing for herpes simplex virus type 2 seropositivity. PLoS ONE 2019, 14, e0225365. [Google Scholar] [CrossRef] [PubMed]
- Danthanarayana, A.N.; Finley, E.; Vu, B.; Kourentzi, K.; Willson, R.C.; Brgoch, J. A multicolor multiplex lateral flow assay for high-sensitivity analyte detection using persistent luminescent nanophosphors. Anal. Methods 2020, 12, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Salazar, J.R.; Rodrigues Cruz, K.; Materón Vásques, E.M.; de Oliveira, O.N., Jr. Microfluidic Point-of-Care Devices: New Trends and Future Prospects for eHealth Diagnostics. Sensors 2020, 20, 1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalal, U.M.; Jin, G.J.; Shim, J.S. Paper–Plastic Hybrid Microfluidic Device for Smartphone-Based Colorimetric Analysis of Urine. Anal. Chem. 2017, 89, 13160–13166. [Google Scholar] [CrossRef] [PubMed]
- Brangel, P.; Sobarzo, A.; Parolo, C.; Miller, B.S.; Howes, P.D.; Gelkop, S.; Lutwama, J.J.; Dye, J.M.; McKendry, R.A.; Lobel, L.; et al. A Serological Point-of-Care Test for the Detection of IgG Antibodies against Ebola Virus in Human Survivors. ACS Nano 2018, 12, 63–73. [Google Scholar] [CrossRef]
- Versek, C.; Woodworth, P.; Meckes, A.; Blair, D. Review of Hamamatsu C128880 Microspec Module. Available online: https://impfs.github.io/review/ (accessed on 15 August 2022).
- Hitchner, D. Serial Port Communication. Available online: http://www.thescarms.com/vbasic/CommIO.aspx (accessed on 15 August 2022).
- Vu, B. Mini-Spectrometer, Github. Available online: https://github.com/willsonlab (accessed on 15 August 2022).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Wacker, A. White LED flash—Texas instruments. 2016. Available online: https://studylib.net/doc/18175978/white-led-flash (accessed on 15 August 2022).
- Löfberg, H.; Grubb, A.O. Quantitation of γ-trace in human biological fluids: Indications for production in the central nervous system. Scand. J. Clin. Lab. Investig. 1979, 39, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, O.; Grubb, A.; Thysell, H. The blood serum concentration of cystatin C (γ-trace) as a measure of the glomerular filtration rate. Scand. J. Clin. Lab. Investig. 1985, 45, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Kyhse-Andersen, J.; Schmidt, C.; Nordin, G.; Andersson, B.; Nilsson-Ehle, P.; Lindström, V.; Grubb, A. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin. Chem. 1994, 40, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Grubb, A.; Simonsen, O.; Sturfelt, G.; Truedsson, L.; Thysell, H. Serum Concentration of Cystatin C, Factor D and β2-Microglobulin as a Measure of Glomerular Filtration Rate. Acta Medica Scand. 2009, 218, 499–503. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, B.V.; Lei, R.; Mohan, C.; Kourentzi, K.; Willson, R.C. Flash Characterization of Smartphones Used in Point-of-Care Diagnostics. Biosensors 2022, 12, 1060. https://doi.org/10.3390/bios12121060
Vu BV, Lei R, Mohan C, Kourentzi K, Willson RC. Flash Characterization of Smartphones Used in Point-of-Care Diagnostics. Biosensors. 2022; 12(12):1060. https://doi.org/10.3390/bios12121060
Chicago/Turabian StyleVu, Binh V., Rongwei Lei, Chandra Mohan, Katerina Kourentzi, and Richard C. Willson. 2022. "Flash Characterization of Smartphones Used in Point-of-Care Diagnostics" Biosensors 12, no. 12: 1060. https://doi.org/10.3390/bios12121060
APA StyleVu, B. V., Lei, R., Mohan, C., Kourentzi, K., & Willson, R. C. (2022). Flash Characterization of Smartphones Used in Point-of-Care Diagnostics. Biosensors, 12(12), 1060. https://doi.org/10.3390/bios12121060




