Review of Thin-Layer Chromatography Tandem with Surface-Enhanced Raman Spectroscopy for Detection of Analytes in Mixture Samples
Abstract
1. Introduction
2. SERS
3. TLC
4. TLC-SERS
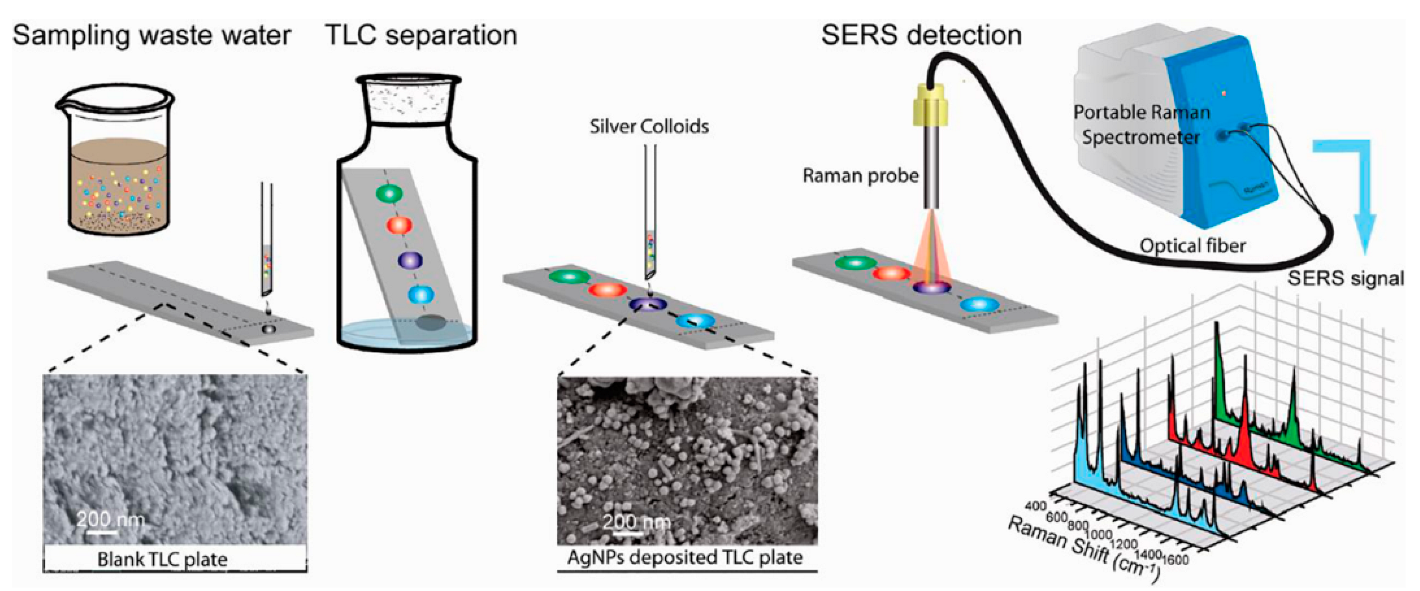
4.1. Environmental Pollutants
4.2. Illegal Additives
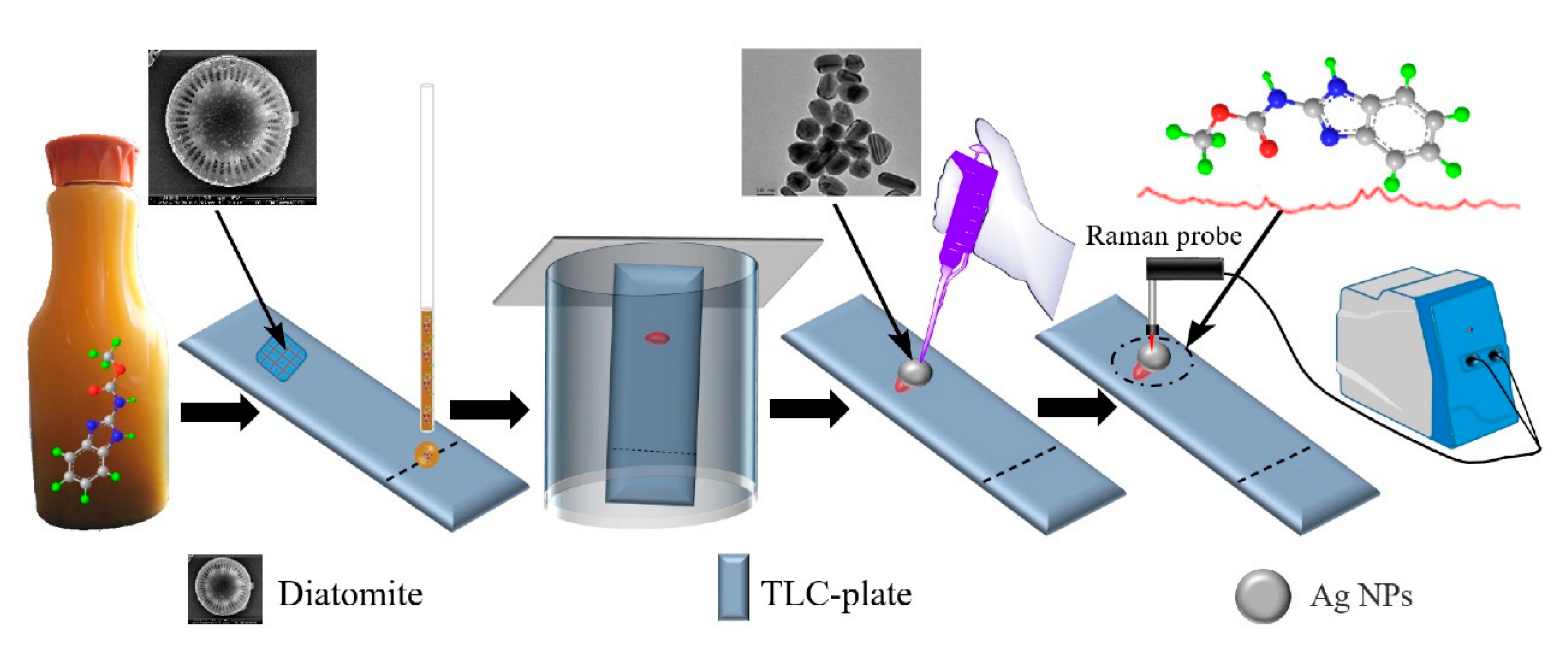
4.3. Pesticide Residues
4.4. Toxic Ingredients
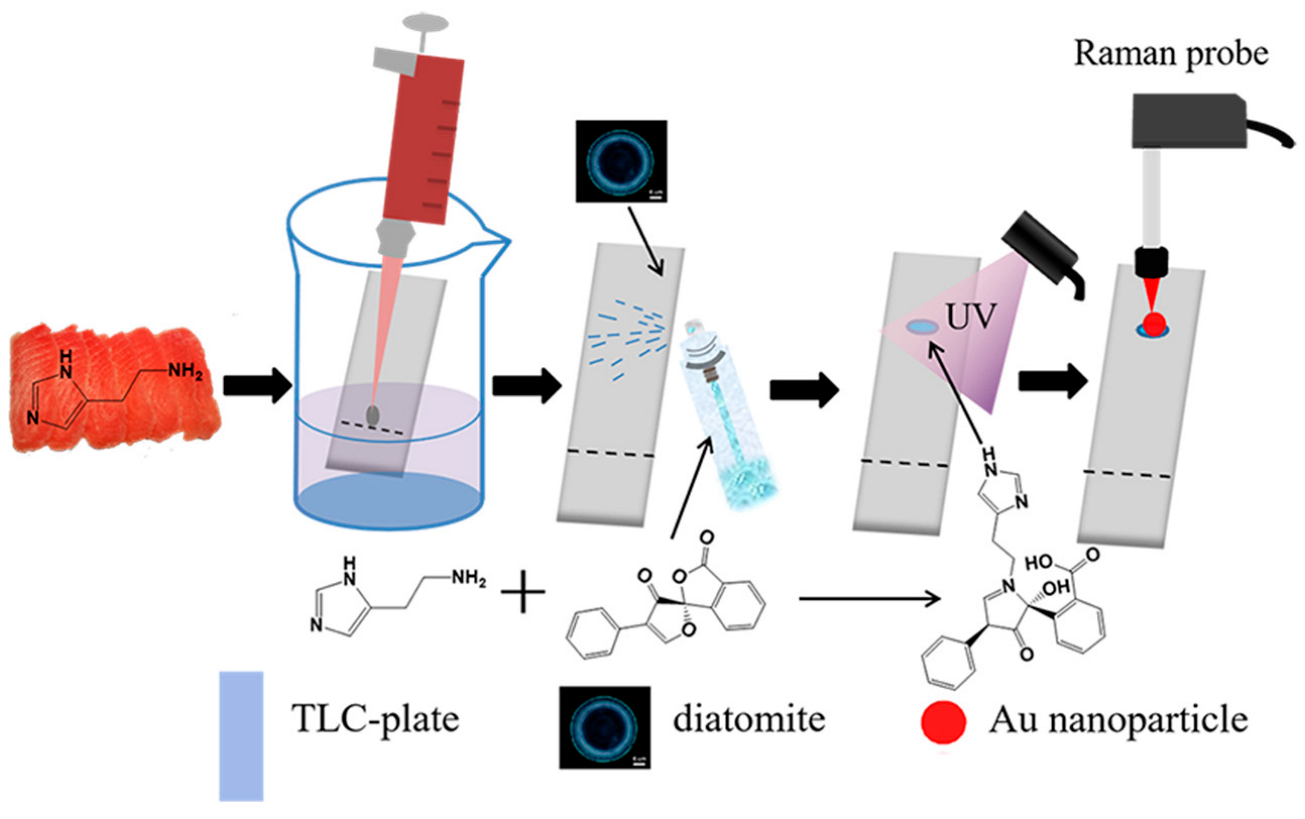
4.5. Biological Molecules
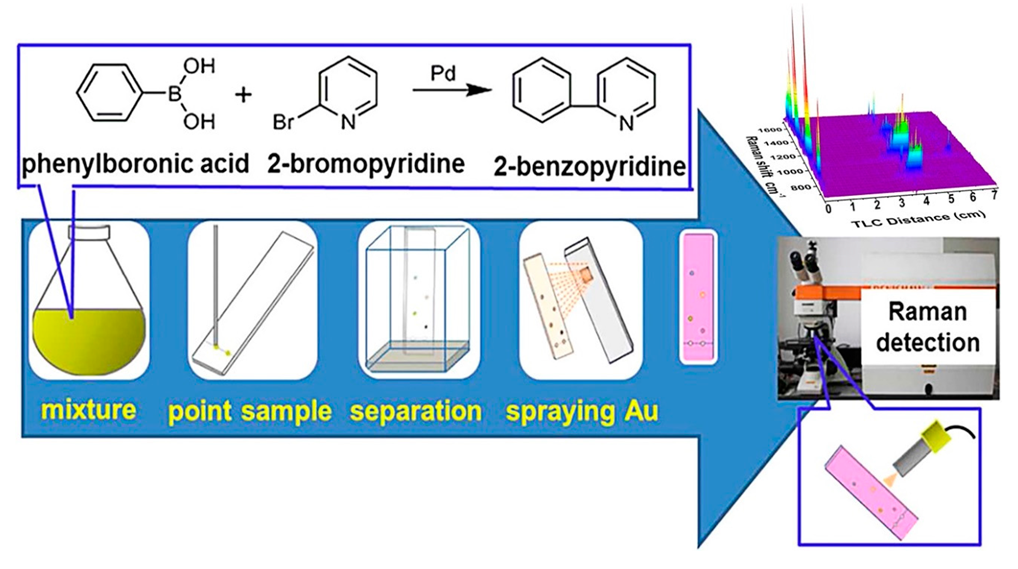
4.6. Chemical Substances
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gillibert, R.; Huang, J.Q.; Zhang, Y.; Fu, W.L.; Lamy de la Chapelle, M. Food quality control by Surface Enhanced Raman Scattering. TrAC Trends Anal. Chem. 2018, 105, 185–190. [Google Scholar] [CrossRef]
- Guo, H.; He, L.; Xing, B. Applications of surface-enhanced Raman spectroscopy in the analysis of nanoparticles in the environment. Environ. Sci. Nano 2017, 4, 2093–2107. [Google Scholar] [CrossRef]
- Tadesse, L.F.; Safir, F.; Ho, C.S.; Hasbach, X.; Khuri-Yakub, B.P.; Jeffrey, S.S.; Saleh, A.A.E.; Dionne, J. Toward rapid infectious disease diagnosis with advances in surface-enhanced Raman spectroscopy. J. Chem. Phys. 2020, 152, 240902. [Google Scholar] [CrossRef]
- Wei, W.; Du, Y.; Zhang, L.; Yang, Y.; Gao, Y. Improving SERS hot spots for on-site pesticide detection by combining silver nanoparticles with nanowires. J. Mater. Chem. C 2018, 6, 8793–8803. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Hong, J.; Zhou, X. Highly selective determination of acid phosphatase in biological samples using a biomimetic recognition-based SERS sensor. Sens. Actuators B Chem. 2018, 276, 421–428. [Google Scholar] [CrossRef]
- Ding, S.Y.; Zhang, X.M.; You, E.M.; Ren, B.; Tian, Z.Q. Surface-Enhanced Raman Spectroscopy: General Introduction. In Encyclopedia of Analytical Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 1–42. [Google Scholar]
- Cindric, M.; Cepo, T.; Marinc, S.; Paskvan, I.; Mijic, I.; Bindila, L.; Peter-Katalinic, J. Determination of dithiothreitol in complex protein mixtures by HPLC-MS. J. Sep. Sci. 2008, 31, 3489–3496. [Google Scholar] [CrossRef]
- Barba, A.I.O.; Hurtado, M.C.; Mata, M.C.S.; Ruiz, V.F.; Tejada, M.L.S.d. Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Wei, X.; Koo, I.; Kim, S.; Zhang, X. Compound identification in GC-MS by simultaneously evaluating the mass spectrum and retention index. Analyst 2014, 139, 2507. [Google Scholar] [CrossRef]
- Khajuria, H.; Nayak, B.P. Detection and accumulation of morphine in hair using GC–MS. Egypt J. Forensic Sci. 2016, 6, 337–341. [Google Scholar] [CrossRef]
- Takei, H.; Saito, J.; Kato, K.; Vieker, H.; Beyer, A.; Gölzhäuser, A. TLC-SERS Plates with a Built-In SERS Layer Consisting of Cap-Shaped Noble Metal Nanoparticles Intended for Environmental Monitoring and Food Safety Assurance. J. Nanomater. 2015, 2015, 9. [Google Scholar] [CrossRef]
- Zhu, Q.; Cao, Y.; Cao, Y.; Chai, Y.; Lu, F. Rapid on-site TLC-SERS detection of four antidiabetes drugs used as adulterants in botanical dietary supplements. Anal. Bioanal. Chem. 2014, 406, 1877–1884. [Google Scholar] [CrossRef]
- Oriňák, A.; Talian, I.; Efremov, E.V.; Ariese, F.; Oriáaková, R. Diterpenoic Acids Analysis Using a Coupled TLC-Surface-Enhanced Raman Spectroscopy System. Chromatographia 2007, 67, 315–319. [Google Scholar] [CrossRef]
- Li, D.; Qu, L.; Zhai, W.; Xue, J.; Fossey, J.S.; Long, Y. Facile on-site detection of substituted aromatic pollutants in water using thin layer chromatography combined with surface-enhanced Raman spectroscopy. Env. Sci. Technol. 2011, 45, 4046–5402. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Grant, M.A.; Creighton, J.A. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. 1977, 3, 5215–5217. [Google Scholar]
- Jeanmaire, D.L.; Van Duyne, R.P. Heterrocyclic, Aromatic, And Aliphatic Amines Adsorbed on The Anodized Silver Electrode. J. Electroanal. Chem. 1977, 84, 20. [Google Scholar]
- Yamamoto, Y.S.; Ishikawa, M.; Ozaki, Y.; Itoh, T. Fundamental studies on enhancement and blinking mechanism of surface-enhanced Raman scattering (SERS) and basic applications of SERS biological sensing. Front. Phys. 2013, 9, 31–46. [Google Scholar] [CrossRef]
- Yilmaz, M.; Babur, E.; Ozdemir, M.; Gieseking, R.L.; Dede, Y.; Tamer, U.; Schatz, G.C.; Facchetti, A.; Usta, H.; Demirel, G. Nanostructured organic semiconductor films for molecular detection with surface-enhanced Raman spectroscopy. Nat. Mater. 2017, 16, 918–924. [Google Scholar] [CrossRef]
- Bilmes, S.A. SERS of pyridine adsorbed on rhodium electrodes. Chem. Phys. Lett. 1990, 171, 6. [Google Scholar] [CrossRef]
- Lin, S.; Lin, X.; Shang, Y.; Han, S.; Hasi, W.; Wang, L. Self-Assembly of Faceted Gold Nanocrystals for Surface-Enhanced Raman Scattering Application. J. Phys. Chem. C 2019, 123, 24714–24722. [Google Scholar] [CrossRef]
- Lin, X.; Fang, G.; Liu, Y.; He, Y.; Wang, L.; Dong, B. Marangoni Effect-Driven Transfer and Compression at Three-Phase Interfaces for Highly Reproducible Nanoparticle Monolayers. J. Phys. Chem. Lett. 2020, 11, 3573–3581. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.; Xu, L.; Wang, W.; Du, J.; Qu, M.; Han, X.; Yang, L.; Zhao, B. Ultrasensitive SERS detection of antitumor drug methotrexate based on modified Ag substrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118589. [Google Scholar] [CrossRef]
- Hou, X.; Sivashanmugan, K.; Zhao, Y.; Zhang, B.; Wang, A.X. Multiplex sensing of complex mixtures by machine vision analysis of TLC-SERS images. Sens. Actuators B Chem. 2022, 357, 131355. [Google Scholar] [CrossRef]
- Ji, W.; Li, L.; Song, W.; Wang, X.; Zhao, B.; Ozaki, Y. Enhanced Raman scattering by ZnO superstructures: Synergistic effect of charge transfer and Mie resonances. Angew. Chem. Int. Ed. 2019, 58, 14452–14456. [Google Scholar] [CrossRef]
- Cho, W.J.; Kim, Y.; Kim, J.K. Ultrahigh-Density Array of Silver Nanoclusters for SERS Substrate with High Sensitivity and Excellent Reproducibility. ACS Nano 2012, 6, 7. [Google Scholar]
- Betz, J.F.; Yu, W.W.; Cheng, Y.; White, I.M.; Rubloff, G.W. Simple SERS substrates: Powerful, portable, and full of potential. Phys. Chem. Chem. Phys. 2014, 16, 2224–2239. [Google Scholar] [CrossRef]
- Ouyang, L.; Ren, W.; Zhu, L.; Irudayaraj, J. Prosperity to challenges: Recent approaches in SERS substrate fabrication. Rev. Anal. Chem. 2017, 36, 20160027. [Google Scholar] [CrossRef]
- Wang, A.X.; Kong, X. Review of Recent Progress of Plasmonic Materials and Nano-Structures for Surface-Enhanced Raman Scattering. Materials 2015, 8, 3024–3052. [Google Scholar] [CrossRef]
- Fu, Z.; Shen, Z.; Fan, Q.; Hao, S.; Wang, Y.; Liu, X.; Tong, X.; Kong, X.; Yang, Z. Preparation of multi-functional magnetic–plasmonic nanocomposite for adsorption and detection of thiram using SERS. J. Hazard. Mater. 2020, 392, 122356. [Google Scholar] [CrossRef]
- Zhang, C.; You, T.; Yang, N.; Gao, Y.; Jiang, L.; Yin, P. Hydrophobic paper-based SERS platform for direct-droplet quantitative determination of melamine. Food Chem. 2019, 287, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Wang, G.; Zhang, X.N.; Geng, H.P.; Shen, J.L.; Wang, L.S.; Zhao, J.; Xu, L.F.; Zhang, L.J.; Wu, Y.Q.; et al. Geometrical and morphological optimizations of plasmonic nanoarrays for high-performance SERS detection. Nanoscale 2015, 7, 15487–15494. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Yu, Z.; Li, G.; Zhang, Z. Advanced sample preparation techniques for rapid surface-enhanced Raman spectroscopy analysis of complex samples. J. Chromatogr. A 2022, 1675, 463181. [Google Scholar] [CrossRef]
- Lai, H.; Li, G.; Zhang, Z. Advanced materials on sample preparation for safety analysis of aquatic products. J. Sep. Sci. 2021, 44, 1174–1194. [Google Scholar] [CrossRef] [PubMed]
- Starek, M.; Plenis, A.; Zagrobelna, M.; Dabrowska, M. Assessment of Lipophilicity Descriptors of Selected NSAIDs Obtained at Different TLC Stationary Phases. Pharmaceutics 2021, 13, 440. [Google Scholar] [CrossRef] [PubMed]
- Bebenek, E.; Bober-Majnusz, K.; Siudak, S.; Chrobak, E.; Kadela-Tomanek, M.; Wietrzyk, J.; Boryczka, S. Application of TLC to Evaluate the Lipophilicity of Newly Synthesized Betulin Derivatives. J. Chromatogr. Sci. 2020, 58, 323–333. [Google Scholar] [CrossRef]
- Oellig, C. Screening for Ricinoleic Acid as a Chemical Marker for Secale cornutum in Rye by High-Performance Thin-Layer Chromatography with Fluorescence Detection. J. Agric. Food Chem. 2016, 64, 8246–8253. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cheng, G.; Zao, F.; Lin, Y.; Huang, J.; Shanks, R. Separation and identification of multicomponent mixture by thin-layer chromatography coupled with Fourier transform–infrared microscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1965–1970. [Google Scholar] [CrossRef]
- Sun, J.M.; Guo, Y.; Zhang, J.; Zhang, H.; Lin, Z. Screening and Isolation of Natural Antioxidants from Acer Ginnala Max by TLC-MS/MS Guided Bioautographic Method. Iran J. Pharm. Res. 2019, 18, 914–921. [Google Scholar]
- Zhang, N.; Wang, M.; Li, Y.; Zhou, M.; Wu, T.; Cheng, Z. TLC-MS identification of alkaloids in Leonuri Herba and Leonuri Fructus aided by a newly developed universal derivatisation reagent optimised by the response surface method. Phytochem. Anal. 2021, 32, 242–251. [Google Scholar] [CrossRef]
- Hu, B.; Xin, G.-z.; So, P.-K.; Yao, Z.-P. Thin layer chromatography coupled with electrospray ionization mass spectrometry for direct analysis of raw samples. J. Chromatogr. A 2015, 1415, 155–160. [Google Scholar] [CrossRef]
- István, K.; Keresztury, G.; Szép, A. Normal Raman and surface enhanced Raman spectroscopic experiments with thin layer chromatography spots of essential amino acids using different laser excitation sources. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 1709–1723. [Google Scholar] [CrossRef]
- Minh, D.T.C.; Thi, L.A.; Huyen, N.T.T.; Van Vu, L.; Anh, N.T.K.; Ha, P.T.T. Detection of sildenafil adulterated in herbal products using thin layer chromatography combined with surface enhanced Raman spectroscopy: “Double coffee-ring effect” based enhancement. J. Pharm. Biomed. Anal. 2019, 174, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tan, A.; Squire, K.; Sivashanmugan, K.; Wang, A.X. Quaternion-based parallel feature extraction: Extending the horizon of quantitative analysis using TLC-SERS sensing. Sens. Actuators B Chem. 2019, 299, 126902. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, C.; Yu, H.; Guo, Y.; Cheng, Y.; Yao, W.; Xie, Y. Establishment of the thin-layer chromatography-surface-enhanced Raman spectroscopy and chemometrics method for simultaneous identification of eleven illegal drugs in anti-rheumatic health food. Food Biosci. 2022, 49, 101842. [Google Scholar] [CrossRef]
- Yao, H.; Dong, X.; Xiong, H.; Liu, J.; Zhou, J.; Ye, Y. Functional cotton fabric-based TLC-SERS matrix for rapid and sensitive detection of mixed dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121464. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Li, E.; Squire, K.; Liu, Y.; Wu, B.; Cheng, L.J.; Wang, A.X. Plasmonic nanoparticles-decorated diatomite biosilica: Extending the horizon of on-chip chromatography and label-free biosensing. J. Biophotonics 2017, 10, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Liu, J.F.; Liu, R.; Sun, J.F.; Wei, G.H. Thin layer chromatography coupled with surface-enhanced Raman scattering as a facile method for on-site quantitative monitoring of chemical reactions. Anal. Chem. 2014, 86, 7286–7292. [Google Scholar] [CrossRef]
- Hezel, U.B.; Zeiss, C. Potential and experience in quantitative “high performance thin-layer chromatography” HPTLC. J. Chromatogr. Libr. 1977, 9, 147–188. [Google Scholar]
- Poole, C.F. Thin-layer chromatography: Challenges and opportunities. J. Chromatogr. A 2003, 1000, 963–984. [Google Scholar] [CrossRef]
- Qu, L.L.; Jia, Q.; Liu, C.; Wang, W.; Duan, L.; Yang, G.; Han, C.Q.; Li, H. Thin layer chromatography combined with surface-enhanced raman spectroscopy for rapid sensing aflatoxins. J. Chromatogr. A 2018, 1579, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yu, Q.; Li, E.; Wang, R.; Liu, Q.; Wang, A.X. Diatomite Photonic Crystals for Facile On-Chip Chromatography and Sensing of Harmful Ingredients from Food. Materials 2018, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wu, T.; Han, X.; Gu, H.; Zhang, X. A needle-like reusable surface-enhanced Raman scattering substrate, and its application to the determination of acetamiprid by combining SERS and thin-layer chromatography. Mikrochim. Acta 2018, 185, 504. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lu, Y.; Zhu, D.; Rosa, L.; Han, F.; Ma, M.; Su, W.; Francis, P.S.; Zheng, Y. Plasmonic nanopapers: Flexible, stable and sensitive multiplex PUF tags for unclonable anti-counterfeiting applications. Nanoscale 2020, 12, 9471–9480. [Google Scholar] [CrossRef]
- Cai, G.; Ge, K.; Ouyang, X.; Hu, Y.; Li, G. Thin-layer chromatography combined with surface-enhanced Raman scattering for rapid detection of benzidine and 4-aminobiphenyl in migration from food contact materials based on gold nanoparticle doped metal-organic framework. J. Sep. Sci. 2020, 43, 2834–2841. [Google Scholar] [CrossRef]
- Muscalu, A.M.; Górecki, T. Comprehensive two-dimensional gas chromatography in environmental analysis. TrAC Trends Anal. Chem. 2018, 106, 225–245. [Google Scholar] [CrossRef]
- Li, D.-W.; Zhai, W.-L.; Li, Y.-T.; Long, Y.-T. Recent progress in surface enhanced Raman spectroscopy for the detection of environmental pollutants. Microchim. Acta 2013, 181, 23–43. [Google Scholar] [CrossRef]
- Al-Hamaiedh, H.D.; Maaitah, O.N. Treatment of oil polluted soil using electrochemical method. Alex. Eng. J. 2011, 50, 105–110. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, Y.; Chen, H.; Zhu, Q.; Lu, F.; Li, Y. A separable surface-enhanced Raman scattering substrate modified with MIL-101 for detection of overlapping and invisible compounds after thin-layer chromatography development. Anal. Chim. Acta 2018, 997, 35–43. [Google Scholar] [CrossRef]
- Rebane, R.; Leito, I.; Yurchenko, S.; Herodes, K. A review of analytical techniques for determination of Sudan I–IV dyes in food matrixes. J. Chromatogr. A 2010, 1217, 2747–2757. [Google Scholar] [CrossRef]
- Calbiani, F.; Careri, M.; Elviri, L.; Mangia, A.; Pistara, L.; Zagnoni, I. Development and in-house validation of a liquid chromatography–electrospray–tandem mass spectrometry method for the simultaneous determination of Sudan I, Sudan II, Sudan III and Sudan IV in hot chilli products. J. Chromatogr. A 2004, 1042, 123–130. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Su, Y.; Shen, X.; Zeng, Z.; Liu, Y. Determination of Sudan dye residues in eggs by liquid chromatography and gas chromatography—Mass spectrometry. Anal. Chim. Acta 2007, 594, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Cao, Y.; Lou, Z.; Li, S.; Chen, X.; Chai, Y.; Lu, F. Rapid on-site detection of ephedrine and its analogues used as adulterants in slimming dietary supplements by TLC-SERS. Anal. Bioanal. Chem. 2015, 407, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Cao, Y.; Li, D.; Fang, F.; Lu, F.; Yuan, Y. A fast response TLC-SERS substrate for on-site detection of hydrophilic and hydrophobic adulterants in botanical dietary supplements. New J. Chem. 2019, 43, 13873–13880. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Y.; Chen, D.; Li-Chan, E.C.Y.; Grant, E.; Lu, X. Determination of Sudan I in paprika powder by molecularly imprinted polymers-thin layer chromatography-surface enhanced Raman spectroscopic biosensor. Talanta 2015, 143, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Squire, K.; Chong, X.; Wang, A.X. Ultra-sensitive lab-on-a-chip detection of Sudan I in food using plasmonics-enhanced diatomaceous thin film. Food Control 2017, 79, 258–265. [Google Scholar] [CrossRef]
- Wu, X.; Chen, J.; Park, B.; Huang, Y.-W.; Zhao, Y. The Use of Silver Nanorod Array-Based Surface-Enhanced Raman Scattering Sensor for Food Safety Applications. In Advances in Applied Nanotechnology for Agriculture; American Chemical Society: Washington, DC, USA, 2013; pp. 85–108. [Google Scholar]
- Chen, J.; Abell, J.; Huang, Y.-w.; Zhao, Y. On-Chip Ultra-Thin Layer Chromatography and Surface Enhanced Raman Spectroscopy. Lab Chip 2012, 12, 3096–3102. [Google Scholar] [CrossRef]
- Hu, X.; Fang, G.; Han, A.; Liu, J.; Wang, S. Rapid detection of Pericarpium papaveris in hot pot condiments using thin-layer chromatography and surface enhanced Raman spectroscopy combined with a support vector machine. Anal. Methods 2017, 9, 2177–2182. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, M.; Han, L.; Yuan, Y.; Lu, F. High efficiency screening of nine lipid-lowering adulterants in herbal dietary supplements using thin layer chromatography coupled with surface enhanced Raman spectroscopy. Anal. Methods 2017, 9, 1595–1602. [Google Scholar] [CrossRef]
- Cimpoiu, C.; Casoni, D.; Hosu, A.; Miclaus, V.; Hodisan, T.; Damian, G. Separation and Identification of Eight Hydrophilic Vitamins Using a New TLC Method and Raman Spectroscopy. J. Liq. Chromatogr. Relat. Technol. 2007, 28, 2551–2559. [Google Scholar] [CrossRef]
- Shen, Z.; Fan, Q.; Yu, Q.; Wang, R.; Wang, H.; Kong, X. Facile detection of carbendazim in food using TLC-SERS on diatomite thin layer chromatography. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 247, 119037. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Cheng, F.; Wang, C.; Wang, Y.; Guo, X.; Gong, Z.; Fan, M.; Zhang, Z. Separation, identification and fast determination of organophosphate pesticide methidathion in tea leaves by thin layer chromatography–surface-enhanced Raman scattering. Anal. Methods 2013, 5, 5560–5564. [Google Scholar] [CrossRef]
- Fang, F.; Qi, Y.; Lu, F.; Yang, L. Highly sensitive on-site detection of drugs adulterated in botanical dietary supplements using thin layer chromatography combined with dynamic surface enhanced Raman spectroscopy. Talanta 2016, 146, 351–357. [Google Scholar] [CrossRef]
- Kang, Y.; Wu, T.; Chen, W.; Li, L.; Du, Y. A novel metastable state nanoparticle-enhanced Raman spectroscopy coupled with thin layer chromatography for determination of multiple pesticides. Food Chem. 2019, 270, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, Q.; Guo, J.; Jiao, X.; Kong, X.; Yu, Q. Surface-enhanced Raman spectroscopy tandem with derivatized thin-layer chromatography for ultra-sensitive on-site detection of histamine from fish. Food Control 2022, 138, 108987. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Y.; Chen, Y.; Xu, X.; Jin, Z.; Ding, Y.; Yang, N.; Wu, F. Tuneable surface enhanced Raman spectroscopy hyphenated to chemically derivatized thin-layer chromatography plates for screening histamine in fish. Food Chem. 2017, 230, 547–552. [Google Scholar] [CrossRef]
- Tan, A.; Zhao, Y.; Sivashanmugan, K.; Squire, K.; Wang, A.X. Quantitative TLC-SERS detection of histamine in seafood with support vector machine analysis. Food Control 2019, 103, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lucotti, A.; Tommasini, M.; Casella, M.; Morganti, A.; Gramatica, F.; Zerbi, G. TLC–surface enhanced Raman scattering of apomorphine in human plasma. Vib. Spectrosc. 2012, 62, 286–291. [Google Scholar] [CrossRef]
- Kong, X.; Chong, X.; Squire, K.; Wang, A.X. Microfluidic diatomite analytical devices for illicit drug sensing with ppb-Level sensitivity. Sens. Actuators B Chem. 2018, 259, 587–595. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Zhao, Y.; Wang, A.X. Tetrahydrocannabinol Sensing in Complex Biofluid with Portable Raman Spectrometer Using Diatomaceous SERS Substrates. Biosensors 2019, 9, 125. [Google Scholar] [CrossRef]
- Durucan, O.; Wu, K.; Viehrig, M.; Rindzevicius, T.; Boisen, A. Nanopillar-Assisted SERS Chromatography. ACS Sens. 2018, 3, 2492–2498. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Han, S.; Li, X. Detection of tobacco-related biomarkers in urine samples by surface-enhanced Raman spectroscopy coupled with thin-layer chromatography. Anal. Bioanal. Chem. 2013, 405, 6815–6822. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; White, I.M. Inkjet Printed Surface Enhanced Raman Spectroscopy Array on Cellulose Paper. Anal. Chem. 2010, 82, 9626–9630. [Google Scholar] [CrossRef]
- Yu, W.W.; White, I.M. Inkjet-printed paper-based SERS dipsticks and swabs for trace chemical detection. Analyst 2013, 138, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; White, I.M. Chromatographic separation and detection of target analytes from complex samples using inkjet printed SERS substrates. Analyst 2013, 138, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, C.L.; Gambardella, A.; Casadio, F.; Grzywacz, C.M.; Wouters, J.; Duyne, R.P.V. Ad-hoc Surface-Enhanced Raman Spectroscopy Methodologies for the Detection of Artist Dyestuffs Thin Layer Chromatography-Surface Enhanced Raman Spectroscopy and in Situ On the Fiber Analysis. Am. Chem. Soc. 2009, 81, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Lin, D.Z.; Huang, C.H.; Yen, T.J. A high-performance multifunctional substrate of ultrathin-layer chromatography (UTLC) and surface-enhancedRaman scattering (SERS) for rapid biochemical mixture screening. J. Raman Spectrosc. 2018, 49, 1920–1927. [Google Scholar] [CrossRef]
- Freye, C.E.; Crane, N.A.; Kirchner, T.B.; Sepaniak, M.J. Surface Enhanced Raman Scattering Imaging of Developed Thin-Layer Chromatography Plates. Anal. Chem. 2013, 85, 3991–3998. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Q.; Guo, J.; Yuan, C.; Kong, X. On-site detection of pyrene from mixture with ppb level sensitivity by plasmonic TLC-DSERS. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 280, 121547. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.-W.; Zhao, Y. Detection of polycyclic aromatic hydrocarbons from cooking oil using ultra-thin layer chromatography and surface enhanced Raman spectroscopy. J. Mater. Chem. B 2015, 3, 1898–1906. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Yu, Q.; Guo, J.; Wu, B.; Kong, X. Review of Thin-Layer Chromatography Tandem with Surface-Enhanced Raman Spectroscopy for Detection of Analytes in Mixture Samples. Biosensors 2022, 12, 937. https://doi.org/10.3390/bios12110937
Zhang M, Yu Q, Guo J, Wu B, Kong X. Review of Thin-Layer Chromatography Tandem with Surface-Enhanced Raman Spectroscopy for Detection of Analytes in Mixture Samples. Biosensors. 2022; 12(11):937. https://doi.org/10.3390/bios12110937
Chicago/Turabian StyleZhang, Meizhen, Qian Yu, Jiaqi Guo, Bo Wu, and Xianming Kong. 2022. "Review of Thin-Layer Chromatography Tandem with Surface-Enhanced Raman Spectroscopy for Detection of Analytes in Mixture Samples" Biosensors 12, no. 11: 937. https://doi.org/10.3390/bios12110937
APA StyleZhang, M., Yu, Q., Guo, J., Wu, B., & Kong, X. (2022). Review of Thin-Layer Chromatography Tandem with Surface-Enhanced Raman Spectroscopy for Detection of Analytes in Mixture Samples. Biosensors, 12(11), 937. https://doi.org/10.3390/bios12110937




