Nanomaterials-Based Electrochemiluminescence Biosensors for Food Analysis: Recent Developments and Future Directions
Abstract
1. Introduction
2. Fundamentals of ECL Biosensors
2.1. Composition of ECL Biosensors
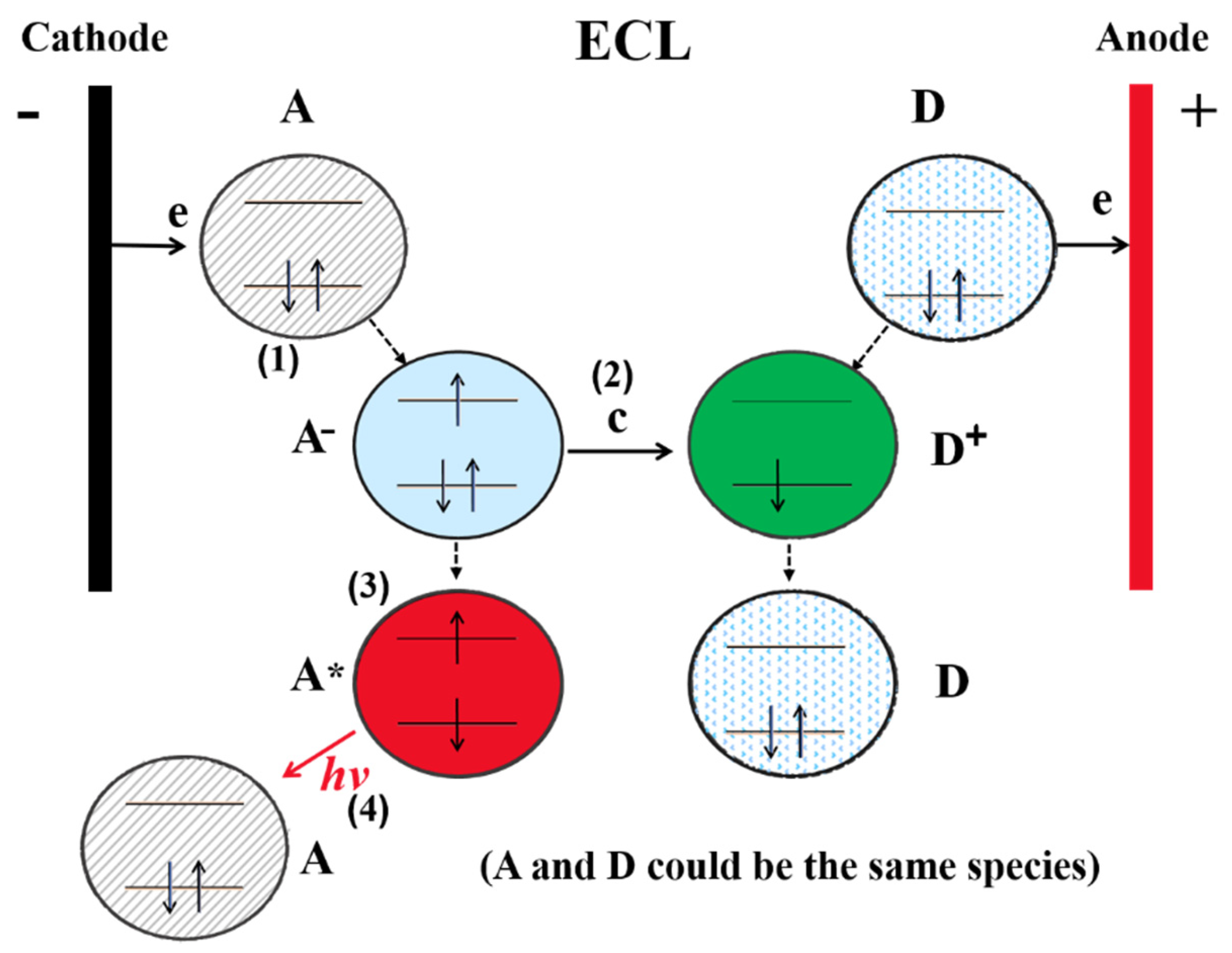
2.2. Mechanism of ECL
2.3. ECL Biosensing Strategies
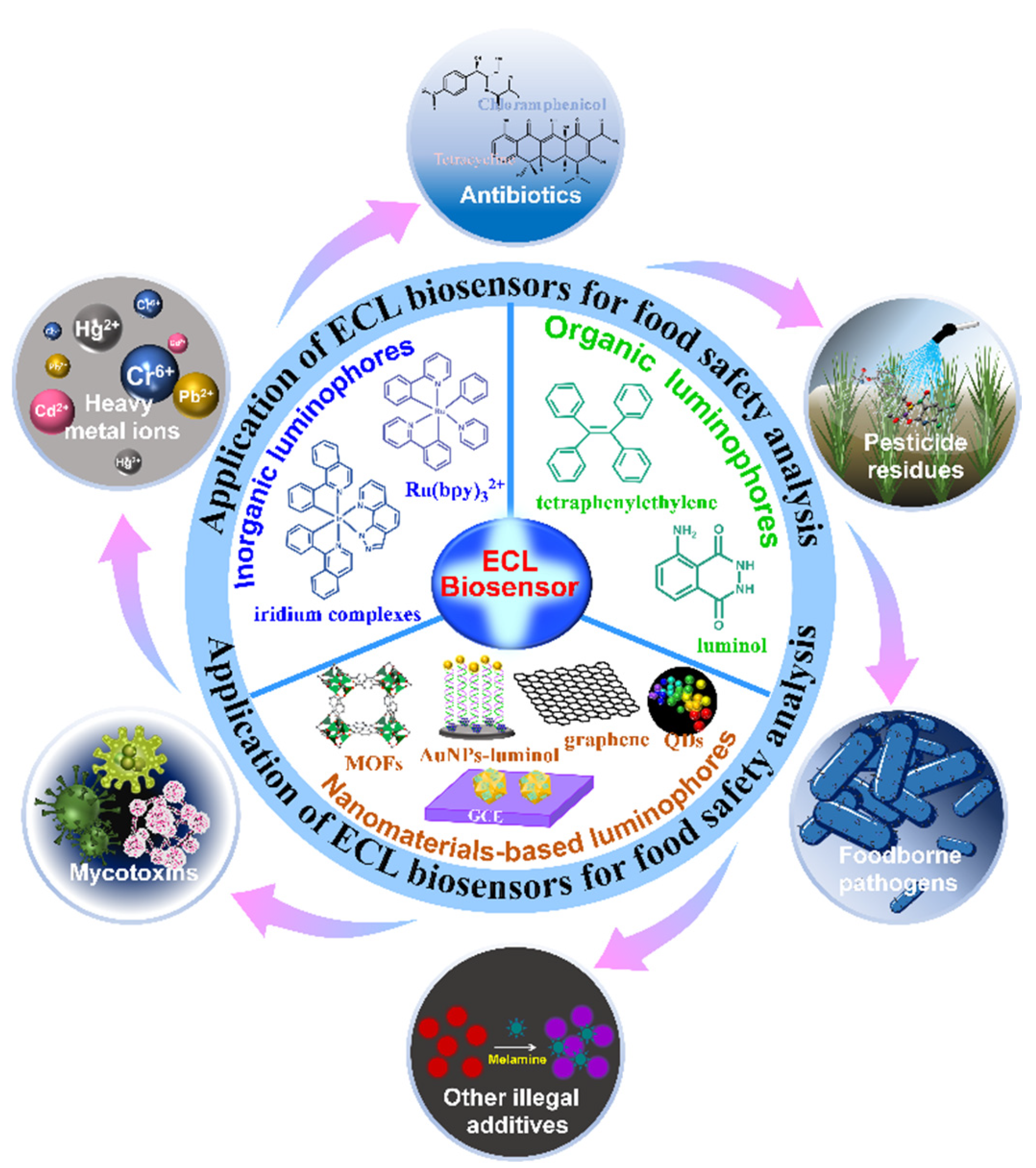
3. ECL Luminophores
3.1. Inorganic Luminophores
3.2. Organic Luminophores
3.3. Nanomaterials-Based Luminophores
4. Applications of ECL Biosensors in Food Analysis
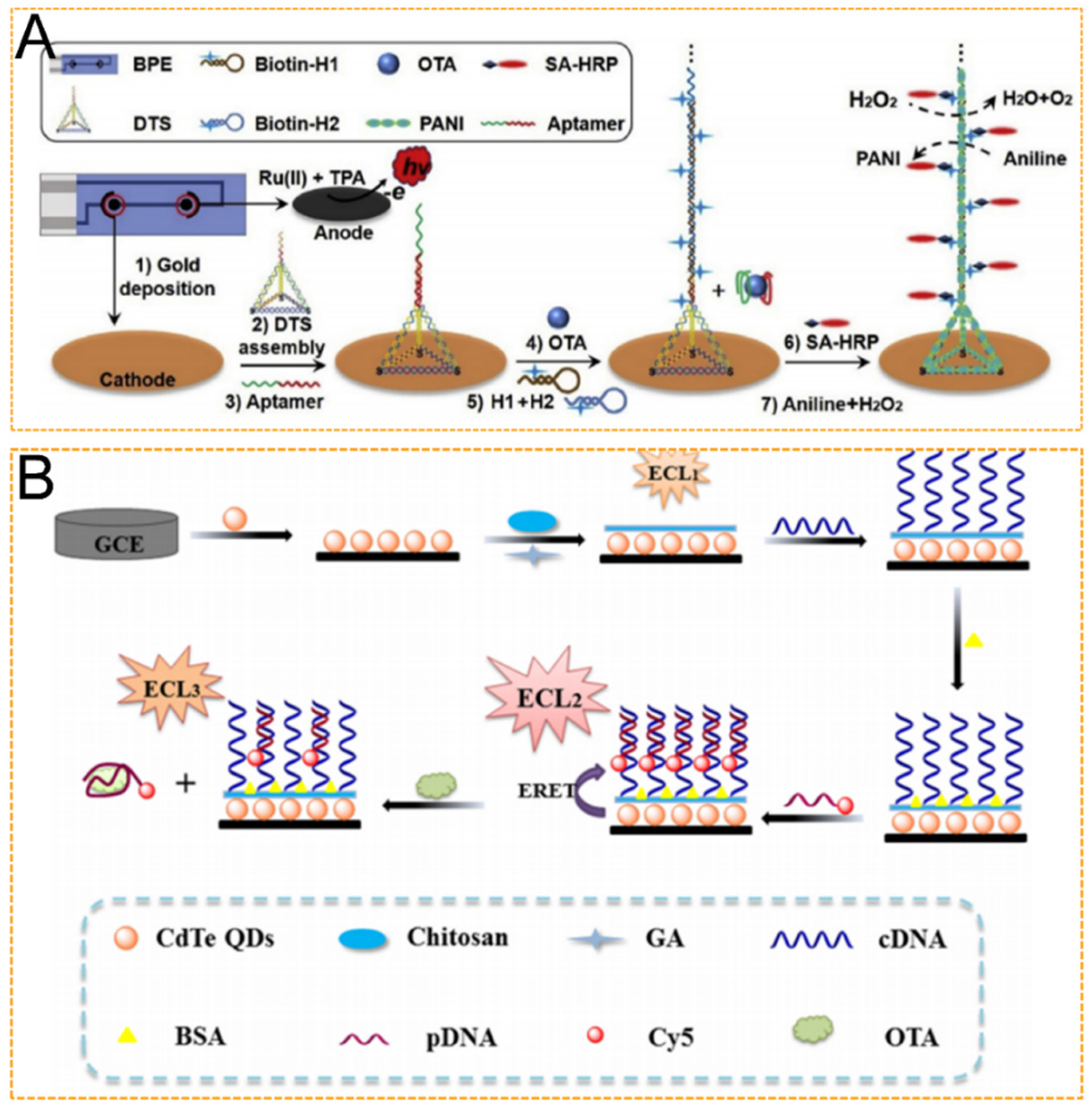
4.1. Mycotoxins
| Analytes | Sample Matrix | Limit of Detection | Linear Range | Ref. |
|---|---|---|---|---|
| Aflatoxin B1 | peanut | 0.17 ng/mL | 3.13–125.00 ng/mL | [46] |
| Aflatoxin B1 | milk | 3.9 pg/mL | 0.01–100 ng/mL | [47] |
| Aflatoxin B1 | peanut, wheat | 0.27 pg/mL | 1 pg/mL–5 ng/mL | [48] |
| Ochratoxin A | corn | 0.17 pg/mL | 0.0005–50 ng/mL | [49] |
| Ochratoxin A | grain | 3 pg/mL | 0.01–500 ng/mL | [50] |
| Ochratoxin A | wine, beer | 0.012 nM | 0.05 nM–5 nM | [51] |
| Zearalenone | corn flour | 1 fg/mL | 10 fg/mL–10 ng/mL | [54] |
| Zearalenone | coconut milk | 3.3 fg/mL | 10 fg/mL–0.1 ng/mL | [55] |
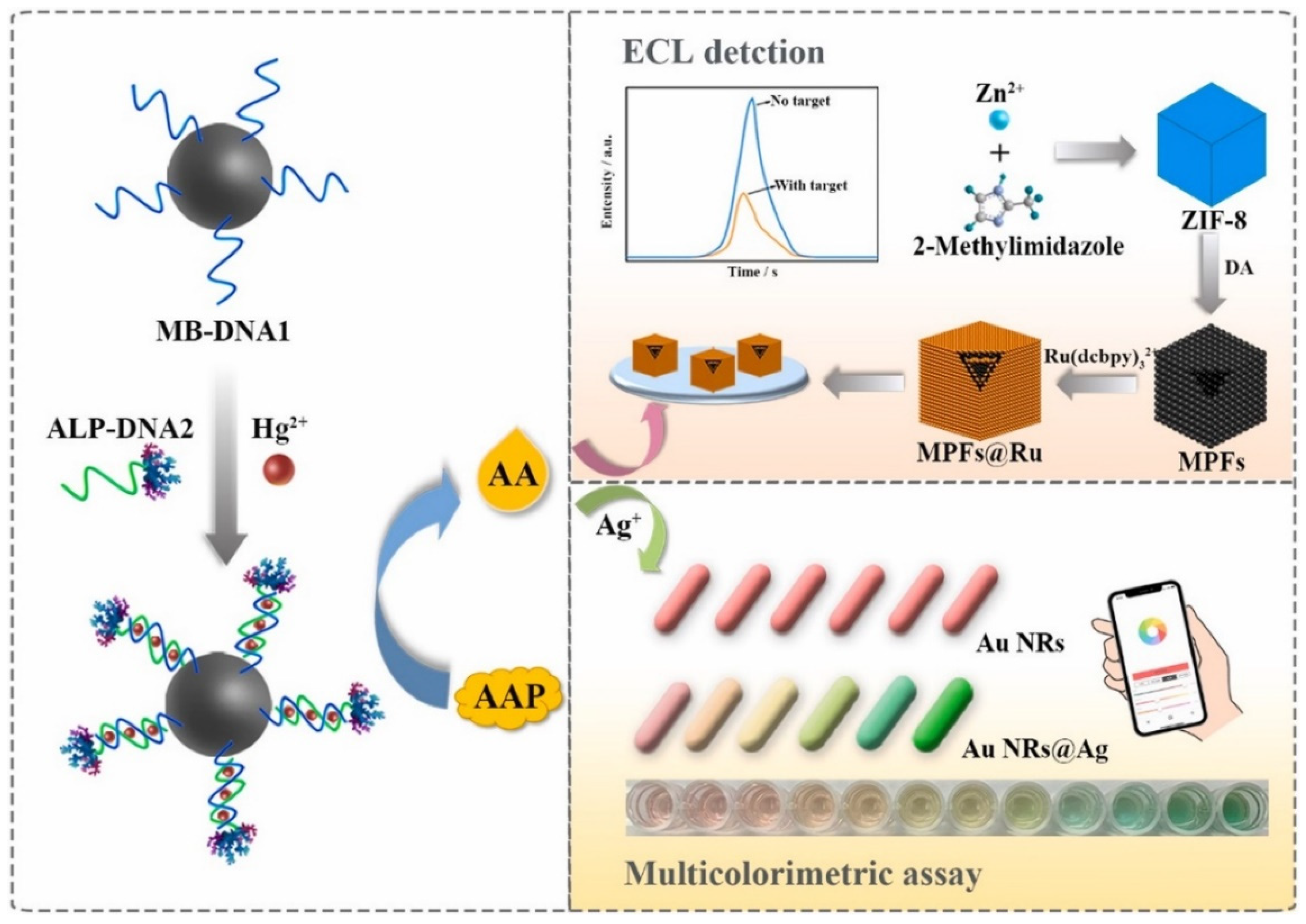
4.2. Heavy Metal Ions
| Analytes | Sample Matrix | Limit of Detection | Linear Range | Ref. |
|---|---|---|---|---|
| Cr(VI) | lake water | 0.83 pM | 10 pM–0.1 mM | [67] |
| Hg2+ | lake water | 0.32 pM | 2 pM–500 nM | [57] |
| Hg2+ | water | 0.04 pM | 0.1–10 pM | [58] |
| Pb2+ | tap water, lake water | 0.27 pM | 0.5 pM–5 nM | [63] |
| Pb2+ | water | 1.9 fM | 10 fM–10 nM. | [64] |
| Pb2+ | drinkable water | 1.2 pM | 10 pM–1 μM | [61] |
| Pb2+ | tap water, river water | 0.14 nM | 0.5–2000 nM | [62] |
| Cd2+ and Cu2+ | - | Cd2+: 0.094 μM Cu2+: 0.008 μM | Cd2+: 1 μM–75 μM, Cu2+: 0.1–1.75 μM | [68] |
4.3. Antibiotics
4.4. Pesticide Residues
4.5. Foodborne Pathogens
4.6. Other Illegal Additives
5. Challenges and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Chen, Y.; Zhu, J. Recent Advances in Electrochemiluminescence Analysis. Anal. Chem. 2017, 89, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, J.; Zhu, Y.; Xu, J.; Chen, H. Quantum dots: Electrochemiluminescent and photoelectrochemical bioanalysis. Anal. Chem. 2015, 87, 9520–9531. [Google Scholar] [CrossRef] [PubMed]
- Babamiri, B.; Bahari, D.; Salimi, A. Highly sensitive bioaffinity electrochemiluminescence sensors: Recent advances and future directions. Biosens. Bioelectron. 2019, 142, 111530. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, L.; Huang, W.; Yang, Y.; Liang, W.; Yuan, R.; Xiao, D. Overcoming Aggregation-Induced Quenching by Metal-Organic Framework for Electrochemiluminescence (ECL) Enhancement: Zn-PTC as a New ECL Emitter for Ultrasensitive MicroRNAs Detection. ACS Appl. Mater. Interfaces 2021, 13, 44079–44085. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Ma, Q. Nanoparticle-based electrochemiluminescence cytosensors for single cell level detection. Trends Anal. Chem. 2019, 110, 277–292. [Google Scholar] [CrossRef]
- Husain, R.A.; Barman, S.R.; Chatterjee, S.; Khan, I.; Lin, Z. Enhanced biosensing strategies using electrogenerated chemiluminescence: Recent progress and future prospects. J. Mater. Chem. B 2020, 8, 3192–3212. [Google Scholar] [CrossRef]
- Lv, W.; Ye, H.; Yuan, Z.; Liu, X.; Chen, X.; Yang, W. Recent advances in electrochemiluminescence-based simultaneous detection of multiple targets. Trends Anal. Chem. 2020, 123, 115767. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Chen, H.; Yang, L.; Cai, A.; Ji, H.; Wang, Q.; Zhou, X.; Li, G.; Wu, M.; et al. Bifunctional Pdots-Based Novel ECL Nanoprobe with Qualitative and Quantitative Dual Signal Amplification Characteristics for Trace Cytokine Analysis. Anal. Chem. 2022, 94, 7115–7122. [Google Scholar] [CrossRef]
- Hu, L.; Xu, G. Applications and trends in electrochemiluminescence. Chem. Soc. Rev. 2010, 39, 3275–3304. [Google Scholar] [CrossRef]
- Miao, W. Electrogenerated Chemiluminescence and Its Biorelated Applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef]
- Zou, R.; Teng, X.; Lin, Y.; Lu, C. Graphitic carbon nitride-based nanocomposites electrochemiluminescence systems and their applications in biosensors. Trends Anal. Chem. 2020, 132, 116054. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent Progress in Nanomaterial-Based Optical Aptamer Assay for the Detection of Food Chemical Contaminants. ACS Appl. Mater. Interfaces 2017, 9, 23287–23301. [Google Scholar] [CrossRef]
- Hejabri Kandeh, S.; Amini, S.; Ebrahimzadeh, H. PVA/Stevia/MIL-88A@AuNPs composite nanofibers as a novel sorbent for simultaneous extraction of eight agricultural pesticides in food and vegetable samples followed by HPLC-UV analysis. Food Chem. 2022, 386, 132734. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, X.; Chen, X.; Wang, D.; Yu, X.; Jiang, W. HPLC-MS/MS analysis of zinc-thiazole residues in foods of plant origin by a modified derivatization-QueChERS method. Food Chem. 2022, 386, 132752. [Google Scholar] [CrossRef]
- Han, C.; Hu, B.; Jin, N.; Jin, J.; Yu, Z.; Huang, C.; Shen, Y. Accelerated solvent extraction-gel permeation chromatography-gas chromatography-tandem mass spectrometry to rapid detection of clotrimazole residue in animal-derived food. LWT-Food Sci. Technol. 2021, 144, 111248. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, J.; Zhang, J.; Pan, A.; Quan, S.; Zhang, D.; Kim, H.; Li, X.; Zhou, S.; Yang, L. Development and inter-laboratory transfer of a decaplex polymerase chain reaction assay combined with capillary electrophoresis for the simultaneous detection of ten food allergens. Food Chem. 2016, 199, 799–808. [Google Scholar] [CrossRef]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef]
- Yang, E.; Zhang, Y.; Shen, Y. Quantum dots for electrochemiluminescence bioanalysis—A review. Anal. Chim. Acta 2022, 1209, 339140. [Google Scholar] [CrossRef]
- Hao, N.; Wang, K. Recent development of electrochemiluminescence sensors for food analysis. Anal. Bioanal. Chem. 2016, 408, 7035–7048. [Google Scholar] [CrossRef]
- Adhikari, J.; Rizwan, M.; Keasberry, N.A.; Ahmed, M.U. Current progresses and trends in carbon nanomaterials-based electrochemical and electrochemiluminescence biosensors. J. Chin. Chem. Soc. 2020, 67, 937–960. [Google Scholar] [CrossRef]
- Peng, L.; Li, P.; Chen, J.; Deng, A.; Li, J. Functional Nanomaterials and strategies of nanomaterials-based ultrasensitive electrochemiluminescence biosensors for food safety and disease diagnosis. Talanta 2022, 253, 123906. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, K.S.V.; Bard, A.J. Chemiluminescence of Electrogenerated 9,10-Diphenylanthracene Anion Radical1. J. Am. Chem. Soc. 1965, 87, 139–140. [Google Scholar] [CrossRef]
- Hercules David, M. Chemiluminescence Resulting from Electrochemically Generated Species. Science 1964, 145, 808–809. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, S.; Li, L.; Zhu, J. Nanomaterials-based sensitive electrochemiluminescence biosensing. Nano Today 2017, 12, 98–115. [Google Scholar] [CrossRef]
- Carrara, S.; Aliprandi, A.; Hogan, C.F.; Cola, L.D. Aggregation-Induced Electrochemiluminescence of Platinum(II) Complexes. J. Am. Chem. Soc. 2017, 139, 14605–14610. [Google Scholar] [CrossRef]
- Guo, W.; Ding, H.; Gu, C.; Liu, Y.; Jiang, X.; Su, B.; Shao, Y. Potential-Resolved Multicolor Electrochemiluminescence for Multiplex Immunoassay in a Single Sample. J. Am. Chem. Soc. 2018, 140, 15904–15915. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, J.; Yan, R.; Cao, D.; Jiang, D.; Ye, D. Aggregation-Induced Electrochemiluminescence from a Cyclometalated Iridium(III) Complex. Inorg. Chem. 2018, 57, 4310–4316. [Google Scholar] [CrossRef]
- Jiang, H.; Li, S.; Zhong, X.; Liang, W.; Chai, Y.; Zhuo, Y.; Yuan, R. Electrochemiluminescence Enhanced by Restriction of Intramolecular Motions (RIM): Tetraphenylethylene Microcrystals as a Novel Emitter for Mucin 1 Detection. Anal. Chem. 2019, 91, 3710–3716. [Google Scholar] [CrossRef]
- Ma, C.; Cao, Y.; Gou, X.; Zhu, J. Recent Progress in Electrochemiluminescence Sensing and Imaging. Anal. Chem. 2020, 92, 431–454. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, W.; Xu, G. Recent advances in electrochemiluminescence. Chem. Soc. Rev. 2015, 44, 3117–3142. [Google Scholar] [CrossRef]
- Mayer, M.; Takegami, S.; Neumeier, M.; Rink, S.; Wangelin, A.J.; Schulte, S.; Vollmer, M.; Griesbeck, A.G.; Duerkop, A.; Baeumner, A.J. Electrochemiluminescence Bioassays with a Water-Soluble Luminol Derivative Can Outperform Fluorescence Assays. Angew. Chem. Int. Ed. Engl. 2018, 57, 408–411. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; Wu, Y.; Li, Z.; Bai, L.; Huo, S.; Lu, X. Substituent-Induced Aggregated State Electrochemiluminescence of Tetraphenylethene Derivatives. Anal. Chem. 2019, 91, 8676–8682. [Google Scholar] [CrossRef]
- Ding, Z.; Quinn, B.M.; Haram, S.K.; Pell, L.E.; Korgel, B.A.; Bard, A.J. Electrochemistry and electrogenerated chemiluminescence from silicon nanocrystal quantum dots. Science 2002, 296, 1293–1297. [Google Scholar] [CrossRef]
- Yang, L.; Bin, Z.; Fu, L.; Fu, K.; Zou, G. Efficient and Monochromatic Electrochemiluminescence of Aqueous-Soluble Au Nanoclusters via Host-Guest Recognition. Angew. Chem. Int. Ed. Engl. 2019, 58, 6901–6905. [Google Scholar] [CrossRef]
- Peng, H.; Huang, Z.; Deng, H.; Wu, W.; Huang, K.; Li, Z.; Chen, W.; Liu, J. Dual Enhancement of Gold Nanocluster Electrochemiluminescence: Electrocatalytic Excitation and Aggregation-Induced Emission. Angew. Chem. Int. Ed. Engl. 2020, 59, 9982–9985. [Google Scholar] [CrossRef]
- Zhai, Q.; Xing, H.; Zhang, X.; Li, J.; Wang, E. Enhanced Electrochemiluminescence Behavior of Gold-Silver Bimetallic Nanoclusters and Its Sensing Application for Mercury(II). Anal. Chem. 2017, 89, 7788–7794. [Google Scholar] [CrossRef]
- Pang, X.; Li, J.; Zhao, Y.; Wu, D.; Zhang, Y.; Du, B.; Ma, H.; Wei, Q. Label-Free Electrochemiluminescent Immunosensor for Detection of Carcinoembryonic Antigen Based on Nanocomposites of GO/MWCNTs-COOH/Au@CeO(2). ACS Appl. Mater. Interfaces 2015, 7, 19260–19267. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Li, J.; Ma, H.; Zhang, Y.; Wu, D.; Du, B.; Wei, Q. A CeO2-matrical enhancing ECL sensing platform based on the Bi2S3-labeled inverted quenching mechanism for PSA detection. J. Mater. Chem. B 2016, 4, 2963–2971. [Google Scholar] [CrossRef]
- Jiang, D.; Du, X.; Liu, Q.; Zhou, L.; Qian, J.; Wang, K. One-step thermal-treatment route to fabricate well-dispersed ZnO nanocrystals on nitrogen-doped graphene for enhanced electrochemiluminescence and ultrasensitive detection of pentachlorophenol. ACS Appl. Mater. Interfaces 2015, 7, 3093–3100. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, D.; Li, P.; Yao, Y.; Chen, S.; Yuan, R.; Xu, W. An electrochemiluminescent sensor based on functionalized conjugated polymer dots for the ultrasensitive detection of Cu2+. Chem. Commun. 2018, 54, 2777–2780. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Wang, W.; Tan, X.; Lu, Z.; Han, H. Metal-organic frameworks-based sensitive electrochemiluminescence biosensing. Biosens. Bioelectron. 2020, 164, 112332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, L.; Yang, Y.; Liang, W.; Yuan, R.; Xiao, D. Conductive Covalent Organic Frameworks with Conductivity- and Pre-Reduction-Enhanced Electrochemiluminescence for Ultrasensitive Biosensor Construction. Biosens. Bioelectron. 2022, 94, 3685–3692. [Google Scholar] [CrossRef]
- Lv, L.; Wang, X. Recent Advances in Ochratoxin A Electrochemical Biosensors: Recognition Elements, Sensitization Technologies, and Their Applications. J. Agric. Food Chem. 2020, 68, 4769–4787. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, X.; Zhu, Y.; Shen, M.; Lu, Y.; Cheng, J.; Xu, Y. Rapid detection of four mycotoxins in corn using a microfluidics and microarray-based immunoassay system. Talanta 2018, 186, 299–305. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Zikankuba, V.L. Mycotoxins contamination in maize alarms food safety in sub-Sahara Africa. Food Control 2018, 90, 372–381. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Wang, S.; Ye, Z.; Wei, L.; Xiao, L. Single-Particle LRET Aptasensor for the Sensitive Detection of Aflatoxin B1 with Upconversion Nanoparticles. Anal. Chem. 2019, 91, 11856–11863. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Li, X.; Liu, L.; Cao, W.; Wei, Q. Electrochemiluminescent competitive immunosensor based on polyethyleneimine capped SiO2 nanomaterials as labels to release Ru(bpy)32+ fixed in 3D Cu/Ni oxalate for the detection of aflatoxin B1. Biosens. Bioelectron. 2018, 101, 290–296. [Google Scholar] [CrossRef]
- Yan, C.; Yang, L.; Yao, L.; Xu, J.; Yao, B.; Liu, G.; Cheng, L.; Chen, W. Ingenious Electrochemiluminescence Bioaptasensor Based on Synergistic Effects and Enzyme-Driven Programmable 3D DNA Nanoflowers for Ultrasensitive Detection of Aflatoxin B1. Anal. Chem. 2020, 92, 14122–14129. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Z.; Mao, L.; Zhang, W.; Wen, W.; Zhang, X.; Wang, S. Electrochemiluminescent aptasensor based on resonance energy transfer system between CdTe quantum dots and cyanine dyes for the sensitive detection of Ochratoxin, A. Talanta 2019, 199, 178–183. [Google Scholar] [CrossRef]
- Lu, L.; Yuan, W.; Xiong, Q.; Wang, M.; Liu, Y.; Cao, M.; Xiong, X. One-step grain pretreatment for ochratoxin A detection based on bipolar electrode-electrochemiluminescence biosensor. Anal. Chim. Acta 2021, 1141, 83–90. [Google Scholar] [CrossRef]
- Wei, M.; Wang, C.; Xu, E.; Chen, J.; Xu, X.; Wei, W.; Liu, S. A simple and sensitive electrochemiluminescence aptasensor for determination of ochratoxin A based on a nicking endonuclease-powered DNA walking machine. Food Chem. 2019, 282, 141–146. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Nan, M.; Li, Y.; Yun, J.; Wang, Y.; Bi, Y. Novel colorimetric aptasensor based on unmodified gold nanoparticle and ssDNA for rapid and sensitive detection of T-2 toxin. Food Chem. 2021, 348, 129128. [Google Scholar] [CrossRef]
- Conceicao, R.R.P.; Simeone, M.L.F.; Queiroz, V.A.V.; Medeiros, E.P.; Araujo, J.B.; Coutinho, W.M.; Silva, D.D.; Miguel, R.A.; Lana, U.G.P.; Stoianoff, M.A.R. Application of near-infrared hyperspectral (NIR) images combined with multivariate image analysis in the differentiation of two mycotoxicogenic Fusarium species associated with maize. Food Chem. 2021, 348, 129128. [Google Scholar]
- Luo, L.; Ma, S.; Li, L.; Liu, X.; Zhang, J.; Li, X.; Liu, D.; You, T. Monitoring zearalenone in corn flour utilizing novel self-enhanced electrochemiluminescence aptasensor based on NGQDs-NH2-Ru@SiO2 luminophore. Food Chem. 2019, 292, 98–105. [Google Scholar] [CrossRef]
- Fang, D.; Zhang, S.; Dai, H.; Liu, X.; Hong, Z.; Lin, Y. Electrochemiluminescent competitive immunoassay for zearalenone based on the use of a mimotope peptide, Ru(II)(bpy)3-loaded NiFe2O4 nanotubes and TiO2 mesocrystals. Mikrochim. Acta 2019, 186, 608. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, S.; Li, J.; Chen, L. Nanomaterial-based optical sensors for mercury ions. TrAC Trends Anal. Chem. 2016, 82, 175–190. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, Y.; Mu, X.; Yu, C.; Zhou, Y.; Chen, J.; Zheng, S.; He, J. Enzyme-induced multicolor colorimetric and electrochemiluminescence sensor with a smartphone for visual and selective detection of Hg2+. J. Hazard. Mater. 2021, 415, 125538. [Google Scholar] [CrossRef]
- Ma, F.; Chen, Y.; Zhu, Y.; Liu, J. Electrogenerated chemiluminescence biosensor for detection of mercury (II) ion via target-triggered manipulation of DNA three-way junctions. Talanta 2019, 194, 114–118. [Google Scholar] [CrossRef]
- Huang, K.; Li, B.; Zhou, F.; Mei, S.; Zhou, Y.; Jing, T. Selective Solid-Phase Extraction of Lead Ions in Water Samples Using Three-Dimensional Ion-Imprinted Polymers. Anal. Chem. 2016, 88, 6820–6826. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, J.; Tang, M.; Huang, L.; Zhang, Z.; Liu, C.; Lu, X.; Hunter, K.W.; Chen, G. A New Electrochemical System Based on a Flow-Field Shaped Solid Electrode and 3D-Printed Thin-Layer Flow Cell: Detection of Pb2+ Ions by Continuous Flow Accumulation Square-Wave Anodic Stripping Voltammetry. Anal. Chem. 2017, 89, 5024–5029. [Google Scholar] [CrossRef]
- Han, Q.; Wang, C.; Liu, P.; Zhang, G.; Song, L.; Fu, Y. Three kinds of porphyrin dots as near-infrared electrochemiluminescence luminophores: Facile synthesis and biosensing. Chem. Eng. J. 2021, 421, 129761. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Li, L.; Kong, Q.; Zhang, L.; Ge, S.; Yu, J. Colorimetric and Electrochemiluminescence Dual-Mode Sensing of Lead Ion Based on Integrated Lab-on-Paper Device. ACS Appl. Mater. Interfaces 2018, 10, 3431–3440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, H.; Zhang, L.; Ge, S.; Meiling, X.; Wang, X.; Yu, J. Two-dimensional black phosphorus nanoflakes: A coreactant-free electrochemiluminescence luminophors for selective Pb2+ detection based on resonance energy transfer. J. Hazard. Mater. 2021, 403, 123601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Jie, G. An “on-off” electrochemiluminescence biosensor based on DNA nanotweezer probe coupled with tripod capture DNA for high sensitive detection of Pb2+. Sens. Actuators B 2021, 326, 128985. [Google Scholar] [CrossRef]
- Shi, W.; He, M.; Li, W.; Wei, X.; Bui, B.; Chen, M.; Chen, W. Cu-Based Metal–Organic Framework Nanoparticles for Sensing Cr(VI) Ions. ACS Appl. Nano Mater. 2021, 4, 802–810. [Google Scholar] [CrossRef]
- Borthakur, P.; Das, M.R.; Szunerits, S.; Boukherroub, R. CuS Decorated Functionalized Reduced Graphene Oxide: A Dual Responsive Nanozyme for Selective Detection and Photoreduction of Cr(VI) in an Aqueous Medium. ACS Sustain. Chem. Eng. 2019, 7, 16131–16143. [Google Scholar] [CrossRef]
- Guo, J.; Feng, W.; Du, P.; Zhang, R.; Liu, J.; Liu, Y.; Wang, Z.; Lu, X. Aggregation-Induced Electrochemiluminescence of Tetraphenylbenzosilole Derivatives in an Aqueous Phase System for Ultrasensitive Detection of Hexavalent Chromium. Anal. Chem. 2020, 92, 14838–14845. [Google Scholar] [CrossRef]
- Moghaddam, M.R.; Carrara, S.; Hogan, C.F. Multi-colour bipolar electrochemiluminescence for heavy metal ion detection. Chem. Commun. 2019, 55, 1024–1027. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Alipour, Z.; Moharramnezhad, M. Amplified cathodic electrochemiluminescence of luminol based on zinc oxide nanoparticle modified Ni-foam electrode for ultrasensitive detection of amoxicillin. J. Solid State Electr. 2020, 25, 445–456. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Pang, C.; Li, H.; Liu, C.; Xu, Z.; Luo, J.; Yang, Y. Novel molecularly imprinted amoxicillin sensor based on a dual recognition and dual detection strategy. Anal. Chim. Acta 2020, 1127, 69–78. [Google Scholar] [CrossRef]
- Li, P.; Yu, J.; Zhao, K.; Deng, A.; Li, J. Efficient enhancement of electrochemiluminescence from tin disulfide quantum dots by hollow titanium dioxide spherical shell for highly sensitive detection of chloramphenicol. Biosens. Bioelectron. 2020, 147, 111790. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, L.; Jiang, Y. Target-initiated autonomous synthesis of metal-ion dependent DNAzymes for label-free and amplified fluorescence detection of kanamycin in milk samples. Anal. Chim. Acta 2021, 1148, 238195. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Z.; Chen, Y.; Xu, L.; Xie, Q.; Deng, H.; Chen, W.; Peng, H. Regulating Valence States of Gold Nanocluster as a New Strategy for the Ultrasensitive Electrochemiluminescence Detection of Kanamycin. Anal. Chem. 2021, 93, 4635–4640. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, D.; Zhu, J.; Ding, S. Photonic Crystal of Polystyrene Nanomembrane: Signal Amplification and Low Triggered Potential Electrochemiluminescence for Tetracycline Detection. Anal. Chem. 2021, 93, 2959–2967. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Z.; Xiao, S.; Huang, C.; Li, Y. Terbium(III) Organic Gels: Novel Antenna Effect-Induced Enhanced Electrochemiluminescence Emitters. Anal. Chem. 2018, 90, 12191–12197. [Google Scholar] [CrossRef]
- Liu, J.; Chen, P.; Xia, F.; Liu, Z.; Liu, H.; Yi, J.; Zhou, C. Sensitive electrochemiluminescence aptasensor for chlorpyrifos detection based on resonance energy transfer between MoS2/CdS nanospheres and Ag/CQDs. Sens. Actuators B Chem. 2020, 315, 128098. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Moharramnezhad, M. An enzyme-free electrochemiluminescence sensing probe based on ternary nanocomposite for ultrasensitive determination of chlorpyrifos. Food Chem. 2021, 351, 129252. [Google Scholar] [CrossRef]
- Liu, H.; Chen, P.; Liu, Z.; Liu, J.; Yi, J.; Xia, F.; Zhou, C. Electrochemical luminescence sensor based on double suppression for highly sensitive detection of glyphosate. Sens. Actuators B Chem. 2020, 304, 127304. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, F.; Yao, Y.; Li, J.; Cheng, S.; Dong, H.; Zhang, H.; Xiang, Y.; Sun, X. Novel Au-tetrahedral aptamer nanostructure for the electrochemiluminescence detection of acetamiprid. J. Hazard. Mater. 2021, 401, 123794. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Yi, J.; Ma, D.; Xia, F.; Tian, D.; Zhou, C. A dual-signal electroluminescence aptasensor based on hollow Cu/Co-MOF-luminol and g-C3N4 for simultaneous detection of acetamiprid and malathion. Sens. Actuators B Chem. 2021, 331, 129412. [Google Scholar] [CrossRef]
- Wang, T.; Song, X.; Lin, H.; Hao, T.; Hu, Y.; Wang, S.; Su, X.; Guo, Z. A Faraday cage-type immunosensor for dual-modal detection of Vibrio parahaemolyticus by electrochemiluminescence and anodic stripping voltammetry. Anal. Chim. Acta 2019, 1062, 124–130. [Google Scholar] [CrossRef]
- Wei, W.; Lin, H.; Hao, T.; Su, X.; Jiang, X.; Wang, S.; Hu, Y.; Guo, Z. Dual-mode ECL/SERS immunoassay for ultrasensitive determination of Vibrio vulnificus based on multifunctional MXene. Sens. Actuators B Chem. 2021, 332, 129525. [Google Scholar] [CrossRef]
- Han, D.; Yuan, Y.; Wang, J.; Zhao, M.; Duan, X.; Kong, L.; Wu, H.; Cheng, W.; Min, X.; Ding, S. An enzyme-free electrochemiluminesce aptasensor for the rapid detection of Staphylococcus aureus by the quenching effect of MoS2-PtNPs-vancomycin to S2O82−/O2 system. Sens. Actuators B Chem. 2019, 288, 586–593. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, D.; Cao, Y.; Ma, W.; Yu, Y.; Guo, M.; Xing, X. A novel universal signal amplification probe-based electrochemiluminescence assay for sensitive detection of pathogenic bacteria. Electrochem. Commun. 2017, 85, 11–14. [Google Scholar] [CrossRef]
- Huo, X.; Chen, Y.; Bao, N.; Shi, C. Electrochemiluminescence integrated with paper chromatography for separation and detection of environmental hormones. Sens. Actuators B Chem. 2021, 334, 129662. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Y.; Kang, T.; Zhang, X.; Liu, H.; Ming, A.; Cheng, S.; Wei, F. Sandwich magnetically imprinted immunosensor for electrochemiluminescence ultrasensing diethylstilbestrol based on enhanced luminescence of Ru@SiO2 by CdTe@ZnS quantum dots. Biosens. Bioelectron. 2020, 155, 112102. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Yao, Y.; Yang, B.; Tian, T.; Miao, Y.; Liu, B. An electrochemiluminescence sensor for 17beta-estradiol detection based on resonance energy transfer in alpha-FeOOH@CdS/Ag NCs. Talanta 2021, 221, 121479. [Google Scholar] [CrossRef]
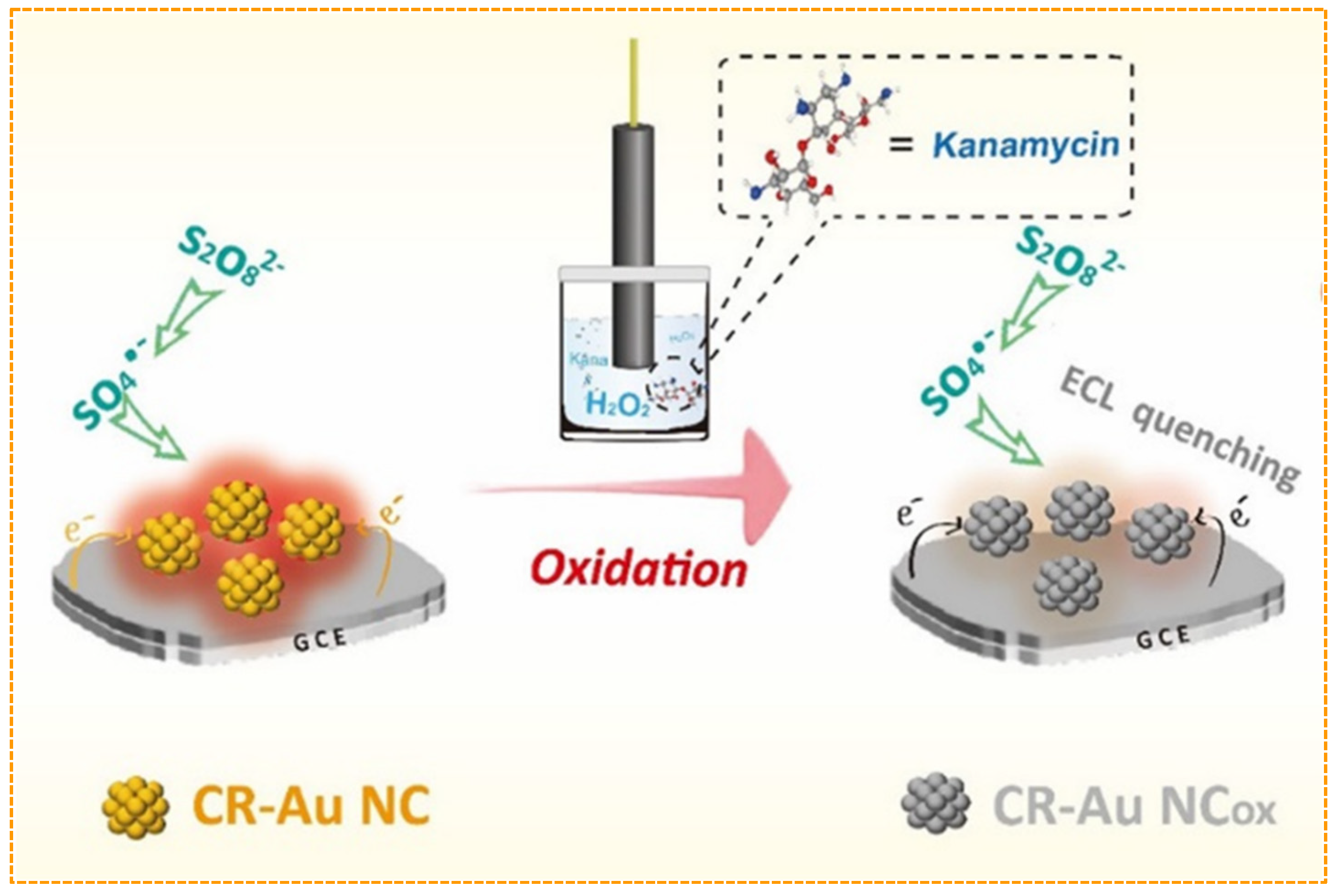
| Analytes | Sample Matrix | Limit of Detection | Linear Range | Ref. |
|---|---|---|---|---|
| amoxicillin | raw and pasteurized milk | 8.3 pM | 40 pM–65 μM | [69] |
| amoxicillin | pork, chicken, and beef | 8.3 pM | 50 pM to 15 nM | [70] |
| chloramphenicol | honey and shrimp | 3.1 pg/mL | 0.01–100 ng/mL | [71] |
| kanamycin | milk | 0.36 nM | 1–500 nM | [72] |
| kanamycin | milk | 1.5 nM | 10 nM–33 μM | [73] |
| tetracycline | pond water, milk, and honey | 0.075 pg/mL | 0.224–1.953 pg/mL | [74] |
| tetracycline | milk | 32.4 nM | 0.1–25 μM | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Lv, X.; Jia, J.; Din, Z.-u.; Cai, S.; He, J.; Xie, F.; Cai, J. Nanomaterials-Based Electrochemiluminescence Biosensors for Food Analysis: Recent Developments and Future Directions. Biosensors 2022, 12, 1046. https://doi.org/10.3390/bios12111046
Zhou J, Lv X, Jia J, Din Z-u, Cai S, He J, Xie F, Cai J. Nanomaterials-Based Electrochemiluminescence Biosensors for Food Analysis: Recent Developments and Future Directions. Biosensors. 2022; 12(11):1046. https://doi.org/10.3390/bios12111046
Chicago/Turabian StyleZhou, Jiaojiao, Xuqin Lv, Jilai Jia, Zia-ud Din, Shiqi Cai, Jiangling He, Fang Xie, and Jie Cai. 2022. "Nanomaterials-Based Electrochemiluminescence Biosensors for Food Analysis: Recent Developments and Future Directions" Biosensors 12, no. 11: 1046. https://doi.org/10.3390/bios12111046
APA StyleZhou, J., Lv, X., Jia, J., Din, Z.-u., Cai, S., He, J., Xie, F., & Cai, J. (2022). Nanomaterials-Based Electrochemiluminescence Biosensors for Food Analysis: Recent Developments and Future Directions. Biosensors, 12(11), 1046. https://doi.org/10.3390/bios12111046







