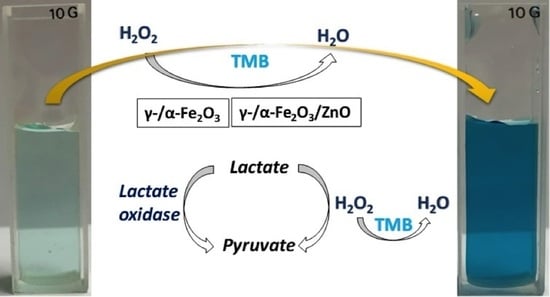
Comparative Colorimetric Sensor Based on Bi-Phase γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO Nanoparticles for Lactate Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Fe2O3 and Fe2O3/ZnO
2.3. Peroxidase-like Activity Measurements
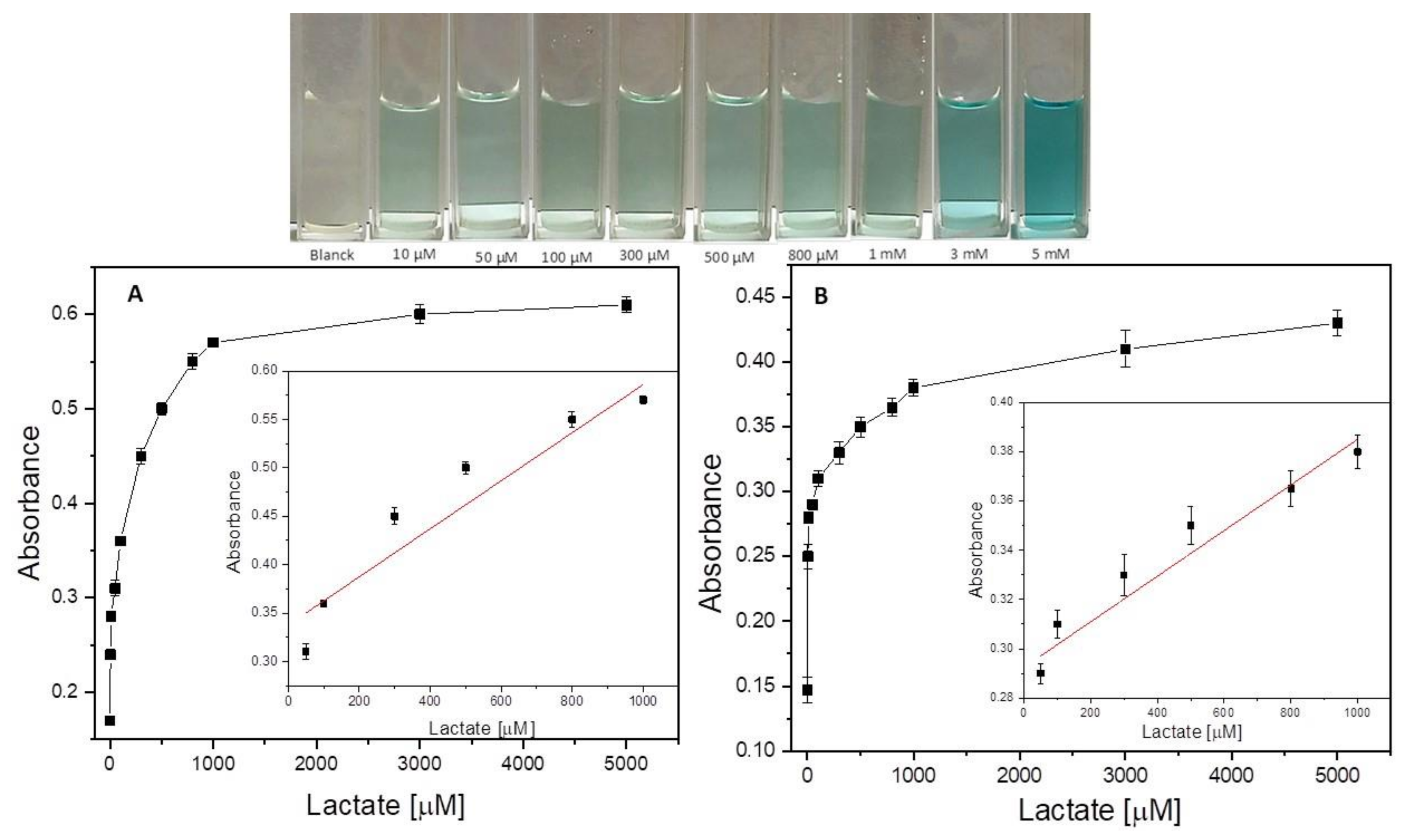
2.4. Colorimetric Measurement of Lactate Using γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO NPs
2.5. Colorimetric Evaluation of Lactate Using γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO in the Presence of Interferents and Real Samples
2.6. Characterization of γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO NPs
3. Results and Discussion
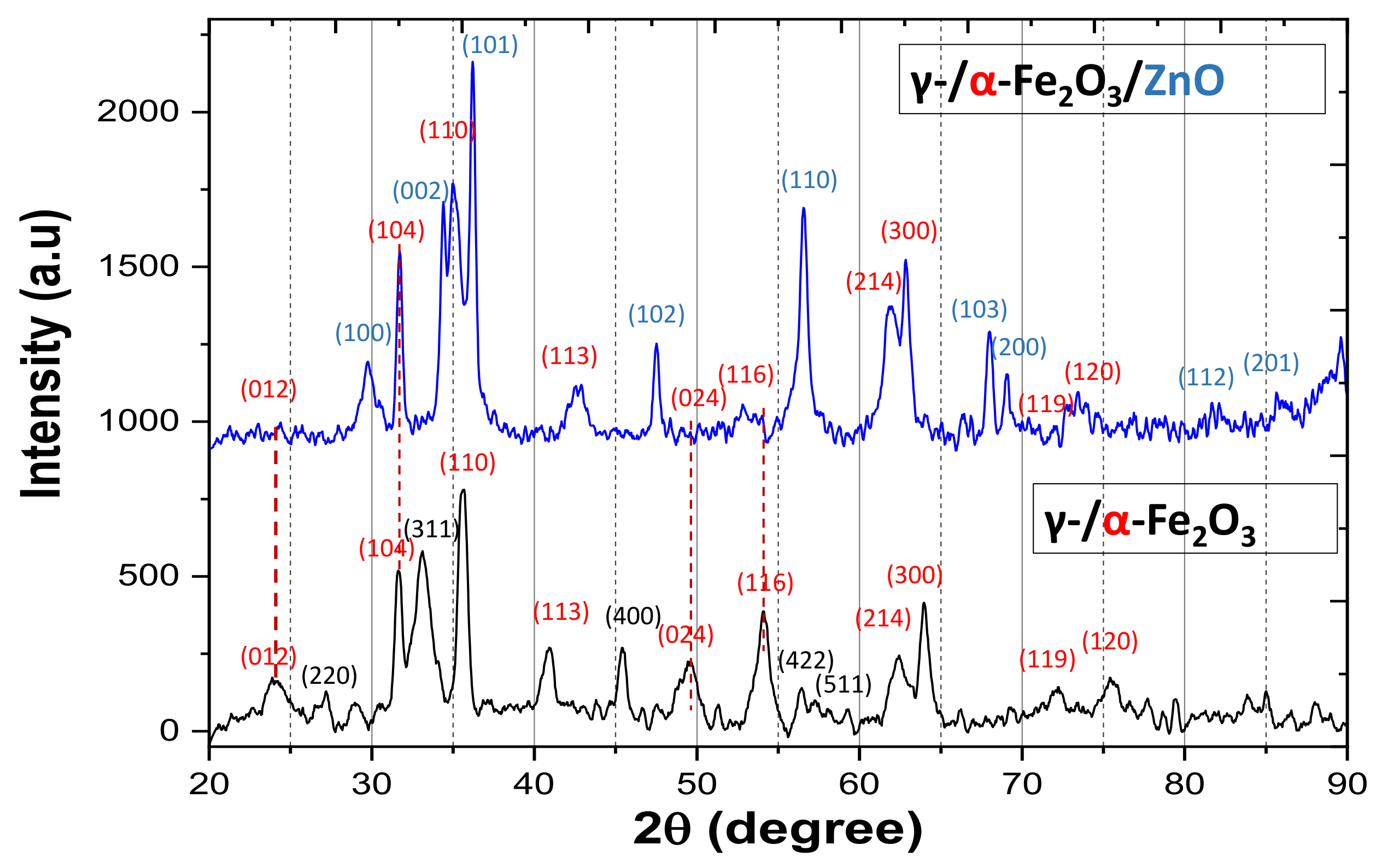
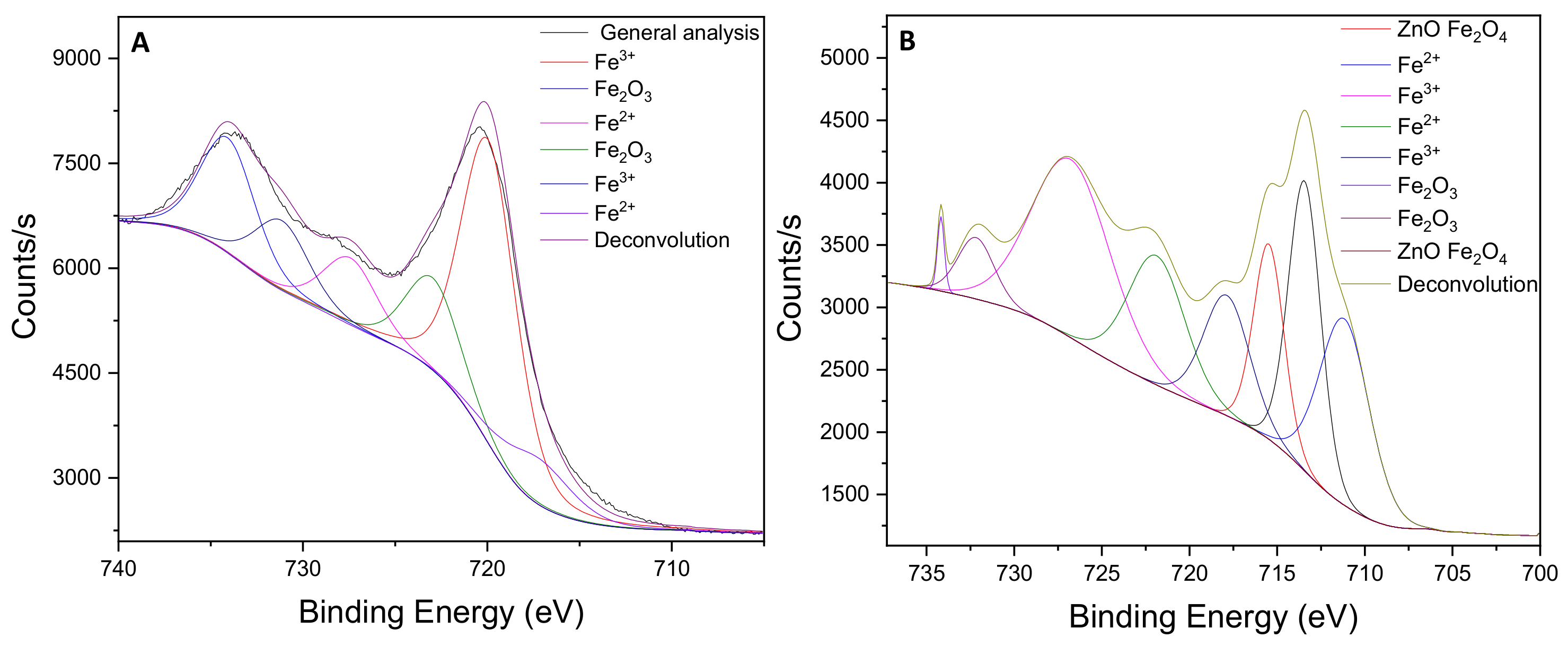
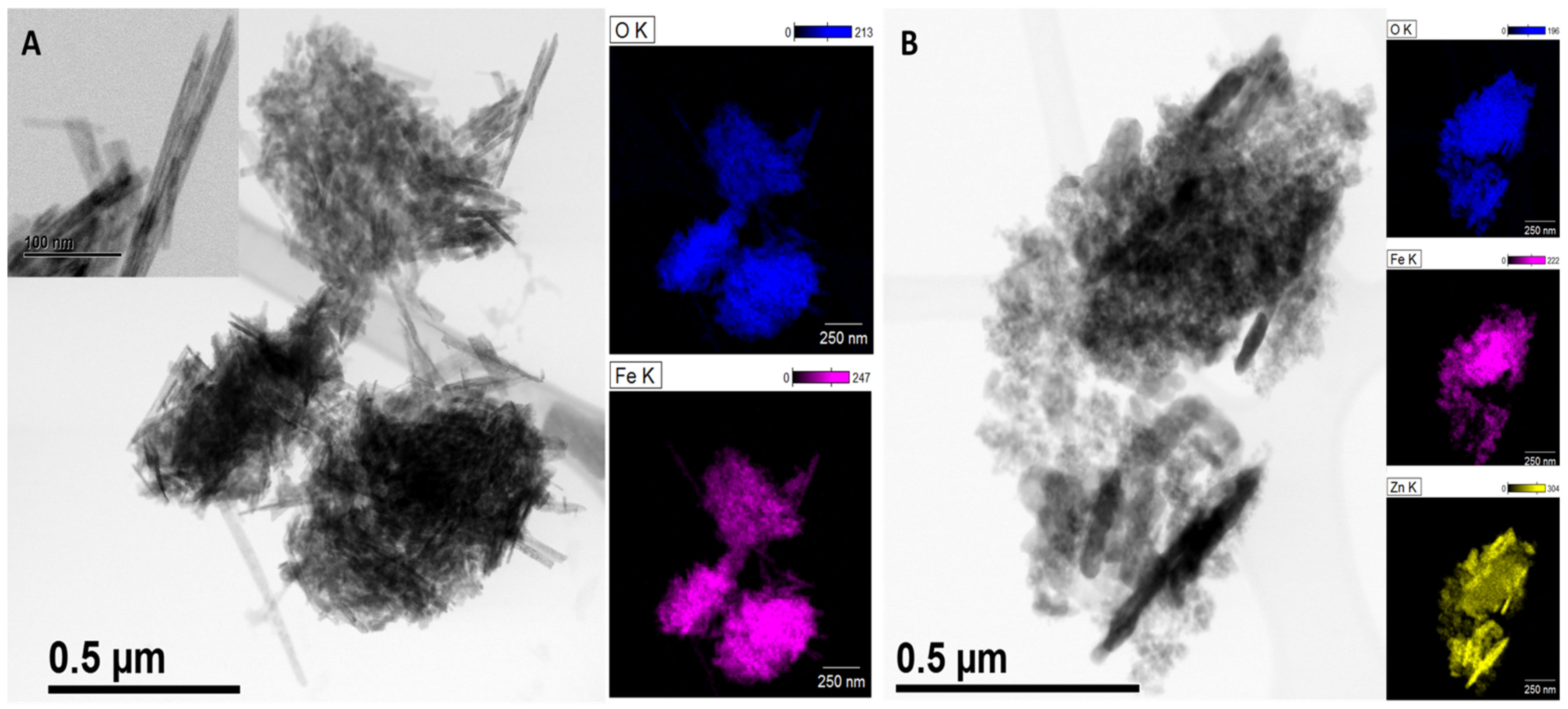
3.1. Characterization of γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO
3.2. Evaluation of Peroxidase-like Activity of γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO
| Catalyst | Km (mM) | Vmax (M s−1) | Ref. |
|---|---|---|---|
| γ-/α-Fe2O3 | 0.02 | 6.0 × 10−8 | This work |
| γ-/α-Fe2O3/ZnO | 0.03 | 8.9 × 10−8 | This work |
| Fe3O4@C | 0.014 | 13.35 × 10−8 | [43] |
| Fe3O4 | 0.013 | 2.95 × 10−8 | [17] |
| H2TCPP-γ-Fe2O3 | 0.013 | 21.14 × 10−9 | [17] |
| GO-Fe2O3 | 0.71 | 5.31 × 10−8 | [21] |
| γ-Fe2O3-SiO2 | 0.63 | 2.25 × 10−5 | [19] |
| PB-Fe2O3 | 91.54 | 8.31 × 10−8 | [44] |
| ZnFe2O4 | 1.66 | 7.74 × 10−8 | [18] |
3.3. Lactate Detection Using LOx, γ-/α-Fe2O3, and γ-/α-Fe2O3/ZnO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kucherenko, I.S.; Topolnikova, Y.V.; Soldatkin, O.O. Advances in the Biosensors for Lactate and Pyruvate Detection for Medical Applications: A Review. TrAC Trends Anal. Chem. 2019, 110, 160–172. [Google Scholar] [CrossRef]
- Heinemann, B. A Rapid Colorimetric Method for the Determination of Lactic Acid in Milk. J. Dairy Sci. 1940, 23, 969–972. [Google Scholar] [CrossRef]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors Based on Electrochemical Lactate Detection: A Comprehensive Review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.B.; Summerson, W.H. The Colorimetric Determination of Lactic Acid in Biological Material. J. Biol. Chem. 1941, 138, 535–554. [Google Scholar] [CrossRef]
- Rattu, G.; Murali Krishna, P. Enzyme-Free Colorimetric Nanosensor for the Rapid Detection of Lactic Acid in Food Quality Analysis. J. Agric. Food Res. 2022, 7, 100268. [Google Scholar] [CrossRef]
- Harvey, C.J.; LeBouf, R.F.; Stefaniak, A.B. Formulation and Stability of a Novel Artificial Human Sweat under Conditions of Storage and Use. Toxicol. In Vitro 2010, 24, 1790–1796. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, H.; Naw, H.P.P.; Lammy, A.V.; Goh, C.H.; Boujday, S.; Steele, T.W.J. Real-Time Colorimetric Hydration Sensor for Sport Activities. Mater. Des. 2016, 90, 1181–1185. [Google Scholar] [CrossRef]
- Petropoulos, K.; Piermarini, S.; Bernardini, S.; Palleschi, G.; Moscone, D. Development of a Disposable Biosensor for Lactate Monitoring in Saliva. Sens. Actuators B Chem. 2016, 237, 8–15. [Google Scholar] [CrossRef]
- Deng, H.-H.; Li, G.-W.; Hong, L.; Liu, A.-L.; Chen, W.; Lin, X.-H.; Xia, X.-H. Colorimetric Sensor Based on Dual-Functional Gold Nanoparticles: Analyte-Recognition and Peroxidase-like Activity. Food Chem. 2014, 147, 257–261. [Google Scholar] [CrossRef]
- Çelik, S.E.; Bekdeşer, B.; Apak, R. A Novel Colorimetric Sensor for Measuring Hydroperoxide Content and Peroxyl Radical Scavenging Activity Using Starch-Stabilized Gold Nanoparticles. Talanta 2019, 196, 32–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.-J.; Xia, X.-D.; Hou, X.-Q.; Feng, C.-T.; Wang, J.-X.; Deng, L. A Quantitative Colorimetric Assay of H2O2 and Glucose Using Silver Nanoparticles Induced by H2O2 and UV. Chin. Chem. Lett. 2013, 24, 1053–1058. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Wu, Q.; Li, K.; Tan, L. Application of Triangular Silver Nanoplates for Colorimetric Detection of H2O2. Sens. Actuators B Chem. 2015, 220, 314–317. [Google Scholar] [CrossRef]
- Liu, S.; Lu, F.; Xing, R.; Zhu, J.-J. Structural Effects of Fe3O4 Nanocrystals on Peroxidase-like Activity. Chem. A Eur. J. 2011, 17, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Puvvada, N.; Panigrahi, P.K.; Mandal, D.; Pathak, A. Shape Dependent Peroxidase Mimetic Activity towards Oxidation of Pyrogallol by H2O2. RSC Adv. 2012, 2, 3270–3273. [Google Scholar] [CrossRef]
- Tan, Y.; Deng, W.; Ge, B.; Xie, Q.; Huang, J.; Yao, S. Biofuel Cell and Phenolic Biosensor Based on Acid-Resistant Laccase–Glutaraldehyde Functionalized Chitosan–Multiwalled Carbon Nanotubes Nanocomposite Film. Biosens. Bioelectron. 2009, 24, 2225–2231. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, L.; Li, H.; Jia, Q.; Jiang, Y.; Yang, Y.; Zhu, R. One-Pot Synthesis of Porphyrin Functionalized γ-Fe2O3 Nanocomposites as Peroxidase Mimics for H2O2 and Glucose Detection. Mater. Sci. Eng. C 2015, 55, 193–200. [Google Scholar] [CrossRef]
- Su, L.; Feng, J.; Zhou, X.; Ren, C.; Li, H.; Chen, X. Colorimetric Detection of Urine Glucose Based ZnFe2O4 Magnetic Nanoparticles. Anal. Chem. 2012, 84, 5753–5758. [Google Scholar] [CrossRef]
- Lu, C.; Liu, X.; Li, Y.; Yu, F.; Tang, L.; Hu, Y.; Ying, Y. Multifunctional Janus Hematite–Silica Nanoparticles: Mimicking Peroxidase-like Activity and Sensitive Colorimetric Detection of Glucose. ACS Appl. Mater. Interfaces 2015, 7, 15395–15402. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. ZnO-Based Nanostructured Electrodes for Electrochemical Sensors and Biosensors in Biomedical Applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, H.; Rahman, Z.U.; Su, L.; Chen, X.; Hu, J.; Chen, X. Graphene Oxide–Fe3O4 Magnetic Nanocomposites with Peroxidase-like Activity for Colorimetric Detection of Glucose. Nanoscale 2012, 4, 3969–3976. [Google Scholar] [CrossRef]
- Ming, J.; Wu, Y.; Wang, L.; Yu, Y.; Zhao, F. CO2-Assisted Template Synthesis of Porous Hollow Bi-Phase γ-/α-Fe2O3 Nanoparticles with High Sensor Property. J. Mater. Chem. 2011, 21, 17776–17782. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, B.; Wang, Q.; Xing, S. Facile Synthesis of α-FeOOH/γ-Fe2O3 by a PH Gradient Method and the Role of γ-Fe2O3 in H2O2 Activation under Visible Light Irradiation. Chem. Eng. J. 2018, 354, 75–84. [Google Scholar] [CrossRef]
- Shooshtari, N.M.; Ghazi, M.M. An Investigation of the Photocatalytic Activity of Nano α-Fe2O3/ZnO on the Photodegradation of Cefixime Trihydrate. Chem. Eng. J. 2017, 315, 527–536. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Hong, M.; Su, Y.; Zhou, C.; Yao, L.; Hu, J.; Yang, Z.; Zhang, L.; Zhou, Z.; Hu, N.; Zhang, Y. Scalable Synthesis of γ-Fe2O3/CNT Composite as High-Performance Anode Material for Lithium-Ion Batteries. J. Alloys Compd. 2019, 770, 116–124. [Google Scholar] [CrossRef]
- Flak, D.; Chen, Q.; Mun, B.S.; Liu, Z.; Rękas, M.; Braun, A. In Snanoparticlesitu Ambient Pressure XPS Observation of Surface Chemistry and Electronic Structure of α-Fe2O3 and γ-Fe2O3. Appl. Surf. Sci. 2018, 455, 1019–1028. [Google Scholar] [CrossRef]
- Zhao, D.; Xiao, Y.; Wang, X.; Gao, Q.; Cao, M. Ultra-High Lithium Storage Capacity Achieved by Porous ZnFe2O4/α-Fe2O3 Micro-Octahedrons. Nano Energy 2014, 7, 124–133. [Google Scholar] [CrossRef]
- Yao, J.; Yan, J.; Huang, Y.; Li, Y.; Xiao, S.; Xiao, J. Preparation of ZnFe2O4/α-Fe2O3 Nanocomposites from Sulfuric Acid Leaching Liquor of Jarosite Residue and Their Application in Lithium-Ion Batteries. Front. Chem. 2018, 6, 442. [Google Scholar] [CrossRef]
- Li, X.Q.; Li, D.P.; Xu, J.C.; Han, Y.B.; Jin, H.X.; Hong, B.; Ge, H.L.; Wang, X.Q. Calcination-Temperature-Dependent Gas-Sensing Properties of Mesoporous α-Fe2O3 Nanowires as Ethanol Sensors. Solid State Sci. 2017, 69, 38–43. [Google Scholar] [CrossRef]
- Huang, B.; Tai, K.; Dillon, S.J. Structural Evolution of α-Fe2O3 Nanowires during Lithiation and Delithiation. J. Power Sources 2014, 245, 308–314. [Google Scholar] [CrossRef]
- Xie, X.; Yang, Y.; Xiao, Y.-H.; Huang, X.; Shi, Q.; Zhang, W.-D. Enhancement of Photoelectrochemical Activity of Fe2O3 Nanowires Decorated with Carbon Quantum Dots. Int. J. Hydrog. Energy 2018, 43, 6954–6962. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Qiu, Z.; Shi, C.; Li, X. A Facile Biomolecule-Assisted Approach for Fabricating α-Fe2O3 Nanowires in Solution. Solid State Commun. 2006, 140, 267–269. [Google Scholar] [CrossRef]
- Tadic, M.; Trpkov, D.; Kopanja, L.; Vojnovic, S.; Panjan, M. Hydrothermal Synthesis of Hematite (α-Fe2O3) Nanoparticle Forms: Synthesis Conditions, Structure, Particle Shape Analysis, Cytotoxicity and Magnetic Properties. J. Alloys Compd. 2019, 792, 599–609. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, L.; Li, M.; Hao, Y.; Lian, Y.; Li, Z.; Wei, Y. α-Fe2O3 Modified ZnO Flower-like Microstructures with Enhanced Photocatalytic Performance for Pentachlorophenol Degradation. Ceram. Int. 2015, 41, 9420–9425. [Google Scholar] [CrossRef]
- Bu, X.; Liu, F.; Zhang, Z.; Wang, Z.; Liu, J.; Liu, W. Facile Synthesis of Flower-like ZnO@Fe2O3 Hierarchical Nanostructures with Enhanced Catalytic Activity on the Thermal Decomposition of Ammonium Perchlorate. Mater. Lett. 2018, 219, 33–36. [Google Scholar] [CrossRef]
- Huang, L.; Fan, H. Room-Temperature Solid State Synthesis of ZnO/α-Fe2O3 Hierarchical Nanostructures and Their Enhanced Gas-Sensing Properties. Sens. Actuators B Chem. 2012, 171–172, 1257–1263. [Google Scholar] [CrossRef]
- Fu, R.; Wang, W.; Han, R.; Chen, K. Preparation and Characterization of γ-Fe2O3/ZnO Composite Particles. Mater. Lett. 2008, 62, 4066–4068. [Google Scholar] [CrossRef]
- Yan, W.; Fan, H.; Yang, C. Ultra-Fast Synthesis and Enhanced Photocatalytic Properties of Alpha-Fe2O3/ZnO Core-Shell Structure. Mater. Lett. 2011, 65, 1595–1597. [Google Scholar] [CrossRef]
- Typek, J.; Wardal, K.; Zolnierkiewicz, G.; Guskos, N.; Sibera, D.; Narkiewicz, U. Magnetic Study of 0.20(Fe2O3)/0.80(ZnO) Nanocomposite. J. Magn. Magn. Mater. 2014, 361, 12–18. [Google Scholar] [CrossRef]
- Si, S.; Li, C.; Wang, X.; Peng, Q.; Li, Y. Fe2O3/ZnO Core–Shell Nanorods for Gas Sensors. Sens. Actuators B Chem. 2006, 119, 52–56. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Wu, J.; Fang, T.; Cai, R.; Wei, A. Preparation and Photocatalytic Activity of ZnO/Fe2O3 Nanotube Composites. Mater. Sci. Eng. B 2015, 194, 9–13. [Google Scholar] [CrossRef]
- Li, Q.; Tang, G.; Xiong, X.; Cao, Y.; Chen, L.; Xu, F.; Tan, H. Carbon Coated Magnetite Nanoparticles with Improved Water-Dispersion and Peroxidase-like Activity for Colorimetric Sensing of Glucose. Sens. Actuators B Chem. 2015, 215, 86–92. [Google Scholar] [CrossRef]
- Dutta, A.K.; Maji, S.K.; Biswas, P.; Adhikary, B. New Peroxidase-Substrate 3,5-Di-Tert-Butylcatechol for Colorimetric Determination of Blood Glucose in Presence of Prussian Blue-Modified Iron Oxide Nanoparticles. Sens. Actuators B Chem. 2013, 177, 676–683. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, W.; Lu, Q.; Liu, M.; Chen, C.; Zhang, Y.; Yao, S. Colorimetric Detection of Hydrogen Peroxide and Lactate Based on the Etching of the Carbon Based Au-Ag Bimetallic Nanocomposite Synthesized by Carbon Dots as the Reductant and Stabilizer. Anal. Chim. Acta 2016, 947, 23–31. [Google Scholar] [CrossRef]
- Hou, W.; Liu, X.; Lu, Q.; Liu, M.; Zhang, Y.; Yao, S. Etching and Anti-Etching Strategy for Sensitive Colorimetric Sensing of H2O2 and Biothiols Based on Silver/Carbon Nanomaterial. Colloids Surf. B Biointerfaces 2018, 162, 118–125. [Google Scholar] [CrossRef]
- Su, C.K.; Li, T.W.; Sun, Y.C. Peroxidase-Mimicking PtNP-Coated, 3D-Printed Multi-Well Plate for Rapid Determination of Glucose and Lactate in Clinical Samples. Sens. Actuators B Chem. 2018, 269, 46–53. [Google Scholar] [CrossRef]
- Kim, H.; Park, I.; Pack, S.; Lee, G.; Hong, Y. Colorimetric Sensing of Lactate in Human Sweat Using Polyaniline Nanoparticles-Based Sensor Platform and Colorimeter. Biosensors 2022, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, Y.; Gholami, A.; Bordbar, M.M. A Distance-Based Paper Sensor for Rapid Detection of Blood Lactate Concentration Using Gold Nanoparticles Synthesized by Satureja Hortensis. Sens. Actuators B Chem. 2021, 345, 130445. [Google Scholar] [CrossRef]
- Alipour, N.; Namazi, H. Chelating ZnO-Dopamine on the Surface of Graphene Oxide and Its Application as PH-Responsive and Antibacterial Nanohybrid Delivery Agent for Doxorubicin. Mater. Sci. Eng. C 2020, 108, 110459. [Google Scholar] [CrossRef]
- Seddik, B.; Salima, B.; Houda, G. Fe Doped ZnO Nanostructures Prepared via Sol-Gel Dip-Coating Technique for Iso-Butane (i-C4H10) Sensing. Mater. Today Commun. 2021, 29, 102805. [Google Scholar] [CrossRef]
- Yüzer, E.; Doğan, V.; Kılıç, V.; Şen, M. Smartphone Embedded Deep Learning Approach for Highly Accurate and Automated Colorimetric Lactate Analysis in Sweat. Sens. Actuators B Chem. 2022, 371, 132489. [Google Scholar] [CrossRef]
- Shitanda, I.; Mitsumoto, M.; Loew, N.; Yoshihara, Y.; Watanabe, H.; Mikawa, T.; Tsujimura, S.; Itagaki, M.; Motosuke, M. Continuous Sweat Lactate Monitoring System with Integrated Screen-Printed Mgo-Templated Carbon-Lactate Oxidase Biosensor and Microfluidic Sweat Collector. Electrochim. Acta 2021, 368, 137620. [Google Scholar] [CrossRef]
- Zhu, D.D.; Zheng, L.W.; Duong, P.K.; Cheah, R.H.; Liu, X.Y.; Wong, J.R.; Wang, W.J.; Tien Guan, S.T.; Zheng, X.T.; Chen, P. Colorimetric Microneedle Patches for Multiplexed Transdermal Detection of Metabolites. Biosens. Bioelectron. 2022, 212, 114412. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lu, F.; Liu, Y.; Ning, Y.; Tian, S.; Zuo, P.; Ji, X.; He, Z. Quantum Dots-Based Hydrogel Microspheres for Visual Determination of Lactate and Simultaneous Detection Coupled with Microfluidic Device. Microchem. J. 2021, 171, 106801. [Google Scholar] [CrossRef]
- Mengarda, P.; Dias, F.A.L.; Peixoto, J.V.C.; Osiecki, R.; Bergamini, M.F.; Marcolino-Junior, L.H. Determination of Lactate Levels in Biological Fluids Using a Disposable Ion-Selective Potentiometric Sensor Based on Polypyrrole Films. Sens. Actuators B Chem. 2019, 296, 126663. [Google Scholar] [CrossRef]
- Derbyshire, P.J.; Barr, H.; Davis, F.; Higson, S.P.J. Lactate in Human Sweat: A Critical Review of Research to the Present Day. J. Physiol. Sci. 2012, 62, 429–440. [Google Scholar] [CrossRef]
- Lin, C.E.; Hiraka, K.; Matloff, D.; Johns, J.; Deng, A.; Sode, K.; La Belle, J. Development toward a Novel Integrated Tear Lactate Sensor Using Schirmer Test Strip and Engineered Lactate Oxidase. Sens. Actuators B Chem. 2018, 270, 525–529. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Dong, Y.; Gong, J.; Li, Z.; Zhang, J. Core-Shell Structured Gold Nanorods on Thread-Embroidered Fabric-Based Microfluidic Device for Ex Situ Detection of Glucose and Lactate in Sweat. Sens. Actuators B Chem. 2022, 353, 131154. [Google Scholar] [CrossRef]
- Promphet, N.; Rattanawaleedirojn, P.; Siralertmukul, K.; Soatthiyanon, N.; Potiyaraj, P.; Thanawattano, C.; Hinestroza, J.P.; Rodthongkum, N. Non-Invasive Textile Based Colorimetric Sensor for the Simultaneous Detection of Sweat PH and Lactate. Talanta 2019, 192, 424–430. [Google Scholar] [CrossRef]
- Ogata, K.; Dobashi, H.; Koike, K.; Sasa, S.; Inoue, M.; Yano, M. Patterned Growth of ZnO Nanorods and Enzyme Immobilization toward the Fabrication of Glucose Sensors. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 42, 2880–2883. [Google Scholar] [CrossRef]



| Sample | Estimated Lactate Concentration-mmol L−1 | Lactate Concentration-mmol L−1 (Lactometer) | Relative Error % | ||
|---|---|---|---|---|---|
| LOx/γ-/α-Fe2O3 | LOx/γ-/α-Fe2O3/ZnO | LOx/γ-/α-Fe2O3 | LOx/γ-/α-Fe2O3/ZnO | ||
| 1 | 6.01 | 6.48 | 6.4 | 6.1 | 1.3 |
| 2 | 5.9 | 6.26 | 6.4 | 7.8 | 2.2 |
| 3 | 8.25 | 8.42 | 8.7 | 5.2 | 3.2 |
| 4 | 6.77 | 6.75 | 6.9 | 1.9 | 2.2 |
| 5 | 4.66 | 5.14 | 5.4 | 13.7 | 4.8 |
| 6 | 5.17 | 5.89 | 5.7 | 9.3 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escalona-Villalpando, R.A.; Viveros-Palma, K.; Espinosa-Lagunes, F.I.; Rodríguez-Morales, J.A.; Arriaga, L.G.; Macazo, F.C.; Minteer, S.D.; Ledesma-García, J. Comparative Colorimetric Sensor Based on Bi-Phase γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO Nanoparticles for Lactate Detection. Biosensors 2022, 12, 1025. https://doi.org/10.3390/bios12111025
Escalona-Villalpando RA, Viveros-Palma K, Espinosa-Lagunes FI, Rodríguez-Morales JA, Arriaga LG, Macazo FC, Minteer SD, Ledesma-García J. Comparative Colorimetric Sensor Based on Bi-Phase γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO Nanoparticles for Lactate Detection. Biosensors. 2022; 12(11):1025. https://doi.org/10.3390/bios12111025
Chicago/Turabian StyleEscalona-Villalpando, Ricardo A., Karen Viveros-Palma, Fabiola I. Espinosa-Lagunes, José A. Rodríguez-Morales, Luis G. Arriaga, Florika C. Macazo, Shelley D. Minteer, and Janet Ledesma-García. 2022. "Comparative Colorimetric Sensor Based on Bi-Phase γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO Nanoparticles for Lactate Detection" Biosensors 12, no. 11: 1025. https://doi.org/10.3390/bios12111025
APA StyleEscalona-Villalpando, R. A., Viveros-Palma, K., Espinosa-Lagunes, F. I., Rodríguez-Morales, J. A., Arriaga, L. G., Macazo, F. C., Minteer, S. D., & Ledesma-García, J. (2022). Comparative Colorimetric Sensor Based on Bi-Phase γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO Nanoparticles for Lactate Detection. Biosensors, 12(11), 1025. https://doi.org/10.3390/bios12111025