An Electrochemical Sensor for the Detection of Albendazole Using Glassy Carbon Electrode Modified with Platinum-Palladium Nanocomposites
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Chemicals
2.2. Instruments
2.3. Measurements and Procedure
2.4. Pt-Pd Catalyst Preparation and Glassy Carbon Electrode Modification
2.5. Pharmaceutical Samples Preparation
2.6. Animal Products Preparation
3. Results and Discussion
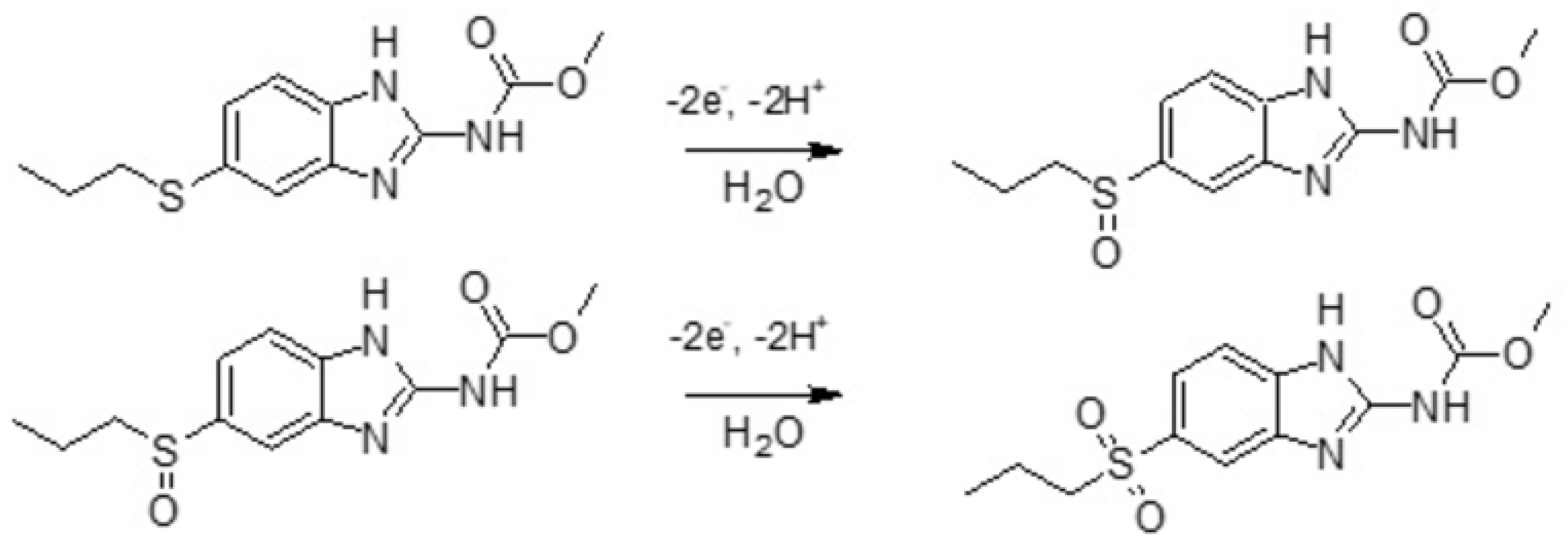
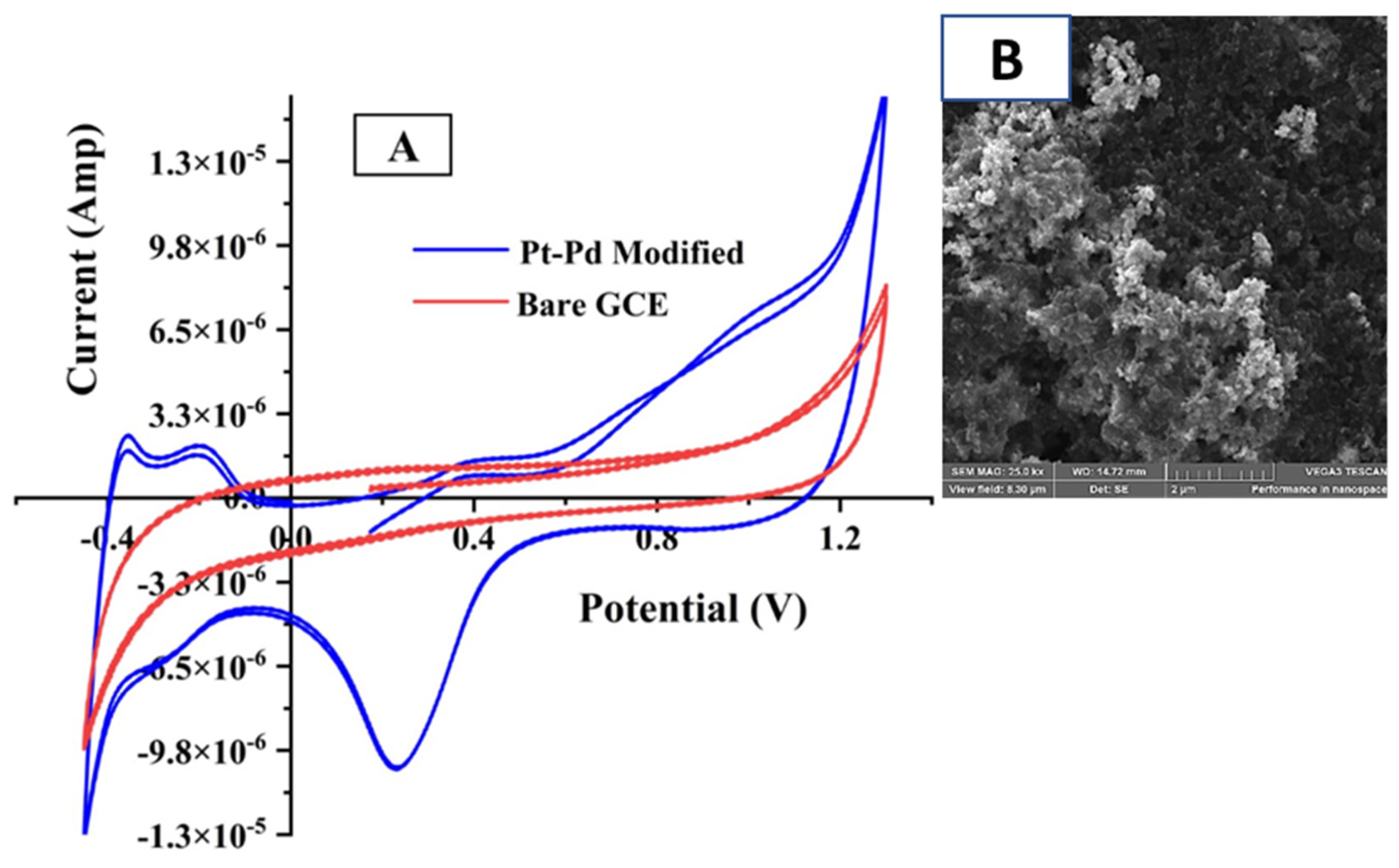
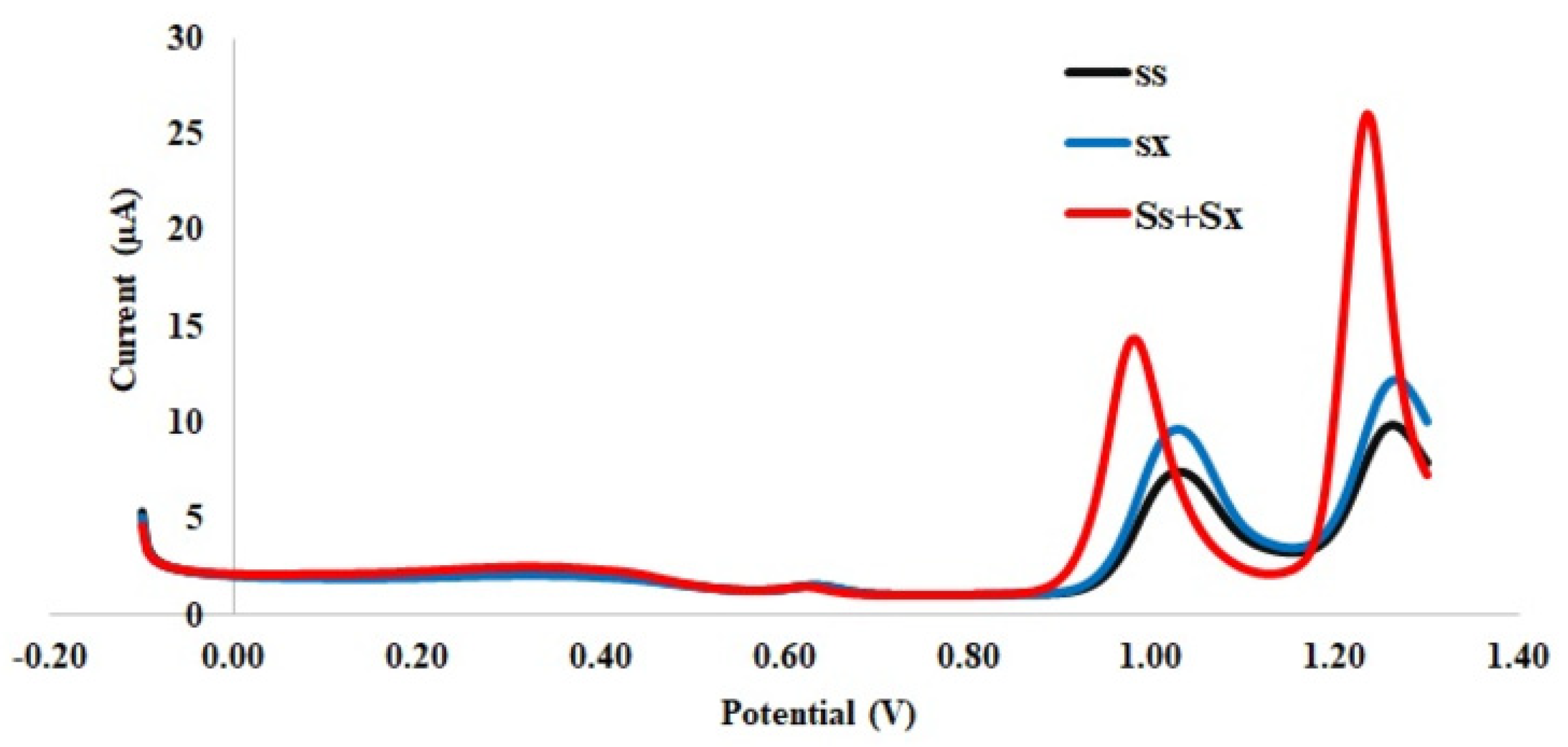
3.1. Electrochemical Behavior of the Pt-Pd-Modified Glassy Carbon Electrode
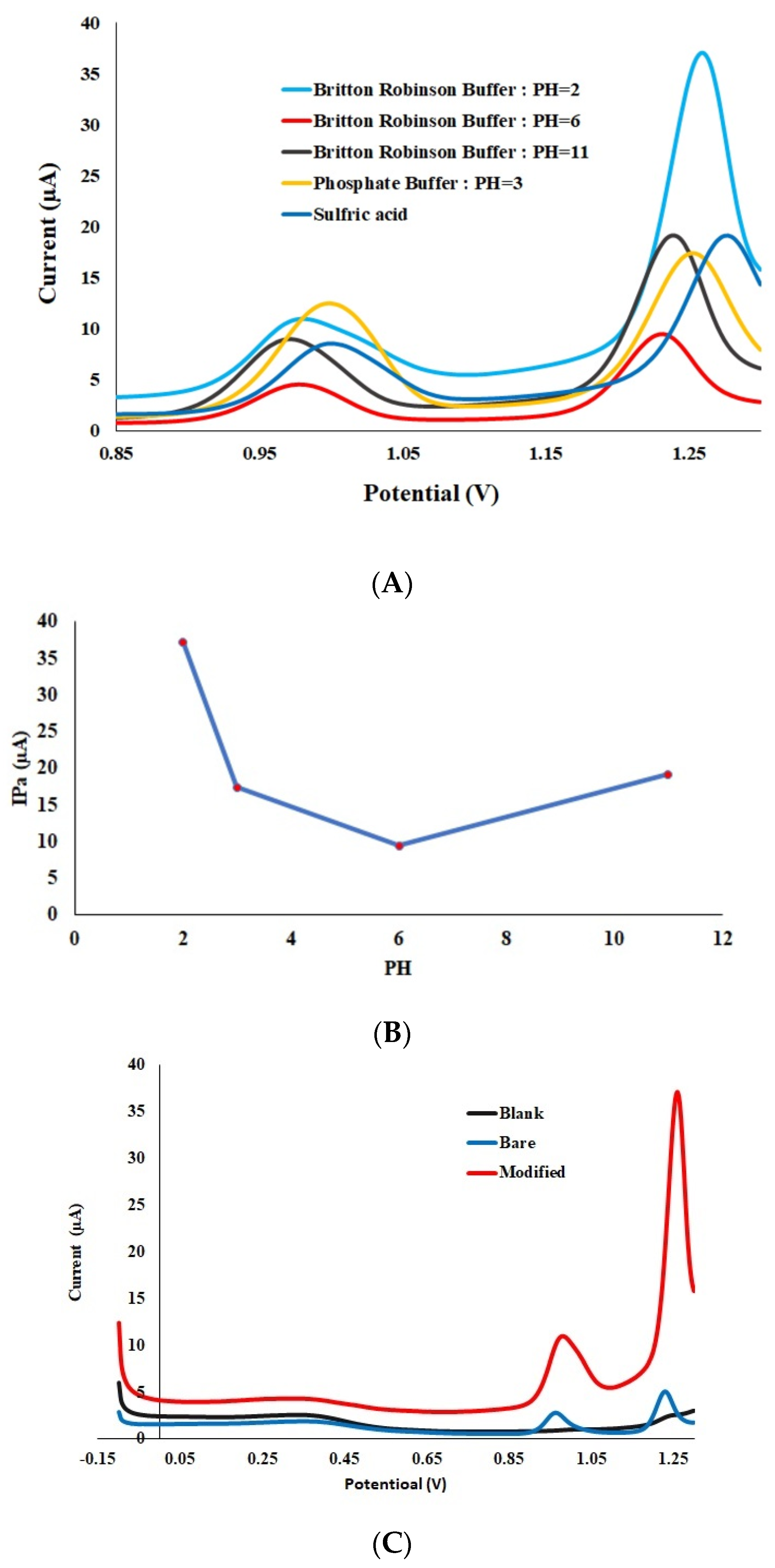
3.2. Effect of Different Electrolytes Compositions and pH Values on the Voltammetric Response of the Pt-Pd-Modified GCE
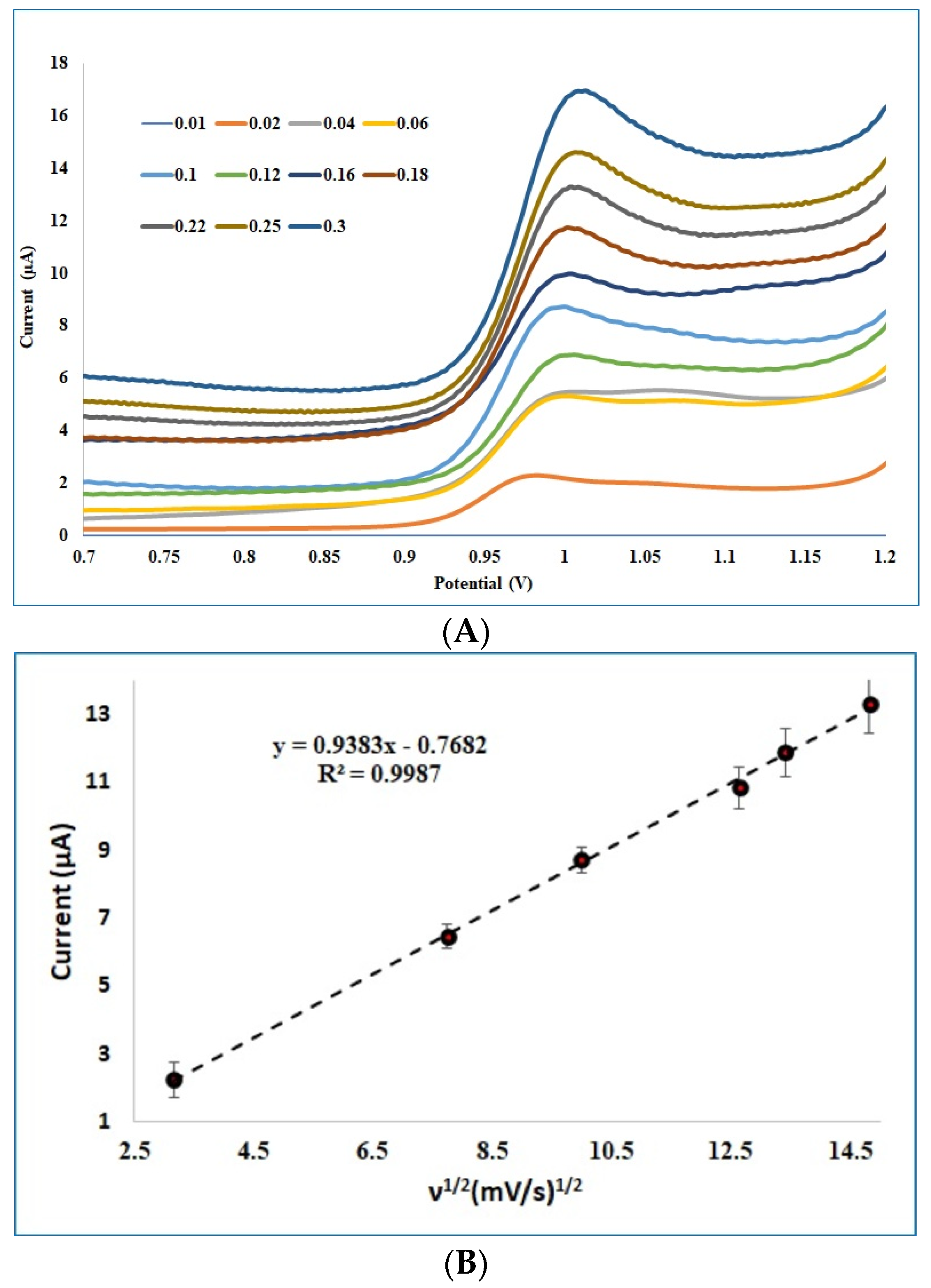
3.3. Effect of Scan Rate on ABZ Detection at the Pt-Pd GCE
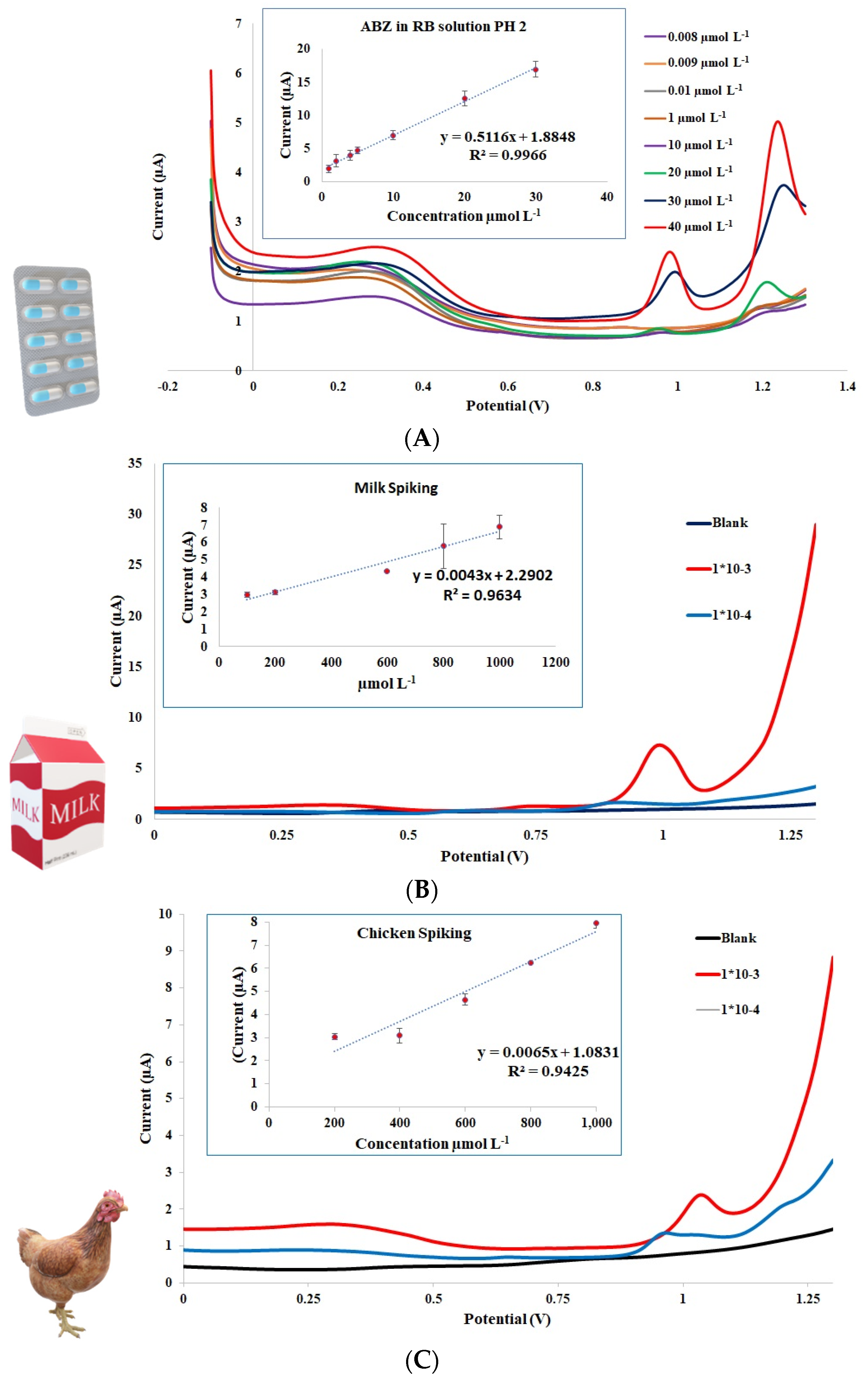
3.4. Analytical Performance of ABZ Detection Using Pt-Pd-Modified GCE
3.5. Determination of ABZ in Local Pharmaceuticals Dosage Form
3.6. Detection of ABZ Contaminated in Animal Product Matrices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palit, R.; Kumar, R.; Saraswat, N.; Wal, A.; Upadhyaya, P. Benzimidazole: An Overview. Int. J. Res. Ayurveda Pharm. 2017, 7, 68–73. [Google Scholar] [CrossRef]
- Yadav, G.; Ganguly, S. Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review. Eur. J. Med. Chem. 2015, 97, 419–443. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.-S.; Lee, S.-E.; Ng, C.L.; Kim, J.-K.; Park, J.-S. Exploring the Preparation of Albendazole-Loaded Chitosan-Tripolyphosphate Nanoparticles. Materials 2015, 8, 486–498. [Google Scholar] [CrossRef]
- Safarpour, H.; Dehghani, S.; Nosrati, R.; Zebardast, N.; Alibolandi, M.; Mokhtarzadeh, A.; Ramezani, M. Optical and electrochemical-based nano-aptasensing approaches for the detection of circulating tumor cells (CTCs). Biosens. Bioelectron. 2020, 148, 111833. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, A.; Stadelmann, B.; Scholl, S.; Müller, J.; Spiliotis, M.; Müller, N.; Gottstein, B.; Siles-Lucas, M. Echinococcusmetacestodes as laboratory models for the screening of drugs against cestodes and trematodes. Parasitology 2010, 137, 569–587. [Google Scholar] [CrossRef]
- Horton, J. Albendazole: A review of anthelmintic efficacy and safety in humans. Parasitology 2000, 121, S113–S132. [Google Scholar] [CrossRef]
- Venkatesan, P. Albendazole. J. Antimicrob. Chemother. 1998, 41, 145–147. [Google Scholar] [CrossRef]
- Piloiu, C.; Dumitrascu, D.L. Albendazole-Induced Liver Injury. Am. J. Ther. 2021, 28, e335–e340. [Google Scholar] [CrossRef]
- Carlsson, G.; Patring, J.; Ullerås, E.; Oskarsson, A. Developmental toxicity of albendazole and its three main metabolites in zebrafish embryos. Reprod. Toxicol. 2011, 32, 129–137. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Maximum residue limits for veterinary drugs in foods. In Proceedings of the 35th Session of the Codex Alimentarius Commission, Rome, Italy, 2–7 July 2012. [Google Scholar]
- European Commission. Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, 15, 1–72. [Google Scholar]
- He, Z.; Diao, Z.; Guo, Y.; Xie, K.; Chen, L.; Xue, C.; Lu, Y.; Chen, J.; Zhang, T. Simultaneous Determination of Albendazole and Its Three Metabolites in Pig and Poultry Muscle by Ultrahigh-Performance Liquid Chromatography-Fluorescence Detection. Foods 2021, 10, 2350. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Castillo, D.; Hernández, E.D.L.C.; Márquez, D.F.; Tilley, R.; Gloag, L.; Owen, P.; González, R.L.; Lemus, M.A. Albendazole Release from Silica-Chitosan Nanospheres. In Vitro Study on Cervix Cancer Cell Lines. Polymers 2021, 13, 1945. [Google Scholar] [CrossRef] [PubMed]
- Laurentis, N.d.; Milillo, M.A.; Bruno, S. Determination of albendazole as raw material and in tablets by nonaqueous titration with sodium methylate solution. Rev. Farm. Bioquim. Univ. Säo Paulo 1997, 33, 23–27. [Google Scholar]
- Basavaiah, K.; Prameela, H.C. Use of an oxidation reaction for the quantitative determination of albendazole with chloramine-T and acid dyes. Anal. Sci. 2003, 19, 779–784. [Google Scholar] [CrossRef][Green Version]
- Basavaiah, K.; Prameela, H.C. Titrimetric and spectrophotometric determination of albendazole with bromate and methyl orange. Oxid. Commun. 2004, 27, 177–185. [Google Scholar]
- Basavaiah, K.; Ramakrishna, V.; Somashekar, B.C. Assay of albendazole using N-bromosuccinimide. Indian Pharm. 2006, 5, 129–136. [Google Scholar]
- Brandon, D.L.; Binder, R.G.; Bates, A.H.; Montague, W.C., Jr. Monoclonal antibody for multiresidue ELISA of benzimidazole anthelmintics in liver. J. Agric. Food Chem. 1994, 42, 1588–1594. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Zhang, H.; Guo, Y.; Dai, Z.; Chen, X. Simultaneous determination of albendazole and its metabolites in fish muscle tissue by stable isotope dilution ultra-performance liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 727–734. [Google Scholar] [CrossRef]
- Hu, X.-Z.; Wang, J.-X.; Feng, Y.-Q. Determination of Benzimidazole Residues in Edible Animal Food by Polymer Monolith Microextraction Combined with Liquid Chromatography−Mass Spectrometry. J. Agric. Food Chem. 2010, 58, 112–119. [Google Scholar] [CrossRef]
- Chiap, P.; Evrard, B.; Bimazubute, M.; De Tullio, P.; Hubert, P.; Delattre, L.; Crommen, J. Determination of albendazole and its main metabolites in ovine plasma by liquid chromatography with dialysis as an integrated sample preparation technique. J. Chromatogr. A 2000, 870, 121–134. [Google Scholar] [CrossRef]
- Yu, D.; Rummel, N.; Shaikh, B. Development of a method to determine albendazole and its metabolites in the muscle tissue of yellow perch using high-performance liquid chromatography with fluorescence detection. J. AOAC Int. 2011, 94, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Suaifan, G.A.R.Y.; Esseghaier, C.; Ng, A.; Zourob, M. Wash-less and highly sensitive assay for prostate specific antigen detection. Anal. 2012, 137, 5614–5619. [Google Scholar] [CrossRef] [PubMed]
- Revanappa, S.K.; Soni, I.; Siddalinganahalli, M.; Jayaprakash, G.K.; Flores-Moreno, R.; Nanjegowda, C.B. A Fukui Analysis of an Arginine-Modified Carbon Surface for the Electrochemical Sensing of Dopamine. Materials 2022, 15, 6337. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2017/852 of the European Parliament and of the Council of 17 May 2017 on mercury, and repealing Regulation (EC) No 1102/2008. 2017. Available online: https://www.legislation.gov.uk/eur/2017/852/contents# (accessed on 2 October 2022).
- Jakubczyk, M.; Michalkiewicz, S. First voltammetric method of phenoxyethanol determination in pharmaceutical and cosmetic preparations. J. Electrochem. Soc. 2019, 166, H291. [Google Scholar] [CrossRef]
- Kaur, R.; Kushwaha, J.P.; Singh, N. Amoxicillin electro-catalytic oxidation using Ti/RuO2 anode: Mechanism, oxidation products and degradation pathway. Electrochim. Acta 2019, 296, 856–866. [Google Scholar] [CrossRef]
- Sahoo, S.; Dekel, D.R.; Maric, R.; Alpay, S.P. Atomistic Insights into the Hydrogen Oxidation Reaction of Palladium-Ceria Bifunctional Catalysts for Anion-Exchange Membrane Fuel Cells. ACS Catal. 2021, 11, 2561–2571. [Google Scholar] [CrossRef]
- Chang, R.; Zheng, L.; Wang, C.; Yang, D.; Zhang, G.; Sun, S. Synthesis of hierarchical platinum-palladium-copper nanodendrites for efficient methanol oxidation. Appl. Catal. B Environ. 2017, 211, 205–211. [Google Scholar] [CrossRef]
- Santos, A.L.; Takeuchi, R.M.; Mariotti, M.P.; De Oliveira, M.F.; Zanoni, M.V.; Stradiotto, N.R. Study of electrochemical oxidation and determination of albendazole using a glassy carbon-rotating disk electrode. Il Farm. 2005, 60, 671–674. [Google Scholar] [CrossRef]
- Msagati, T.A.; Ngila, C.J. Voltammetric determination of a benzimidazole anthelmintic mixture at a poly (3-methylthiophene)-modified glassy carbon electrode. S. Afr. J. Chem. 2003, 56, 5–9. [Google Scholar]
- Suaifan, G.A.; Alhogail, S.; Zourob, M. Paper-based magnetic nanoparticle-peptide probe for rapid and quantitative colorimetric detection of Escherichia coli O157:H7. Biosens. Bioelectron. 2017, 92, 702–708. [Google Scholar] [CrossRef]
- Suaifan, G.; Alhogail, S.; Zourob, M. Rapid and low-cost biosensor for the detection of Staphylococcus aureus. Biosens. Bioelectron. 2017, 90, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, H.; Eid, K.; Alshehri, S.M.; Malgras, V.; Yamauchi, Y.; Wang, L. One-step synthesis of dendritic bimetallic PtPd nanoparticles on reduced graphene oxide and its electrocatalytic properties. Electrochim. Acta. 2016, 188, 845–851. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, G.; Shu, H.; Oyama, M.; Liu, X.; He, Y. Synthesis of Pt–Pd bimetallic nanoparticles anchored on graphene for highly active methanol electro-oxidation. J. Power Sources 2014, 262, 279–285. [Google Scholar] [CrossRef]
- Qi, H.; Song, J.; Fu, Y.; Wu, X.; Qi, H. Highly dispersive Pt–Pd nanoparticles on graphene oxide sheathed carbon fiber microelectrodes for electrochemical detection of H2O2 released from living cells. Nanotechnology 2020, 31, 135503. [Google Scholar] [CrossRef] [PubMed]
- Pletcher, D.; Greff, R.; Peat, R.; Peter, L.M.; Robinson, J. Instrumental Methods in Electrochemistry; Ellis Horwood Ltd.: Chichester, UK, 1985. [Google Scholar]
- O’Dea, J.J.; Ribes, A.; Osteryoung, J.G. Square-wave voltammetry applied to the totally irreversible reduction of adsorbate. J. Electroanal. 1999, 345, 287–301. [Google Scholar] [CrossRef]
- De Macedo, I.Y.L.; Garcia, L.F.; de Souza, A.R.; da Silva, A.M.L.; Fernandez, C.; Santos, M.D.G.; Magalhaes, R.S.; Torres, I.M.S.; de Souza Gil, E. Differential pulse voltammetric determination of albendazole and mebendazole in pharmaceutical formulations based on modified sonogel carbon paste electrodes with perovskite-type LaFeO3 nanoparticles. J. Electrochem. Soc. 2016, 163, B428. [Google Scholar] [CrossRef]
- Jadali, S.; Kamyabi, M.A.; Solla-Gullón, J.; Herrero, E. Effect of pd on the electrocatalytic activity of pt towards oxidation of ethanol in alkaline solutions. Appl. Sci. 2021, 11, 1315. [Google Scholar] [CrossRef]
- Basu, D.; Basu, S. Performance studies of Pd–Pt and Pt–Pd–Au catalyst for electro-oxidation of glucose in direct glucose fuel cell. Int. J. Hydrog. 2012, 37, 4678–4684. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, L.; Chai, Y.; Yuan, R. Electrochemistry of cholesterol biosensor based on a novel Pt–Pd bimetallic nanoparticle decorated graphene catalyst. Talanta 2013, 109, 167–172. [Google Scholar] [CrossRef]
- Chen, K.-J.; Pillai, K.C.; Rick, J.; Pan, C.-J.; Wang, S.-H.; Liu, C.-C.; Hwang, B.-J. Bimetallic PtM (M=Pd, Ir) nanoparticle decorated multi-walled carbon nanotube enzyme-free, mediator-less amperometric sensor for H2O2. Biosens. Bioelectron. 2012, 33, 120–127. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Z.; Liu, J.; Wang, C. Pt-Pd bimetallic nanoparticles decorated nanoporous graphene as a catalytic amplification platform for electrochemical detection of xanthine. Electroanalysis 2017, 29, 1258–1266. [Google Scholar] [CrossRef]
- Küçükkolbaşı. S; Gündüz, B; Kılıç, E. Development of a spectrofluorimetric method for determination of albendazole in tablets. Anal. Lett. 2008, 41, 104–118. [Google Scholar] [CrossRef]
- Atkoşar, Z.; Altiokka, G. The Determination of Albendazole by Flow Injection Analysis Method Using UV-Detection and HPLC Method in Suspensions. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 849–856. [Google Scholar] [CrossRef]
- Swamy, N.; Basavaiah, K. Simple and rapid spectrophotometric assay of albendazole in pharmaceuticals using iodine and picric acid as CT complexing agents. Braz. J. Pharm. Sci. 2014, 50, 839–850. [Google Scholar] [CrossRef]
- Wu, Z.; Medlicott, N.J.; Razzak, M.; Tucker, I.G. Development and optimization of a rapid HPLC method for analysis of ricobendazole and albendazole sulfone in sheep plasma. J. pharm. biomed. 2005, 39, 225–232. [Google Scholar] [CrossRef]
- Waldia, A.; Gupta, S.; Issarani, R.; Nagori, B.P. Validated liquid chromatographic method for simultaneous estimation of albendazole and ivermectin in tablet dosage form. Indian J. Chem. Technol. 2008, 15, 617–620. [Google Scholar]
- Vignaduzzo, S.E.; Operto, M.A.; Castellano, P.M. Development and validation of a dissolution test method for albendazole and praziquantel in their combined dosage form. J. Braz. Chem. Soc. 2015, 26, 729–735. [Google Scholar] [CrossRef]
- Shah, S.R.; Dey, S.; Pradhan, P.; Jain, H.; Upadhyay, U.M. Method development and validation for simulataneous estimation of albendazole and praziquantel in bulk and in a synthetic mixture. J. Taibah Univ. Med. Sci. 2014, 8, 54–63. [Google Scholar] [CrossRef]
- De Oliveira, M.F.; Stradiotto, N.R. Voltammetric assay of albendazole in pharmaceutical dosage forms. Anal. Lett. 2001, 34, 377–387. [Google Scholar] [CrossRef]
- Abu Zuhri, A.Z.; Hussein, A.I.; Musmar, M.; Yaish, S. Adsorptive stripping voltammetric determination of albendazole at a hanging mercury drop electrode. Anal. Lett. 1999, 2965–2975. [Google Scholar] [CrossRef]
- Gowda, J.I.; Hurakadli, G.S.; Nandibewoor, S.T. Pretreated graphite pencil electrode based voltammetric sensing of albendazole. Anal. Chem. 2017, 7, 389–401. [Google Scholar] [CrossRef]
- Mersal, G.A.; Alharthi, S.S.; Al Maliki, N.A.; Zayed, S.I. Electrochemical Behavior and Voltammetric Determination of Albendazole Using Carbon Paste Electrode. Int. J. Electrochem. 2021, 16. [Google Scholar]
- Lourencao, B.C.; Baccarin, M.; Medeiros, R.A.; Rocha-Filho, R.C.; Fatibello-Filho, O. Differential pulse voltammetric determination of albendazole in pharmaceutical tablets using a cathodically pretreated boron-doped diamond electrode. J. Electroanal. Chem. 2013, 707, 15–19. [Google Scholar] [CrossRef]
- Hughes, G.; Westmacott, K.; Honeychurch, K.C.; Crew, A.; Pemberton, R.M.; Hart, J.P. Recent advances in the fabrication and application of screen-printed electrochemical (bio) sensors based on carbon materials for biomedical, agri-food and environmental analyses. Biosensors 2016, 6, 50. [Google Scholar] [CrossRef]


| Detection Method | LOD (μg mL−1) * | Reference |
|---|---|---|
| Titrimetric and spectrophotometric methods | 0.237 μg mL−1 & 0.711 μg mL−1 | [17] |
| Spectrophotometric | 7.42 & 9.94 μg mL−1 | [45] |
| Flow injection analysis (FIA) using UV detection & HPLC method | 0.00318 μg mL−1 (FIA), 0.00297 μg mL−1 (HPLC) | [46] |
| Pectrophotometric (2 methods) | 0.69 & 0.10 μg mL−1 0.001438 μg mL−1 | [47] |
| RP-HPLC–Mobile Phase (Methanol:acetonitrile:phosphate buffer (22:22:56)) | 3 ng mL−1 | [48] |
| RP-HPLC–Mobile Phase (Acetonitrile:methanol:water (60:30:10)) | N.A. | [49] |
| RP-HPLC–Mobile Phase (Acetonitrile:phosphate buffer (50:50)) | 0.001 μg mL−1 | [50] |
| RP-HPLC–Mobile Phase (Acetonitrile:water:phosphate buffer (60:40:10)) | 0.0167 μg mL−1 | [51] |
| Voltammetric method linear sweep (LSV), square-wave (SWV) and differential pulse voltammetry (DPV) | 7.95 μg mL−1 (LSV), 16.45 μg mL−1 (SWV), 10.61 μg mL−1 (DPV) | [52] |
| Differential pulse cathodic stripping voltammetry (DPCSV) at the hanging drop mercury electrode (HMDE) | 0.003 μg mL−1 | [53] |
| Pencil graphite electrode Voltammetry | 0.001438 μg mL−1 | [54] |
| Modified sonogel with perovskite-type LaFeO3 nanoparticles | 79.6 μg mL−1 | [39] |
| Carbon paste electrode | 0.056 μg mL−1 | [55] |
| Cathodically pretreated at the boron doped diamond electrode | 16.5 μg mL-1 (DPV), 43 μg mL−1 (SWV) | [56] |
| Electrochemical oxidation at the glassy carbon-rotating disk electrode | 6.367 μg mL−1 | [30] |
| Current study | 21.7 μg mL−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suaifan, G.A.R.Y.; Khanfar, M.F.; Shehadeh, M.B.; Alnajajrah, A.; Abuhamdan, R.; Hasan, S.A. An Electrochemical Sensor for the Detection of Albendazole Using Glassy Carbon Electrode Modified with Platinum-Palladium Nanocomposites. Biosensors 2022, 12, 1026. https://doi.org/10.3390/bios12111026
Suaifan GARY, Khanfar MF, Shehadeh MB, Alnajajrah A, Abuhamdan R, Hasan SA. An Electrochemical Sensor for the Detection of Albendazole Using Glassy Carbon Electrode Modified with Platinum-Palladium Nanocomposites. Biosensors. 2022; 12(11):1026. https://doi.org/10.3390/bios12111026
Chicago/Turabian StyleSuaifan, Ghadeer A. R. Y., Mohammad F. Khanfar, Mayadah B. Shehadeh, Asmaa Alnajajrah, Raghad Abuhamdan, and Sameer Ahmad Hasan. 2022. "An Electrochemical Sensor for the Detection of Albendazole Using Glassy Carbon Electrode Modified with Platinum-Palladium Nanocomposites" Biosensors 12, no. 11: 1026. https://doi.org/10.3390/bios12111026
APA StyleSuaifan, G. A. R. Y., Khanfar, M. F., Shehadeh, M. B., Alnajajrah, A., Abuhamdan, R., & Hasan, S. A. (2022). An Electrochemical Sensor for the Detection of Albendazole Using Glassy Carbon Electrode Modified with Platinum-Palladium Nanocomposites. Biosensors, 12(11), 1026. https://doi.org/10.3390/bios12111026






