Abstract
This review highlights the recent advancements in the field of nanozymes and their applications in the development of point-of-care biosensors. The use of nanozymes as enzyme-mimicking components in biosensing systems has led to improved performance and miniaturization of these sensors. The unique properties of nanozymes, such as high stability, robustness, and surface tunability, make them an attractive alternative to traditional enzymes in biosensing applications. Researchers have explored a wide range of nanomaterials, including metals, metal oxides, and metal–organic frameworks, for the development of nanozyme-based biosensors. Different sensing strategies, such as colorimetric, fluorescent, electrochemical and SERS, have been implemented using nanozymes as signal-producing components. Despite the numerous advantages, there are also challenges associated with nanozyme-based biosensors, including stability and specificity, which need to be addressed for their wider applications. The future of nanozyme-based biosensors looks promising, with the potential to bring a paradigm shift in biomolecular sensing. The development of highly specific, multi-enzyme mimicking nanozymes could lead to the creation of highly sensitive and low-biofouling biosensors. Integration of nanozymes into point-of-care diagnostics promises to revolutionize healthcare by improving patient outcomes and reducing costs while enhancing the accuracy and sensitivity of diagnostic tools.
Keywords:
nanozyme; point of care; peroxidase; oxidase; SOD; catalase; colorimetric; fluorescence; electrochemical; SERS 1. Introduction
Recent years have seen a surge in interest in early disease detection as a new area of medical research due to its apparent ability to reduce mortality rates and raise survival rates. Modern research progress has played a significant role in sustaining high levels of healthcare and general well-being. Although conventional technologies like real-time polymerase chain reaction (RT-PCR) [1,2], enzyme-linked immunosorbent assay (ELISA) [3], high-performance liquid chromatography (HPLC) [4], gas chromatography–mass spectrometry (GCMS) [5], and so on demonstrate high sensitivity and accuracy, the complex and costly equipment required could cause a delay in response time. This highlights the essential need in biosensor and bioassay research for the development of rapid, portable, and user-friendly assays that could accelerate the diagnostic process and alleviate treatment delay [6,7]. Point-of-care (POC) testing is a catch-all word for diagnostic treatments that are conducted directly at the site of a patient.
Consequently, there has been an effort to develop advanced biosensing technologies that can accurately detect biomarkers, the naturally occurring molecules that indicate the presence of a disease and can be utilized to monitor disease outbreaks and facilitate timely diagnosis [8]. When a patient’s blood, serum, urine, saliva, or tears are placed on a biosensor’s surface, the target biomarker reacts with the bioreceptor (an enzyme, an antibody, a protein receptor, DNA, or whole cells) attached to the sensor, and the presence or absence of disease is determined based on the resulting signal change. Bio-threat agents, chemical contaminants, toxins, bio-molecular targets, and pathogens are all within the detection range of biosensors [9,10,11]. Over the past decade, many biosensors have emerged as viable complementary or alternative detection equipment to traditional methods, allowing for faster and more precise detection in the aforementioned areas of study.
Enzymes play the role of catalysts, speeding up the biochemical processes that take place in the biological molecules [12]. While proteins are typically thought of as enzymes, some RNA molecules have enzymatic activity, and both of them exhibit remarkable efficiency and substrate specificity. By lowering the activation energy needed for a reaction to take place, enzymes speed up chemical processes. Due to their exceptional biocatalytic activity, natural enzymes have been widely used as a key component, the bioreceptor in the development of point-of-care biosensors [13]. The biological component of a biosensor interacts with the target analyte to provide a signal that is proportional to the concentration of the analyte. The transducer turns this signal into a measured output that can be shown on a readout or sent to a computer for analysis [9]. Natural enzymes such as horseradish peroxidase (HRP) [14], glucose oxidase [15], alcohol oxidase [16], lactate oxidase [17], cholesterol oxidase [17], cytochrome c reductase [18], and acetylcholinesterase [19] have been used as the bioreceptor in biosensing applications. Variations in environmental factors, such as temperature, pH, and ionic strength, can diminish the enzyme’s stability and activity over time [20]. Enzymes can also be difficult to store effectively and may require specialized storage conditions to preserve their activity and they may also have a short shelf life, leading to a brief lifespan for biosensors that employ them. Enzymes may also be susceptible to interference from other substances in the sample, resulting in either false-positive or false-negative results [19]. Natural enzymes have limited use in fields such as biomedicine, environmental protection, biosensing, and food processing due to the aforementioned limitations. As a result, researchers have devoted enormous resources to the study of artificial enzyme mimics to circumvent these restrictions. Current developments in nanotechnology have led to the development of functional nanomaterials with natural enzyme-like activity. These nanomaterials are called “nanozymes” because they mimic the catalytic action of enzymes. Nanozymes can perform the same kinetic behaviors as natural enzymes and catalyze the conversion of substrates to oxidized coloring products [21,22]. In 2004, Pasquato and coworkers coined the term “nanozymes” to represent the ribonuclease-mimicking activity of triazacyclononane functionalized gold nanoparticles (NPs) in the transphosphorylation reaction. Numerous nanomaterials have been discovered to have biocatalytic properties since the first nanozyme (Fe3O4 NPs) was discovered [23]. For the oxidation of chromogenic substrates like o-phenylenediamine dihydrochloride (OPD), 3,3′-diaminobenzidine (DAB), and 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), nanomaterials including metals, metal oxides, carbon nanomaterials (CNMs), and metal–organic frameworks (MOFs) mimic the behavior of natural enzymes [24,25]. Each substrate’s oxidation can produce a different color in aqueous solutions; these solutions can be examined visually, and the absorption spectrum can be identified with a spectrophotometer. Nanozymes are often seen as functionally equivalent replacements for natural enzymes because of their customizable catalytic activity, adaptability, surface area, cost, and manufacturing scale. As a result of their unique features, nanozymes can also serve as recognition receptors [26] or signal tags [27]. Signal amplification by nanozymes has allowed for improvements in the performance and sensitivity of a wide variety of biosensor platforms, including colorimetric, fluorometric, chemiluminescent, surface-enhanced Raman scattering, and electrochemical biosensors [21]. Up until now, there has been a dearth of comprehensive reviews on the use of nanozyme-based biosensors, particularly as they relate to personalized diagnostics. This article aims to address that gap by providing a detailed and comprehensive overview of the use of nanozyme-based biosensors in POC settings. In doing so, we aim to explore the catalytic mechanisms employed by nanozymes for biosensing and provide an overview of the various types of nanozyme-based biosensors currently in use.
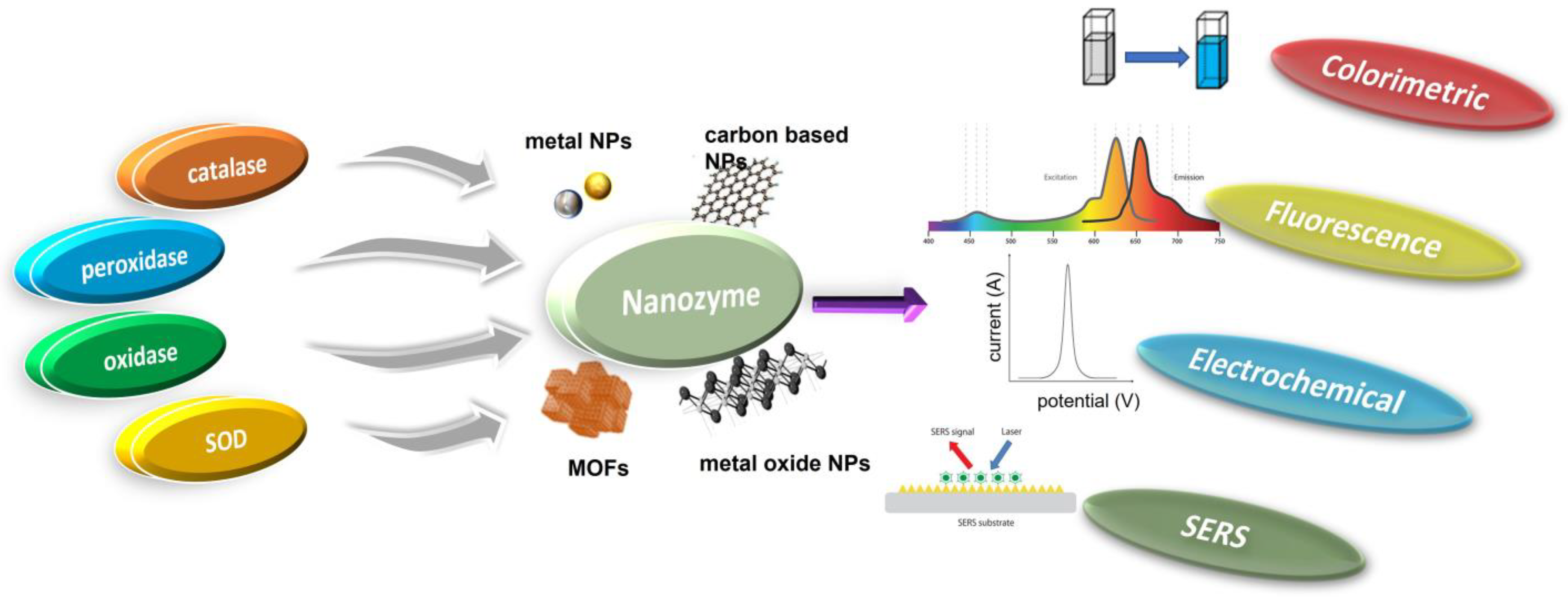
2. Classification of Nanozymes
Natural enzymes play a crucial role in the biochemical processes that sustain life, but they also have some significant limitations that make it important to investigate potential substitutes [28]. Numerous nanomaterials have been proposed as possible enzyme candidates for practical applications by researchers. Although other characteristics such as size, shape, coating, surface modification, pH, and temperature can have a major influence, the atomic composition of nanozymes is the most relevant since the atoms on the surface and inside the core of the NPs are responsible for the enzymatic activity of the nanozyme [29,30]. Therefore, the incorporation of various NPs may either modify the basic characteristics of nanozymes or provide for their multifunctionality. According to their enzymatic activity, nanozymes fall into two broad categories: oxidoreductase and hydrolase. Family members of the oxidoreductase class perform redox catalysis, much as catalase, superoxide dismutase (SOD), oxidase, peroxidase, and nitrate reductase. Similar to phosphatases, proteases, nucleases, esterases, and silicatein, hydrolases catalyze hydrolysis processes [31]. Nanozymes based on peroxidase, superoxide dismutase (SOD), catalase, and oxidase are commonly used in biosensing applications [32,33,34,35,36]. Recent years have seen the publication of a variety of articles addressing the topic of nanomaterial-based enzyme mimics, with subjects from peroxidase mimics and oxidase mimics to catalase mimics and sulfite oxidase mimics [25,26,29,36,37]. However, the purpose of this review is to provide readers with an idea of the state of the art in the burgeoning field of nanozyme-based biosensors. Since recent developments in chemical synthetic methods have led to the formation of nanomaterials with precise controls of size, shape, and compositions, we hope this review article can highlight the various new nanomaterials used and thereby facilitate the research in enzyme mimics. In this section, we explore the plethora of nanomaterials that exhibit these enzyme-mimicking properties, as well as the method by which they function in biosensing applications (Figure 1).
Figure 1.
Schematic illustration of various mimicking types of enzymes and the nanozyme base.
2.1. Peroxidases
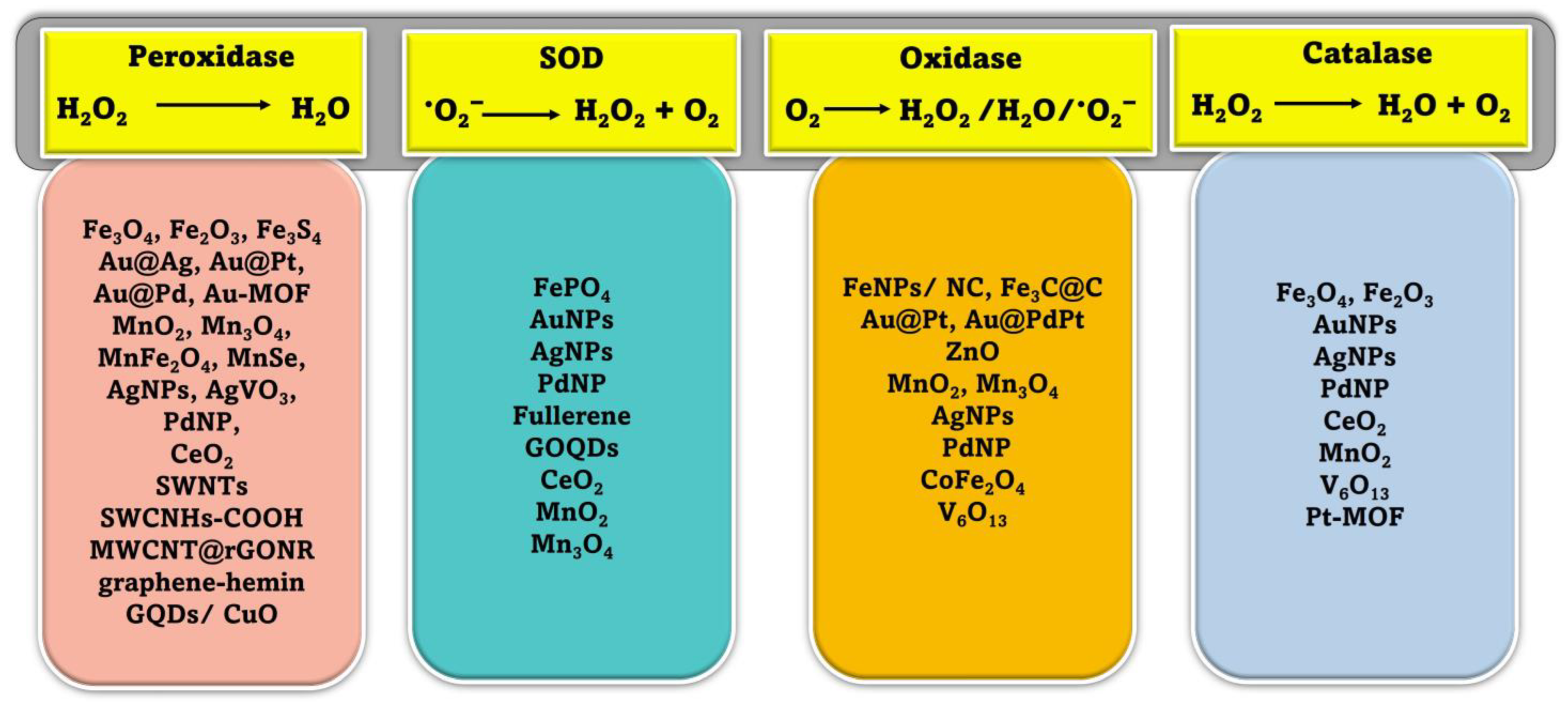
Peroxidases are a broad family of isoenzymes found in various sources, such as plants, animals, and microbes. They generally contain an iron-porphyrin derivative (heme) in their active site, which can accelerate biological oxidation events. In these reactions, the organic hydroperoxides or hydrogen peroxide act as electron acceptors and collaborate with oxidized redox substrates, which serve as electron donors during the reduction process. (Figure 2). The ping-pong mechanism is the recognized mechanism for peroxidase activity (double-displacement reaction). There are two common catalysis pathways for nanozymes in a peroxidase mimic reaction: an electron transfer pathway and a hydroxyl radical production pathway based on the Fenton reaction [38]. The Fenton reaction is mainly responsible for the peroxidase-like activity of nanomaterials [39]. In the Fenton process, the hydrogen peroxide (H2O2) is catalytically broken down by the ferrous ion (Fe2+) in a sequence of reactions in solution (summarized by Equations (1)–(3), leading to the generation of reactive oxygen species [40].
Fe2+ + H2O2 + H+ → Fe3+ + •OH + H2O
Fe3+ + H2O2 → Fe2+ + •OOH + H+
Fe3+ + OOH → Fe2+ + O2 + H+
In 2007, it was demonstrated that magnetic nanoparticles made of iron oxide (Fe3O4) possess the ability to catalyze the oxidation of TMB, o-phenylenediamine (OPD), and diazoaminobenzene (DAB) in the presence of H2O2 under acidic pH conditions. The resulting reaction produced a range of colored products, such as blue, orange, and brown, resembling the outcomes of the natural enzyme HRP [23]. More recently, an electron transfer pathway has also been identified to increase the peroxidase activity [41]. Their peroxidase-like activity was determined by the number of oxidized products produced and H2O2 consumed. As possible peroxidase (POD) mimics, transition metal dichalcogenides (TMDs) are a type of 2D materials with considerable potential. Several properties of PODs, such as their active edge locations and surface electron transfer capabilities, contribute to this. TMDs, such as molybdenum disulfide (MoS2), tungsten diselenide (WSe2), and tungsten ditelluride (WTe2), have comparable active sites and electron transfer abilities to PODs [42,43,44]. The maximum turnover number (Kcat), maximum reaction speed (Vmax), and Michaelis–Menten constant (Km) are determined for enzyme kinetics studies using the Michaelis–Menten equation. In addition to the Fenton reaction, H2O2 can be transformed into reactive hydroxyl radicals (OH) and superoxide anion (O2) via “Haber–Weiss reactions” in the presence of strong catalytic metal ions (often iron ions) [45]. Nanozymes made of metallic NPs have numerous applications. The detection of inherent peroxidase activity in Fe3O4 nanoparticles [23], which closely resembles the peroxidase system found in nature (specifically, the horseradish peroxidase enzyme), has inspired the exploration of peroxidase-mimicking biosensors based on nanomaterials. These biosensors have received significant attention over time [46]. CNMs possess several appealing characteristics as peroxidase mimics, including a high specific surface area, high water solubility, stability, biocompatibility, and non-toxicity [47]. It was shown that single-walled carbon nanotubes (SWCNTs) have peroxidase-like activity, just as natural HRP [48]. Similar to HRP, SWNTs catalyze the peroxidase substrate 3,3,5,5-tetramethylbenzidine (TMB), resulting in a change in color that is highly sensitive to changes in pH, temperature, and H2O2 concentration. These intriguing results have encouraged the study of different carbon-based nanomaterials as peroxidase mimics in the field of biosensors, including GQDs [49], carbon dots [50], and graphitic carbon nitride [51].
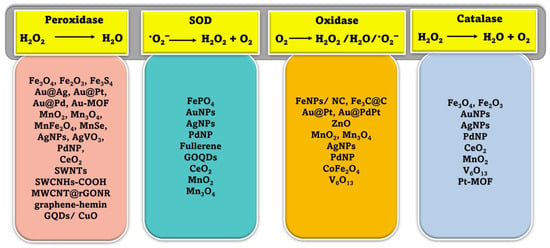
Figure 2.
Schematic representation of different enzyme-mimicking actions of nanozymes with examples [24,52,53].
Figure 2.
Schematic representation of different enzyme-mimicking actions of nanozymes with examples [24,52,53].
2.2. Superoxide Dismutase (SOD)
When it comes to protecting mammalian cells from damage, natural SOD enzymes are indispensable because of their ability to catalyze the dismutation reaction that converts superoxide anion O2 (one of the reactive oxygen species, (ROS)) into harmless hydrogen peroxide (H2O2) and oxygen (O2) (Figure 2). Increased oxidative stress and negative health impacts may result from defective deregulation of ROS formation at the cellular level [54]. Some inorganic NMs have been shown to have scavenging •O2− activity, similar to that of SOD, and mostly they are more reliable and less expensive than SOD [55,56]. Additionally, unlike SOD, these nanozymes exhibit both electrical and magnetic properties. Therefore, NMs with SOD-like catalytic activity have shown the potential to develop novel biosensors in various areas [53,56]. While these NMs have some catalytic activity toward removing •O2−, it is significantly less potent than that of SODs, which limits their utility [57]. In recent years, there have been multiple attempts to develop a highly effective nanozyme that can mimic the behavior of superoxide dismutase (SOD). After discovering fullerene’s radical sponge properties, researchers began using it and its derivatives to prevent oxidative damage to neurons [58]. The water-soluble C60 fullerene was proposed to catalyze the removal of •O2− in two stages. In the presence of protons in the solution, the catalyst is reduced by taking one electron from •O2−, and then that electron is transferred to another •O2−, resulting in the production of •O2− and H2O2 [59]. The ceria nanoparticles (CeNPs) are the most researched of the nanozymes that mimic SOD. CeNPs mimicking SOD activity with enhanced catalytic efficiency were originally reported by the Self group [60]. Numerous studies have indicated that the ability of CeNPs to mimic the behavior of superoxide dismutase (SOD) is primarily linked to the presence of an electron shuttle between their mixed oxidation states, which consist of Ce3+ and Ce4+ [61].
2.3. Oxidase Mimics
Enzymes known as natural oxidases can facilitate the oxidation of a substrate, or electron donor, to produce its corresponding oxidized product in the presence of oxygen, typically resulting in the formation of H2O, H2O2, or •O2−, as shown in Figure 2. Recent research has shown that a variety of NMs are capable of catalyzing the oxidation of single or multiple substrates in oxygen-rich environments, demonstrating properties that are identical to those of natural oxidases [33,62,63].
To evaluate the oxidase-like activity of different nanomaterials, organic substrates containing amino groups, including polyamines and aromatic amines, as well as TMB, OPD, and ABTS, are commonly used because they produce a noticeable change in color and a sensitive signal response when exposed to UV-visible light. A variety of biomedical uses for citrate-capped Au NPs have prompted much research. Extensive research has been conducted on the catalytic properties of “naked” or citrate-capped gold nanoparticles (Au NPs). Rossi and colleagues showed that water-dispersed gold sol may facilitate the oxidation of beta-D-glucose by O2 without the need of conventional supports like carbon or protectors such as PVP [64]. Contrary to what could be expected based on control tests, other metal nanoparticles did not exhibit pronounced catalytic ability on the glucose oxidation reaction. It was discovered, in 2009, that nanoceria, which contains a Ce3+/Ce4+ redox pair, could catalyze the oxidation of organic molecules in ambient conditions [65]. Adsorption of O2 is preferentially favored by nanoceria defect sites. This results in the oxidation of TMB and the reduction of Ce4+ to Ce3+ on the nanoceria surface from the adsorbed O2. Afterwards, the newly formed •O2− will re-oxidize the Ce3+ to Ce4+. Nanoceria’s oxidase-mimetic properties can be traced back to the redox switching of Ce3+/Ce4+ and the production of •O2− radicals [66]. Nanomaterials based on manganese (Mn) were also frequently described as acting as oxidase mimics (MnO2 and Mn3O4). An example of a nanomaterial with oxidase-like properties is MnO2 nanoparticles, which have been shown to promote the oxidation of substrate molecules such as TMB and OPD using O2, resulting in a color change reaction [67].
2.4. Catalase Mimics
The ability of natural catalase enzymes to catalyze the cellular degradation of H2O2 into the water and molecular oxygen is of great importance (Figure 2). An abundance of nanomaterials, including metals and metal oxides, showed catalase-like activity. In most cases, the reported nanomaterials contained catalase-like activities in addition to other enzyme-mimicking activities, and the main enzyme-mimicking activity depended on the pH or temperature [68]. It is vital to remember that if H2O2 levels are not monitored closely, it can contribute to the spread of several different diseases. Consequently, catalase enzymes are crucial for getting rid of H2O2 buildup in the cytoplasm by dismutating it into harmless water and oxygen molecules. Researchers have discovered a wide variety of metal-based nanomaterials (Au, Ag, Pd, Pt) [69] and metal oxide-based NPs (cerium oxide, iron oxides, and cobalt oxide nanoparticles) [69,70,71] displaying catalase enzyme-like activity in recent years. Most nanomaterials had catalase-like and other enzyme-mimicking properties. The catalytic reaction’s pH and temperature determine whether these switchable enzyme-mimicking features coexist [69]. Metal nanoparticles can act as mimics of different enzymes depending on the conditions they are in. Under basic pH conditions, they can act as mimics of catalase, which decomposes hydrogen peroxide (H2O2) into water (H2O) and oxygen gas (O2). However, in acidic pH conditions, certain metal nanoparticles can exhibit peroxidase-like activity, similar to the natural enzyme horseradish peroxidase, which catalyzes the oxidation of substrates, with hydrogen peroxide as the oxidant [72].
3. Nanozyme-Based Biosensors
The use of nanozymes for developing biosensors that can mimic enzyme-like catalytic activity and amplify signals has gained popularity in recent times. They have been found to be an excellent substitute for biological enzymes in the fabrication of innovative biosensors. We discuss the wide range of nanozyme-based biosensors that have been developed and utilized successfully, employing various techniques, such as colorimetry, fluorescence, electrochemistry, surface enhanced Raman, and scattering (Table 1).

Table 1.
Different biosensors based on nanozymes used in biosensing applications.
3.1. Colorimetric Biosensors
As a promising technique for point-of-care detections, colorimetric biosensors allow for the quantitative identification of a specific analyte by color changes using only one’s eyes or a simple portable optical detector. A catalyst’s catalytic activity may also influence the sensing efficiency. Natural enzymes are often used in colorimetric detection methods due to their great sensitivity and specificity [92]. However, their utilization is constrained by inherent restrictions such as high cost, complex treatments, low stability, and challenging storage. Nanozymes, which combine the benefits of natural enzymes with those of nanomaterials, have gained a lot of attention as a viable alternative to natural enzymes because of their low synthesis cost, ease of recycling, and the aforementioned advantages [10]. Enzymes and nanozymes provide colorimetric output signals when they react with chromogenic substrates like 3,3′,5,5′-tetramethylbenzidine (TMB), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), and o-phenylenediamine (OPD) [21]. We have chosen a few publications to serve as examples of the mechanism of nanozyme-based colorimetric biosensors.
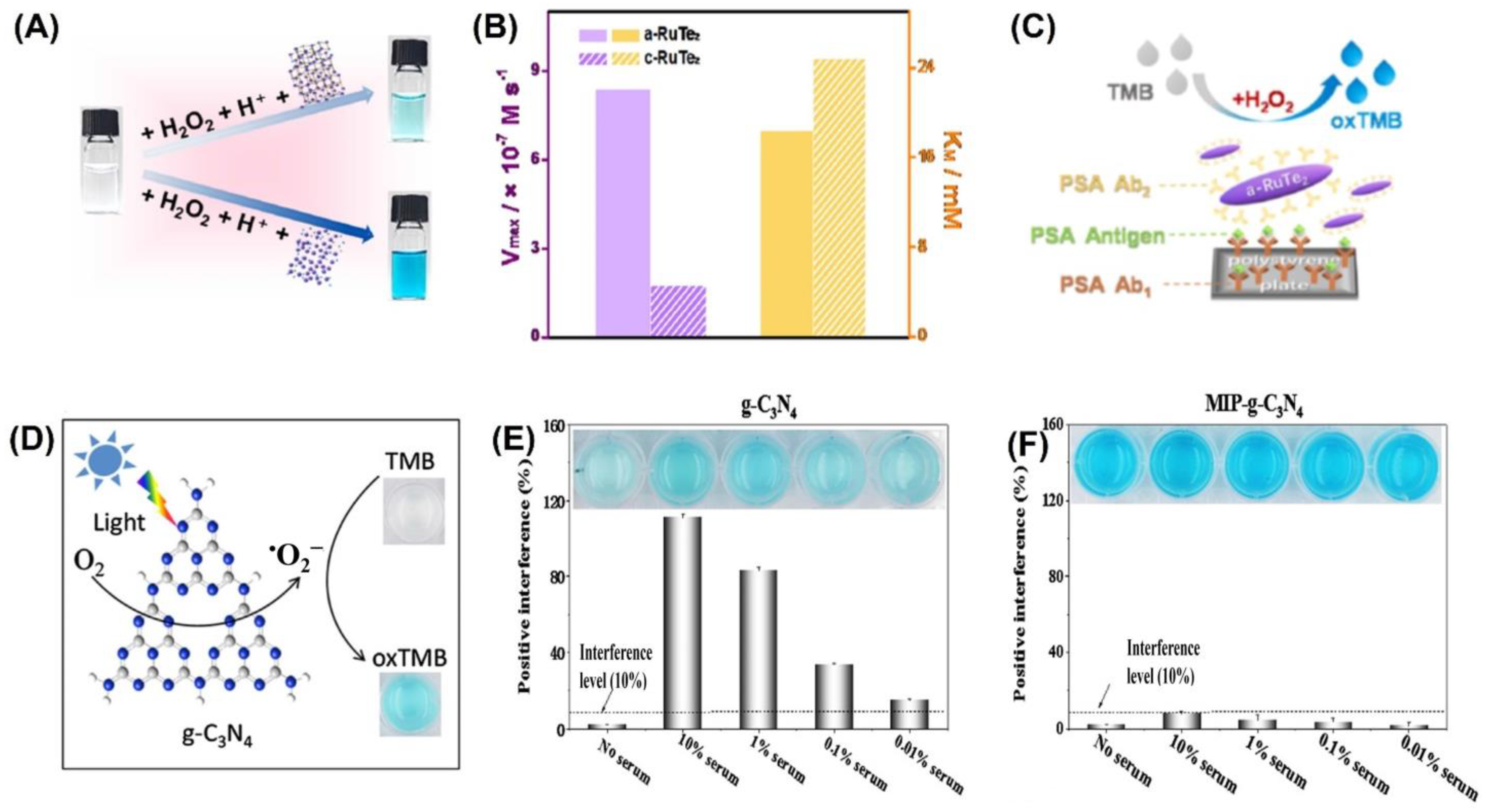
Colorimetric sensors were developed using a variety of inorganic nanomaterials with peroxidase-like activity, including transition metal oxides (e.g., ferromagnetic nanoparticles, MOFs), metals (nanohybrids of gold nanoparticles and MoS2 nanoribbons), and carbon-based nanomaterials (e.g., graphene dots, graphene, and carbon nanotubes). Liu et al. were able to successfully develop a simple and cost-effective approach for the simultaneous detection of three liver-related biomarkers—aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP)—from human plasma employing Au-decorated CoAl-layered double oxide (Au/LDO) nanozymes [93]. Two-dimensional (2D) nanomaterial layered double hydroxides (LDHs) are also known as anionic clays or hydrotalcite-like compounds. One of the most researched LDHs, CoAl-LDHs convert between divalent Co2+ and trivalent Al3+ ions, which could aid in the faradaic redox process [94]. The nanozyme Au/LDO, combined with the agarose hydrogel, acted as a peroxidase mimic, expediting the transformation of colorless 3,3′,5,5′-tetramethylbenzidine (TMB) to blue oxTMB in the presence of hydrogen peroxide (H2O2). Significant interest has been drawn to the specific advantages of the amorphous structure over the crystalline structure in the realm of catalysis. Improved activity and enhanced catalytic selectivity are two of the benefits [95]. Peroxidase mimetic amorphous ruthenium hexamine/tellurium nanorod (a-RuTe2) has a catalytic constant (Kcat) 3.77 times higher than that of crystalline RuTe (c-RuTe2), as indicated by the work of Yan et al. (Figure 3A,B) [96]. Based on this, an enzyme-linked immunosorbent assay (ELISA) was developed for the detection of prostate-specific antigen (PSA) using a-RuTe2 nanorods as labels (Figure 3C). The proposed ELISA based on a-RuTe2 nanorods has a very high sensitivity, at approximately an order of magnitude lower than that of a standard ELISA based on natural horseradish peroxidase.
Similarly, an ultrasensitive immunosensor was constructed for ApoA1 detection using POD-mimicking nanozymes synthesized from Prussian Blue (PB) and magnetic graphene oxide (MGO, PMGO). By catalyzing the oxidation of a colorimetric substrate, TMB, the produced nanozyme could be used as a signal-generating material [97]. Increasing interest in metal–organic frameworks (MOF) can be attributed to the materials’ many desirable qualities, such as their high specific surface area and pore volume, their malleable composition, and their remarkable thermal stability [98]. Recently, MOF-818, a nanozyme-mimicking catechol oxidase, was homogeneously prepared and characterized on the surface of carbon cloth (CC) fibers using a hydrothermal method. The nanozyme displayed high catalytic activity toward the oxidation of pale yellow 3,5-di-tert-butylcatechol (3,5-DTBC) to bright yellow 3,5-di-tert-butyl-(3,5-DTBQ) [73]. Following aptamer modification on the MOF-818/CC surface, thrombin was detected, which hindered the catalytic activity of the nanozyme composite. The aptamer-MOF-818/CC selectively and sensitively measured thrombin with an LOD of 6.4 pM.
The active surface of nanozymes allows for rapid interaction with a range of biomolecules, from low-molecular-weight compounds to macromolecules. However, interference from the biological sample matrix is a persistent issue. To address this concern, molecularly imprinted polymers (MIPs) have been used as they can create large-scale target molecule-binding sites, reducing interference from other molecules [99,100]. In their research, Wu et al. [101] discovered that the MIP graphitic carbon nitride (MIP-g-C3N4) nanozyme possessed intrinsic photooxidase activity (Figure 3D), which led to an increase in the enzyme’s bioactivity and target specificity. They also found that this nanozyme reduced matrix interference from serum samples by a factor of 1000 (Figure 3E,F). During colorimetric sensing, MIP-g-C3N4 displayed enzyme activity that was four times higher than that of bare g-C3N4. Using the novel nanozyme, the group was able to detect L-cysteine, a biomarker associated with a range of diseases like cancer and Alzheimer’s. Nanozyme-based colorimetric biosensors have proven to be highly useful owing to their ease of use, quick response time, portability, and adaptability. However, their detection accuracy and sensitivity can be compromised by the background color of the sample, which can cause interference [21,22].
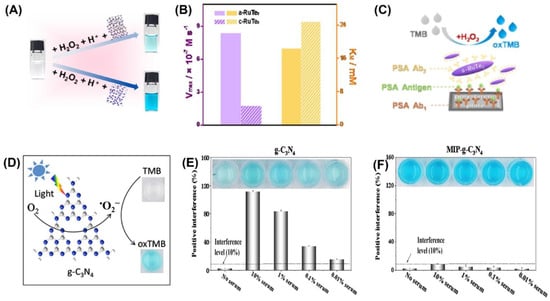
Figure 3.
(A) RuTe2 nanorods demonstrate peroxidase-like activity by catalyzing the oxidation of TMB similar to POD. (B) Comparison of the enzymatic parameters Vmax and KM of amorphous and crystalline RuTe2 using H2O2 as a substrate. (C) Representation of the colorimetric a-RuTe2-ELISA for detecting PSA. (D) Illustration of g-C3N4-catalyzed oxidation of TMB. Experimental conditions: g-C3N4 concentration of 20 mg/mL, solution pH of 4.0, TMB concentration of 0.5 mmol/L, and blue LED color. Interfering matrices from serum samples are demonstrated in (E) g-C3N4 and (F) MIP-g-C3N4. Reprinted with permission from refs. [96,101].
Figure 3.
(A) RuTe2 nanorods demonstrate peroxidase-like activity by catalyzing the oxidation of TMB similar to POD. (B) Comparison of the enzymatic parameters Vmax and KM of amorphous and crystalline RuTe2 using H2O2 as a substrate. (C) Representation of the colorimetric a-RuTe2-ELISA for detecting PSA. (D) Illustration of g-C3N4-catalyzed oxidation of TMB. Experimental conditions: g-C3N4 concentration of 20 mg/mL, solution pH of 4.0, TMB concentration of 0.5 mmol/L, and blue LED color. Interfering matrices from serum samples are demonstrated in (E) g-C3N4 and (F) MIP-g-C3N4. Reprinted with permission from refs. [96,101].
3.2. Fluorescence Biosensors
Fluorescent biosensors, which consist of small scaffolds, can be attached to molecules using one or more fluorescent probes through enzymatic, chemical, or genetic methods. This technology has emerged as a sensitive and effective approach for biosensing due to its ability to improve sensitivity and reduce matrix effects. The biosensor’s detection function relies on the activation or deactivation of its fluorescence by the target analytes, which can either turn it on or turn it off [102]. Fluorescence is a form of luminescence that occurs when a substance absorbs light with high energy (shorter wavelength) and subsequently emits light with lower energy (longer wavelength). This process occurs rapidly, typically taking place within 10−8 to 10−9 s [103]. Nanozyme-based fluorescence approaches have been developed for a number of diagnostic applications and have recently attracted a lot of attention in biosensing research. One type of these sensors is fluorescence resonance energy transfer (FRET) biosensors, which rely on the direct excitation of a fluorophore donor by electromagnetic emission at the appropriate wavelength [104]. The substances that prevent excited fluorophores from emitting further light and instead turn that energy into heat are called quenchers. Quenchers are effective energy acceptors in FRET pairs because they maintain their darkness by releasing the absorbed energy as heat.
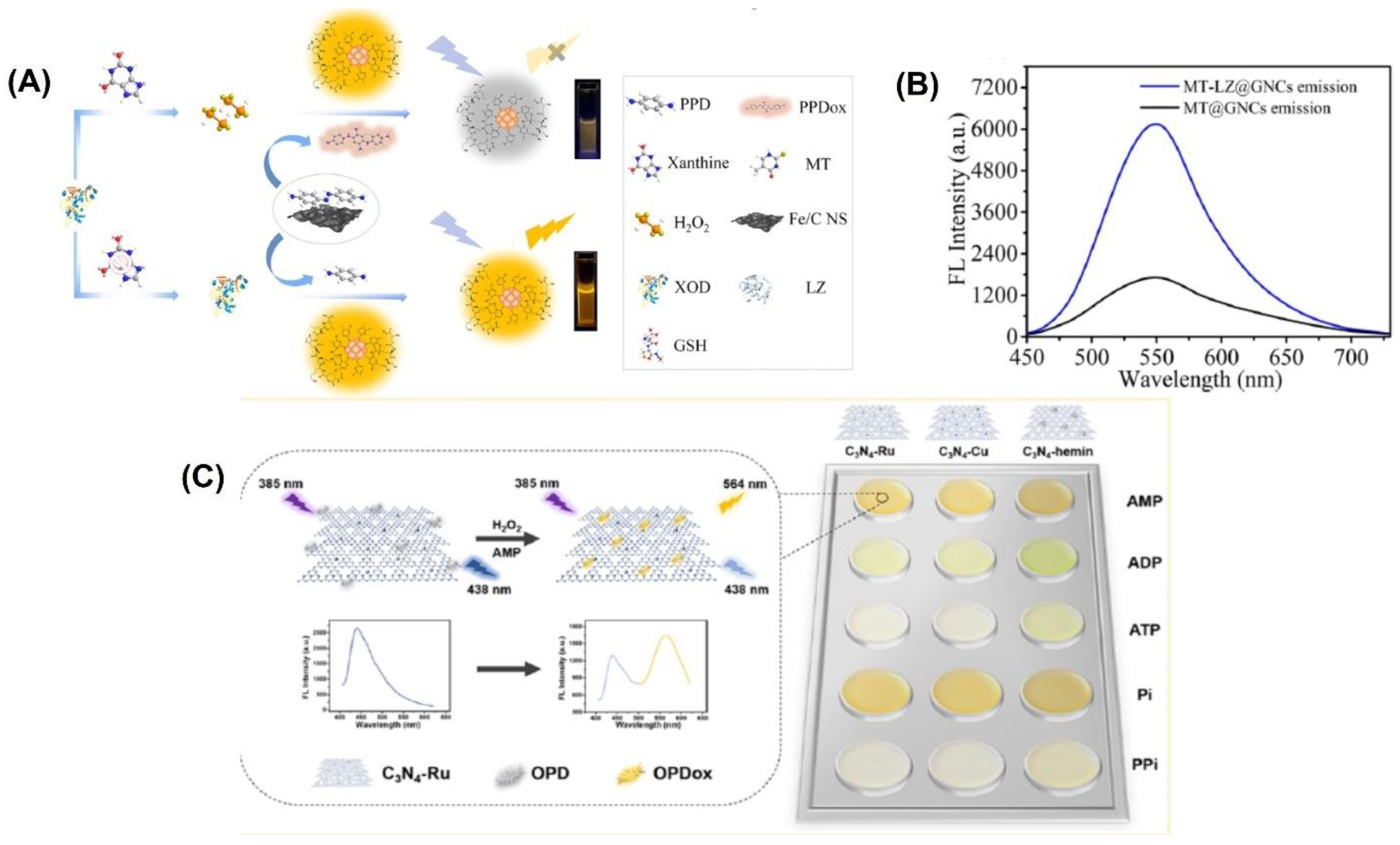
For example, gold nanoclusters were modified by Wang et al. with lysozyme-functionalized 5-methyl-2-thiouracil gold nanoclusters (MT-LZ@GNCs) (Figure 4A) to enhance the fluorescence activity. [105]. The MT-LZ@GNCs exhibited a yellow fluorescence when measured at a wavelength of 550 nm (Figure 4B). To create a fluorescent nanoprobe for detecting xanthine, MT-LZ@GNCs was combined with an iron-doped carbon nanosheet (Fe/CNS), which has similar activity to the enzyme peroxidase. The resulting MT-LZ@GNCs/Fe/CNS fluorescent nanoprobe was able to detect xanthine, and it showed a 3.67-fold increase in fluorescence intensity compared to MT@GNCs. The nanoprobe was able to detect xanthine in human serum samples with a low detection limit of 0.23 µmol L−1 and a recovery rate between 98.72% and 109.27%.
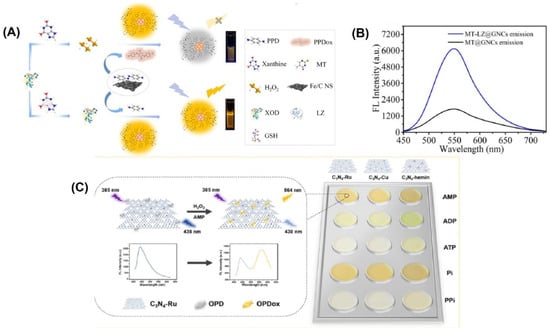
Figure 4.
(A) Schematic representation of the fabrication of MT-LZ@GNCs. (B) Fluorescence emission spectra of MT@GNCs and MT-LZ@GNCs. (C) Schematic representation of the principle behind the assay and the color change obtained with the three different materials. Reprinted with permission from refs. [51,105].
In another study, a fluorescence biosensor was fabricated based on the dual functions of MIL-101(Fe) particles, peroxidase-mimicking activity and fluorescent emission, for the simultaneous detection of choline and acetylcholine (ACh) [106]. This method of fluorescence sensing uses acetylcholinesterase (AChE) to break down ACh into choline, which is then oxidized by choline oxidase (ChOx) to create H2O2. The H2O2 is then broken down into hydroxyl radicals using MIL-101(Fe) nanozymes, resulting in the oxidation of the non-fluorescent terephthalic acid of MIL-101(Fe) to form a highly fluorescent 2-hydroxy terephthalic acid. The MIL-101(Fe) nanozyme has a much greater affinity for H2O2 than the enzyme HRP, as shown by its Km value being around 67 times lower. This indicates that the MIL-101(Fe) nanozyme is more effective at detecting H2O2 than HRP. Using this biosensor, choline in milk and ACh in human plasma were detected, with recoveries ranging from 99.63% to 102.00% and 97.20% to 102.91%, respectively.
A ratiometric fluorescence sensing system requires the integration of two emitting components, which can be accomplished through the production of nanoparticles or various organic dye composites and the manufacture of intrinsic dual-emission fluorophores [13,14]. Unfortunately, the general application of such approaches is hampered by the fact that they typically necessitate elaborate and time-consuming pretreatment steps [15]. Graphitic carbon nitrides (C3N4) have been utilized to create highly active nanozymes for biosensing applications. These nanozymes have abundant pyridinic nitrogen moieties and a π-conjugated framework that provides potential binding sites for further modifications to enhance their catalytic activity. Wang et al. [51] developed three fluorescent C3N4-based nanozymes, namely C3N4-Ru, C3N4-Cu, and C3N4-hemin, with excellent peroxidase-like activities. They combined ruthenium and copper ions into the nanosheets through coordination with pyridinic nitrogen moieties, while hemin was linked to C3N4 through π-π interaction. These fluorescent nanozymes emitted a fluorescence at 438 nm when excited at 385 nm. An intriguing observation was made when the nanozymes were present during the catalytic oxidation of o-phenylenediamine (OPD) to oxidized OPD (OPDox) in the presence of H2O2. In addition to the emission of a new fluorescence at 564 nm by the OPDox, the fluorescence at 438 nm of the nanozymes was also quenched. As a result, the researchers used the ratio between the fluorescent intensity at 564 and 438 nm (F564/F438) as the signal output to create a ratiometric biosensing system. To create a ratiometric H2O2 sensing system, the C3N4-Ru nanozyme was used (Figure 4C). In order to detect and differentiate between five phosphates, a ratiometric sensor array was built using three distinct C3N4-based nanozymes.
Li and colleagues developed a dual-emission ratiometric fluorescence sensing system using MnO2 nanosheets (MnO2 NSs) as quenchers for blue fluorescent carbon dots (BCDs) to determine multiple H2O2-related biomarkers with high accuracy and reliability [107]. The system avoids the need for synthesizing dual-emission fluorophores, making it simple, sensitive, and versatile. MnO2 NSs possess oxidase-like activity that can convert non-fluorescent o-phenylenediamine (OPD) to 2,3-diaminophenazine (DAP), producing a fluorescence signal at 562 nm. As mediators, MnO2 NSs decompose in the presence of H2O2, resulting in the fluorescence recovery of BCDs and a decrease in DAP. Sarcosine, a prostate cancer biomarker, generates H2O2 when catalyzed by sarcosine oxidase. Under optimal conditions, sarcosine can be detected as low as 0.36 μM. The system’s practicability was demonstrated by successfully detecting sarcosine in human urine samples with satisfactory recoveries of 94.9–98.6%.
3.3. Electrochemical Biosensors
Incorporating the natural bioselectivity of the biological component, electrochemical biosensors combine the sensitivity of electroanalytical methods with the analytical precision of the chemical component. Once the analyte has been recognized by the biological component of the sensor, a catalytic or binding event will follow, resulting in an electrical signal that is measured by a transducer and will be proportionate to the analyte concentration [108,109,110,111,112,113]. Electrochemical biosensors have been widely used in numerous industries, including clinical diagnostics, environmental monitoring, food safety analysis, etc., because of their ease of use, low cost, exceptional stability, and sensitive response [6,114]. Since the nanozymes have a large surface area and a high density of capture sites, they may be able to increase the loading of the electroactive species at their surfaces, leading to better electrochemical reactions [21,29,115]. Thus, nanozymes can serve either as an electrode material [116] or as a tracer tag [117] for signal amplification in electrochemical biosensors. Graphene oxides, fullerenes, carbon nanotubes (CNTs), and AuNPs Zr and Cu-based MOFs are just some of the nanozymes employed in EC biosensors due to their exceptional catalytic activity [10,118].
It was worth noting that Gugoasa’s team developed the first in situ synthesis method for a hybrid material made of gold nanoparticles and reduced graphene oxide (Au-rGO), which combines the unique features of each nanomaterial [119]. Laccase-like catalytic activity was demonstrated by the Au-rGO on a phenolic substrate (catechol). Compared to unmodified SPE 0.073 cm2, the electroactive surface area of Au-rGO/SPE was increased to 0.215 cm2. Au-rGO’s surface area, efficient electro-catalysis, and high conductivity aided in electron transfer between the analyte and electrode, boosting the response signal of the SPE-modified electrode.
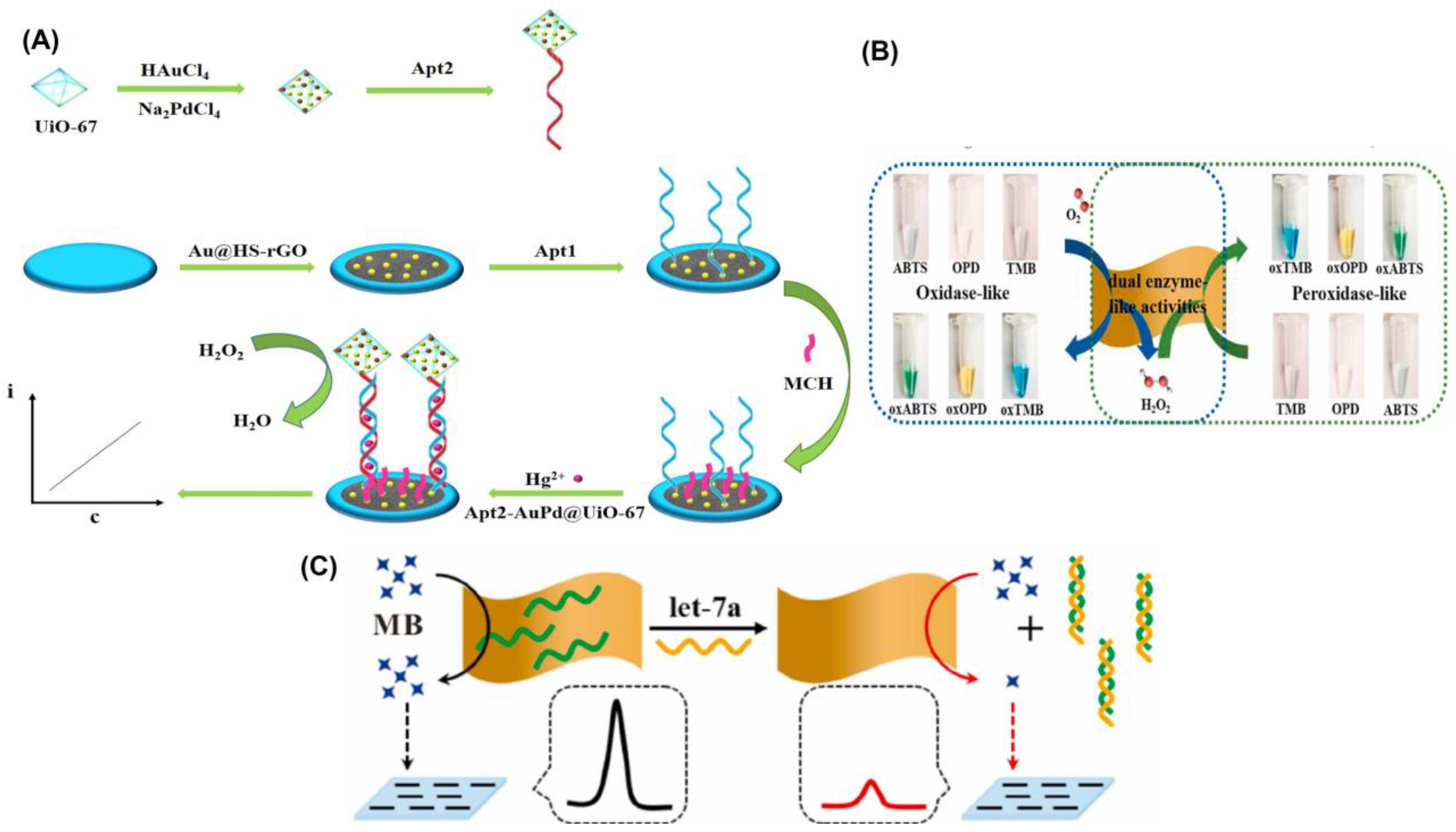
Recent years have seen a surge in interest in metal–organic frameworks (MOFs) as promising new materials due to their tailorable pore size, functional groups, and biocompatibility. In comparison to previous porous solid supports (such as zeolites, mesoporous silica, sol-gel hydrogels, and porous polymers), MOFs have greatly broadened the possibilities for immobilizing enzymes, and are seen as a highly promising platform for researching enzyme–host material interactions [120,121]. Therefore, for the immobilizing of nanoparticles, they are also widely utilized as a matrix [122]. For the sensitive detection of Hg2+, Wang’s team decorated zirconium MOFs with a complex of gold and palladium (AuPd@UiO-67), which served as a nanozyme to amplify the signal [32]. Electrode modification was accomplished by the use of gold-modified thiol graphene (Au@HS-rGO). As part of the platform construction, an Au-S bond was made to the substrate strand (Apt1). Nanozyme AuPd@UiO-67 was used to label Apt2, and it exhibited catalase-like characteristics (Figure 5A). AuPd@UiO-67 nanozyme’s catalytic action toward H2O2 allowed for the recording of the current signal. It was reported that the designed electrochemical aptasensor for Hg2+ has a low detection limit of 0.16 nmol/L and a wide linear range of 1.0 nmol/L to 1.0 mmol/L.
DNA-based homogeneous electrochemical sensing is a novel approach among electrochemical sensors since it enables target identification in a single, diluted solution [123]. Unlike traditional heterogeneous sensors, homogeneous sensors do not require the immobilization of DNA recognition elements, the laborious functionalization of electrodes, or the washing stages [124]. In a dynamic system, where target identification, real-time assay, and regeneration will be conducted in succession, homogeneous electrochemistry provides additional benefits. Nanomaterials with a uniform size and shape can be produced through cost-effective and sustainable wet chemical synthesis, which is guided by a soft template [85]. 2D MnO2 nanoflakes were synthesized by this method by Wu and colleagues [125], and their functionality resembled that of oxidase and peroxidase enzymes (Figure 5B). As a result of their enzyme-like properties, 2D MnO2 nanoflakes have been shown to be highly active in catalyzing the oxidation of O2 into ROS and greatly lowering the differential pulse voltammetry (DPV) peak current by removing methylene blue (MB). More so, 2D MnO2 nanoflakes showed off a peculiar reaction to ssDNA. A homogeneous electrochemical 2D MnO2 nanoflake-based biosensor for miRNA, let-7a, was created (Figure 5C), with a linear range of 0.4–100 nM and a LOD of 0.25 nM, due to its sensitivity to ssDNA and dual enzyme-like activities. Similarly, Wang et al. [110] proposed a flow homogenous electrochemical microRNA detection devoid of immobilized DNA recognition components and time-consuming electrode functionalization. This article describes the ultrasonic production of a 2D MOF nanozyme with a thickness of roughly 1 nm with peroxidase-like activity. As part of the DNA-based homogeneous electrochemical sensing system, Co-MOF nanozymes were used as ssDNA collectors and signal amplifiers. Even after being subjected to six cycles of regeneration, the system maintained its peak performance. A 0.12 pM LOD and successful detection of the target microRNA in biological samples demonstrated the system’s exceptional long-term stability. As their sensitivity extends over large dynamic ranges, electrochemical biosensors find widespread use in initial, semi-quantitative, and qualitative screening. In spite of their excellent accuracy and practicality, these biosensors still have some ways to go before they are entirely problem-free because of their poor replication and low stability.
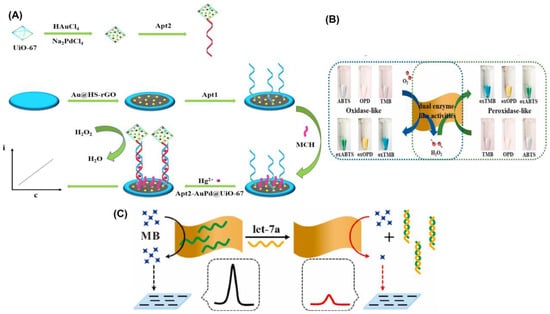
Figure 5.
(A) Schematic diagram of the synthesis of Apt2-AuPd@UiO-67 and illustration of the construction of the electrochemical aptasensor. (B) Graphical representation of 2D MnO2 nanoflake interaction with enzymatic substrates through oxidase mimetic and peroxidase mimetic activities. The substrate concentrations of TMB, OPD, and ABTS are all 0.5 mM, and the 2D MnO2 nanoflake concentration is 2.6 μg/mL. (C) Illustration of the detection concept of a homogeneous electrochemical biosensor for miRNA assay. Reprinted with permission from refs. [32,125].
Figure 5.
(A) Schematic diagram of the synthesis of Apt2-AuPd@UiO-67 and illustration of the construction of the electrochemical aptasensor. (B) Graphical representation of 2D MnO2 nanoflake interaction with enzymatic substrates through oxidase mimetic and peroxidase mimetic activities. The substrate concentrations of TMB, OPD, and ABTS are all 0.5 mM, and the 2D MnO2 nanoflake concentration is 2.6 μg/mL. (C) Illustration of the detection concept of a homogeneous electrochemical biosensor for miRNA assay. Reprinted with permission from refs. [32,125].
3.4. Surface-Enhanced Raman Spectroscopy (SERS) Biosensors
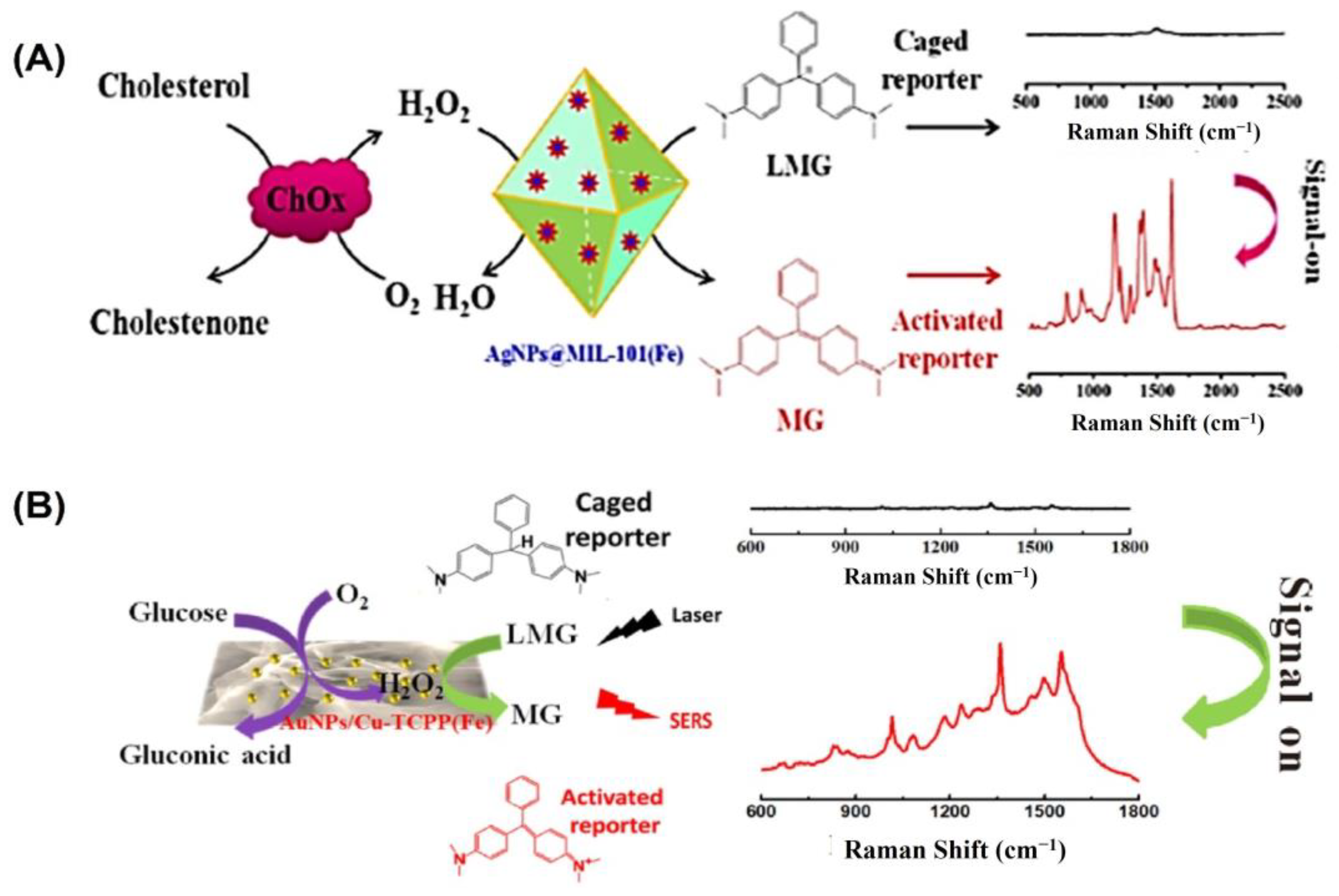
In the field of bioanalysis and detection, surface-enhanced Raman spectroscopy (SERS)-based biosensors have recently attracted a lot of interest due to their high sensitivity, efficiency, accuracy, low sample demand, and non-destructive nature [126,127]. Raman spectroscopy receives its signal from Raman scattering produced by a Raman reporter on the surface of plasmonic nanomaterials like gold, silver, silicon, porous alumina, or their combinations [128]. The SERS signal can be modified through the reaction of reporter molecules by incorporating peroxidase nanozymes with the plasmonic nanostructures [129]. For example, Wu and colleagues grew silver nanoparticles (AgNPs) on the surface of a metal–organic framework (MOF) named MIL-101 (Fe) to develop a peroxidase-mimicking nanozyme AgNPs@MOF [88] (Figure 6A). The high adsorption capacity of MOFs makes them suitable for usage as SERS substrates. The AgNPs@MOF were used as a substrate for surface-enhanced Raman spectroscopy (SERS) and as peroxidase mimics to convert inert leucomalachite green to Raman-active malachite green. A sensitive and precise SERS sensing platform was proposed for cholesterol monitoring based on the strong peroxidase-mimicking activity and high SERS enhancement of AgNPs@MOF. The constructed biosensor was able to detect concentrations as low as 0.36 µM under the best possible conditions, with a dynamic detection range of 1.0–100 µM.
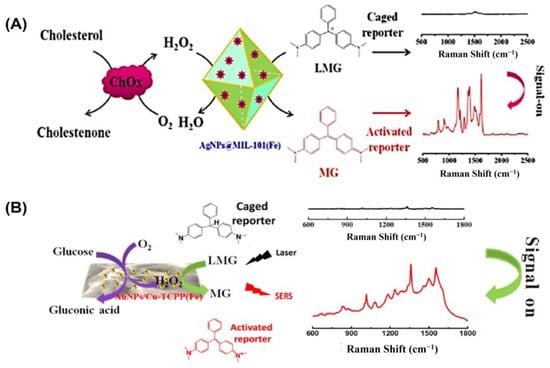
Figure 6.
(A) Illustration of the cholesterol-detecting SERS biosensor based on AgNPs@MIL-101(Fe). (B) Schematic representation of the enzyme-free tandem reaction method for SERS glucose measurement. Reprinted with permission from refs. [87,88].
Likewise, Hu and colleagues developed a non-invasive method of measuring blood glucose levels in saliva [87]. Metalloporphyrin-based metal–organic frameworks are a recently discovered class of peroxidase mimic enzymes that can catalyze the interaction between hydrogen peroxide and 3,3′,5,5′-tetramethylbenzidine (TMB) to generate oxidized TMB (oxTMB). Incorporating AuNPs into MOF-based hybrid nanomaterials has been shown to improve their performance in SERS measurement (Figure 6B). Susceptible to water but not sinking, after synthesizing water-stable 2D metalloporphyrin Cu-TCPP(Fe) nanosheets, hybrid nanosheets of AuNPs/Cu-TCPP(Fe) were formed through in situ growth. It was found that in the presence of oxygen, glucose may be oxidized catalytically by AuNPs, yielding gluconic acid and H2O2. On the other hand, the Cu-TCPP(Fe) nanosheets can act as a catalyst for transforming oxide-caged leucomalachite green (LMG), which lacks Raman activity, into MG by utilizing H2O2 that is generated on-site, resulting in a Raman-active signal. GOx-like activities were observed in “naked” gold nanoparticles (Au NPs) that were prepared without stabilizers or protectors [64]. However, it has been observed that the usage of protectors may prevent GOx-like activity. Peroxidase-like activities and SERS activities were both found to be high in Ag nanoparticles [130]. To catalyze the sequential oxidation of glucose, Xia’s group has created synthetic tandem nanozyme Au@Ag NPs that mimic the actions of both GOx and peroxidase [131]. In the realm of surface-enhanced Raman scattering (SERS), core–shell plasmonic nanostructures have recently emerged as an exciting new area of study [132,133]. Shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS), which uses plasmonic nanoparticles (NPs) as the core and inert or semiconductor materials as the shell, has emerged as a promising and potent technique in a wide range of chemical and biological investigations. Some of the most promising SHINERS built for biological research, including biosensing, imaging, and treatment, are graphene-isolated metal nanoparticles (GIMNs). Jin’s group designed a monodispersed Ag/oxidized GQDs (o-GQDs) nanohybrid with a small core–shell configuration (ca. 10 nm). Small Ag/o-GQDs have improved biocompatibility and good nucleus-mitochondria dual-targeting capacity without change to the targeting ligand, presenting unparalleled potential for intracellular applications. In addition, the SERS-active Ag/o-GQDs demonstrate a peroxidase (POD)-like response, allowing accurate subcellular detection of intracellular H2O2 [134].
4. Market Opportunities and Commercialization
Bringing nanozyme-based biosensors to the market is a challenging process that involves considerable industry input and market analysis. Despite the clear potential benefits of these biosensors, such as personalized medicine and cost savings, it may take up to a decade to commercialize a biosensor and another decade or more before it becomes profitable. Biosensors have to adhere to a number of different regulations and requirements before they can be commercially supplied, and this is a costly and time-consuming process. It may be difficult to reliably and consistently scale up the manufacturing of biosensors built in the lab to industrial levels. Furthermore, researchers may have limited access to real samples and limited expertise in commercialization, both of which might make validating novel biosensor concepts developed in the lab difficult in the real world. Competing technologies and conventional platforms have lower development costs and a guaranteed market share, due to their longer experience in product formulation. However, advances in micro- and nano-systems, such as polymers and hydrogels, and 3D printing manufacturing, have led to a surge in interest in these technologies despite their higher development costs. Investors have shown a willingness to invest in the healthcare industry when the benefits of new technologies, such as shortened assay times, improved specificity, and point-of-care testing, offset the high research costs. The profitability of nanozyme-based biosensor R&D is also dependent on the supporting technologies and infrastructure. To conclude, successfully commercializing nanozyme-based biosensors requires a comprehensive understanding of market trends, development of enabling technologies, and significant industry involvement. Although there are challenges, the potential benefits of these biosensors make them a promising area for research and development in the healthcare industry.
5. Challenges in the Development of Personalized Biosensors
Personalized biosensors provide comprehensive data on an individual’s health and physical activities. This promising development is made possible by the superior precision and robustness of detecting biomarkers in the body [135]. To better translate and use personalized biosensors, there has been an increase in the usage of collaborative system design, which brings together user-defined needs with technological innovation. Moreover, the utilization of personalized biosensors is expanding as sensor technologies progress beyond the conventional biomarker classes of nucleic acids and proteins to encompass metabolites and direct detection of infections. Nanozymes have several properties that make them attractive for personalized diagnostics. Firstly, (a) nanozymes can mimic the catalytic activities of enzymes and can be designed to have high selectivity and sensitivity towards specific biomolecules. This allows for the detection and quantification of biomarkers in biological samples, which can provide valuable information about an individual’s health status. Additionally, (b) nanozymes are highly stable and robust, making them ideal for use in point-of-care diagnostic devices. This could enable real-time monitoring of biomarkers, allowing for early detection and intervention in diseases. Furthermore, (c) nanozymes can be engineered to have unique surface properties, which can enable their integration into personalized diagnostic platforms. For example, nanozymes can be functionalized with targeting moieties such as antibodies or aptamers, allowing for the selective detection of specific biomolecules in complex biological matrices.
Despite significant progress, designing personalized biosensors still faces critical challenges and technological gaps that need to be addressed. One of the biggest challenges is the extremely complex nature of biological samples. Sample matrices can contain a diverse range of non-target compounds at varying concentrations, which can lead to inaccurate detection of the target analyte, either overestimating or underestimating its concentration. This can be further complicated by sensor fouling, especially when the sample matrix includes proteins. Detecting both low and high concentrations with a single sensor presents a significant challenge. As a result, it may be necessary to design multiple sensors to cover the full concentration range. Another option is to create a sensor that functions optimally in the medium-to-low concentration range, with the caveat that samples with higher analyte levels will need to be diluted. As the sample gets more diluted, it becomes increasingly important to consider the sensing threshold.
6. Conclusions and Outlook
Clinical diagnosis specialists and medical offices are not the only ones who need these simple and cutting-edge tools; regular people using them in their homes or the field with limited resources also need them. When compared to using natural enzymes as key functional components for analyte detection, the application of nanomaterials exhibiting enzyme-mimicking activity (nanozymes) incorporated in POC-based biosensor systems shows various advantages. This review has covered a wide range of topics pertaining to the use of nanozymes for biosensor creation. The most up-to-date examples of each kind of nanozymes used in POC biosensor development are summarized in this article. Recent advances in these instances show that the study of nanozymes and their biosensing applications has increased steadily. The huge potential of nanozymes in POC-based biosensor development, however, has yet to be realized because of various research gaps and hurdles that have been recognized and need to be handled at this frontier. To produce high-performance nanozymes, it is essential to comprehend their catalytic mechanism and the structure–activity relationship. This understanding can be achieved by combining experimental and computational research. Despite testing several nanomaterials for their ability to imitate natural enzymes, peroxidase mimics, primarily redox enzyme mimics, are still the most popular choice for biosensing. Furthermore, understanding the catalytic mechanism of nanozymes is crucial in determining their efficacy. Given the diversity of natural enzymes, more work needs to go into engineering nanozymes with new catalytic capabilities like synthetase and hydrolase. Similarly, enzyme-active sites are the primary focus of current research. The protein scaffold of an enzyme is crucial to the selectivity and effectiveness of an enzymatic reaction, yet its analogues have not been well investigated. This might be accomplished by taking a page out of the book of building new functional proteins and combining experimental methods with computational and/or theoretical strategies for designing nanozymes. If this were to happen, not only would the versatility of nanozyme applications in biosensor development be greatly increased, but the cost of detecting a wider variety of analytes would also be reduced. Substrate specificity is a vital characteristic of natural enzymes, and the reduced specificity of nanozymes poses a significant challenge. Combining natural enzymes with nanozymes may help mitigate this issue, but it could compromise the stability and cost of the entire catalyst system. While nanozymes show promise as an enzyme replacement, further study is needed before they can compete with normal enzymes. Since chemical processes are typically employed to generate nanozymes, it is essential to increase batch-to-batch reproducibility if these products are to be used in industrial or clinical contexts. Nanozyme-based point-of-care (POC) biosensors will only be widely used if scientists can design them to be easy to use, highly automated, and require minimal user input. Scalability, mobility, and ease of application are all crucial early design considerations that must be made before mass manufacturing can begin.
Author Contributions
C.P.K.: conceptualization, investigation, methodology, visualization, writing—original draft, and writing—review and editing; M.U.A.: conceptualization, writing—review and editing, project administration, funding acquisition, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by the Universiti Brunei Darussalam’s Grants UBD/RSCH/1.4/FICBF(b)/2020/025 and UBD/RSCH/1.4/FICBF(b)/2021/032 and Brunei Research Council Grant-10.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data were used for the research described in the article.
Acknowledgments
C.P.K. thanks the Ministry of Education of Brunei and Universiti Brunei Darussalam for her Ph.D. and post-doctoral fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Navarro, E.; Serrano-Heras, G.; Castaño, M.J.; Solera, J. Real-Time PCR Detection Chemistry. Clin. Chim. Acta 2015, 439, 231–250. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, K.A.; Nonaka, C.K.; de Ávila Mendonça, R.N.; Mascarenhas, V.N.; Weber, T.G.; Regis Silva, C.G.; Mendes, A.V.; Khouri, R.; Souza, B.S.; Gurgel Rocha, C.A. SARS-CoV-2 Detection via RT-PCR in Matched Saliva and Nasopharyngeal Samples Reveals High Concordance in Different Commercial Assays. Diagnostics 2023, 13, 329. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.A.; AL-maqati, T.N.; Alnaam, Y.A.; Alharbi, S.S.; Khaneen, R.; Almutairi, H.; AL-harbi, M. The Association between Brain-Derived Neurotrophic Factor (BDNF) Protein Level and Body Mass Index. Medicina 2023, 59, 99. [Google Scholar] [CrossRef] [PubMed]
- Mpupa, A.; Nqombolo, A.; Mizaikoff, B.; Nomngongo, P.N. Beta-Cyclodextrin-Decorated Magnetic Activated Carbon as a Sorbent for Extraction and Enrichment of Steroid Hormones (Estrone, β-Estradiol, Hydrocortisone and Progesterone) for Liquid Chromatographic Analysis. Molecules 2022, 27, 248. [Google Scholar] [CrossRef]
- Incocciati, A.; Di Fabio, E.; Boffi, A.; Bonamore, A.; Macone, A. Rapid and Simultaneous Determination of Free Aromatic Carboxylic Acids and Phenols in Commercial Juices by GC-MS after Ethyl Chloroformate Derivatization. Separations 2022, 9, 9. [Google Scholar] [CrossRef]
- Kurup, C.P.; Mohd-Naim, N.F.; Ahmed, M.U. Recent Trends in Nanomaterial-Based Signal Amplification in Electrochemical Aptasensors. Crit. Rev. Biotechnol. 2022, 42, 794–812. [Google Scholar] [CrossRef]
- Adhikari, J.; Rizwan, M.; Keasberry, N.A.; Ahmed, M.U. Current Progresses and Trends in Carbon Nanomaterials-based Electrochemical and Electrochemiluminescence Biosensors. J. Chinese Chem. Soc. 2020, 67, 937–960. [Google Scholar] [CrossRef]
- Baehner, T.; Rohner, M.; Heinze, I.; Schindler, E.; Wittmann, M.; Strassberger-Nerschbach, N.; Kim, S.-C.; Velten, M. Point-of-Care Ultrasound-Guided Protocol to Confirm Central Venous Catheter Placement in Pediatric Patients Undergoing Cardiothoracic Surgery: A Prospective Feasibility Study. J. Clin. Med. 2021, 10, 5971. [Google Scholar] [CrossRef]
- Rizwan, M.; Mohd-Naim, N.; Ahmed, M. Trends and Advances in Electrochemiluminescence Nanobiosensors. Sensors 2018, 18, 166. [Google Scholar] [CrossRef]
- Arshad, F.; Arrigan, D.W.M.; Ahmed, M.U. Recent Developments in Nanozyme Based Sensors for Detection of Clinical Biomarkers-A Review. IEEE Sens. J. 2022, 22, 15622–15634. [Google Scholar] [CrossRef]
- Yan, S.; Foroughi, M.M.; Safaei, M.; Jahani, S.; Ebrahimpour, N.; Borhani, F.; Rezaei Zade Baravati, N.; Aramesh-Boroujeni, Z.; Foong, L.K. A Review: Recent Advances in Ultrasensitive and Highly Specific Recognition Aptasensors with Various Detection Strategies. Int. J. Biol. Macromol. 2020, 155, 184–207. [Google Scholar] [CrossRef]
- Tandon, S.; Sharma, A.; Singh, S.; Sharma, S.; Sarma, S.J. Therapeutic Enzymes: Discoveries, Production and Applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102455. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization Strategies to Develop Enzymatic Biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Chen, Z.; Lai, G.; Liu, S.; Yu, A. Ultrasensitive Electrochemical Aptasensing of Kanamycin Antibiotic by Enzymatic Signal Amplification with a Horseradish Peroxidase-Functionalized Gold Nanoprobe. Sens. Actuators B Chem. 2018, 273, 1762–1767. [Google Scholar] [CrossRef]
- Amara, U.; Mahmood, K.; Riaz, S.; Nasir, M.; Hayat, A.; Hanif, M.; Yaqub, M.; Han, D.; Niu, L.; Nawaz, M.H. Self-Assembled Perylene-Tetracarboxylic Acid/Multi-Walled Carbon Nanotube Adducts Based Modification of Screen-Printed Interface for Efficient Enzyme Immobilization towards Glucose Biosensing. Microchem. J. 2021, 165, 106109. [Google Scholar] [CrossRef]
- Barsan, M.M.; Brett, C.M.A. An Alcohol Oxidase Biosensor Using PNR Redox Mediator at Carbon Film Electrodes. Talanta 2008, 74, 1505–1510. [Google Scholar] [CrossRef]
- Parra, A.; Casero, E.; Vázquez, L.; Pariente, F.; Lorenzo, E. Design and Characterization of a Lactate Biosensor Based on Immobilized Lactate Oxidase onto Gold Surfaces. Anal. Chim. Acta 2006, 555, 308–315. [Google Scholar] [CrossRef]
- Santharaman, P.; Venkatesh, K.A.; Vairamani, K.; Benjamin, A.R.; Sethy, N.K.; Bhargava, K.; Karunakaran, C. ARM-Microcontroller Based Portable Nitrite Electrochemical Analyzer Using Cytochrome c Reductase Biofunctionalized onto Screen Printed Carbon Electrode. Biosens. Bioelectron. 2017, 90, 410–417. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Y.; Han, G.; Li, M. Porous-Reduced Graphene Oxide for Fabricating an Amperometric Acetylcholinesterase Biosensor. Sens. Actuators B Chem. 2013, 185, 706–712. [Google Scholar] [CrossRef]
- Genet, J.-P. Asymmetric Catalytic Hydrogenation. Design of New Ru Catalysts and Chiral Ligands: From Laboratory to Industrial Applications. Acc. Chem. Res. 2003, 36, 908–918. [Google Scholar] [CrossRef]
- Mohamad, A.; Teo, H.; Keasberry, N.A.; Uddin, M. Critical Reviews in Biotechnology Recent Developments in Colorimetric Immunoassays Using Nanozymes and Plasmonic Nanoparticles. Crit. Rev. Biotechnol. 2019, 39, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Mohd-Naim, N.F.; Chandrawati, R.; Cozzolino, D.; Ahmed, M.U. Nanozyme-Based Sensors for Detection of Food Biomarkers: A Review. RSC Adv. 2022, 12, 26160–26175. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sharifi, M.; Bloukh, S.H.; Edis, Z.; Siddique, R.; Falahati, M. In Vivo Guiding Inorganic Nanozymes for Biosensing and Therapeutic Potential in Cancer, Inflammation and Microbial Infections. Talanta 2021, 224, 121805. [Google Scholar] [CrossRef]
- Wang, W.; Gunasekaran, S. Nanozymes-Based Biosensors for Food Quality and Safety. TrAC—Trends Anal. Chem. 2020, 126, 115841. [Google Scholar] [CrossRef]
- Su, Z.; Du, T.; Liang, X.; Wang, X.; Zhao, L.; Sun, J.; Wang, J.; Zhang, W. Nanozymes for Foodborne Microbial Contaminants Detection: Mechanisms, Recent Advances, and Challenges. Food Control. 2022, 141, 109165. [Google Scholar] [CrossRef]
- Zhong, Y.; Tang, X.; Li, J.; Lan, Q.; Min, L.; Ren, C.; Hu, X.; Torrente-Rodríguez, R.M.; Gao, W.; Yang, Z. A Nanozyme Tag Enabled Chemiluminescence Imaging Immunoassay for Multiplexed Cytokine Monitoring. Chem. Commun. 2018, 54, 13813–13816. [Google Scholar] [CrossRef]
- Economou, A.; Karapetis, S.K.; Nikoleli, G.-P.; Nikolelis, D.P.; Bratakou, S.; Varzakas, T.H. Enzyme-Based Sensors. In Advances in Food Diagnostics, 2nd ed.; Toldrá, F., Nollet, L.M.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 231–250. [Google Scholar] [CrossRef]
- Stasyuk, N.; Smutok, O.; Demkiv, O.; Prokopiv, T.; Gayda, G.; Nisnevitch, M.; Gonchar, M. Synthesis, Catalytic Properties and Application in Biosensorics of Nanozymes and Electronanocatalysts: A Review. Sensors 2020, 20, 4509. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Wei, H. Light-Responsive Nanozymes for Biosensing. Analyst 2020, 145, 4388–4397. [Google Scholar] [CrossRef]
- Wu, Y.; Darland, D.C.; Zhao, J.X. Nanozymes-Hitting the Biosensing “Target”. Sensors 2021, 21, 5201. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, F.; Chi, H.; Zhao, G.; Zhang, Y.; Li, T.; Wei, Q. Electrochemical Aptasensor Based on Gold Modified Thiol Graphene as Sensing Platform and Gold-Palladium Modified Zirconium Metal-Organic Frameworks Nanozyme as Signal Enhancer for Ultrasensitive Detection of Mercury Ions. J. Colloid Interface Sci. 2022, 606, 510–517. [Google Scholar] [CrossRef]
- Chong, Y.; Liu, Q.; Ge, C. Advances in Oxidase-Mimicking Nanozymes: Classification, Activity Regulation and Biomedical Applications. Nano Today 2021, 37, 101076. [Google Scholar] [CrossRef]
- Navyatha, B.; Singh, S.; Nara, S. AuPeroxidase Nanozymes: Promises and Applications in Biosensing. Biosens. Bioelectron. 2021, 175, 112882. [Google Scholar] [CrossRef]
- Wang, X.; Wei, H. Peroxidase-like Nanozyme Sensing Arrays for Versatile Analytes. J. Nanoparticle Res. 2020, 22, 22. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Bytesnikova, Z.; Barek, J.; Richtera, L.; Adam, V. A Critical Comparison of Natural Enzymes and Nanozymes in Biosensing and Bioassays. Biosens. Bioelectron. 2021, 192, 113494. [Google Scholar] [CrossRef]
- Mahmudunnabi, R.G.; Farhana, F.Z.; Kashaninejad, N.; Firoz, S.H.; Shim, Y.-B.; Shiddiky, M.J.A. Nanozyme-Based Electrochemical Biosensors for Disease Biomarker Detection. Analyst 2020, 145, 4398–4420. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, Y.; Ma, X.; Guo, L.; Qiu, B.; Chen, G.; Lin, Z. Multicolor Biosensor for Fish Freshness Assessment with the Naked Eye. Sens. Actuators B Chem. 2017, 252, 201–208. [Google Scholar] [CrossRef]
- Campos, S.; Salazar, R.; Arancibia-Miranda, N.; Rubio, M.A.; Aranda, M.; García, A.; Sepúlveda, P.; Espinoza, L.C. Nafcillin Degradation by Heterogeneous Electro-Fenton Process Using Fe, Cu and Fe/Cu Nanoparticles. Chemosphere 2020, 247, 125813. [Google Scholar] [CrossRef]
- Walling, C. Fenton’s Reagent Revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Wang, M.; Tian, B.; Xue, Y.; Li, R.; Zhai, T.; Tan, L. Determination of Aminophylline Based on Fluorescence Quenching of Amino-Functionalized Graphene Quantum Dots Induced by Photoilluminated Riboflavin-Aminophylline System. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 235, 118306. [Google Scholar] [CrossRef]
- Chen, T.M.; Wu, X.J.; Wang, J.X.; Yang, G.W. WSe2 Few Layers with Enzyme Mimic Activity for High-Sensitive and High-Selective Visual Detection of Glucose. Nanoscale 2017, 9, 11806–11813. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, F.; Wang, A.; Chen, X.; Li, H.; Zhang, X.; Zheng, H.; Ji, R.; Li, B.; Yu, X.; et al. Defect-Rich Adhesive Molybdenum Disulfide/RGO Vertical Heterostructures with Enhanced Nanozyme Activity for Smart Bacterial Killing Application. Adv. Mater. 2020, 32, 2005423. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, Y.; Xu, H.; Du, C.; Wu, X.; Yang, G. Superior Peroxidase Mimetic Activity Induced by Topological Surface States of Weyl Semimetal WTe2. Nano Today 2022, 43, 101421. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss Reaction and Mechanisms of Toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Chen, Y.; Liu, Z.; Huang, C.; Li, Y.; Kong, D.; Shen, W.; Tang, S. Modification and Application of Fe3O4 Nanozymes in Analytical Chemistry: A Review. Chinese Chem. Lett. 2023, 34, 107820. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew. Chemie Int. Ed. 2018, 57, 9224–9237. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.; Zhao, C.; Qu, K.; Ren, J.; Qu, X. Label-Free Colorimetric Detection of Single Nucleotide Polymorphism by Using Single-Walled Carbon Nanotube Intrinsic Peroxidase-like Activity. Chem. —A Eur. J. 2010, 16, 3617–3621. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Liu, Y.; Cui, J.; Liu, H.; Wang, P.; Li, Y.; Chen, L.; Zhao, Z.; Dong, Y. A Novel Label-Free Electrochemical Immunosensor Based on Functionalized Nitrogen-Doped Graphene Quantum Dots for Carcinoembryonic Antigen Detection. Biosens. Bioelectron. 2017, 90, 31–38. [Google Scholar] [CrossRef]
- Lu, W.; Guo, Y.; Zhang, J.; Yue, Y.; Fan, L.; Li, F.; Dong, C.; Shuang, S. A High Catalytic Activity Nanozyme Based on Cobalt-Doped Carbon Dots for Biosensor and Anticancer Cell Effect. ACS Appl. Mater. Interfaces 2022, 14, 57206–57214. [Google Scholar] [CrossRef]
- Wang, X.; Qin, L.; Lin, M.; Xing, H.; Wei, H. Fluorescent Graphitic Carbon Nitride-Based Nanozymes with Peroxidase-Like Activities for Ratiometric Biosensing. Anal. Chem. 2019, 91, 10648–10656. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.-F.; Teixeira, M.; Valentine, J.S. Superoxide Dismutases and Superoxide Reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, J.; Qu, X. Catalytically Active Nanomaterials: A Promising Candidate for Artificial Enzymes. Acc. Chem. Res. 2014, 47, 1097–1105. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Das, B.; Franco, J.L.; Logan, N.; Balasubramanian, P.; Kim, M.I.; Cao, C. Nanozymes in Point-of-Care Diagnosis: An Emerging Futuristic Approach for Biosensing. Nano-Micro Lett. 2021, 13. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, B.; Yang, R.; Liu, J. Filling in the Gaps between Nanozymes and Enzymes: Challenges and Opportunities. Bioconjug. Chem. 2017, 28, 2903–2909. [Google Scholar] [CrossRef]
- Dugan, L.L.; Turetsky, D.M.; Du, C.; Lobner, D.; Wheeler, M.; Almli, C.R.; Shen, C.K.-F.; Luh, T.-Y.; Choi, D.W.; Lin, T.-S. Carboxyfullerenes as Neuroprotective Agents. Proc. Natl. Acad. Sci. USA 1997, 94, 9434–9439. [Google Scholar] [CrossRef]
- Ali, S.S.; Hardt, J.I.; Quick, K.L.; Sook Kim-Han, J.; Erlanger, B.F.; Huang, T.; Epstein, C.J.; Dugan, L.L. A Biologically Effective Fullerene (C60) Derivative with Superoxide Dismutase Mimetic Properties. Free Radic. Biol. Med. 2004, 37, 1191–1202. [Google Scholar] [CrossRef]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The Role of Cerium Redox State in the SOD Mimetic Activity of Nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef]
- Li, D.; Liu, B.; Huang, P.-J.J.; Zhang, Z.; Liu, J. Highly Active Fluorogenic Oxidase-Mimicking NiO Nanozymes. Chem. Commun. 2018, 54, 12519–12522. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Huang, Z.; Sun, Y.-M.; Xu, Z.-L.; Liu, J. The Most Active Oxidase-Mimicking Mn2O3 Nanozyme for Biosensor Signal Generation. Chem.—A Eur. J. 2021, 27, 9597–9604. [Google Scholar] [CrossRef] [PubMed]
- Comotti, M.; Della Pina, C.; Matarrese, R.; Rossi, M. The Catalytic Activity of “Naked” Gold Particles. Angew. Chemie Int. Ed. 2004, 43, 5812–5815. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-Like Activity of Polymer-Coated Cerium Oxide Nanoparticles. Angew. Chemie Int. Ed. 2009, 48, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Vinothkumar, G.; Arunkumar, P.; Mahesh, A.; Dhayalan, A.; Suresh Babu, K. Size- and Defect-Controlled Anti-Oxidant Enzyme Mimetic and Radical Scavenging Properties of Cerium Oxide Nanoparticles. New J. Chem. 2018, 42, 18810–18823. [Google Scholar] [CrossRef]
- Chen, M.; Shu, J.; Wang, Z.; Ren, C. Porous Surface MnO2 Microspheres as Oxidase Mimetics for Colorimetric Detection of Sulfite. J. Porous Mater. 2017, 24, 973–977. [Google Scholar] [CrossRef]
- Lopez-Cantu, D.O.; González-González, R.B.; Melchor-Martínez, E.M.; Martínez, S.A.H.; Araújo, R.G.; Parra-Arroyo, L.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Iqbal, H.M.N. Enzyme-Mimicking Capacities of Carbon-Dots Nanozymes: Properties, Catalytic Mechanism, and Applications—A Review. Int. J. Biol. Macromol. 2022, 194, 676–687. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Wu, X.; Gao, X. Mechanism of PH-Switchable Peroxidase and Catalase-like Activities of Gold, Silver, Platinum and Palladium. Biomaterials 2015, 48, 37–44. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Singh, S. Recent Advances and Future Prospects of Iron Oxide Nanoparticles in Biomedicine and Diagnostics. 3 Biotech 2018, 8, 279. [Google Scholar] [CrossRef]
- Mu, J.; Zhang, L.; Zhao, M.; Wang, Y. Co3O4 Nanoparticles as an Efficient Catalase Mimic: Properties, Mechanism and Its Electrocatalytic Sensing Application for Hydrogen Peroxide. J. Mol. Catal. A Chem. 2013, 378, 30–37. [Google Scholar] [CrossRef]
- Mu, J.; Wang, Y.; Zhao, M.; Zhang, L. Intrinsic Peroxidase-like Activity and Catalase-like Activity of Co3O4 Nanoparticles. Chem. Commun. 2012, 48, 2540–2542. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Kan, X. A Facile Nanozyme Based Catalytic Platform for the Selective and Sensitive Detection of Thrombin. Microchem. J. 2022, 172, 106965. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Zhang, C.; Lin, Y.; Lv, J.; Hu, S.; Zhang, S.; Li, M. Colorimetric Determination of Amyloid-β Peptide Using MOF-Derived Nanozyme Based on Porous ZnO-Co3O4 Nanocages. Microchim. Acta 2021, 188, 56. [Google Scholar] [CrossRef]
- Wang, S.; Ma, R.; Zhang, H.; Li, L.; Li, X.; Zhao, Y.; Wang, L.; Mao, X. Construction of a High Affinity Aptamer and an Aptasensor with Chitosan Oligosaccharide-AuNPs@Fe2+ Nanozyme for Highly Sensitive Detection of Phosphatidylserine. Sens. Actuators B Chem. 2022, 362, 131800. [Google Scholar] [CrossRef]
- Wu, H.; Liu, J.; Chen, Z.; Lin, P.; Ou, W.; Wang, Z.; Xiao, W.; Chen, Y.; Cao, D. Mechanism and Application of Surface-Charged Ferrite Nanozyme-Based Biosensor toward Colorimetric Detection of l-Cysteine. Langmuir 2022, 38, 8266–8279. [Google Scholar] [CrossRef]
- Sun, W.; Wang, N.; Zhou, X.; Sheng, Y.; Su, X. Co, N Co-Doped Porous Carbon-Based Nanozyme as an Oxidase Mimic for Fluorescence and Colorimetric Biosensing of Butyrylcholinesterase Activity. Microchim. Acta 2022, 189, 363. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.K.; Omer, K.M. Ultrasensitive Aptamer-Functionalized Cu-MOF Fluorescent Nanozyme as an Optical Biosensor for Detection of C-Reactive Protein. Anal. Biochem. 2022, 658, 114928. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.K.; Omer, K.M. Nanozyme and Stimulated Fluorescent Cu-Based Metal–Organic Frameworks (Cu-MOFs) Functionalized with Engineered Aptamers as a Molecular Recognition Element for Thrombin Detection in the Plasma of COVID-19 Patients. ACS Omega 2022, 7, 36804–36810. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhou, X.; Wang, M.; Su, X. Nanozyme-Based Sensitive Ratiometric Fluorescence Detection Platform for Glucose. Anal. Chim. Acta 2022, 1216, 339993. [Google Scholar] [CrossRef]
- Hou, L.; Huang, Y.; Lin, T.; Ye, F.; Zhao, S. A FRET Ratiometric Fluorescence Biosensor for the Selective Determination of Pyrophosphate Ion and Pyrophosphatase Activity Based on Difunctional Cu-MOF Nanozyme. Biosens. Bioelectron. X 2022, 10, 100101. [Google Scholar] [CrossRef]
- Tian, L.; Qi, J.; Oderinde, O.; Yao, C.; Song, W.; Wang, Y. Planar Intercalated Copper (II) Complex Molecule as Small Molecule Enzyme Mimic Combined with Fe3O4 Nanozyme for Bienzyme Synergistic Catalysis Applied to the MicroRNA Biosensor. Biosens. Bioelectron. 2018, 110, 110–117. [Google Scholar] [CrossRef]
- Yang, Q.; Li, N.; Li, Q.; Chen, S.; Wang, H.-L.; Yang, H. Amperometric Sarcosine Biosensor Based on Hollow Magnetic Pt–Fe3O4@C Nanospheres. Anal. Chim. Acta 2019, 1078, 161–167. [Google Scholar] [CrossRef]
- Tian, L.; Qi, J.; Qian, K.; Oderinde, O.; Liu, Q.; Yao, C.; Song, W.; Wang, Y. Copper (II) Oxide Nanozyme Based Electrochemical Cytosensor for High Sensitive Detection of Circulating Tumor Cells in Breast Cancer. J. Electroanal. Chem. 2018, 812, 1–9. [Google Scholar] [CrossRef]
- Wu, J.; Lv, W.; Yang, Q.; Li, H.; Li, F. Label-Free Homogeneous Electrochemical Detection of MicroRNA Based on Target-Induced Anti-Shielding against the Catalytic Activity of Two-Dimension Nanozyme. Biosens. Bioelectron. 2021, 171, 112707. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Meng, Y.; Zhao, L.; Shi, W.; Wang, X.; He, Z.; Chao, J.; Li, C. Electrochemical/Visual Microfluidic Detection with a Covalent Organic Framework Supported Platinum Nanozyme-Based Device for Early Diagnosis of Pheochromocytoma. Biosens. Bioelectron. 2022, 207, 114208. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, Y.; Wu, Y.; Guo, X.; Ying, Y.; Wen, Y.; Yang, H. Enzyme-Free Tandem Reaction Strategy for Surface-Enhanced Raman Scattering Detection of Glucose by Using the Composite of Au Nanoparticles and Porphyrin-Based Metal–Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 55324–55330. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.-Y.; He, W.-M. Surface-Enhanced Raman Spectroscopy Biosensor Based on Silver Nanoparticles@metal-Organic Frameworks with Peroxidase-Mimicking Activities for Ultrasensitive Monitoring of Blood Cholesterol. Sens. Actuators B Chem. 2022, 365, 131939. [Google Scholar] [CrossRef]
- Huang, Y.; Gu, Y.; Liu, X.; Deng, T.; Dai, S.; Qu, J.; Yang, G.; Qu, L. Reusable Ring-like Fe3O4/Au Nanozymes with Enhanced Peroxidase-like Activities for Colorimetric-SERS Dual-Mode Sensing of Biomolecules in Human Blood. Biosens. Bioelectron. 2022, 209, 114253. [Google Scholar] [CrossRef]
- Tan, Y.; Jiang, H.; Wang, B.; Zhang, X. MoS2-Based Composite Nanozymes with Superior Peroxidase-like Activity for Ultrasensitive SERS Detection of Glucose. New J. Chem. 2021, 45, 19593–19604. [Google Scholar] [CrossRef]
- Zhao, X.; Niu, R.; Fan, S.; Jing, X.; Gao, R.; Yang, H.; Wang, H.; Wang, D.; Yang, Z.; Xie, Y.; et al. A Dual-Mode NADH Biosensor Based on Gold Nanostars Decorated CoFe2 Metal–Organic Frameworks to Reveal Dynamics of Cell Metabolism. ACS Sens. 2022, 7, 2671–2679. [Google Scholar] [CrossRef]
- Attaallah, R.; Amine, A. Highly Selective and Sensitive Detection of Cadmium Ions by Horseradish Peroxidase Enzyme Inhibition Using a Colorimetric Microplate Reader and Smartphone Paper-Based Analytical Device. Microchem. J. 2022, 172, 106940. [Google Scholar] [CrossRef]
- Liu, X.; Mei, X.; Yang, J.; Li, Y. Hydrogel-Involved Colorimetric Platforms Based on Layered Double Oxide Nanozymes for Point-of-Care Detection of Liver-Related Biomarkers. ACS Appl. Mater. Interfaces 2022, 14, 6985–6993. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liao, Y.; Gao, A.; Hao, J.; Shu, D.; Huang, Y.; Zhong, J.; He, C.; Zeng, R. Supercapacitive Behavior of Electrostatic Self-Assembly Reduced Graphene Oxide/CoAl-Layered Double Hydroxides Nanocomposites. J. Alloys Compd. 2016, 669, 146–155. [Google Scholar] [CrossRef]
- Nai, J.; Yin, H.; You, T.; Zheng, L.; Zhang, J.; Wang, P.; Jin, Z.; Tian, Y.; Liu, J.; Tang, Z.; et al. Efficient Electrocatalytic Water Oxidation by Using Amorphous Ni–Co Double Hydroxides Nanocages. Adv. Energy Mater. 2015, 5, 1401880. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Y.; Jiao, L.; Gu, W.; Zhu, C. Amorphous RuTe2 Nanorods as Efficient Peroxidase Mimics for Colorimetric Immunoassay. Sens. Actuators B Chem. 2021, 341, 130007. [Google Scholar] [CrossRef]
- Peng, C.; Hua, M.; Li, N.; Hsu, Y.; Chen, Y.; Chuang, C. Biosensors and Bioelectronics A Colorimetric Immunosensor Based on Self-Linkable Dual-Nanozyme for Ultrasensitive Bladder Cancer Diagnosis and Prognosis Monitoring. Biosens. Bioelectron. 2019, 126, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, Z.; Guan, Y.; Chen, Y.; Liu, W.; Liu, Y. Zeolitic Imidazolate Frameworks-Derived Hollow Co/N-Doped CNTs as Oxidase-Mimic for Colorimetric-Fluorescence Immunoassay of Ochratoxin A. Sens. Actuators B Chem. 2022, 359, 131609. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical Affinity Biosensors Based on Selected Nanostructures for Food and Environmental Monitoring. Sensors 2020, 20, 5125. [Google Scholar] [CrossRef]
- Wang, X.; Qin, L.; Zhou, M.; Lou, Z.; Wei, H. Nanozyme Sensor Arrays for Detecting Versatile Analytes from Small Molecules to Proteins and Cells. Anal. Chem. 2018, 90, 11696–11702. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Q.; Liu, S.; Xiao, H.; Zhang, M.; Zhang, X. Surface Molecular Imprinting on G-C3N4 Photooxidative Nanozyme for Improved Colorimetric Biosensing. Chinese Chem. Lett. 2019, 30, 2186–2190. [Google Scholar] [CrossRef]
- Zhang, X.; Khan, I.M.; Ji, H.; Wang, Z.; Tian, H.; Cao, W.; Mi, W. A Label-Free Fluorescent Aptasensor for Detection of Staphylococcal Enterotoxin A Based on Aptamer-Functionalized Silver Nanoclusters. Polymers 2020, 12, 152. [Google Scholar] [CrossRef]
- Serrano-Andrés, L.; Serrano-Pérez, J.J. Calculation of Excited States: Molecular Photophysics and Photochemistry on Display BT. In Handbook of Computational Chemistry; Leszczynski, J., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 483–560. ISBN 978-94-007-0711-5. [Google Scholar]
- Furukawa, K.; Ueno, Y.; Takamura, M.; Hibino, H. Graphene FRET Aptasensor. ACS Sens. 2016, 1, 710–716. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Zhou, X.; Sun, H.; Su, X. Sensors and Actuators: B. Chemical Fluorescence Sensing Strategy for Xanthine Assay Based on Gold Nanoclusters and Nanozyme. Sens. Actuators B. Chem. 2022, 358, 131488. [Google Scholar] [CrossRef]
- Guo, J.; Wu, S.; Wang, Y.; Zhao, M. Sensors and Actuators B: Chemical A Label-Free Fl Uorescence Biosensor Based on a Bifunctional MIL-101(Fe) Nanozyme for Sensitive Detection of Choline and Acetylcholine at Nanomolar Level. Sens. Actuators B. Chem. 2020, 312, 128021. [Google Scholar] [CrossRef]
- Li, W.; Li, T.; Chen, S.; Deng, D.; Ji, Y.; Li, R. Sensors and Actuators: B. Chemical Nanozyme-Mediated Cascade Reaction System for Ratiometric Fluorescence Detection of Sarcosine. Sens. Actuators B. Chem. 2022, 355, 131341. [Google Scholar] [CrossRef]
- Rizwan, M.; Elma, S.; Lim, S.A.; Ahmed, M.U. Biosensors and Bioelectronics AuNPs/CNOs/SWCNTs/Chitosan-Nanocomposite Modi Fi Ed Electrochemical Sensor for the Label-Free Detection of Carcinoembryonic Antigen. Biosens. Bioelectron. 2018, 107, 211–217. [Google Scholar] [CrossRef]
- Kurup, C.P.; Mohd-Naim, N.F.; Tlili, C.; Ahmed, M.U. A Highly Sensitive Label-Free Aptasensor Based on Gold Nanourchins and Carbon Nanohorns for the Detection of Lipocalin-2 (LCN-2). Anal. Sci. 2021, 37, 825–831. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Wang, X.; Han, L. Flow-Homogeneous Electrochemical Sensing System Based on 2D Metal-Organic Framework Nanozyme for Successive MicroRNA Assay. Biosens. Bioelectron. 2022, 206, 114120. [Google Scholar] [CrossRef]
- Lim, S.A.; Ahmed, M.U. A Label Free Electrochemical Immunosensor for Sensitive Detection of Porcine Serum Albumin as a Marker for Pork Adulteration in Raw Meat. Food Chem. 2016, 206, 197–203. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Rao, Z.; Dhanjai; Wu, J.; Shen, Y.; Boddula, R.; Huang, Y. Electrochemical (Bio) Sensors Go Green. Biosens. Bioelectron. 2020, 163, 112270. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhu, A.; Tian, Y. In Vivo Electrochemical Biosensors: Recent Advances in Molecular Design, Electrode Materials, and Electrochemical Devices. Anal. Chem. 2023, 95, 388–406. [Google Scholar] [CrossRef]
- Hong, S.P.; Zakaria, S.N.A.; Ahmed, M.U. Trends in the Development of Immunoassays for Mycotoxins and Food Allergens Using Gold and Carbon Nanostructured Material. Food Chem. Adv. 2022, 1, 100069. [Google Scholar] [CrossRef]
- Ansari, A.A.; Malhotra, B.D. Current Progress in Organic–Inorganic Hetero-Nano-Interfaces Based Electrochemical Biosensors for Healthcare Monitoring. Coord. Chem. Rev. 2022, 452, 214282. [Google Scholar] [CrossRef]
- Mohamad, A.; Rizwan, M.; Keasberry, N.A.; Nguyen, A.S.; Lam, T.D.; Ahmed, M.U. Gold-Microrods/Pd-Nanoparticles/Polyaniline-Nanocomposite-Interface as a Peroxidase-Mimic for Sensitive Detection of Tropomyosin. Biosens. Bioelectron. 2020, 155, 112108. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Zhang, Q.; Wang, H.; Zhang, Y.; Cao, W.; Zhang, N.; Du, B.; Wei, Q. Electrochemical Aptasensor Based on Gold Modified Graphene Nanocomposite with Different Morphologies for Ultrasensitive Detection of Pb2+. Sens. Actuators B Chem. 2019, 288, 325–331. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Nanozymes in Electrochemical Affinity Biosensing. Microchim. Acta 2020, 187, 423. [Google Scholar] [CrossRef]
- Dinu Gugoasa, L.A.; Pogacean, F.; Kurbanoglu, S.; Tudoran, L.-B.; Serban, A.B.; Kacso, I.; Pruneanu, S. Graphene-Gold Nanoparticles Nanozyme-Based Electrochemical Sensor with Enhanced Laccase-Like Activity for Determination of Phenolic Substrates. J. Electrochem. Soc. 2021, 168, 067523. [Google Scholar] [CrossRef]
- Meilikhov, M.; Yusenko, K.; Esken, D.; Turner, S.; Van Tendeloo, G.; Fischer, R.A. Metals@MOFs—Loading MOFs with Metal Nanoparticles for Hybrid Functions. Eur. J. Inorg. Chem. 2010, 2010, 3701–3714. [Google Scholar] [CrossRef]
- Wang, X.; Lan, P.C.; Ma, S. Metal–Organic Frameworks for Enzyme Immobilization: Beyond Host Matrix Materials. ACS Cent. Sci. 2020, 6, 1497–1506. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, Y.; Li, X.; Yue, Q.; Dong, X.; Du, B.; Cao, W.; Wei, Q. Dual-Quenching Electrochemiluminescence Strategy Based on Three-Dimensional Metal–Organic Frameworks for Ultrasensitive Detection of Amyloid-β. Anal. Chem. 2019, 91, 1989–1996. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Homogeneous Assays Using Aptamers. Analyst 2011, 136, 257–274. [Google Scholar] [CrossRef]
- Zhang, F.-T.; Cai, L.-Y.; Zhou, Y.-L.; Zhang, X.-X. Immobilization-Free DNA-Based Homogeneous Electrochemical Biosensors. TrAC Trends Anal. Chem. 2016, 85, 17–32. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Ye, Z.; Zhao, M.; Huang, J. Synthesis of ZnO Micro-Pompons by Soft Template-Directed Wet Chemical Method and Their Application in Electrochemical Biosensors. Electrochim. Acta 2014, 115, 277–282. [Google Scholar] [CrossRef]
- Das, R.S.; Agrawal, Y.K. Raman Spectroscopy: Recent Advancements, Techniques and Applications. Vib. Spectrosc. 2011, 57, 163–176. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Yaraki, M.T.; Tan, Y.N. Metal Nanoparticles-Enhanced Biosensors: Synthesis, Design and Applications in Fluorescence Enhancement and Surface-Enhanced Raman Scattering. Chem.—An Asian J. 2020, 15, 3180–3208. [Google Scholar] [CrossRef]
- Mu, M.; Wen, S.; Hu, S.; Zhao, B.; Song, W. Putting Surface-Enhanced Raman Spectroscopy to Work for Nanozyme Research: Methods, Materials and Applications. TrAC—Trends Anal. Chem. 2022, 152, 116603. [Google Scholar] [CrossRef]
- Yao, D.; Li, C.; Liang, A.; Jiang, Z. A Facile SERS Strategy for Quantitative Analysis of Trace Glucose Coupling Glucose Oxidase and Nanosilver Catalytic Oxidation of Tetramethylbenzidine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 216, 146–153. [Google Scholar] [CrossRef]
- Xia, X.; Weng, Y.; Zhang, L.; Tang, R.; Zhang, X. A Facile SERS Strategy to Detect Glucose Utilizing Tandem Enzyme Activities of Au@Ag Nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 259, 119889. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhang, Y.-J.; Ding, S.-Y.; Panneerselvam, R.; Tian, Z.-Q. Core–Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Jin, J.; Song, W.; Wang, J.; Li, L.; Tian, Y.; Zhu, S.; Zhang, Y.; Xu, S.; Yang, B.; Zhao, B. A Highly Sensitive SERS Platform Based on Small-Sized Ag/GQDs Nanozyme for Intracellular Analysis. Chem. Eng. J. 2022, 430, 132687. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Saaem, I.; Wu, P.C.; Brown, A.S. Personalized Diagnostics and Biosensors: A Review of the Biology and Technology Needed for Personalized Medicine. Crit. Rev. Biotechnol. 2014, 34, 180–196. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).