Generation of Dynamic Concentration Profile Using A Microfluidic Device Integrating Pneumatic Microvalves
Abstract
1. Introduction
2. Materials and Methods
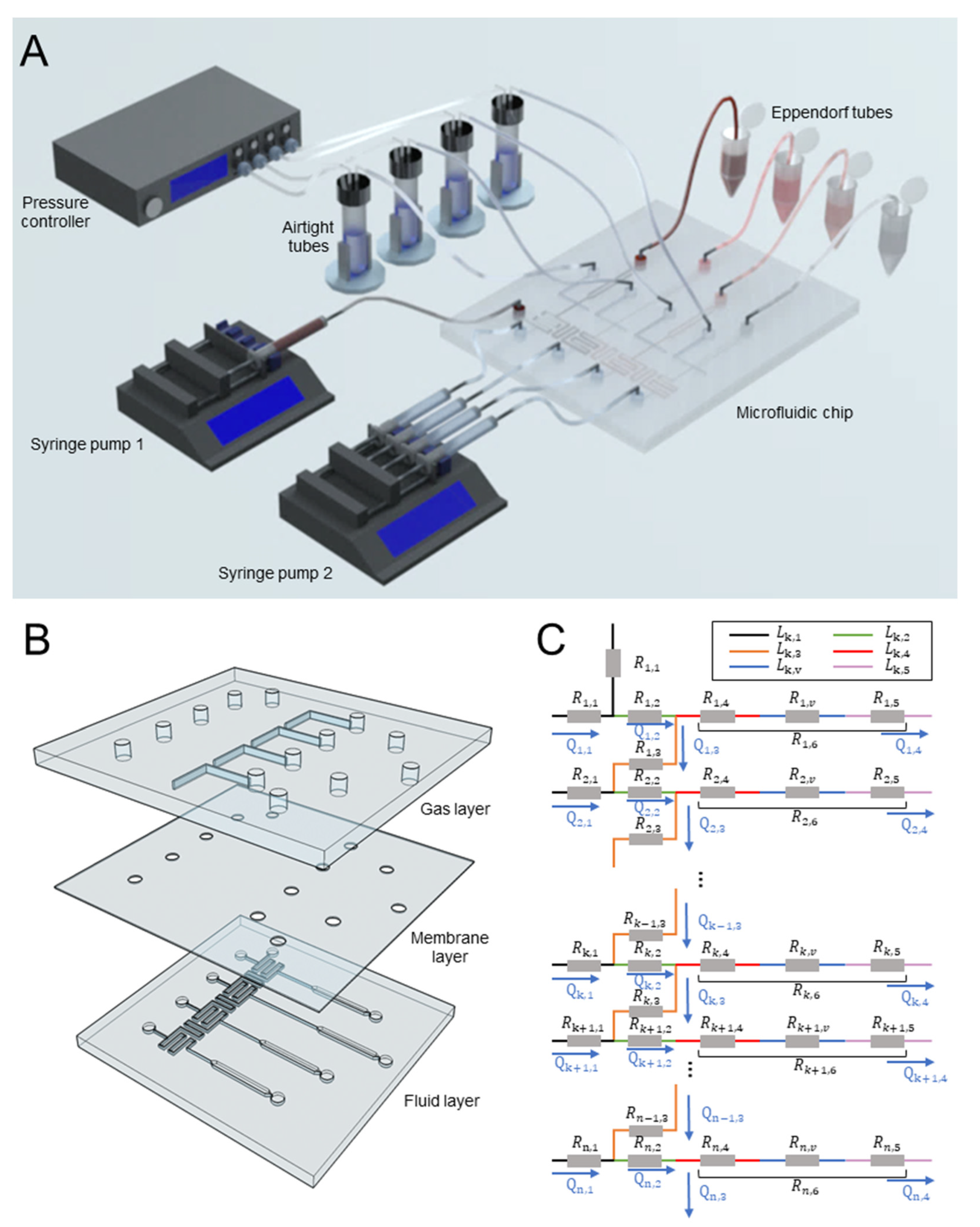
2.1. Design and Fabrication
2.2. Concentration Prediction
2.3. Simulation of The Valve Deformation and Flow Resistance
2.4. Flow Resistance Measurement
2.5. Characterization of the Flow in Microvalves
2.6. Mixing Characterization
2.7. Generation of Dynamic Concentration and Data Analysis
3. Results and Discussion
3.1. Flow Resistance Calculation for Dynamic Concentration Profile
3.2. Simulation of The Valve Deformation and Flow Resistance
3.3. Flow Resistance Measurement
3.4. Characterization of The Flow in Microvalves
3.5. Mixing Characterization
3.6. Generation of Dynamic Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucchetta, E.; Lee, J.; Fu, L.; Patel, N.H.; Ismagilov, R.F. Dynamics of Drosophila embryonic patterning network perturbed in space and time using microfluidics. Nature 2005, 434, 1134–1138. [Google Scholar] [CrossRef]
- Wang, X.; Christov, I.C. Theory of the flow-induced deformation of shallow compliant microchannels with thick walls. Proc. Math. Phys. Eng. Sci. 2019, 475, 20190513. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, Z.; Bai, S.; Lu, H.; Xu, D.; Chen, C.; Liu, D.; Zhu, Y. Microfluidic models of physiological or pathological flow shear stress for cell biology, disease modeling and drug development. TrAC Trends Anal. Chem. 2019, 117, 186–199. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Virumbrales-Muñoz, M.; Lang, J.M.; Beebe, D.J. A role for microfluidic systems in precision medicine. Nat. Commun. 2022, 13, 3086. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Xiong, R.; Braeckmans, K.; Scheid, B.; Huang, C.; Sauvage, F.; De, S.C. Concentration Gradients in Material Sciences: Methods to Design and Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2009005. [Google Scholar] [CrossRef]
- Berthier, E.; Beebe, D.J. Gradient generation platforms: New directions for an established microfluidic technology. Lab Chip 2014, 14, 3241–3247. [Google Scholar] [CrossRef]
- Boneschansker, L.; Yan, J.; Wong, E.; Briscoe, D.M.; Irimia, D. Microfluidic platform for the quantitative analysis of leukocyte migration signatures. Nat. Commun. 2014, 5, 4787. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.; Zielinski, A.; Weber, C.; Arcizet, D.; Youssef, S.; Franosch, T.; Rädler, J.O.; Heinrich, D. Chemotactic cell trapping in controlled alternating gradient fields. Proc. Natl. Acad. Sci. USA 2011, 108, 11417–11422. [Google Scholar] [CrossRef]
- Berendsen, J.T.W.; Kruit, S.A.; Atak, N.; Willink, E.; Segerink, L.I. Flow-Free Microfluidic Device for Quantifying Chemotaxis in Spermatozoa. Anal. Chem. 2020, 92, 3302–3306. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Nsamela, A.; Garlan, B.; Simmchen, J. A Platform for Stop-Flow Gradient Generation to Investigate Chemotaxis. Angew. Chem. Int. Ed. Engl. 2022, 61, e202117768. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.L.; Dertinger, S.K.; Chiu, D.T.; Choi, I.S.; Stroock, A.D.; Whitesides, G.M. Generation of Solution and Surface Gradients Using Microfluidic Systems. Langmuir 2000, 16, 8311–8316. [Google Scholar] [CrossRef]
- Hattori, K.; Sugiura, S.; Kanamori, T. Generation of arbitrary monotonic concentration profiles by a serial dilution microfluidic network composed of microchannels with a high fluidic-resistance ratio. Lab Chip 2009, 9, 1763–1772. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, Q.; Dertinger, S.K.; Stroock, A.D.; Fu, T.M.; Whitesides, G.M. A general method for patterning gradients of biomolecules on surfaces using microfluidic networks. Anal. Chem. 2005, 77, 2338–2347. [Google Scholar] [CrossRef]
- Mao, H.; Cremer, P.S.; Manson, M.D. A sensitive, versatile microfluidic assay for bacterial chemotaxis. Proc. Natl. Acad. Sci. USA 2003, 100, 5449–5454. [Google Scholar] [CrossRef]
- Holden, M.A.; Kumar, S.; Castellana, E.T.; Beskok, A.; Cremer, P.S. Generating fixed concentration arrays in a microfluidic device. Sens. Actuators B Chem. 2003, 92, 199–207. [Google Scholar] [CrossRef]
- Walker, G. Cell infection within a microfluidic device using virus gradients. Sens. Actuators B Chem. 2004, 98, 347–355. [Google Scholar] [CrossRef]
- Keenan, T.M.; Frevert, C.W.; Wu, A.; Wong, V.; Folch, A. A new method for studying gradient-induced neutrophil desensitization based on an open microfluidic chamber. Lab Chip 2010, 10, 116–122. [Google Scholar] [CrossRef]
- Shamloo, A.; Ma, N.; Poo, M.M.; Sohn, L.L.; Heilshorn, S.C. Endothelial cell polarization and chemotaxis in a microfluidic device. Lab Chip 2008, 8, 1292–1299. [Google Scholar] [CrossRef]
- Diao, J.; Young, L.; Kim, S.; Fogarty, E.A.; Heilman, S.M.; Zhou, P.; Shuler, M.L.; Wu, M.; DeLisa, M.P. A three-channel microfluidic device for generating static linear gradients and its application to the quantitative analysis of bacterial chemotaxis. Lab Chip 2006, 6, 381–388. [Google Scholar] [CrossRef]
- Xu, H.; Ferreira, M.M.; Heilshorn, S.C. Small-molecule axon-polarization studies enabled by a shear-free microfluidic gradient generator. Lab Chip 2014, 14, 2047–2056. [Google Scholar] [CrossRef]
- Kim, M.; Kim, T. Diffusion-based and long-range concentration gradients of multiple chemicals for bacterial chemotaxis assays. Anal. Chem. 2010, 82, 9401–9409. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Folch, A. Large-scale microfluidic gradient arrays reveal axon guidance behaviors in hippocampal neurons. Microsyst. Nanoeng. 2017, 3, 17003. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Ellett, F.; Jorgensen, J.; Irimia, D. Temporal gradients limit the accumulation of neutrophils towards sources of chemoattractant. Microsyst. Nanoeng. 2017, 3, 16067. [Google Scholar] [CrossRef]
- Lin, S.J.; Chao, P.H.; Cheng, H.W.; Wang, J.K.; Wang, Y.L.; Han, Y.Y.; Huang, N.T. An antibiotic concentration gradient microfluidic device integrating surface-enhanced Raman spectroscopy for multiplex antimicrobial susceptibility testing. Lab Chip 2022, 22, 1805–1814. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, P.; Li, S.; Sha, Q.; Li, P.; Zeng, X.; Feng, X.; Du, W.; Liu, B.F. Hand-powered vacuum-driven microfluidic gradient generator for high-throughput antimicrobial susceptibility testing. Biosens. Bioelectron. 2022, 205, 114100. [Google Scholar] [CrossRef]
- Oh, K.W.; Lee, K.; Ahn, B.; Furlani, E.P. Design of pressure-driven microfluidic networks using electric circuit analogy. Lab Chip 2012, 12, 515–545. [Google Scholar] [CrossRef]
- Yamada, M.; Hirano, T.; Yasuda, M.; Seki, M. A microfluidic flow distributor generating stepwise concentrations for high-throughput biochemical processing. Lab Chip 2006, 6, 179–184. [Google Scholar] [CrossRef]
- Walker, G.M.; Monteiro-Riviere, N.; Rouse, J.; O’Neill, A.T. A linear dilution microfluidic device for cytotoxicity assays. Lab Chip 2007, 7, 226–232. [Google Scholar] [CrossRef]
- Paduthol, G.; Korma, T.S.; Agrawal, A.; Paul, D. Dynamic generation of power function gradient profiles in a universal microfluidic gradient generator by controlling the inlet flow rates. Lab Chip 2022, 22, 592–604. [Google Scholar] [CrossRef]
- Liu, X.; Jia, Y.; Han, Z.; Hou, Q.; Zhang, W.; Zheng, W.; Jiang, X. Integrating a Concentration Gradient Generator and a Single-Cell Trapper Array for High-Throughput Screening the Bioeffects of Nanomaterials. Angew. Chem. Int. Ed. Engl. 2021, 60, 12319–12322. [Google Scholar] [CrossRef]
- Dertinger, S.K.W.; Chiu, D.T.; Jeon, N.L.; Whitesides, G.M. Generation of Gradients Having Complex Shapes Using Microfluidic Networks. Anal. Chem. 2001, 73, 1240–1246. [Google Scholar] [CrossRef]
- Irimia, D.; Geba, D.A.; Toner, M. Universal Microfluidic Gradient Generator. Anal. Chem. 2006, 78, 3472–3477. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Groisman, A. Generation of complex concentration profiles in microchannels in a logarithmically small number of steps. Lab Chip 2007, 7, 264–272. [Google Scholar] [CrossRef]
- Amarie, D.; Glazier, J.A.; Jacobson, S.C. Compact Microfluidic Structures for Generating Spatial and Temporal Gradients. Anal. Chem. 2007, 79, 9471. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Saadi, W.; Rhee, S.W.; Wang, S.J.; Mittal, S.; Jeon, N.L. Generation of dynamic temporal and spatial concentration gradients using microfluidic devices. Lab Chip 2004, 4, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, C.; Ahn, B.; Panchapakesan, R.; Full, A.R.; Nordee, L.; Kang, J.Y.; Oh, K.W. Generalized serial dilution module for monotonic and arbitrary microfluidic gradient generators. Lab Chip 2009, 9, 709–717. [Google Scholar] [CrossRef]
- Hosokawa, M.; Hayashi, T.; Mori, T.; Yoshino, T.; Nakasono, S.; Matsunaga, T. Microfluidic device with chemical gradient for single-cell cytotoxicity assays. Anal. Chem. 2011, 83, 3648–3654. [Google Scholar] [CrossRef]
- Zheng, G.; Lu, L.; Yang, Y.; Wei, J.; Han, B.; Zhang, Q.; Wang, Y. Development of Microfluidic Dilution Network-Based System for Lab-on-a-Chip Microalgal Bioassays. Anal. Chem. 2018, 90, 13280–13289. [Google Scholar] [CrossRef]
- Skoge, M.; Yue, H.; Erickstad, M.; Bae, A.; Levine, H.; Groisman, A.; Loomis, W.F.; Rappel, W.J. Cellular memory in eukaryotic chemotaxis. Proc. Natl. Acad. Sci. USA 2014, 111, 14448–14453. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef]
- Chen, H.; Cornwell, J.; Zhang, H.; Lim, T.; Resurreccion, R.; Port, T.; Rosengarten, G.; Nordon, R.E. Cardiac-like flow generator for long-term imaging of endothelial cell responses to circulatory pulsatile flow at microscale. Lab Chip 2013, 13, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Li, B.; Qin, J.; Lin, B. Microvalve-actuated precise control of individual droplets in microfluidic devices. Lab Chip 2009, 9, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.H.; Jin, S.H.; Lee, B.J.; Kim, T.; Lee, C.S. Microfluidic static droplet array for analyzing microbial communication on a population gradient. Lab Chip 2015, 15, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, M.; Jensen, E.C.; Mathies, R.A. Lifting Gate Polydimethylsiloxane Microvalves and Pumps for Microfluidic Control. Anal. Chem. 2012, 84, 2067–2071. [Google Scholar] [CrossRef]
- Chen, C.; Xu, D.; Bai, S.; Yu, Z.; Zhu, Y.; Xing, X.; Chen, H. Dynamic screening and printing of single cells using a microfluidic chip with dual microvalves. Lab Chip 2020, 20, 1227–1237. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Xu, D.; Alam, M.M.; Shui, L.; Chen, H. Cell elasticity measurement using a microfluidic device with real-time pressure feedback. Lab Chip 2020, 20, 2343–2353. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J.; Wolvetang, E.; Cooper-White, J. High-throughput, deterministic single cell trapping and long-term clonal cell culture in microfluidic devices. Lab Chip 2014, 15, 1072–1083. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Y.; Ho, J.W.; Chen, H. The method to dynamically screen and print single cells using microfluidics with pneumatic microvalves. MethodsX 2021, 8, 101190. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Sugiura, S.; Hattori, K.; Kanamori, T. Microfluidic Serial Dilution Cell-Based Assay for Analyzing Drug Dose Response over a Wide Concentration Range. Anal. Chem. 2010, 82, 8278–8282. [Google Scholar] [CrossRef]
- Irimia, D.; Liu, S.Y.; Tharp, W.G.; Samadani, A.; Toner, M.; Poznansky, M.C. Microfluidic system for measuring neutrophil migratory responses to fast switches of chemical gradients. Lab Chip 2006, 6, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Bruus, H. Theoretical Microfluidics; Oxford University Press: New York, NY, USA, 2008. [Google Scholar]
- Suteria, N.S.; Nekouei, M.; Vanapalli, S.A. Microfluidic bypass manometry: Highly parallelized measurement of flow resistance of complex channel geometries and trapped droplets. Lab Chip 2018, 18, 343–355. [Google Scholar] [CrossRef]
- Kim, C.; Lee, K.; Kim, J.H.; Shin, K.S.; Lee, K.-J.; Kim, T.S.; Kang, J.Y. A serial dilution microfluidic device using a ladder network generating logarithmic or linear concentrations. Lab Chip 2008, 8, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, C.; Bai, S.; Gao, Y.; Metcalfe, G.; Cheng, W.; Zhu, Y. Multiplexed detection of cancer biomarkers using a microfluidic platform integrating single bead trapping and acoustic mixing techniques. Nanoscale 2018, 10, 20196–20206. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Chan, C.Y.; Lin, S.-C.S.; Muddana, H.S.; Nama, N.; Benkovic, S.J.; Jun Huang, T. Tunable, pulsatile chemical gradient generation via acoustically driven oscillating bubbles. Lab Chip 2013, 13, 328–331. [Google Scholar] [CrossRef]
- Liu, B.; Ma, Z.; Yang, J.; Gao, G.; Liu, H. A Concentration Gradients Tunable Generator with Adjustable Position of the Acoustically Oscillating Bubbles. Micromachines 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, K.; Xia, Y.; Gui, J.; Wu, Z.; Cui, H.; Wu, Z.; Liu, W.; Zhao, X.; Guo, S. A localized surface acoustic wave applied spatiotemporally controllable chemical gradient generator. Biomicrofluidics 2020, 14, 024106. [Google Scholar] [CrossRef]
- Amini, H.; Sollier, E.; Masaeli, M.; Xie, Y.; Ganapathysubramanian, B.; Stone, H.A.; di Carlo, D. Engineering fluid flow using sequenced microstructures. Nat. Commun. 2013, 4, 1826. [Google Scholar] [CrossRef]
- Marschewski, J.; Jung, S.; Ruch, P.; Prasad, N.; Mazzotti, S.; Michel, B.; Poulikakos, D. Mixing with herringbone-inspired microstructures: Overcoming the diffusion limit in co-laminar microfluidic devices. Lab Chip 2015, 15, 1923–1933. [Google Scholar] [CrossRef]
- Jiang, X.; Ng, J.M.K.; Stroock, A.; Dertinger, S.K.W.; Whitesides, G.M.J. A miniaturized, parallel, serially diluted immunoassay for analyzing multiple antigens. J. Am. Chem. Soc. 2003, 125, 5294–5295. [Google Scholar] [CrossRef]
- Chen, C.; Meng, H.; Guo, T.; Deshpande, S.; Chen, H. Development of Paper Microfluidics with 3D-Printed PDMS Barriers for Flow Control. ACS Appl. Mater. Interfaces 2022, 14, 40286–40296. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, L.; Zhang, H.; Shen, X.; Zhu, Y.; Chen, H. Novel Wax Valves To Improve Distance-Based Analyte Detection in Paper Microfluidics. Anal. Chem. 2019, 91, 5169–5175. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Chen, C.; Zhu, Y.; Li, Z.; Ye, F.; Ho, J.W.; Chen, H. Automatic flow delay through passive wax valves for paper-based analytical devices. Lab Chip 2021, 21, 4166–4176. [Google Scholar] [CrossRef]
- Liu, B.; Ran, B.; Chen, C.; Shi, L.; Liu, Y.; Chen, H.; Zhu, Y. A low-cost and high-performance 3D micromixer over a wide working range and its application for high-sensitivity biomarker detection. React. Chem. Eng. 2022. [Google Scholar] [CrossRef]
- Isaksson, J.; Kjäll, P.; Nilsson, D.; Robinson, N.; Berggren, M.; Richter-Dahlfors, A. Electronic control of Ca2+ signalling in neuronal cells using an organic electronic ion pump. Nat. Mater. 2007, 6, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Roh, H.; Park, J.-K. Finger-Actuated Microfluidic Concentration Gradient Generator Compatible with a Microplate. Micromachines 2019, 10, 174. [Google Scholar] [CrossRef]
- Moorjani, S.; Nielson, R.; Chang, X.A.; Shear, J.B. Dynamic remodeling of subcellular chemical gradients using a multi-directional flow device. Lab Chip 2010, 10, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Holmes, W.R.; Wang, C.J.; Ueno, T.; Harwell, A.; Edelstein-Keshet, L.; Inoue, T.; Levchenko, A. Synthetic spatially graded Rac activation drives cell polarization and movement. Proc. Natl. Acad. Sci. USA 2012, 109, E3668–E3677. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Li, P.; Guo, T.; Chen, S.; Xu, D.; Chen, H. Generation of Dynamic Concentration Profile Using A Microfluidic Device Integrating Pneumatic Microvalves. Biosensors 2022, 12, 868. https://doi.org/10.3390/bios12100868
Chen C, Li P, Guo T, Chen S, Xu D, Chen H. Generation of Dynamic Concentration Profile Using A Microfluidic Device Integrating Pneumatic Microvalves. Biosensors. 2022; 12(10):868. https://doi.org/10.3390/bios12100868
Chicago/Turabian StyleChen, Chang, Panpan Li, Tianruo Guo, Siyuan Chen, Dong Xu, and Huaying Chen. 2022. "Generation of Dynamic Concentration Profile Using A Microfluidic Device Integrating Pneumatic Microvalves" Biosensors 12, no. 10: 868. https://doi.org/10.3390/bios12100868
APA StyleChen, C., Li, P., Guo, T., Chen, S., Xu, D., & Chen, H. (2022). Generation of Dynamic Concentration Profile Using A Microfluidic Device Integrating Pneumatic Microvalves. Biosensors, 12(10), 868. https://doi.org/10.3390/bios12100868





