Highly Sensitive Plasmonic Biosensors with Precise Phase Singularity Coupling on the Metastructures
Abstract
1. Introduction
2. Materials and Methods
3. Results
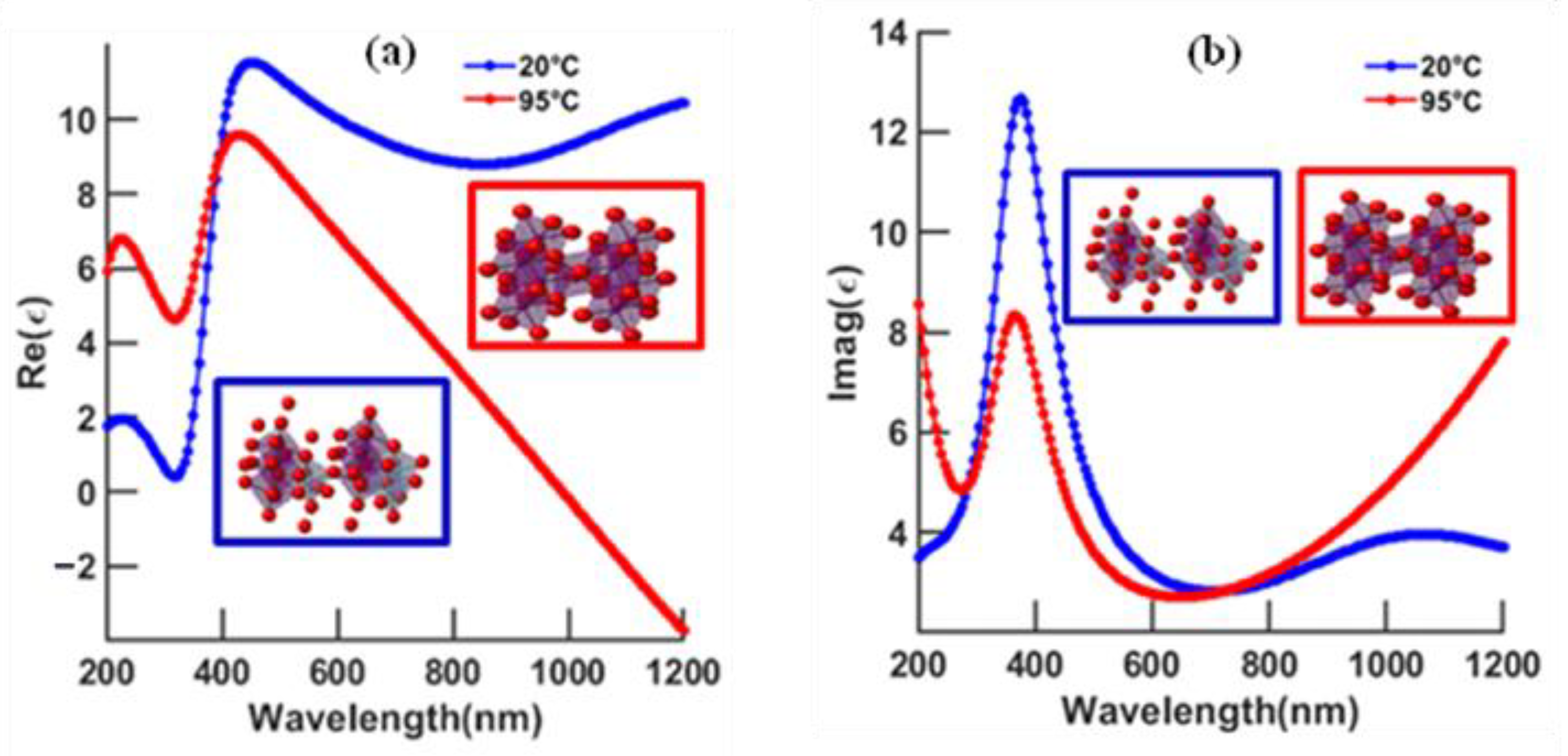
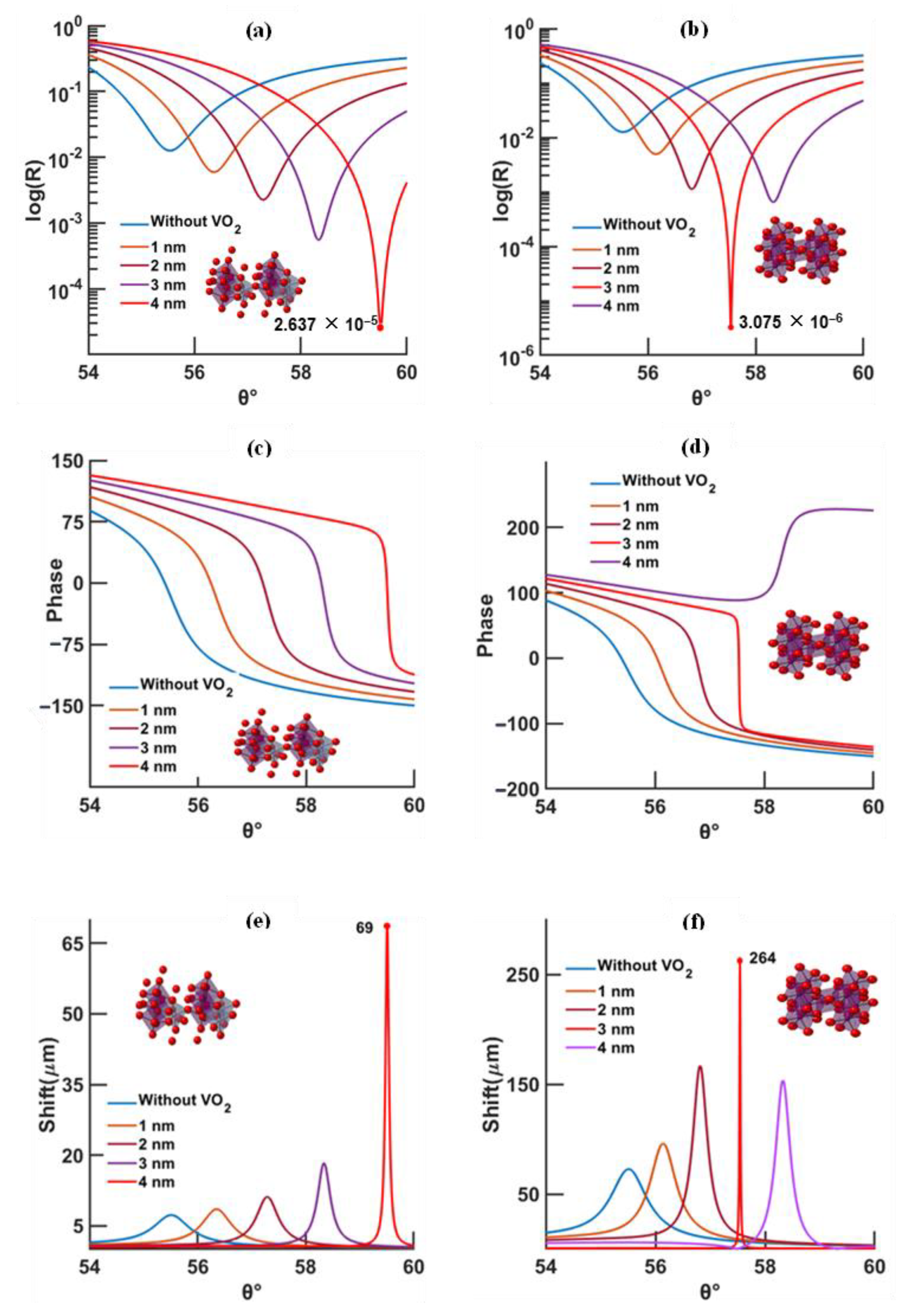
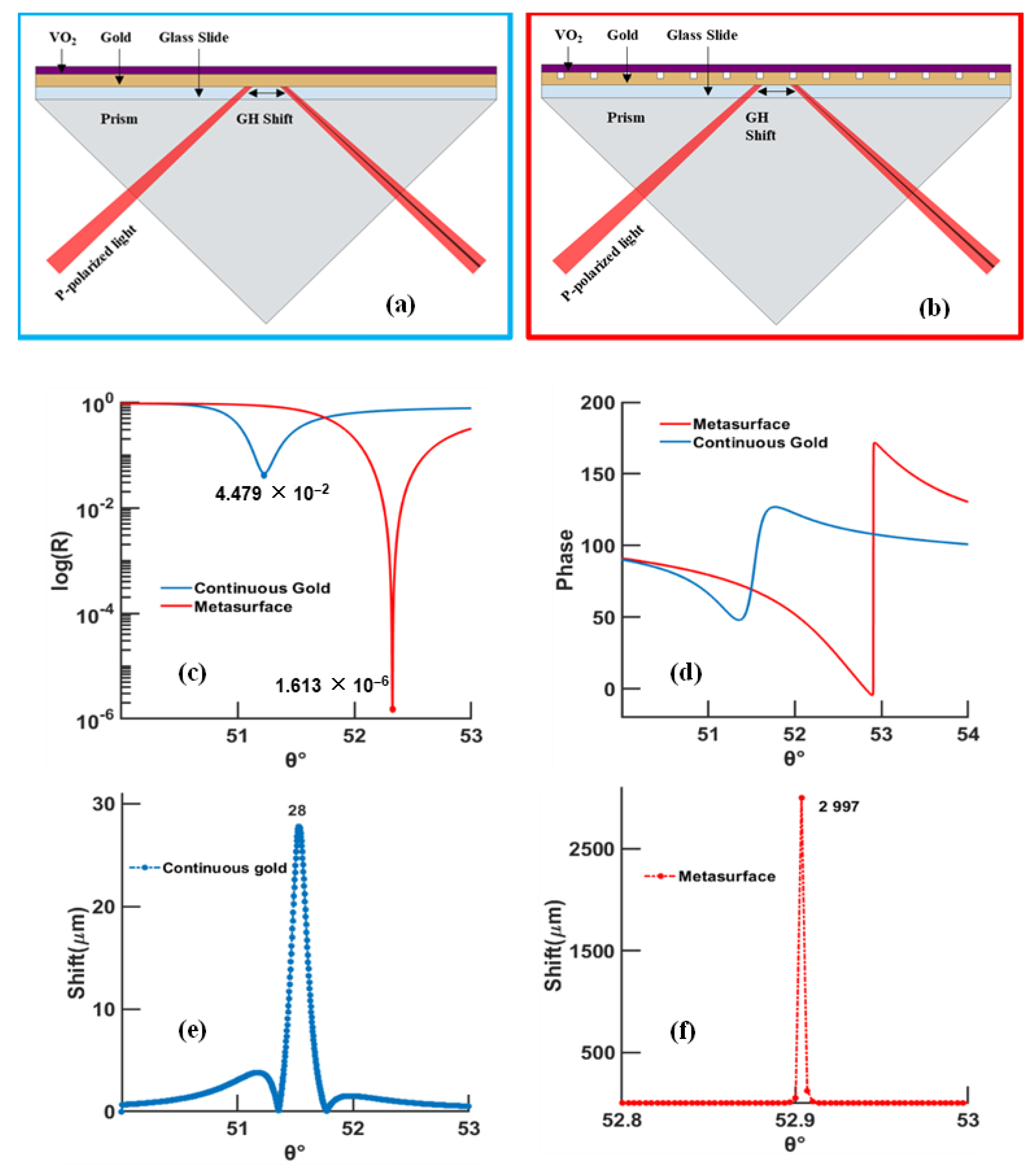
3.1. Continuous Gold Model
- At higher temperatures and wavelengths, decreases in the resonance angles for each thickness of the phase-change material are shown;
- At higher temperature, less VO2 thickness is needed to reach the minimum reflectivity compared to low temperature;
- At longer wavelength, the minimum reflectivity occurs for less VO2 thickness than for a shorter wavelength;
- The lowest reflectivity and highest GH shift occur at 630 nm and 95 °C, and, at a longer wavelength (785 nm), the lowest values occur at 20 °C.
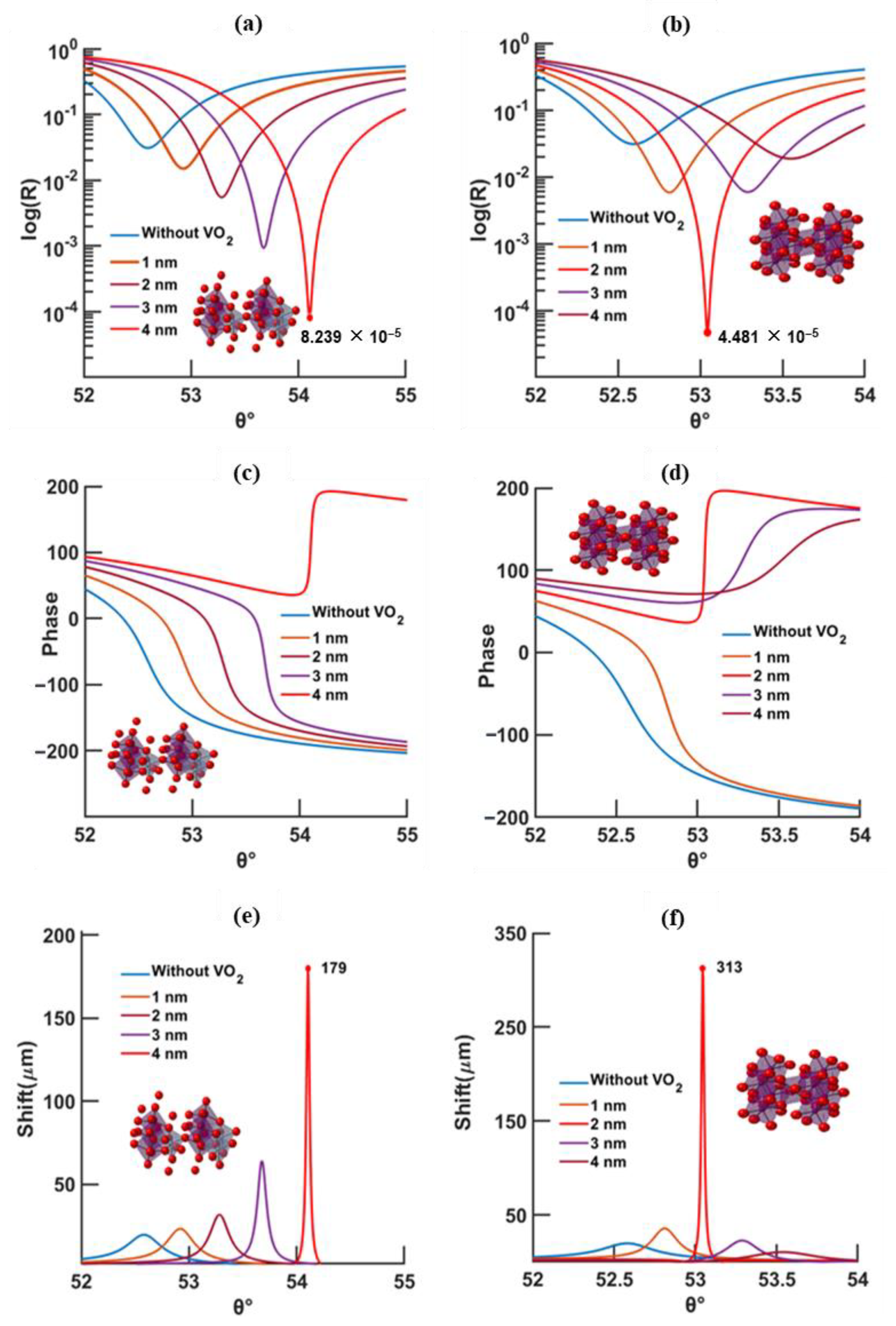
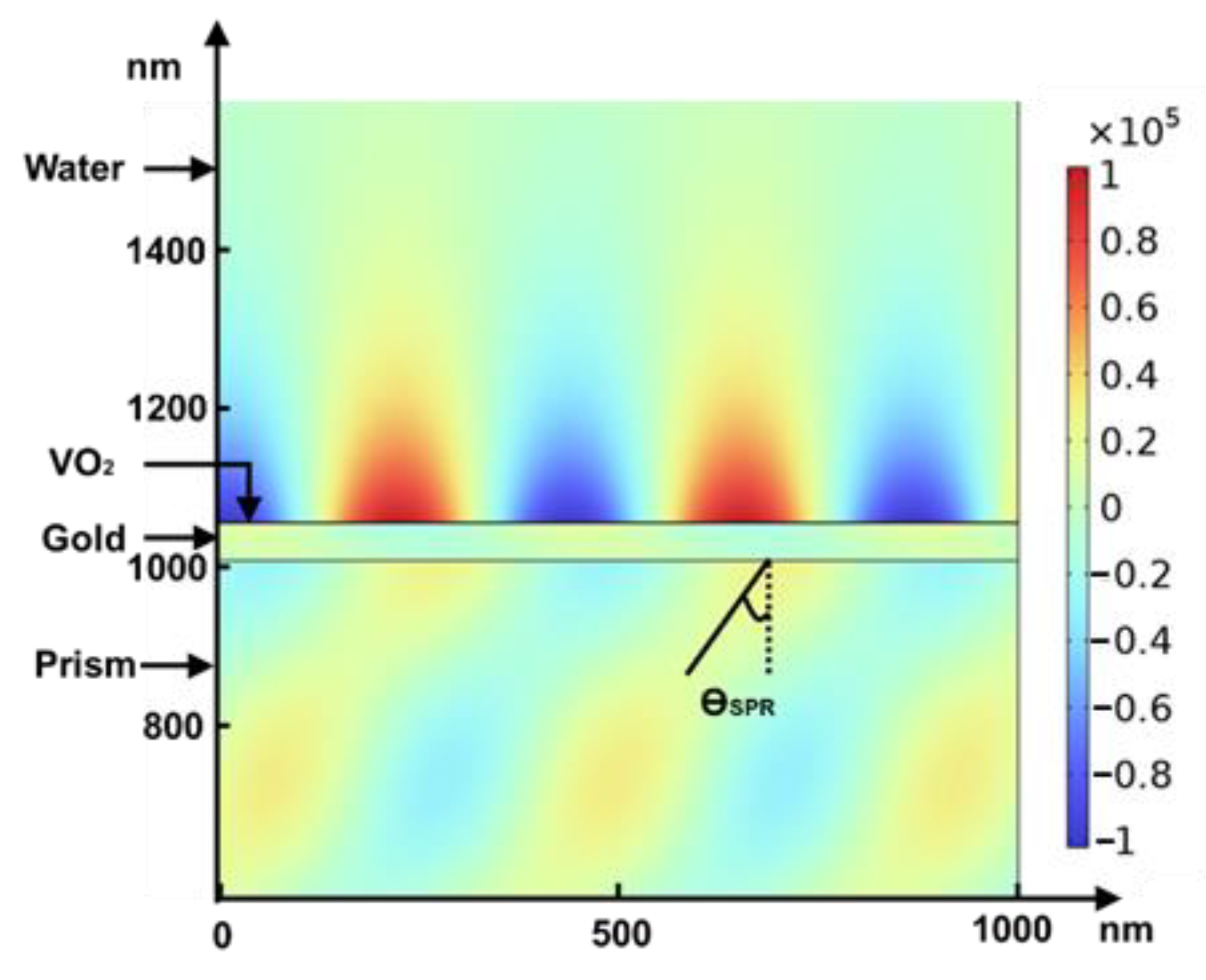
3.2. Design with Metasurface
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 18 June 2022).
- Cancer Statistics—NCI. Available online: https://www.cancer.gov/about-cancer/understanding/statistics (accessed on 9 May 2022).
- Wang, Y.; Zeng, S.; Crunteanu, A.; Xie, Z.; Humbert, G.; Ma, L.; Wei, Y.; Brunel, A.; Bessette, B.; Orlianges, J.-C.; et al. Targeted Sub-Attomole Cancer Biomarker Detection Based on Phase Singularity 2D Nanomaterial-Enhanced Plasmonic Biosensor. Nano-Micro Lett. 2021, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Understanding Laboratory Tests Fact Sheet—NCI. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/understanding-lab-tests-fact-sheet (accessed on 18 June 2022).
- Mousa, S. Biosensors: The new wave in cancer diagnosis. NSA 2010, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kooyman, R.P.H. Chapter 2. Physics of Surface Plasmon Resonance. In Handbook of Surface Plasmon Resonance; Schasfoort, R.B.M., Tudos, A.J., Eds.; Royal Society of Chemistry: Cambridge, UK, 2008; pp. 15–34. ISBN 978-0-85404-267-8. [Google Scholar]
- Zeng, S.; Sreekanth, K.V.; Shang, J.; Yu, T.; Chen, C.-K.; Yin, F.; Baillargeat, D.; Coquet, P.; Ho, H.-P.; Kabashin, A.V.; et al. Graphene-Gold Metasurface Architectures for Ultrasensitive Plasmonic Biosensing. Adv. Mater. 2015, 27, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Liang, G.; Gheno, A.; Vedraine, S.; Ratier, B.; Ho, H.-P.; Yu, N. Plasmonic Metasensors Based on 2D Hybrid Atomically Thin Perovskite Nanomaterials. Nanomaterials 2020, 10, 1289. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Zeng, S. Recent Advances in Surface Plasmon Resonance for Biosensing Applications and Future Prospects. In Nanophotonics in Biomedical Engineering; Zhao, X., Lu, M., Eds.; Springer: Singapore, 2021; pp. 21–48. ISBN 9789811561368. [Google Scholar]
- Lv, R.; Robinson, J.A.; Schaak, R.E.; Sun, D.; Sun, Y.; Mallouk, T.E.; Terrones, M. Transition Metal Dichalcogenides and Beyond: Synthesis, Properties, and Applications of Single- and Few-Layer Nanosheets. Acc. Chem. Res. 2015, 48, 56–64. [Google Scholar] [CrossRef]
- Kravets, V.G.; Schedin, F.; Jalil, R.; Britnell, L.; Gorbachev, R.V.; Ansell, D.; Thackray, B.; Novoselov, K.S.; Geim, A.K.; Kabashin, A.V.; et al. Singular phase nano-optics in plasmonic metamaterials for label-free single-molecule detection. Nat. Mater. 2013, 12, 304–309. [Google Scholar] [CrossRef]
- Yin, X.; Hesselink, L. Goos-Hänchen shift surface plasmon resonance sensor. Appl. Phys. Lett. 2006, 89, 261108. [Google Scholar] [CrossRef]
- Jiang, L.; Zeng, S.; Xu, Z.; Ouyang, Q.; Zhang, D.-H.; Chong, P.H.J.; Coquet, P.; He, S.; Yong, K.-T. Multifunctional Hyperbolic Nanogroove Metasurface for Submolecular Detection. Small 2017, 13, 1700600. [Google Scholar] [CrossRef]
- Yasumoto, K.; Ōishi, Y. A new evaluation of the Goos–Hänchen shift and associated time delay. J. Appl. Phys. 1983, 54, 2170–2176. [Google Scholar] [CrossRef]
- Zeng, S.; Hu, S.; Xia, J.; Anderson, T.; Dinh, X.-Q.; Meng, X.-M.; Coquet, P.; Yong, K.-T. Graphene–MoS2 hybrid nanostructures enhanced surface plasmon resonance biosensors. Sens. Actuators B Chem. 2015, 207, 801–810. [Google Scholar] [CrossRef]
- Berguiga, L.; Ferrier, L.; Jamois, C.; Benyattou, T.; Letartre, X.; Cueff, S. Ultimate phase sensitivity in surface plasmon resonance sensors by tuning critical coupling with phase change materials. Opt. Express 2021, 29, 42162. [Google Scholar] [CrossRef]
- Cueff, S.; John, J.; Zhang, Z.; Parra, J.; Sun, J.; Orobtchouk, R.; Ramanathan, S.; Sanchis, P. VO2 nanophotonics. APL Photonics 2020, 5, 110901. [Google Scholar] [CrossRef]
- Lei, D.Y.; Appavoo, K.; Ligmajer, F.; Sonnefraud, Y.; Haglund, R.F.; Maier, S.A. Optically-Triggered Nanoscale Memory Effect in a Hybrid Plasmonic-Phase Changing Nanostructure. ACS Photonics 2015, 2, 1306–1313. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; Das, C.M.; Medwal, R.; Mishra, M.; Ouyang, Q.; Rawat, R.S.; Yong, K.; Singh, R. Electrically Tunable Singular Phase and Goos–Hänchen Shifts in Phase-Change-Material-Based Thin-Film Coatings as Optical Absorbers. Adv. Mater. 2021, 33, 2006926. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, K.V.; Ouyang, Q.; Sreejith, S.; Zeng, S.; Lishu, W.; Ilker, E.; Dong, W.; ElKabbash, M.; Ting, Y.; Lim, C.T.; et al. Phase-Change-Material-Based Low-Loss Visible-Frequency Hyperbolic Metamaterials for Ultrasensitive Label-Free Biosensing. Adv. Opt. Mater. 2019, 7, 1900081. [Google Scholar] [CrossRef]
- Orlianges, J.C.; Leroy, J.; Crunteanu, A.; Mayet, R.; Carles, P.; Champeaux, C. Electrical and Optical Properties of Vanadium Dioxide Containing Gold Nanoparticles Deposited by Pulsed Laser Deposition. Appl. Phys. Lett. 2012, 101, 133102. Available online: https://aip.scitation.org/doi/10.1063/1.4754708 (accessed on 5 May 2022). [CrossRef]
- Vieira, N.S.; de Souza, F.A.; da Rocha, R.C.F.; Cestarolli, D.T.; Guerra, E.M. Development of amperometric biosensors using VO2/GOx films for detection of glucose. Mater. Sci. Semicond. Process. 2021, 121, 105337. [Google Scholar] [CrossRef]
- Ko, C.; Yang, Z.; Ramanathan, S. Work Function of Vanadium Dioxide Thin Films Across the Metal-Insulator Transition and the Role of Surface Nonstoichiometry. ACS Appl. Mater. Interfaces 2011, 3, 3396–3401. [Google Scholar] [CrossRef]
- Akazawa-Ogawa, Y.; Nagai, H.; Hagihara, Y. Heat denaturation of the antibody, a multi-domain protein. Biophys. Rev. 2018, 10, 255–258. [Google Scholar] [CrossRef]
- Piccirillo, C.; Binions, R.; Parkin, I.P. Nb-Doped VO2 Thin Films Prepared by Aerosol-Assisted Chemical Vapour Deposition. Eur. J. Inorg. Chem. 2007, 2007, 4050–4055. [Google Scholar] [CrossRef]
- Khan, G.R.; Asokan, K.; Ahmad, B. Room temperature tunability of Mo-doped VO2 nanofilms across semiconductor to metal phase transition. Thin Solid Film. 2017, 625, 155–162. [Google Scholar] [CrossRef]
- Piccirillo, C.; Binions, R.; Parkin, I.P. Synthesis and characterisation of W-doped VO2 by Aerosol Assisted Chemical Vapour Deposition. Thin Solid Film. 2008, 516, 1992–1997. [Google Scholar] [CrossRef]
- Wang, N.; Duchamp, M.; Dunin-Borkowski, R.E.; Liu, S.; Zeng, X.; Cao, X.; Long, Y. Terbium-Doped VO2 Thin Films: Reduced Phase Transition Temperature and Largely Enhanced Luminous Transmittance. Langmuir 2016, 32, 759–764. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, D.; Yang, Y.; Liu, Y.; Zhong, A.; He, Q.; Qi, J.; Fan, P. A Novel Method for Notable Reducing Phase Transition Temperature of VO2 Films for Smart Energy Efficient Windows. Nanomaterials 2019, 10, 58. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.-W.; Chen, F.; Yu, L.; Chen, X. Tuning phase transition temperature of VO2 thin films by annealing atmosphere. J. Phys. D Appl. Phys. 2015, 48, 265104. [Google Scholar] [CrossRef]
- Yuan, N.; Li, J.; Li, G.; Chen, X. The large modification of phase transition characteristics of VO2 films on SiO2/Si substrates. Thin Solid Film. 2006, 515, 1275–1279. [Google Scholar] [CrossRef]
- Pinto, H.M.; Correia, J.; Binions, R.; Piccirillo, C.; Parkin, I.P.; Teixeira, V. Determination of the Optical Constants of VO2 and Nb-Doped VO2 Thin Films. MSF 2008, 587–588, 640–644. [Google Scholar] [CrossRef]
- Muller, A.; Khadar, R.A.; Abel, T.; Negm, N.; Rosca, T.; Krammer, A.; Cavalieri, M.; Schueler, A.; Qaderi, F.; Bolten, J.; et al. Radio-Frequency Characteristics of Ge-Doped Vanadium Dioxide Thin Films with Increased Transition Temperature. ACS Appl. Electron. Mater. 2020, 2, 1263–1272. [Google Scholar] [CrossRef]

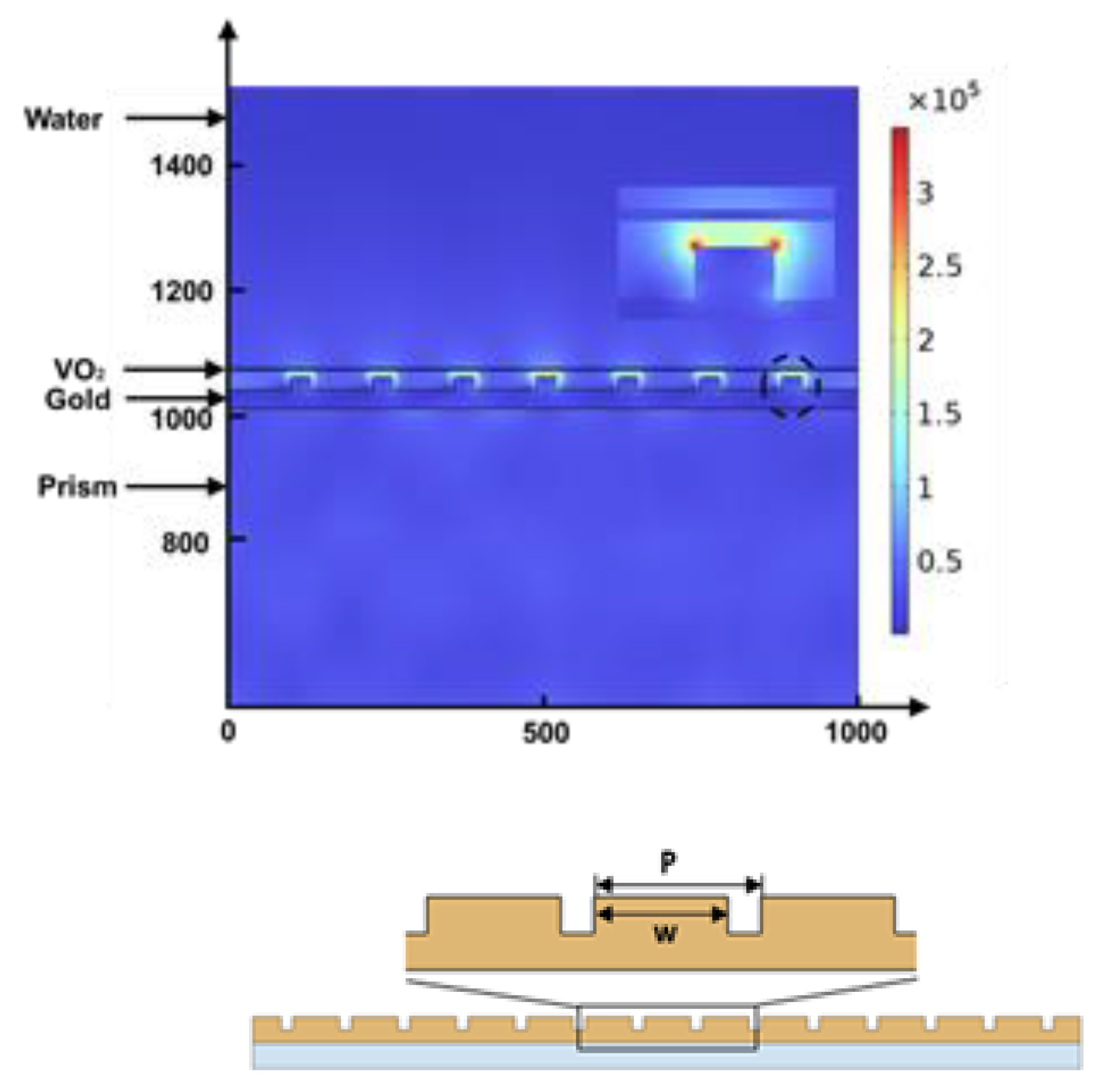
| λ (nm) | T (°C) | P (nm) | w (nm) | VO2 (nm) | Reflectivity | FWHM (°) | GH Shift (μm) | SGH (μm/RIU) ∆n = 1.2 × 10−6 | SGH (μm/RIU) ∆n = 1 × 10−10 |
|---|---|---|---|---|---|---|---|---|---|
| 785 | 95 | 140 | 20 | 1 | 1.549 × 10−6 | 1.657 | 2.248 × 103 | 2.253 × 106 | 1.738 × 106 |
| 785 | 20 | 130 | 20 | 2 | 3.542 × 10−7 | 1.954 | 1.359 × 103 | 5.046 × 107 | 4.885 × 107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, J.; Zhu, S.; Crunteanu, A.; Orlianges, J.-C.; Ho, H.-P.; Bachelot, R.; Zeng, S. Highly Sensitive Plasmonic Biosensors with Precise Phase Singularity Coupling on the Metastructures. Biosensors 2022, 12, 866. https://doi.org/10.3390/bios12100866
Youssef J, Zhu S, Crunteanu A, Orlianges J-C, Ho H-P, Bachelot R, Zeng S. Highly Sensitive Plasmonic Biosensors with Precise Phase Singularity Coupling on the Metastructures. Biosensors. 2022; 12(10):866. https://doi.org/10.3390/bios12100866
Chicago/Turabian StyleYoussef, Joelle, Shaodi Zhu, Aurelian Crunteanu, Jean-Christophe Orlianges, Ho-Pui Ho, Renaud Bachelot, and Shuwen Zeng. 2022. "Highly Sensitive Plasmonic Biosensors with Precise Phase Singularity Coupling on the Metastructures" Biosensors 12, no. 10: 866. https://doi.org/10.3390/bios12100866
APA StyleYoussef, J., Zhu, S., Crunteanu, A., Orlianges, J.-C., Ho, H.-P., Bachelot, R., & Zeng, S. (2022). Highly Sensitive Plasmonic Biosensors with Precise Phase Singularity Coupling on the Metastructures. Biosensors, 12(10), 866. https://doi.org/10.3390/bios12100866







