In Situ Growth Intercalation Structure MXene@Anatase/Rutile TiO2 Ternary Heterojunction with Excellent Phosphoprotein Detection in Sweat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Characterizations
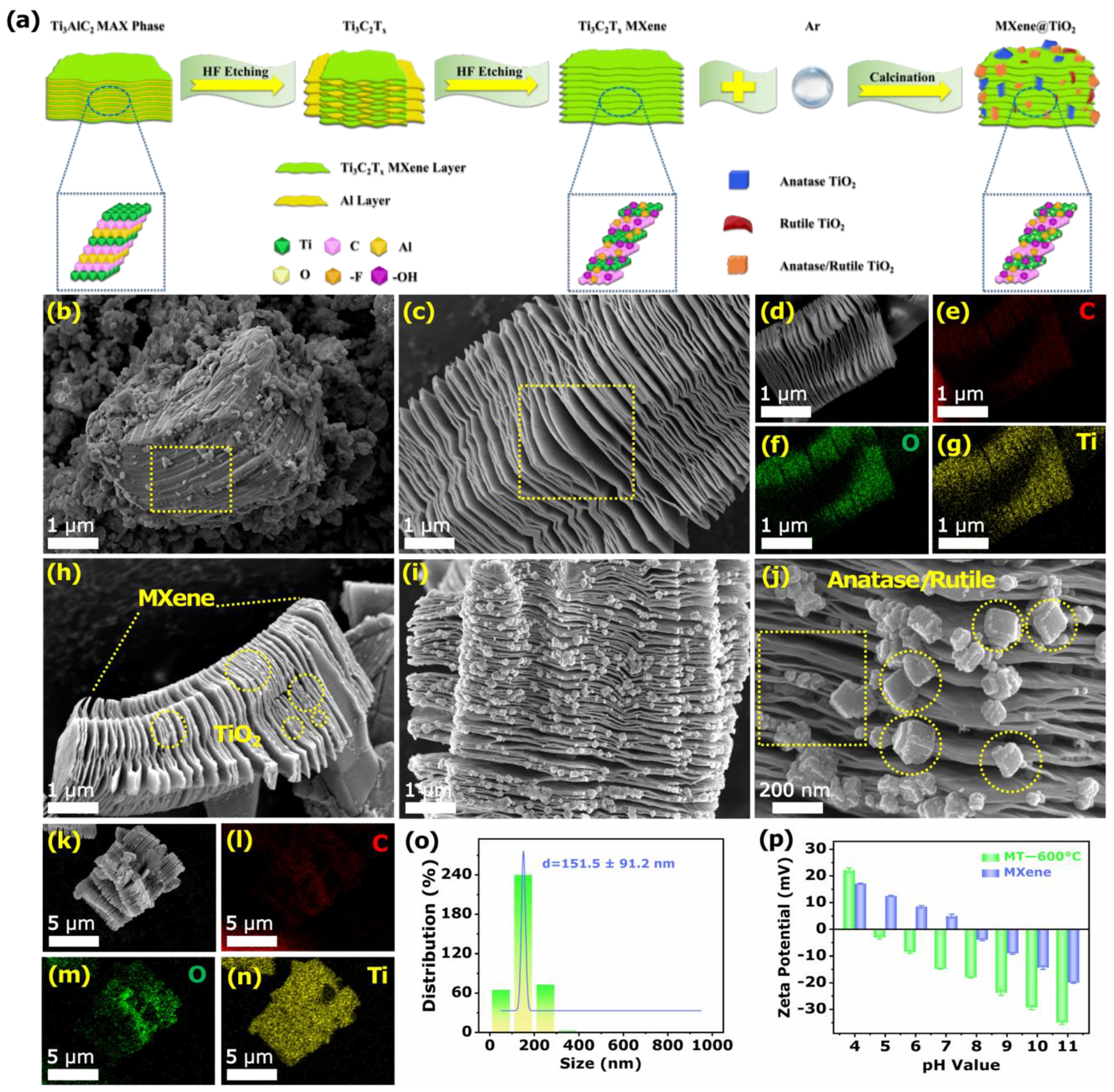
2.3. Synthesis of MXene
2.4. Synthesis of MXene@TiO2
2.5. Electrochemical Measurement
2.6. Preparation of Sensing Array Construction
3. Results and Discussion
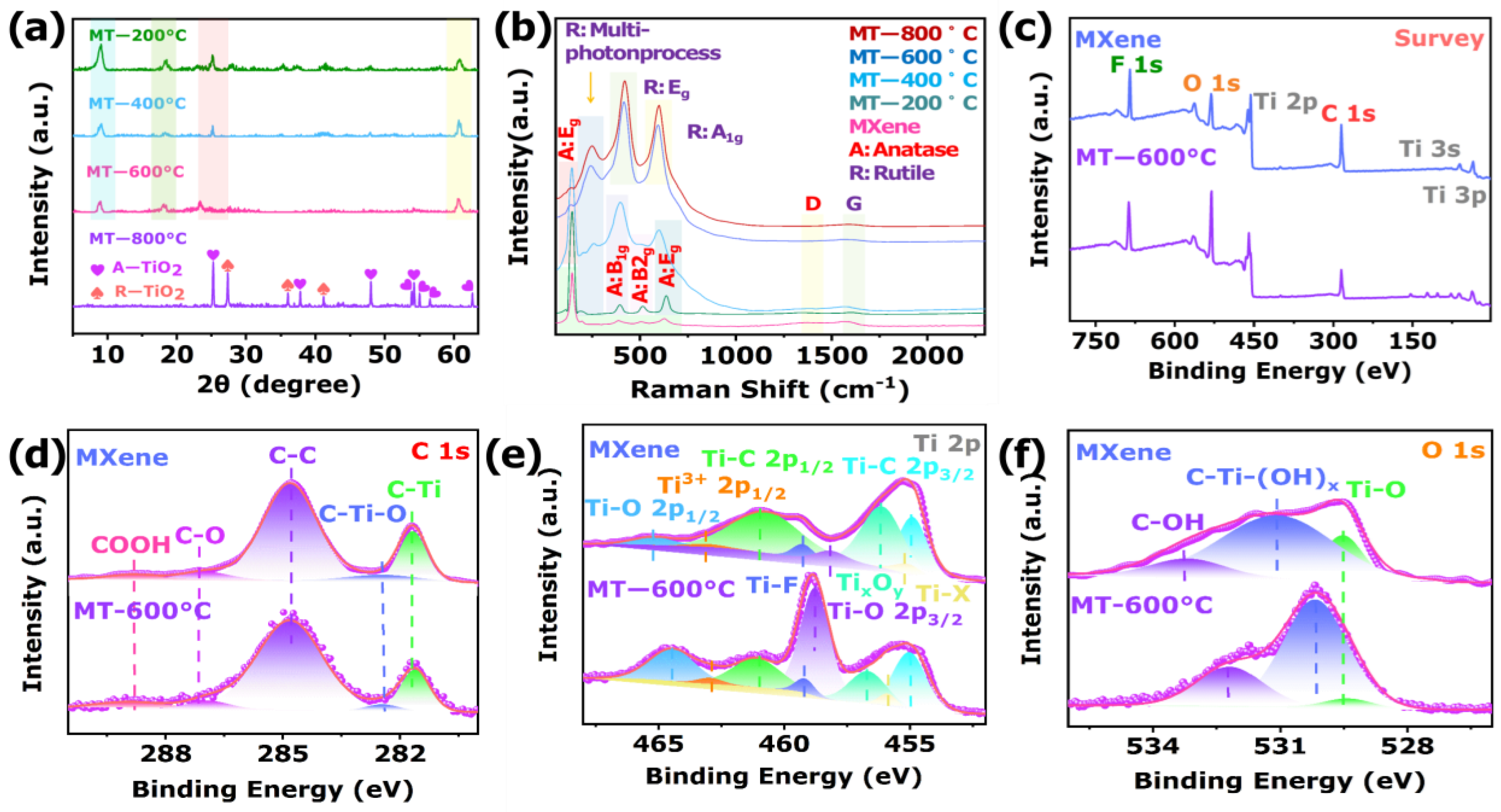
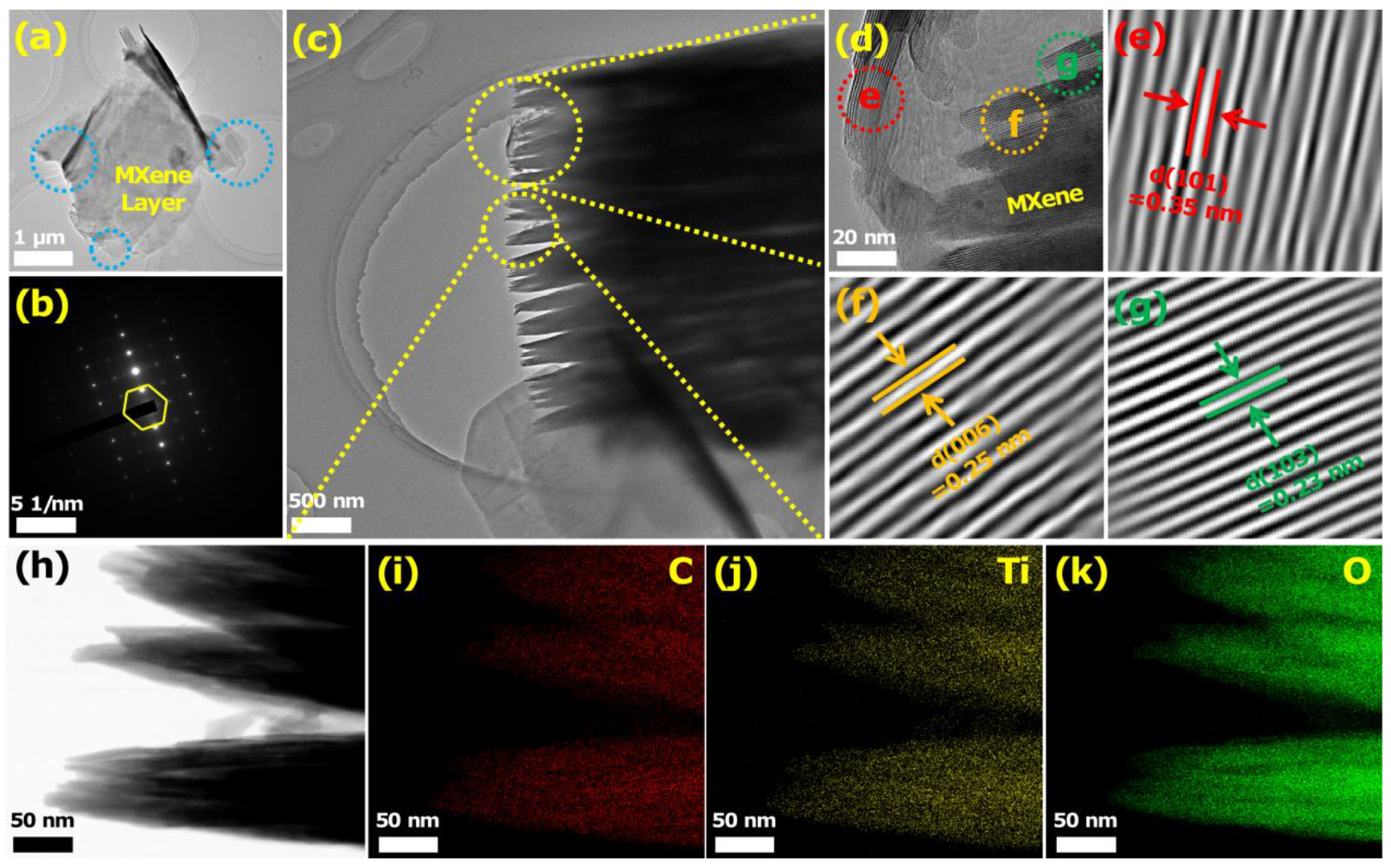
3.1. Characterization of the Sensing Materials
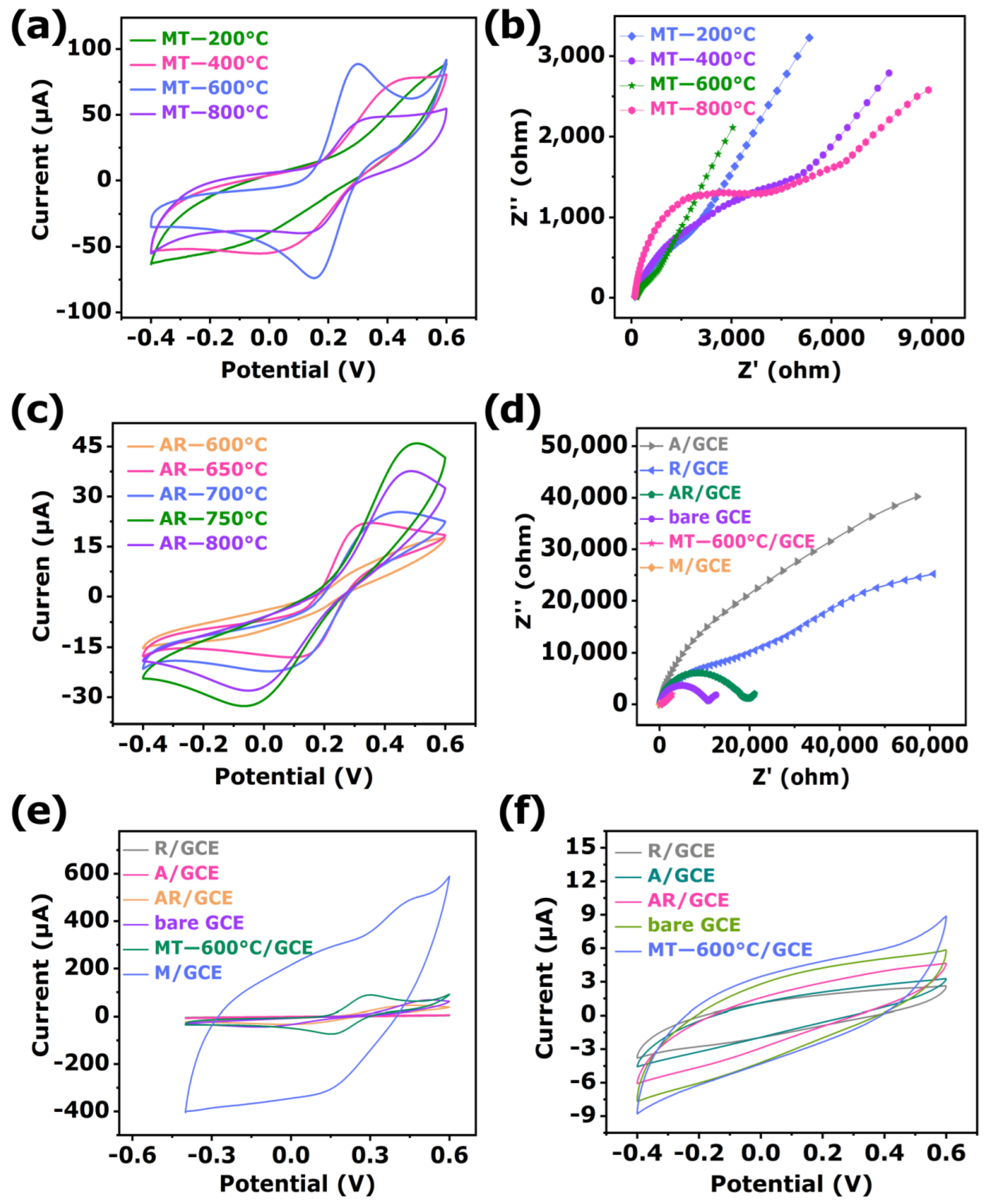
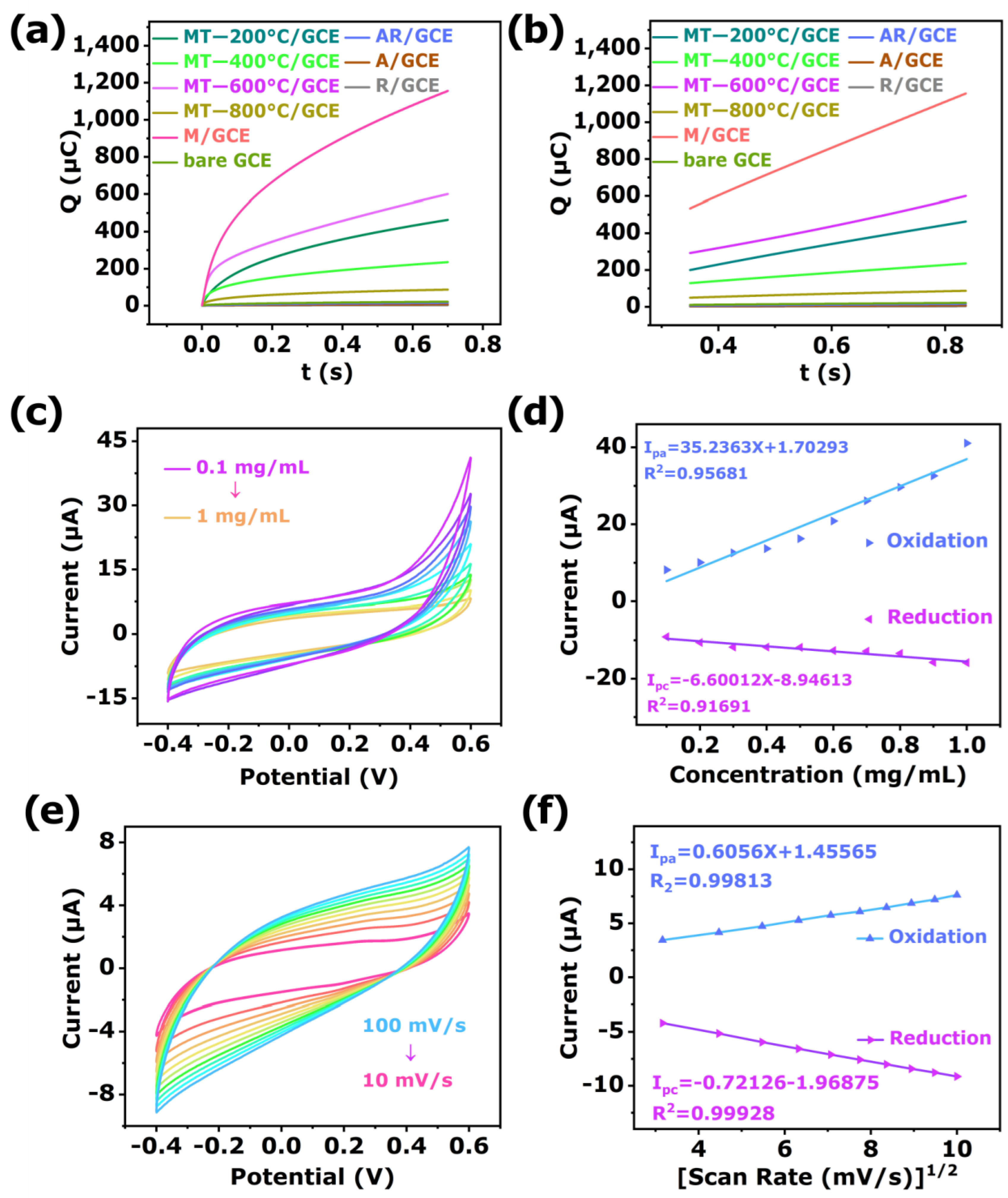
3.2. Phosphoprotein Sensing
3.3. Selectivity, Repeatability, Reproducibility, and Stability
3.3.1. Stability
3.3.2. Selectivity
3.3.3. Repeatability
3.3.4. Reproducibility
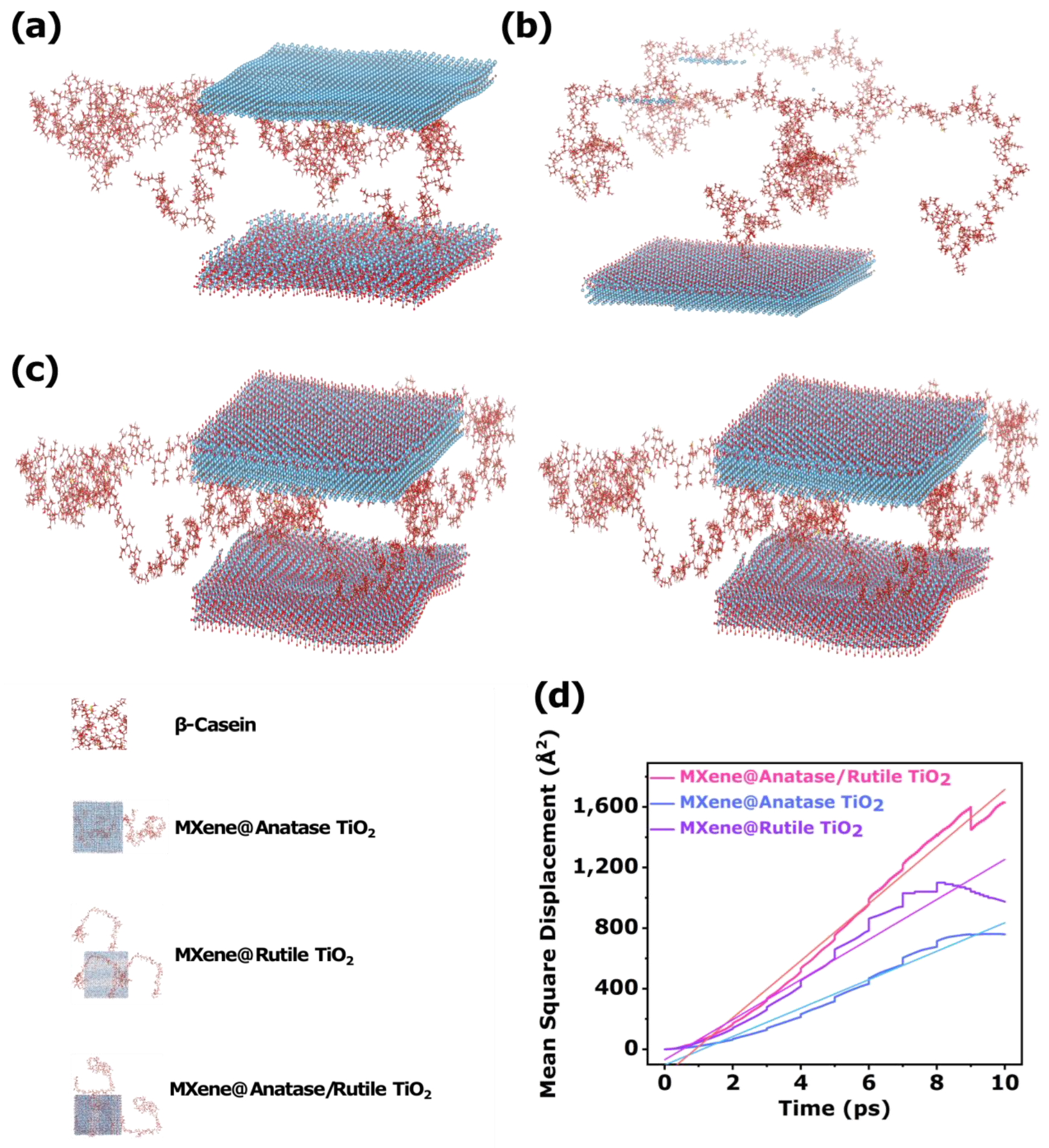
3.4. Sensing Mechanism Analysis
3.5. Practical Application Sensor Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiao, Y.; Qia, L.; Chen, Z.; Liu, B.; Gao, L.; Zhang, L. Wearable Sensor for Continuous Sweat Biomarker Monitoring. Chemosensors 2022, 10, 273. [Google Scholar] [CrossRef]
- Herrmann, W.; Habbig, J. Immunological studies on the protems of human eccrine sweat. Arch. Dermatol. Res. 1976, 255, 123–127. [Google Scholar] [CrossRef]
- Medagedara, M.; Peiris, T.; Wanasekara, N. Review of Recent Advances in Non-invasive, Flexible, Wearable Sweat Monitoring Sensors. Instrumentation 2020, 7, 36–50. [Google Scholar]
- Zhang, H.; Pelech, S. Using protein microarrays to study phosphorylation-mediated signal transduction. Semin. Cell Dev. Biol. 2012, 23, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, M.; Zhang, T.; Cao, L.; Li, Z.; Du, Y.; Hai, Y.; Gao, X.; Ji, J.; Wu, J. Dual roles of β-arrestin 1 in mediating cell metabolism and proliferation in gastric cancer. Proc. Natl. Acad. Sci. USA 2022, 11, e2123231119. [Google Scholar] [CrossRef]
- Su, T.; Wang, T.; Zhang, N.; Shen, Y.; Li, W.; Xing, H.; Yang, M. Long non-coding RNAs in gastrointestinal cancers: Implications for protein phosphorylation. Biochem. Pharmacol. 2022, 197, 114907. [Google Scholar] [CrossRef]
- Kenji, K.; Kenya, T.; Nobunori, T.; Yutaka, Y.; Yoshifumi, O.; Kyosuke, H.; Daisuke, K.; Masataka, M.; Toshihisa, K.; Shiro, I. Metabolic changes in synovial cells in early inflammation: Involvement of CREB phosphorylation in the anti-inflammatory effect of 2-deoxyglucose. Arch. Biochem. Biophys. 2021, 708, 108962. [Google Scholar]
- Thiago, B.; Ashok, J.; Nicolai, W.; Salvatore, I.; Jasmin, L.; Hui, P.; Jonathan, D.; Anna, K.; Juleen, Z.; Matthias, M.; et al. A Cell-Autonomous Signature of Dysregulated Protein Phosphorylation Underlies Muscle Insulin Resistance in Type 2 Diabetes. Cell Metab. 2020, 32, 844–859. [Google Scholar]
- Kelly, S.; Michael, D.; Paul, B.; Julian, H. Role of phosphorylation clusters in the biology of the coronavirus infectious bronchitis virus nucleocapsid protein. Virology 2008, 370, 373–381. [Google Scholar]
- Wang, X.; Zhou, W.; Gao, Z.; Lv, X. Mass spectrometry analysis of S-nitrosylation of proteins and its role in cancer, cardiovascular and neurodegenerative diseases. TrAC Trends Anal. Chem. 2022, 152, 116625. [Google Scholar] [CrossRef]
- Qing, L.; Guo, L.; Jun, F. Direct Electron Transfer for Heme Proteins Assembled on Nanocrystalline TiO2 Film. Electroanalysis 2001, 13, 359–363. [Google Scholar]
- Meyer, J.; Dick, P. Fluorescent protein-based redox probes. Antioxid. Redox Signal. 2010, 13, 621–650. [Google Scholar] [CrossRef] [PubMed]
- Dalila, R.; Arshad, M.; Subash, G.; Conlathan, I.; Nuzaihan, N.; Fathil, M.; Azmi, U.; Periasamy, A. Faradaic electrochemical impedimetric analysis on MoS2/Au-NPs decorated surface for C-reactive protein detection. J. Taiwan Inst. Chem. Eng. 2022, 138, 104450. [Google Scholar] [CrossRef]
- Liang, J.; Teng, P.; Hu, L.; He, G.; Song, Q.; Zhang, Y.; Peng, B.; Li, G.; Xiao, W.; Cao, D.; et al. Platinum nanoparticles (PtNPs)-based CRISPR/Cas12a platform for detection of nucleic acid and protein in clinical samples. Anal. Chim. Acta 2022, 1225, 340203. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Li, Y.; Wei, X.; Li, P.; Jiang, J.; Chen, Y.; Duan, P.; Wang, X.; Deng, P.; Li, X. Sensitive photoelectrochemical biosensors based on AuNPs/MXenes electrode coupled with light-harvesting UiO-66-NH2 probes for protein kinase detection. Biosens. Bioelectron. X 2022, 11, 100204. [Google Scholar] [CrossRef]
- Lian, M.; Shi, Y.; Chen, L.; Qin, Y.; Zhang, W.; Zhao, J.; Chen, D. A Cell Membrane and V2C MXene-Based Electrochemical Immunosensor with Enhanced Antifouling Capability for Detection of CD44. ACS Appl. Mater. Interfaces 2022, 5, 11352–11360. [Google Scholar]
- Li, X.; Zhang, N.; Tang, R.; Lyu, J.; Liu, Z.; Ma, S.; Ou, J.; Ye, M. Comparative Evaluation of MAX-Ti3AlC2 and MXene-Ti3C2 as Affinity Chromatographic Materials for Highly Selective Enrichment of Phosphopeptides. Nanoscale 2021, 13, 2923–2930. [Google Scholar] [CrossRef]
- Fang, G.; Gao, W.; Deng, Q.; Qian, K.; Han, H.; Wang, S. Highly selective capture of phosphopeptides using a nano titanium dioxide–multiwalled carbon nanotube nanocomposite. Anal. Biochem. 2012, 423, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, M.; Deng, C.; Zhang, X. Facile synthesis of Fe3O4@mesoporous TiO2 microspheres for selective enrichment of phosphopeptides for phosphoproteomics analysis. Talanta 2013, 105, 20–27. [Google Scholar] [CrossRef]
- Yu, Q.; Li, X.; Xiao, Y.; Guo, L.; Zhang, F.; Cai, Q.; Feng, Y.; Yuan, B.; Wang, Y. Sequential enrichment with titania-coated magnetic mesoporous hollow silica microspheres and zirconium arsenate-modifified magnetic nanoparticles for the study of phosphoproteome of HL60 cells. J. Chromatogr. A 2014, 1365, 54–60. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, J.; Deng, C.; Zhang, X. Facile synthesis of titania nanoparticles coated carbon nanotubes for selective enrichment of phosphopeptides for mass spectrometry analysis. Talanta 2013, 107, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Qiu, P.; Liang, Z.; Xue, Y.; Zhang, X.; Yang, L.; Cui, H.; Tian, J. The fabrication of 1D/2D CdS nanorod@Ti3C2 MXene composites for good photocatalytic activity of hydrogen generation and ammonia synthesis. Chem. Eng. J. 2021, 406, 127177. [Google Scholar] [CrossRef]
- Su, Y.; Ma, K.; Yuan, F.; Tang, J.; Liu, M.; Xu, Z. High-Performance Flexible Piezoresistive Sensor Based on Ti3C2Tx MXene with a Honeycomb-like Structure for Human Activity Monitoring. Micromachines 2022, 13, 821. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Qu, X.; Chen, C.; Zhang, L.; Sun, D. Ti3C2 MXene derived (001)TiO2/Ti3C2 heterojunctions for enhanced visible-light photocatalytic degradation of tetracycline. Mater. Today Commun. 2022, 33, 104216. [Google Scholar] [CrossRef]
- Liu, P.; Vincent, N.; Yao, Z.; Zhou, Z.; Ling, K. Ultrasmall Fe3O4 nanoparticles on MXenes with high microwave absorption performance. Mater. Lett. 2018, 229, 286–289. [Google Scholar] [CrossRef]
- Li, R.; Ma, X.; Li, J.; Cao, J.; Gao, H.; Li, T.; Zhang, X.; Wang, L.; Zhang, Q.; Wang, G.; et al. Flexible and high-performance electrochromic devices enabled by self-assembled 2D TiO2/MXene heterostructures. Nat. Commun. 2021, 12, 1587. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, L.; Zhou, A.; Li, Z.; Chen, J.; Bala, H.; Hu, Q.; Cao, X. Hydrothermal synthesis of TiO2/Ti3C2 nanocomposites with enhanced photocatalytic activity. Mater. Lett. 2015, 150, 62–64. [Google Scholar] [CrossRef]
- Razium, S.; Sana, J.; Nazar, K.; Mawada, T.; Selcan, K.; Ayben, K.; Magnus, W. In-situ engineered MXene-TiO2/BiVO4 hybrid as an efficient photoelectrochemical platform for sensitive detection of soluble CD44 proteins. Biosens. Bioelectron. 2020, 166, 112439. [Google Scholar]
- Bao, D.; Zhang, Q.; Meng, F.; Zhong, H.; Shi, M.; Zhang, Y.; Yan, J.; Jiang, Q.; Zhang, X. Electrochemical Reduction of N2 under Ambient Conditions for Artificial N2 Fixation and Renewable Energy Storage Using N2/NH3 Cycle. Adv. Mater. 2017, 29, 1604799. [Google Scholar] [CrossRef] [PubMed]
- Junghoon, C.; Yong, K.; Soo, C.; Kangho, P.; Hohyung, K.; Seon, K.; Hee, J. In situ formation of multiple schottky barriers in a Ti3C2 MXene film and its application in highly sensitive gas sensors. Adv. Funct. Mater. 2020, 30, 2003998. [Google Scholar]
- Olha, M.; Michael, N.; Vadym, M.; Yohan, A.; Min, H.; Michel, B.; Yury, G. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar]
- Yang, W.; Jia, L.; Wu, P.; Zhai, H.; He, J.; Liu, C.; Jiang, W. Effect of thermal program on structure–activity relationship of g-C3N4 prepared by urea pyrolysis and its application for controllable production of g-C3N4. J. Solid State Chem. 2021, 304, 122545. [Google Scholar] [CrossRef]
- Wang, X.; Fan, X.; Li, M.; Zhu, W.; Xue, J.; Fang, Y.; Cheng, L. Structure and electromagnetic properties of Ti3C2Tx MXene derived from Ti3AlC2 with different microstructures. Ceram. Int. 2021, 47, 13628–13634. [Google Scholar] [CrossRef]
- Shuck, E.; Sarycheva, A.; Anayee, M.; Levitt, S.; Gogotsil, Y. Scalable Synthesis of Ti3C2Tx MXene. Adv. Eng. Mater. 2020, 22, 1901241. [Google Scholar] [CrossRef]
- Michael, N.; Murat, K.; Jun, L.; Jun, N.; Min, H.; Lars, H.; Yury, G.; Michel, B. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 42, 4248–4253. [Google Scholar]
- Song, Y.; Sun, Z.; Fan, Z.; Cai, W.; Shao, Y.; Sheng, G.; Wang, M.; Song, L.; Liu, Z.; Zhang, Q.; et al. Rational design of porous nitrogen-doped Ti3C2 MXene as a multifunctional electrocatalyst for Li–S chemistry. Nano Energy 2020, 70, 104555. [Google Scholar] [CrossRef]
- Peng, C.; Zhou, T.; Wei, P.; Ai, H.; Zhou, B.; Pan, H.; Xu, W.; Jia, J.; Zhang, K.; Wang, H.; et al. Regulation of the rutile/anatase TiO2 phase junction in-situ grown on –OH terminated Ti3C2Tx (MXene) towards remarkably enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 439, 135685. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Bi, F.; Zhang, X.; Yang, Y.; Wang, Y. A facile synthesis for uniform tablet-like TiO2/C derived from Materials of Institut Lavoisier-125(Ti) (MIL-125(Ti)) and their enhanced visible light-driven photodegradation of tetracycline. J. Colloid Interface Sci. 2020, 571, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Jiang, L.; Lu, C.; Yu, Z.; Li, F.; Jing, X.; Rui, X.; Wei, Z.; Jin, S. Large-scale two-dimensional titanium carbide MXene as SERS-active substrate for reliable and sensitive detection of organic pollutants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 236, 118336. [Google Scholar] [CrossRef]
- Xia, X.; Peng, S.; Bao, Y.; Wang, Y.; Lei, B.; Wang, Z.; Huang, Z.; Gao, Y. Control of interface between anatase TiO2 nanoparticles and rutile TiO2 nanorods for efficient photocatalytic H2 generation. J. Power Sources 2018, 376, 11–17. [Google Scholar] [CrossRef]
- Hanaor, H.; Sorrell, C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef]
- Bassi, A.; Cattaneo, D.; Russo, V.; Bottani, C. Raman spectroscopy characterization of titania nanoparticles produced by flame pyrolysis: The influence of size and stoichiometry. J. Appl. Phys. 2005, 98, 074305. [Google Scholar] [CrossRef]
- Choi, H.; Jung, Y.; Kim, S. Size effects in the Raman spectra of TiO2 nanoparticles. Vib. Spectrosc. 2005, 37, 33–38. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Wu, H.; Hou, Z.; Song, C.; Li, X.; Zhang, L.; Cheng, L. Ti3C2 MXenes with Modified Surface for High-Performance Electromagnetic Absorption and Shielding in the X-Band. ACS Appl. Mater. Interfaces 2016, 8, 21011–21019. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, H.; Bibi, R.; Shen, Q.; Ngulube, R.; Zhou, J.; Liu, M. Noble-metal-free MOF derived hollow CdS/TiO2 decorated with NiS cocatalyst for efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 476, 378–386. [Google Scholar] [CrossRef]
- Qu, T.; Ha, T.; Thanh, D.M.; Le, V.; Viet, M.; Nham, T.; Thang, Q. Advanced synthesis of MXene-derived nanoflower-shaped TiO2@Ti heterojunction to enhance photocatalytic degradation of Rhodamine B. Environ. Technol. Innov.Volume 2021, 21, 101286. [Google Scholar]
- Wang, F.; He, X.; Sun, L.; Chen, J.; Wang, X.; Xu, J.; Han, X. Engineering an N-doped TiO2@N-doped C butterfly-like nanostructure with long-lived photo-generated carriers for efficient photocatalytic selective amine oxidation. J. Mater. Chem. A 2018, 6, 2091–2099. [Google Scholar] [CrossRef]
- Deng, L.; Chang, B.; Shi, D.; Yao, X.; Shao, Y.; Shen, J.; Zhang, B.; Wu, Y.; Hao, X. MXene decorated by phosphorus-doped TiO2 for photo-enhanced electrocatalytic hydrogen evolution reaction. Renew. Energy 2021, 170, 858–865. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Z.; Ji, L.; Liang, Z.; Xue, Y.; Guo, Y.; Tian, J.; Wang, Z.; Cui, H. 2D/2D/2D heterojunction of Ti3C2 MXene/MoS2 nanosheets/TiO2 nanosheets with exposed (001) facets toward enhanced photocatalytic hydrogen production activity. Appl. Catal. B Environ. 2019, 246, 12–20. [Google Scholar] [CrossRef]
- Liu, H.; Ma, Y.; Chen, J.; Wen, M.; Li, G.; An, T. Highly efficient visible-light-driven photocatalytic degradation of VOCs by CO2-assisted synthesized mesoporous carbon confined mixed-phase TiO2 nanocomposites derived from MOFs. Appl. Catal. B Environ. 2019, 250, 337–346. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Y.; Sun, X.; Zhang, Y.; Hou, L.; Zhang, Q.; Yuan, C. In-situ construction of hierarchical accordion-like TiO2/Ti3C2 nanohybrid as anode material for lithium and sodium ion batteries. Electrochim. Acta 2018, 271, 165–172. [Google Scholar] [CrossRef]
- Ingemar, P.; Lars-Åke, N.; Joseph, H.; Michel, B.; Vanya, D.; Justinas, P.; Johanna, R.; Per, P. On the organization and thermal behavior of functional groups on Ti3C2 MXene surfaces in vacuum. 2D Materials 2018, 5, 015002. [Google Scholar]
- Jai, K.; Rana, N.; Ayman, N.; Munirah, A.; Razium, S.; Marwa, B.; Nazeer, A.; Selcan, K. Robust Electrochemical Sensors for Detection of Isoprenaline Using Hexagonal Co3O4 Nanoplates Embedded in Few-Layer Ti3C2Tx Nanosheets. ACS Appl. Nano Mater. 2022, 5, 11352–11360. [Google Scholar]
- Tran, N.; Ta, Q.; Noh, J. Unusual synthesis of safflower-shaped TiO2/Ti3C2 heterostructures initiated from two-dimensional Ti3C2 MXene. Appl. Surf. Sci. 2021, 538, 148023. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, L.; An, Y.; Lv, S.; Wu, M.; Fan, X.; Zhang, Y.; Guo, X.; Tang, J. Laminated Hybrid Junction of Sulfur-Doped TiO2 and a Carbon Substrate Derived from Ti3C2 MXenes: Toward Highly Visible Light-Driven Photocatalytic Hydrogen Evolution. Adv. Sci. 2018, 5, 1700870. [Google Scholar] [CrossRef]
- Qin, Y.; Guo, Y.; Liang, Z.; Xue, Y.; Zhang, X.; Yang, L.; Tian, J. Au nanorods decorated TiO2 nanobelts with enhanced full solar spectrum photocatalytic antibacterial activity and the sterilization file cabinet application. Chin. Chem. Lett. 2021, 32, 1523–1526. [Google Scholar] [CrossRef]
- Dong, L.; Wei, Y.; Li, L.; Zhang, T.; Wang, H. MXene molecular sieving membranes for highly efficient gas separation. Nat. Commun. 2018, 9, 155. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An Effective 2D Light-to-Heat Conversion Material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Dong, S.; Ye, Z.; Guo, Y. One-step hydrothermal synthesis of a TiO2-Ti3C2Tx nanocomposite with small sized TiO2 nanoparticles. Ceram. Int. 2017, 43, 11065–11070. [Google Scholar] [CrossRef]
- Ghassemi, H.; Harlow, W.; Mashtalir, O.; Beidaghi, M.; Lukatskaya, M.; Gogotsi, Y.; Taheri, L. In situ environmental transmission electron microscopy study of oxidation of two-dimensional Ti3C2 and formation of carbon-supported TiO2. J. Mater. Chem. A 2014, 35, 14339. [Google Scholar] [CrossRef]
- Wang, X.; Meng, S.; Zhang, S.; Zheng, X.; Chen, S. 2D/2D MXene/g-C3N4 for photocatalytic selective oxidation of 5-hydroxymethylfurfural into 2,5-formylfuran. Catal. Commun. 2020, 147, 106152. [Google Scholar] [CrossRef]
- Liu, G.; Xu, L.; Li, Y.; Guo, D.; Wu, N.; Yuan, C.; Qin, A.; Cao, A.; Liu, X. Metal-organic frameworks derived anatase/rutile heterostructures with enhanced reaction kinetics for lithium and sodium storage. Chem. Eng. J. 2022, 430, 132689. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, X.; Chen, T.; Xu, G.; Liu, M.; Liu, J.; Xu, Y. Two-dimensional titanium carbide (MXene)-based solid-state electrochemiluminescent sensor for label-free single-nucleotide mismatch discrimination in human urine. Sens. Actuators B Chem. 2018, 263, 400–407. [Google Scholar] [CrossRef]
- Peng, C.; Xie, X.; Xu, W.; Zhou, T.; Wei, P.; Jia, J.; Zhang, K.; Cao, Y.; Wang, H.; Peng, F.; et al. Engineering highly active Ag/Nb2O5@Nb2CTx (MXene) photocatalysts via steering charge kinetics strategy. Chem. Eng. J. 2021, 415, 128766. [Google Scholar] [CrossRef]
- Khan, M.M.; Ali Ansari, S.; Jintae, L.; Moo, C. Novel Ag@TiO2 nanocomposite synthesized by electrochemically active biofilm for nonenzymatic hydrogen peroxide sensor. Mater. Sci. Eng. C 2013, 33, 4692–4699. [Google Scholar] [CrossRef] [PubMed]
- Veera, R.; Jae, S.; Jaehyeon, H.; Dae, K.; Chang, C.; Kyeongsoon, P.; Sun, K.; Madhavi, G.; Hyunmin, Y.; Jong, P. Fine-tuning of MXene-nickel oxide-reduced graphene oxide nanocomposite bioelectrode: Sensor for the detection of influenza virus and viral protein. Biosens. Bioelectron. 2022, 214, 114511. [Google Scholar]
- Tan, Y.; Jin, J.; Zhang, S.; Shi, Z.; Wang, J.; Zhang, J.; Pu, W.; Yang, C. Electrochemical determination of bisphenol A using a molecularly imprinted chitosan-acetylene black composite film modified glassy carbon electrode. Electroanalysis 2016, 28, 189–196. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, N.; Ni, Y.; Xu, J.; Shao, S. Electrochemical sensor based on Nbim/CNT composite for selective determination of luteolin in the flavonoids. J. Electroanal. Chem. 2015, 754, 94–99. [Google Scholar] [CrossRef]
- Armbruster, D.; Tillman, M.; Hubbs, L. Limit of detection (LQD)/limit of quantitation (LOQ): Comparison of the empirical and the statistical methods exemplified with GC-MS assays of abused drugs. Clin. Chem. 1994, 7, 1233–1238. [Google Scholar] [CrossRef]
- Qiao, Y.; Qiao, L.; Zhao, P.; Zhang, P.; Wu, F.; Zhang, J.; Gao, L.; Liu, B.; Zhang, L. Phosphoprotein Detection in Sweat Realized by Intercalation Structure 2D@3D g-C3N4@Fe3O4 Wearable Sensitive Motif. Biosensors 2022, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Tsukuru, M.; Tsuyoshi, M.; Petr, K.; Anzenbacher, P., Jr.; Shi, T. Antibody- and Label-Free Phosphoprotein Sensor Device Based on an Organic Transistor. Anal. Chem. 2016, 88, 1092–1095. [Google Scholar]
- Saima, N.; Mubarak, A.; Ishtiaq, A.; Christof, N.; Wolfgang, E. Phosphoprotein Detection with a Single Nanofluidic Diode Decorated with Zinc Chelates. ChemPlusChem 2020, 85, 587–594. [Google Scholar]
- Ali, M.; Nasir, S.; Ahmed, I.; Niemeyer, C.M.; Ensinger, W. Biomolecular Detection with a Single Nanofluidic Diode Decorated with Metal Chelates. ChemPlusChem 2020, 85, 101002. [Google Scholar]
- James, W.; Aurora, A.; Nicholas, V.; Hongi, Y.; Mark, J.; Ryan, B. Rapid, Multiplexed Phosphoprotein Profiling Using Silicon Photonic Sensor Arrays. ACS Cent. Sci. 2015, 1, 374–382. [Google Scholar]
- Liu, X.; Er, S.; Cao, X. Metal-organic framework/3,30,5,50-tetramethylbenzidine based multidimensional spectral array platform for sensitive discrimination of protein phosphorylation. J. Colloid Interface Sci. 2021, 602, 513–519. [Google Scholar]
- Guo, J.; Li, J.; Wang, S.; Wang, J. Determination of Trace Phosphoprotein in Food Based on Fluorescent Probe-Triggered Target-Induced Quench by Electrochemiluminescence. J. Agric. Food Chem. 2020, 68, 12738–12748. [Google Scholar] [CrossRef]
- Guo, j.; Fang, G.; Wang, S.; Wang, J. Quartz crystal microbalance sensor based on 11-mercaptoundecanoic acid self-assembly and amidated nano-titanium film for selective and ultrafast detection of phosphoproteins in food. Food Chem. 2021, 344, 128656. [Google Scholar] [CrossRef]
- Mayuko, O.; Hideyuki, K.; Tomoteru, F.; Tetsuya, H. DFT-based ab initio MD simulation of the ionic conduction in doped ZrO2 systems under epitaxial strain. Phys. Chem. Chem. Phys. PCCP 2015, 17, 29057–29063. [Google Scholar]
- Chen, Q.; Ma, J.; Zhang, Y.; Wu, C.; Xu, J. Effects of temperature and ionic concentration on nanodroplets electrocoalescence. Langmuir 2018, 35, 750–759. [Google Scholar] [CrossRef]
- Ilham, E.; Jean, B.; Arnaud, J.; Armand, S.; Anthony, S.; Patrice, M.; Aziz, G. Molecular simulation contact angle and surface tension of water on a hexagonal boron nitride monolayer: A methodological investigation contact angle and surface tension of water on a hexagonal boron nitride monolayer: A methodological investigation. Mol. Simul. 2019, 45, 454–461. [Google Scholar]
- Gui, L.; Lin, J.; Liu, J.; Zuo, J.; Wang, Q.; Jiang, W.; Feng, T.; Liu, S.; Wang, S.; Liu, Z. Difference and association of antibacterial and bacterial anti-adhesive performances between smart Ag/AgCl/TiO2 composite surfaces with switchable wettability. Chem. Eng. J. 2022, 431, 134103. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set-ScienceDirect. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 13115–13118. [Google Scholar] [CrossRef] [PubMed]
- Perdew, P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Blöchl, E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Shu, R.; Run, F.; Shou, C.; Qing, W.; Zhi, Z. Synergistic Catalytic Acceleration of MXene/MWCNTs as Decorating Materials for Ultrasensitive Detection of Morphine. Electroanalysis 2021, 33, 1471–1483. [Google Scholar]
- Zhou, L.; Wu, F.; Yu, J.; Deng, Q.; Zhang, F.; Wang, G. Titanium carbide (Ti3C2Tx) MXene: A novel precursor to amphiphilic carbide-derived graphene quantum dots for fluorescent ink, light-emitting composite and bioimaging. Carbon 2017, 118, 50–57. [Google Scholar] [CrossRef]
- Peng, C.; Wang, H.; Yu, H.; Peng, F. (111) TiO2-x/Ti3C2: Synergy of active facets, interfacial charge transfer and Ti3+ doping for enhance photocatalytic activity. Mater. Res. Bull. 2017, 89, 16–25. [Google Scholar] [CrossRef]
- Meng, L.; Jia, L.; Rui, B.; Xin, W.; Ying, J.; Xiao, Z.; Jian, T.; Feng, S.; Hong, C. ZnO@Ti3C2 MXene interfacial Schottky junction for boosting spatial charge separation in photocatalytic degradation. J. Alloys Compd. 2022, 905, 164025. [Google Scholar]
- Xin, D.; Ya, W.; Ming, J.; Zhao, N.; Jun, C.; Xi, Y.; Xue, K.; Jian, Y.; Xing, Z. Sustainable and scalable in-situ synthesis of hydrochar-wrapped Ti3AlC2-derived nanofibers as adsorbents to remove heavy metals. Bioresour. Technol. 2019, 282, 222–227. [Google Scholar]
- Wang, K.; Zhou, Y.; Xu, W.; Huang, D.; Wang, Z.; Hong, M. Fabrication and thermal stability of two-dimensional carbide Ti3C2 nanosheets. Ceram. Int. 2016, 42, 8419–8424. [Google Scholar] [CrossRef]
- Li, A.; Wang, Z.; Yin, H.; Wang, S.; Yan, P.; Huang, B.; Wang, X.; Li, R.; Zong, X.; Han, H.; et al. Understanding the anatase-rutile phase junction in charge separation and transfer in a TiO2 electrode for photoelectrochemical water splitting. Chem. Sci. 2016, 7, 6076–6082. [Google Scholar] [CrossRef]
- Zhu, S.; Xie, S.; Liu, Z. Nature of Rutile Nuclei in Anatase-to-Rutile Phase Transition. J. Am. Chem. Soc. 2015, 137, 11532–11539. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Sun, D.; Zhang, Y.; Liu, B.; Hu, Q.; Zhou, A. Synthesis and thermal stability of two-dimensional carbide MXene Ti3C2. Mater. Sci. Eng. B 2015, B191, 33–40. [Google Scholar] [CrossRef]
- Aihu, F.; Yun, Y.; Feng, J.; Yong, W.; Le, M.; Yang, Y.; Li, S. Fabrication and thermal stability of NH4HF2-etched Ti3C2 MXene. Ceram. Int. 2017, 43, 6322–6328. [Google Scholar]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, P.; Chen, T.; Lv, Q.; Gao, L.; Liu, B.; Duan, J.; Wu, Z.; Li, J. Construction of flexible and wearable 3D TiO2 NTs@Ti mesh for physiological detection based on sweat. JCIS Open 2021, 2, 100007. [Google Scholar] [CrossRef]
- Gao, C.; Yu, R.; Li, E.; Zhang, C.; Zou, Y.; Chen, H.; Lin, Z.; Guo, T. Adaptive immunomorphic hardware based on organic semiconductors and oxidized MXene heterostructures for feature information recognition. Cell Rep. Phys. Sci. 2022, 3, 100930. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Sun, M.; Liu, Z.; Liu, H.; Xiong, S.; Li, S.; Song, J.; Wang, K. Functionalized carbon fibers with MXene via electrochemistry aryl diazonium salt reaction to improve the interfacial properties of carbon fiber/epoxy composites. J. Mater. Res. Technol. 2022, 19, 3699–3712. [Google Scholar] [CrossRef]
- Lukatskaya, R.; Mashtalir, O.; Ren, E.; Dall’Agnese, Y.; Rozier, P.; Taberna, L.; Naguib, M.; Simon, P.; Barsoum, W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Jiang, S.; Zhang, X.; Bi, F.; Yang, Y.; Wang, Y.; Wang, Z. Enhanced photocatalytic degradation of gaseous toluene and liquidus tetracycline by anatase/rutile titanium dioxide with heterophase junction derived from materials of Institut Lavoisier-125(Ti): Degradation pathway and mechanism studies. J. Colloid Interface Sci. 2020, 588, 122–137. [Google Scholar] [CrossRef]



| Samples | Modified Materials | Liner Range | LOD | References |
|---|---|---|---|---|
| Sweat | g-C3N4@Fe3O4 | 0.01–1 mg/mL | 9.7 μM | [70] |
| Water | ZnII-DPA a | 0.22 ppm | [71] | |
| Electrolyte | DPA-NH2 b | ≥1 nM | [72] | |
| Electrolyte | DPA-Zn2+ c | ≥1 nM | [73] | |
| Glioblastoma cell | Silicon photonic microring resonator arrays | 3.55−log | 0.6 pM | [74] |
| Cancer cell | Zr-FeTCPP d-MOF | 0.1–40 nM/40–150 nM | [75] | |
| Food | NH2-TiO2/UCNPs e-rGO | 0–1 mg/mL | 9.2 × 10−5 mg/mL | [76] |
| Food | NH2-TiO2/MUA f/AuE g-QCM h | 1.0 × 10−3–1.0 mg/mL | 0.09 mM | [77] |
| Sweat | MXene@TiO2 | 0.01–1 mg/mL | 1.52 μM | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Liu, X.; Jia, Z.; Zhang, P.; Gao, L.; Liu, B.; Qiao, L.; Zhang, L. In Situ Growth Intercalation Structure MXene@Anatase/Rutile TiO2 Ternary Heterojunction with Excellent Phosphoprotein Detection in Sweat. Biosensors 2022, 12, 865. https://doi.org/10.3390/bios12100865
Qiao Y, Liu X, Jia Z, Zhang P, Gao L, Liu B, Qiao L, Zhang L. In Situ Growth Intercalation Structure MXene@Anatase/Rutile TiO2 Ternary Heterojunction with Excellent Phosphoprotein Detection in Sweat. Biosensors. 2022; 12(10):865. https://doi.org/10.3390/bios12100865
Chicago/Turabian StyleQiao, Yuting, Xianrong Liu, Zhi Jia, Peng Zhang, Li Gao, Bingxin Liu, Lijuan Qiao, and Lei Zhang. 2022. "In Situ Growth Intercalation Structure MXene@Anatase/Rutile TiO2 Ternary Heterojunction with Excellent Phosphoprotein Detection in Sweat" Biosensors 12, no. 10: 865. https://doi.org/10.3390/bios12100865
APA StyleQiao, Y., Liu, X., Jia, Z., Zhang, P., Gao, L., Liu, B., Qiao, L., & Zhang, L. (2022). In Situ Growth Intercalation Structure MXene@Anatase/Rutile TiO2 Ternary Heterojunction with Excellent Phosphoprotein Detection in Sweat. Biosensors, 12(10), 865. https://doi.org/10.3390/bios12100865





