Polydopamine-Functionalized Copper Peroxide/ZIF-8 Nanoparticle-Based Fluorescence-Linked Immunosorbent Assay for the Sensitive Determination of Carcinoembryonic Antigen by Self-Supplied H2O2 Generation
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Apparatus
2.3. Synthesis of CP/ZIF-8/PDA
2.4. Synthesis of the CP/ZIF-8/PDA/Aptamer Immunoprobe
2.5. The 96-Well Plate Trimmed with CEA Antibody
2.6. FLISA Determination of CEA
3. Results and Discussion
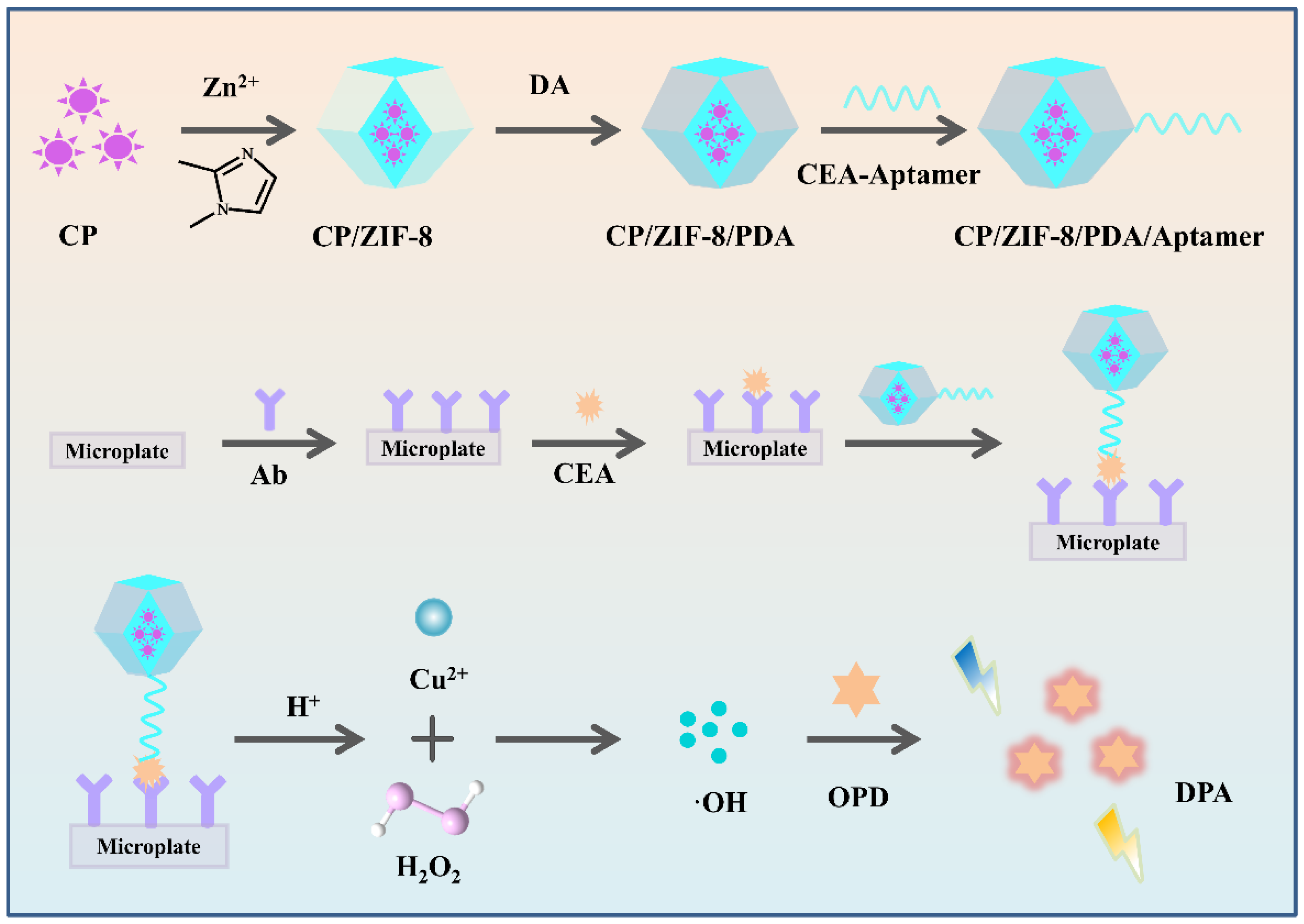
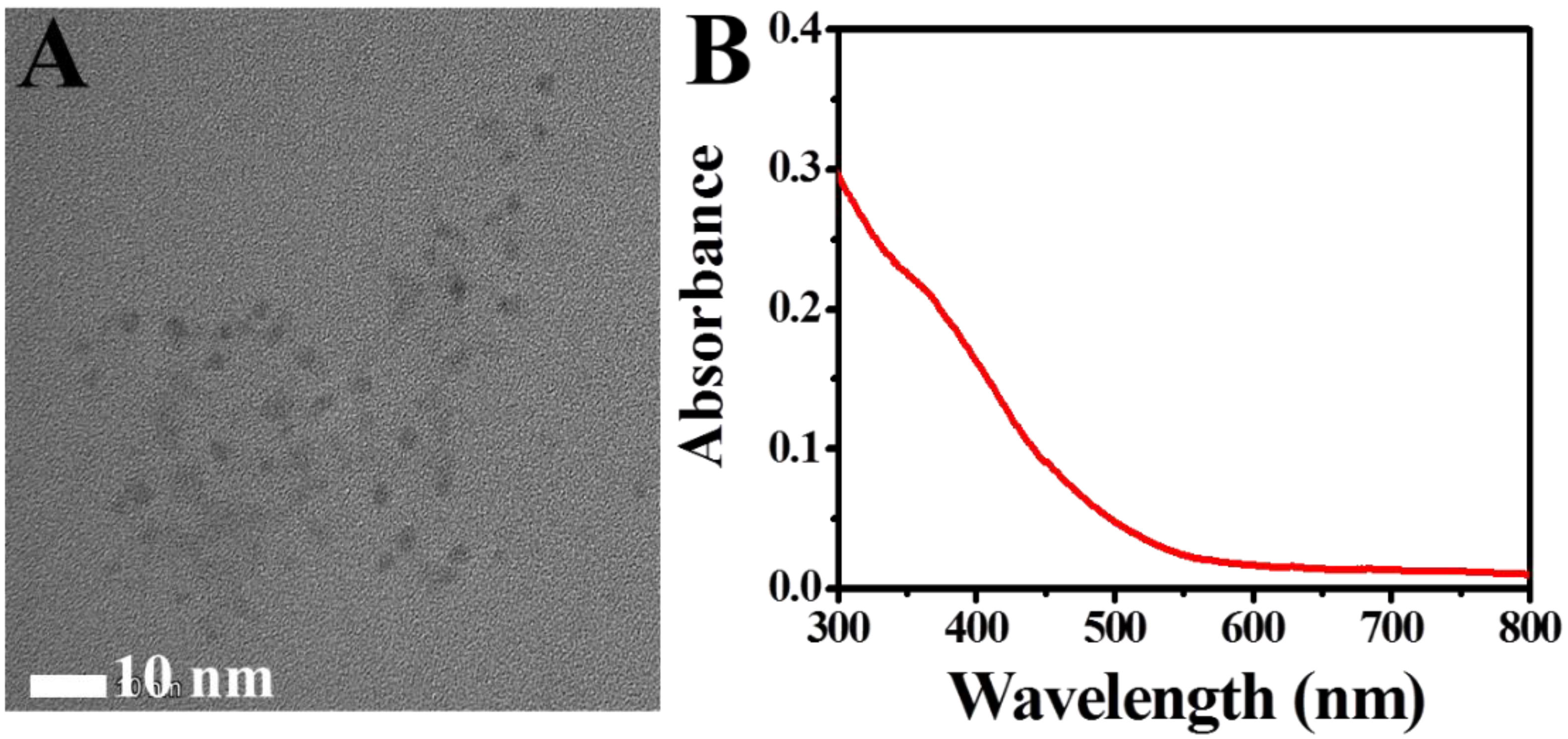
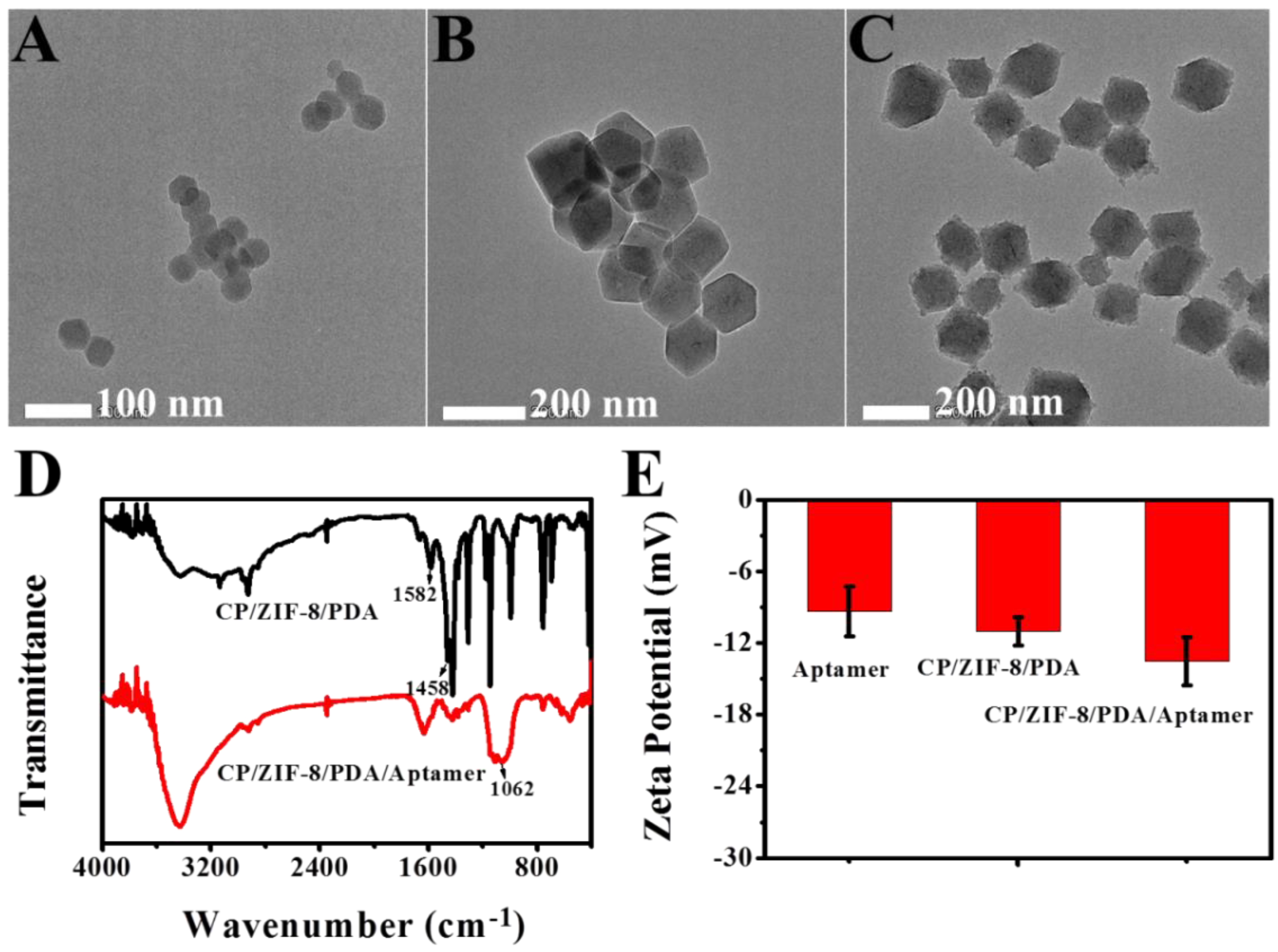
3.1. Characterization of the CP and CP/ZIF-8/PDA/Aptamer
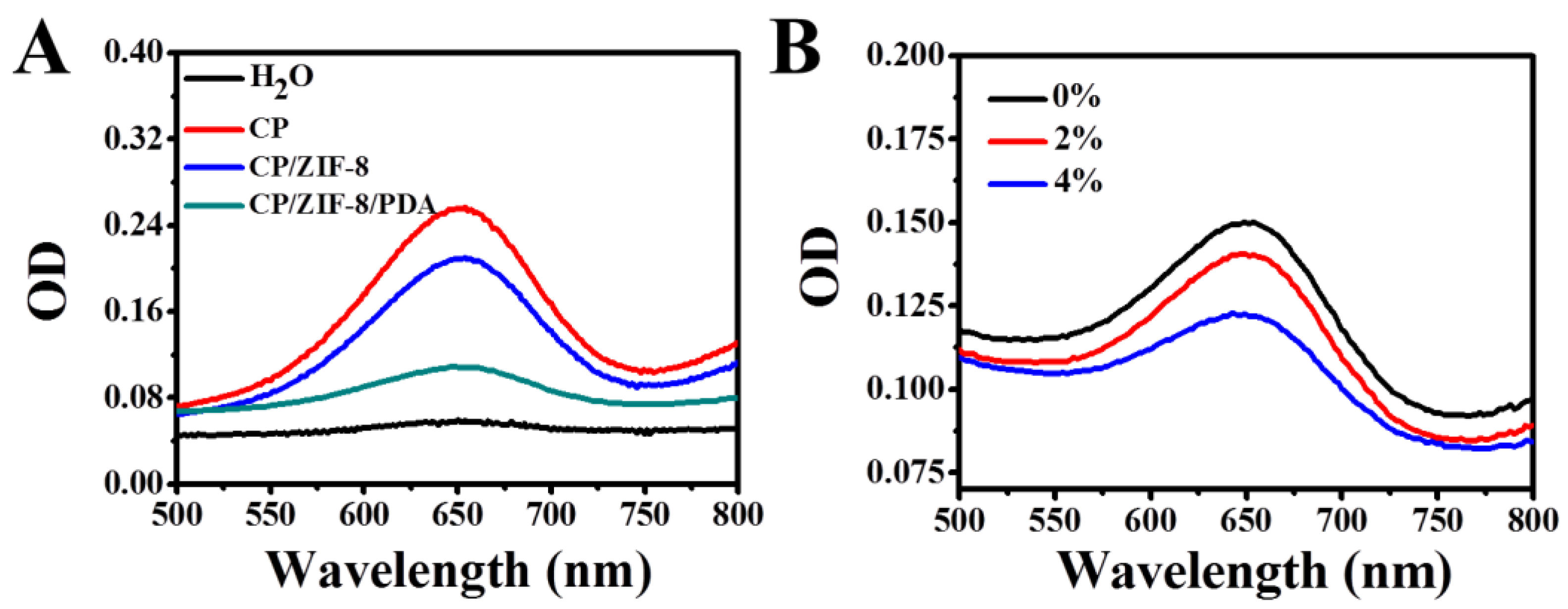
3.2. Feasibility of the Proposed FLISA Platform
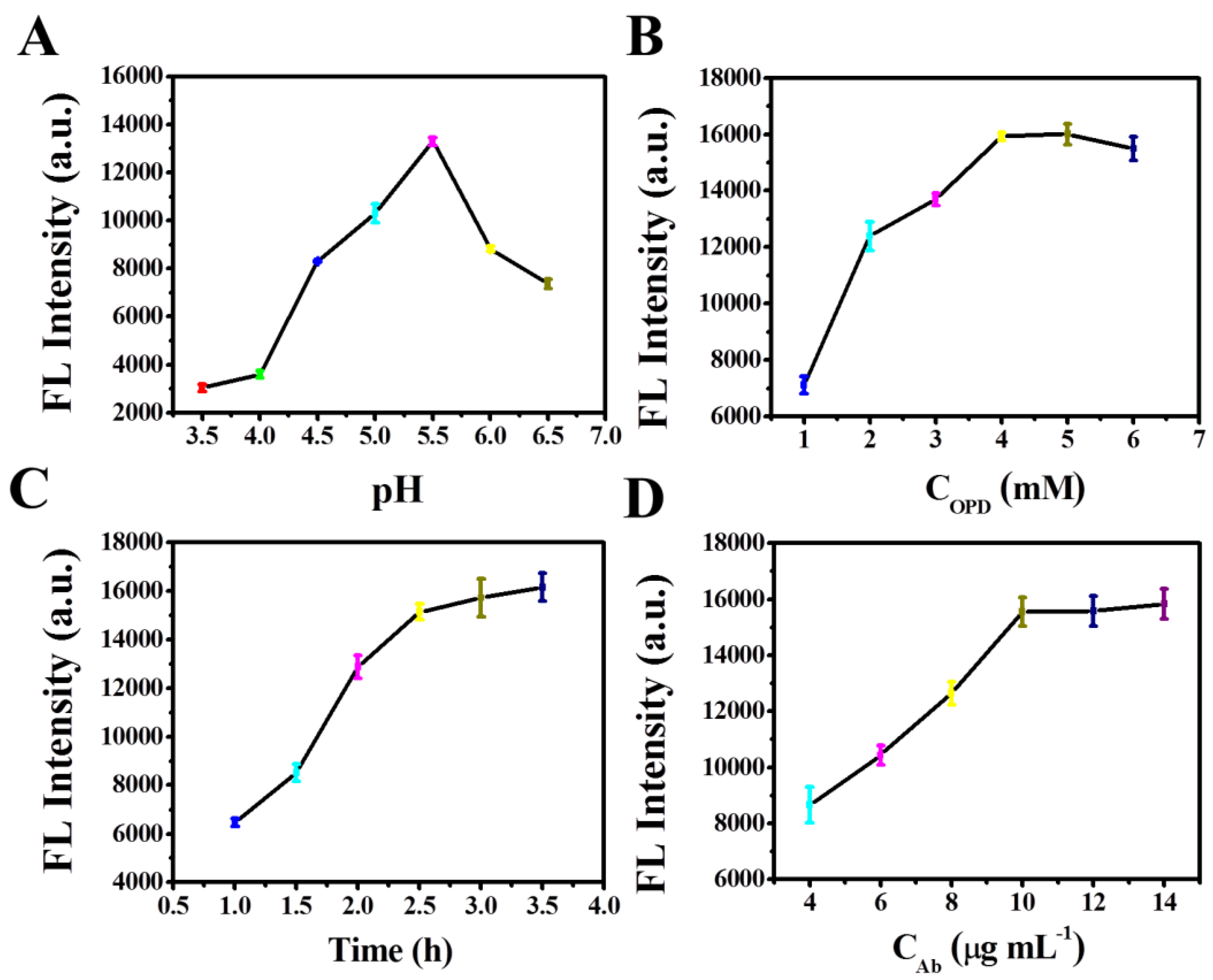
3.3. Effect of Reaction Parameters
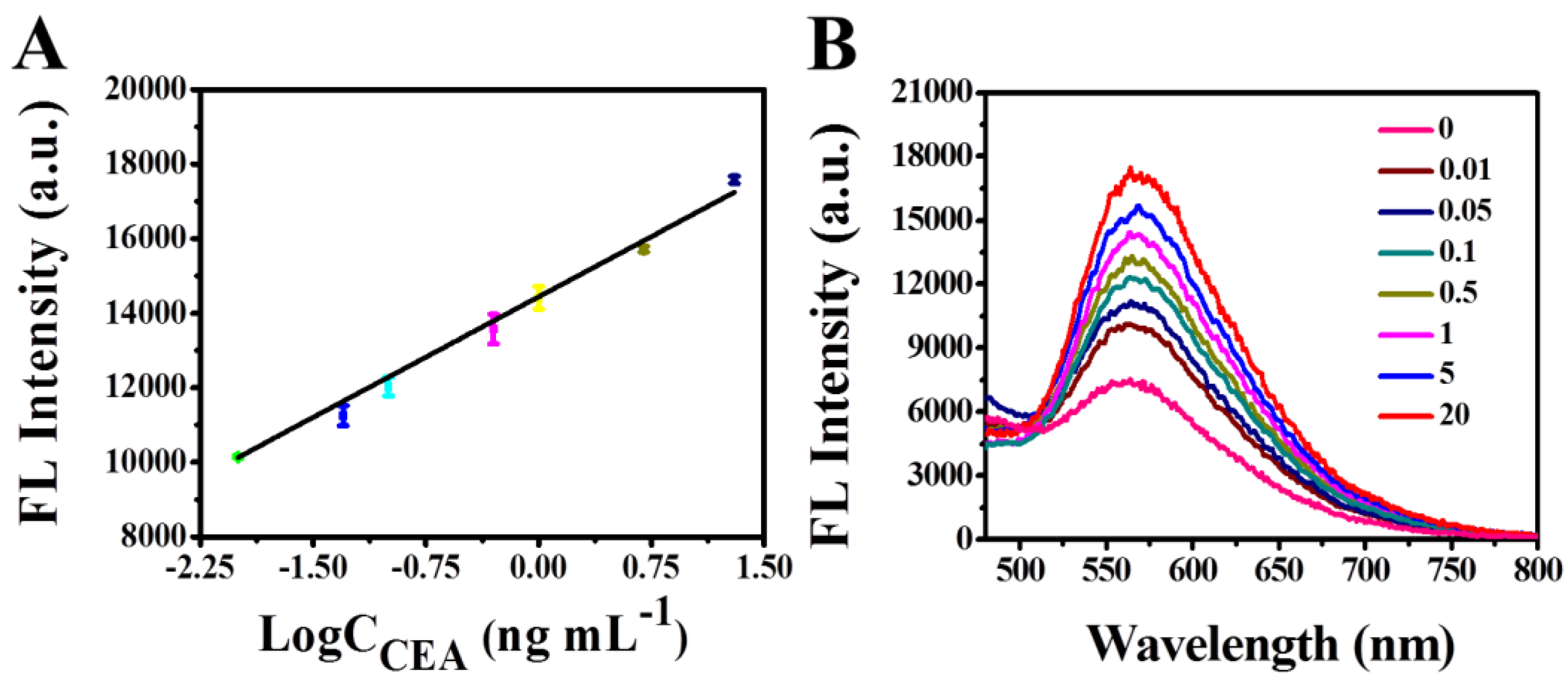
3.4. Analytical Performance
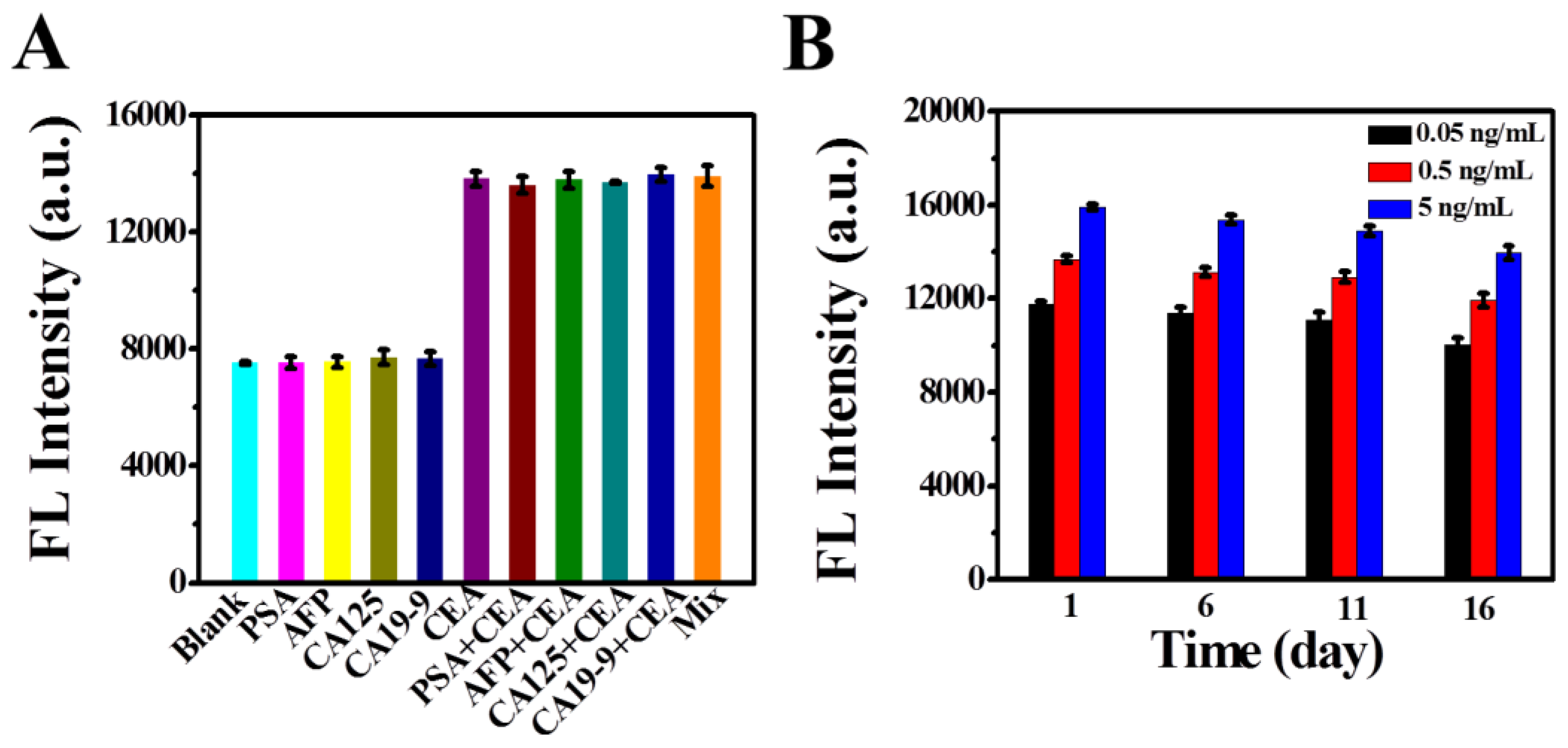
3.5. Selectivity and Stability Investigation
3.6. Analysis of Real Samples and Evaluation of Method Accuracy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.; Chen, J.; Yu, Z.; Tang, D. Self-powered temperature sensor with seebeck effect transduction for photothermal-thermoelectric coupled immunoassay. Anal. Chem. 2020, 92, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gong, H.; Xu, J.; Li, Y.; Zeng, Y.; Liu, X.; Tang, D. Exploiting photoelectric activities and piezoelectric properties of NaNbO3 semiconductors for point-of-care immunoassay. Anal. Chem. 2022, 94, 3418–3426. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Xi, Z.; Vlasov, S.; Ayala, J.; Xia, X.H. Nanocrystals of platinum-group metals as peroxidase mimics for in vitro diagnostics. Chem. Commun. 2020, 56, 14962–14975. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Liu, C.; Li, Z.D.; Xue, Z.R.; Mao, P.; Hu, J.; Xu, F.; Yao, C.Y.; You, M.L. Emerging ELISA derived technologies for in vitro diagnostics. TrAC Trends Anal. Chem. 2022, 152, 116605. [Google Scholar] [CrossRef]
- Ruan, Y.; Xu, H.H.; Yu, J.L.; Chen, Q.; Gu, L.H.; Guo, A.L. A fluorescence immunoassay based on CdTe: Zn/ZnS quantum dots for the rapid detection of bacteria, taking Delftia tsuruhatensis CM’13 as an example. RSC Adv. 2020, 10, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Liu, X.Y.; Shen, M.F.; Shen, L.S.; Ke, X.; Cui, D.X.; Li, W.W. Multicolor quantum dot nanobeads based fluorescence-linked immunosorbent assay for highly sensitive multiplexed detection. Sens. Actuators B 2021, 338, 129827. [Google Scholar] [CrossRef]
- Smitha, P.K.; Bathula, C.; Chandrashekara, K.N.; Das, M. Usage of graphene oxide in fluorescence quenching-linked immunosorbent assay for the detection of Cry2Ab protein present in transgenic plants. J. Agric. Food Chem. 2020, 68, 3656–3662. [Google Scholar] [CrossRef]
- Lv, S.; Tang, Y.; Zhang, K.; Tang, D. Wet NH3-triggered NH2-MIL-125(Ti) structural switch for svisible fluorescence immunoassay impregnated on paper. Anal. Chem. 2018, 90, 14121–14125. [Google Scholar] [CrossRef]
- Zhan, Y.J.; Yang, S.T.; Luo, F.; Guo, L.H.; Zeng, Y.B.; Qiu, B.; Lin, Z.Y. Emission wavelength switchable carbon dots combined with biomimetic inorganic nanozymes for a two-photon fluorescence immunoassay. ACS Appl. Mater. Interfaces 2020, 12, 30085–30094. [Google Scholar] [CrossRef]
- Taron, W.; Phooplub, K.; Sanchimplee, S.; Piyanamvanich, K.; Jamnongkan, W.; Techasen, A.; Phetcharaburanin, J.; Klanrit, P.; Namwat, N.; Khuntikeo, N.; et al. Smartphone-based fluorescent ELISA with simple fluorescent enhancement strategy for Opisthorchis viverrini (Ov) antigen detection in urine samples. Sens. Actuators B Chem. 2021, 348, 130705. [Google Scholar] [CrossRef]
- Lv, X.; Huang, Y.M.; Liu, D.F.; Liu, C.W.; Shan, S.; Li, G.Q.; Duan, M.L.; Lai, W.H. Multicolor and ultrasensitive enzyme-linked immunosorbent assay based on the fluorescence hybrid chain reaction for simultaneous detection of pathogens. J. Agric. Food Chem. 2019, 67, 9390–9398. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.S.; Liu, X.; Niu, C.J.; Pang, Q.; Li, J.T.; Liu, F.; Liu, Z.A.; Mai, L.Q. Advances in metal-organic framework coatings: Versatile synthesis and broad applications. Chem. Soc. Rev. 2020, 49, 3142–3186. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, K.; Zhu, L.; Tang, D. ZIF-8-assisted NaYF4:Yb, Tm@ZnO converter with exonuclease III-powered DNA walker for near-infrared light responsive biosensor. Anal. Chem. 2020, 92, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.L.; Chi, J.T.; Zhang, C.; Wang, M.L.; Liang, H.; Hou, J.Y.; Ai, S.Y.; Li, X.Y. A simple and sensitive sensor for lactose based on cascade reactions in Au nanoclusters and enzymes co-encapsulated metal-organic frameworks. Food Chem. 2021, 339, 127863. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Ma, Z.F. Copper peroxide/ZIF-8 self-producing H2O2 triggered cascade reaction for amperometric immunoassay of carbohydrate antigen 19-9. Biosens. Bioelectron. 2020, 169, 112644. [Google Scholar] [CrossRef]
- Lin, L.S.; Huang, T.; Song, J.B.; Ou, X.Y.; Wang, Z.T.; Deng, H.Z.; Tian, R.; Liu, Y.J.; Wang, J.F.; Liu, Y.; et al. Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J. Am. Chem. Soc. 2019, 141, 9937–9945. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Du, Y.; Wang, H.; Ma, H.M.; Wu, D.; Ren, X.; Wei, Q.; Xu, J.J. Self-supply of H2O2 and O2 by hydrolyzing CaO2 to enhance the electrochemiluminescence of luminol based on a closed bipolar electrode. Anal. Chem. 2020, 92, 12693–12699. [Google Scholar] [CrossRef]
- Ross, P.K.; Solomon, E.I. An electronic structural comparison of copper-peroxide complexes of relevance to hemocyanin and tyrosinase active sites. J. Am. Chem. Soc. 1991, 113, 3246–3259. [Google Scholar] [CrossRef]
- Wan, W.; Han, Q.; Zhang, X.Q.; Xie, Y.M.; Sun, J.P.; Ding, M.Y. Selective enrichment of proteins for MALDI-TOF MS analysis based on molecular imprinting. Chem. Commun. 2015, 51, 3541–3544. [Google Scholar] [CrossRef]
- Shuai, C.J.; Zan, J.; Deng, F.; Yang, Y.W.; Peng, S.P.; Zhao, Z.Y. Core-shell-structured ZIF-8@PDA-HA with controllable zinc ion release and superior bioactivity for improving a poly-l-lactic acid Scaffold. ACS Sustain. Chem. Eng. 2021, 9, 1814–1825. [Google Scholar] [CrossRef]
- Fan, Y.C.; Xing, H.H.; Xue, Y.; Peng, C.; Li, J.; Wang, E.K. Universal platform for ratiometric sensing based on catalytically induced inner-filter effect by Cu2+. Anal. Chem. 2020, 92, 16066–16071. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, J.P.; Song, D.D.; Xu, J.L.; Zhang, M. Electrochemical aptasensor of carcinoembryonic antigen based on concanavalin a-functionalized magnetic copper silicate carbon microtubes and gold-nanocluster-assisted signal amplification. ACS Appl. Nano Mater. 2020, 3, 3449–3458. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.H.; Wang, Y.; Zhao, F.Q.; Zeng, B.Z. Electrochemiluminescence immunosensor for the detection of carcinoembryonic antigen based on oxygen vacancy-rich Co3O4 nanorods and luminol. ACS Appl. Nano Mater. 2021, 4, 7264–7271. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, C.T.; Xu, Q.S.; Lou, Z.C.; Xu, Z.F.; Thierry, B.; Gu, N. Gold nanoparticle probe-assisted antigen-counting chip using SEM. ACS Appl. Mater. Interfaces 2019, 11, 6769–6776. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Su, Z.Y.; Zhang, J.; Cai, M.J.; Wu, L.L. A novel electrochemical immunoassay for carcinoembryonic antigen based on glucose oxidase-encapsulated nanogold hollow spheres with a pH meter readout. Analyst 2018, 143, 5271–5277. [Google Scholar] [CrossRef]
- Fan, Y.C.; Lv, M.M.; Xue, Y.; Li, J.; Wang, E.K. In situ fluorogenic reaction generated via ascorbic acid for the construction of universal sensing platform. Anal. Chem. 2021, 93, 6873–6880. [Google Scholar] [CrossRef]
- Xu, S.H.; Feng, X.Y.; Gao, T.; Liu, G.F.; Mao, Y.N.; Lin, J.H.; Yu, X.J.; Luo, X.L. Aptamer induced multicoloured Au NCs-MoS2 "switch on" fluorescence resonance energy transfer biosensor for dual color simultaneous detection of multiple tumor markers by single wavelength excitation. Anal. Chim. Acta 2017, 983, 173–180. [Google Scholar] [CrossRef]
- Qiu, Z.; Shu, J.; Tang, D. Bioresponsive release system for visual fluorescence detection of carcinoembryonic antigen from mesoporous silica nanocontainers mediated optical color on quantum dot-enzyme-impregnated paper. Anal. Chem. 2017, 89, 5152–5160. [Google Scholar] [CrossRef]
| Sample Number | Found by the Electrochemiluminescence Method [ng mL−1] | Found by Self-Supplied H2O2 Fluorescent Immunosensor [mean ± SD (RSD), ng mL−1], n = 3 |
|---|---|---|
| 1 | 0.05 | 0.052 ± 0.001 (2.54%) |
| 2 | 0.5 | 0.51 ± 0.03 (5.84%) |
| 3 | 1.5 | 1.55 ± 0.13 (8.16%) |
| 4 | 2.1 | 1.99 ± 0.14 (7.20%) |
| 5 | 5.4 | 5.94 ± 0.17 (5.24%) |
| 6 | 13.7 | 13.05 ± 0.68 (5.24%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Yao, Y.; Chen, Y.; Lin, T.; Hou, L.; Tang, D. Polydopamine-Functionalized Copper Peroxide/ZIF-8 Nanoparticle-Based Fluorescence-Linked Immunosorbent Assay for the Sensitive Determination of Carcinoembryonic Antigen by Self-Supplied H2O2 Generation. Biosensors 2022, 12, 830. https://doi.org/10.3390/bios12100830
Huang J, Yao Y, Chen Y, Lin T, Hou L, Tang D. Polydopamine-Functionalized Copper Peroxide/ZIF-8 Nanoparticle-Based Fluorescence-Linked Immunosorbent Assay for the Sensitive Determination of Carcinoembryonic Antigen by Self-Supplied H2O2 Generation. Biosensors. 2022; 12(10):830. https://doi.org/10.3390/bios12100830
Chicago/Turabian StyleHuang, Juanjuan, Yiyun Yao, Yanling Chen, Tianran Lin, Li Hou, and Dianping Tang. 2022. "Polydopamine-Functionalized Copper Peroxide/ZIF-8 Nanoparticle-Based Fluorescence-Linked Immunosorbent Assay for the Sensitive Determination of Carcinoembryonic Antigen by Self-Supplied H2O2 Generation" Biosensors 12, no. 10: 830. https://doi.org/10.3390/bios12100830
APA StyleHuang, J., Yao, Y., Chen, Y., Lin, T., Hou, L., & Tang, D. (2022). Polydopamine-Functionalized Copper Peroxide/ZIF-8 Nanoparticle-Based Fluorescence-Linked Immunosorbent Assay for the Sensitive Determination of Carcinoembryonic Antigen by Self-Supplied H2O2 Generation. Biosensors, 12(10), 830. https://doi.org/10.3390/bios12100830






