New Horizons for MXenes in Biosensing Applications
Abstract
1. Introduction
2. Synthesis and Structures of MXenes
2.1. Synthesis of MXenes
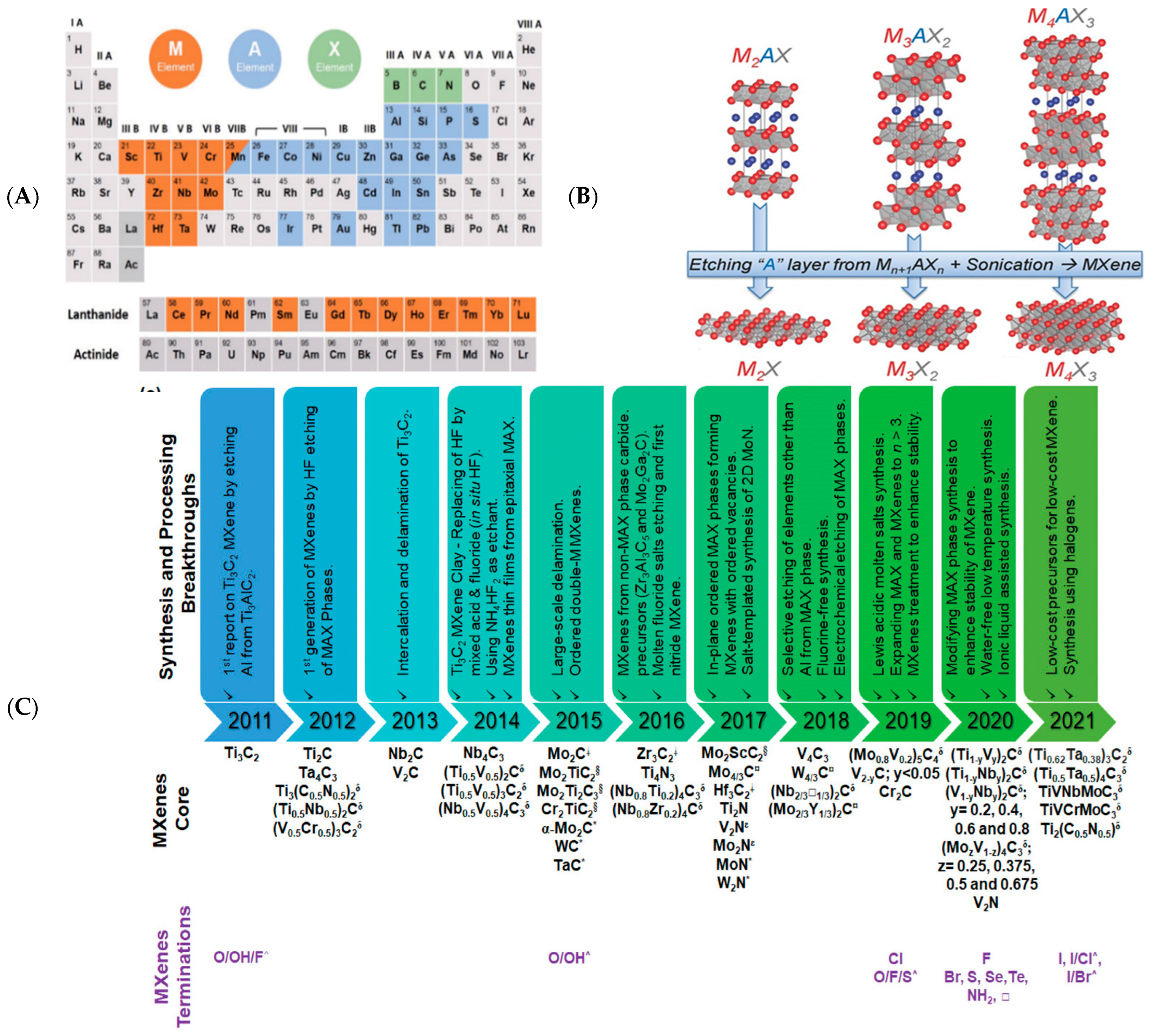
2.1.1. Top-Down Method
2.1.2. Bottom-Up Method

2.2. Strustures of Mxenes
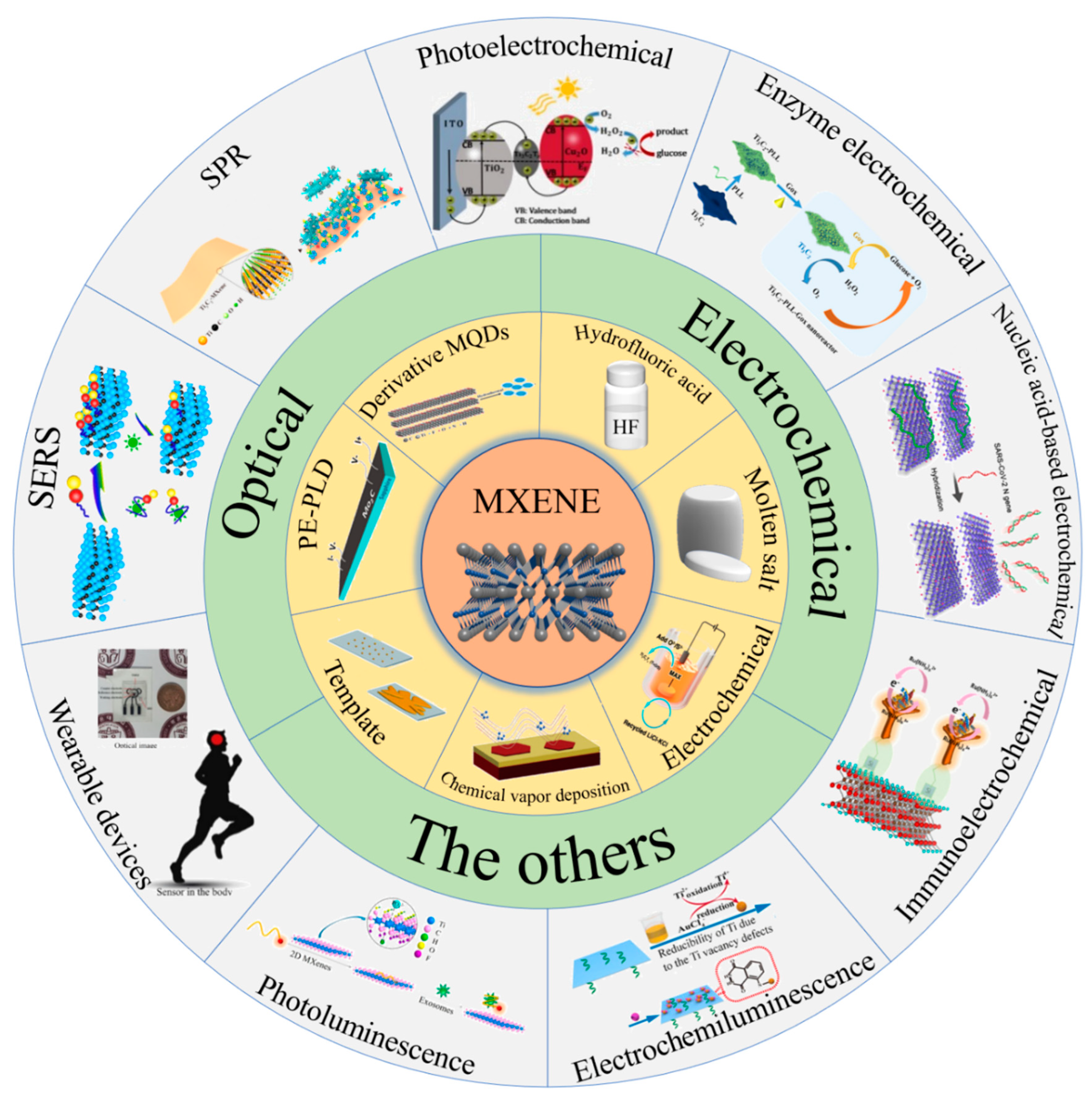
3. MXenes in Biosensing
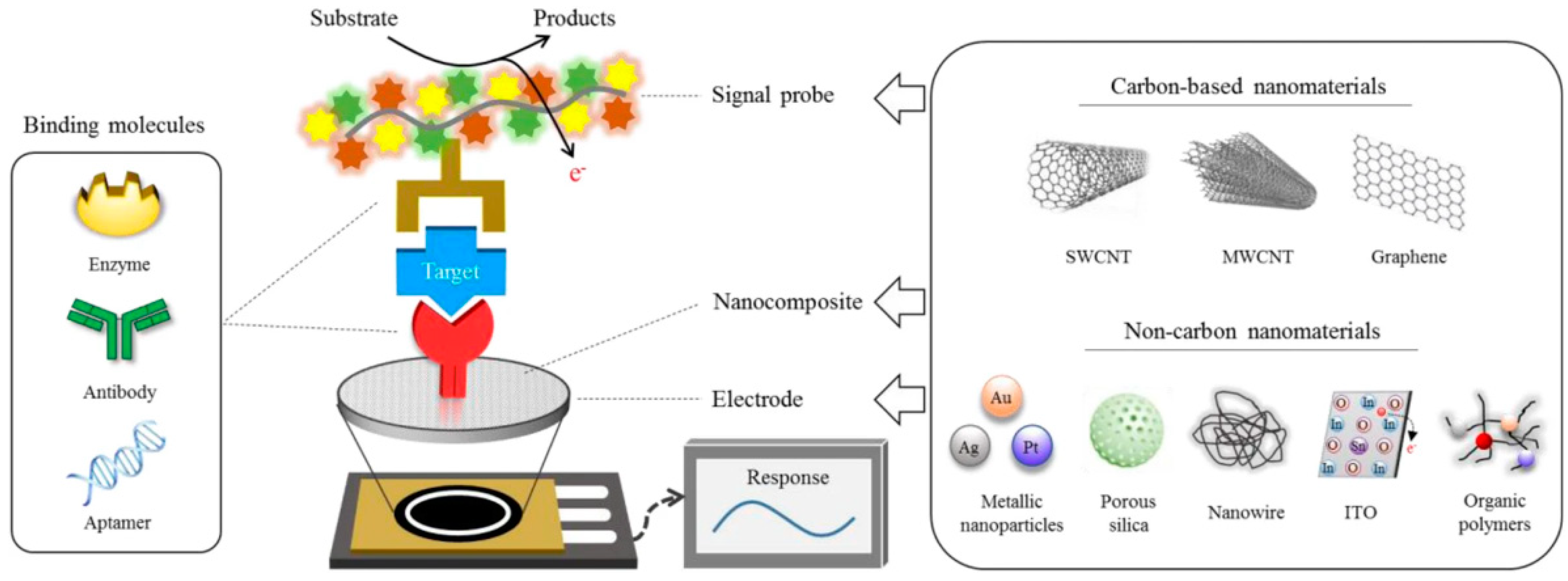
3.1. Electrochemical Biosensing
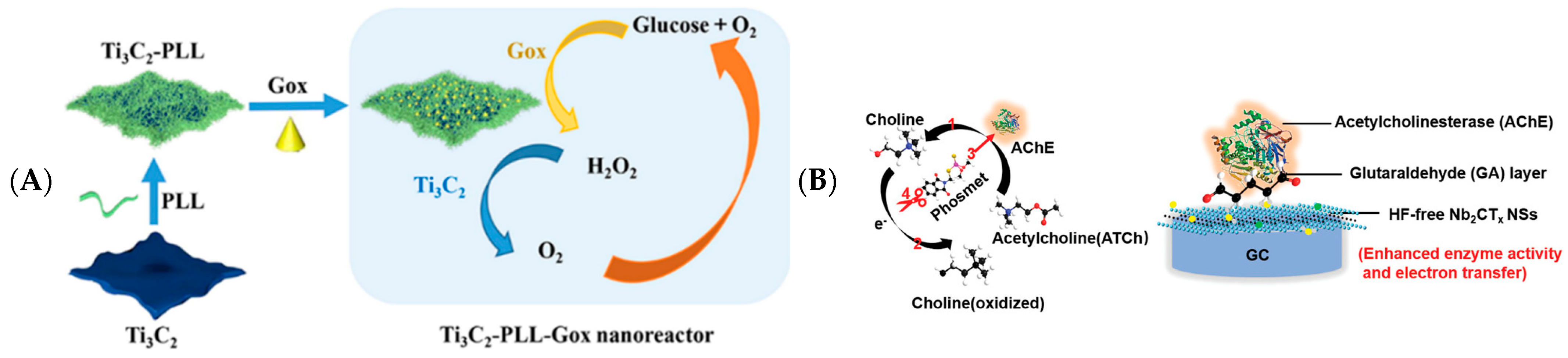
3.1.1. Enzyme-Based Electrochemical Biosensing
| MXenes Composite | Identify Units | Target | LOD | Range | Ref. |
|---|---|---|---|---|---|
| Au/Ti3C2 | glucose oxidase | glucose | 5.9 μM | 0.1–18 mM | [55] |
| PLL/Ti3C2 | glucose oxidase | glucose | 2.6 μM | 4.0–20 µM | [56] |
| PEDOT: SCX/Ti3C2Tx | glucose oxidase | glucose | 22.5 μM | 0.5–8 mM | [57] |
| Ti3C2/Nafions | Horse Radish Peroxidase | H2O2 | 1 μM | 5–8000 μM | [53] |
| MXene/chitosan | Horse Radish Peroxidase | H2O2 | 0.74 μM | 5–1650 μM | [54] |
| Chit/ChOx/Ti3C2Tx | cholesterol oxidase | cholesterol | 0.11 nM | 0.3–4.5 nM | [58] |
| Ti3C2 | tyrosinase | phenol | 12 nmol L−1 | 50 nM–15.5 μM | [59] |
| CS-Ti3C2Tx | acetylcholinesterase | acetylthiocholine chloride | 3 fM | 10 nM–10 fM | [60] |
| GA/Nb2CTx | acetylcholinesterase | phosmet | 144 pM | 200 pM–1 μM | [26] |
3.1.2. Nucleic Acid-Based Electrochemical Biosensing
| MXenes Composite | Identify Units | Target | LOD | Range | Ref. |
|---|---|---|---|---|---|
| MoS2 /Au NPs/Ti3C2 | DNA probe | miRNA-182 | 0.43 fM | 1 fM–0.1 nM | [63] |
| Au/Ti3C2 | DNA probe | miRNA-21, 141 | 204 aM 138 aM | 500 aM–50 nM | [73] |
| Ti3C2Tx @FePcQDs | DNA probe | miRNA-155 | 4.3 aM | 0.01 fM–10 pM | [72] |
| MCH/CP/AuNPs/Ti3C2Tx | DNA probe | BCR/ABL fusion gene | 0.05 fM | 0.2 fM–20 nM | [75] |
| Ti3C2Tx | DNA probe | SARS-Cov-2 N gene | 105 copies mL−1 | 105–109 copies mL−1 | [74] |
| PMo12/PPy@Ti3C2Tx | Aptamer | Osteopontin | 0.98 fg mL−1 | 0.05–10,000 pg mL−1 | [64] |
| AuNPs/Ti3C2 | Aptamer | Mucin 1 | 0.72 pg mL−1 | 5 pg mL−1–50 ng mL−1 | [76] |
| Ti3C2 | Aptamer | gliotoxin | 5 pM | 5 pM–10 nM | [65] |
| Ti3C2 | Aptamer | HER2-positive CTCs | 47 cell mL−1 | 20–200 cells mL−1 | [77] |
| CoCu-ZIF@ Ti3C2 CDs | Aptamer | B16-F10 cell | 33 cells∙mL−1 | 1 × 102–1× 105 cells∙mL−1 | [67] |
| Au@Nb4C3Tx | Aptamer | Pb2+ | 4 nM | 10 nM–5 μM | [66] |

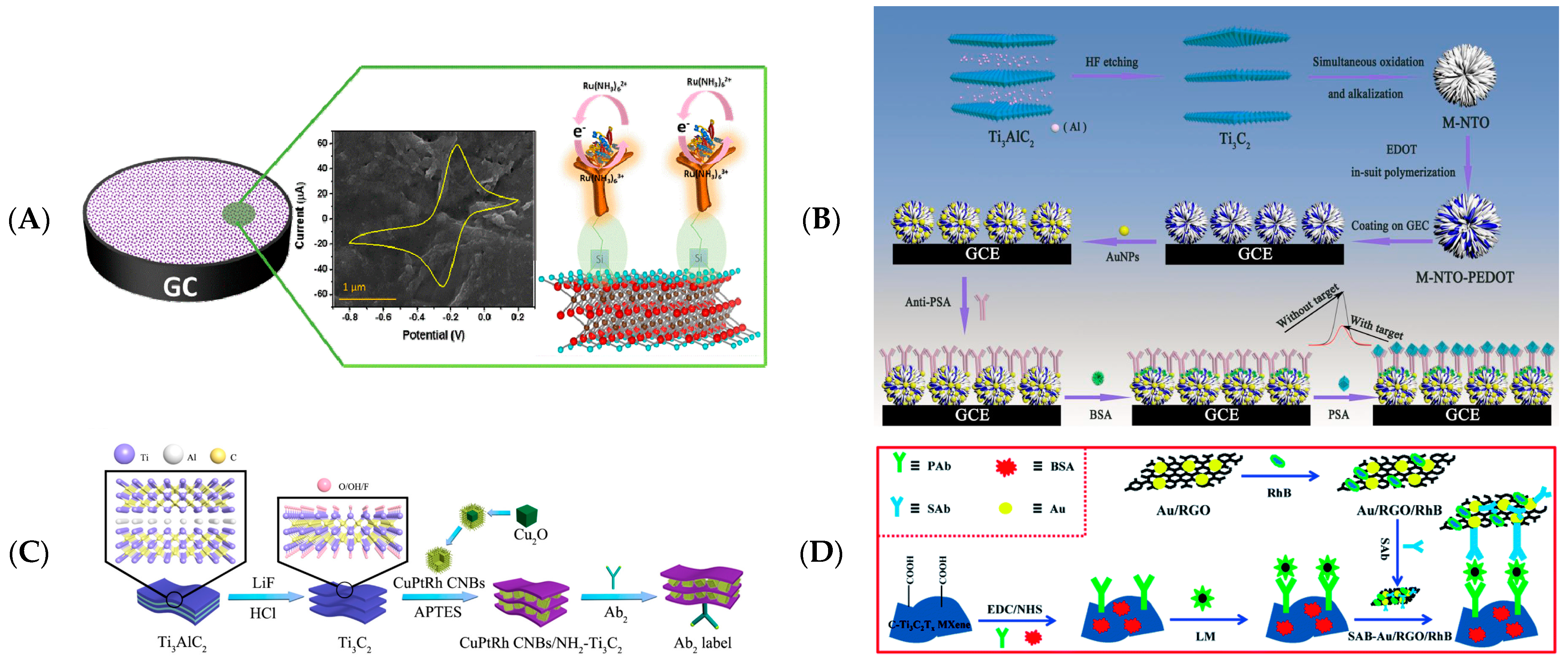
3.1.3. Immunoelectrochemical Biosensing
3.2. Optical Biosensing
3.2.1. Photoluminescence (PL)
3.2.2. Electrochemiluminescence (ECL)
| MXenes Composite | Identify Units | Target | LOD | Range | Ref. |
|---|---|---|---|---|---|
| g-C3N4/Ti3C2 | Ti3C2 | Protein Kinase | 1.0 mU mL−1 | 0.015–40 U mL−1 | [115] |
| Ti3C2Tx | Ru(bpy)32+ | nucleotide mismatch | 5 nM | - | [111] |
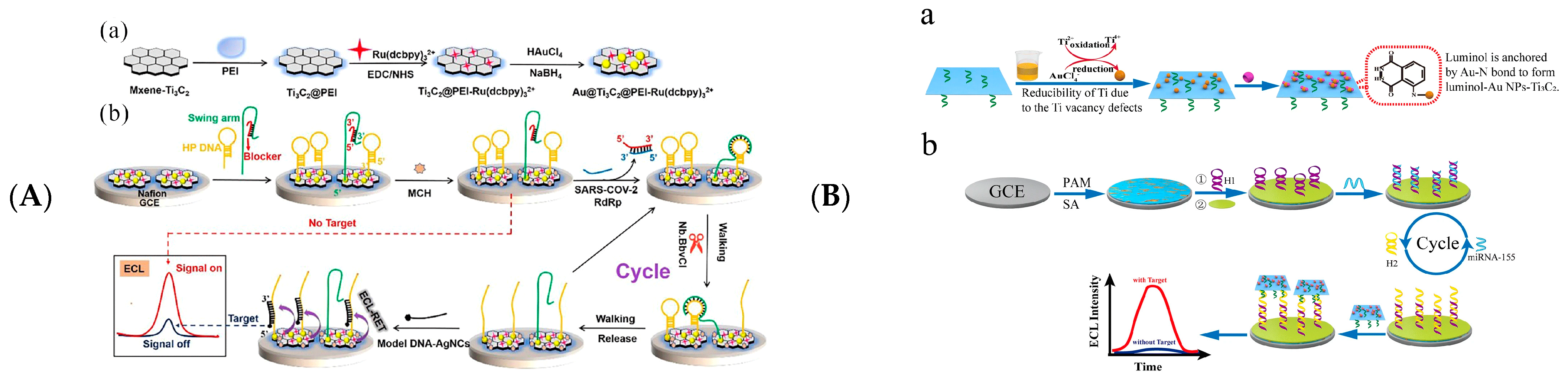
| Au@Ti3C2@PEI-Ru(dcbpy)32+ | Model DNA-AgNCs | SARS-Cov-2 Gene | 0.21 fM | 1 fM–100 pM | [113] |
| AuNPs/Ti3C2/Luminol | sDNA | miRNA-155 | 0.15 fM | 0.3 fM–1 nM | [112] |
| Ru@Ti3C2@AuNPs | Fc-DNA | SARS-Cov-2 gene | 12.8 aM | - | [114] |
| Ti3C2/PEI | aptamer | MCF-7 | 125 particles μL−1 | 5 × 102–5 × 106 particles μL−1 | [116] |
| Ti3C2/Au | aptamer | CD63 | 30 particles μL−1 | 102–105 particles μL−1 | [117] |
| AuNPs/Ti3C2 | aptamer | cardiac troponin I | 0.04 fM | 0.1 fM–1 pM | [118] |
| AuNPs-Ru-Arg@Ti3C2 | antibody | CEA | 1.5 pg mL−1 | 0.01–150 ng mL−1 | [119] |
| R6G-Ti3C2Tx@AuNRs/ABEI | antibody | Vibrio vulnificus | 1 CFU mL−1 | 1–108 CFU mL−1 | [120] |

3.2.3. Photoelectrochemical (PEC)
3.3. Other Biosensing
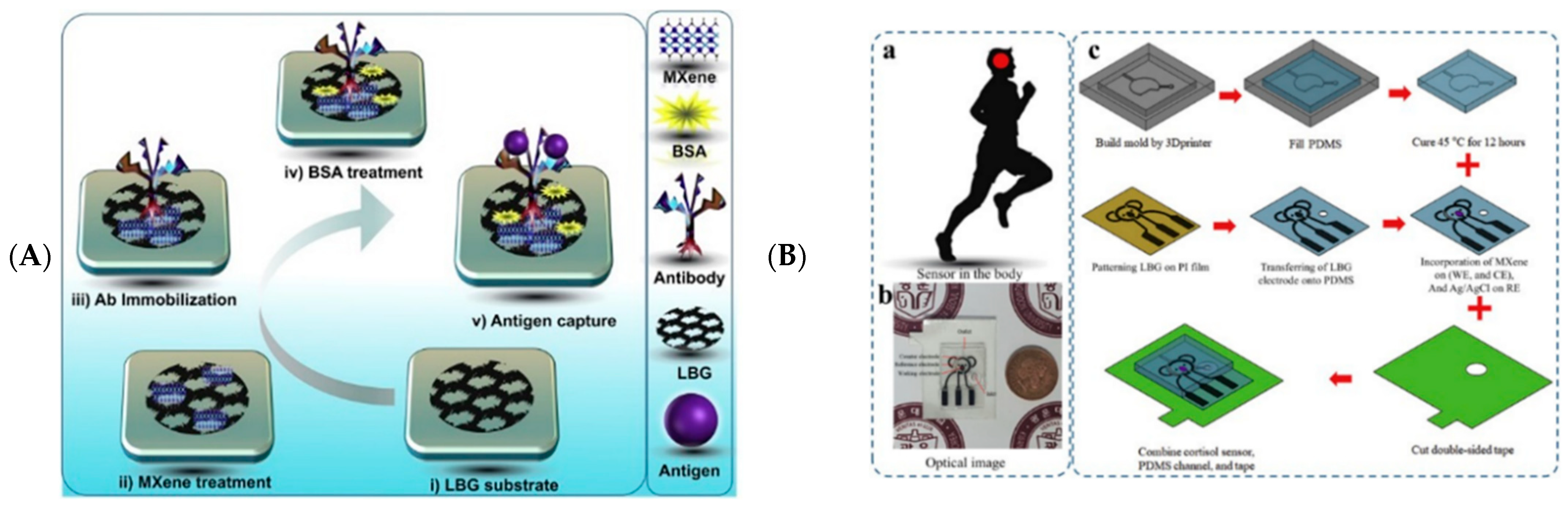
3.3.1. Wearable Biosensing
3.3.2. Surface-Enhanced Raman Spectroscopy (SERS)
3.3.3. Surface Plasmon Resonance (SPR)
4. Conclusions and Outlook
- (1)
- MXenes and MQDs synthesis methods are supposed to develop with adjustable size and surface groups. It is necessary to study further study the interaction between the composition, structure, properties, and biomolecules of MXenes in order to understand the appropriate MXene-adaptation-related biosensors that can be developed according to the actual situation. Furthermore, it can provide theoretical support for the development and development of MXenes morphological structure, size, and surface groups, and the study of interaction with biomolecules with the help of machine learning methods.
- (2)
- The direct construction of biosensors on MXenes surfaces: The abundant surface groups of MXenes can be complexed or combined with many biomolecules, and enzyme-based electrochemical sensors have been partially verified. Nevertheless, there are relatively few studies on nucleic acid, antibodies, and other biomolecules combined with MXenes and constructed in sensors. Moreover, the natural binding process can shorten the experimental time and reduce errors caused by too many experimental procedures. At the same time, it is also very convenient for developed point-of-care tests. During the COVID-19 epidemic, point-of-care was necessary for rapid diagnoses and the timely treatment of patients.
- (3)
- Utilise the catalytic properties and the reduction of MXenes: For electrochemical biosensing, MXenes can reduce precious metal nanoparticles in situ. They can also be used to catalyse redox reactions of O2 in systems that promote free radical reactions in ECL, and improving the ECL signal is well worth exploring. MXenes are also used as semiconductor materials during electrochemical reactions and can be used to enhance the electrochemical signal. Therefore, reducing the detection limit, extending the detection range, and improving the sensitivity during electrochemical biosensing are possible.
- (4)
- Develop MQDs with high fluorescence efficiency, quantum yield, and different fluorescence emission wavelengths: As a result, the sensitivity and detection limit of fluorescence detections can be improved, and they can be used in optical biosensors with different wavelengths. MQDs can be extended to cell imaging, photothermal therapy, and other biomedical tissue applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, F.; Song, P.; Ruan, M.; Xu, W. Recent progress in two-dimensional nanomaterials: Synthesis, engineering, and applications. FlatChem 2019, 18, 100133. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kim, I.-D. Recent Developments in 2D Nanomaterials for Chemiresistive-Type Gas Sensors. Electron. Mater. Lett. 2018, 14, 221–260. [Google Scholar] [CrossRef]
- Deshmukh, K.; Kovářík, T.; Khadheer Pasha, S.K. State of the art recent progress in two dimensional MXenes based gas sensors and biosensors: A comprehensive review. Coord. Chem. Rev. 2020, 424, 213514. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, P. A Facile, High-Yield, and Freeze-and-Thaw-Assisted Approach to Fabricate MXene with Plentiful Wrinkles and Its Application in On-Chip Micro-Supercapacitors. Adv. Funct. Mater. 2020, 30, 1910048. [Google Scholar] [CrossRef]
- Ma, Y.; Li, B.; Yang, S. Ultrathin two-dimensional metallic nanomaterials. Mater. Chem. Front. 2018, 2, 456–467. [Google Scholar] [CrossRef]
- Ghazaly, A.E.; Ahmed, H.; Rezk, A.R.; Halim, J.; Persson, P.O.Å.; Yeo, L.Y.; Rosen, J. Ultrafast, One-Step, Salt-Solution-Based Acoustic Synthesis of Ti3C2 MXene. ACS Nano 2021, 15, 4287–4293. [Google Scholar] [CrossRef]
- Qing, H.; Mian, L.I. Recent Progress and Prospects of Ternary Layered Carbides/Nitrides MAX Phases and Their Derived Two-Dimensional Nanolaminates MXenes. J. Inorg. Mater. 2019, 35, 1–7. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Liu, Q. Electrochemical and optical biosensors based on multifunctional MXene nanoplatforms: Progress and prospects. Talanta 2021, 235, 122726. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Zhuang, T.; Liu, J.; Sojic, N.; Wang, Z. Sensitive electrochemiluminescence biosensing of polynucleotide kinase using the versatility of two-dimensional Ti3C2TX MXene nanomaterials. Anal. Chim. Acta 2022, 1191, 339346. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten Years of Progress in the Synthesis and Development of MXenes. Adv. Mater. 2021, 33, 2103393. [Google Scholar] [CrossRef] [PubMed]
- Roointan, A.; Ahmad Mir, T.; Ibrahim Wani, S.; Mati Ur, R.; Hussain, K.K.; Ahmed, B.; Abrahim, S.; Savardashtaki, A.; Gandomani, G.; Gandomani, M.; et al. Early detection of lung cancer biomarkers through biosensor technology: A review. J. Pharm. Biomed. Anal. 2019, 164, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Kubota, L.T. Trends in Electrochemical Sensing. ChemElectroChem 2020, 7, 3684–3685. [Google Scholar] [CrossRef]
- Mohammadniaei, M.; Nguyen, H.V.; Tieu, M.V.; Lee, M.-H. 2D Materials in Development of Electrochemical Point-of-Care Cancer Screening Devices. Micromachines 2019, 10, 662. [Google Scholar] [CrossRef]
- Wrobel, T.P.; Bhargava, R. Infrared Spectroscopic Imaging Advances as an Analytical Technology for Biomedical Sciences. Anal. Chem. 2018, 90, 1444–1463. [Google Scholar] [CrossRef]
- Kumar, J.A.; Prakash, P.; Krithiga, T.; Amarnath, D.J.; Premkumar, J.; Rajamohan, N.; Vasseghian, Y.; Saravanan, P.; Rajasimman, M. Methods of synthesis, characteristics, and environmental applications of MXene: A comprehensive review. Chemosphere 2022, 286 Pt 1, 131607. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Mao, Y.; Li, Z. Progress and biomedical applications of MXenes. Nano Sel. 2021, 2, 1480–1508. [Google Scholar] [CrossRef]
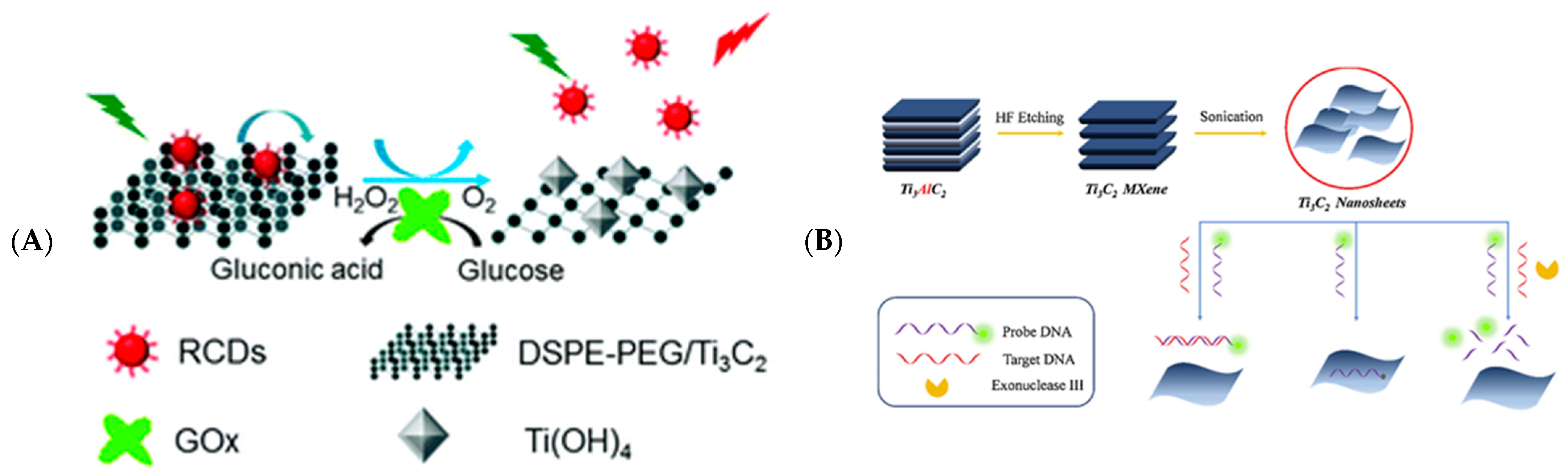
- Zhang, J.; Li, Y.; Duan, S.; He, F. Highly electrically conductive two-dimensional Ti3C2 Mxenes-based 16S rDNA electrochemical sensor for detecting Mycobacterium tuberculosis. Anal. Chim. Acta 2020, 1123, 9–17. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Lin, J.; Zhang, Y.; Qiu, M.; Chen, Y.; Li, M.; Tang, D. Ultrasensitive fluorometric biosensor based on Ti3C2 MXenes with Hg(2+)-triggered exonuclease III-assisted recycling amplification. Analyst 2021, 146, 2664–2669. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ’clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Sang, X.; Xie, Y.; Lin, M.W.; Alhabeb, M.; Van Aken, K.L.; Gogotsi, Y.; Kent, P.R.C.; Xiao, K.; Unocic, R.R. Atomic Defects in Monolayer Titanium Carbide (Ti3C2Tx) MXene. ACS Nano 2016, 10, 9193–9200. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Alhabeb, M.; Hatter Christine, B.; Anasori, B.; Man Hong, S.; Koo Chong, M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Pang, S.Y.; Guo, F.; Wong, M.C.; Hao, J. Fluoride-Free 2D Niobium Carbide MXenes as Stable and Biocompatible Nanoplatforms for Electrochemical Biosensors with Ultrahigh Sensitivity. Adv. Sci 2020, 7, 2001546. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, C.; Huang, W.; Mei, S.; Lin, H.; Ou, Q.; Zhang, Y.; Guo, J.; Zhang, F.; Xu, S.; et al. MXene/Polymer Membranes: Synthesis, Properties, and Emerging Applications. Chem. Mater. 2020, 32, 1703–1747. [Google Scholar] [CrossRef]
- Tian, Z.; Wei, C.; Sun, J. Recent advances in the template-confined synthesis of two-dimensional materials for aqueous energy storage devices. Nanoscale Adv. 2020, 2, 2220–2233. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Wang, H.; Chan, C.H.; Chan, N.Y.; Chen, X.X.; Dai, J.-Y. Plasma-enhanced pulsed-laser deposition of single-crystalline Mo2C ultrathin superconducting films. Phys. Rev. Mater. 2017, 1, 034002. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Sun, W.; Wang, X.; Feng, J.; Li, T.; Huan, Y.; Qiao, J.; He, L.; Ma, D. Controlled synthesis of 2D Mo2C/graphene heterostructure on liquid Au substrates as enhanced electrocatalytic electrodes. Nanotechnology 2019, 30, 385601. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Mishra, A.; Mizuseki, H.; Lee, K.R.; Singh, A.K. Mechanistic Insight into the Chemical Exfoliation and Functionalization of Ti3C2 MXene. ACS Appl Mater. Interfaces 2016, 8, 24256–24264. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Z.; Zhi, C. Environmental Stability of MXenes as Energy Storage Materials. Front. Mater. 2019, 6, 312. [Google Scholar] [CrossRef]
- Huang, S.; Mochalin, V.N. Hydrolysis of 2D Transition-Metal Carbides (MXenes) in Colloidal Solutions. Inorg. Chem. 2019, 58, 1958–1966. [Google Scholar] [CrossRef]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.-H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Tan, T.L.; Jin, H.M.; Sullivan, M.B.; Anasori, B.; Gogotsi, Y. High-Throughput Survey of Ordering Configurations in MXene Alloys Across Compositions and Temperatures. ACS Nano 2017, 11, 4407–4418. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-W.; Dai, J.-H.; Zhang, Y.-M.; Song, Y. Two-Dimensional, Ordered, Double Transition Metal Carbides (MXenes): A New Family of Promising Catalysts for the Hydrogen Evolution Reaction. J. Phys. Chem. C 2018, 122, 28113–28122. [Google Scholar] [CrossRef]
- Tao, Q.; Dahlqvist, M.; Lu, J.; Kota, S.; Meshkian, R.; Halim, J.; Palisaitis, J.; Hultman, L.; Barsoum, M.W.; Persson, P.O.A.; et al. Two-dimensional Mo1.33C MXene with divacancy ordering prepared from parent 3D laminate with in-plane chemical ordering. Nat. Commun. 2017, 8, 14949. [Google Scholar] [CrossRef]
- Halim, J.; Palisaitis, J.; Lu, J.; Thörnberg, J.; Moon, E.J.; Precner, M.; Eklund, P.; Persson, P.O.Å.; Barsoum, M.W.; Rosen, J. Synthesis of Two-Dimensional Nb1.33C (MXene) with Randomly Distributed Vacancies by Etching of the Quaternary Solid Solution (Nb2/3Sc1/3)2AlC MAX Phase. ACS Appl. Nano Mater. 2018, 1, 2455–2460. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.J.; Li, P.; Zhao, Y.; Niu, Q.J. Fabrication of novel MXene (Ti3C2)/polyacrylamide nanocomposite hydrogels with enhanced mechanical and drug release properties. Soft Matter 2020, 16, 162–169. [Google Scholar] [CrossRef]
- Khaledialidusti, R.; Anasori, B.; Barnoush, A. Temperature-dependent mechanical properties of Tin+1CnO2 (n = 1, 2) MXene monolayers: A first-principles study. Phys. Chem Chem Phys. 2020, 22, 3414–3424. [Google Scholar] [CrossRef]
- Ronchi, R.M.; Arantes, J.T.; Santos, S.F. Synthesis, structure, properties and applications of MXenes: Current status and perspectives. Ceram. Int. 2019, 45, 18167–18188. [Google Scholar] [CrossRef]
- Wu, X.; Ma, P.; Sun, Y.; Du, F.; Song, D.; Xu, G. Application of MXene in Electrochemical Sensors: A Review. Electroanalysis 2021, 33, 1827–1851. [Google Scholar] [CrossRef]
- Manzanares-Palenzuela, C.L.; Pourrahimi, A.M.; Gonzalez-Julian, J.; Sofer, Z.; Pykal, M.; Otyepka, M.; Pumera, M. Interaction of single- and double-stranded DNA with multilayer MXene by fluorescence spectroscopy and molecular dynamics simulations. Chem. Sci. 2019, 10, 10010–10017. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, B.; Liu, J. Mn(2+)-Assisted DNA Oligonucleotide Adsorption on Ti2C MXene Nanosheets. Langmuir 2019, 35, 9858–9866. [Google Scholar] [CrossRef]
- Vural, M.; Zhu, H.; Pena-Francesch, A.; Jung, H.; Allen, B.D.; Demirel, M.C. Self-Assembly of Topologically Networked Protein–Ti3C2Tx MXene Composites. ACS Nano 2020, 14, 6956–6967. [Google Scholar] [CrossRef]
- Huang, R.; Chen, X.; Dong, Y.; Zhang, X.; Wei, Y.; Yang, Z.; Li, W.; Guo, Y.; Liu, J.; Yang, Z.; et al. MXene Composite Nanofibers for Cell Culture and Tissue Engineering. ACS Appl. Bio Mater. 2020, 3, 2125–2131. [Google Scholar] [CrossRef]
- Shahzad, F.; Zaidi, S.A.; Naqvi, R.A. 2D Transition Metal Carbides (MXene) for Electrochemical Sensing: A Review. Crit Rev. Anal. Chem. 2020, 52, 848–864. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef]
- Verger, L.; Natu, V.; Carey, M.; Barsoum, M.W. MXenes: An Introduction of Their Synthesis, Select Properties, and Applications. Trends Chem. 2019, 1, 656–669. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.L.; Mayorga-Martinez, C.C.; Antonatos, N.; Sofer, Z.; Gonzalez-Julian, J.J.; Webster, R.D.; Pumera, M. MXene Titanium Carbide-based Biosensor: Strong Dependence of Exfoliation Method on Performance. Anal. Chem. 2020, 92, 2452–2459. [Google Scholar] [CrossRef]
- Xu, W.; Sakran, M.; Fei, J.; Li, X.; Weng, C.; Yang, W.; Zhu, G.; Zhu, W.; Zhou, X. Electrochemical Biosensor Based on HRP/Ti3C2/Nafion Film for Determination of Hydrogen Peroxide in Serum Samples of Patients with Acute Myocardial Infarction. ACS Biomater. Sci. Eng. 2021, 7, 2767–2773. [Google Scholar] [CrossRef]
- Ma, B.K.; Li, M.; Cheong, L.Z.; Weng, X.C.; Shen, C.; Huang, Q. Enzyme-MXene Nanosheets: Fabrication and Application in Electrochemical Detection of H2O2. J. Inorg. Mater. 2020, 35, 131. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Nayak, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Q.; Fang, Y.; Deng, C.; Zhou, F.; Zhang, Y.; Wang, X.; Tang, Y.; Wang, Y. Polylysine-modified MXene nanosheets with highly loaded glucose oxidase as cascade nanoreactor for glucose decomposition and electrochemical sensing. J. Colloid Interface Sci. 2021, 586, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Murugan, P.; Annamalai, J.; Atchudan, R.; Govindasamy, M.; Nallaswamy, D.; Ganapathy, D.; Reshetilov, A.; Sundramoorthy, A.K. Electrochemical Sensing of Glucose Using Glucose Oxidase/PEDOT:4-Sulfocalix [4]arene/MXene Composite Modified Electrode. Micromachines 2022, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Liu, G.; Wang, J.; Hou, S.; Hou, S. MXene-based enzymatic sensor for highly sensitive and selective detection of cholesterol. Biosens. Bioelectron. 2021, 183, 113243. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lu, X.; Dhanjai; Wu, Z.-S.; Dong, Y.; Wang, X.; Zheng, S.; Chen, J. 2D transition metal carbide MXene as a robust biosensing platform for enzyme immobilization and ultrasensitive detection of phenol. Biosens. Bioelectron. 2018, 107, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Zhang, X.; Ma, L.; Gao, J.; Jiang, Y.J. Acetylcholinesterase/chitosan-transition metal carbides nanocomposites-based biosensor for the organophosphate pesticides detection. Biochem. Eng. J. 2017, 128, 243–249. [Google Scholar] [CrossRef]
- Du, Y.; Dong, S. Nucleic Acid Biosensors: Recent Advances and Perspectives. Anal. Chem. 2017, 89, 189–215. [Google Scholar] [CrossRef]
- Wu, Y.; Arroyo-Currás, N. Advances in nucleic acid architectures for electrochemical sensing. Curr. Opin. Electrochem. 2021, 27, 100695. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Y.; Jiao, S.; Zhu, S.; Liu, X. A novel label-free strategy for the ultrasensitive miRNA-182 detection based on MoS2/Ti3C2 nanohybrids. Biosens. Bioelectron. 2019, 137, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Gu, C.; Li, Z.; Yang, L.; He, L.; Wang, M.; Huang, X.; Zhou, N.; Zhang, Z. Ti3C2Tx MXene and polyoxometalate nanohybrid embedded with polypyrrole: Ultra-sensitive platform for the detection of osteopontin. Appl. Surf. Sci. 2019, 498, 143889. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Huang, Y.; Xiong, M.; Wang, F.; Li, C. A label-free electrochemical biosensor for highly sensitive detection of gliotoxin based on DNA nanostructure/MXene nanocomplexes. Biosens. Bioelectron. 2019, 142, 111531. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Jabbar, K.A.; Mahmoud, K.A. Nb4C3Tx(MXene)/Au/DNA Aptasensor for the Ultraselective Electrochemical Detection of Lead in Water Samples. Electroanalysis 2022, 34, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.J.; Li, J.N.; Wang, M.H.; Wang, C.B.; Hu, B.; Zhou, N.; Zhang, Z.H. 0D/2D heteronanostructure-integrated bimetallic CoCu-ZIF nanosheets and MXene-derived carbon dots for impedimetric cytosensing of melanoma B16-F10 cells. Microchim. Acta 2021, 188, 69. [Google Scholar] [CrossRef]
- Hu, Z.; Suo, Z.; Liu, W.; Zhao, B.; Xing, F.; Zhang, Y.; Feng, L. DNA conformational polymorphism for biosensing applications. Biosens. Bioelectron. 2019, 131, 237–249. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lim, J.; Lee, J.Y.; Choi, J.W. Recent Advances in MXene Nanocomposite-Based Biosensors. Biosensors 2020, 10, 185. [Google Scholar] [CrossRef]
- Khan, R.; Ben Aissa, S.; Sherazi, T.A.; Catanante, G.; Hayat, A.; Marty, J.L. Development of an Impedimetric Aptasensor for Label Free Detection of Patulin in Apple Juice. Molecules 2019, 24, 1017. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Duan, F.; Guo, C.; Hu, M.; Song, Y.; Wang, M.; He, L.; Zhang, Z.; Pettinari, R.; Zhou, L. Construction of the 0D/2D heterojunction of Ti3C2Tx MXene nanosheets and iron phthalocyanine quantum dots for the impedimetric aptasensing of microRNA--155. Sens. Actuators B Chem. 2020, 310, 127844. [Google Scholar] [CrossRef]
- Mohammadniaei, M.; Koyappayil, A.; Sun, Y.; Min, J.; Lee, M.H. Gold nanoparticle/MXene for multiple and sensitive detection of oncomiRs based on synergetic signal amplification. Biosens. Bioelectron. 2020, 159, 112208. [Google Scholar] [CrossRef]
- Chen, W.Y.; Lin, H.; Barui, A.K.; Gomez, A.M.U.; Wendt, M.K.; Stanciu, L.A. DNA-Functionalized Ti3C2Tx MXenes for Selective and Rapid Detection of SARS-CoV-2 Nucleocapsid Gene. ACS Appl. Nano Mater. 2021, 5, 1902–1910. [Google Scholar] [CrossRef]
- Yu, R.J.; Xue, J.; Wang, Y.; Qiu, J.F.; Huang, X.Y.; Chen, A.Y.; Xue, J.J. Novel Ti3C2Tx MXene nanozyme with manageable catalytic activity and application to electrochemical biosensor. J. Nanobiotechnol. 2022, 20, 119. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Li, D.Y.; Huang, L.; Hu, R.; Yang, T.; Yang, Y.H. An electrochemical aptasensor based on intelligent walking DNA nanomachine with cascade signal amplification powered by nuclease for Mucin 1 assay. Anal. Chim. Acta 2022, 1214, 339964. [Google Scholar] [CrossRef]
- Vajhadin, F.; Mazloum-Ardakani, M.; Shahidi, M.; Moshtaghioun, S.M.; Haghiralsadat, F.; Ebadi, A.; Amini, A. MXene-based cytosensor for the detection of HER2-positive cancer cells using CoFe2O4@Ag magnetic nanohybrids conjugated to the HB5 aptamer. Biosens. Bioelectron. 2022, 195, 113626. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xiang, J. Aptamers, the Nucleic Acid Antibodies, in Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 2793. [Google Scholar] [CrossRef]
- Ranallo, S.; Porchetta, A.; Ricci, F. DNA-Based Scaffolds for Sensing Applications. Anal. Chem. 2019, 91, 44–59. [Google Scholar] [CrossRef]
- Wei, X.; Wang, S.; Zhan, Y.; Kai, T.; Ding, P. Sensitive Identification of Microcystin-LR via a Reagent-Free and Reusable Electrochemical Biosensor Using a Methylene Blue-Labeled Aptamer. Biosensors 2022, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Zhang, M.; Long, H.; Hu, Z.; Zhao, B.; Feng, L.; Du, J. A reusable neurotransmitter aptasensor for the sensitive detection of serotonin. Anal. Chim. Acta 2021, 1145, 124–131. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. A paper-based electrochemical immunosensor with reduced graphene oxide/thionine/gold nanoparticles nanocomposites modification for the detection of cancer antigen 125. Biosens. Bioelectron. 2019, 135, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Mei, L.S.; Zhang, L.; Wang, X.; Liao, X.C.; Qiao, X.W.; Hong, C.L. Ti3C2 MXene anchors CuAu-LDH multifunctional two-dimensional nanomaterials for dual-mode detection of CEA in electrochemical immunosensors. Bioelectrochemistry 2021, 142, 107943. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lei, Y.; Alshareef, N.H.; Quevedo-Lopez, M.A.; Salama, K.N. Biofunctionalized two-dimensional Ti3C2 MXenes for ultrasensitive detection of cancer biomarker. Biosens. Bioelectron. 2018, 121, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Medetalibeyoglu, H.; Kotan, G.; Atar, N.; Yola, M.L. A novel and ultrasensitive sandwich-type electrochemical immunosensor based on delaminated MXene@AuNPs as signal amplification for prostate specific antigen (PSA) detection and immunosensor validation. Talanta 2020, 220, 121403. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, J.K.; Jia, H.Y.; Tian, Q.Y.; Liu, P.; Chen, S.X.; Cai, Y.; Lu, X.Y.; Duan, X.M.; Lu, L.M. Hierarchical Ti3C2 MXene-derived sodium titanate nanoribbons/PEDOT for signal amplified electrochemical immunoassay of prostate specific antigen. J. Electroanal. Chem. 2022, 860, 113869. [Google Scholar] [CrossRef]
- Dong, H.; Cao, L.; Tan, Z.; Liu, Q.; Zhou, J.; Zhao, P.; Wang, P.; Li, Y.; Ma, W.; Dong, Y. A Signal Amplification Strategy of CuPtRh CNB-Embedded Ammoniated Ti3C2 MXene for Detecting Cardiac Troponin I by a Sandwich-Type Electrochemical Immunosensor. ACS Appl. Bio Mater. 2020, 3, 377–384. [Google Scholar] [CrossRef]
- Niu, H.M.; Cai, S.M.; Liu, X.K.; Huang, X.M.; Chen, J.; Wang, S.L.; Zhang, S.H. A novel electrochemical sandwich-like immunosensor based on carboxyl Ti3C2Tx MXene and rhodamine b/gold/reduced graphene oxide for Listeria monocytogenes. Anal. Methods 2022, 14, 843–849. [Google Scholar] [CrossRef]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, K.; Srikanth, N.; Pang, J.H.L.; He, X.; Wang, R. Dependence of elastic and optical properties on surface terminated groups in two-dimensional MXene monolayers: A first-principles study. RSC Adv. 2016, 6, 35731–35739. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Liu, M.; Liu, Y. 2D titanium carbide MXenes as emerging optical biosensing platforms. Biosens. Bioelectron. 2021, 171, 112730. [Google Scholar] [CrossRef]
- Xu, Q.; Ding, L.; Wen, Y.; Yang, W.; Zhou, H.; Chen, X.; Street, J.; Zhou, A.; Ong, W.-J.; Li, N. High photoluminescence quantum yield of 18.7% by using nitrogen-doped Ti3C2 MXene quantum dots. J. Mater. Chem. C 2018, 6, 6360–6369. [Google Scholar] [CrossRef]
- Xu, G.; Niu, Y.; Yang, X.; Jin, Z.; Wang, Y.; Xu, Y.; Niu, H. Preparation of Ti3C2Tx MXene-Derived Quantum Dots with White/Blue-Emitting Photoluminescence and Electrochemiluminescence. Adv. Opt. Mater. 2018, 6, 1800951. [Google Scholar] [CrossRef]
- Zhu, X.; Pang, X.; Zhang, Y.; Yao, S. Titanium carbide MXenes combined with red-emitting carbon dots as a unique turn-on fluorescent nanosensor for label-free determination of glucose. J. Mater. Chem. B 2019, 7, 7729–7735. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Y.; Lu, D.; Guo, Y.; Guo, S. Ultrathin Ti3C2 nanosheets based “off-on” fluorescent nanoprobe for rapid and sensitive detection of HPV infection. Sens. Actuators B Chem. 2019, 286, 222–229. [Google Scholar] [CrossRef]
- Wang, S.; Wei, S.; Wang, S.; Zhu, X.; Lei, C.; Huang, Y.; Nie, Z.; Yao, S. Chimeric DNA-Functionalized Titanium Carbide MXenes for Simultaneous Mapping of Dual Cancer Biomarkers in Living Cells. Anal. Chem. 2019, 91, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Kalkal, A.; Kadian, S.; Kumar, S.; Manik, G.; Sen, P.; Kumar, S.; Packirisamy, G. Ti3C2-MXene decorated with nanostructured silver as a dual-energy acceptor for the fluorometric neuron specific enolase detection. Biosens. Bioelectron. 2022, 195, 113620. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fan, L.; Wang, S.; Lei, C.; Huang, Y.; Nie, Z.; Yao, S. Phospholipid-Tailored Titanium Carbide Nanosheets as a Novel Fluorescent Nanoprobe for Activity Assay and Imaging of Phospholipase D. Anal. Chem. 2018, 90, 6742–6748. [Google Scholar] [CrossRef]
- Shi, Y.E.; Han, F.; Xie, L.; Zhang, C.; Li, T.; Wang, H.; Lai, W.F.; Luo, S.; Wei, W.; Wang, Z.; et al. A MXene of type Ti3C2Tx functionalized with copper nanoclusters for the fluorometric determination of glutathione. Microchim. Acta 2019, 187, 38. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, H.; Zhu, M.; Pei, Z.; Li, H.; Wang, Z.; Huang, Y.; Huang, Y.; Deng, Q.; Zhou, J.; et al. Photoluminescent Ti3C2 MXene Quantum Dots for Multicolor Cellular Imaging. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Guan, Q.; Ma, J.; Yang, W.; Zhang, R.; Zhang, X.; Dong, X.; Fan, Y.; Cai, L.; Cao, Y.; Zhang, Y.; et al. Highly fluorescent Ti3C2 MXene quantum dots for macrophage labeling and Cu(2+) ion sensing. Nanoscale 2019, 11, 14123–14133. [Google Scholar] [CrossRef]
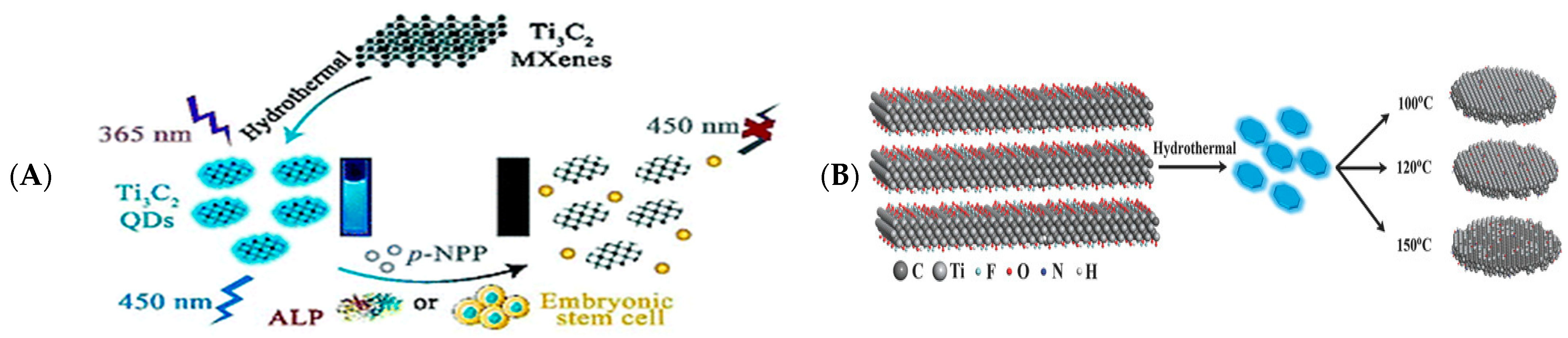
- Guo, Z.; Zhu, X.; Wang, S.; Lei, C.; Huang, Y.; Nie, Z.; Yao, S. Fluorescent Ti3C2 MXene quantum dots for an alkaline phosphatase assay and embryonic stem cell identification based on the inner filter effect. Nanoscale 2018, 10, 19579–19585. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, J.; He, Y.; Cai, Z.; Ge, Y.; Zhou, J.; Song, G. epsilon-Poly-L-lysine-protected Ti3C2 MXene quantum dots with high quantum yield for fluorometric determination of cytochrome c and trypsin. Microchim Acta 2019, 186, 770. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, X.; Xu, W.; Pan, G.; Zhou, D.; Zhu, J.; Wang, H.; Bai, X.; Dong, B.; Song, H. Ratiometric photoluminescence sensing based on Ti3C2 MXene quantum dots as an intracellular pH sensor. Nanoscale 2018, 10, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Zhang, W.L.; Lv, F.; Guo, S. Recent progress in two-dimensional inorganic quantum dots. Chem. Soc. Rev. 2018, 47, 586–625. [Google Scholar] [CrossRef]
- Shao, B.; Liu, Z.; Zeng, G.; Wang, H.; Liang, Q.; He, Q.; Cheng, M.; Zhou, C.; Jiang, L.; Song, B. Two-dimensional transition metal carbide and nitride (MXene) derived quantum dots (QDs): Synthesis, properties, applications and prospects. J. Mater. Chem. A 2020, 8, 7508–7535. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. TrAC Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Wang, Z.; Xuan, J.; Zhao, Z.; Li, Q.; Geng, F. Versatile Cutting Method for Producing Fluorescent Ultrasmall MXene Sheets. ACS Nano 2017, 11, 11559–11565. [Google Scholar] [CrossRef]
- Desai, M.L.; Basu, H.; Singhal, R.K.; Saha, S.; Kailasa, S.K. Ultra-small two dimensional MXene nanosheets for selective and sensitive fluorescence detection of Ag+ and Mn2+ ions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 70–77. [Google Scholar] [CrossRef]
- Zhang, J.; Kerr, E.; Usman, K.A.S.; Doeven, E.H.; Francis, P.S.; Henderson, L.C.; Razal, J.M. Cathodic electrogenerated chemiluminescence of tris(2,2’-bipyridine)ruthenium(ii) and peroxydisulfate at pure Ti3C2Tx MXene electrodes. Chem. Commun. 2020, 56, 10022–10025. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yang, X.; Chen, T.; Xu, G.; Liu, M.; Liu, J.; Xu, Y. Two-dimensional titanium carbide (MXene)-based solid-state electrochemiluminescent sensor for label-free single-nucleotide mismatch discrimination in human urine. Sens. Actuators B Chem. 2018, 263, 400–407. [Google Scholar] [CrossRef]
- Zhuang, T.T.; Zhang, H.X.; Wang, L.; Yu, L.H.; Wang, Z.H. Anchoring luminol based on Ti3C2-mediated in situ formation of Au NPs for construction of an efficient probe for miRNA electrogenerated chemiluminescence detection. Anal. Bioanal. Chem. 2021, 413, 6963–6971. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, J.; Fan, Z.; Ding, Y.; Zhou, B.; Yang, R.; Zhao, J.; Zhang, K. Rational Engineering of the DNA Walker Amplification Strategy by Using a Au@Ti3C2@PEI-Ru(dcbpy)3(2+) Nanocomposite Biosensor for Detection of the SARS-CoV-2 RdRp Gene. ACS Appl. Mater. Interfaces 2021, 13, 19816–19824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fan, Z.; Huang, Y.; Ding, Y.; Xie, M. A strategy combining 3D-DNA Walker and CRISPR-Cas12a trans-cleavage activity applied to MXene based electrochemiluminescent sensor for SARS-CoV-2 RdRp gene detection. Talanta 2022, 236, 122868. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Zhang, H.; Liu, M.; Liu, Y. Integrating Highly Efficient Recognition and Signal Transition of g-C3N4 Embellished Ti3C2 MXene Hybrid Nanosheets for Electrogenerated Chemiluminescence Analysis of Protein Kinase Activity. Anal. Chem. 2020, 92, 10668–10676. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Zhang, Q.; Wang, F.; Liu, Y. Ti3C2 MXenes nanosheets catalyzed highly efficient electrogenerated chemiluminescence biosensor for the detection of exosomes. Biosens. Bioelectron. 2019, 124–125, 184–190. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Wang, F.; Zhang, Y.; Wang, H.; Liu, Y. In Situ Formation of Gold Nanoparticles Decorated Ti3C2 MXenes Nanoprobe for Highly Sensitive Electrogenerated Chemiluminescence Detection of Exosomes and Their Surface Proteins. Anal. Chem. 2020, 92, 5546–5553. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Li, H.; Tan, R.; Feng, B.; Tu, Y. The TDs/aptamer cTnI biosensors based on HCR and Au/Ti3C2-MXene amplification for screening serious patient in COVID-19 pandemic. Biosens. Bioelectron. 2021, 192, 113482. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Ye, Z.; Ma, P.; Wu, Q.; Song, D. Preparation of a disposable electrochemiluminescence sensor chip based on an MXene-loaded ruthenium luminescent agent and its application in the detection of carcinoembryonic antigens. Analyst 2022, 147, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Lin, H.; Hao, T.; Su, X.; Jiang, X.; Wang, S.; Hu, Y.; Guo, Z. Dual-mode ECL/SERS immunoassay for ultrasensitive determination of Vibrio vulnificus based on multifunctional MXene. Sens. Actuators B Chem. 2021, 332, 129525. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, Z.; Miao, Z.; Liu, Y. Dye-Sensitized and Localized Surface Plasmon Resonance Enhanced Visible-Light Photoelectrochemical Biosensors for Highly Sensitive Analysis of Protein Kinase Activity. Anal. Chem. 2016, 88, 922–929. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Wang, X.; Lu, Q.; Li, H.; Zhang, Y.; Yao, S. Ti3C2/Cu2O heterostructure based signal-off photoelectrochemical sensor for high sensitivity detection of glucose. Biosens. Bioelectron. 2019, 142, 111535. [Google Scholar] [CrossRef]
- Chen, G.; Wang, H.; Wei, X.; Wu, Y.; Gu, W.; Hu, L.; Xu, D.; Zhu, C. Efficient Z-Scheme heterostructure based on TiO2/Ti3C2Tx/Cu2O to boost photoelectrochemical response for ultrasensitive biosensing. Sens. Actuators B Chem. 2020, 312, 127951. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, F.; Chen, J.; Liu, M.; Zhang, X.; Du, C.; Si, S. Label-free and near-zero-background-noise photoelectrochemical assay of methyltransferase activity based on a Bi2S3/Ti3C2 Schottky junction. Chem. Commun. 2020, 56, 5799–5802. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.L.; Fan, D.C.; Xue, X.H.; Zhang, J.Y.; Xu, J.L.; Lyu, H.X.; Chen, Y.T. Ti3C2 MXene-anchored photoelectrochemical detection of exosomes by in situ fabrication of CdS nanoparticles with enzyme-assisted hybridization chain reaction. RSC Adv. 2022, 12, 14260–14267. [Google Scholar] [CrossRef]
- Liu, L.; Yao, Y.; Ma, K.J.; Shangguan, C.J.; Jiao, S.L.; Zhu, S.Y.; Xu, X.X. Ultrasensitive photoelectrochemical detection of cancer-related miRNA-141 by carrier recombination inhibition in hierarchical Ti3C2@ReS2. Sens. Actuators B Chem. 2021, 331, 129470. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Zhou, Y.L.; Cui, X.T.; Yin, H.S.; Ai, S.Y. Enhanced photoactivity of CdS nanorods by MXene and ZnSnO3: Application in photoelectrochemical biosensor for the effect of environmental pollutants on DNA hydroxymethylation in wheat tissues. Mater. Today Chem. 2022, 24, 100878. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Pan, G.; Xu, W.; Zhu, J.; Zhou, D.; Li, D.; Chen, C.; Lu, G.; Song, H. Ti3C2 MXene quantum dots/TiO2 inverse opal heterojunction electrode platform for superior photoelectrochemical biosensing. Sens. Actuators B Chem. 2019, 289, 131–137. [Google Scholar] [CrossRef]
- Kurtoglu, M.; Naguib, M.; Gogotsi, Y.; Barsoum, M.W. First principles study of two-dimensional early transition metal carbides. MRS Commun. 2012, 2, 133–137. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- An, H.; Habib, T.; Shah, S.; Gao, H.; Radovic, M.; Green, M.J.; Lutkenhaus, J.L. Surface-agnostic highly stretchable and bendable conductive MXene multilayers. Sci. Adv. 2018, 4, eaaq0118. [Google Scholar] [CrossRef]
- Nah, J.S.; Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Park, J.Y. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuators B Chem. 2021, 329, 129206. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.-H.; Baeumner, A.J.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Alshareef, H.N. A MXene-Based Wearable Biosensor System for High-Performance In Vitro Perspiration Analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Liu, R.; Li, J.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron. 2021, 174, 112828. [Google Scholar] [CrossRef]
- Zhang, S.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, S.; Hui, X.; Chandra Barman, S.; Sharma, S.; Yoon, H.S.; Park, C.; Park, J.Y. A wearable battery-free wireless and skin-interfaced microfluidics integrated electrochemical sensing patch for on-site biomarkers monitoring in human perspiration. Biosens. Bioelectron. 2021, 175, 112844. [Google Scholar] [CrossRef]
- Saleh, A.; Wustoni, S.; Bihar, E.; El-Demellawi, J.K.; Zhang, Y.; Hama, A.; Druet, V.; Yudhanto, A.; Lubineau, G.; Alshareef, H.N.; et al. Inkjet-printed Ti3C2Tx MXene electrodes for multimodal cutaneous biosensing. J. Phys. Mater. 2020, 3, 044004. [Google Scholar] [CrossRef]
- Chen, L.; Chen, F.; Liu, G.; Lin, H.; Bao, Y.; Han, D.; Wang, W.; Ma, Y.; Zhang, B.; Niu, L. Superhydrophobic Functionalized Ti3C2Tx MXene-Based Skin-Attachable and Wearable Electrochemical pH Sensor for Real-Time Sweat Detection. Anal. Chem. 2022, 94, 7319–7328. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, N.; Li, L.; Hu, X.; Zou, Z.; Wang, J.; Luo, S.; Gao, Y. A highly flexible and sensitive piezoresistive sensor based on MXene with greatly changed interlayer distances. Nat. Commun. 2017, 8, 1207. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Shen, J.; Ge, G.; Zhang, Y.; Jin, W.; Huang, W.; Shao, J.; Yang, J.; Dong, X. Stretchable Ti3C2Tx MXene/Carbon Nanotube Composite Based Strain Sensor with Ultrahigh Sensitivity and Tunable Sensing Range. ACS Nano 2018, 12, 56–62. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, C.; Huang, H.; Yang, T.; Tian, G.; Xiong, D.; Chen, N.; Chu, X.; Zhong, S.; Deng, W.; et al. Microchannel-Confined MXene Based Flexible Piezoresistive Multifunctional Micro-Force Sensor. Adv. Funct. Mater. 2020, 30, 1909603. [Google Scholar] [CrossRef]
- Mathew, M.; Rout, C.S. Electrochemical biosensors based on Ti3C2Tx MXene: Future perspectives for on-site analysis. Curr. Opin. Electrochem. 2021, 30, 100782. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab. Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Lei, Y.; Cui, Y.; Huang, Q.; Dou, J.; Gan, D.; Deng, F.; Liu, M.; Li, X.; Zhang, X.; Wei, Y. Facile preparation of sulfonic groups functionalized Mxenes for efficient removal of methylene blue. Ceram. Int. 2019, 45, 17653–17661. [Google Scholar] [CrossRef]
- Sarycheva, A.; Makaryan, T.; Maleski, K.; Satheeshkumar, E.; Melikyan, A.; Minassian, H.; Yoshimura, M.; Gogotsi, Y. Two-Dimensional Titanium Carbide (MXene) as Surface-Enhanced Raman Scattering Substrate. J. Phys. Chem. C 2017, 121, 19983–19988. [Google Scholar] [CrossRef]
- Xie, H.; Li, P.; Shao, J.; Huang, H.; Chen, Y.; Jiang, Z.; Chu, P.K.; Yu, X.F. Electrostatic Self-Assembly of Ti3C2Tx MXene and Gold Nanorods as an Efficient Surface-Enhanced Raman Scattering Platform for Reliable and High-Sensitivity Determination of Organic Pollutants. ACS Sens. 2019, 4, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Ke, W.; Shi, L.; Liu, H.; Zhao, Y. Plasmonic Au-Ag Janus Nanoparticle Engineered Ratiometric Surface-Enhanced Raman Scattering Aptasensor for Ochratoxin A Detection. Anal. Chem. 2019, 91, 11812–11820. [Google Scholar] [CrossRef]
- Liu, R.Y.; Jiang, L.; Yu, Z.Z.; Jing, X.F.; Liang, X.; Wang, D.; Yang, B.; Lu, C.X.; Zhou, W.; Jin, S.Z. MXene (Ti3C2Tx)-Ag nanocomplex as efficient and quantitative SERS biosensor platform by in-situ PDDA electrostatic self-assembly synthesis strategy. Sens. Actuators B Chem. 2021, 333, 129581. [Google Scholar] [CrossRef]
- Su, S.; Sun, Q.; Gu, X.; Xu, Y.; Shen, J.; Zhu, D.; Chao, J.; Fan, C.; Wang, L. Two-dimensional nanomaterials for biosensing applications. TrAC Trends Anal. Chem. 2019, 119, 115610. [Google Scholar] [CrossRef]
- Wu, Q.; Li, N.; Wang, Y.; Liu, Y.; Xu, Y.; Wei, S.; Wu, J.; Jia, G.; Fang, X.; Chen, F.; et al. A 2D transition metal carbide MXene-based SPR biosensor for ultrasensitive carcinoembryonic antigen detection. Biosens. Bioelectron. 2019, 144, 111697. [Google Scholar] [CrossRef]
- Wu, Q.; Li, N.; Wang, Y.; Xu, Y.; Wu, J.; Jia, G.; Ji, F.; Fang, X.; Chen, F.; Cui, X. Ultrasensitive and Selective Determination of Carcinoembryonic Antigen Using Multifunctional Ultrathin Amino-Functionalized Ti3C2-MXene Nanosheets. Anal. Chem. 2020, 92, 3354–3360. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Y.; Kan, L.; Duan, F.H.; He, L.H.; Wang, M.H.; Cui, J.; Zhang, Z.H.; Zhang, Z.H. Surface plasmon resonance aptasensor based on niobium carbide MXene quantum dots for nucleocapsid of SARS-CoV-2 detection. Microchim. Acta 2021, 188, 316. [Google Scholar] [CrossRef] [PubMed]

| MXenes Composite | Identify Units | Target | LOD | Range | Ref. |
|---|---|---|---|---|---|
| Ti3C2@CuAu-LDH | Antibody | Carcinoembryonic antigen | 0.033 pg mL−1 | 0.0001–80 ng mL−1 | [83] |
| Ti3C2 | Antibody | CEA | 18 fg mL−1 | 0.0001–2000 ngmL−1 | [84] |
| Au/Ti3C2Tx | Antibody | PSA | 3.0 fg mL−1 | 0.01–1.0 pg mL−1 | [85] |
| M/NTO/PEDOT/AuNPs | Antibody | PSA | 0.03 pg L−1 | 0.0001–20 ng mL−1 | [86] |
| CuPtRh/NH2-Ti3C2 | Antibody | cardiac troponin I | 8.3 fg mL−1 | 25 fg mL−1–100 ng mL−1 | [87] |
| MXenes Composite | Identify Units | Target | LOD | Range | Ref. |
|---|---|---|---|---|---|
| DSPE-PEG/Ti3C2 | Gox/RCDs | Glucose | 50 μM | 0.1–20 mM | [94] |
| Ti3C2 | DNA probe | HPV-18 DNA | 100 pM | 0.5 nM–50 nM | [95] |
| Ti3C2 | Aptamer, DNA probe | MUC1, miRNA-21 | 6 nM, 0.8 nM | 0–60 nM, 0–25 nM | [96] |
| Ag@Ti3C2/GQDs | Antibody NSE | neuron-specific enolase (NSE) | 0.05 pg mL−1 | 0.0001–1500 ng mL−1 | [97] |
| PL-Ti3C2 | rhodamine B | Phospholipase D | 0.10 U L−1 | 0.5–50 U L−1 | [98] |
| Ti3C2Tx | quantum dots | glutathione | 3.0 μM | 5.0–100 μM | [99] |
| Ti3C2 QD | quantum dots | RAW264.7 cells, Zn2+ | - | - | [100] |
| N,P-Ti3C2 QDs | quantum dots | Cu2+ | 2 μM | 2–100 μM, 250–5000 μM | [101] |
| Ti3C2 MQDs | quantum dots | alkaline phosphatase | 0.02 U L−1 | 0.1–2.0 U L−1 | [102] |
| epsilon-poly-L-lysine (PLL)/Ti3C2 QDs | quantum dots | cytochrome c and trypsin | 20.5 nM and 0.1 μg mL−1 | 0.2–40 μM and 0.5–80.0 μg mL−1 | [103] |
| Ti3C2 QDs | quantum dots | Intracellular pH | - | pH 6.0–8.0 | [104] |
| MXenes Composite | Identify Units | Target | LOD | Range | Ref. |
|---|---|---|---|---|---|
| Ti3C2/Cu2O | non-enzymatic | glucose | 0.17 nM | 0.5 nM–0.5 mM | [122] |
| TiO2/Ti3C2Tx/Cu2O | non-enzymatic | glucose | 33.75 nM | 100 nM–10 μM | [123] |
| Bi2S3/Ti3C2 | DNA probe | methyltransferase | 0.003 U mL−1 | 0.01–30 U mL−1 | [124] |
| Ti3C2/CdS | Aptamer | Exosomes | 7.875 × 104 particles mL−1 | 7.3 × 105–3.285 × 108 particles mL−1 | [125] |
| Ti3C2@ReS2 | DNA probe | miRNA-141 | 2.4 aM | 0.1 fM–1 nM | [126] |
| APTES/ Ti3C2 | Antibody | 5hmCTP | 4.21 pM | 0.008–100 nM | [127] |
| (IOPCs)/Ti3C2 QDs | quantum dots | glutathione | 9.0 nM | 0.1–1000 μM | [128] |
| MXenes Composite | Detection Technology | Target | LOD | Range | Ref. |
|---|---|---|---|---|---|
| Ti3C2Tx/LBG/PDMS | Vitro Perspiration Analysis | cortisol | 88 pM | 0.01–100 nM | [132] |
| Ti3C2Tx/PB | Vitro Perspiration Analysis | glucose and lactate | 35.3 and 11.4 µA mm−1 cm−2 | - | [133] |
| Ti3C2Tx/MB | Vitro Perspiration Analysis | glucose and lactate | 2.4 nA μM−1 and 0.49 μA mM−1 | - | [134] |
| Ti3C2Tx/MWNTS | Vitro Perspiration Analysis | K+ | - | 1–32 mM | [135] |
| Ti3C2Tx | Vitro Perspiration Analysis | Na+, protein | - | - | [136] |
| F-Ti3C2Tx/PANI | Vitro Perspiration Analysis | human sweat pH | - | - | [137] |
| MXenes Composite | Detection Technology | Target | LOD | Range | Ref. |
|---|---|---|---|---|---|
| Ti3C2Tx/AuNRs | - | R6G, crystal violet, malachite green | 1 pM, 1 pM, 10 nM | - | [145] |
| Ti3C2O2 | Aptamer | Ochratoxin A | 1.28 pM | - | [146] |
| Ti3C2Tx-PDDA-AgNPs | Electrostatic | Dopamine | 10 nM | 5 μM–50 nM | [147] |
| R6G-Ti3C2Tx@AuNRs/ABEI | Antibody Vibrio vulnificus | Vibrio vulnificus | 102 CFU mL−1 | 102–108 CFU mL−1 | [120] |
| MXenes Composite | Detection Technology | Target | LOD | Range | Ref. |
|---|---|---|---|---|---|
| Ti3C2/AuNPs | Antibody | carcinoembryonic antigen | 0.2 fM–20 nM | 0.07 fM | [149] |
| APTES/Ti3C2/HGNPs | Antibody | carcinoembryonic antigen | 0.001–1000 pM | 0.15 fM | [150] |
| Nb2C-SH QDs | Aptamer | N-gene of SARS CoV-2 | 4.9 pg mL−1 | 0.05–100 ng mL−1 | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Zhao, H.; Zhang, X.; Chen, Y.; Feng, L. New Horizons for MXenes in Biosensing Applications. Biosensors 2022, 12, 820. https://doi.org/10.3390/bios12100820
Lu D, Zhao H, Zhang X, Chen Y, Feng L. New Horizons for MXenes in Biosensing Applications. Biosensors. 2022; 12(10):820. https://doi.org/10.3390/bios12100820
Chicago/Turabian StyleLu, Decheng, Huijuan Zhao, Xinying Zhang, Yingying Chen, and Lingyan Feng. 2022. "New Horizons for MXenes in Biosensing Applications" Biosensors 12, no. 10: 820. https://doi.org/10.3390/bios12100820
APA StyleLu, D., Zhao, H., Zhang, X., Chen, Y., & Feng, L. (2022). New Horizons for MXenes in Biosensing Applications. Biosensors, 12(10), 820. https://doi.org/10.3390/bios12100820








