State-of-the-Art Advances of Nanomedicine for Diagnosis and Treatment of Bladder Cancer
Abstract
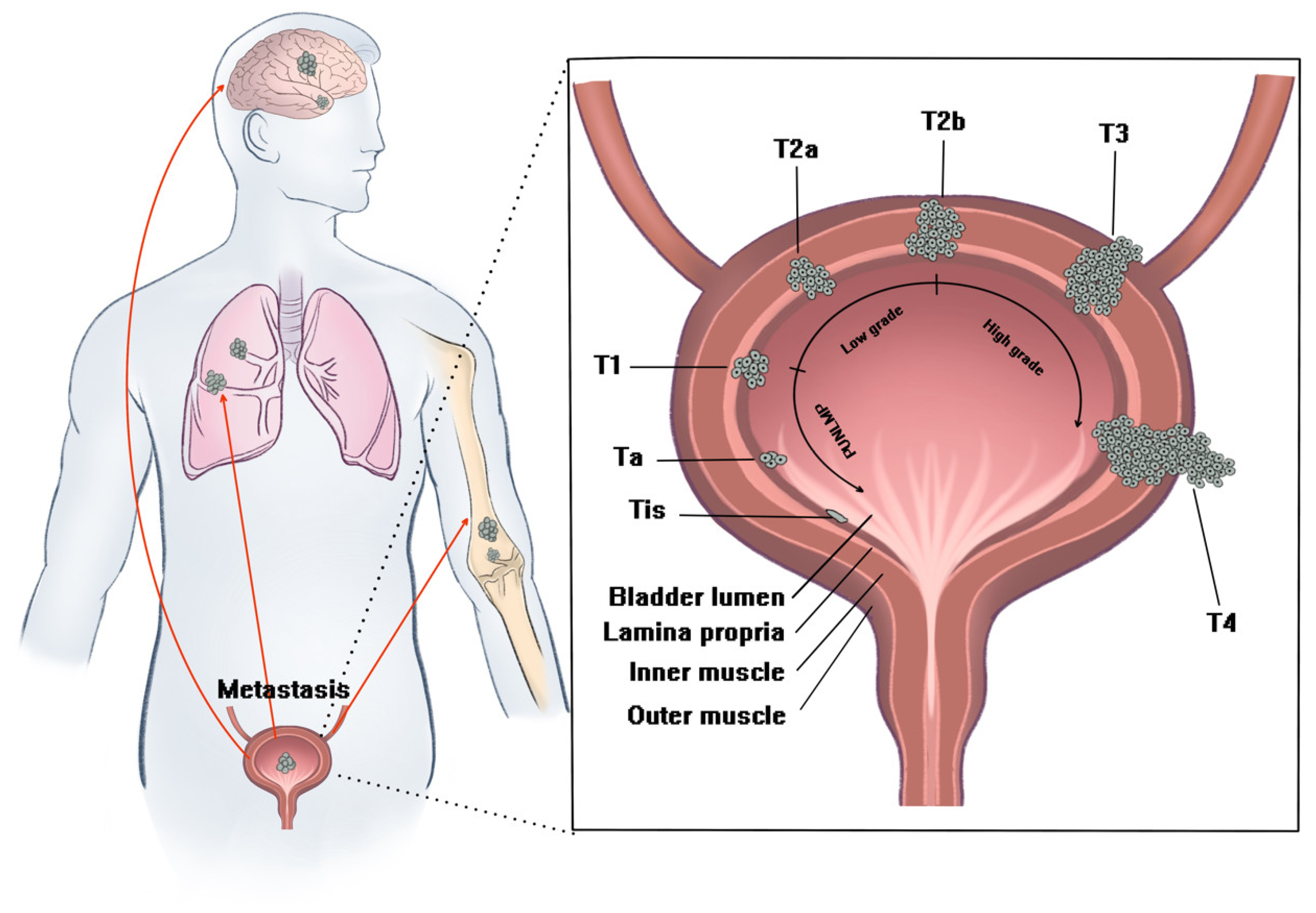
1. Introduction
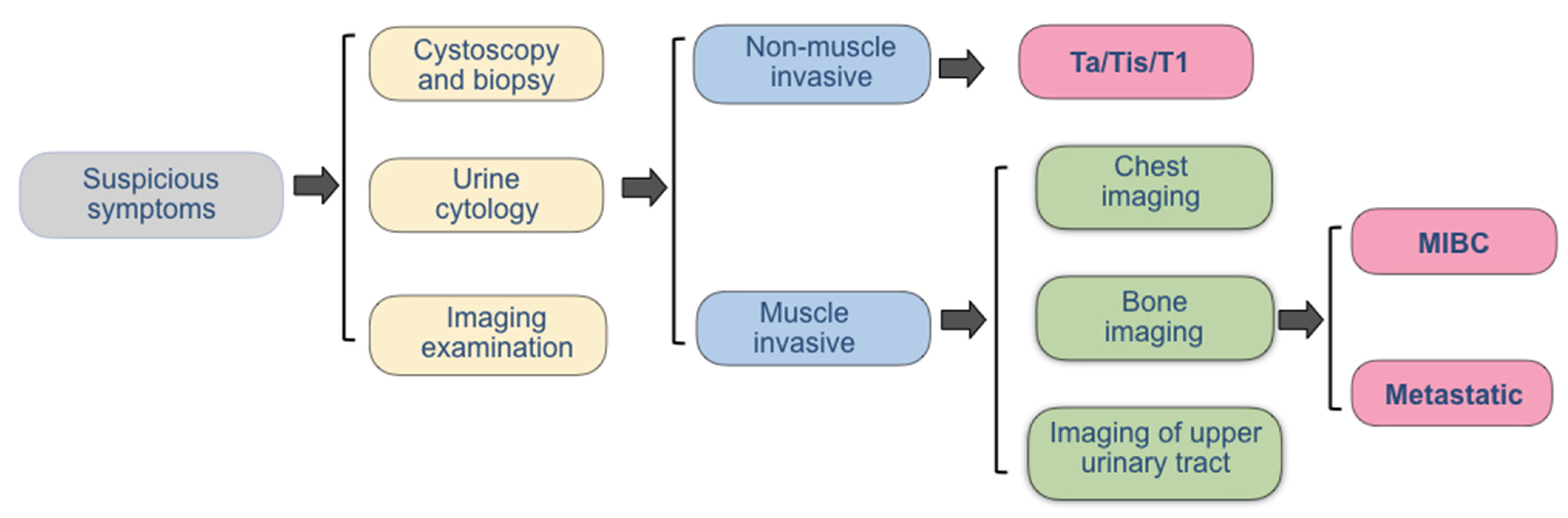
2. The Current Diagnostic Methods
3. The Current Therapies
4. Nanotechnology in Diagnosis
4.1. Nanotechnology in Light-Based Imaging
4.2. Nanotechnology in Urine Test
5. Nanotechnology in Treatment
5.1. Nano-Formulations for Chemotherapy
5.2. Nano-Formulations for Immune Therapy
5.3. Nano-Formulations for Targeted Therapy
5.4. Nano-Formulations for Light-Based Therapy
5.5. Nano-Formulations for Sonodynamic Therapy
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIE | Aggregation induced emission |
| AIE | Aggregation-induced emission |
| AIEgen | AIE luminogens |
| ALA | 5-aminolevulinic acid |
| AMPK | Adenosine phosphate activated protein kinase |
| AP | Penetratin |
| ATF | Amino-terminal fragment |
| AUC | Area under curve |
| AuNRs | Embedded gold nanorods |
| BCG | Bacillus calmette–guérin |
| BCG-CWS | BCG cell wall skeleton |
| BSA | Bovine serum albumin |
| Cat | Catechin |
| Cd | Cadmium |
| CD47 | Cytokine |
| Ce6 | Chlorin e6 |
| Chl | Chlorophy |
| CI | Confidence interval |
| CS | Chitosan |
| CT | Computer tomography |
| CWS-NP | BCG-CWS nanoparticle |
| Dox | Doxorubicin |
| DTX | Docetaxel |
| EGFR | Epidermal growth factor |
| ELISA | Enzyme linked immunosorbent assay |
| ELISA | Enzyme linked immunosorbent assay; |
| EphA2 | Ephrin receptor A2 |
| EPR | Enhanced permeability and retention |
| FAP | Fibronectin attach protein |
| FCS | Fluorinated chitosan |
| FDA | Food and Drug Administration |
| FGFR | Fibroblast growth factor receptors |
| FISH | Fluorescence in situ hybridization |
| GC regimen | Gemcitabine cisplatin/carboplatin |
| GEM | Gemcitabine |
| GO | Graphene oxide |
| GP | B-glycerophosphate |
| HA | Hyaluronic acid |
| HAase | Hyaluronidase |
| HLA | Hexaminolaevulinic acid |
| HSA | Human serum albumin |
| IAP | Integrin-associated protein |
| IONs | Iron oxide nanoparticles |
| LEEL | Liposome evaporated emulsified lipid |
| LK | Lumbrokinase |
| mAb | Monoclonal antibody |
| Met | Metformin |
| MIBC | Muscle-invasive bladder cancer |
| MMC | Mitomycin |
| Mn | Manganese |
| MNP | Magnetic nanoparticles |
| MPI | Polybia-mastoparan I |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger RNA |
| MVAC | Methotrexate, vinblastine, doxorubicin and cisplatin |
| NCCN | National comprehensive cancer network |
| NIR | Near infrared ray |
| NIR-II | Near-infrared-II |
| NK cell | Natural kill cell |
| NMIBC | Non-muscle-invasive bladder cancer |
| NSs | Nanosupensions |
| NTZ | Nitazoxanide |
| OEGMA | Polyethylene glycol ester |
| PAMAM | Poly amidoamine |
| PCI | Photochemical internalization |
| PCI | Photochemical internalization |
| PD-1/L1 | Programmed cell death 1 |
| PD-L1 | Programmed cell death 1 ligand 1 |
| PDT | Photodynamic therapy |
| PDX | Xenograft |
| PEG | Polyethylene glycol |
| PET / CT | Positron emission tomography computed tomography |
| PLGA | Poly (lactic-co-glycolic acid) |
| PS | Photosensitizers |
| PSCA | Prostate stem cell antigen |
| PTT | Photothermal therapy |
| PTX | Paclitaxel |
| QD | Quantum dot |
| ROC | Receiver operating characteristic |
| ROS | Reactive oxygen species |
| SDT | Sonodynamic therapy |
| Se | Selenium |
| SI | Singe-dose immediate intravesical chemotherapy |
| Si | Silicon |
| siRNA | Small interfering RNA |
| TCPP | Meso-tetra(4-carboxyphenyl)porphine |
| TiO2 | Titanium dioxide |
| TME | Tumor microenvironment |
| TURBT | Transurethral resection of the bladder cancer |
| UCNP | Upconversion nanoparticle |
| β- E | Β- elemene |
| δ-FeOOH | Feroxyhyte nanosheets |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.G.; Oh, W.K.; Galsky, M.D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA A Cancer J. Clin. 2020, 70, 404–423. [Google Scholar] [CrossRef] [PubMed]
- Grayson, M. Bladder cancer. Nature 2017, 551, S33. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The global burden of urinary bladder cancer: An update. World J. Urol. 2019, 38, 1895–1904. [Google Scholar] [CrossRef]
- Sloan, F.A.; Yashkin, A.P.; Akushevich, I.; Inman, B. The Cost to Medicare of Bladder Cancer Care. Eur. Urol. Oncol. 2019, 3, 515–522. [Google Scholar] [CrossRef]
- Schmid, S.C.; Zahel, T.; Haller, B.; Horn, T.; Metzger, I.; Holzapfel, K.; Seitz, A.K.; Gschwend, J.E.; Retz, M.; Maurer, T. Prognostic value of computed tomography before radical cystectomy in patients with invasive bladder cancer: Imaging predicts survival. World J. Urol. 2015, 34, 569–576. [Google Scholar] [CrossRef]
- Trinh, T.W.; Glazer, D.I.; Sadow, C.A.; Sahni, V.A.; Geller, N.L.; Silverman, S.G. Bladder cancer diagnosis with CT urography: Test characteristics and reasons for false-positive and false-negative results. Abdom. Radiol. 2018, 43, 663–671. [Google Scholar] [CrossRef]
- Mossanen, M.; Chang, S.L.; Kimm, S.; Sonpavde, G.P.; Kibel, A.S. Current Staging Strategies for Muscle-Invasive Bladder Cancer and Upper Tract Urothelial Cell Carcinoma. Urol. Clin. North Am. 2018, 45, 143–154. [Google Scholar] [CrossRef]
- Leow, J.J.; Martin-Doyle, W.; Rajagopal, P.S.; Patel, C.G.; Anderson, E.M.; Rothman, A.T.; Cote, R.J.; Urun, Y.; Chang, S.L.; Choueiri, T.K.; et al. Adjuvant Chemotherapy for Invasive Bladder Cancer: A 2013 Updated Systematic Review and Meta-Analysis of Randomized Trials. Eur. Urol. 2014, 66, 42–54. [Google Scholar] [CrossRef]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Johnson-Chilla, A. Bladder Cancer, Version 3. 2020, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network: JNCCN 2020, 18, 329–354. [Google Scholar]
- Bosschieter, J.; Nieuwenhuijzen, J.A.; van Ginkel, T.; Vis, A.N.; Witte, B.; Newling, D.; Beckers, G.M.; van Moorselaar, R.J.A. Value of an Immediate Intravesical Instillation of Mitomycin C in Patients with Non–muscle-invasive Bladder Cancer: A Prospective Multicentre Randomised Study in 2243 patients. Eur. Urol. 2017, 73, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Messing, E.M.; Tangen, C.M.; Lerner, S.P.; Sahasrabudhe, D.M.; Koppie, T.M.; Wood, D.P.; Mack, P.C.; Svatek, R.S.; Evans, C.P.; Hafez, K.S.; et al. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non–Muscle-Invasive Bladder Cancer on Tumor Recurrence. JAMA 2018, 319, 1880–1888. [Google Scholar] [CrossRef]
- Chang, S.S. Re: EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-Specific and Overall Survival in Non-Muscle-Invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guérin. J. Urol. 2017, 198, 39–41. [Google Scholar] [CrossRef]
- Jo, A.; Mb, B.; Rs, C.; Ab, D.; Cvdb, E.; Gva, F.; Pg, G.; Wh, H.; Lt, I.; Sm, C. Final Results of an EORTC-GU Cancers Group Randomized Study of Maintenance Bacillus Calmette-Guérin in Intermediate- and High-risk Ta, T1 Papillary Carcinoma of the Urinary Bladder: One-third Dose Versus Full Dose and 1 Year Versus 3 Years of Maintenance. European urology 2013, 63, 462–472. [Google Scholar]
- Barocas, D.A.; Globe, D.R.; Colayco, D.C.; Onyenwenyi, A.; Bruno, A.S.; Bramley, T.J.; Spear, R.J. Surveillance and Treatment of Non-Muscle-Invasive Bladder Cancer in the USA. Adv. Urol. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, A.; Zapała, P.; Zapała, Ł.; Piecha, T.; Radziszewski, P. Prediction of BCG responses in non-muscle-invasive bladder cancer in the era of novel immunotherapeutics. Int. Urol. Nephrol. 2019, 51, 1089–1099. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. New Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Pan, C.-X.; Lin, T.-Y.; Zhang, H.; Luo, J.; Li, Y.; Gao, T.; Lara, P.N., Jr.; White, R.D.V.; Lam, K.S. Multifunctional targeting micelle nanocarriers with both imaging and therapeutic potential for bladder cancer. Int. J. Nanomed. 2012, 7, 2793–2804. [Google Scholar] [CrossRef]
- Tao, K.; Liu, S.; Wang, L.; Qiu, H.; Li, B.; Zhang, M.; Guo, M.; Liu, H.; Zhang, X.; Liu, Y.; et al. Targeted multifunctional nanomaterials with MRI, chemotherapy and photothermal therapy for the diagnosis and treatment of bladder cancer. Biomater. Sci. 2019, 8, 342–352. [Google Scholar] [CrossRef]
- Zhou, Y.; Chan, C.-F.; Kwong, D.W.J.; Law, G.-L.; Cobb, S.; Wong, W.-K. αvβ3-Isoform specific erbium complexes highly specific for bladder cancer imaging and photodynamic therapy. Chem. Commun. 2016, 53, 557–560. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Liu, H.; Ren, F.; Qiu, W.; Sun, Q.; Yan, F.; Zheng, H.; Li, Z.; Gao, M. Second near-infrared photodynamic therapy and chemotherapy of orthotopic malignant glioblastoma with ultra-small Cu2−xSe nanoparticles. Nanoscale 2019, 11, 7600–7608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, C.; Zeng, J.; Sun, Q.; Wang, G.; Wang, Y.; Wu, Y.; Dou, S.; Gao, M.; Li, Z. Ambient Aqueous Synthesis of Ultrasmall PEGylated Cu2-xSe Nanoparticles as a Multifunctional Theranostic Agent for Multimodal Imaging Guided Photothermal Therapy of Cancer. Adv. Mater. 2016, 28, 8927–8936. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Lotan, Y.; Roehrborn, C.G. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: Results of a comprehensive literature review and meta-analyses. Urology 2003, 61, 109–118. [Google Scholar] [CrossRef]
- Daneshmand, S.; Patel, S.; Lotan, Y.; Pohar, K.; Trabulsi, E.; Woods, M.; Downs, T.; Huang, W.; Jones, J.S.; O’Donnell, M.; et al. Efficacy and Safety of Blue Light Flexible Cystoscopy with Hexaminolevulinate in the Surveillance of Bladder Cancer: A Phase III, Comparative, Multicenter Study. J. Urol. 2018, 199, 1158–1165. [Google Scholar] [CrossRef]
- Lotan, Y.; Oʼsullivan, P.; Raman, J.D.; Shariat, S.F.; Kavalieris, L.; Frampton, C.; Guilford, P.; Luxmanan, C.; Suttie, J.; Crist, H.; et al. Clinical comparison of noninvasive urine tests for ruling out recurrent urothelial carcinoma. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 531.e15–531.e22. [Google Scholar] [CrossRef]
- Yafi, F.A.; Brimo, F.; Steinberg, J.; Aprikian, A.G.; Tanguay, S.; Kassouf, W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 66.e25–66.e31. [Google Scholar] [CrossRef]
- Soria, F.; Krabbe, L.-M.; Todenhöfer, T.; Dobruch, J.; Mitra, A.P.; Inman, B.A.; Gust, K.M.; Lotan, Y.; Shariat, S.F. Molecular markers in bladder cancer. World J. Urol. 2018, 37, 31–40. [Google Scholar] [CrossRef]
- Chou, R.; Gore, J.L.; Buckley, D.; Fu, R.; Gustafson, K.; Griffin, J.C.; Grusing, S.; Selph, S. Urinary Biomarkers for Diagnosis of Bladder Cancer. Ann. Intern. Med. 2015, 163, 922–931. [Google Scholar] [CrossRef]
- Mowatt, G.; Zhu, S.; Kilonzo, M.; Boachie, C.; Fraser, C.; Griffiths, T.R.L.; N’Dow, J.; Nabi, G.; Cook, J.; Vale, L. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol. Assess. 2010, 14, 1–331, iii. [Google Scholar] [CrossRef]
- Hong, S.B.; Lee, N.K.; Kim, S.; Son, I.W.; Ha, H.K.; Ku, J.Y.; Kim, K.H.; Park, W.Y. Vesical Imaging–Reporting and Data System for Multiparametric MRI to Predict the Presence of Muscle Invasion for Bladder Cancer. J. Magn. Reson. Imaging 2020, 52, 1249–1256. [Google Scholar] [CrossRef]
- Lin, W.-C.; Chen, J.-H. Pitfalls and Limitations of Diffusion-Weighted Magnetic Resonance Imaging in the Diagnosis of Urinary Bladder Cancer. Transl. Oncol. 2015, 8, 217–230. [Google Scholar] [CrossRef]
- Zhang, S.; Song, M.; Zhao, Y.; Xu, S.; Sun, Q.; Zhai, G.; Liang, D.; Wu, G.; Li, Z.-C. Radiomics nomogram for preoperative prediction of progression-free survival using diffusion-weighted imaging in patients with muscle-invasive bladder cancer. Eur. J. Radiol. 2020, 131, 109219. [Google Scholar] [CrossRef]
- Li, C.; Gu, Z.; Ni, P.; Zhang, W.; Yang, F.; Li, W.; Yao, X.; Chen, Y. The value of contrast-enhanced ultrasound and magnetic resonance imaging in the diagnosis of bladder cancer. Journal of cancer research and therapeutics 2021, 17, 1179–1185. [Google Scholar] [CrossRef]
- McKibben, M.J.; Woods, M.E. Preoperative Imaging for Staging Bladder Cancer. Curr. Urol. Rep. 2015, 16, 1–7. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Oosterlinck, W.; Holmang, S.; Sydes, M.R.; Birtle, A.; Gudjonsson, S.; De Nunzio, C.; Okamura, K.; Kaasinen, E.; Solsona, E.; et al. Systematic Review and Individual Patient Data Meta-analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa–pT1 Urothelial Carcinoma of the Bladder: Which Patients Benefit from the Instillation? Eur. Urol. 2016, 69, 231–244. [Google Scholar] [CrossRef]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-guerin in the Treatment of Superficial Bladder Tumors. J. Urol. 1976, 116, 180–182. [Google Scholar] [CrossRef]
- Ratliff, T.L.; Kavoussi, L.R.; Catalona, W.J. Role of Fibronectin in Intravesical BCG Therapy for Superficial Bladder Cancer. J. Urol. 1988, 139, 410–414. [Google Scholar] [CrossRef]
- Sinn, H.W.; Elzey, B.D.; Jensen, R.J.; Zhao, X.; Zhao, W.; Ratliff, T.L. The fibronectin attachment protein of bacillus Calmette-Guerin (BCG) mediates antitumor activity. Cancer Immunol. Immunother. 2007, 57, 573–579. [Google Scholar] [CrossRef]
- Ratliff, T.L.; Palmer, J.O.; McGarr, J.A.; Brown, E.J. Intravesical Bacillus Calmette-Guérin therapy for murine bladder tumors: Initiation of the response by fibronectin-mediated attachment of Bacillus Calmette-Guérin. Cancer Res. 1987, 47, 1762–1766. [Google Scholar]
- Mora-Bau, G.; Platt, A.M.; Van Rooijen, N.; Randolph, G.J.; Albert, M.L.; Ingersoll, M.A. Macrophages Subvert Adaptive Immunity to Urinary Tract Infection. PLOS Pathog. 2015, 11, e1005044. [Google Scholar] [CrossRef]
- Ingersoll, M.A.; Albert, M.L. From infection to immunotherapy: Host immune responses to bacteria at the bladder mucosa. Mucosal Immunol. 2013, 6, 1041–1053. [Google Scholar] [CrossRef]
- Redelman-Sidi, G.; Iyer, G.; Solit, D.B.; Glickman, M.S. Oncogenic Activation of Pak1-Dependent Pathway of Macropinocytosis Determines BCG Entry into Bladder Cancer Cells. Cancer Res. 2013, 73, 1156–1167. [Google Scholar] [CrossRef]
- Bakhru, P.; Sirisaengtaksin, N.; Soudani, E.; Mukherjee, S.; Khan, A.; Jagannath, C. BCG vaccine mediated reduction in the MHC-II expression of macrophages and dendritic cells is reversed by activation of Toll-like receptors 7 and 9. Cell. Immunol. 2013, 287, 53–61. [Google Scholar] [CrossRef]
- Kamat, A.M.; Briggman, J.; Urbauer, D.L.; Svatek, R.; González, G.M.N.; Anderson, R.; Grossman, H.B.; Prat, F.; Dinney, C.P. Cytokine Panel for Response to Intravesical Therapy (CyPRIT): Nomogram of Changes in Urinary Cytokine Levels Predicts Patient Response to Bacillus Calmette-Guérin. Eur. Urol. 2015, 69, 197–200. [Google Scholar] [CrossRef]
- Oddens, J.R.; De Reijke, T.M. The Current State of Predicting Response on Bacillus Calmette-Guérin Treatment for Nonmuscle Invasive Bladder Cancer is Not Yet Useful for Patients but Attributes to Understanding Its Mechanisms of Action. Eur. Urol. 2018, 73, 749–750. [Google Scholar] [CrossRef]
- Ajili, F.; Kaabi, B.; Darouiche, A.; Tounsi, H.; Kourda, N.; Chebil, M.; Manai, M.; Boubaker, S. Prognostic Value of Bcl-2 and Bax Tumor Cell Expression in Patients with Non Muscle-Invasive Bladder Cancer Receiving Bacillus Calmette-Guerin Immunotherapy. Ultrastruct. Pathol. 2012, 36, 31–39. [Google Scholar] [CrossRef]
- Yu, D.; Wu, C.; Ping, S.; Keng, C.; Shen, K. Bacille Calmette-Guerin can induce cellular apoptosis of urothelial cancer directly through toll-like receptor 7 activation. Kaohsiung J. Med Sci. 2015, 31, 391–397. [Google Scholar] [CrossRef]
- See, W.A.; Zhang, G.; Chen, F.; Cao, Y.; Langenstroer, P.; Sandlow, J. Bacille-Calmette Guèrin induces caspase-independent cell death in urothelial carcinoma cells together with release of the necrosis-associated chemokine high molecular group box protein 1. Br. J. Urol. 2009, 103, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Ryk, C.; Koskela, L.R.; Thiel, T.; Wiklund, N.P.; Steineck, G.; Schumacher, M.C.; de Verdier, P.J. Outcome after BCG treatment for urinary bladder cancer may be influenced by polymorphisms in the NOS2 and NOS3 genes. Redox Biol. 2015, 6, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.F.; Silva, M.; Carrascal, M.; Malagolini, N.; Chiricolo, M.; Venturi, G.; Astolfi, A.; Catera, M.; Videira, P.A.; Dall’Olio, F. Expression of sialyl-Tn sugar antigen in bladder cancer cells affects response to Bacillus Calmette Guérin (BCG) and to oxidative damage. Oncotarget 2017, 8, 54506–54517. [Google Scholar] [CrossRef][Green Version]
- Severino, P.F.; Silva, M.; Carrascal, M.; Malagolini, N.; Chiricolo, M.; Venturi, G.; Forleo, R.B.; Astolfi, A.; Catera, M.; Videira, P.A.; et al. Oxidative damage and response to Bacillus Calmette-Guérin in bladder cancer cells expressing sialyltransferase ST3GAL1. BMC Cancer 2018, 18, 198. [Google Scholar] [CrossRef]
- Bayoumi, Y.; Heikal, T.; Darweish, H. Survival benefit of adjuvant radiotherapy in stage III and IV bladder cancer: Results of 170 patients. Cancer Manag. Res. 2014, 6, 459–465. [Google Scholar] [CrossRef]
- Tey, J.; Soon, Y.Y.; Cheo, T.; Ooi, K.H.; Ho, F.; Vellayappan, B.; Chia, D.; Tai, B.C. Efficacy of Palliative Bladder Radiotherapy for Hematuria in Advanced Bladder Cancer Using Contemporary Radiotherapy Techniques. Vivo 2019, 33, 2161–2167. [Google Scholar] [CrossRef]
- Tey, J.; Ho, F.; Koh, W.Y.; Chia, D.; Ooi, K.H.; Tuan, J.K.L.; Vellayappan, B.; Soon, Y.Y. Palliative radiotherapy for bladder cancer: A systematic review and meta-analysis. Acta Oncol. 2021, 60, 635–644. [Google Scholar] [CrossRef]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Van der Heijden, M.S.; Loriot, Y.; Durán, I.; Ravaud, A.; Retz, M.; Vogelzang, N.J.; Nelson, B.; Wang, J.; Shen, X.; Powles, T. Atezolizumab Versus Chemotherapy in Patients with Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: A Long-term Overall Survival and Safety Update from the Phase 3 IMvigor211 Clinical Trial. Eur. Urol. 2021, 80, 7–11. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. New Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, X.; Wang, L.; Shi, Y.-X.; Yao, X.; Luo, H.; Shi, B.; Liu, J.-Y.; He, Z.; Yu, G.; et al. Open-label, Multicenter, Phase II Study of RC48-ADC, a HER2-Targeting Antibody–Drug Conjugate, in Patients with Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2021, 27, 43–51. [Google Scholar] [CrossRef]
- Diamond, I.; Mcdonagh, A.; Wilson, C.; Granelli, S.; Nielsen, S.; Jaenicke, R. Photodynamic therapy of malignant tumours. Lancet 1972, 300, 1175–1177. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Jichlinski, P.; Leisinger, H.-J. Photodynamic therapy in superficial bladder cancer: Past, present and future. Urol. Res. 2001, 29, 396–405. [Google Scholar] [CrossRef]
- Lee, J.Y.; Diaz, R.R.; Cho, K.S.; Lim, M.S.; Chung, J.S.; Kim, W.T.; Ham, W.S.; Choi, Y.D. Efficacy and Safety of Photodynamic Therapy for Recurrent, High Grade Nonmuscle Invasive Bladder Cancer Refractory or Intolerant to Bacille Calmette-Guérin Immunotherapy. J. Urol. 2013, 190, 1192–1199. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Xiong, W.; Qi, L.; Jiang, N.; Zhao, Q.; Chen, L.; Jiang, X.; Li, Y.; Zhou, Z.; Shen, J. Metformin Liposome-Mediated PD-L1 Downregulation for Amplifying the Photodynamic Immunotherapy Efficacy. ACS Appl. Mater. Interfaces 2021, 13, 8026–8041. [Google Scholar] [CrossRef]
- Pan, Y.; Volkmer, J.-P.; Mach, K.E.; Rouse, R.V.; Liu, J.-J.; Sahoo, D.; Chang, T.C.; Metzner, T.J.; Kang, L.; van de Rijn, M.; et al. Endoscopic molecular imaging of human bladder cancer using a CD47 antibody. Sci. Transl. Med. 2014, 6, 260ra148. [Google Scholar] [CrossRef]
- Pan, Y.; Chang, T.; Marcq, G.; Liu, C.; Kiss, B.; Rouse, R.; Mach, K.E.; Cheng, Z.; Liao, J.C. In vivo biodistribution and toxicity of intravesical administration of quantum dots for optical molecular imaging of bladder cancer. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Ren, Z.J.; Jin, T.; Yang, B.; Dong, Q. Contribution of prostate stem cell antigen variation rs2294008 to the risk of bladder cancer. Medicine 2019, 98, e15179. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Rao, T.; Cheng, F.; Yu, W.; Ruan, Y.; Zhang, X. Quantum dot-based fluorescent probes for targeted imaging of the EJ human bladder urothelial cancer cell line. Exp. Ther. Med. 2018, 16, 4779–4783. [Google Scholar] [CrossRef]
- Yamali, C.; Gul, H.I.; Ozli, G.; Angeli, A.; Kirmizibayrak, P.B.; Tepedelen, B.E.; Sakagami, H.; Bua, S.; Supuran, C.T. Exploring of tumor-associated carbonic anhydrase isoenzyme IX and XII inhibitory effects and cytotoxicities of the novel N-aryl-1-(4-sulfamoylphenyl)-5-(thiophen-2-yl)-1H-pyrazole-3-carboxamides. Bioorganic Chem. 2021, 115, 105194. [Google Scholar] [CrossRef] [PubMed]
- Biagiotti, G.; Angeli, A.; Giacomini, A.; Toniolo, G.; Landini, L.; Salerno, G.; Mannelli, L.D.C.; Ghelardini, C.; Mello, T.; Mussi, S.; et al. Glyco-Coated CdSe/ZnS Quantum Dots as Nanoprobes for Carbonic Anhydrase IX Imaging in Cancer Cells. ACS Appl. Nano Mater. 2021, 4, 14153–14160. [Google Scholar] [CrossRef]
- Li, X.; Wu, T.; Fu, Y.; Ding, X.; Li, Z.; Zhu, G.; Fan, J. A high sensitivity background eliminated fluorescence sensing platform for hyaluronidase activity detection based on Si QDs/HA-δ-FeOOH nanoassembly. Biosens. Bioelectron. 2019, 150, 111928. [Google Scholar] [CrossRef]
- Davis, R.M.; Kiss, B.; Trivedi, D.R.; Metzner, T.J.; Liao, J.C.; Gambhir, S.S. Surface-Enhanced Raman Scattering Nanoparticles for Multiplexed Imaging of Bladder Cancer Tissue Permeability and Molecular Phenotype. ACS Nano 2018, 12, 9669–9679. [Google Scholar] [CrossRef]
- Cho, S.K.; Su, L.-J.; Mao, C.; Wolenski, C.D.; Flaig, T.W.; Park, W. Multifunctional nanoclusters of NaYF4:Yb3+,Er3+ upconversion nanoparticle and gold nanorod for simultaneous imaging and targeted chemotherapy of bladder cancer. Mater. Sci. Eng. C 2018, 97, 784–792. [Google Scholar] [CrossRef]
- Ma, Y.; Mao, G.; Zhong, Y.; Wu, G.; Wu, W.; Zhan, Y.; He, Z.; Huang, W. Highly sensitive ratiometric fluorescent paper sensor for the urine assay of cancer. Talanta 2018, 194, 199–204. [Google Scholar] [CrossRef]
- Gennari, A.; Sun, Z.; Hasler-Strub, U.; Colleoni, M.; Kennedy, M.; Von Moos, R.; Cortés, J.; Vidal, M.; Hennessy, B.; Walshe, J.; et al. A randomized phase II study evaluating different maintenance schedules of nab-paclitaxel in the first-line treatment of metastatic breast cancer: Final results of the IBCSG 42-12/BIG 2-12 SNAP trial. Ann. Oncol. 2017, 29, 661–668. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Krasnojon, D.; Cheporov, S.; Makhson, A.N.; Manikhas, G.M.; Clawson, A.; Bhar, P. Significantly Longer Progression-Free Survival With nab-Paclitaxel Compared With Docetaxel As First-Line Therapy for Metastatic Breast Cancer. J. Clin. Oncol. 2009, 27, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.D.; Hussain, M.; Hafez, K.; Daignault-Newton, S.; Wood, D.; Lee, C.T.; Weizer, A.; Montie, J.E.; Hollenbeck, B.; Montgomery, J.S.; et al. A Phase II Trial of Neoadjuvant nab-paclitaxel, Carboplatin, and Gemcitabine (ACaG) in Patients With Locally Advanced Carcinoma of the Bladder. Urology 2013, 82, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-J.; Canil, C.M.; Mukherjee, S.D.; Winquist, E.; Elser, C.; Eisen, A.; Reaume, M.N.; Zhang, L.; Sridhar, S.S. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: A single group, multicentre, phase 2 study. Lancet Oncol. 2013, 14, 769–776. [Google Scholar] [CrossRef]
- Hu, B.; Yan, Y.; Tong, F.; Xu, L.; Zhu, J.; Xu, G.; Shen, R. Lumbrokinase/paclitaxel nanoparticle complex: Potential therapeutic applications in bladder cancer. Int. J. Nanomed. 2018, 13, 3625–3640. [Google Scholar] [CrossRef]
- Pan, A.; Zhang, H.; Li, Y.; Lin, T.-Y.; Wang, F.; Lee, J.; Cheng, M.; Dall’Era, M.; Li, T.; White, R.D.; et al. Disulfide-crosslinked nanomicelles confer cancer-specific drug delivery and improve efficacy of paclitaxel in bladder cancer. Nanotechnology 2016, 27, 425103. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Hou, J.; Sun, B.; Zhu, B.; Qiao, Z.; Su, Y.; Zhu, X. Paclitaxel/Chitosan Nanosupensions Provide Enhanced Intravesical Bladder Cancer Therapy with Sustained and Prolonged Delivery of Paclitaxel. ACS Appl. Bio Mater. 2018, 1, 1992–2001. [Google Scholar] [CrossRef]
- Xiao, T.; Xiao, Y.; Wang, W.; Tang, Y.Y.; Xiao, Z.; Su, M. Targeting EphA2 in cancer. J. Hematol. Oncol. 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Kamoun, W.S.; Kirpotin, D.B.; Huang, Z.R.; Tipparaju, S.K.; Noble, C.O.; Hayes, M.E.; Luus, L.; Koshkaryev, A.; Kim, J.; Olivier, K.; et al. Antitumour activity and tolerability of an EphA2-targeted nanotherapeutic in multiple mouse models. Nat. Biomed. Eng. 2019, 3, 264–280. [Google Scholar] [CrossRef]
- Kamoun, W.; Swindell, E.; Pien, C.; Luus, L.; Cain, J.; Pham, M.; Kandela, I.; Huang, Z.R.; Tipparaju, S.K.; Koshkaryev, A.; et al. Targeting EphA2 in Bladder Cancer Using a Novel Antibody-Directed Nanotherapeutic. Pharmaceutics 2020, 12, 996. [Google Scholar] [CrossRef]
- Manan, F.A.A.; Yusof, N.A.; Abdullah, J.; Mohammad, F.; Nurdin, A.; Yazan, L.S.; Khiste, S.K.; Al-Lohedan, H.A. Drug Release Profiles of Mitomycin C Encapsulated Quantum Dots–Chitosan Nanocarrier System for the Possible Treatment of Non-Muscle Invasive Bladder Cancer. Pharmaceutics 2021, 13, 1379. [Google Scholar] [CrossRef]
- Sun, R.; Liu, X.; Li, G.; Wang, H.; Luo, Y.; Huang, G.; Wang, X.; Zeng, G.; Liu, Z.; Wu, S. Photoactivated H2 Nanogenerator for Enhanced Chemotherapy of Bladder Cancer. ACS Nano 2020, 14, 8135–8148. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Cao, K.; Lin, T.; Chen, W.; Yuan, A.; Wu, J.; Hu, Y.; Guo, H. Drug delivery system based on dendritic nanoparticles for enhancement of intravesical instillation. Int. J. Nanomed. 2017, 12, 7365–7374. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Wang, L.; Zhu, J.; He, D.; Huang, Y.; Zhang, W.; Wang, Z.; Qin, A.; Hou, J.; Tang, B.Z. Photo-Enhanced Chemotherapy Performance in Bladder Cancer Treatment via Albumin Coated AIE Aggregates. ACS Nano 2022, 16, 7535–7546. [Google Scholar] [CrossRef]
- Chang, Z.; Gao, M.; Zhang, W.; Song, L.; Jia, Y.; Qin, Y. Beta-elemene treatment is associated with improved outcomes of patients with esophageal squamous cell carcinoma. Surg. Oncol. 2017, 26, 333–337. [Google Scholar] [CrossRef]
- Zhai, B.; Zeng, Y.; Zeng, Z.; Zhang, N.; Li, C.; Zeng, Y.; You, Y.; Wang, S.; Chen, X.; Sui, X.; et al. Drug delivery systems for elemene, its main active ingredient β-elemene, and its derivatives in cancer therapy. Int. J. Nanomed. 2018, 13, 6279–6296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Staley, C.; Kooby, D.; El-Rays, B.; Mao, H.; Yang, L. Current status of biomarker and targeted nanoparticle development: The precision oncology approach for pancreatic cancer therapy. Cancer Lett. 2016, 388, 139–148. [Google Scholar] [CrossRef]
- Zhai, E.A.B.; Chen, P.; Wang, W.; Liu, S.; Feng, J.; Duan, T.; Xiang, Y.; Zhang, R.; Zhang, M.; Han, X.; et al. An ATF24 peptide-functionalized β-elemene-nanostructured lipid carrier combined with cisplatin for bladder cancer treatment. Cancer Biol. Med. 2020, 17, 676–692. [Google Scholar] [CrossRef]
- Wang, K.-R.; Zhang, B.-Z.; Zhang, W.; Yan, J.-X.; Li, J.; Wang, R. Antitumor effects, cell selectivity and structure–activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides 2008, 29, 963–968. [Google Scholar] [CrossRef]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2011, 31, 173–194. [Google Scholar] [CrossRef]
- Li, G.; Lei, Q.; Wang, F.; Deng, D.; Wang, S.; Tian, L.; Shen, W.; Cheng, Y.; Liu, Z.; Wu, S. Fluorinated Polymer Mediated Transmucosal Peptide Delivery for Intravesical Instillation Therapy of Bladder Cancer. Small 2019, 15, e1900936. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, A.; Ren, Q.; Xue, Y.; Yu, X.; Ying, Y.; Gao, H.; Tan, H.; Zhang, Z.; Li, W.; et al. Cuprous oxide nanoparticles trigger reactive oxygen species-induced apoptosis through activation of erk-dependent autophagy in bladder cancer. Cell Death Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Folkes, L.K.; Wardman, P. Oxidative activation of indole-3-acetic acids to cytotoxic species— a potential new role for plant auxins in cancer therapy. Biochem. Pharmacol. 2001, 61, 129–136. [Google Scholar] [CrossRef]
- Pereira, F.M.; Melo, M.N.; Santos, K.M.; Oliveira, K.V.; Diz, F.M.; Ligabue, R.A.; Morrone, F.B.; Severino, P.; Fricks, A.T. Hyaluronic acid-coated chitosan nanoparticles as carrier for the enzyme/prodrug complex based on horseradish peroxidase/indole-3-acetic acid: Characterization and potential therapeutic for bladder cancer cells. Enzym. Microb. Technol. 2021, 150, 109889. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, P.; Li, P.; Xue, A.; Zhang, X.; Zhang, H.; Jin, X. A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette–Guérin in the treatment of bladder cancer. Biomaterials 2013, 34, 10258–10266. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Bilim, V.; Yuuki, K.; Naito, S.; Yamanobe, T.; Nagaoka, A.; Yano, I.; Akaza, H.; Tomita, Y. Bacillus Calmette-Guerin and BCG cell wall skeleton suppressed viability of bladder cancer cells in vitro. Anticancer Res. 2010, 30, 4089–4096. [Google Scholar] [PubMed]
- Nakamura, T.; Fukiage, M.; Higuchi, M.; Nakaya, A.; Yano, I.; Miyazaki, J.; Nishiyama, H.; Akaza, H.; Ito, T.; Hosokawa, H.; et al. Nanoparticulation of BCG-CWS for application to bladder cancer therapy. J. Control. Release 2014, 176, 44–53. [Google Scholar] [CrossRef]
- Nakamura, T.; Fukiage, M.; Suzuki, Y.; Yano, I.; Miyazaki, J.; Nishiyama, H.; Akaza, H.; Harashima, H. Mechanism responsible for the antitumor effect of BCG-CWS using the LEEL method in a mouse bladder cancer model. J. Control. Release 2014, 196, 161–167. [Google Scholar] [CrossRef]
- Masuda, H.; Nakamura, T.; Noma, Y.; Harashima, H. Application of BCG-CWS as a systemic adjuvant by using nanoparticulation technology. Mol. Pharm. 2018, 15, 5762–5771. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Yang, H.M.; Kim, C.H.; Goo, Y.T.; Hwang, G.Y.; Chang, I.H.; Whang, Y.M.; Choi, Y.W. Enhanced intracellular delivery of BCG cell wall skeleton into bladder cancer cells using liposomes functionalized with folic acid and pep-1 peptide. Pharmaceutics 2019, 11, 652. [Google Scholar] [CrossRef]
- Whang, Y.M.; Yoon, D.H.; Hwang, G.Y.; Yoon, H.; Park, S.I.; Choi, Y.W.; Chang, I.H. Liposome-Encapsulated Bacillus Calmette–Guérin Cell Wall Skeleton Enhances Antitumor Efficiency for Bladder Cancer In Vitro and In Vivo via Induction of AMP-Activated Protein Kinase. Cancers 2020, 12, 3679. [Google Scholar] [CrossRef]
- Joraku, A.; Homhuan, A.; Kawai, K.; Yamamoto, T.; Miyazaki, J.; Kogure, K.; Yano, I.; Harashima, H.; Akaza, H. Immunoprotection against murine bladder carcinoma by octaarginine-modified liposomes incorporating cell wall ofMycobacterium bovisbacillus Calmette-Guérin. Br. J. Urol. 2009, 103, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Nishiyama, H.; Yano, I.; Nakaya, A.; Kohama, H.; Kawai, K.; Joraku, A.; Nakamura, T.; Harashima, H.; Akaza, H. The therapeutic effects of R8-liposome-BCG-CWS on BBN-induced rat urinary bladder carcinoma. Anticancer Res. 2011, 31, 2065–2071. [Google Scholar] [PubMed]
- Miyazaki, J.; Kawai, K.; Kojima, T.; Oikawa, T.; Joraku, A.; Shimazui, T.; Nakaya, A.; Yano, I.; Nakamura, T.; Harashima, H.; et al. The liposome-incorporating cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guéin can directly enhance the susceptibility of cancer cells to lymphokine-activated killer cells through up-regulation of natural-killer group 2, member D ligands. Br. J. Urol. 2011, 108, 1520–1526. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, T.; Zhou, B.; Wei, J.; Fang, Y.; Lu, J.; Guo, L.; Chen, W.; Liu, Z.-P.; Luo, J. Mg(II)-Catechin nanoparticles delivering siRNA targeting EIF5A2 inhibit bladder cancer cell growth in vitro and in vivo. Biomaterials 2015, 81, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Groner, B.; Weiss, A. Targeting survivin in cancer: Novel drug development approaches. BioDrugs 2013, 28, 27–39. [Google Scholar] [CrossRef]
- García, D.M.; Manero-Rupérez, N.; Quesada, R.; Korrodi-Gregório, L.; Soto-Cerrato, V. Therapeutic strategies involving survivin inhibition in cancer. Med. Res. Rev. 2018, 39, 887–909. [Google Scholar] [CrossRef]
- Krafft, U.; Tschirdewahn, S.; Hess, J.; Harke, N.N.; Hadaschik, B.; Olah, C.; Krege, S.; Nyirády, P.; Szendröi, A.; Szücs, M.; et al. Validation of survivin and HMGA2 as biomarkers for cisplatin resistance in bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 810.e7–810.e15. [Google Scholar] [CrossRef]
- Chen, L.; Liang, L.; Yan, X.; Liu, N.; Gong, L.; Pan, S.; Lin, F.; Zhang, Q.; Zhao, H.; Zheng, F. Survivin Status Affects Prognosis and Chemosensitivity in Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2013, 23, 256–263. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Landry, B.; Mahdipoor, P.; Uludağ, H. Induction of Apoptosis by Survivin Silencing through siRNA Delivery in a Human Breast Cancer Cell Line. Mol. Pharm. 2011, 8, 1821–1830. [Google Scholar] [CrossRef]
- Arista-Romero, M.; Cascante, A.; Fornaguera, C.; Borrós, S. Role of Survivin in Bladder Cancer: Issues to Be Overcome When Designing an Efficient Dual Nano-Therapy. Pharmaceutics 2021, 13, 1959. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.T.; Steinbach, J.M.; Liu, J.; Shimizu, S.; Kaimakliotis, H.Z.; Wheeler, M.A.; Hittelman, A.B.; Saltzman, W.M.; Weiss, R.M. Surface-Modified Nanoparticles Enhance Transurothelial Penetration and Delivery of Survivin siRNA in Treating Bladder Cancer. Mol. Cancer Ther. 2014, 13, 71–81. [Google Scholar] [CrossRef]
- Luo, J.-H.; Xie, D.; Liu, M.-Z.; Chen, W.; Liu, Y.-D.; Wu, G.-Q.; Kung, H.-F.; Zeng, Y.-X.; Guan, X.-Y. Protein expression and amplification of AIB1 in human urothelial carcinoma of the bladder and overexpression of AIB1 is a new independent prognostic marker of patient survival. Int. J. Cancer 2008, 122, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.-T.; Wei, J.-H.; Zhang, J.-X.; Liang, C.-Z.; Liao, B.; Lu, J.; Fan, S.; Chen, Z.-H.; Zhang, F.; Ma, H.-H.; et al. AIB1 predicts bladder cancer outcome and promotes bladder cancer cell proliferation through AKT and E2F1. Br. J. Cancer 2013, 108, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Cheang, T.; Tang, B.; Xia, H.; Xing, Z.; Chen, Z.; Fang, Y.; Chen, W.; Xu, A.; Wang, S.; et al. The inhibition of human bladder cancer growth by calcium carbonate/CaIP6 nanocomposite particles delivering AIB1 siRNA. Biomaterials 2013, 34, 1246–1254. [Google Scholar] [CrossRef]
- Su, S.; Wang, J.; Vargas, E.; Wei, J.; Martinez-Zaguilan, R.; Sennoune, S.R.; Pantoya, M.L.; Wang, S.; Chaudhuri, J.; Qiu, J. Porphyrin Immobilized Nanographene Oxide for Enhanced and Targeted Photothermal Therapy of Brain Cancer. ACS Biomater. Sci. Eng. 2016, 2, 1357–1366. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, H.; Yang, Z.; Zhong, X.; Chen, Y.; Dai, W.; Zhang, X. Aptamer-Conjugated Graphene Quantum Dots/Porphyrin Derivative Theranostic Agent for Intracellular Cancer-Related MicroRNA Detection and Fluorescence-Guided Photothermal/Photodynamic Synergetic Therapy. ACS Appl. Mater. Interfaces 2016, 9, 159–166. [Google Scholar] [CrossRef]
- Menilli, L.; Monteiro, A.R.; Lazzarotto, S.; Morais, F.M.P.; Gomes, A.T.P.C.; Moura, N.M.M.; Fateixa, S.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Trindade, T.; et al. Graphene Oxide and Graphene Quantum Dots as Delivery Systems of Cationic Porphyrins: Photo-Antiproliferative Activity Evaluation towards T24 Human Bladder Cancer Cells. Pharmaceutics 2021, 13, 1512. [Google Scholar] [CrossRef]
- Wang, S.; Jin, S.; Li, G.; Xu, M.; Deng, D.; Xiao, Z.; Sun, H.; Zhang, S.; Zhang, E.; Xie, L.; et al. Transmucosal Delivery of Self-Assembling Photosensitizer–Nitazoxanide Nanocomplexes with Fluorinated Chitosan for Instillation-Based Photodynamic Therapy of Orthotopic Bladder Tumors. ACS Biomater. Sci. Eng. 2021, 7, 1485–1495. [Google Scholar] [CrossRef]
- Li, G.; Yuan, S.; Deng, D.; Ou, T.; Li, Y.; Sun, R.; Lei, Q.; Wang, X.; Shen, W.; Cheng, Y.; et al. Fluorinated Polyethylenimine to Enable Transmucosal Delivery of Photosensitizer-Conjugated Catalase for Photodynamic Therapy of Orthotopic Bladder Tumors Postintravesical Instillation. Adv. Funct. Mater. 2019, 29, 1901932. [Google Scholar] [CrossRef]
- Tan, P.; Cai, H.; Wei, Q.; Tang, X.; Zhang, Q.; Kopytynski, M.; Yang, J.; Yi, Y.; Zhang, H.; Gong, Q.; et al. Enhanced chemo-photodynamic therapy of an enzyme-responsive prodrug in bladder cancer patient-derived xenograft models. Biomaterials 2021, 277, 121061. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Hu, J.-J.; Dai, J.; Lou, X.; Zhao, Z.; Xia, F.; Tang, B.Z. Self-Guiding Polymeric Prodrug Micelles with Two Aggregation-Induced Emission Photosensitizers for Enhanced Chemo-Photodynamic Therapy. ACS Nano 2021, 15, 3026–3037. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, G.; Zhang, X.; Xu, F.; Xiong, X.; Zhou, S. A Step-by-Step Multiple Stimuli-Responsive Nanoplatform for Enhancing Combined Chemo-Photodynamic Therapy. Adv. Mater. 2017, 29, 1605357. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-H.; Yang, W.-H.; Xia, W.; Wei, Y.; Chan, L.-C.; Lim, S.-O.; Li, C.-W.; Kim, T.; Chang, S.-S.; Lee, H.-H.; et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol. Cell 2018, 71, 606–620.e7. [Google Scholar] [CrossRef]
- Munoz, L.E.; Huang, L.; Bommireddy, R.; Sharma, R.; Monterroza, L.; Guin, R.N.; Samaranayake, S.G.; Pack, C.D.; Ramachandiran, S.; Reddy, S.J.; et al. Metformin reduces PD-L1 on tumor cells and enhances the anti-tumor immune response generated by vaccine immunotherapy. J. Immunother. Cancer 2021, 9, e002614. [Google Scholar] [CrossRef]
- Chin, Y.-C.; Yang, L.-X.; Hsu, F.-T.; Hsu, C.-W.; Chang, T.-W.; Chen, H.-Y.; Chen, L.Y.-C.; Chia, Z.C.; Hung, C.-H.; Su, W.-C.; et al. Iron oxide@chlorophyll clustered nanoparticles eliminate bladder cancer by photodynamic immunotherapy-initiated ferroptosis and immunostimulation. J. Nanobiotechnology 2022, 20, 1–18. [Google Scholar] [CrossRef]
- Zhang, S.; Li, G.; Deng, D.; Dai, Y.; Liu, Z.; Wu, S. Fluorinated Chitosan Mediated Synthesis of Copper Selenide Nanoparticles with Enhanced Penetration for Second Near-Infrared Photothermal Therapy of Bladder Cancer. Adv. Ther. 2021, 4, 2100043. [Google Scholar] [CrossRef]
- Ni, W.; Li, M.; Cui, J.; Xing, Z.; Li, Z.; Wu, X.; Song, E.; Gong, M.; Zhou, W. 808 nm light triggered black TiO2 nanoparticles for killing of bladder cancer cells. Mater. Sci. Eng. C 2017, 81, 252–260. [Google Scholar] [CrossRef]
- Self-Assembled Nanoparticle-Mediated Chemophototherapy Reverses the Drug Resistance of Bladder Cancers through Dual AKT/ERK Inhibition. Adv. Ther. 2020, 3, 2000032. [CrossRef]
- Kobayashi, H.; Choyke, P.L. Near-Infrared Photoimmunotherapy of Cancer. Accounts Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef]
- Wu, X.; Wei, Y.; Lin, R.; Chen, P.; Hong, Z.; Zeng, R.; Xu, Q.; Li, T. Multi-responsive mesoporous polydopamine composite nanorods cooperate with nano-enzyme and photosensitiser for intensive immunotherapy of bladder cancer. Immunology 2022. early view. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, L.; Shang, W.; Chen, J.; Chen, Z.; Xiong, F.; Wang, Z.; Tong, Z.; Wang, K.; Yang, L.; et al. Intravesical In Situ Immunostimulatory Gel for Triple Therapy of Bladder Cancer. ACS Appl. Mater. Interfaces 2020, 12, 54367–54377. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; Berg, N.S.V.D.; Ertsey, R.; McKenna, K.; Mach, K.E.; Zhang, C.A.; Volkmer, J.-P.; Weissman, I.L.; Rosenthal, E.L.; Liao, J.C. CD47-Targeted Near-Infrared Photoimmunotherapy for Human Bladder Cancer. Clin. Cancer Res. 2019, 25, 3561–3571. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Yuan, A.; Zhao, X.; Lian, H.; Zhuang, J.; Chen, W.; Zhang, Q.; Liu, G.; Zhang, S.; Cao, W.; et al. Self-assembled tumor-targeting hyaluronic acid nanoparticles for photothermal ablation in orthotopic bladder cancer. Acta Biomater. 2017, 53, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Yumita, N.; Nishigaki, R.; Umemura, K.; Umemura, S.-I. Synergistic Effect of Ultrasound and Hematoporphyrin on Sarcoma 180. Jpn. J. Cancer Res. 1990, 81, 304–308. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Deng, D.; Xiao, Z.; Dong, Z.; Wang, Z.; Lei, Q.; Gao, S.; Huang, G.; Zhang, E.; et al. Fluorinated Chitosan To Enhance Transmucosal Delivery of Sonosensitizer-Conjugated Catalase for Sonodynamic Bladder Cancer Treatment Post-intravesical Instillation. ACS Nano 2020, 14, 1586–1599. [Google Scholar] [CrossRef]
- Duo, Y.; Zhu, D.; Sun, X.; Suo, M.; Zheng, Z.; Jiang, W.; Tang, B.Z. Patient-derived microvesicles/AIE luminogen hybrid system for personalized sonodynamic cancer therapy in patient-derived xenograft models. Biomaterials 2021, 272, 120755. [Google Scholar] [CrossRef]






| Biomarkers and Manufacturer | Detected Biomarkers | Assay Type | Specimen | Sensitivity (CI 95%) | Specificity (CI 95%) | Ref. |
|---|---|---|---|---|---|---|
| NMP22 (Matritech, Inc., Alere, Jena, Thuringia, Germany) | Nuclear mitotic apparatus proteins | ELISA | Urine | 62–75% | 70–83% | [31] |
| NMP22 (Matritech, Inc., Alere, Jena, Thuringia, Germany) | Nuclear mitotic apparatus proteins | Point- of-care test | Urine | 52–59% | 87–89% | [31] |
| BTA Stat (Polymedco, Cortlandt, NY, USA) | Complement factor H-related protein and complement factor H | Point-of-care test | Urine | 58–69% | 73–81% | [31] |
| BTA TRAK (Polymedco, Cortlandt, NY, USA) | Complement factor H-related protein and complement factor H | ELISA | Urine | 54–75% | 64–82% | [31] |
| UroVysion (Abbott Vysis, Chicgo, Illinois, USA) | Alterations in chromosomes 3, 7, 17, and 9p21 | FISH | Urine | 65–84% | 78–92% | [32] |
| uCyt+/Immunocyt (Scimedx, Inc., Dover, New Jersey, USA) | Bladder tumor cell associated mucins/carcinoembryonic antigen | Immunocytochemistry | Urine | 78–90% | 77–87% | [31] |
| Nanomatierials | Detect Target | Properties | Sensitivity (CI 95%) | Specificity (CI 95%) | Applcations | Ref. |
|---|---|---|---|---|---|---|
| QD625 | CD47 | High sensitivity and specificity. | 82.9% | 90.5% | Targeted fluorescent probe for cystoscope | [70] |
| QD605 | PSCA | Specifically targets BC cells and emits stable and long duration fluorescent | - | - | Targeted fluorescent probe for cystoscope | [73] |
| CdSe/ZnS QD | Carbonic anhydrase | Well biocompatibility and dispersion. | - | - | Targeted fluorescent probe for cystoscope | [74,75] |
| Heteroatom-doped graphene QD | Haase | Emits white light and broad excitation-dependent full-color photoluminescence from 463 nm to 672 nm. | - | - | Targeted fluorescent probe for cystoscope | [76] |
| Surface-enhanced Raman scattering nanoparticle | Carbonic anhydrase9, CD47 | Multiple targets and imaging | (ROC AUC: 0.95) | - | Targeted fluorescent probe for Raman endoscopy | [77] |
| UCNP | EGFR | Well ability of tissue penetration | - | - | NIR probe and imaging system | [78] |
| Si QDs/HA-δ-FeOOH | Haase | Detection limit for Haase: 0.02 ng/mL (based on 3σ/S). RSD < 3% (Compared with ELISA method) | Detection limit: 0.02 ng/mL | - | Fluorescence platform for urine test | [76] |
| Rox-DNA functionalized QD | Telomerase | Enabled visual semi-quantitative detection with naked eye. The detection limit was 10 cells and response time was within an hour. | - | - | Sensitive ratiometric fluorescence paper sensor | [79] |
| Nanoparticle | Therapeutic Agents | Condition | Sponsor/Collaborations | States | Study Start | NCT Number |
|---|---|---|---|---|---|---|
| Paclitaxel albumin-stabilized nanoparticle (Nab-paclitaxel) | PTX | Recurrent BC; Stage IV BC | Mayo Clinic/NCI | Phase 2 (Withdrawn) | June 2016 | NCT02718742 |
| Paclitaxel albumin-stabilized nanoparticle (Nab-paclitaxel) | PTX | Bladder cancer | University of Michigan Rogel Cancer Center/Celgene Corporation | Phase 2 | December 2007 | NCT00585689 |
| PLZ4-coated paclitaxel-loaded micelles (PPM) | PTX | NMIBC | VA Office of Research and Development/University of California, Davis | Phase 1 (Not yet recruiting) | - | NCT05519241 |
| Nanoparticle | Size (nm) | Therapeutic Agents | Loading Efficiency | Properties | Application | Ref. |
|---|---|---|---|---|---|---|
| Nab-paclitaxel | 150–200 | PTX | 10% | Low side-effects; good solubility and biocompatibility | Vein injection | [80] |
| LK/PTX/PEGb- (PELG-g-(PZLL-r-PLL)) | 89 ± 3 | LK, PTX | LK (6.74%), PTX (4.13%) | Increasing of the half-life and bioavailability of the drugs | Abdominal subcutaneous injection | [84] |
| DC-PNM-PTX | 23 ± 6 | PTX | >99% | Specifically targeting the bladder cancer PDXs; improvement of the cisplatin resistance; GSH-responsive release | Tail vein injection | [85] |
| PTX/CS NSs | 194.48 ± 86.24 | PTX | 81.4% | Attaching to mucosa of the bladder through electrostatic adsorption | Intravesical instillation | [86] |
| EphA2-ILs-DTXp | 110 ± 10 | DTX prodrug | 90–99% | Specific targeting to tumor; improvement of penetration; minimal haematological toxicity | Tail vein injection | [88,89] |
| MMC@CS -Mn:ZnS | 175 | MMC | 44.52 ± 1.05% | Long retention time | - | [90] |
| [FeFe]TPP/GEM/FCS NPs | 220 | GEM; [FeFe]TPP | GEM (6.9%); [FeFe]TPP (7.7%) | Improvement of penetration capacity; H2 generation under 660nm laser irradiation; inhibition of drug transport capacity of cancer cells | Intravesical instillation | [91] |
| PEG-PAMAM-DOX | 13 | DOX | - | pH-responsive release | Intravesical instillation | [92] |
| BITT@BSA-DSP | 70.2 ± 22.0 | DSP | 35% | Visible drug delivery; photodynamic and photothermal effect | Intravesical instillation | [93] |
| ATF24-PEG-Lipo-β-E | 79.32 ± 1.282 | β-E | 98.37% | Specific targeting to tumor | Intravesical instillation | [97] |
| MPI/F-PEI NPs | 260.67 ± 6.62 | MPI | - | Improved cross-membrane and transmucosal penetration | Intravesical instillation | [100] |
| CONPs | 40~110 | CONPs | - | Activation of ERK-dependent autophagy; synergistic effect with chemo drugs. | Intravesical instillation and in situ injection | [101] |
| IAA-CS/HA NP and HRP-CS/HA NP | 170~200 | HRP, IAA | Both > 90% | Enzyme/prodrug system. | In vitro (T24) | [103] |
| Nanoparticle | Size (nm) | Therapeutic Agents | Loading Efficiency | Properties | Application | Ref. |
|---|---|---|---|---|---|---|
| Fe3O4-BCG-CS/GP gel | - | BCG | 1% (w/v) | Response to magnetic field control; long retention time | Intravesical instillation | [104] |
| CWS-NP/LEEL | 166 | BCG-CWS | 57% | Good water solubility | Intravesical instillation | [106,107] |
| CWS-FPL | <200 | BCG-CWS | 60% | Improvement of tumor targeting by folic acid; improvement of penetration by Pep-1 peptide | Intravesical instillation | [109] |
| R8-liposome-BCG-CW | 230 | BCG-CWS | - | Improvement of cell binding and internalization | Intravesical instillation | [110] |
| Nanoparticle | Size (nm) | Therapeutic Agents | Loading Efficiency | Properties | Application | Ref. |
|---|---|---|---|---|---|---|
| Mg(II)-Cat/siEIF5A2 | 10-20 | Catechin; siEIF5A2 | - | Good biocompatibility and cellular uptake; inhibition of oncogene eukaryotic translation initiation factor | Tail vein injection | [114] |
| Anti-survivin siRNA-1 pbae-NP | 150 | Survivin siRNA | 100% | No synergistic effect with PTX | In virto (T24, RT4) | [110] |
| NP-siSUR-CH2.5 | 137 ± 51 | Survivin siRNA | 70% | Long release time of sirna | In situ injection | [121,122] |
| NP-ACC/caip6/siAIB1 | 80–200 | siAIB1 | - | Well ability of lysosome escape; good biocompatibility | In situ injection | [125] |
| Nanoparticle | Responsive Part | Size (nm) | Therapeutic Agents | Properties | Application | Ref. |
|---|---|---|---|---|---|---|
| Zn-TMPyP@GQDs | An-TMPyP, GQDs | 28.4 | - | Blue light-responsive; good stability of porphyrins in aqueous solutions; multiple targets binding sites and possible photothermal effect | In vitro (T24) | [128] |
| HSA-Ce6/NTZ/FCS | Ce6 | 192 | NTZ | Improvement of tumor hypoxia and drug transmucosal delivery | Intravesical instillation | [129] |
| CAT-Ce6/F-PEI | Ce6 | 220.3 | - | Improvement of tumor hypoxia by catalase and drug transmucosal delivery | Intravesical instillation | [130] |
| Poly (OEGMA)-PTX@Ce6 (NPs@Ce6) | Ce6 | 168.2 ± 1.12 | Polymer-PTX prodrug | Combination of PCI effect and enhanced chemo-PDT | In situ injection | [131,132,133] |
| IR775@Met@Lip | IR775 | - | Metformin | Improvement of tumor hypoxia; down-regulate PD-L1 | Intravesical instillation | [69] |
| Fe3O4@Chl/Fe CNPs | Chl/Fe | 12.8 ± 4.8 | - | Photodynamic immunotherapy-initiated ferroptosis and immune stimulation. | Intravesical instillation | [136] |
| FCS-Cu2-xSe | Cu2-xSe | 30.1 | - | Improvement of drug transmucosal delivery; NIR-II-responsive | Intravesical instillation | [137] |
| Black TiO2 NPs | TiO2 | 20–30 | - | Absorption of visible light and near in- frared | In vitro (T24) | [138] |
| PhD | Pheophorbide a | 71 | DOX | Combination of PDT, PTT and DOX; pH and NIR-responsive. | Tail vein injection | [139] |
| MPDIαW | ICG, MnO2 | 120 | PD-L1 antibody | Combination of PTT and immunotherapy; specific adherence to bladder cancer cell; pH-responsive | Intravesical instillation | [141] |
| AuNRs&IONs@Gel | AuNrs | 80–120 | Iron oxide nanoparticles | Combination of PTT, iron death, and macrophages re-polarization; targeting delivery | In situ injection | [142] |
| Anti-CD47-IR700 | IR700 | - | - | Targeting delivery; long retention time | Tail vein injection | [143] |
| HA-IR780 NPs | IR780 | 171.3 | - | Targeting delivery; good bioavailability and biocompatibility | Tail vein injection | [144] |
| Nanoparticle | Responsive Part | Size (nm) | Therapeutic Agents | Properties | Application | Ref. |
|---|---|---|---|---|---|---|
| CAT-TCPP/FCS NPs | TCPP | 190 ± 12 | - | Improvement of tumor hypoxia by catalase and drug transmucosal delivery | Intravesical instillation | [146] |
| AMVs | AIEgen | 300 | - | Good internalization and personalized tumor targeting ability | Tail vein injection | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, C.; Zhang, S.; Lei, Q.; Wu, S. State-of-the-Art Advances of Nanomedicine for Diagnosis and Treatment of Bladder Cancer. Biosensors 2022, 12, 796. https://doi.org/10.3390/bios12100796
Kong C, Zhang S, Lei Q, Wu S. State-of-the-Art Advances of Nanomedicine for Diagnosis and Treatment of Bladder Cancer. Biosensors. 2022; 12(10):796. https://doi.org/10.3390/bios12100796
Chicago/Turabian StyleKong, Chenfan, Shaohua Zhang, Qifang Lei, and Song Wu. 2022. "State-of-the-Art Advances of Nanomedicine for Diagnosis and Treatment of Bladder Cancer" Biosensors 12, no. 10: 796. https://doi.org/10.3390/bios12100796
APA StyleKong, C., Zhang, S., Lei, Q., & Wu, S. (2022). State-of-the-Art Advances of Nanomedicine for Diagnosis and Treatment of Bladder Cancer. Biosensors, 12(10), 796. https://doi.org/10.3390/bios12100796




