Spectral Properties Echoing the Tautomerism of Milrinone and Its Application to Fe3+ Ion Sensing and Protein Staining
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Instruments
2.2. Measurement Procedure
2.3. Preparation of Real Samples
2.4. Protein Staining
2.5. Computational Methods
3. Results and Discussion
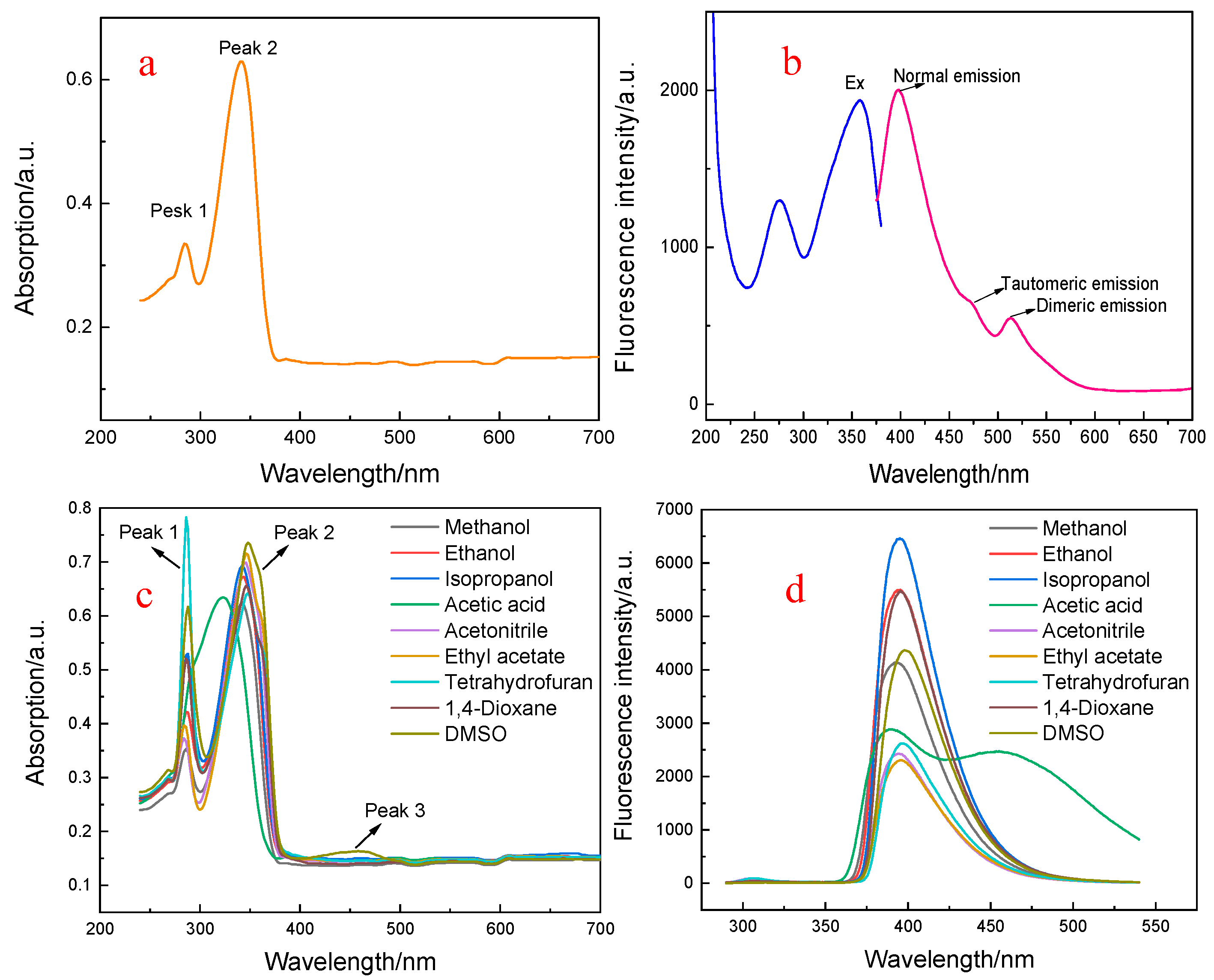
3.1. Spectroscopic Characteristics of MLR
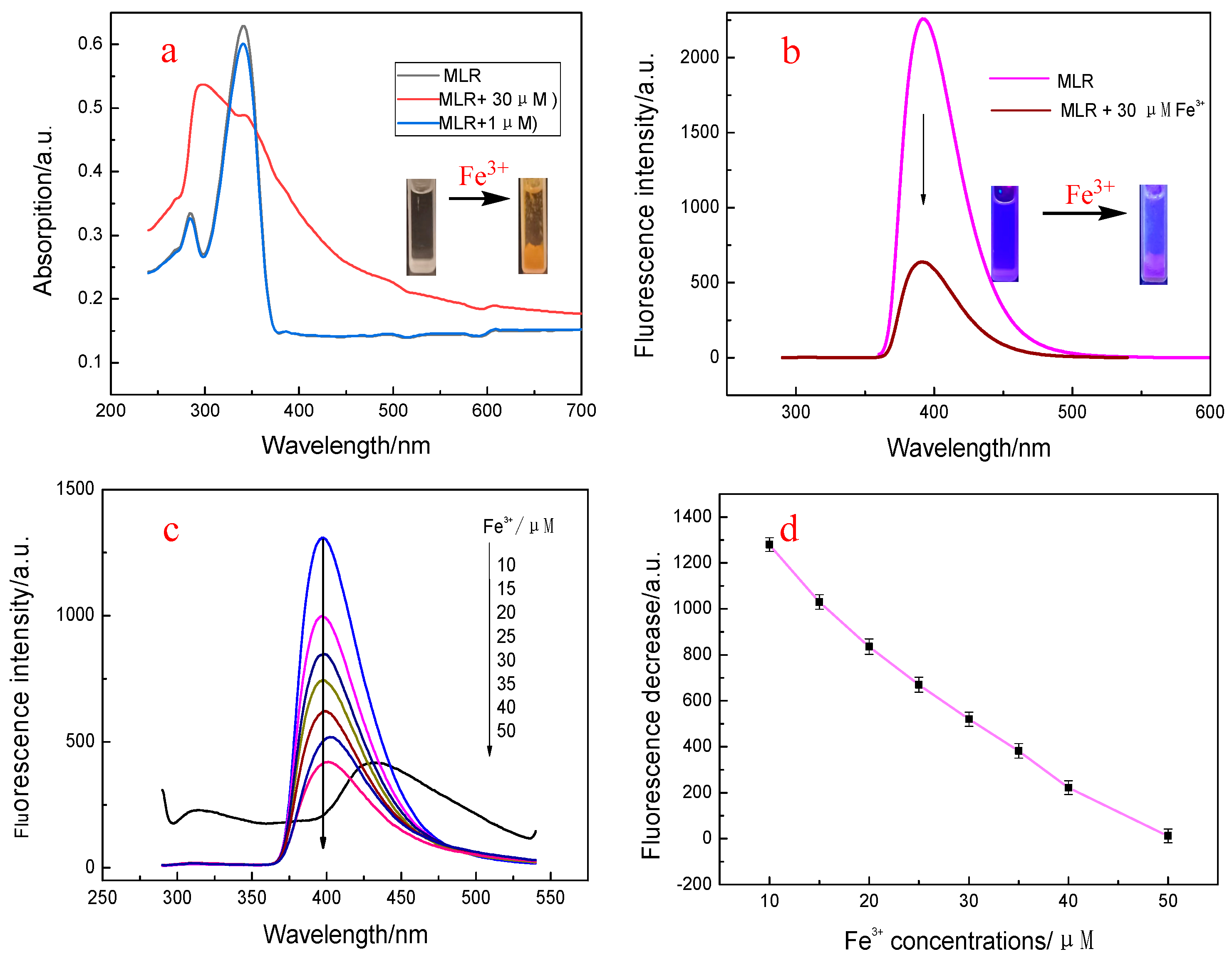
3.2. Spectroscopic Response of MLR to Fe3+
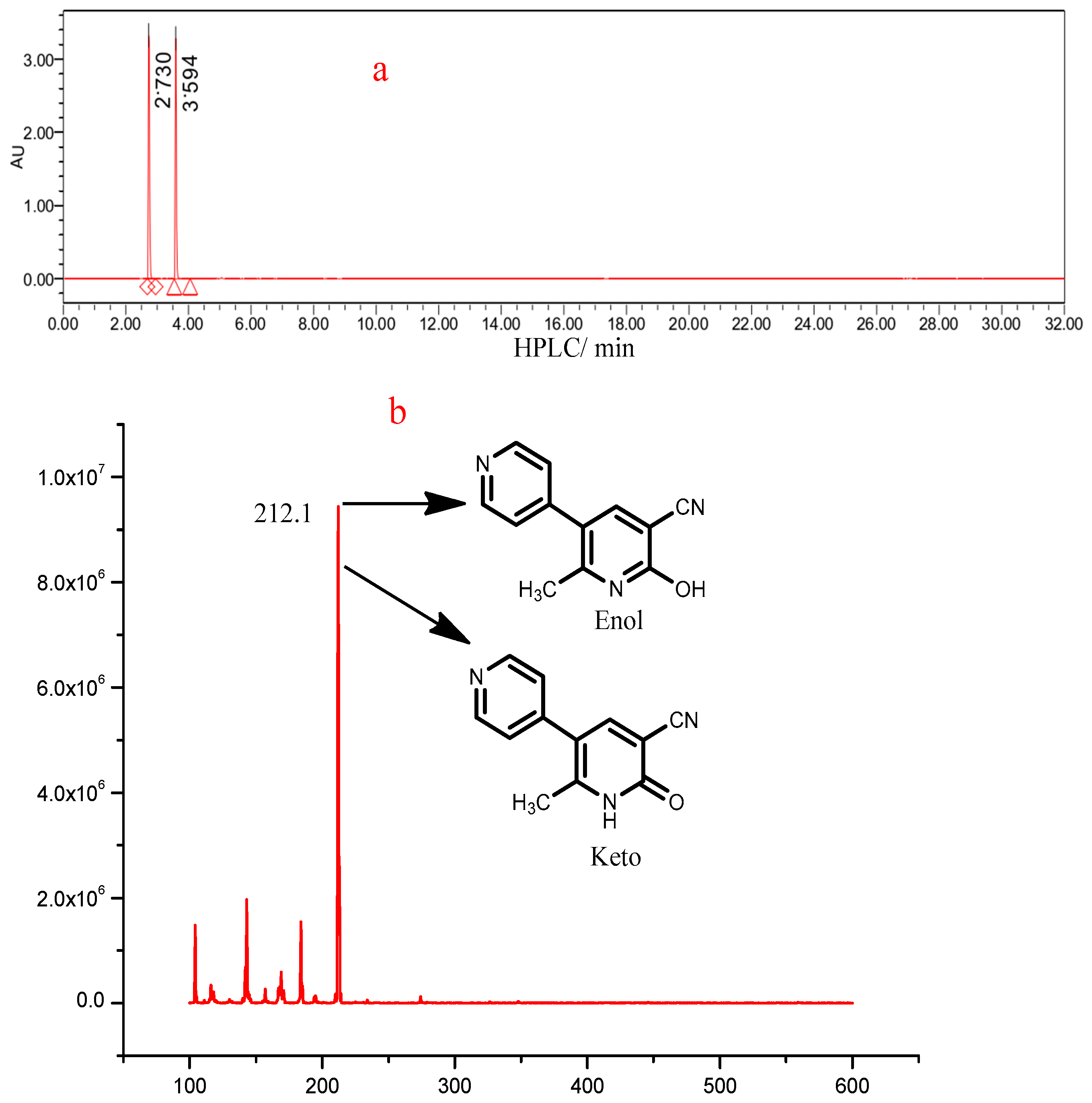
3.3. Effect of pH on the Tautomerization of MLR
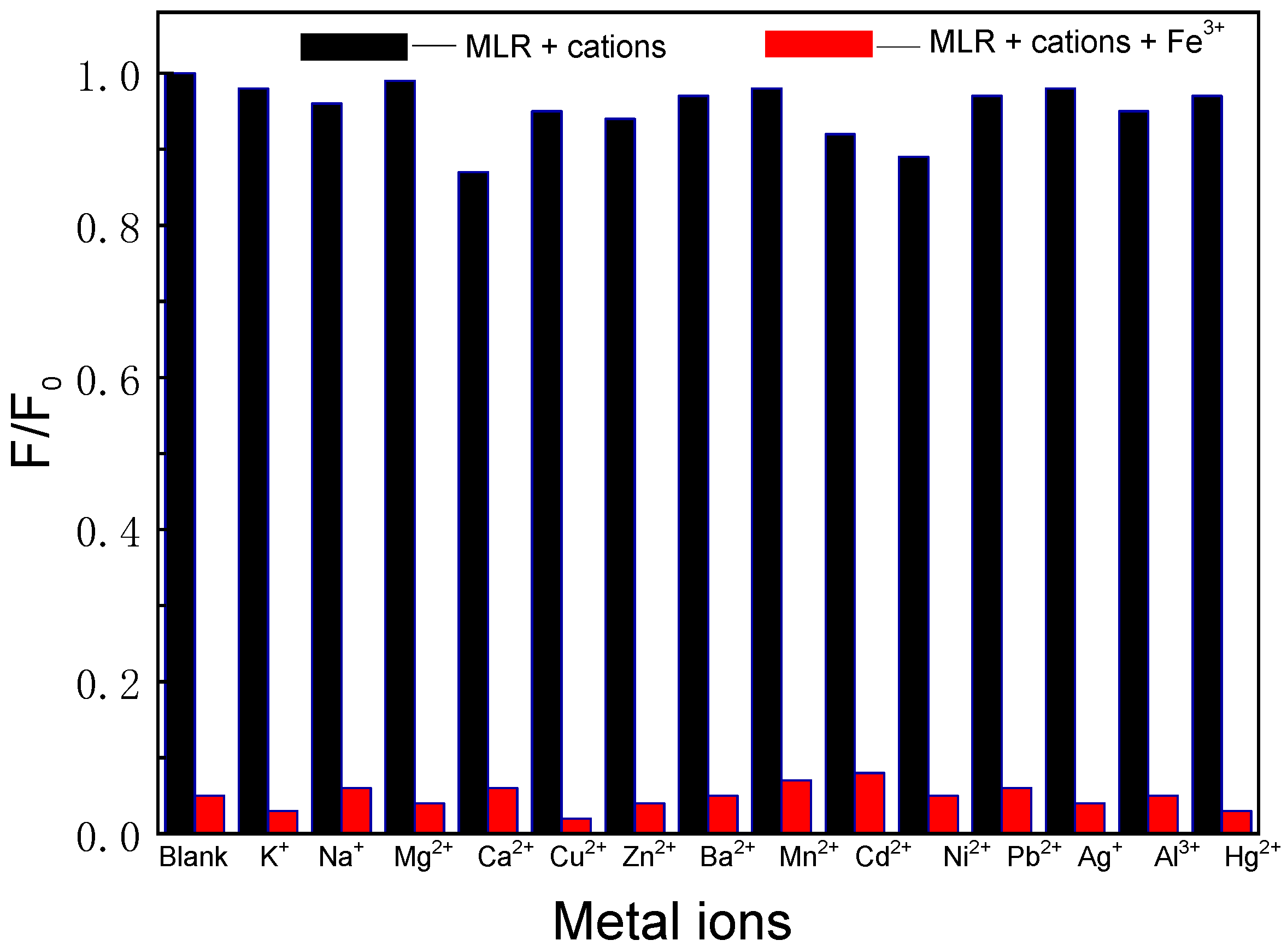
3.4. Selectivity of MLR towards Fe3+
3.5. Detection of Fe3+ in Real Samples Using MLR
3.6. Theoretical Calculation and Analysis
3.7. Protein Staining Using MLR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castle-Kirszbaum, M.; Lai, L.; Maingard, J.; Asadi, H.; Danks, R.A.; Goldschlager, T.; Chandra, R.V. Intravenous milrinone for treatment of delayed cerebral ischaemia following subarachnoid haemorrhage: A pooled systematic review. Neurosurg. Rev. 2021, 44, 3107–3124. [Google Scholar] [CrossRef] [PubMed]
- Bork, N.I.; Kuret, A.; Cruz Santos, M.; Molina, C.E.; Reiter, B.; Reichenspurner, H.; Friebe, A.; Skryabin, B.V.; Rozhdestvensky, T.S.; Kuhn, M.; et al. Rise of cGMP by partial phosphodiesterase-3A degradation enhances cardioprotection during hypoxia. Redox. Biol. 2021, 48, 102179. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Sugiura, M.; Fukaya, C.; Yokoyama, K.; Hashimoto, Y.; Kawanishi, K.; Moriyasu, M. Periandradulcins A, B and C: Phosphodiesterase Inhibitors from Periandra dulcis MART. Chem. Pharm. Bull. 1991, 39, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Nadur, N.F.; de Azevedo, L.L.; Caruso, L.; Graebin, C.S.; Lacerda, R.B.; Kümmerle, A.E. The long and winding road of designing phosphodiesterase inhibitors for the treatment of heart failure. Eur. J. Med. Chem. 2021, 212, 113123. [Google Scholar] [CrossRef] [PubMed]
- Larsson, R.; Liedholm, H.; Andersson, K.E.; Keane, M.A.; Henry, G. Pharmacokinetics and effects on blood pressure of a single oral dose of milrinone in healthy subjects and in patients with renal impairment. Eur. J. Clin. Pharmacol. 1986, 29, 549–553. [Google Scholar] [CrossRef]
- Burniston, J.G.; Ellison, G.M.; Clark, W.A.; Goldspink, D.F.; Tan, L.-B. Relative toxicity of cardiotonic agents. Cardiovasc. Toxicol. 2005, 5, 355–364. [Google Scholar] [CrossRef]
- Charisopoulou, D.; Leaver, N.; Banner, N.R. Milrinone in Advanced Heart Failure: Dose and Therapeutic Monitor Outside Intensive Care Unit. Angiology 2013, 65, 343–349. [Google Scholar] [CrossRef]
- Ayres, J.K.; Maani, C.V. Milrinone. In StatPearls [Internet]; StatPearls Publishing: San Francisco, CA, USA, 2022. [Google Scholar]
- De Candia, M.; Fossa, P.; Cellamare, S.; Mosti, L.; Carotti, A.; Altomare, C. Insights into structure–activity relationships from lipophilicity profiles of pyridin-2(1H)-one analogs of the cardiotonic agent milrinone. Eur. J. Pharm. Sci. 2005, 26, 78–86. [Google Scholar] [CrossRef]
- El-Kemary, M.; Organero, J.A.; Douhal, A. Fast Relaxation Dynamics of the Cardiotonic Drug Milrinone in Water Solutions. J. Med. Chem. 2006, 49, 3086–3091. [Google Scholar] [CrossRef]
- Krauze, A.; Vītoliņa, R.; Garaliene, V.; Sīle, L.; Kluša, V.; Duburs, G. 3,4-trans-4-Aryl-3-(1-pyridinio)-1,2,3,4-tetrahydropyridine-6-thiolates—new group of potential cardiotonic drugs. Eur. J. Med. Chem. 2005, 40, 1163–1167. [Google Scholar] [CrossRef]
- El-Kemary, M.; Organero, J.A.; Douhal, A. Assessment of solvent effect on the relaxation dynamics of milrinone. J. Photochem. Photobiol. A 2007, 187, 339–347. [Google Scholar] [CrossRef]
- El-Kemary, M.; Organero, J.A.; Santos, L.; Douhal, A. Effect of Cyclodextrin Nanocavity Confinement on the Photorelaxation of the Cardiotonic Drug Milrinone. J. Phys. Chem. B 2006, 110, 14128–14134. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, J.; Norell, M.; Canepa-Anson, R.; Reuben, S.R.; Poole-Wilson, P.A.; Sutton, G.C. Acute haemodynamic comparison of amrinone and pirbuterol in chronic heart failure. Additional effects of isosorbide dinitrate. Br. Heart J. 1983, 49, 214. [Google Scholar] [CrossRef] [PubMed]
- Altomare, C.; Cellamare, S.; Summo, L.; Fossa, P.; Mosti, L.; Carotti, A. Ionization behaviour and tautomerism-dependent lipophilicity of pyridine-2(1H)-one cardiotonic agents. Bioorg. Med. Chem. 2000, 8, 909–916. [Google Scholar] [CrossRef]
- Noguchi, A.; Kondo, S.-i.; Kuzuya, M. Quantum Chemical Study on Conformational Properties of Bipyridine Cardiotonics. Chem. Pharm. Bull. 1993, 41, 1331–1336. [Google Scholar] [CrossRef]
- Gil, M.; Douhal, A. Femtosecond dynamics of a cardiotonic medicine (milrinone) in neutral water. Chem. Phys. Lett. 2006, 428, 174–177. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.; Tang, W.; Hu, C. The intra-annular acylamide chelate-coordinated compound: The keto-tautomer of metal (II)–milrinone complex. J. Mol. Struct. 2008, 875, 113–120. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, Y.; Farajtabar, A. Milrinone solubility in aqueous cosolvent solutions revisited: Inter/intra-molecular interactions, enthalpy-entropy compensation, and preferential solvation. J. Mol. Liq. 2022, 360, 119452. [Google Scholar] [CrossRef]
- Shen, J.-Y.; Chao, W.-C.; Liu, C.; Pan, H.-A.; Yang, H.-C.; Chen, C.-L.; Lan, Y.-K.; Lin, L.-J.; Wang, J.-S.; Lu, J.-F.; et al. Probing water micro-solvation in proteins by water catalysed proton-transfer tautomerism. Nat. Commun. 2013, 4, 2611. [Google Scholar] [CrossRef]
- Paterson, M.J.; Robb, M.A.; Blancafort, L.; DeBellis, A.D. Mechanism of an Exceptional Class of Photostabilizers: A Seam of Conical Intersection Parallel to Excited State Intramolecular Proton Transfer (ESIPT) in o-Hydroxyphenyl-(1,3,5)-triazine. J. Phys. Chem. A 2005, 109, 7527–7537. [Google Scholar] [CrossRef]
- Todorov, A.R.; Nieger, M.; Helaja, J. Tautomeric Switching and Metal-Cation Sensing of Ligand-Equipped 4-Hydroxy-/4-oxo-1,4-dihydroquinolines. Chem. Eur. J. 2012, 18, 7269–7277. [Google Scholar] [CrossRef] [PubMed]
- Mata, S.; Cortijo, V.; Caminati, W.; Alonso, J.L.; Sanz, M.E.; López, J.C.; Blanco, S. Tautomerism and Microsolvation in 2-Hydroxypyridine/2-Pyridone. J. Phys. Chem. A 2010, 114, 11393–11398. [Google Scholar] [CrossRef]
- Wu, D.-l.; Liu, L.; Liu, G.-f.; Jia, D.-z. Ab Initio/DFT and AIM Studies on Dual Hydrogen-Bonded Complexes of 2-Hydroxypyridine/2-Pyridone Tautomerism. J. Phys. Chem. A 2007, 111, 5244–5252. [Google Scholar] [CrossRef]
- Li, Q.-S.; Fang, W.-H.; Yu, J.-G. Theoretical Studies of Proton-Transfer Reactions of 2-Hydroxypyridine−(H2O)n (n = 0−2) in the Ground and Excited States. J. Phys. Chem. A 2005, 109, 3983–3990. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, D.; Zheng, F.; Zhou, H.; Jiang, S.; Wu, Y. Water-Soluble and Lowly Toxic Sulphur Quantum Dots. Adv. Funct. Mater. 2014, 24, 7133–7138. [Google Scholar] [CrossRef]
- Qin, C.; Troya, D.; Shang, C.; Hildreth, S.; Helm, R.; Xia, K. Surface Catalyzed Oxidative Oligomerization of 17β-Estradiol by Fe3+-Saturated Montmorillonite. Environ. Sci. Technol. 2015, 49, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Zeng, M.; Xu, J.; Wang, X.; Hu, W. Retracted Article: Polymer nanodots of graphitic carbon nitride as effective fluorescent probes for the detection of Fe3+ and Cu2+ ions. Nanoscale 2014, 6, 4157–4162. [Google Scholar] [CrossRef]
- Yiannikourides, A.; Latunde-Dada, G.O. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef]
- Duan, N.; Guo, F.; Deng, B.; Yang, S.; Tian, H.; Sun, B. Application of a luminous intensity variation fluorescent probe for the detection of ferric ions. Luminescence 2022, 37, 803–809. [Google Scholar] [CrossRef]
- Qin, Z.; Su, W.; Liu, P.; Ma, J.; Zhang, Y.; Jiao, T. Facile Preparation of a Rhodamine B Derivative-Based Fluorescent Probe for Visual Detection of Iron Ions. ACS Omega 2021, 6, 25040–25048. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y.; Yin, H.; Wang, Y.; Cong, L.; Jin, M.-X.; Ding, D.-J. New insights into the solvent-assisted excited-state double proton transfer of 2-(1H-pyrazol-5-yl)-pyridine with alcoholic partners: A TDDFT investigation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-S.; Du, R.; Zhao, Y.-Y.; Xue, J.-D.; Wang, H.-G.; Zheng, X. Solvent-dependent structural dynamics of 2(1H)-pyridinone in light absorbing S4(ππ*) state. J. Raman Spectrosc. 2016, 47, 299–309. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, S.; Zhang, K.; Zhou, T.; Ban, X.; Duan, Y.; Jia, D.; Zhu, Q.; Zhang, T. A novel thermally-activated delayed fluorescent probe based on hydroxyl as identify group for detection of iron ions. J. Mol. Struct. 2022, 1251, 132074. [Google Scholar] [CrossRef]
- Lee, J.-S.; Warkad, S.D.; Shinde, P.B.; Kuwar, A.; Nimse, S.B. A highly selective fluorescent probe for nanomolar detection of ferric ions in the living cells and aqueous media. Arab. J. Chem. 2020, 13, 8697–8707. [Google Scholar] [CrossRef]



| Samples a | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| River water | 0 | 1.93 | 100.1 | 3.2 |
| 5 | 8.98 | 99.7 | 3.4 | |
| 10 | 17.82 | 98.5 | 3.6 | |
| Tap water | 0 | 5.21 | 99.6 | 3.7 |
| 5 | 10.42 | 100.2 | 3.6 | |
| 10 | 15.45 | 98.3 | 3.5 | |
| Well water | 0 | 4.93 | 103.4 | 3.5 |
| 3 | 8.81 | 102.3 | 3.2 | |
| 6 | 11.02 | 99.7 | 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Ma, P.; Qian, Y.; Xia, J.; Gong, F.; Chen, L.; Xu, L. Spectral Properties Echoing the Tautomerism of Milrinone and Its Application to Fe3+ Ion Sensing and Protein Staining. Biosensors 2022, 12, 777. https://doi.org/10.3390/bios12100777
Zhu H, Ma P, Qian Y, Xia J, Gong F, Chen L, Xu L. Spectral Properties Echoing the Tautomerism of Milrinone and Its Application to Fe3+ Ion Sensing and Protein Staining. Biosensors. 2022; 12(10):777. https://doi.org/10.3390/bios12100777
Chicago/Turabian StyleZhu, Hanming, Pan Ma, You Qian, Jiaoyun Xia, Fuchun Gong, Lusen Chen, and Lujie Xu. 2022. "Spectral Properties Echoing the Tautomerism of Milrinone and Its Application to Fe3+ Ion Sensing and Protein Staining" Biosensors 12, no. 10: 777. https://doi.org/10.3390/bios12100777
APA StyleZhu, H., Ma, P., Qian, Y., Xia, J., Gong, F., Chen, L., & Xu, L. (2022). Spectral Properties Echoing the Tautomerism of Milrinone and Its Application to Fe3+ Ion Sensing and Protein Staining. Biosensors, 12(10), 777. https://doi.org/10.3390/bios12100777




