Label-Free Aptasensor for Detection of Fipronil Based on Black Phosphorus Nanosheets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. Preparation of BPNSs
2.4. Fabrication of PLL-BPNSs Composition
2.5. Preparation of Apt/AuNPs/PLL- BPNSs/GCE Electrode
2.6. Electrochemical Detection of Fipronil
3. Results and Discussion
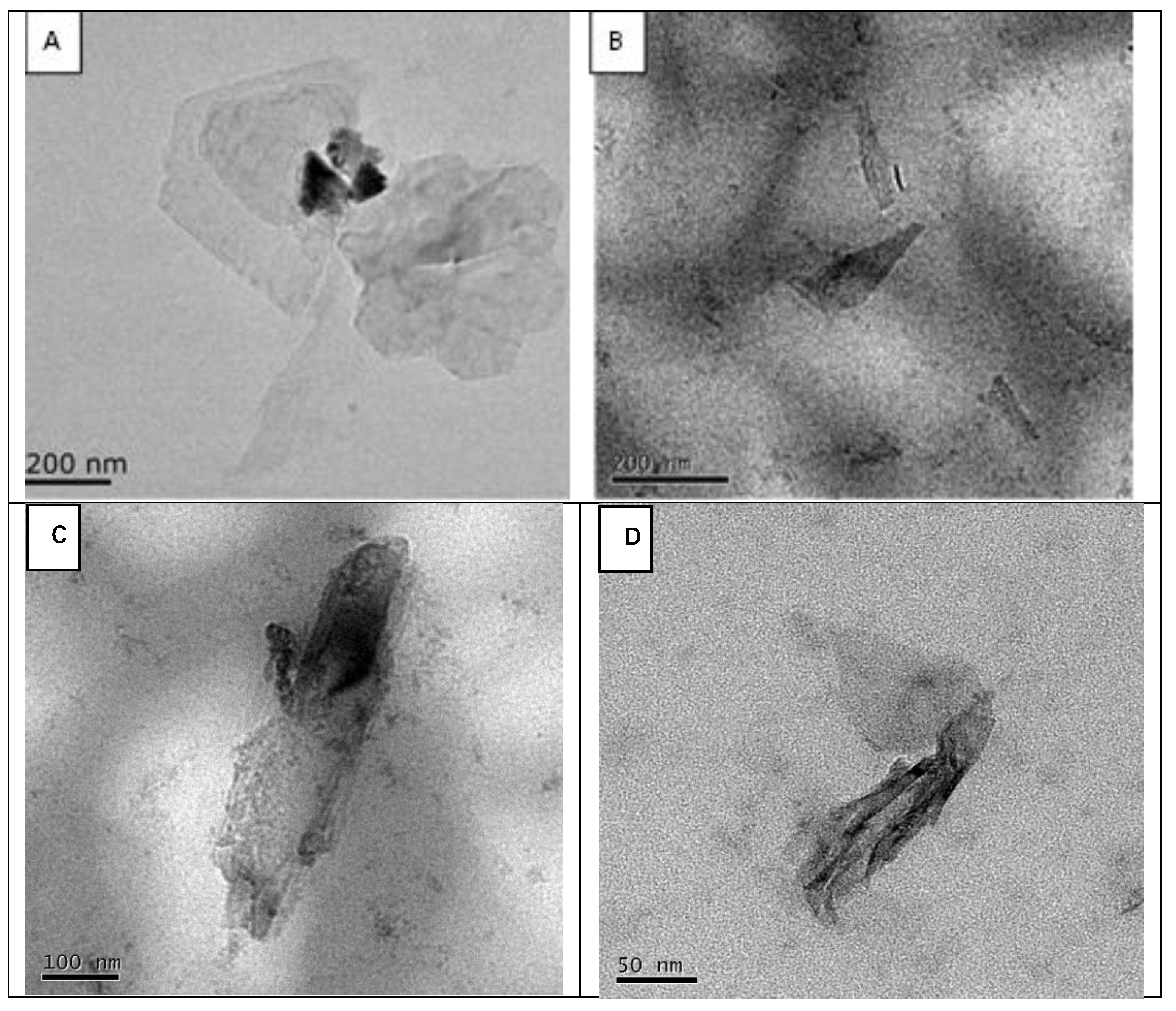
3.1. Characterization of PLL–BPNSs Nanocomposite
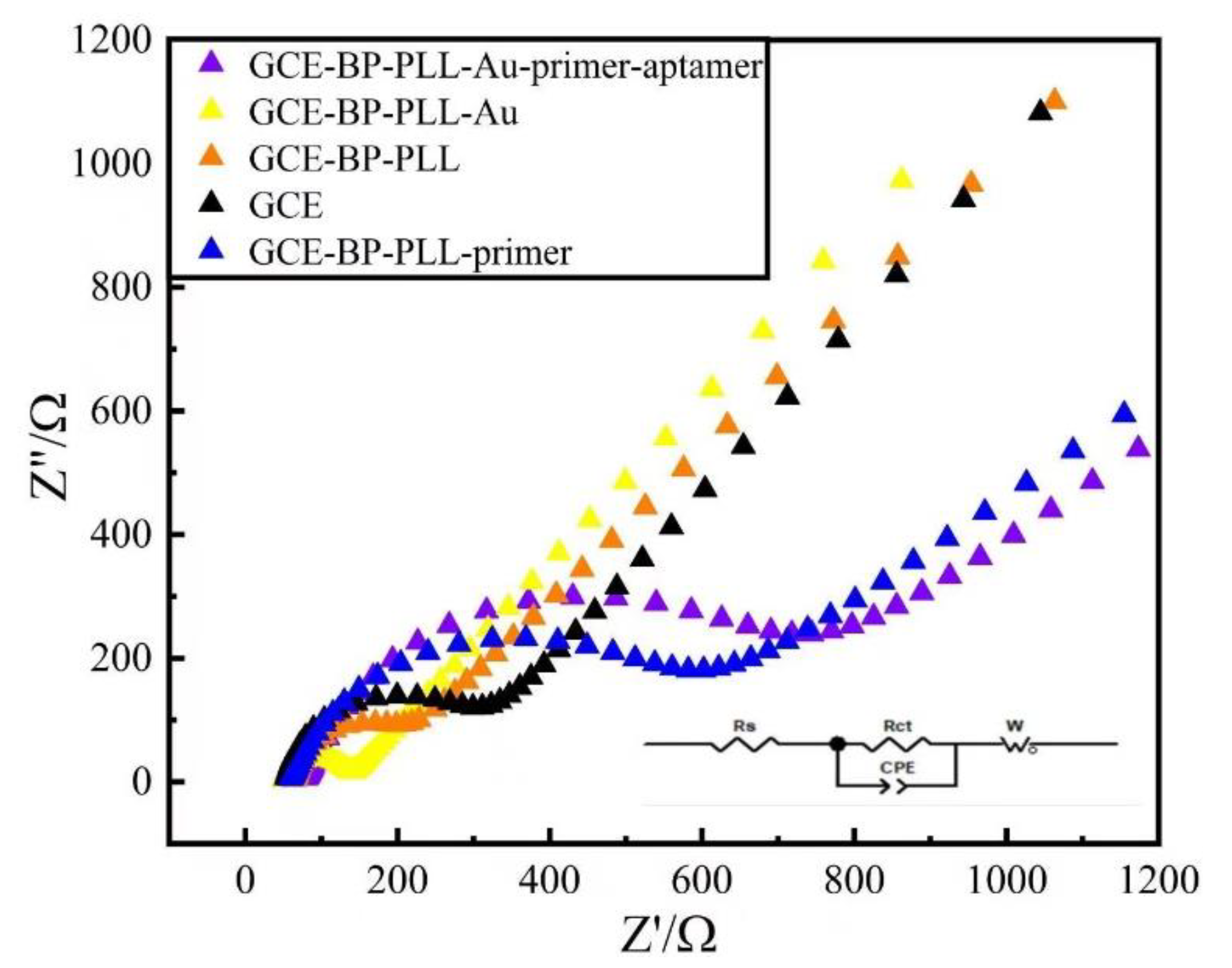
3.2. Electrochemical Characterization of Modified Electrodes
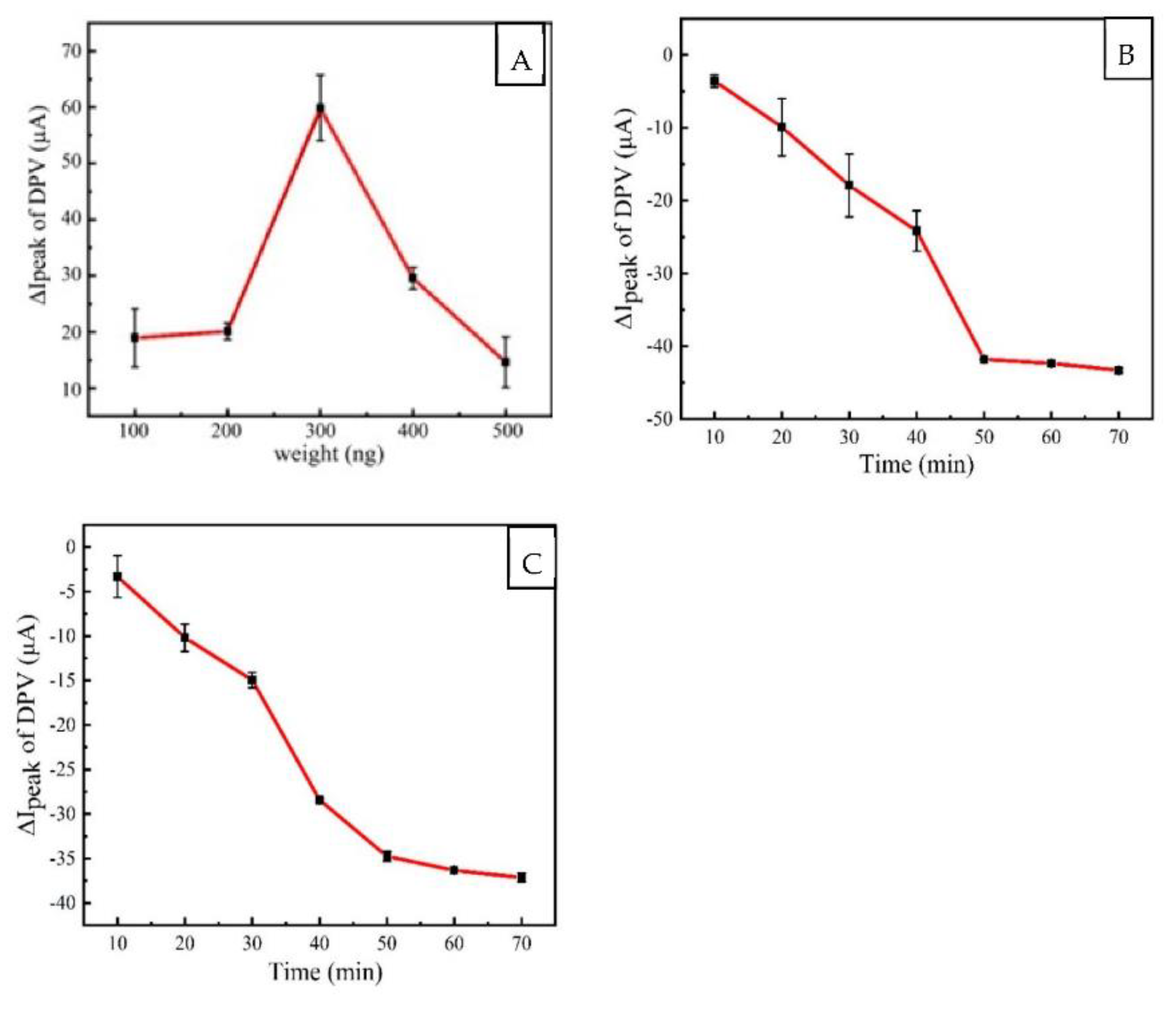
3.3. Optimization of Recognition Conditions
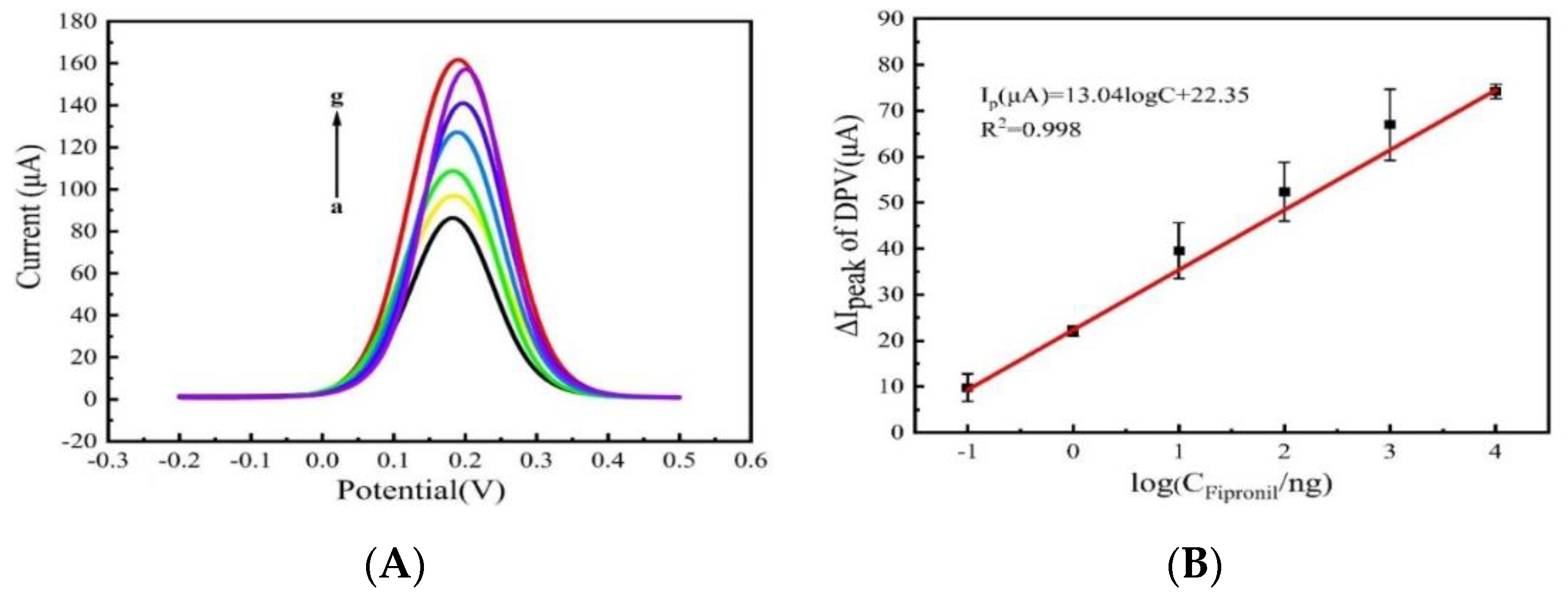
3.4. Detection of Fipronil on Apt/Primer/AuNPs/PLL-BP/GCE
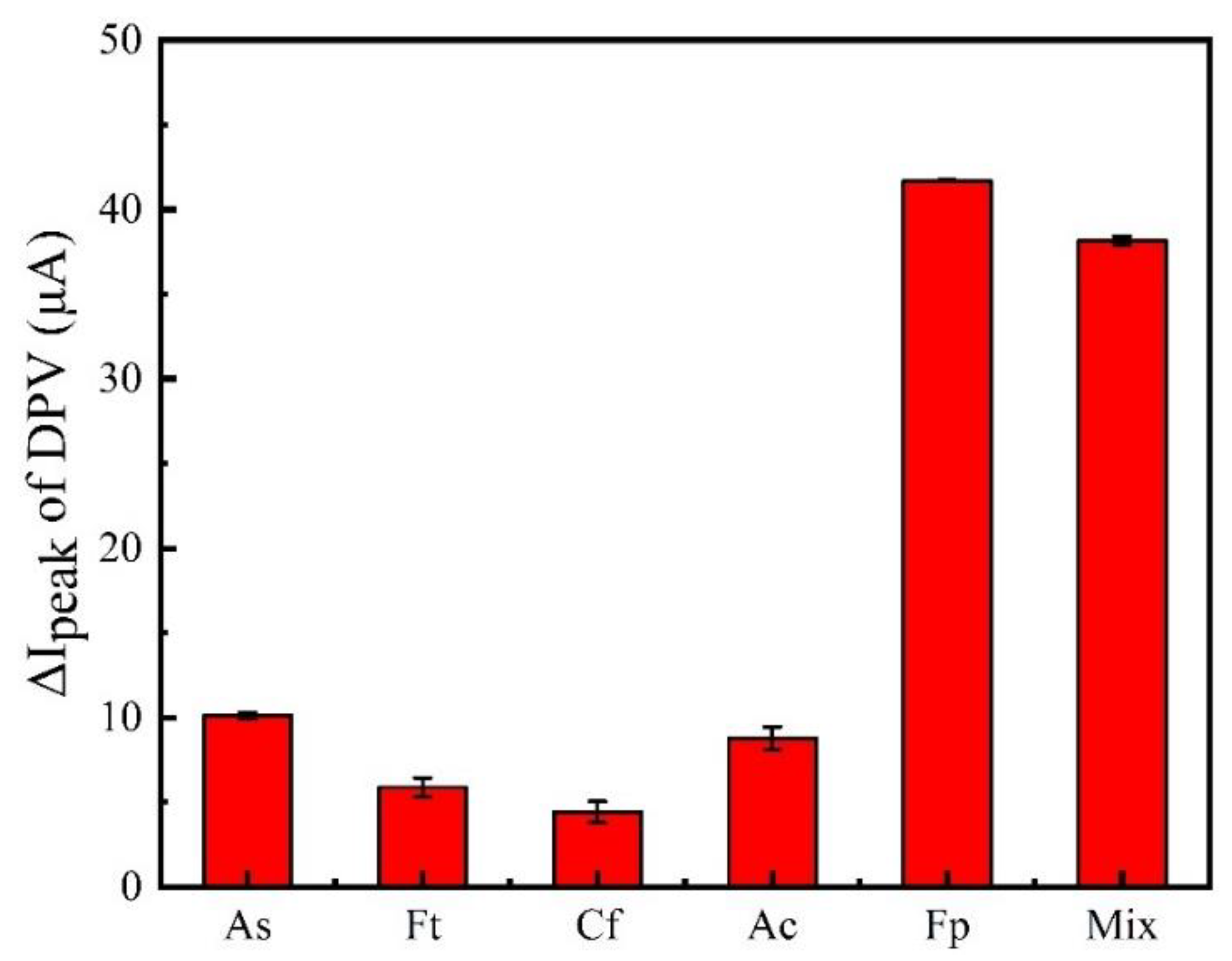
3.5. Stability, Reproducibility and Specificity
3.6. Sample Analysis and Analyte Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Chen, S.W.; Zhang, B.W.; Tu, Q.; Wang, J.Y.; Yuan, M.S. Non-biological fluorescent chemosensors for pesticides detection. Talanta 2022, 240, 123200. [Google Scholar] [CrossRef] [PubMed]
- Tingle, C.C.; Rother, J.A.; Dewhurst, C.F.; Lauer, S.; King, W.J. Fipronil: Environmental fate, ecotoxicology, and human health concerns. Rev. Environ. Contam. Toxicol. 2003, 176, 1–66. [Google Scholar] [PubMed]
- Ichikawa, H. Neurotoxicology of pesticides. Brain Nerve 2015, 6739–6748. [Google Scholar] [CrossRef]
- Gutta, S.; Prasad, J.D.; Gunasekaran, K.; Iyadurai, R. Hepatotoxicity and neurotoxicity of Fipronil poisoning in human: A case report. J. Fam. Med. Prim. Care 2019, 8, 3437. [Google Scholar]
- Khalaf, A.A.; Ibrahim, M.A.; Galal, M.K.; Abdallah, A.A.; Mansour, R.; Afify, M.M. The protective effects of Terminalia laxiflora extract on hepato-nephrotoxicity induced by fipronil in male rats. Environ. Sci. Pollut. R. 2020, 27, 39507–39515. [Google Scholar] [CrossRef]
- Wang, X.; Martínez, M.A.; Wu, Q.H.; Ares, I.; Martinez-Larranaga, M.R.; Anadón, A.; Yuan, Z.H. Fipronil insecticide toxicology: Oxidative stress and metabolism. Crit. Rev. Toxicol. 2016, 46, 876–899. [Google Scholar] [CrossRef]
- Kim, Y.A.; Yoon, Y.S.; Kim, H.S.; Jeon, S.J.; Cole, E.; Lee, J.S.; Kho, Y.; Cho, Y.H. Distribution of fipronil in humans, and adverse health outcomes of in utero fipronil sulfone exposure in newborns. Int.J. Hyg. Environ. Health 2019, 222, 524–532. [Google Scholar] [CrossRef]
- Araujo, L.; Troconis, M.E.; Cubillán, D.; Mercado, J.; Villa, N.; Prieto, A. Single drop microextraction and gas chromatography–mass spectrometry for the determination of diflufenican, mepanipyrim, fipronil, and pretilachlor in water samples. Environ. Monit. Assess. 2013, 185, 10225–10233. [Google Scholar] [CrossRef]
- Wang, T.L.; Hu, J.Y.; Liu, C.L. Simultaneous determination of insecticide fipronil and its metabolites in maize and soil by gas chromatography with electron capture detection. Environ. Monit. Assess. 2014, 186, 2767–2774. [Google Scholar] [CrossRef]
- Cheng, Y.P.; Dong, F.S.; Liu, X.G.; Xu, J.; Meng, W.; Liu, N.; Chen, Z.L.; Tao, Y.; Zheng, Y.Q. Simultaneous determination of fipronil and its major metabolites in corn and soil by ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Methods-UK 2014, 6, 1788–1795. [Google Scholar] [CrossRef]
- Ghambarian, M.; Ghashghaee, M.; Azizi, Z.; Balar, M. Molecular interactions of MeOH and EtOH with black phosphorus monolayer: A periodic density functional study. Phys. Chem. Res. 2019, 7, 435–447. [Google Scholar]
- Li, C.; Si, L.; Feng, X.W.; Wang, L.; Huang, X.; Tee, B.C.-K.; Ang, K.W. Gigahertz integrated circuits based on complementary black phosphorus transistors. Adv. Electron. Mater. 2018, 4, 1800274. [Google Scholar]
- Cho, S.Y.; Lee, Y.H.; Koh, H.J.; Jung, H.; Kim, J.S.; Yoo, H.W.; Kim, J.; Jung, H.T. Superior chemical sensing performance of black phosphorus: Comparison with MoS2 and graphene. Adv. Mater. 2016, 28, 7020–7028. [Google Scholar] [CrossRef]
- Rong, X.M.; Yu, Z.Z.; Wu, Z.W.; Li, J.J.; Wang, B.; Wang, Y. First principles modeling of pure black phosphorus devices under pressure. Beilstein J. Nanotech. 2019, 10, 1943–1951. [Google Scholar] [CrossRef]
- Luo, W.; Zemlyanov, D.Y.; Milligan, C.A.; Du, Y.C.; Yang, L.M.; Wu, Y.Q.; Peide, D.Y. Surface chemistry of black phosphorus under a controlled oxidative environment. Nanotechnology 2016, 27, 434002. [Google Scholar] [CrossRef]
- Brent, J.R.; Savjani, N.; Lewis, E.A.; Haigh, S.J.; Lewis, D.J.; O’Brien, P. Production of few-layer phosphorene by liquid exfoliation of black phosphorus. Chem. Commun. 2014, 50, 13338–13341. [Google Scholar] [CrossRef]
- Gusmao, R.; Sofer, Z.; Pumera, M. Black phosphorus rediscovered: From bulk material to monolayers. Angew. Chem. Int. Ed. 2017, 56, 8052–8072. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wu, L.P.; Kattel, B.; Li, C.H.; Yong, Z.; Hou, Y.B.; Wu, J.; Chan, W.L. Using bulk heterojunctions and selective electron trapping to enhance the responsivity of perovskite–graphene photodetectors. Adv. Funct. Mater. 2017, 27, 1704173. [Google Scholar] [CrossRef]
- Favron, A.; Gaufrès, E.; Fossard, F.; Lévesque, P.L.; Phaneuf-L’Heureux, A.; Tang, N.Y.; Loiseau, A.; Leonelli, R.; Francoeur, S.; Martel, R. Photooxidation and quantum confinement effects in exfoliated black phosphorus. Nature Mater. 2015, 14, 826–832. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.H.; Zhuge, Z.; Tang, Y.H. Synthesis of a Poly-L-Lysine/Black Phosphorus Hybrid for Biosensors. Anal. Chem. 2018, 90, 3149–3155. [Google Scholar] [CrossRef]
- Kumar, V.; Brent, J.R.; Shorie, M.; Kaur, H.; Chadha, G.; Thomas, A.G.; Lewis, E.A.; Rooney, A.P.; Nguyen, L.; Zhong, X.L.; et al. Nanostructured Aptamer-Functionalized Black Phosphorus Sensing Platform for Label-Free Detection of Myoglobin, a Cardiovascular Disease Biomarker. ACS Appl. Mater. Interfaces 2016, 8, 22860–22868. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Liu, Y.; Deng, Y.; Chen, Z.; Chen, H.; Li, S.; He, N.Y. Selection of a high-affinity DNA aptamer for the recognition of cadmium ions. J. Biomed. Nanotechnol. 2021, 17, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical applications of aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef]
- Song, S.P.; Wang, L.H.; Li, J.; Fan, C.H.; Zhao, J.L. Aptamer-based biosensors. TrAC-Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Sun, H.G.; Zu, Y.L. A highlight of recent advances in aptamer technology and its application. Molecules 2015, 20, 11959. [Google Scholar] [CrossRef]
- Guo, W.F.; Zhang, C.X.; Ma, T.T.; Liu, X.Y.; Chen, Z.; Li, S.; Deng, Y. Advances in aptamer screening and aptasensors’ detection of heavy metal ions. J. Nanobiotechnol. 2021, 19, 166. [Google Scholar] [CrossRef]
- Liu, M.; Khan, A.; Wang, Z.F.; Liu, Y.; Yang, G.J.; Deng, Y.; He, N.Y. Aptasensors for pesticide detection. Biosens. Bioelectron. 2019, 130, 174–184. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.J.; Li, T.T.; Deng, Y.; Chen, Z.; He, N.Y. Selection of a DNA aptamer for the development of fluorescent aptasensor for carbaryl detection. Chin. Chem. Lett. 2021, 32, 1957–1962. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.T.; Yang, G.J.; Deng, Y.; Mou, X.B.; He, N.Y. A simple AuNPs-based colorimetric aptasensor for chlorpyrifos detection. Chin. Chem. Lett. 2022, 33, 1913–1916. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. Nanomaterials in electrochemical biosensors for pesticide detection: Advances and challenges in food analysis. Microchim. Acta 2016, 183, 2063–2083. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Du, Y.; Chen, C.G.; Li, B.L.; Zhou, M.; Dong, S.J. Au NPs-enhanced surface plasmon resonance for sensitive detection of mercury (II) ions. Biosen. Bioelectron. 2010, 25, 2622–2626. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, T.T.; Ling, C.X.; Wang, Z.L.; Jin, L.; Zhao, Y.X.; Chen, Z.; Li, S.; Deng, Y.; He, N.Y. A simple visual method for DNA detection based on the formation of gold nanoparticles. Chin. Chem. Lett. 2019, 30, 2359–2362. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.; Li, T.T.; Chen, Z.; Chen, H.; Li, S.; Liu, H.N. Aptamer-based electrochemical biosensor for mercury ions detection using AuNPs-modified glass carbon electrode. J. Biomed. Nanotechnol. 2018, 14, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Huang, H.; Xiao, Z.Q.; Zhang, C.X.; Guo, W.F.; Ma, T.T.; Ma, L.; Chen, Z.; Deng, Y. A novel strategy for liquid exfoliation of ultrathin black phosphorus nanosheets. J. Biomed. Nanotechnol. 2020, 16, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Vasylieva, N.; Singh, V.; Hammock, B.; Singh, S.G. A facile, sensitive and rapid sensing platform based on CoZnO for detection of fipronil; an environmental toxin. Electroanalysis 2020, 32, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Li, S.Y.; Jiang, Z.J.; Li, J.P. A versatile and ultrasensitive molecularly imprinted electrochemiluminescence sensor with HRP-encapsulated liposome labeled by light-triggered click reaction for pesticide residues. Microchim. Acta 2022, 189, 33. [Google Scholar] [CrossRef]
- El-Akaad, S.; Morozov, R.; Golovin, M.; Bol’shakov, O.; Saeger, S.D.; Beloglazova, N. A novel electrochemical sensor for the detection of fipronil and its toxic metabolite fipronil sulfone using TiO2-polytriazine imide submicrostructured composite as an efficient electrocatalyst. Talanta 2022, 238, 123025. [Google Scholar] [CrossRef]
- Qin, T.Y.; Zhao, X.F.; Jia, T.H.; Du, X.F.; Lv, T.Y.; Tian, Y.Q.; Zhang, Z.X.; Liu, B.; Xu, H.H.; Zhao, C. A novel protein-based supramolecular recognition approach for ratiometric fluorescence detection of fipronil. Sens. Actuators B-Chem. 2022, 369, 132358. [Google Scholar] [CrossRef]
- El-Akaad, S.; Mohamed, M.A.; Elmasri, M.M.; Abdelwahab, N.S.; Abdelaleem, E.A.; Saeger, S.D.; Beloglazova, N. 3D bismuth ferrite microflowers electrochemical sensor for the multiple detection of pesticides. J. Electrochem. Soc. 2020, 167, 027543. [Google Scholar] [CrossRef]
- Zhang, J.X.; Feng, T.T.; Zhang, J.Y.; Liang, N.; Zhao, L.S. Fluorescence assay for the sensitive detection offipronil based on an “on–off” oxidized SWCNH/aptamer sensor. Anal. Methods 2021, 13, 3282. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.H.; Chen, X.H.; Chen, Z.D. Quenched electrochemiluminescence sensor of ZnO@g-C3N4 modified glassy carbon electrode for fipronil determination. Microchem. J. 2019, 145, 295–300. [Google Scholar] [CrossRef]
- Maulidiyah, M.; Azis, T.; Lindayani, L.; Wibowo, D.; Salim, L.O.A.; Aladin, A.; Nurdin, M. Sol-gel TiO2/carbon paste electrode nanocomposites for electrochemical-assisted sensing of fipronil pesticide. J. Electrochem. Sci. Technol. 2019, 10, 394–401. [Google Scholar] [CrossRef]
- Yang, S.L.; Lu, J.N.; Zhang, S.J.; Zhang, C.X.; Wang, Q.L. 2D europium coordination polymer as a regenerable fluorescence probe for efficiently detecting fipronil. Analyst 2018, 143, 4901–4906. [Google Scholar] [CrossRef] [PubMed]



| Sample | Added (ng/mL) | Found (ng/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| River water | 10 | 10.79 | 107.9% | 0.61% |
| 100 | 100.69 | 100.69% | 8.23% | |
| 10,000 | 8410 | 84.1% | 5.58% |
| Methods | Sample | Linear Range (nM) | LOD (nM) | References |
|---|---|---|---|---|
| GCE/CoZnONF/Anti-fipronil | _ | 100 ag/mL–100 μg/mL, 0.23 fM–0.23 mM | 112 ag/mL, 0.25 fM | [36] |
| Molecularly imprinted electrochemiluminescence sensor | Egg, Banana, Oilseed rape, Orange | 10 fM–1 nM | 0.78 fM | [37] |
| A screen-printed electrode (SPE) | Eggs, Water | 0.01 to 10 μM | 8.42 nM | [38] |
| Fluorescence detection | Arabidopsis thaliana | 0–500 μM | 22 nM | [39] |
| BiFeO3/CPE | Environmental water | 1.0–100.0 μM | 0.81 μM | [40] |
| Aptamer-based Fluorescence assay | Corn, Honey, Tap water | 10–100 nM | 3 nM | [41] |
| Electrochemiluminscence (ECL) sensor | Eggs | 5–1000 nM | 1.5 nM | [42] |
| Electrochemical sensor | Fipronil pesticide | - | 34 nM | [43] |
| Fluorescent-based probe | Eggs | 1 μM–0.1 mM | 0.8 M | [44] |
| PLL-BP-Apt sensor | Lake water | 0.1 ng/mL–10 μg/mL, 0.23 nM–0.23 μM | 74 pg/mL, 0.17 nM | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Zhang, C.; Zhou, J.; Wei, D.; Ma, T.; Guo, W.; Liu, X.; Li, S.; Deng, Y. Label-Free Aptasensor for Detection of Fipronil Based on Black Phosphorus Nanosheets. Biosensors 2022, 12, 775. https://doi.org/10.3390/bios12100775
Huang H, Zhang C, Zhou J, Wei D, Ma T, Guo W, Liu X, Li S, Deng Y. Label-Free Aptasensor for Detection of Fipronil Based on Black Phosphorus Nanosheets. Biosensors. 2022; 12(10):775. https://doi.org/10.3390/bios12100775
Chicago/Turabian StyleHuang, Hao, Chuanxiang Zhang, Jie Zhou, Dan Wei, Tingting Ma, Wenfei Guo, Xueying Liu, Song Li, and Yan Deng. 2022. "Label-Free Aptasensor for Detection of Fipronil Based on Black Phosphorus Nanosheets" Biosensors 12, no. 10: 775. https://doi.org/10.3390/bios12100775
APA StyleHuang, H., Zhang, C., Zhou, J., Wei, D., Ma, T., Guo, W., Liu, X., Li, S., & Deng, Y. (2022). Label-Free Aptasensor for Detection of Fipronil Based on Black Phosphorus Nanosheets. Biosensors, 12(10), 775. https://doi.org/10.3390/bios12100775




