A Fiber-Optic Surface Plasmon Resonance Sensor for Bio-Detection in Visible to Near-Infrared Images
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
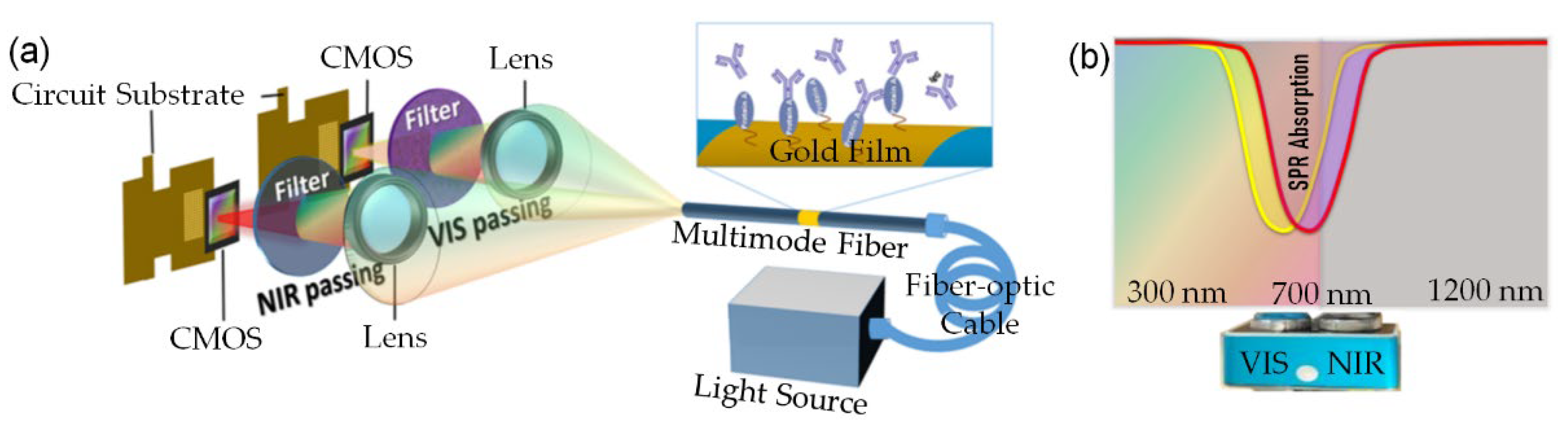
2.2. Fabrication of FO-SPR Sensing Element
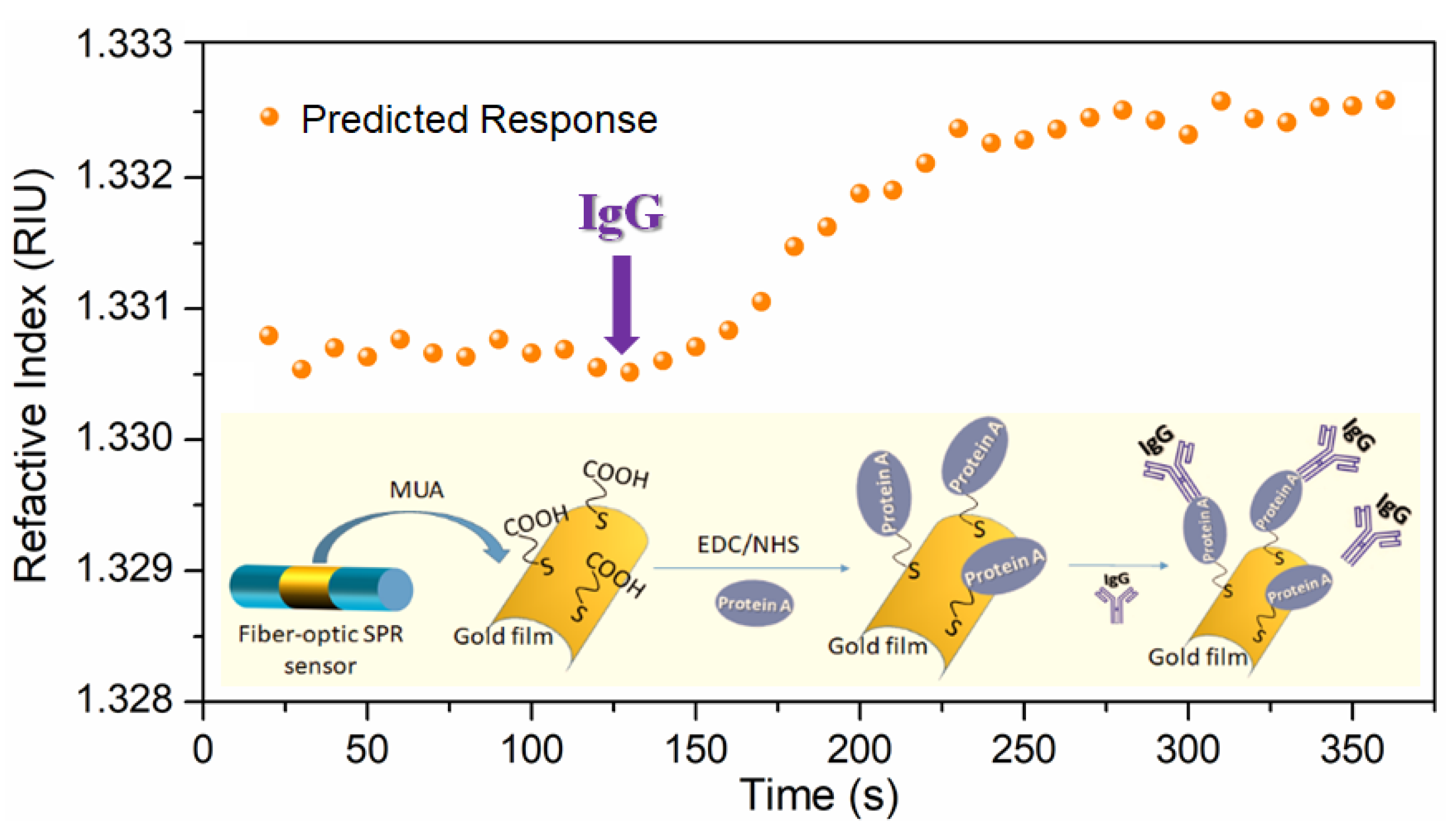
2.3. Anti-Body Immobilization
2.4. Sensor Interrogation
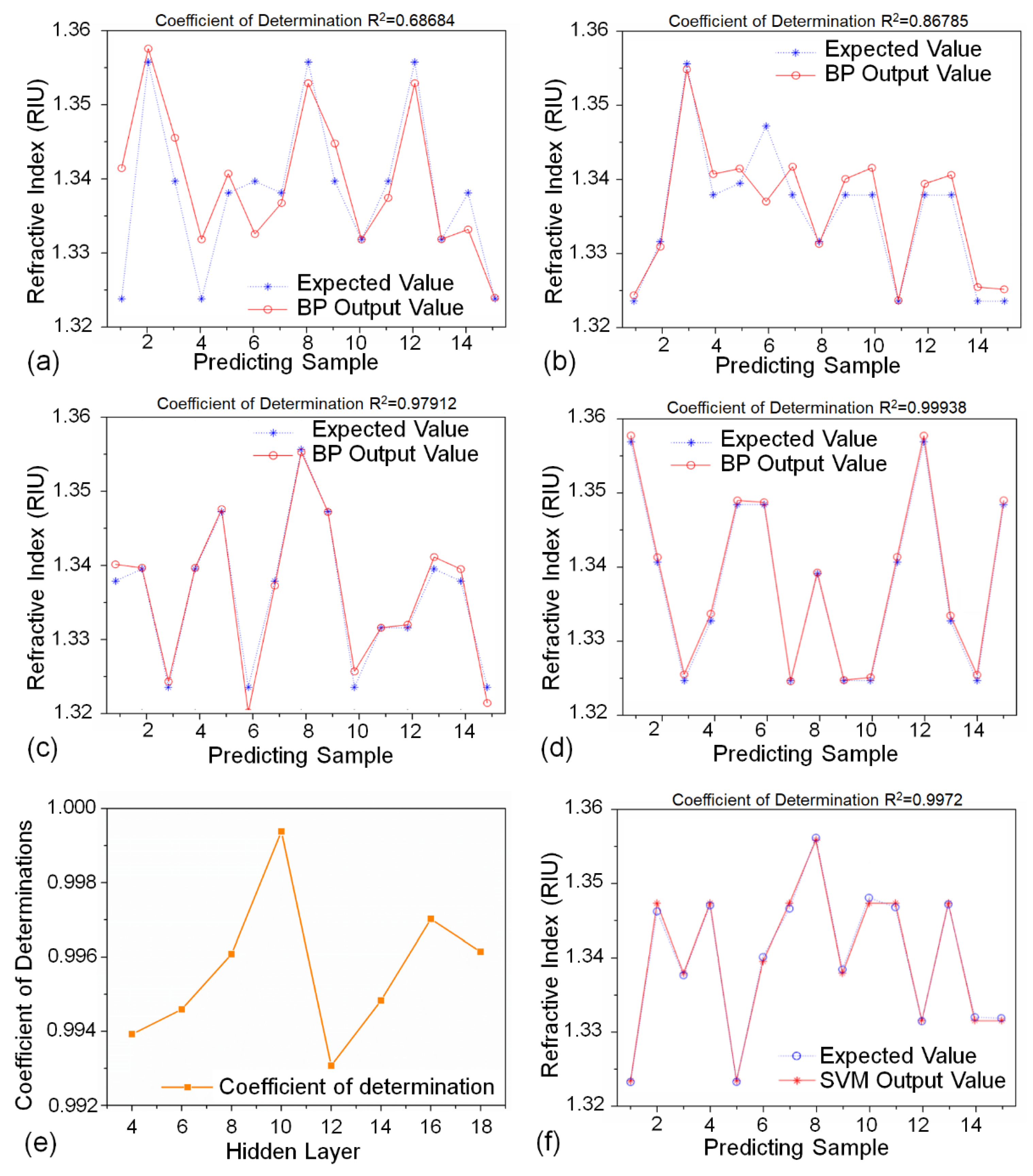
2.5. Machine Learning Algorithm
3. Results and Discussion
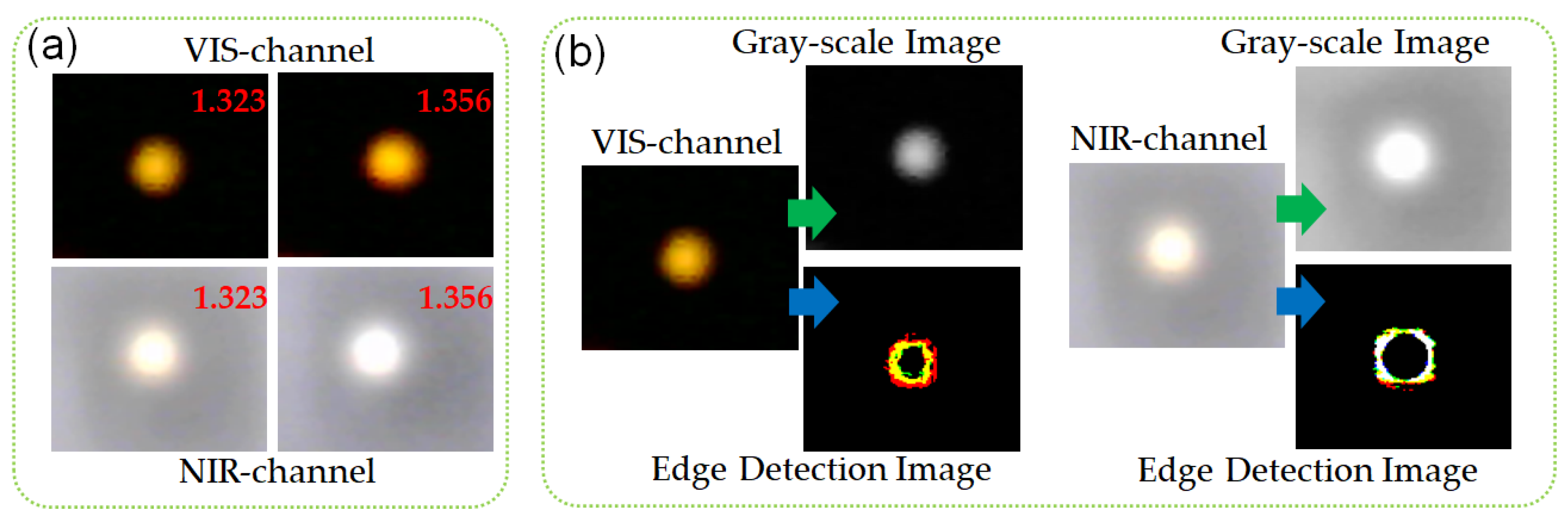
3.1. SPR Image Processing and Sensing
3.2. Response to RI Variation of Aqueous Solution
3.3. Response to Antigen–Antibody Binding
3.4. Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raether, H. Surface plasmons on smooth surfaces. In Surface Plasmons on Smooth and Rough Surfaces and on Gratings. Springer: Berlin, HDB, Germany, 1988; pp. 4–39. [Google Scholar]
- Zhao, Y.; Tong, R.J.; Xia, F.; Peng, Y. Current status of optical fiber biosensor based on surface plasmon resonance. Biosens. Bioelectron. 2019, 142, 111505. [Google Scholar] [CrossRef]
- Prabowo, B.A.; Purwidyantri, A.; Liu, K.C. Surface plasmon resonance optical sensor: A review on light source technology. Biosensors 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Liedberg, B.; Nylander, C.; Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators 1983, 4, 299–304. [Google Scholar] [CrossRef]
- Masson, J.F. Surface plasmon resonance clinical biosensors for medical diagnostics. ACS Sens. 2017, 2, 16–30. [Google Scholar] [CrossRef]
- Zeni, L.; Perri, C.; Cennamo, N.; Arcadio, F.; D’Agostino, G.; Salmona, M.; Beeg, M.; Gobbi, M. A portable optical-fibre-based surface plasmon resonance biosensor for the detection of therapeutic antibodies in human serum. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Raj, D.R.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic sensor for mercury detection using gold nanoparticles PVA hybrid. Opt. Commun. 2016, 367, 102–107. [Google Scholar]
- Pan, M.; Yang, J.; Li, S.; Wen, W.; Wang, J.; Ding, Y.; Wang, S. A reproducible surface plasmon resonance immunochip for the label-free detection of amantadine in animal-derived foods. Food Anal. Methods 2019, 12, 1007–1016. [Google Scholar] [CrossRef]
- Nguyen, H.; Park, J.; Kang, S.; Kim, M. Surface plasmon resonance: A versatile technique for biosensor applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef] [Green Version]
- Gangwar, R.K.; Singh, V.K. Highly sensitive surface plasmon resonance based D-shaped photonic crystal fiber refractive index sensor. Plasmonics 2017, 12, 1367–1372. [Google Scholar] [CrossRef]
- Drescher, D.G.; Selvakumar, D.; Drescher, M.J. Analysis of protein interactions by surface plasmon resonance. Adv. Protein Chem. Struct. Biol. 2018, 110, 1–30. [Google Scholar]
- Breveglieri, G.; D’Aversa, E.; Cosenza, L.C.; Boutou, E.; Balassopoulou, A.; Voskaridou, E.; Gambari, R.; Borgatti, M. Detection of the sickle hemoglobin allele using a surface plasmon resonance based biosensor. Sens. Actuators B Chem. 2019, 296, 126604. [Google Scholar] [CrossRef]
- Trzaskowski, M.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Ciach, T. Detection of tuberculosis in patients with the use of portable SPR device. Sens. Actuators B Chem. 2018, 260, 786–792. [Google Scholar] [CrossRef]
- Schasfoort, R.B. Surface Plasmon Resonance Instruments. In Handbook of Surface Plasmon Resonance; Richard, B.M.S., Ed.; Royal Society of Chemistry: London, UK, 2017; pp. 60–105. [Google Scholar]
- Zhao, S.S.; Bukar, N.; Toulouse, J.L.; Pelechacz, D.; Robitaille, R.; Pelletier, J.N.; Masson, J.F. Miniature multi-channel SPR instrument for methotrexate monitoring in clinical samples. Biosens. Bioelectron. 2015, 64, 664–670. [Google Scholar] [CrossRef]
- Qiu, P.; Zhang, D.; Jing, M.; Lu, T.; Yu, B.; Zhan, Q.; Zhuang, S. Dynamic tailoring of surface plasmon polaritons through incident angle modulation. Opt. Express 2018, 26, 9772–9783. [Google Scholar] [CrossRef]
- Sinha, R.K. Wavelength modulation based surface plasmon resonance sensor for detection of cardiac marker proteins troponin I and troponin T. Sensor. Actuat. A Phys. 2021, 332, 113104. [Google Scholar] [CrossRef]
- Li, K.; Zhou, W.; Zeng, S. Optical micro/nanofiber-based localized surface plasmon resonance biosensors: Fiber diameter dependence. Sensors 2018, 18, 3295. [Google Scholar] [CrossRef] [Green Version]
- Kaňok, R.; Ciprian, D.; Hlubina, P. Surface Plasmon Resonance-Based Sensing Utilizing Spatial Phase Modulation in an Imaging Interferometer. Sensors 2020, 20, 1616. [Google Scholar] [CrossRef] [Green Version]
- Puiu, M.; Bala, C. SPR and SPR imaging: Recent trends in developing nanodevices for detection and real-time monitoring of biomolecular events. Sensors 2016, 16, 870. [Google Scholar] [CrossRef]
- Chen, S.; Liu, C.; Liu, Y.; Liu, Q.; Lu, M.; Bi, S.; Jing, Z.; Peng, W. Label-Free Near-Infrared Plasmonic Sensing Technique for DNA Detection at Ultralow Concentrations. Adv. Sci. 2020, 7, 2000763. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; Li, X.; Zhou, H.; Hong, X.; Geng, Y. Fiber-optic urine specific gravity sensor based on surface plasmon resonance. Sens. Actuators B Chem. 2016, 226, 412–418. [Google Scholar] [CrossRef]
- Wang, W.; Mai, Z.; Chen, Y.; Wang, J.; Li, L.; Su, Q.; Li, X.; Hong, X. A label-free fiber optic SPR biosensor for specific detection of C-reactive protein. Sci. Rep. 2017, 7, 16904. [Google Scholar] [CrossRef]
- Mai, Z.; Zhang, J.; Chen, Y.; Wang, J.; Hong, X.; Su, Q.; Li, X. A disposable fiber optic SPR probe for immunoassay. Biosens. Bioelectron. 2019, 144, 111621. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Liu, Q.; Masson, J.F.; Peng, W. Compact multi-channel surface plasmon resonance sensor for real-time multi-analyte biosensing. Opt. Express 2015, 23, 20540–20548. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Liu, Q.; Liu, Z.; Peng, W. Simple method for self-referenced and lable-free biosensing by using a capillary sensing element. Opt. Express 2017, 25, 11750–11759. [Google Scholar] [CrossRef]
- Ding, S.; Su, C.; Yu, J. An optimizing BP neural network algorithm based on genetic algorithm. Artif. Intell. Rev. 2011, 36, 153–162. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Chen, S.; Cheng, F.; Wang, H.; Peng, W. Surface plasmon resonance biosensor based on smart phone platforms. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Liu, Y.; Chen, S.; Wang, F.; Peng, W. A low-cost and portable dual-channel fiber optic surface plasmon resonance system. Sensors 2017, 17, 2797. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, C. Color image to grayscale image conversion. In Proceedings of the 2010 Second International Conference on Computer Engineering and Applications, Bali, Indonesia, 19–21 March 2010. [Google Scholar]
- Al-Amri, S.S.; Kalyankar, N.V.; Khamitkar, S.D. Image segmentation by using edge detection. Int. J. Comput. Sci. Eng. 2010, 2, 804. [Google Scholar]
- MacDougall, D.; Crummett, W.B. Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal. Chem. 1980, 52, 2242–2249. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of detection. A closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar]
- Coelho, L.; de Almeida, J.M.M.M.; Santos, J.L.; Ferreira, R.A.S.; André, P.S.; Viegas, D. Sensing structure based on surface plasmon resonance in chemically etched single mode optical fibres. Plasmonics 2015, 10, 319–327. [Google Scholar] [CrossRef]
- Dhara, P.; Singh, V.K.; Olivero, M.; Perrone, G. Reflectance-based low-cost disposable optical fiber surface plasmon resonance probe with enhanced biochemical sensitivity. Opt. Eng. 2016, 55, 046114. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Lang, T.; Cao, B.; Jin, J.; Dong, R.; Feng, H. Fiber optic SPR sensor for human Immunoglobulin G measurement based on the MMF-NCF-MMF structure. Opt. Fiber Technol. 2018, 46, 179–185. [Google Scholar] [CrossRef]
- Shi, S.; Wang, L.; Su, R.; Liu, B.; Huang, R.; Qi, W.; He, Z. A polydopamine-modified optical fiber SPR biosensor using electroless-plated gold films for immunoassays. Biosens. Bioelectron. 2015, 74, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, Y.; Wu, Q.; Ma, P.; Zhang, H.; Wang, Y.; Song, D. Enhancing sensitivity of surface plasmon resonance biosensor by Ag nanocubes/chitosan composite for the detection of mouse IgG. Talanta 2016, 146, 364–368. [Google Scholar] [CrossRef]
- Lang, T.; Cao, B.; Shen, C.; Shi, G. Multimode-coreless-multimode fiber biosensor based on surface plasmon resonance. J. Phys. D Appl. Phys. 2019, 52, 195204. [Google Scholar] [CrossRef]


| Strategy | Resolution | LOD of IgG | Ref. |
|---|---|---|---|
| Chemically etched, single-mode fiber probe | 1.6 × 10−3 RIU | [34] | |
| Dual-Channel SPR System | 4.3 × 10−4 RIU | [29] | |
| Reflectance-based disposable fiber probe | 9.3 × 10−5 RIU | [35] | |
| Smart phone SPR biosensor | 7.4 × 10−5 RIU | 47.4 nM | [28] |
| MMF-NCF-MMF structure | 11.7 nM | [36] | |
| A polydopamine-modified FO-SPR biosensor | 6.0 nM | [37] | |
| Ag nanocubes/chitosan composite | 4.0 nM | [38] | |
| Multimode-coreless-multimode fiber probe | 3.1 nM | [39] | |
| Miniature commercial SPR instrument | 2.7 × 10−5 RIU | 15.7 nM | Biosuplar6 |
| FO-SPR based on (BP) neural network | 6.3 × 10−5 RIU | 0.9 nM | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wu, H.; Song, Y.; Peng, W.; Liu, Y. A Fiber-Optic Surface Plasmon Resonance Sensor for Bio-Detection in Visible to Near-Infrared Images. Biosensors 2022, 12, 9. https://doi.org/10.3390/bios12010009
Chen S, Wu H, Song Y, Peng W, Liu Y. A Fiber-Optic Surface Plasmon Resonance Sensor for Bio-Detection in Visible to Near-Infrared Images. Biosensors. 2022; 12(1):9. https://doi.org/10.3390/bios12010009
Chicago/Turabian StyleChen, Shimeng, Haojun Wu, Yongxin Song, Wei Peng, and Yun Liu. 2022. "A Fiber-Optic Surface Plasmon Resonance Sensor for Bio-Detection in Visible to Near-Infrared Images" Biosensors 12, no. 1: 9. https://doi.org/10.3390/bios12010009
APA StyleChen, S., Wu, H., Song, Y., Peng, W., & Liu, Y. (2022). A Fiber-Optic Surface Plasmon Resonance Sensor for Bio-Detection in Visible to Near-Infrared Images. Biosensors, 12(1), 9. https://doi.org/10.3390/bios12010009




