A Review of Recent Advances in Flexible Wearable Sensors for Wound Detection Based on Optical and Electrical Sensing
Abstract
:1. Introduction
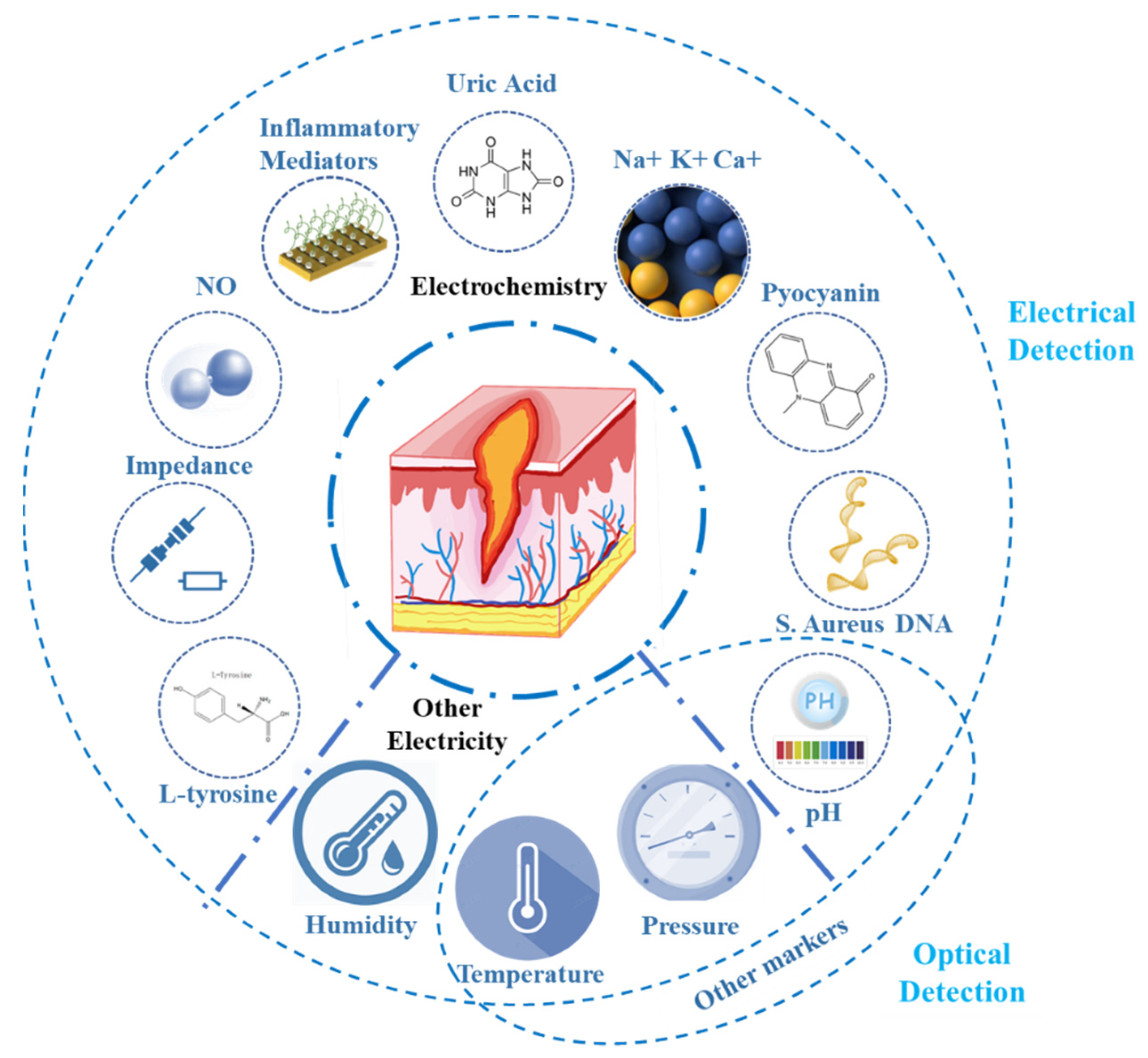
2. Key Markers of Wounds and Their Physiological Significance
2.1. The Physiological Significance of Biochemical Markers in Wounds
2.1.1. The Physiological Significance of Uric Acid in Wounds
2.1.2. The Physiological Significance of pH in Wounds
2.1.3. The Physiological Significance of Other Biochemical Markers in Wounds
2.2. The Physiological Significance of Physical Parameters in Wounds
3. Key Wound Marker Detection
3.1. Electrical Detection of Wound Markers
3.1.1. Electrochemical Detection
The Detection of Uric Acid
The Detection of pH
The Detection of Skin and Wound Impedance
The Detection of Other Biomarkers
The Detection of Multiple Parameters
3.1.2. Other Electrical Detection
3.2. Optical Detection of Wound Markers
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFU | diabetic foot ulcer |
| ATP | adenosine triphosphate |
| S. aureus | Staphylococcus aureus |
| P. aeruginosa | Pseudomonas aeruginosa |
| UOx | uricase |
| EIS | electrochemical impedance spectroscopy |
| PB | Prussian blue |
| FCA | ferrocene carboxylic acid |
| MWCNTs | multiwalled carbon nanotubes |
| AuNPs | Au nanoparticles |
| HRP | horseradish peroxidase |
| LGG | laser-guided graphene |
| PDMS | polydimethylsiloxane |
| IPA | iso-propyl alcohol |
| CNT | carbon nanotube |
| PA | polyacrylamide |
| PANI | polyaniline |
| p-BC | pyrolyzed bacterial cellulose |
| PEDOT | poly(3,4ethylenedioxythiophene) |
| PSS | poly (styrene sulfonate) |
| WSI | wound status index |
| PA/CNT | polyacrylamide-coated carbon nanotube |
| HPLC | high-performance liquid chromatography |
| rGO | reduced graphene oxide |
| PEDOT:PSS | poly (3,4-ethylenedioxythiophene) polystyrene sulfonate |
| PU | polyurethane |
| NIPAAm | N-isopropyl acrylamide |
| MPBA | methylacrylamide phenyl boric acid |
| BSA | bovine serum albumin |
| PET | polyethylene terephthalate |
| PI | polyimide |
| TINT | TiO2 nanotube |
| AgNW | silver nanowire |
| O-CDs | orange-emissive carbon quantum dots |
| CQDs | carbon quantum dots |
| NFC | near-field communication |
| VTFs | reversible thermochromic fibers |
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Clayton, W.; Elasy, T.A. A Review of the Pathophysiology, Classification, and Treatment of Foot Ulcers in Diabetic Patients. Clin. Diabetes 2009, 27, 52. [Google Scholar] [CrossRef] [Green Version]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health Care-Associated Infections A Meta-analysis of Costs and Financial Impact on the US Health Care System. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef]
- Phillips, C.J.; Humphreys, I.; Fletcher, J.; Harding, K.; Chamberlain, G.; Macey, S. Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int. Wound J. 2016, 13, 1193–1197. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef]
- Williams, J.Z.; Barbul, A. Nutrition and wound healing. Surg. Clin. N. Am. 2003, 83, 571–596. [Google Scholar] [CrossRef]
- Serra, M.B.; Barroso, W.A.; Silva, N.N.D.; Silva, S.D.N.; Borges, A.C.R.; Abreu, I.C.; Borges, M.O.D.R. From Inflammation to Current and Alternative Therapies Involved in Wound Healing. Int. J. Inflamm. 2017, 2017, 3406215. [Google Scholar] [CrossRef] [Green Version]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Tonnesen, M.G.; Feng, X.; Clark, R.A.F. Angiogenesis in Wound Healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Menke, N.B.; Ward, K.R.; Witten, T.M.; Bonchev, D.G.; Diegelmann, R.F. Impaired wound healing. Clin. Dermatol. 2007, 25, 19–25. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Mehmood, N.; Hariz, A.; Fitridge, R.; Voelcker, N.H. Applications of modern sensors and wireless technology in effective wound management. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 885–895. [Google Scholar] [CrossRef]
- Liu, Y.C.; Margolis, D.J.; Rivkah Isseroff, R. Does Inflammation Have a Role in the Pathogenesis of Venous Ulcers?: A Critical Review of the Evidence. J. Investig. Dermatol. 2011, 131, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.R.; Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef]
- Wagner, D.R.; Jeter, K.F.; Tintle, T.; Martin, M.S.; Long, J.M., 3rd. Bioelectrical impedance as a discriminator of pressure ulcer risk. Adv. Wound Care J. Prev. Health 1996, 9, 30–37. [Google Scholar]
- Mao, N.; Russell, S.J. Nonwoven Wound Dressings. Text. Prog. 2004, 36, 1–57. [Google Scholar] [CrossRef]
- Abdelrahman, T.; Newton, H. Wound dressings: Principles and practice. Surgery 2011, 29, 491–495. [Google Scholar] [CrossRef]
- Seaman, S. Dressing selection in chronic wound management. J. Am. Podiatr. Med. Assoc. 2002, 92, 24–33. [Google Scholar] [CrossRef]
- Cutting, K.F. Wound dressings: 21st century performance requirements. J. Wound Care 2010, 19, 4–9. [Google Scholar] [CrossRef]
- Barros Almeida, I.; Garcez Barretto Teixeira, L.; Oliveira de Carvalho, F.; Ramos Silva, E.; Santos Nunes, P.; Viana Dos Santos, M.R.; Antunes de Souza Araujo, A. Smart Dressings for Wound Healing: A Review. Adv. Ski. Wound Care 2021, 34, 1–8. [Google Scholar] [CrossRef]
- Xu, G.; Lu, Y.; Cheng, C.; Li, X.; Xu, J.; Liu, Z.; Liu, J.; Liu, G.; Shi, Z.; Chen, Z.; et al. Battery-Free and Wireless Smart Wound Dressing for Wound Infection Monitoring and Electrically Controlled On-Demand Drug Delivery. Adv. Funct. Mater. 2021, 31, 2100852. [Google Scholar] [CrossRef]
- McLister, A.; McHugh, J.; Cundell, J.; Davis, J. New Developments in Smart Bandage Technologies for Wound Diagnostics. Adv. Mater 2016, 28, 5732–5737. [Google Scholar] [CrossRef]
- McLister, A.; Phair, J.; Cundell, J.; Davis, J. Electrochemical approaches to the development of smart bandages: A mini-review. Electrochem. Commun. 2014, 40, 96–99. [Google Scholar] [CrossRef]
- McLister, A.; Mathur, A.; Davis, J. Wound diagnostics: Deploying electroanalytical strategies for point of care sensors and smart dressings. Curr. Opin. Electrochem. 2017, 3, 40–45. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Ma, X.; Zheng, J.; Zheng, J.; Wu, A. Nanoparticle-Based Wound Dressing: Recent Progress in the Detection and Therapy of Bacterial Infections. Bioconjug. Chem. 2020, 31, 1708–1723. [Google Scholar] [CrossRef]
- Gong, M.; Zhang, L.; Wan, P. Polymer nanocomposite meshes for flexible electronic devices. Prog. Polym. Sci. 2020, 107, 101279. [Google Scholar] [CrossRef]
- Scott, C.; Cameron, S.; Cundell, J.; Mathur, A.; Davis, J. Adapting resistive sensors for monitoring moisture in smart wound dressings. Curr. Opin. Electrochem. 2020, 23, 31–35. [Google Scholar] [CrossRef]
- Tang, N.; Zheng, Y.; Cui, D.; Haick, H. Multifunctional Dressing for Wound Diagnosis and Rehabilitation. Adv. Healthc Mater 2021, 10, e2101292. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Jimenez, M.A.; Aguilar-Garcia, J.; Valdes-Rodriguez, R.; Metlich-Medlich, M.A.; Dietsch, L.J.P.; Gaitan-Gaona, F.I.; Kolosovas-Machuca, E.S.; Gonzalez, F.J.; Sanchez-Aguilar, J.M. Local Use of Insulin in Wounds of Diabetic Patients: Higher Temperature, Fibrosis, and Angiogenesis. Plast. Reconstr. Surg. 2013, 132, 1015E–1019E. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.V.; Perelshtein, I.; Perkas, N.; Gedanken, A.; Cunha, J.; Cavaco-Paulo, A. Detection of human neutrophil elastase (HNE) on wound dressings as marker of inflammation. Appl. Microbiol. Biotechnol. 2017, 101, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and characterization of immobilized antibodies for improved immunoassays (Review). Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.-T.; Li, Y.; Gu, J.; Wang, H.; Zhu, Z.; Hong, X.; Zhang, Z.; Lu, Q.; Qiu, J.; Wang, X.; et al. A Conductive Nanowire-Mesh Biosensor for Ultrasensitive Detection of Serum C-Reactive Protein in Melanoma. Adv. Funct. Mater. 2018, 28, 1802482. [Google Scholar] [CrossRef]
- James, T.J.; Hughes, M.A.; Cherry, G.W.; Taylor, R.P. Evidence of oxidative stress in chronic venous ulcers. Wound Repair Regen. 2003, 11, 172–176. [Google Scholar] [CrossRef]
- McCord, J.M. Oxygen-derived free-radicals in postischemic tissue-injury. Clin. Chem. 1989, 35, 1049–1050. [Google Scholar]
- Fernandez, M.L.; Upton, Z.; Edwards, H.; Finlayson, K.; Shooter, G.K. Elevated uric acid correlates with wound severity. Int. Wound J. 2012, 9, 139–149. [Google Scholar] [CrossRef]
- Wlaschek, M.; Scharffetter-Kochanek, K. Oxidative stress in chronic venous leg ulcers. Wound Repair Regen. 2005, 13, 452–461. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Upton, Z.; Shooter, G.K. Uric Acid and Xanthine Oxidoreductase in Wound Healing. Curr. Rheumatol. Rep. 2014, 16. [Google Scholar] [CrossRef]
- Sismaet, H.J.; Pinto, A.J.; Goluch, E.D. Electrochemical sensors for identifying pyocyanin production in clinical Pseudomonas aeruginosa isolates. Biosens. Bioelectron. 2017, 97, 65–69. [Google Scholar] [CrossRef]
- Trengove, N.J.; Langton, S.R.; Stacey, M.C. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair Regen. 1996, 4, 234–239. [Google Scholar] [CrossRef]
- Basu, P.; Kim, J.H.; Saeed, S.; Martins-Green, M. Using systems biology approaches to identify signalling pathways activated during chronic wound initiation. Wound Repair Regen. 2021, 29, 881–898. [Google Scholar] [CrossRef]
- Rouf, M.A.; Lomprey, R.F. Degradation of uric acid by certain aerobic bacteria. J. Bacteriol. 1968, 96, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.F. Nitrogen requirements and uricolytic activity of cutaneous bacteria. Appl. Microbiol. 1970, 19, 643–648. [Google Scholar] [CrossRef]
- Bongaerts, G.P.A.; Sin, I.L.; Peters, A.L.J.; Vogels, G.D. Purine degradation in Pseudomonas aeruginosa and Pseudomonas testosteroni. Biochim. Biophys. Acta Gen. Subj. 1977, 499, 111–118. [Google Scholar] [CrossRef]
- Abdel-Fattah, Y.R.; Saeed, H.M.; Gohar, Y.M.; El-Baz, M.A. Improved production of Pseudomonas aeruginosa uricase by optimization of process parameters through statistical experimental designs. Process. Biochem. 2005, 40, 1707–1714. [Google Scholar] [CrossRef]
- Adamek, V.; Kralova, B.; Suchova, M.; Valentova, O.; Demnerova, K. PURIFICATION OF MICROBIAL URICASE. J. Chromatogr. -Biomed. Appl. 1989, 497, 268–275. [Google Scholar]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 48, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, R.; Ochoa, M.; Tarnayol, A.; Khalili, S.; Khademhosseini, A.; Ziaie, B. Highly Stretchable Potentiometric pH Sensor Fabricated via Laser Carbonization and Machining of Carbon-Polyaniline Composite. Acs Appl. Mater. Interfaces 2017, 9, 9015–9023. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Kekonen, A.; Bergelin, M.; Johansson, M.; Joon, N.K.; Bobacka, J.; Viik, J. Bioimpedance Sensor Array for Long-Term Monitoring of Wound Healing from Beneath the Primary Dressings and Controlled Formation of H2O2 Using Low-Intensity Direct Current. Sensors 2019, 19, 2505. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of pH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef]
- Kuo, S.H.; Shen, C.J.; Shen, C.F.; Cheng, C.M. Role of pH Value in Clinically Relevant Diagnosis. Diagnostics 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Greener, B.; Hughes, A.A.; Bannister, N.P.; Douglass, J. Proteases and pH in chronic wounds. J. Wound Care 2005, 14, 59–61. [Google Scholar] [CrossRef]
- Leveen, H.H.; Falk, G.; Borek, B.; Diaz, C.; Lynfield, Y.; Wynkoop, B.J.; Mabunda, G.A.; Rubricius, J.L.; Christoudias, G.C. Chemical acidification of wounds—Adjuvant to healing and unfavorable action of alkalinity and ammonia. Ann. Surg. 1973, 178, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, D.; Eswaramoorthy, S.; Furey, W.; Sax, M.; Swaminathan, S. Structure of staphylococcal enterotoxin C2 at various pH levels. Acta Crystallogr. Sect. D 2001, 57, 1270–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alatraktchi, F.A.; Johansen, H.K.; Molin, S.; Svendsen, W.E. Electrochemical sensing of biomarker for diagnostics of bacteria-specific infections. Nanomedicine 2016, 11, 2185–2195. [Google Scholar] [CrossRef] [Green Version]
- Kassal, P.; Kim, J.; Kumar, R.; de Araujo, W.R.; Steinberg, I.M.; Steinberg, M.D.; Wang, J. Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status. Electrochem. Commun. 2015, 56, 6–10. [Google Scholar] [CrossRef]
- Dowd, S.E.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Smith, E.; Rhoads, D. Polymicrobial Nature of Chronic Diabetic Foot Ulcer Biofilm Infections Determined Using Bacterial Tag Encoded FLX Amplicon Pyrosequencing (bTEFAP). PLoS ONE 2008, 3, e3326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.J.; Forbes, A.; Perkins, A.V.; Davey, A.K.; Chess-Williams, R.; Kiefel, M.J.; Arora, D.; et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins 2016, 8, 236. [Google Scholar] [CrossRef]
- McGhee, J.R.; Denning Gerene, M.; Wollenweber Laura, A.; Railsback Michelle, A.; Cox Charles, D.; Stoll Lynn, L.; Britigan Bradley, E. Pseudomonas Pyocyanin Increases Interleukin-8 Expression by Human Airway Epithelial Cells. Infect. Immun. 1998, 66, 5777–5784. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, L.E.P.; Price-Whelan, A.; Petersen, A.; Whiteley, M.; Newman, D.K. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 2006, 61, 1308–1321. [Google Scholar] [CrossRef]
- Römling, U. Innate Immune Mechanisms with a Focus on Small-Molecule Microbe-Host Cross Talk. J. Innate Immun. 2019, 11, 191–192. [Google Scholar] [CrossRef]
- Usher, L.R.; Lawson, R.A.; Geary, I.; Taylor, C.J.; Bingle, C.D.; Taylor, G.W.; Whyte, M.K.B. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: A potential mechanism of persistent infection. J. Immunol. 2002, 168, 1861–1868. [Google Scholar] [CrossRef] [Green Version]
- Mavrodi, D.V.; Blankenfeldt, W.; Thomashow, L.S. Phenazine Compounds in Fluorescent Pseudomonas Spp. Biosynthesis and Regulation. Annu. Rev. Phytopathol. 2006, 44, 417–445. [Google Scholar] [CrossRef]
- Pierson, L.S.; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Jarosova, R.; McClure, S.E.; Gajda, M.; Jovic, M.; Girault, H.H.; Lesch, A.; Maiden, M.; Waters, C.; Swain, G.M. Inkjet-Printed Carbon Nanotube Electrodes for Measuring Pyocyanin and Uric Acid in a Wound Fluid Simulant and Culture Media. Anal. Chem. 2019, 91, 8835–8844. [Google Scholar] [CrossRef]
- Roy, S.; Bisaria, K.; Nagabooshanam, S.; Selvam, A.; Chakrabarti, S.; Wadhwa, S.; Singh, R.; Mathur, A.; Davis, J. An Electroanalytical Paper-Based Wound Dressing Using ZIF-67/C3 N4 Nanocomposite Towards the Monitoring of Staphylococcus aureus in Diabetic Foot Ulcer. IEEE Sens. J. 2021, 21, 1215–1221. [Google Scholar] [CrossRef]
- Kundu, Z.S.; Tanwar, M.; Singh, K.; Singh, B. Clinical assessment, risk factors and classification of diabetic foot: An overview. J. Foot Ankle Surg. 2017, 4, 35–39. [Google Scholar]
- Brown, M.S.; Ashley, B.; Koh, A. Wearable Technology for Chronic Wound Monitoring: Current Dressings, Advancements, and Future Prospects. Front. Bioeng. Biotechnol. 2018, 6, 47. [Google Scholar] [CrossRef]
- Waite, R.D.; Stewart, J.E.; Stephen, A.S.; Allaker, R.P. Activity of a nitric oxide-generating wound treatment system against wound pathogen biofilms. Int. J. Antimicrob. Agents 2018, 52, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Duay, J.; Simoska, O.; Shear, J.B.; Stevenson, K.J. Gold Nanoparticle Modified Transparent Carbon Ultramicroelectrode Arrays for the Selective and Sensitive Electroanalytical Detection of Nitric Oxide. Anal. Chem. 2017, 89, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
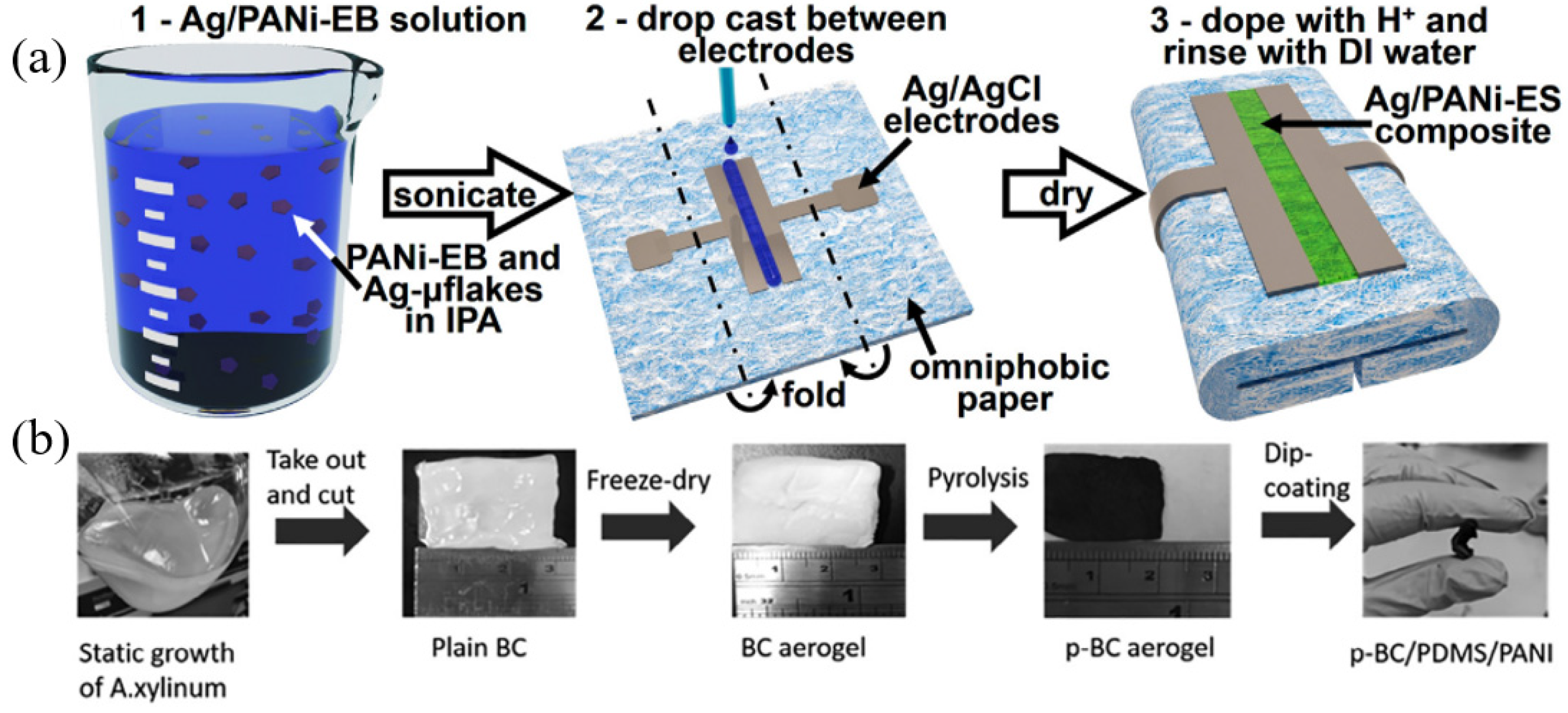
- Pal, A.; Goswami, D.; Cuellar, H.E.; Castro, B.; Kuang, S.; Martinez, R.V. Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages. Biosens. Bioelectron. 2018, 117, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Simoska, O.; Duay, J.; Stevenson, K.J. Electrochemical Detection of Multianalyte Biomarkers in Wound Healing Efficacy. ACS Sens. 2020, 5, 3547–3557. [Google Scholar] [CrossRef]
- Barraud, N.; Hassett Daniel, J.; Hwang, S.-H.; Rice Scott, A.; Kjelleberg, S.; Webb Jeremy, S. Involvement of Nitric Oxide in Biofilm Dispersal of Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7344–7353. [Google Scholar] [CrossRef] [Green Version]
- Arora, D.P.; Hossain, S.; Xu, Y.; Boon, E.M. Nitric Oxide Regulation of Bacterial Biofilms. Biochemistry 2015, 54, 3717–3728. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Sun, T.; Zeng, D.; Yang, C.; Wang, H.; Yang, C.; Guo, J.; Wu, Q.; Chen, H.J.; et al. Integrated Multiplex Sensing Bandage for In Situ Monitoring of Early Infected Wounds. ACS Sens. 2021, 6, 3112–3124. [Google Scholar] [CrossRef]
- Gao, Y.J.; Nguyen, D.T.; Yeo, T.; Lim, S.B.; Tan, W.X.; Madden, L.E.; Jin, L.; Long, J.; Aloweni, F.A.; Liew, Y.J.A.; et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 2021, 7, eabg9614. [Google Scholar] [CrossRef]
- Bass, M.J.; Phillips, L.G. Pressure Sores. Curr. Probl. Surg. 2007, 44, 101–143. [Google Scholar] [CrossRef]
- Ayello, E.A.; Lyder, C.H. A new era of pressure ulcer accountability in acute care. Adv. Ski. Wound Care 2008, 21, 134–140, quiz 140–132. [Google Scholar] [CrossRef]
- Honda, W.; Harada, S.; Arie, T.; Akita, S.; Takei, K. Wearable, Human-Interactive, Health-Monitoring, Wireless Devices Fabricated by Macroscale Printing Techniques. Adv. Funct. Mater. 2014, 24, 3299–3304. [Google Scholar] [CrossRef]
- Wong, V. Skin Blood Flow Response to 2-Hour Repositioning in Long-term Care Residents A Pilot Study. J. Wound Ostomy Cont. Nurs. 2011, 38, 529–537. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Moore, M. Bioelectrical impedance assessment of wound healing. J. Diabetes Sci. Technol. 2012, 6, 209–212. [Google Scholar] [CrossRef] [Green Version]
- Wijlens, A.M.; Holloway, S.; Bus, S.A.; van Netten, J.J. An explorative study on the validity of various definitions of a 2 center dot 2 degrees C temperature threshold as warning signal for impending diabetic foot ulceration. Int. Wound J. 2017, 14, 1346–1351. [Google Scholar] [CrossRef] [Green Version]
- Gaiziunas, A.G.; Hast, M.H. Temperature gradients and prediction of flap viability. J. Otolaryngol. 1976, 5, 399–402. [Google Scholar]
- Wilmore, D.W.; Aulick, L.H.; Mason, A.D.; Pruitt, B.A. Influence of burn wound on local and systemic responses to injury. Ann. Surg. 1977, 186, 444–458. [Google Scholar] [CrossRef]
- Cutting, K.F.; White, R.J. Maceration of the skin and wound bed 1: Its nature and causes. J. Wound Care 2002, 11, 275–278. [Google Scholar] [CrossRef]
- McGill, S.L.; Yung, Y.; Hunt, K.A.; Henson, M.A.; Hanley, L.; Carlson, R.P. Pseudomonas aeruginosa reverse diauxie is a multidimensional, optimized, resource utilization strategy. Sci. Rep. 2021, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.G.; Morris, H.L.; Patel, G.K. Science, medicine, and the future—Healing chronic wounds. Br. Med. J. 2002, 324, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Lee, J.; Lewis, S.W.; Silvester, D.S. Detection of 2,4,6-Trinitrotoluene Using a Miniaturized, Disposable Electrochemical Sensor with an Ionic Liquid Gel-Polymer Electrolyte Film. Anal. Chem. 2017, 89, 4729–4736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, D.; Forsythe, S.; Davis, J. Carbon fibre composites: Integrated electrochemical sensors for wound management. J. Biochem. 2008, 144, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Sharp, D.; Davis, J. Integrated urate sensors for detecting wound infection. Electrochem. Commun. 2008, 10, 709–713. [Google Scholar] [CrossRef]
- Phair, J.; Leach, C.P.; Cardosi, M.F.; Davis, J. Atmospheric pressure plasma treated carbon fibre weave: A flexible approach to wound monitoring. Electrochem. Commun. 2013, 33, 99–101. [Google Scholar] [CrossRef]
- Phair, J.; Joshi, M.; Benson, J.; McDonald, D.; Davis, J. Laser patterned carbon–polyethylene mesh electrodes for wound diagnostics. Mater. Chem. Phys. 2014, 143, 991–995. [Google Scholar] [CrossRef]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- RoyChoudhury, S.; Umasankar, Y.; Hutcheson, J.D.; Lev-Tov, H.A.; Kirsner, R.S.; Bhansali, S. Uricase Based Enzymatic Biosensor for Non-invasive Detection of Uric Acid by Entrapment in PVA-SbQ Polymer Matrix. Electroanalysis 2018, 30, 2374–2385. [Google Scholar] [CrossRef]
- Bhushan, P.; Umasankar, Y.; RoyChoudhury, S.; Hirt, P.A.; MacQuhaec, F.E.; Borda, L.J.; Lev-Tov, H.A.; Kirsner, R.S.; Bhansali, S. Biosensor for Monitoring Uric Acid in Wound and Its Proximity: A Potential Wound Diagnostic Tool. J. Electrochem. Soc. 2019, 166, B830–B836. [Google Scholar] [CrossRef]
- Liu, X.; Lillehoj, P.B. Embroidered electrochemical sensors on gauze for rapid quantification of wound biomarkers. Biosens. Bioelectron. 2017, 98, 189–194. [Google Scholar] [CrossRef]
- Sharifuzzaman, M.; Chhetry, A.; Zahed, M.A.; Yoon, S.H.; Park, C.I.; Zhang, S.; Chandra Barman, S.; Sharma, S.; Yoon, H.; Park, J.Y. Smart bandage with integrated multifunctional sensors based on MXene-functionalized porous graphene scaffold for chronic wound care management. Biosens. Bioelectron. 2020, 169, 112637. [Google Scholar] [CrossRef]
- Bartlett, P.N. Modified electrode surface in amperometric biosensors. Med. Biol. Eng. Comput. 1990, 28, B10–B17. [Google Scholar] [CrossRef]
- Punjiya, M.; Nejad, H.R.; Mostafalu, P.; Sonkusale, S. pH sensing threads with CMOS readout for Smart Bandages. In Proceedings of the IEEE International Symposium on Circuits and Systems (ISCAS), Baltimore, MD, USA, 28–31 May 2017. [Google Scholar]
- Jin, Z.; Su, Y.X.; Duan, Y.X. An improved optical pH sensor based on polyaniline. Sens. Actuators B-Chem. 2000, 71, 118–122. [Google Scholar] [CrossRef]
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B.; et al. Smart Bandage for Monitoring and Treatment of Chronic Wounds. Small 2018, 14, e1703509. [Google Scholar] [CrossRef] [Green Version]
- Lyu, B.Y.; Punjiya, M.; Matharu, Z.; Sonkusale, S. An improved pH mapping bandage with thread-based sensors for chronic wound monitoring. In Proceedings of the IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018. [Google Scholar]
- Yang, M.; Choy, K.-l. A nature-derived, flexible and three dimensional (3D) nano-composite for chronic wounds pH monitoring. Mater. Lett. 2021, 288, 129335. [Google Scholar] [CrossRef]
- McLister, A.; Davis, J. Molecular Wiring in Smart Dressings: Opening a New Route to Monitoring Wound pH. Healthcare 2015, 3, 466–477. [Google Scholar] [CrossRef] [Green Version]
- Mariani, F.; Serafini, M.; Gualandi, I.; Arcangeli, D.; Decataldo, F.; Possanzini, L.; Tessarolo, M.; Tonelli, D.; Fraboni, B.; Scavetta, E. Advanced Wound Dressing for Real-Time pH Monitoring. ACS Sens. 2021, 6, 2366–2377. [Google Scholar] [CrossRef]
- Swisher, S.L.; Lin, M.C.; Liao, A.; Leeflang, E.J.; Khan, Y.; Pavinatto, F.J.; Mann, K.; Naujokas, A.; Young, D.; Roy, S.; et al. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat. Commun. 2015, 6, 6575. [Google Scholar] [CrossRef]
- Kekonen, A.; Bergelin, M.; Eriksson, J.E.; Vaalasti, A.; Ylanen, H.; Kielosto, S.; Viik, J. Bioimpedance method for monitoring venous ulcers: Clinical proof-of-concept study. Biosens. Bioelectron. 2021, 178, 112974. [Google Scholar] [CrossRef]
- Kekonen, A.; Bergelin, M.; Eriksson, J.E.; Johansson, M.; Vesa, M.; Viik, J. Long-term monitoring of acute wound healing from beneath the primary wound dressings. In Proceedings of the 16th Biennial Baltic Electronics Conference (BEC), TALTECH Univ Technol, Tallinn, Estonia, 8–10 October 2018. [Google Scholar]
- Pei, X.; Jin, H.; Dong, S.; Lou, D.; Ma, L.; Wang, X.; Cheng, W.; Wong, H. Flexible wireless skin impedance sensing system for wound healing assessment. Vacuum 2019, 168, 108808. [Google Scholar] [CrossRef]
- Gianino, E.; Miller, C.; Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Kanthakumar, K.; Taylor, G.; Tsang, K.W.T.; Cundell, D.R.; Rutman, A.; Smith, S.; Jeffery, P.K.; Cole, P.J.; Wilson, R. Mechanisms of action of Pseudomonas aeruginosa pyocyanin on human ciliary beat in vitro. Infect. Immun. 1993, 61, 2848–2853. [Google Scholar] [CrossRef] [Green Version]
- Muller, M. Premature cellular senescence induced by pyocyanin, a redox-active Pseudomonas aeruginosa toxin. Free Radic. Biol. Med. 2006, 41, 1670–1677. [Google Scholar] [CrossRef]
- Sharp, D.; Gladstone, P.; Smith, R.B.; Forsythe, S.; Davis, J. Approaching intelligent infection diagnostics: Carbon fibre sensor for electrochemical pyocyanin detection. Bioelectrochemistry 2010, 77, 114–119. [Google Scholar] [CrossRef]
- Oyibo, S.O.; Jude, E.B.; Tarawneh, I.; Nguyen, H.C.; Armstrong, D.G.; Harkless, L.B.; Boulton, A.J.M. The effects of ulcer size and site, patient’s age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabet. Med. 2001, 18, 133–138. [Google Scholar] [CrossRef]
- Salvo, P.; Dini, V.; Di Francesco, F.; Romanelli, M. The role of biomedical sensors in wound healing. Wound Med. 2015, 8, 15–18. [Google Scholar] [CrossRef]
- Peat, J.; Garg, U. Determination of Phenylalanine and Tyrosine by High Performance Liquid Chromatography-Tandem Mass Spectrometry. In Clinical Applications of Mass Spectrometry in Biomolecular Analysis: Methods and Protocols; Garg, U., Ed.; Springer: New York, NY, USA, 2016; pp. 219–225. [Google Scholar]
- Letellier, S.; Garnier, J.P.; Spy, J.; Bousquet, B. Determination of the l-DOPA/l-tyrosine ratio in human plasma by high-performance liquid chromatography: Usefulness as a marker in metastatic malignant melanoma. J. Chromatogr. B Biomed. Sci. Appl. 1997, 696, 9–17. [Google Scholar] [CrossRef]
- Roy, S.; John, A.; Nagabooshanam, S.; Mishra, A.; Wadhwa, S.; Mathur, A.; Narang, J.; Singh, J.; Dilawar, N.; Davis, J. Self-aligned TiO2—Photo reduced graphene oxide hybrid surface for smart bandage application. Appl. Surf. Sci. 2019, 488, 261–268. [Google Scholar] [CrossRef]
- Roy, S.; Nagabooshanam, S.; Krishna, K.; Wadhwa, S.; Chauhan, N.; Jain, U.; Kumar, R.; Mathur, A.; Davis, J. Electroanalytical Sensor for Diabetic Foot Ulcer Monitoring with Integrated Electronics for Connected Health Application. Electroanalysis 2020, 32, 2082–2089. [Google Scholar] [CrossRef]
- Bala, J.; Roy, S.; John, A.T.; Wadhwa, S.; Mathur, A.; Singh, D.; Devi, D.; Tripathi, A. Ion beam modified TiO2 nanotubular bio-interface for electrochemical detection of L-tyrosine towards smart bandage application. Colloids Surf. B Biointerfaces 2020, 195, 111239. [Google Scholar] [CrossRef]
- Mannello, F.; Ligi, D.; Canale, M.; Raffetto, J.D. Omics profiles in chronic venous ulcer wound fluid: Innovative applications for translational medicine. Expert Rev. Mol. Diagn. 2014, 14, 737–762. [Google Scholar] [CrossRef]
- Edsberg, L.E.; Wyffels, J.T.; Brogan, M.S.; Fries, K.M. Analysis of the proteomic profile of chronic pressure ulcers. Wound Repair Regen. 2012, 20, 378–401. [Google Scholar] [CrossRef]
- Löffler, M.W.; Schuster, H.; Bühler, S.; Beckert, S. Wound Fluid in Diabetic Foot Ulceration: More Than Just an Undefined Soup? Int. J. Low. Extrem. Wounds 2013, 12, 113–129. [Google Scholar] [CrossRef]
- Descent, P.; Izquierdo, R.; Fayomi, C. Printing of temperature and humidity sensors on flexible substrates for biomedical applications. In Proceedings of the IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018. [Google Scholar]
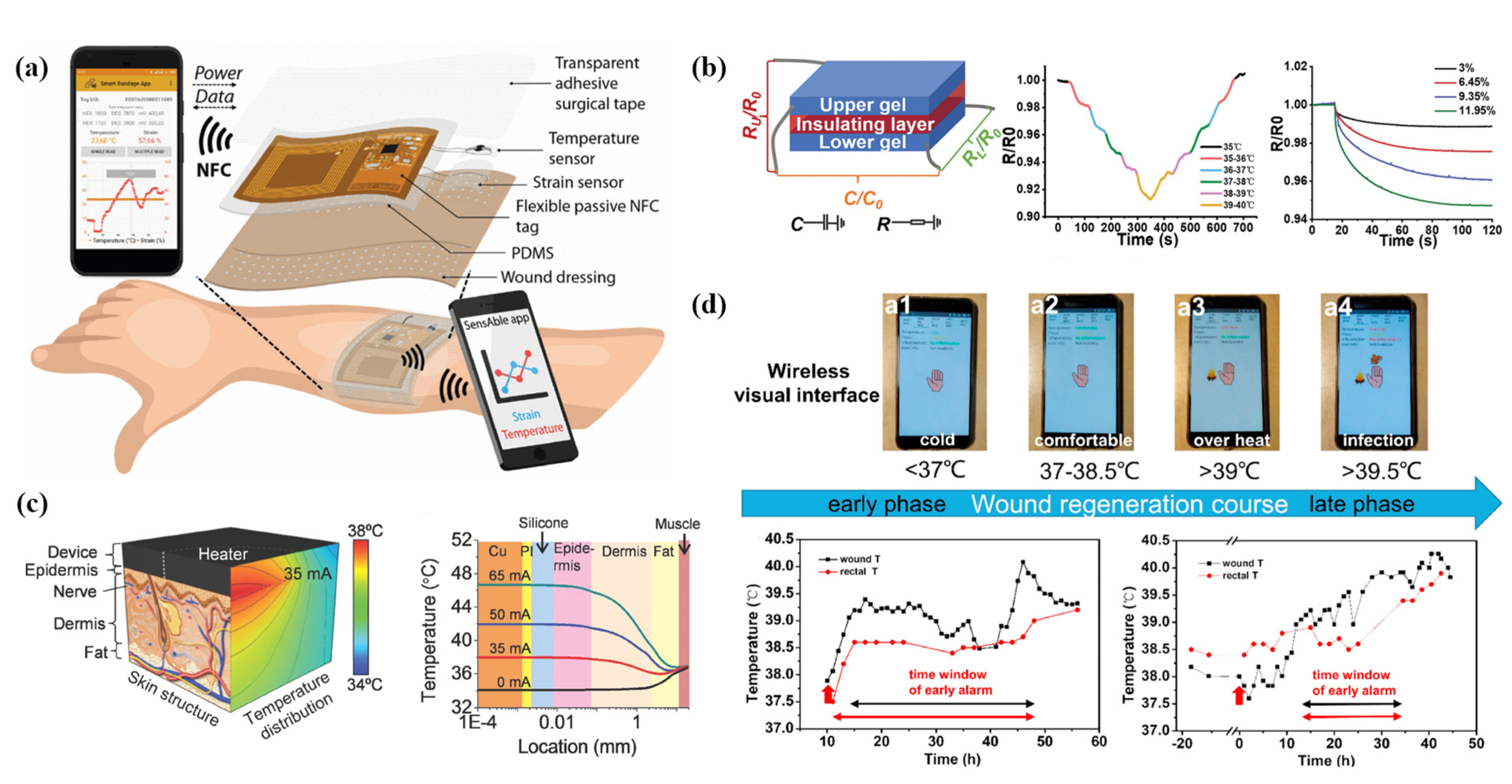
- Escobedo, P.; Bhattacharjee, M.; Nikbakhtnasrabadi, F.; Dahiya, R. Smart Bandage with Wireless Strain and Temperature Sensors and Batteryless NFC Tag. IEEE Internet Things J. 2021, 8, 5093–5100. [Google Scholar] [CrossRef]
- Guo, H.; Bai, M.; Zhu, Y.; Liu, X.; Tian, S.; Long, Y.; Ma, Y.; Wen, C.; Li, Q.; Yang, J.; et al. Pro-Healing Zwitterionic Skin Sensor Enables Multi-Indicator Distinction and Continuous Real-Time Monitoring. Adv. Funct. Mater. 2021, 31, 2106406. [Google Scholar] [CrossRef]
- Hattori, Y.; Falgout, L.; Lee, W.; Jung, S.Y.; Poon, E.; Lee, J.W.; Na, I.; Geisler, A.; Sadhwani, D.; Zhang, Y.; et al. Multifunctional skin-like electronics for quantitative, clinical monitoring of cutaneous wound healing. Adv. Healthc Mater. 2014, 3, 1597–1607. [Google Scholar] [CrossRef]
- Lou, D.; Pang, Q.; Pei, X.; Dong, S.; Li, S.; Tan, W.Q.; Ma, L. Flexible wound healing system for pro-regeneration, temperature monitoring and infection early warning. Biosens. Bioelectron. 2020, 162, 112275. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Manjakkal, L.; Dahiya, R. Bio-organic Glycine based Flexible Piezoelectric Stress Sensor for Wound Monitoring. In Proceedings of the 17th IEEE SENSORS Conference, New Delhi, India, 28–31 October 2018; pp. 1314–1317. [Google Scholar]
- Wang, J.; Jiu, J.; Nogi, M.; Sugahara, T.; Nagao, S.; Koga, H.; He, P.; Suganuma, K. A highly sensitive and flexible pressure sensor with electrodes and elastomeric interlayer containing silver nanowires. Nanoscale 2015, 7, 2926–2932. [Google Scholar] [CrossRef]
- Deng, W.-J.; Wang, L.-F.; Dong, L.; Huang, Q.-A. LC Wireless Sensitive Pressure Sensors With Microstructured PDMS Dielectric Layers for Wound Monitoring. IEEE Sens. J. 2018, 18, 4886–4892. [Google Scholar] [CrossRef]
- Farooq, M.; Iqbal, T.; Vazquez, P.; Farid, N.; Thampi, S.; Wijns, W.; Shahzad, A. Thin-Film Flexible Wireless Pressure Sensor for Continuous Pressure Monitoring in Medical Applications. Sensors 2020, 20, 6653. [Google Scholar] [CrossRef]
- Charkhabi, S.; Jackson, K.J.; Beierle, A.M.; Carr, A.R.; Zellner, E.M.; Reuel, N.F. Monitoring Wound Health through Bandages with Passive LC Resonant Sensors. ACS Sens. 2021, 6, 111–122. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, Z. Preliminary study on the new wound monitoring technology using co-planar waveguide sensor: Modeling and simulation. Technol. Health Care 2021, 29, 463–473. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Zhao, C.; Li, J.; Huang, R.; Zhang, Q.; Li, Y.; Li, X. An Integrated Smart Sensor Dressing for Real-Time Wound Microenvironment Monitoring and Promoting Angiogenesis and Wound Healing. Front Cell Dev. Biol. 2021, 9, 701525. [Google Scholar] [CrossRef]
- Pang, Q.; Lou, D.; Li, S.; Wang, G.; Qiao, B.; Dong, S.; Ma, L.; Gao, C.; Wu, Z. Smart Flexible Electronics-Integrated Wound Dressing for Real-Time Monitoring and On-Demand Treatment of Infected Wounds. Adv. Sci. 2020, 7, 1902673. [Google Scholar] [CrossRef]
- Cho, H.-W.; Jo, S.-H.; Yoon, J.H.; Goh, T.-S.; Choi, B.G.; Yoo, H.-J. A Batteryless Chronic Wound Monitoring System with 13.56-MHz Energy Harvesting. IEEE Sens. J. 2019, 19, 9431–9440. [Google Scholar] [CrossRef]
- Saminathan, J.; Sasikala, M.; Narayanamurthy, V.; Rajesh, K.; Arvind, R. Computer aided detection of diabetic foot ulcer using asymmetry analysis of texture and temperature features. Infrared Phys. Technol. 2020, 105, 103219. [Google Scholar] [CrossRef]
- Gamerith, C.; Luschnig, D.; Ortner, A.; Pietrzik, N.; Guse, J.-H.; Burnet, M.; Haalboom, M.; van der Palen, J.; Heinzle, A.; Sigl, E.; et al. pH-responsive materials for optical monitoring of wound status. Sens. Actuators B Chem. 2019, 301, 126966. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Zhang, T.; Zhang, W.; Chen, W.; Cao, Y.; Chen, M.; Zhou, X. Orange-Emissive Carbon Quantum Dots: Toward Application in Wound pH Monitoring Based on Colorimetric and Fluorescent Changing. Small 2019, 15, e1902823. [Google Scholar] [CrossRef] [PubMed]
- Panzarasa, G.; Osypova, A.; Toncelli, C.; Buhmann, M.T.; Rottmar, M.; Ren, Q.; Maniura-Weber, K.; Rossi, R.M.; Boesel, L.F. The pyranine-benzalkonium ion pair: A promising fluorescent system for the ratiometric detection of wound pH. Sens. Actuators B Chem. 2017, 249, 156–160. [Google Scholar] [CrossRef]
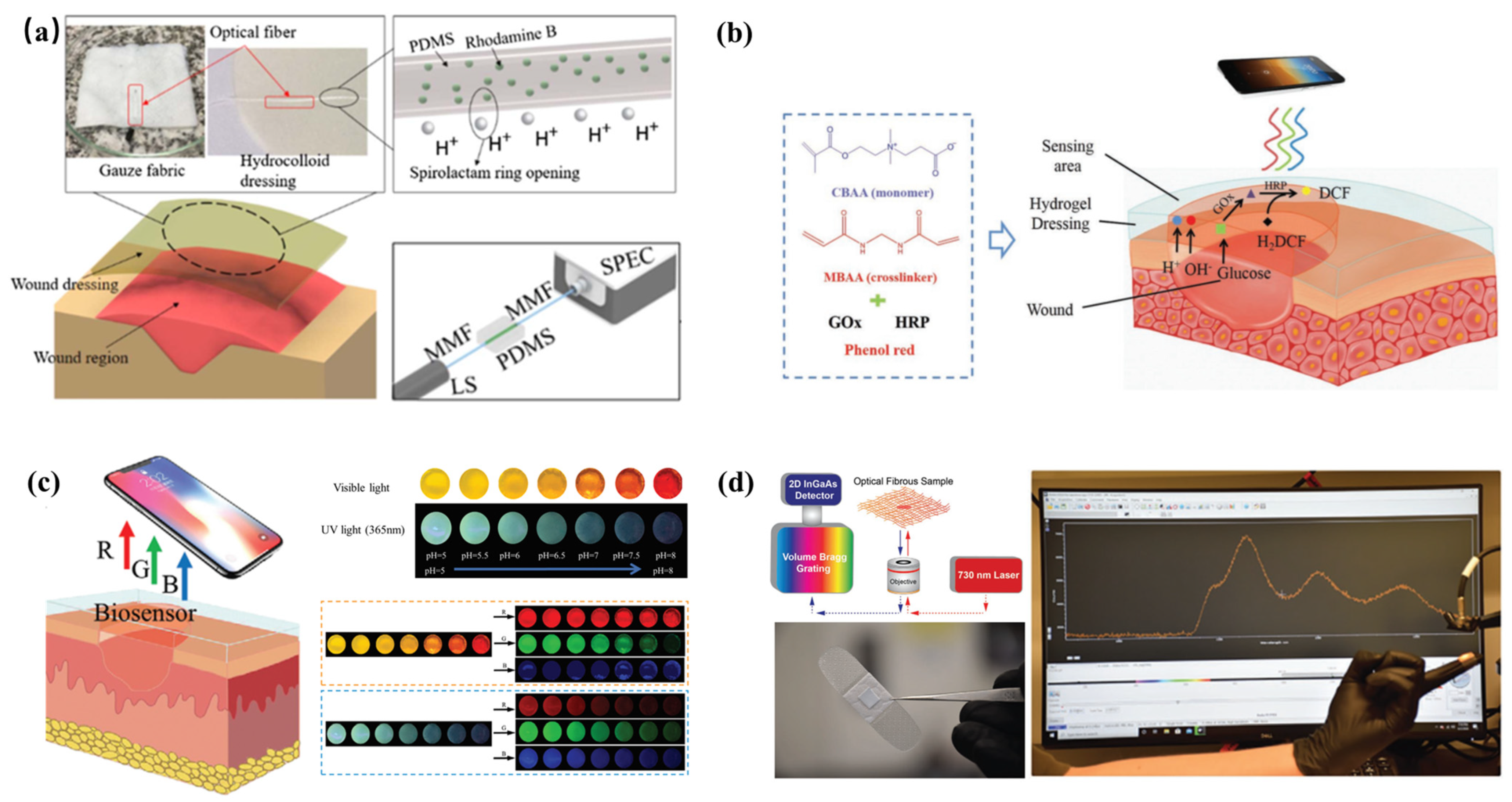
- Leal-Junior, A.; Guo, J.; Min, R.; Fernandes, A.J.; Frizera, A.; Marques, C. Photonic smart bandage for wound healing assessment. Photonics Res. 2021, 9, 272. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Zhang, J.M.; Song, J.Y.; Yang, J.; Du, Z.; Zhao, W.Q.; Guo, H.S.; Wen, C.Y.; Li, Q.S.; Sui, X.J.; et al. A Multifunctional Pro-Healing Zwitterionic Hydrogel for Simultaneous Optical Monitoring of pH and Glucose in Diabetic Wound Treatment. Adv. Funct. Mater. 2020, 30, 1905493. [Google Scholar] [CrossRef]
- Guembe-García, M.; Santaolalla-García, V.; Moradillo-Renuncio, N.; Ibeas, S.; Reglero, J.A.; García, F.C.; Pacheco, J.; Casado, S.; García, J.M.; Vallejos, S. Monitoring of the evolution of human chronic wounds using a ninhydrin-based sensory polymer and a smartphone. Sens. Actuators B Chem. 2021, 335, 129688. [Google Scholar] [CrossRef]
- Zheng, K.; Tong, Y.; Zhang, S.; He, R.; Xiao, L.; Iqbal, Z.; Zhang, Y.; Gao, J.; Zhang, L.; Jiang, L.; et al. Flexible Bicolorimetric Polyacrylamide/Chitosan Hydrogels for Smart Real-Time Monitoring and Promotion of Wound Healing. Adv. Funct. Mater. 2021, 31, 2102599. [Google Scholar] [CrossRef]
- Wang, S.C.; Anderson, J.A.E.; Evans, R.; Woo, K.; Beland, B.; Sasseville, D.; Moreau, L. Point-of-care wound visioning technology: Reproducibility and accuracy of a wound measurement app. PLoS ONE 2017, 12, e0183139. [Google Scholar] [CrossRef] [Green Version]
- Carriere, M.E.; de Haas, L.E.M.; Pijpe, A.; Meij-de Vries, A.; Gardien, K.L.M.; van Zuijlen, P.P.M.; Jaspers, M.E.H. Validity of thermography for measuring burn wound healing potential. Wound Repair Regen. 2020, 28, 347–354. [Google Scholar] [CrossRef]
- Safaee, M.M.; Gravely, M.; Roxbury, D. A Wearable Optical Microfibrous Biomaterial with Encapsulated Nanosensors Enables Wireless Monitoring of Oxidative Stress. Adv. Funct. Mater. 2021, 31, 2006254. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Hu, Z.X.; Xiang, H.X.; Zhai, G.X.; Zhu, M.F. Fabrication of visual textile temperature indicators based on reversible thermochromic fibers. Dye. Pigment. 2019, 162, 705–711. [Google Scholar] [CrossRef]


| Wound Marker | Detection Method | Sensor Material * | Fabrication Method | Feature | Ref. |
|---|---|---|---|---|---|
| Uric acid | SWV | Insulating laminate/carbon fiber mesh/cellulose acetate | Laser etching | Easy integration and stabilization | [97] |
| Uric acid | CV | Insulator/Prussian blue carbon ink/UOx | Screen printing | Wireless communication, applicable to mechanical deformation, good selectivity | [62] |
| Uric acid | CV | Commercial screen-printed carbon electrode/UOx entrapped in PVA-SbQ, FCA | - | Good selectivity and stability, (maintain 90% activity until the 5th day) | [103] |
| Uric acid | CV | Commercial screen-printed carbon electrode/nanocomposite of MWCNTs and AuNPs/UOx, HRP | - | High sensitivity and low detection limit | [104] |
| Uric acid | CV | Gauze/polyester thread soaked in carbon ink/UOx | Embroidery fabrication process | Wearing comfort, soft, good flexibility, and applicable to mechanical deformation | [105] |
| (a) Uric acid, (b) PH, (c) temperature | (a) DPV, (b) potentiometric measurement, (c) thermistor measurement | PDMS/LGG-Mxene/(a) UOx, BSA, (b) PANI | Laser scribing | Multi-marker detection, in-situ detection, smart stretchable, and flexible multifunctional | [106] |
| (a) Uric acid, (b) pH, (c) impedance | (a) Chronocoulometry, (b) impedance measurement, (c) EIS | Whatman #1 paper/(a) carbon/UOx, (b) carbon/silver microflakes and PANI polymeric composite, (c) Ag/AgCl ink/conductive hydrogel | Stencil printing | Low cost, flexible, breathable, multi-marker detection, detachable, and disposable | [78] |
| Uric acid, pyocyanin | SWV | Kapton substrate/carbon nanotube/nanoporous PA hydrogel | Inkjet printing | Good selectivity, repeatability, and service life | [72] |
| pH | Potentiometric measurement | Patch substrate/carbon/PANI | Laser-machining and screen-printing | Support drug release, Bluetooth communication | [110] |
| pH | Potentiometric measurement | Polyester threads/carbon/PANI | Stitching process | Low cost, biocompatible, soft, perception of deep and uneven wounds | [111] |
| pH | Potentiometric measurement | Nanocomposites of p-BC, PDMS and PANI | Pyrolysis aerogel | Lost cost, soft, easy fabrication, and mechanical robust 3D carbon nano-network structures | [112] |
| pH | SWV | Polyester laminate/carbon fiber/poly-l-tryptophan | - | Biocompatible | [113] |
| pH | Voltammetry | Conducting ink/chemically synthesized IrOx particles embedded in a PEDOT:PSS thin film | Screen printing | Good reproducibility, stability, and accuracy | [114] |
| Impedance | EIS | Polyethylene naphthalate substrate/gold nanoparticle ink/hydrogel | Inkjet printing | Flexible, stretchable, mechanical robustness, and in vivo detection of rat models | [115] |
| Impedance | EIS | Thermoplastic polyurethane substrate/silver ink/biomedical grade carbon ink | Screen printing | Long-term monitoring, in vivo detection of human | [55,117] |
| Impedance | EIS | PET/biomedical-grade carbon ink | Screen printing | Clinical applications, long-term-monitoring and sensitive | [116] |
| Impedance | EIS | PI substrate/Cu film/PDMS | Magnetron sputtering | Accurate, reliable, wireless communication, and in vivo detection of pig models | [118] |
| Pyocyanin | SWV | Insulating polyester sheath/carbon fiber | Laser-etched | Application to aerobic and anaerobic environments | [122] |
| Uric acid, pyocyanin, NO | SWV | Poly(ethylene terephthalate) (PET) substrate/layers of pyrolyzed photoresist film | Electron beam deposition, atomic layer deposition | Flexible, good selectivity, and multi-marker detection | [79] |
| the DNA molecules of S. aureus | EIS | Cellulose paper/carbon ink/composite of zeolitic imidazolate framework (ZIF 67) and carbon nitride (C3N4) conjugated with Staphylococcus aureus probe DNA | Screen printing | Cost-effective, disposable, portable, and specific | [73] |
| l-Tyrosine | CV, EIS | TINT-rGO/tyrosinase | Electrodeposition | High conductivity, robustness, biocompatibility | [127] |
| l-Tyrosine | CV | Commercial band-aids/carbon conductive ink/α-MnO2/tyrosinase bio-enzyme | Screen-printed | Good selectivity, wireless communication, stable | [128] |
| l-Tyrosine | EIS | TINT film/low-energy ion beam containing nitrogen ions and gold ions | Ion beam technique | Sensitive and wide detection range | [129] |
| (a) Na+, K+, Ca+, (b) pH, (c) uric acid, and (d) temperature | (a), (b) Potentiometric measurement, (c) chronoamperometry, (d) thermistor measurement | PET/Au/(a) PEDOT:PSS/ion-selective membrane,(b) PEDOT:PSS/polyaniline emeraldine (c) chitosan-Prussian blue mediator layer/UOx, (d) graphene | Magnetron sputtering | Multi-marker detection, sensitive, wireless communication, in vivo detection of rat models | [82] |
| (a) Tumor necrosis factor-α, interleukin-6, interleukin-8, transforming growth fac-tor-β1, S. aureus, (b) pH, (c) temperature | (a) SWV, (b) potentiometric measurement, (c) thermistor measurement | PU film/AuNPs-GP/(a) aptamer, (b) PANI/medical-grade PU film | Sputtering and photolithography | Multi-marker detection, biocompatibility, collection of wound exudates, wireless communication, in vivo detection of rat models | [83] |
| (a) pH, (b) uric acid, (c) temperature | (a) potentiometric measurement, (b) DPV, (c) thermistor measurement | PI substrate/carbon/(a) AuNPs/PANI, (b) rGO/AuNPs | Screen printing and laser cutting | Multi-marker detection, stabilized, supports drug release, wireless communication, in vivo detection of rat models | [25] |
| Parameter | Detection Principle | Sensitive Material | Feature | Ref. |
|---|---|---|---|---|
| Temperature and pressure | Resistive and capacitive sensing | PEDOT:PSS/CNT hybrid material | Pioneer in the realization of printed sensors | [86] |
| Temperature and humidity | Resistive sensing | Graphene oxide | Printed interdigitated electrodes using thermal transfer technic | [133] |
| Temperature and strain | Resistive and capacitive sensing | PEDOT:PSS | High strain resolution | [134] |
| Temperature, strain, and glucose | Resistive and capacitive sensing | Zwitterionic thermo glucose-sensitive skin-like hydrogel | Continuous real-time monitoring of three indicators infection, swelling, and blood glucose | [135] |
| Strain | Piezoelectric effect | Piezoelectric γ-glycine micro-crystals | Biodegradable, potential for self-powered and autonomous electrical stimulation | [138] |
| Strain | Capacitive sensing | AgNW and PU | Flexible, suitable for different parts of the body | [139] |
| Strain | Capacitive sensing | Pyramidal PDMS elastomers | Fabricated using a silicon anisotropic etching mold | [140] |
| Dielectric properties of wound tissues | Capacitive sensing | Wound tissues | Detection of wound skin as self-capacitance medium | [142] |
| Dielectric properties of wound tissues | Coplanar waveguide | Wound tissues | A novel approach to wound assessment by transmission line theory | [143] |
| Wound Marker | Detection Method | Sensor Material | Fabrication Method | Feature | Ref. |
|---|---|---|---|---|---|
| Temperature | Thermal imaging | FLIR E60 thermal imaging camera | - | High accuracy and sensitivity | [147] |
| pH | Color indicator | Swabs or dressings | A silane-based coupling agent for immobilization of bromocresol purple | Low cost and convenience | [148] |
| pH | Colorimetric | Cotton cloth | Microwave-assisted heating of 1,2,4-triaminobenzene and urea aqueous solution | Biocompatibility, drug compatibility, resistance leachability, and high reversibility | [149] |
| pH | Fluorescence | Membranes and commercial wound dressings | Pyranine was incorporated in wound dressing via benzalkonium | Portable and semi-quantitative | [150] |
| pH and pressure | Spectroscopy | Spectrometer USB2000+ | An intrinsically pH-sensitive optical fiber was fabricated using a polydimethylsiloxane precursor doped with rhodamine B dye | Portable and quantitative | [151] |
| pH and glucose | Colorimetry | Multifunctional zwitterionic hydrogel | Phenol red, GOx, and HRP were encapsulated in the polycarboxybetaine hydrogel matrix | Portable and quantitative | [152] |
| Amino acid | Colorimetry | Colorimetric sensory polymer film | Test kit with colorimetric sensory polymer film | Portable and quantitative | [153] |
| pH | Colorimetry | Hydrogels | Double colorimetry-integrated polyacrylamide–quaternary ammonium chitosan–carbon quantum dots (CQDs)–phenol red hydrogels | Portable, quantitative, real-time, and remote | [154] |
| Temperature | Infrared method | FLIR™ infrared camera | Combined with smartphone and the Swift Wound app | Inexpensive, easy to use, Reliable, and accurate | [155] |
| Temperature | Infrared method | Infrared thermography | Containing a lepton thermal sensor and a visible VGA imager | Small, low-priced, and handheld | [156] |
| Reactive oxygen species | Fluorescence | SWCNT nanosensors | Optical core–shell microfibrous textiles incorporating single-walled carbon nanotubes (SWCNTs) | Portable, wearable, and real-time | [157] |
| Temperature | Colorimetry | Reversible thermochromic fibers | Thermochromic microcapsules and polypropylene (PP) was used as color indicator and polymer fiber matrix | Portable and reusable | [158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Zhang, Y.; Ma, C.; Yuan, Q.; Wang, X.; Wan, H.; Wang, P. A Review of Recent Advances in Flexible Wearable Sensors for Wound Detection Based on Optical and Electrical Sensing. Biosensors 2022, 12, 10. https://doi.org/10.3390/bios12010010
Sun X, Zhang Y, Ma C, Yuan Q, Wang X, Wan H, Wang P. A Review of Recent Advances in Flexible Wearable Sensors for Wound Detection Based on Optical and Electrical Sensing. Biosensors. 2022; 12(1):10. https://doi.org/10.3390/bios12010010
Chicago/Turabian StyleSun, Xianyou, Yanchi Zhang, Chiyu Ma, Qunchen Yuan, Xinyi Wang, Hao Wan, and Ping Wang. 2022. "A Review of Recent Advances in Flexible Wearable Sensors for Wound Detection Based on Optical and Electrical Sensing" Biosensors 12, no. 1: 10. https://doi.org/10.3390/bios12010010
APA StyleSun, X., Zhang, Y., Ma, C., Yuan, Q., Wang, X., Wan, H., & Wang, P. (2022). A Review of Recent Advances in Flexible Wearable Sensors for Wound Detection Based on Optical and Electrical Sensing. Biosensors, 12(1), 10. https://doi.org/10.3390/bios12010010







