Stretchable Capacitive Pressure Sensing Sleeve Deployable onto Catheter Balloons towards Continuous Intra-Abdominal Pressure Monitoring
Abstract
1. Introduction
- (1)
- A potential method of fabrication altering the sensitivity for capacitance-based pressure sensors.
- (2)
- Incorporation of a pressure sensor into a stretchable pressure sensing sleeve.
- (3)
- Introduction of a pressure sensing sleeve on a Foley catheter for continuous monitoring of the intra-abdominal pressure.
2. Materials and Methods
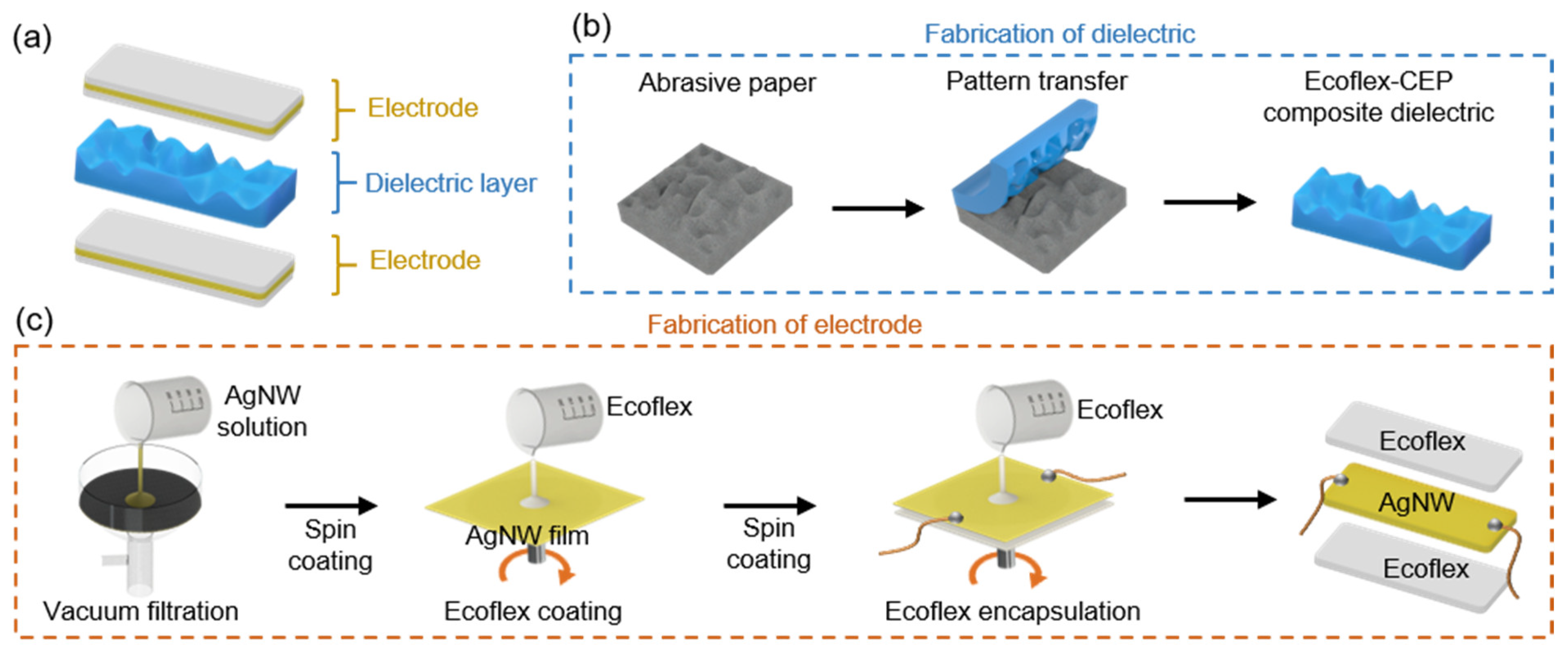
2.1. Pressure Sensor Fabrication
2.2. Sensing Sleeve Fabrication
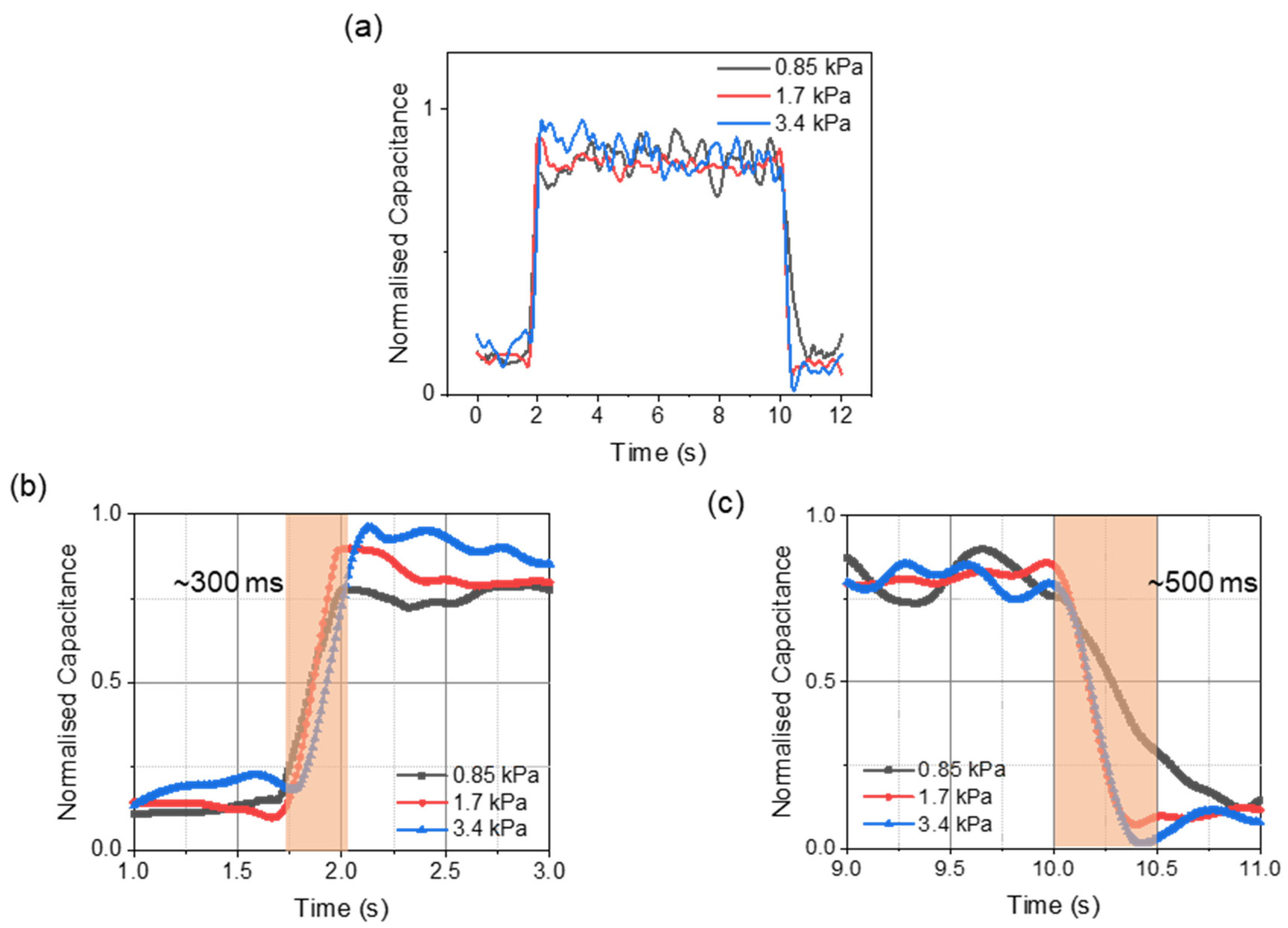
3. Results and Discussion
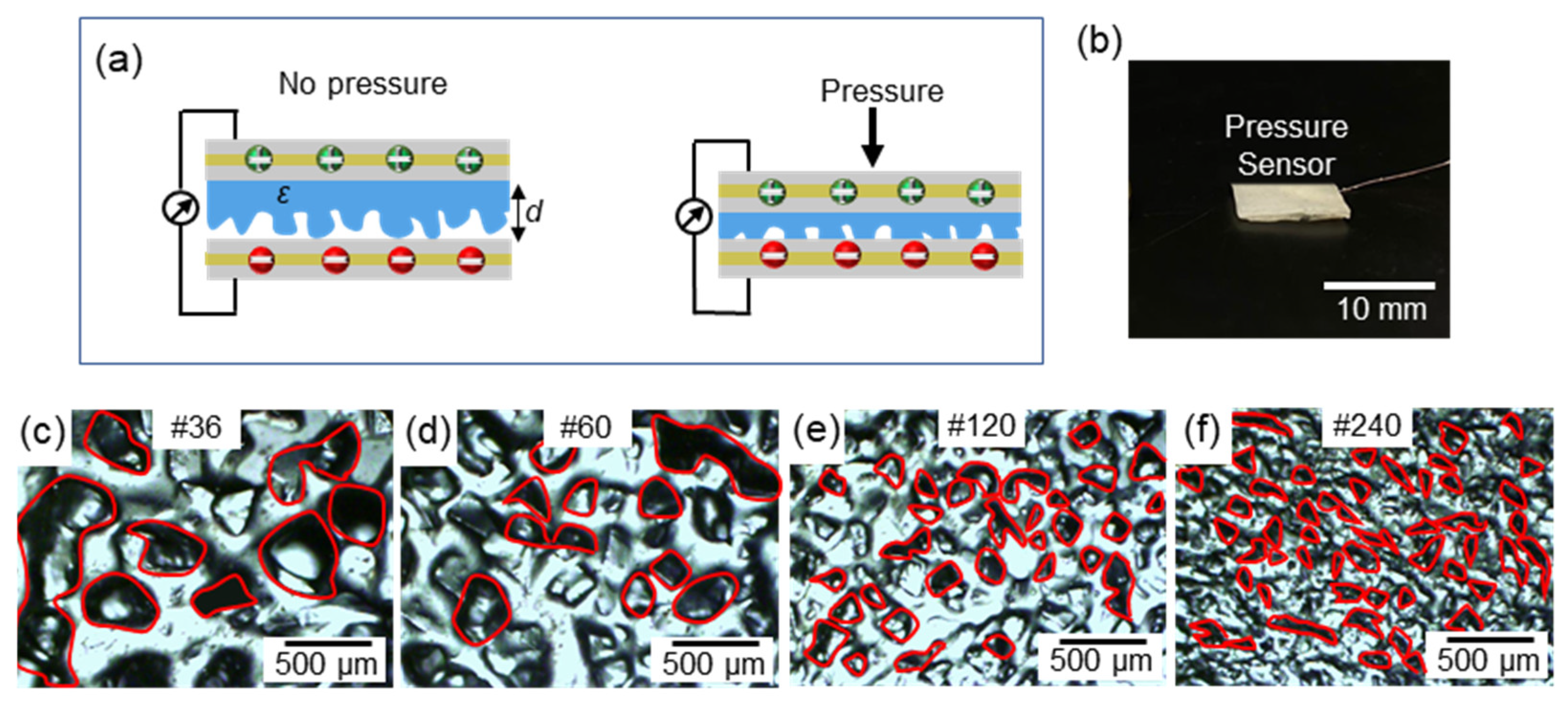
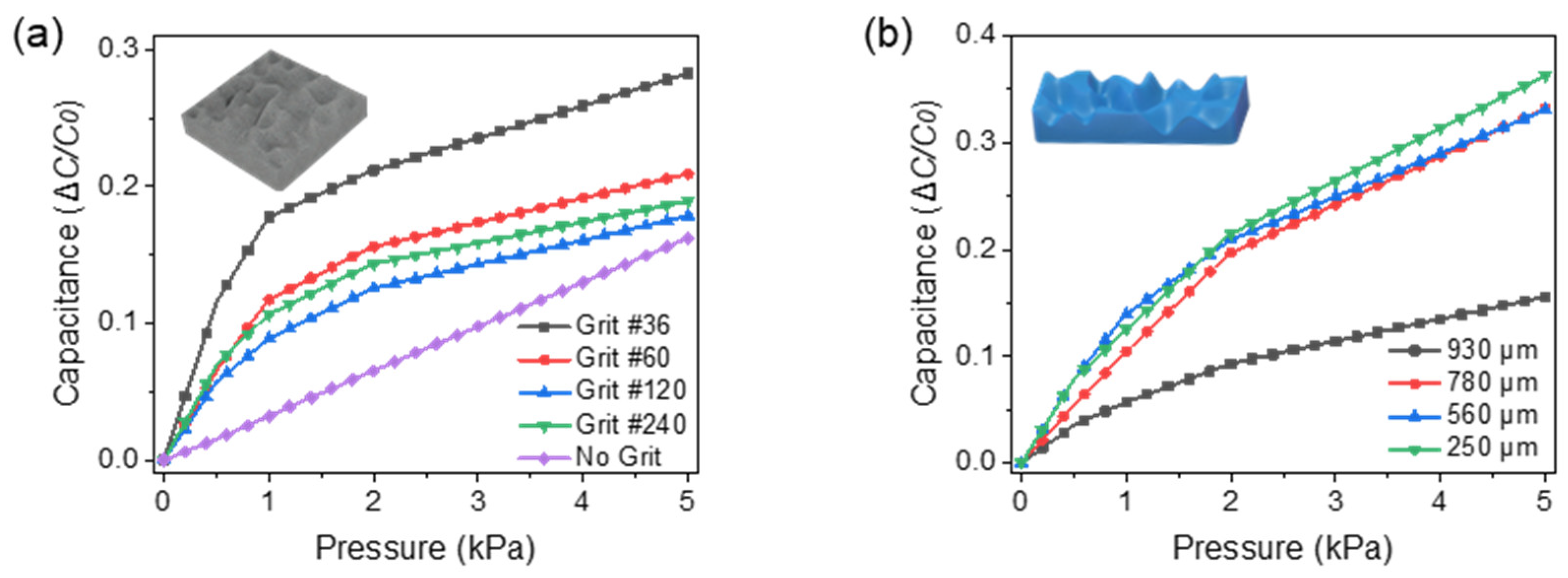
3.1. Dielectric Optimization
3.2. In-Vitro Characterizations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, G.R.; Rao, J.V.S.; Aziz, A.; Rashmi, T.M. The role of routine measurement of intra-abdominal pressure in preventing abdominal compartment syndrome. J. Indian Assoc. Pediatr. Surg. 2017, 22, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Al-Abassi, A.A.; Al Saadi, A.S.; Ahmed, F. Is intra-bladder pressure measurement a reliable indicator for raised intra-abdominal pressure? A prospective comparative study. BMC Anesthesiol. 2018, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.N.G.; Cheatham, M.L.; Kirkpatrick, A.; Sugrue, M.; Parr, M.; De Waele, J.; Balogh, Z.; Leppäniemi, A.; Olvera, C.; Ivatury, R.; et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensiv. Care Med. 2006, 32, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- De Laet, I.E.; Malbrain, M.L.; De Waele, J.J. A clinician’s guide to management of intra-abdominal hypertension and abdominal compartment syndrome in critically ill patients. Crit. Care 2020, 24, 1–9. [Google Scholar] [CrossRef]
- De Laet, I.; Malbrain, M. Current insights in intra-abdominal hypertension and abdominal compartment syndrome. Med. Intensiv. 2007, 31, 88–99. [Google Scholar] [CrossRef]
- De Keulenaer, B.L.; Regli, A.; Malbrain, M.L.N.G. Intra-abdominal measurement techniques: Is there anything new? Am. Surg. 2011, 77, s17–s22. [Google Scholar]
- Hunt, L.; Frost, S.; Hillman, K.; Newton, P.J.; Davidson, P.M. Management of intra-abdominal hypertension and abdominal compartment syndrome: A review. J. Trauma Manag. Outcomes 2014, 8, 2. [Google Scholar] [CrossRef]
- Kron, I.L.; Harman, P.K.; Nolan, S.P. The Measurement of Intra-abdominal Pressure as a Criterion for Abdominal Re-exploration. Ann. Surg. 1984, 199, 28–30. [Google Scholar] [CrossRef]
- Sugrue, M.; De Waele, J.J.; De Keulenaer, B.L.; Roberts, D.J.; Malbrain, M.L. A user’s guide to intra-abdominal pressure measurement. Anestezjol. Intensywna Ter. 2015, 47, 241–251. [Google Scholar] [CrossRef]
- Balogh, Z.; Jones, F.; D’Amours, S.; Parr, M.; Sugrue, M. Continuous intra-abdominal pressure measurement technique. Am. J. Surg. 2004, 188, 679–684. [Google Scholar] [CrossRef]
- Liao, C.-H.; Cheng, C.-T.; Chen, C.-C.; Jow, U.-M.; Chen, C.-H.; Lai, Y.-L.; Chen, Y.-C.; Ho, D.-R. An Ingestible Electronics for Continuous and Real-Time Intraabdominal Pressure Monitoring. J. Pers. Med. 2020, 11, 12. [Google Scholar] [CrossRef]
- Niederauer, S.; De Gennaro, J.; Nygaard, I.; Petelenz, T.; Hitchcock, R. Development of a novel intra-abdominal pressure transducer for large scale clinical studies. Biomed. Microdevices 2017, 19, 80. [Google Scholar] [CrossRef]
- Jiang, H.; Woodhouse, I.B.; Selvamani, V.; Ma, J.L.; Tang, R.; Goergen, C.J.; Soleimani, T.; Rahimi, R. A Wireless Implantable Passive Intra-Abdominal Pressure Sensing Scheme via Ultrasonic Imaging of a Microfluidic Device. IEEE Trans. Biomed. Eng. 2021, 68, 747–758. [Google Scholar] [CrossRef]
- Van Ramshorst, G.H.; Lange, J.F.; Goossens, R.H.M.; Agudelo, N.L.; Kleinrensink, G.J.; Verwaal, M.; Flipsen, S.F.J.; Hop, W.C.J.; Wauben, L.S.G.L.; Jeekel, J. Non-invasive measurement of intra-abdominal pressure: A preliminary study. Physiol. Meas. 2008, 29, N41–N47. [Google Scholar] [CrossRef]
- Meena, K.V.; Sankar, A.R. Biomedical Catheters with Integrated Miniature Piezoresistive Pressure Sensors: A Review. IEEE Sens. J. 2021, 1. [Google Scholar] [CrossRef]
- Han, M.; Chen, L.; Aras, K.; Liang, C.; Chen, X.; Zhao, H.; Li, K.; Faye, N.R.; Sun, B.; Kim, J.-H.; et al. Catheter-integrated soft multilayer electronic arrays for multiplexed sensing and actuation during cardiac surgery. Nat. Biomed. Eng. 2020, 4, 1–13. [Google Scholar] [CrossRef]
- Kumar, K.S.; Chen, P.-Y.; Ren, H. A Review of Printable Flexible and Stretchable Tactile Sensors. Research 2019, 2019, 1–32. [Google Scholar] [CrossRef]
- Jin, T.; Sun, Z.; Li, L.; Zhang, Q.; Zhu, M.; Zhang, Z.; Yuan, G.; Chen, T.; Tian, Y.; Hou, X.; et al. Triboelectric nanogenerator sensors for soft robotics aiming at digital twin applications. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Booth, J.W.; Shah, D.; Case, J.C.; White, E.L.; Yuen, M.C.; Cyr-Choiniere, O.; Kramer-Bottiglio, R. OmniSkins: Robotic skins that turn inanimate objects into multifunctional robots. Sci. Robot. 2018, 3, eaat1853. [Google Scholar] [CrossRef]
- Ponraj, G.; Kirthika, S.K.; Thakor, N.V.; Yeow, C.-H.; Kukreja, S.L.; Ren, H. Development of flexible fabric based tactile sensor for closed loop control of soft robotic actuator. In Proceedings of the 2017 13th IEEE Conference on Automation Science and Engineering (CASE), Xi’an, China, 20–23 August 2017; pp. 1451–1456. [Google Scholar]
- Ponraj, G.; Kirthika, S.K.; Lim, C.M.; Ren, H. Soft Tactile Sensors with Inkjet-Printing Conductivity and Hydrogel Biocompatibility for Retractors in Cadaveric Surgical Trials. IEEE Sens. J. 2018, 18, 9840–9847. [Google Scholar] [CrossRef]
- Boutry, C.M.; Kaizawa, Y.; Schroeder, B.C.; Chortos, A.; Legrand, A.; Wang, Z.; Chang, J.; Fox, P.; Bao, Z. A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat. Electron. 2018, 1, 314–321. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ren, H.; Chan, Y.H. Soft Tactile Sensors for Rehabilitation Robotic Hand with 3D Printed Folds; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; pp. 55–60. [Google Scholar]
- Kirthika, S.K.; Ponraj, G.; Ren, H. Fabrication and Comparative Study on Sensing Characteristics of Soft Textile-Layered Tactile Sensors. IEEE Sensors Lett. 2017, 1, 1–4. [Google Scholar] [CrossRef]
- Gao, Y.; Ota, H.; Schaler, E.W.; Chen, K.; Zhao, A.; Gao, W.; Fahad, H.M.; Leng, Y.; Zheng, A.; Xiong, F.; et al. Wearable Microfluidic Diaphragm Pressure Sensor for Health and Tactile Touch Monitoring. Adv. Mater. 2017, 29, 1701985. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, X.; Shi, Y.; Li, L.; Wang, W.; He, L.; Liu, R. Recent Developments for Flexible Pressure Sensors: A Review. Micromachines 2018, 9, 580. [Google Scholar] [CrossRef]
- Saccomandi, P.; Schena, E.; Oddo, C.M.; Zollo, L.; Silvestri, S.; Guglielmelli, E. Microfabricated Tactile Sensors for Biomedical Applications: A Review. Biosensors 2014, 4, 422–448. [Google Scholar] [CrossRef]
- Boutry, C.M.; Beker, L.; Kaizawa, Y.; Vassos, C.; Tran, H.; Hinckley, A.C.; Pfattner, R.; Niu, S.; Li, J.; Claverie, J.; et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 2019, 3, 47–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Ahmadi, M.; Timm, G.; Sezen, S.; Rajamani, R. An Instrumented Urethral Catheter with a Distributed Array of Iontronic Force Sensors. Ann. Biomed. Eng. 2021, 49, 149–161. [Google Scholar] [CrossRef]
- Ruth, S.R.A.; Feig, V.R.; Tran, H.; Bao, Z. Microengineering Pressure Sensor Active Layers for Improved Performance. Adv. Funct. Mater. 2020, 30, 2003491. [Google Scholar] [CrossRef]
- Chen, S.; Zhuo, B.; Guo, X.-J. Large Area One-Step Facile Processing of Microstructured Elastomeric Dielectric Film for High Sensitivity and Durable Sensing over Wide Pressure Range. ACS Appl. Mater. Interfaces 2016, 8, 20364–20370. [Google Scholar] [CrossRef]
- Kwon, D.; Lee, T.-I.; Kim, M.; Kim, S.; Kim, T.-S.; Park, I. Porous dielectric elastomer based ultra-sensitive capacitive pressure sensor and its application to wearable sensing device. In Proceedings of the 2015 Transducers—2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 299–302. [Google Scholar]
- Lee, B.-Y.; Kim, J.; Kim, H.; Kim, C.; Lee, S.-D. Low-cost flexible pressure sensor based on dielectric elastomer film with micro-pores. Sens. Actuators A Phys. 2016, 240, 103–109. [Google Scholar] [CrossRef]
- Kang, S.; Lee, J.; Lee, S.; Kim, S.; Kim, J.-K.; Algadi, H.; Al-Sayari, S.; Kim, D.-E.; Lee, T. Highly Sensitive Pressure Sensor Based on Bioinspired Porous Structure for Real-Time Tactile Sensing. Adv. Electron. Mater. 2016, 2, 1600356. [Google Scholar] [CrossRef]
- Wan, S.; Bi, H.; Zhou, Y.; Xie, X.; Su, S.; Yin, K.; Sun, L. Graphene oxide as high-performance dielectric materials for capacitive pressure sensors. Carbon 2017, 114, 209–216. [Google Scholar] [CrossRef]
- Yang, W.; Li, N.-W.; Zhao, S.; Yuan, Z.; Wang, J.; Du, X.; Wang, B.; Cao, R.; Li, X.; Xu, W.; et al. A Breathable and Screen-Printed Pressure Sensor Based on Nanofiber Membranes for Electronic Skins. Adv. Mater. Technol. 2018, 3, 1700241. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Q.; Bi, Y.; Cao, S.; Xia, X.; Yang, A.; Li, S.; Xiao, X. Research progress of flexible capacitive pressure sensor for sensitivity enhancement approaches. Sens. Actuators A Phys. 2021, 321, 112425. [Google Scholar] [CrossRef]
- Ruth, S.R.A.; Bao, Z. Designing Tunable Capacitive Pressure Sensors Based on Material Properties and Microstructure Geometry. ACS Appl. Mater. Interfaces 2020, 12, 58301–58316. [Google Scholar] [CrossRef]
- Ruth, S.R.A.; Beker, L.; Tran, H.; Feig, V.R.; Matsuhisa, N.; Bao, Z. Rational design of capacitive pressure sensors based on pyramidal microstructures for specialized monitoring of biosignals. Adv. Funct. Mater. 2020, 30, 1903100. [Google Scholar] [CrossRef]
- Lee, S.L.; Anderson, J.T.; Kraut, E.J.; Wisner, D.H.; Wolfe, B.M. A Simplified Approach to the Diagnosis of Elevated Intra-abdominal Pressure. J. Trauma: Inj. Infect. Crit. Care 2002, 52, 1169–1172. [Google Scholar] [CrossRef]
- Kwak, J.W.; Han, M.; Xie, Z.; Chung, H.U.; Lee, J.Y.; Avila, R.; Yohay, J.; Chen, X.; Liang, C.; Patel, M.; et al. Wireless sensors for continuous, multimodal measurements at the skin interface with lower limb prostheses. Sci. Transl. Med. 2020, 12, eabc4327. [Google Scholar] [CrossRef]
- Stannard, A.; Eliason, J.L.; Rasmussen, T.E. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) as an Adjunct for Hemorrhagic Shock. J. Trauma: Inj. Infect. Crit. Care 2011, 71, 1869–1872. [Google Scholar] [CrossRef]
- Junior, M.A.F.R.; Feng, C.Y.D.; Nguyen, A.T.M.; Rodrigues, V.C.; Bechara, G.E.K.; De-Moura, R.R.; Brenner, M. The complications associated with Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). World J. Emerg. Surg. 2018, 13, 1–6. [Google Scholar] [CrossRef]
- Tokas, T.; Training and Research in Urological Surgery and Technology (T.R.U.S.T.) Group; Skolarikos, A.; Herrmann, T.R.W.; Nagele, U. Pressure matters 2: Intrarenal pressure ranges during upper-tract endourological procedures. World J. Urol. 2018, 37, 133–142. [Google Scholar] [CrossRef]
- Ganpule, A.P.; Rawandale-Patil, A.V.; Patni, L.G. Development of an innovative intrarenal pressure regulation system for mini-PCNL: A preliminary study. Indian J. Urol. 2019, 35, 197–201. [Google Scholar] [CrossRef]
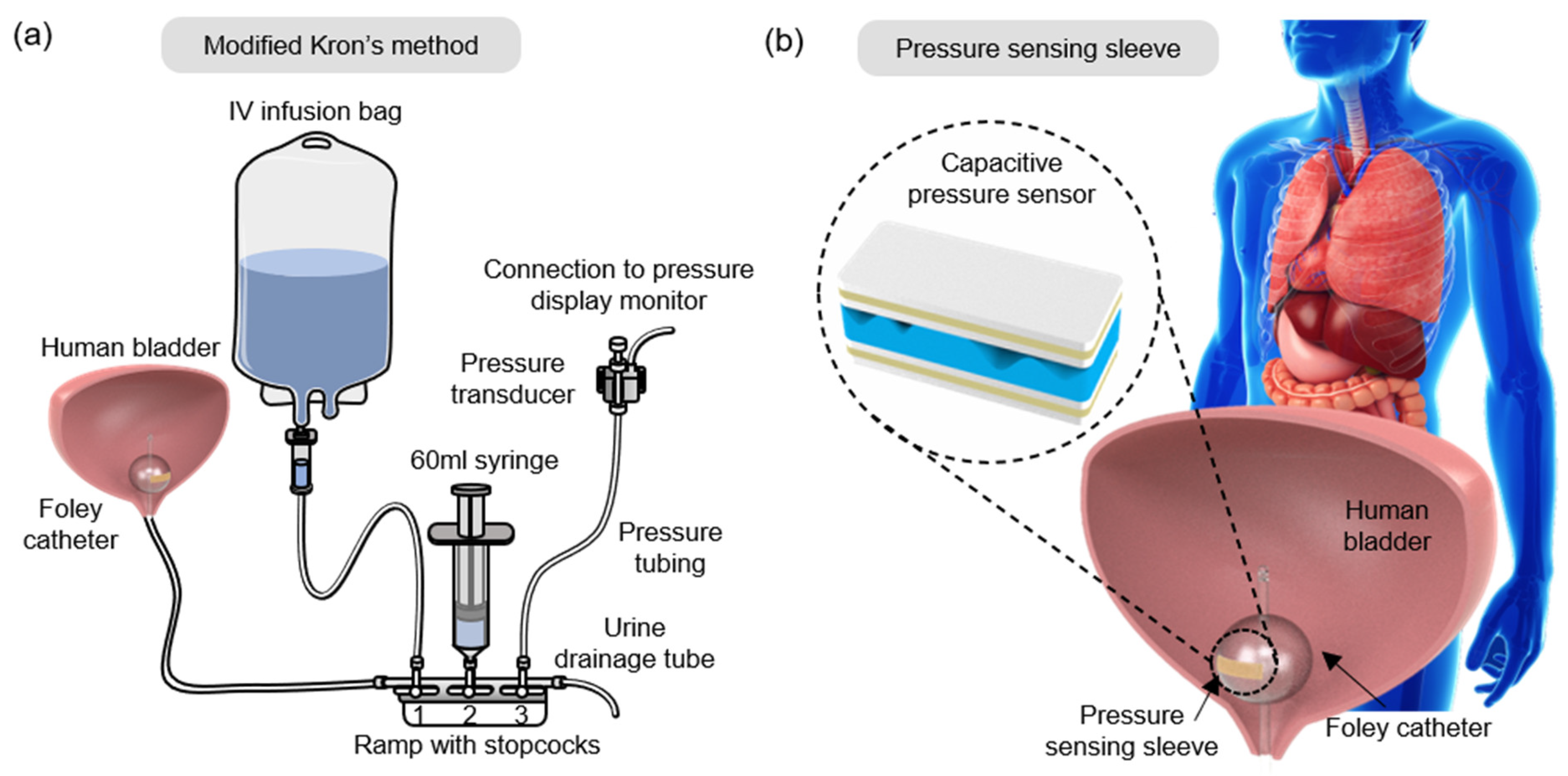




| Steps | Current Practice |
|---|---|
| 1 | A ramp with three stopcocks is connected to the drainage tubing of the Foley catheter. |
| 2 | An IV infusion bag, 60 mL syringe, and a pressure transducer are connected to the ramp. |
| 3 | The bladder and the system are flushed with normal saline. |
| 4 | The pressure transducer is fixed at the top of the patient’s symphysis pubis bone or thigh. |
| 5 | Zero-point calibration of the pressure transducer is done upon stabilization. |
| 6 | Urine drainage tubing is clamped. |
| 7 | Bladder filled with <25 mL saline solution. |
| 8 | The IVP value is recorded as shown on the monitor. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senthil Kumar, K.; Xu, Z.; Sivaperuman Kalairaj, M.; Ponraj, G.; Huang, H.; Ng, C.-F.; Wu, Q.H.; Ren, H. Stretchable Capacitive Pressure Sensing Sleeve Deployable onto Catheter Balloons towards Continuous Intra-Abdominal Pressure Monitoring. Biosensors 2021, 11, 156. https://doi.org/10.3390/bios11050156
Senthil Kumar K, Xu Z, Sivaperuman Kalairaj M, Ponraj G, Huang H, Ng C-F, Wu QH, Ren H. Stretchable Capacitive Pressure Sensing Sleeve Deployable onto Catheter Balloons towards Continuous Intra-Abdominal Pressure Monitoring. Biosensors. 2021; 11(5):156. https://doi.org/10.3390/bios11050156
Chicago/Turabian StyleSenthil Kumar, Kirthika, Zongyuan Xu, Manivannan Sivaperuman Kalairaj, Godwin Ponraj, Hui Huang, Chi-Fai Ng, Qing Hui Wu, and Hongliang Ren. 2021. "Stretchable Capacitive Pressure Sensing Sleeve Deployable onto Catheter Balloons towards Continuous Intra-Abdominal Pressure Monitoring" Biosensors 11, no. 5: 156. https://doi.org/10.3390/bios11050156
APA StyleSenthil Kumar, K., Xu, Z., Sivaperuman Kalairaj, M., Ponraj, G., Huang, H., Ng, C.-F., Wu, Q. H., & Ren, H. (2021). Stretchable Capacitive Pressure Sensing Sleeve Deployable onto Catheter Balloons towards Continuous Intra-Abdominal Pressure Monitoring. Biosensors, 11(5), 156. https://doi.org/10.3390/bios11050156








