Electrochemical Trimethylamine N-Oxide Biosensor with Enzyme-Based Oxygen-Scavenging Membrane for Long-Term Operation under Ambient Air
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus and Procedure
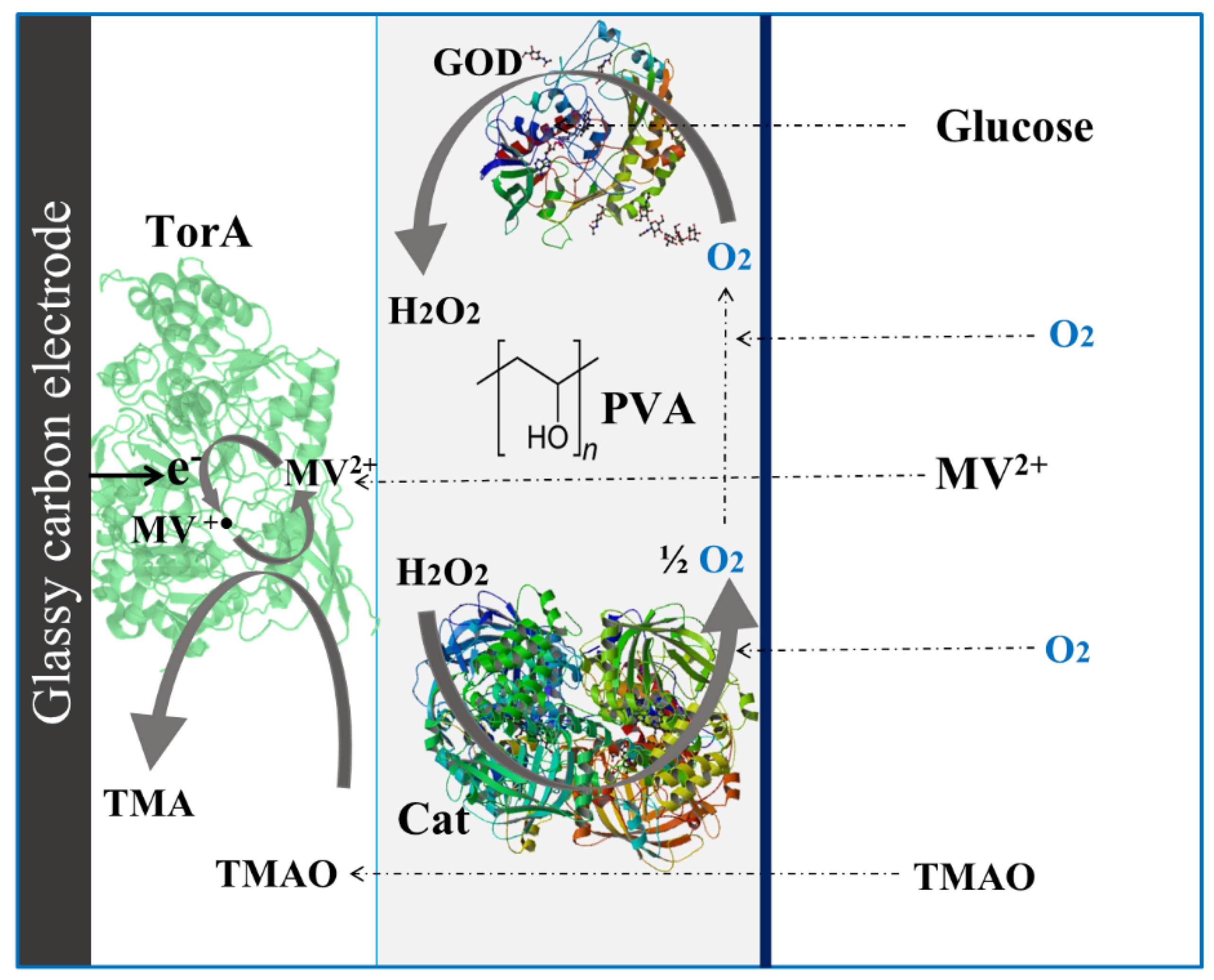
2.2.1. Oxygen Scavenging Membrane Preparation and Optimization
2.2.2. TMAO Biosensor
3. Results and Discussion
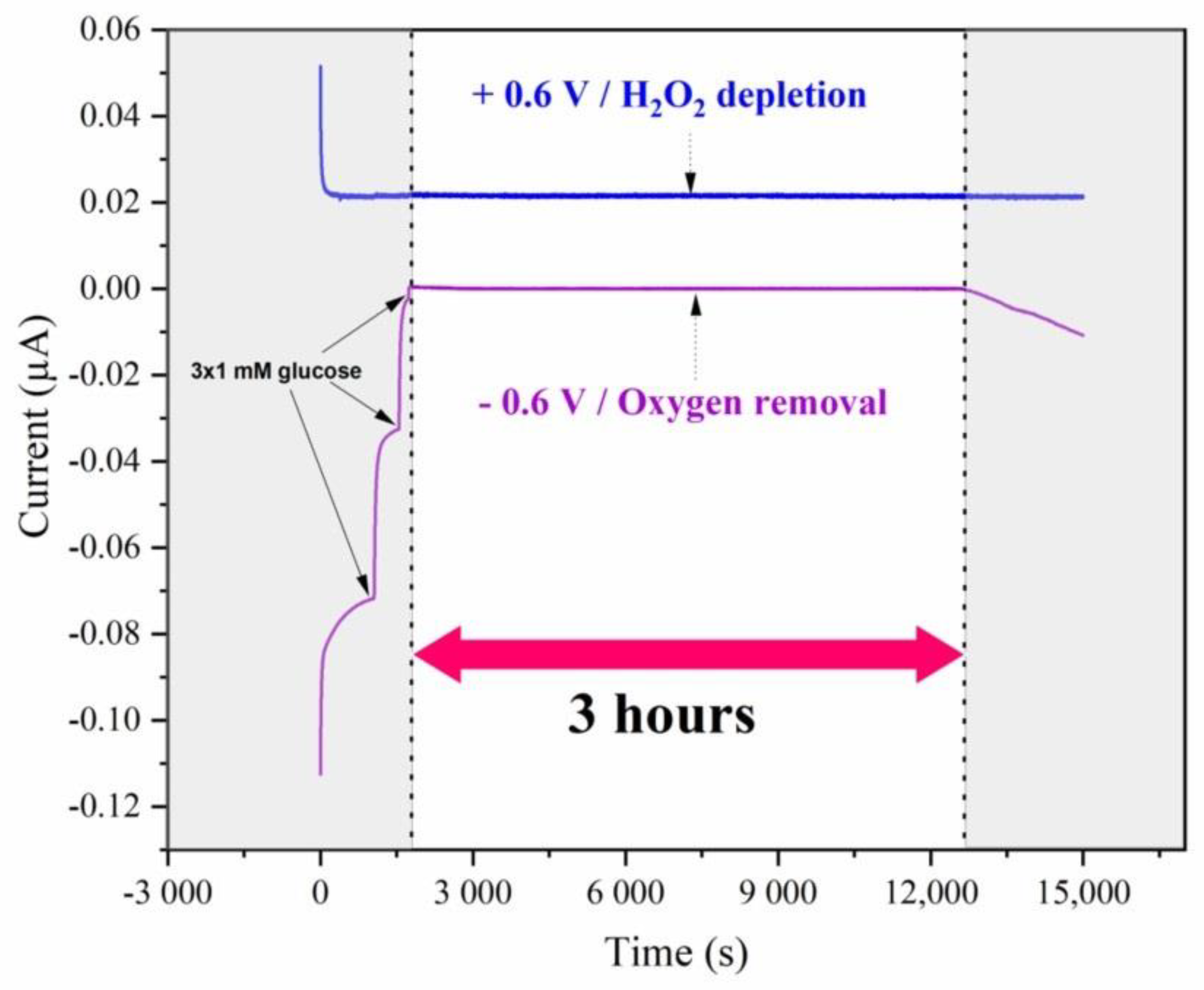
3.1. Optimization of the Oxygen-Scavenging Membrane
3.2. TMAO Biosensor
3.2.1. Assembly of a TMAO Biosensor
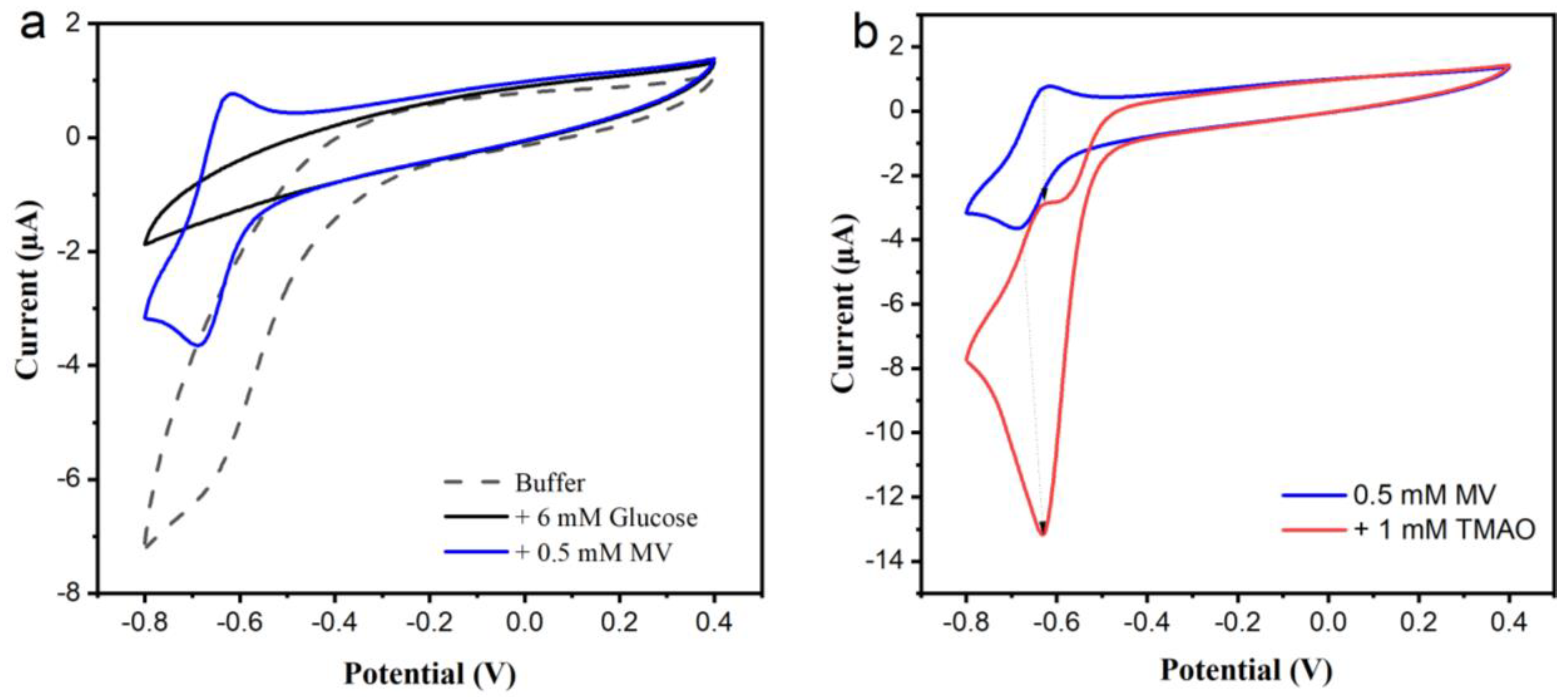
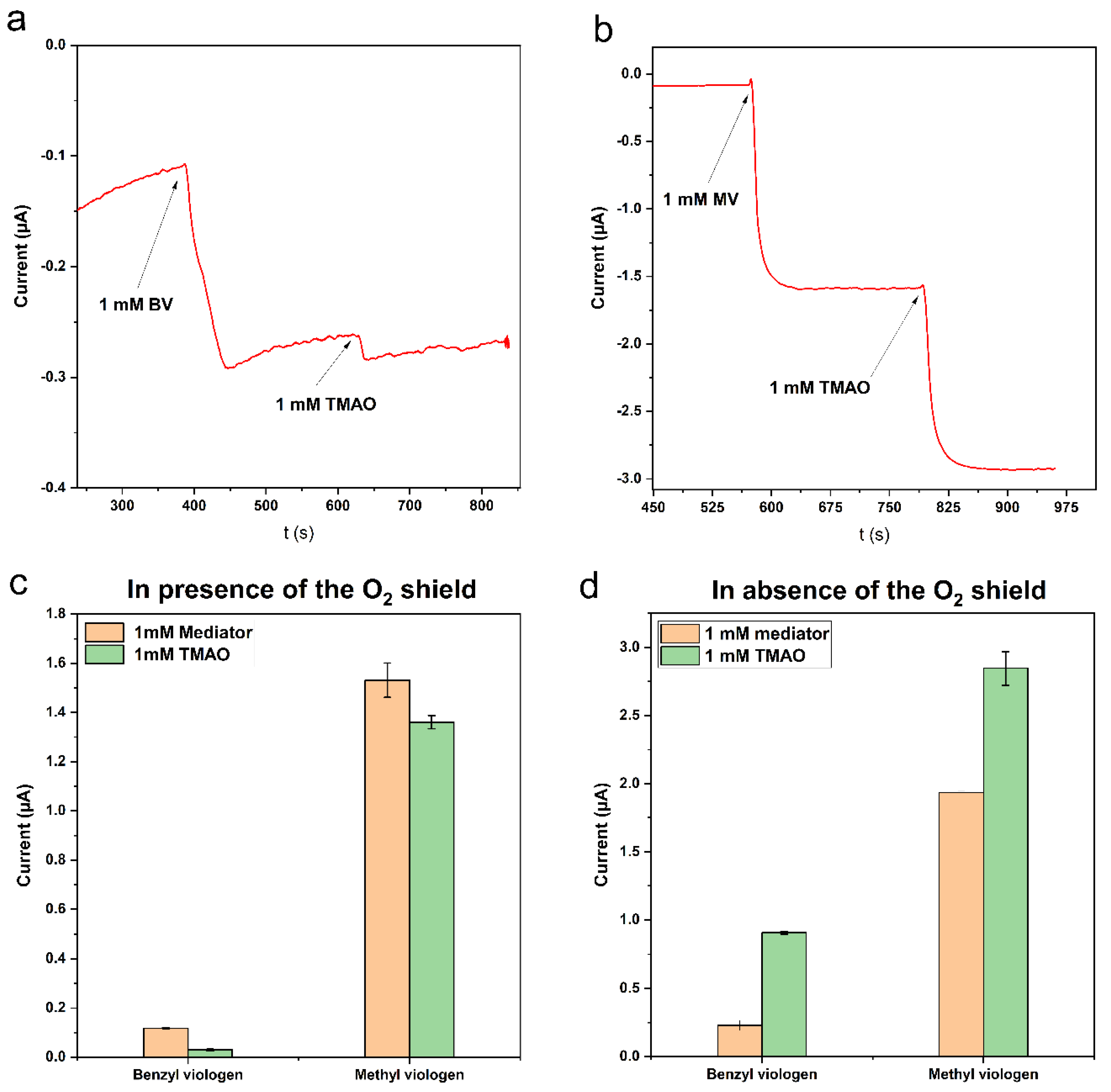
3.2.2. Amperometry for the Selection of a Mediator
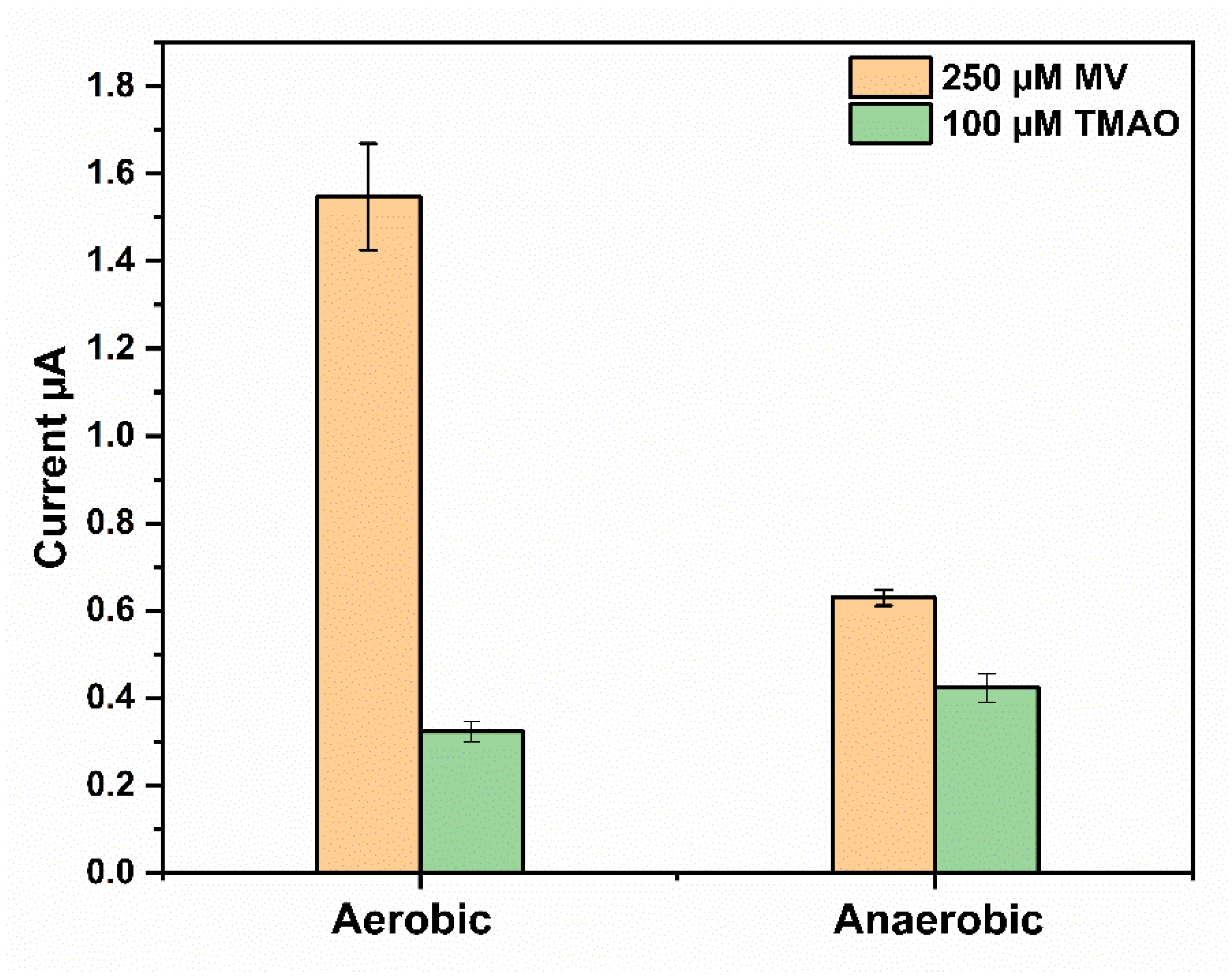
3.3. Amperometric TMAO Biosensor
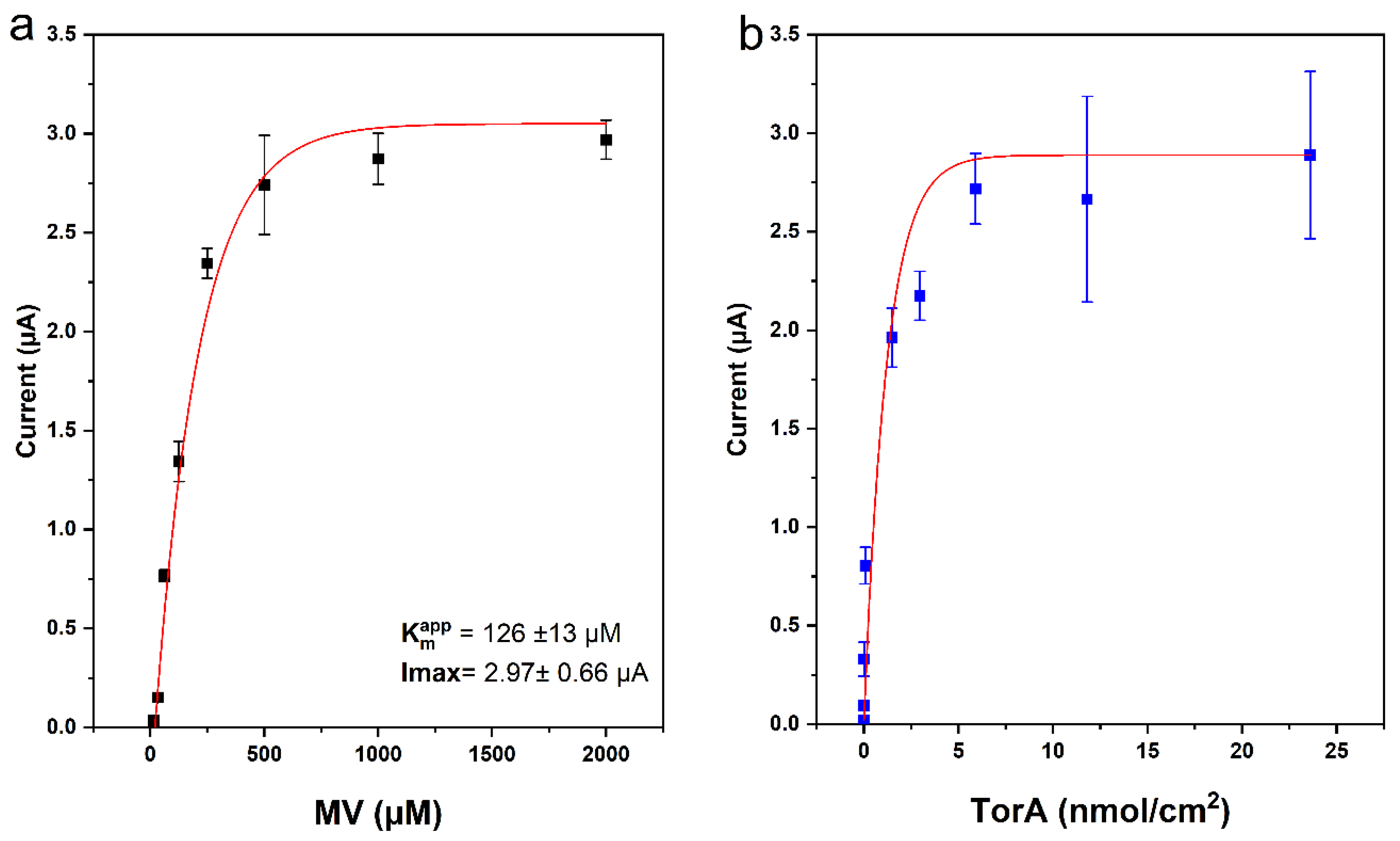
Optimization of Mediator and Immobilized TorA Concentrations
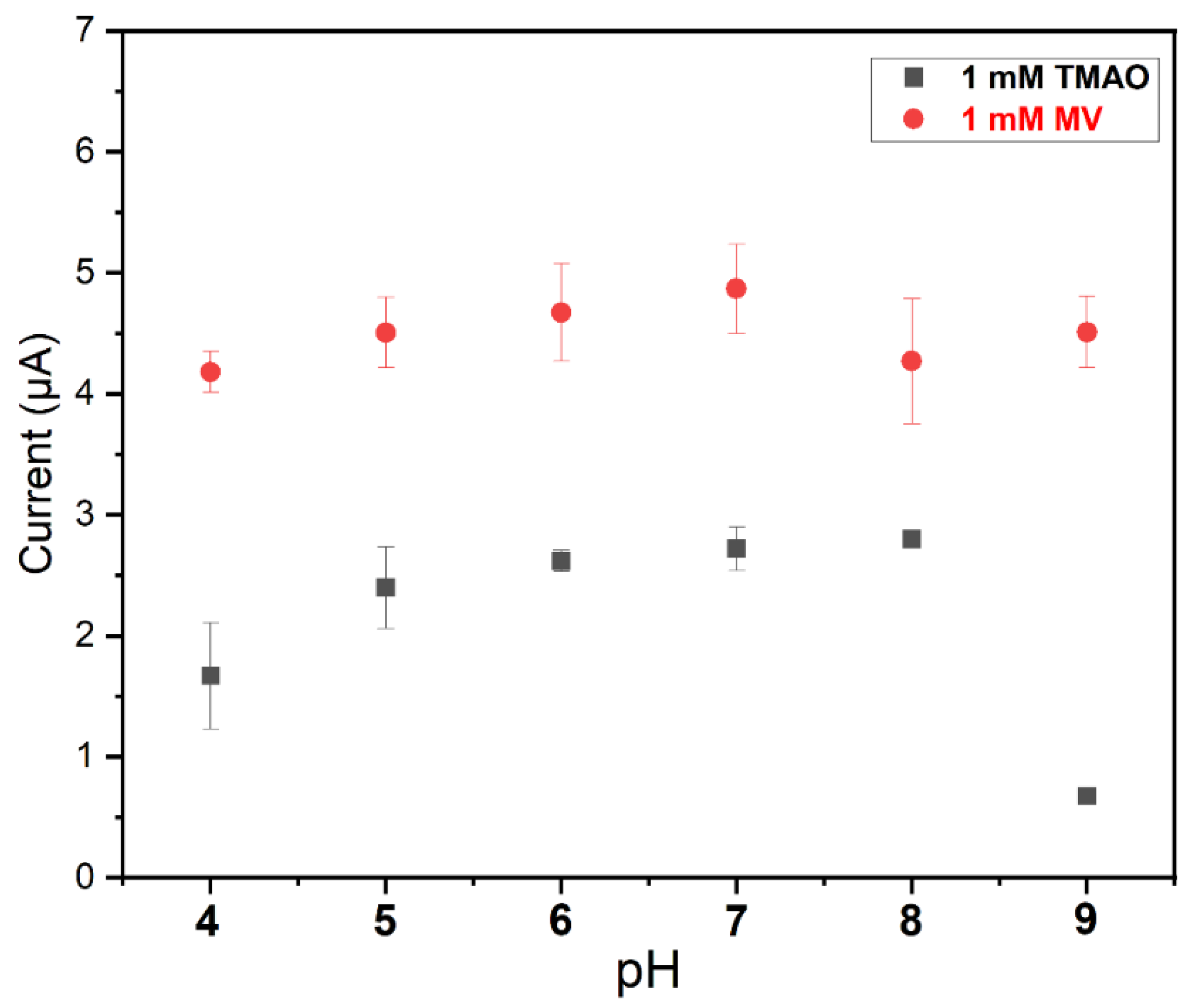
3.4. PH Dependency
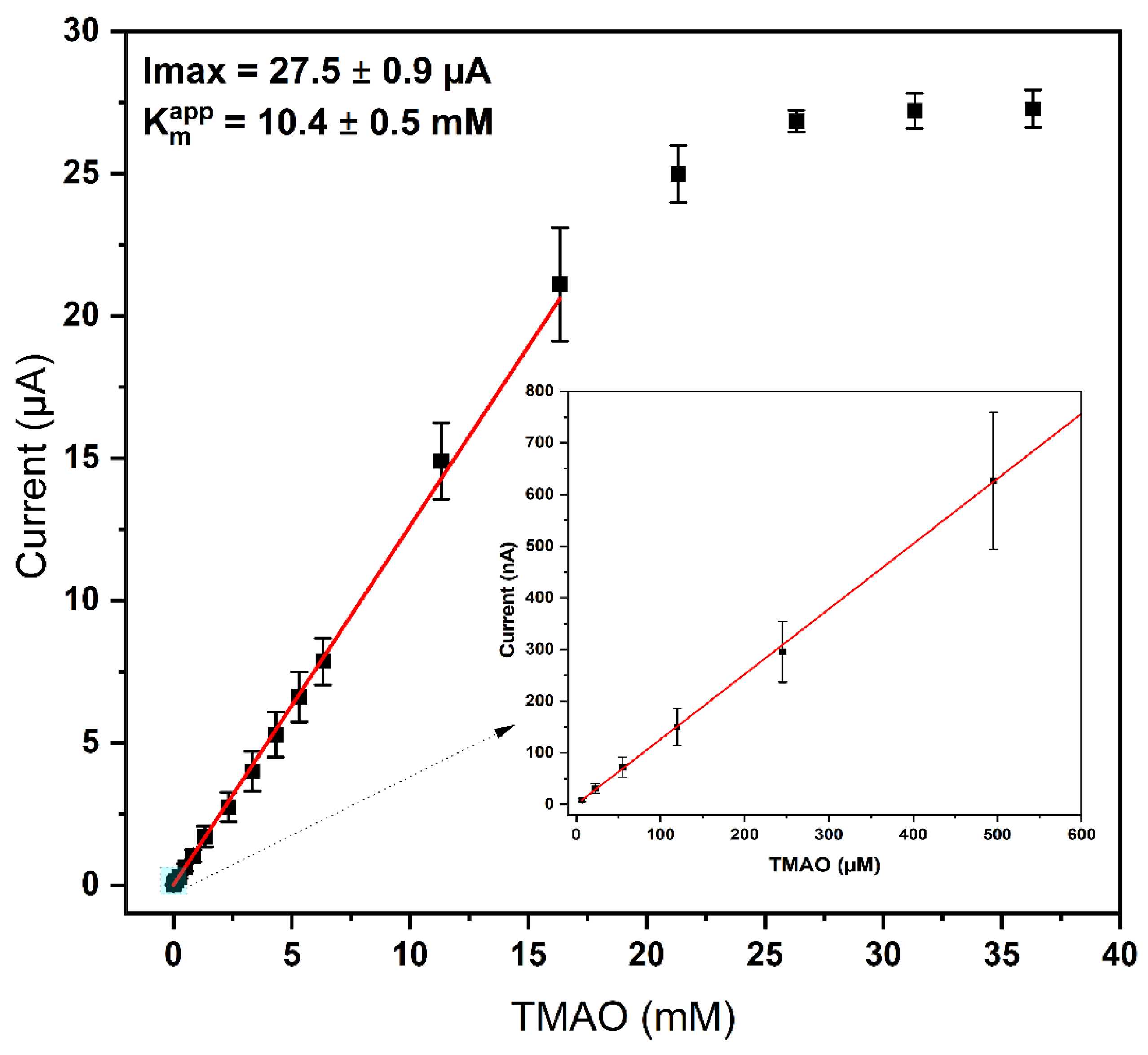
3.5. Biosensor Performance
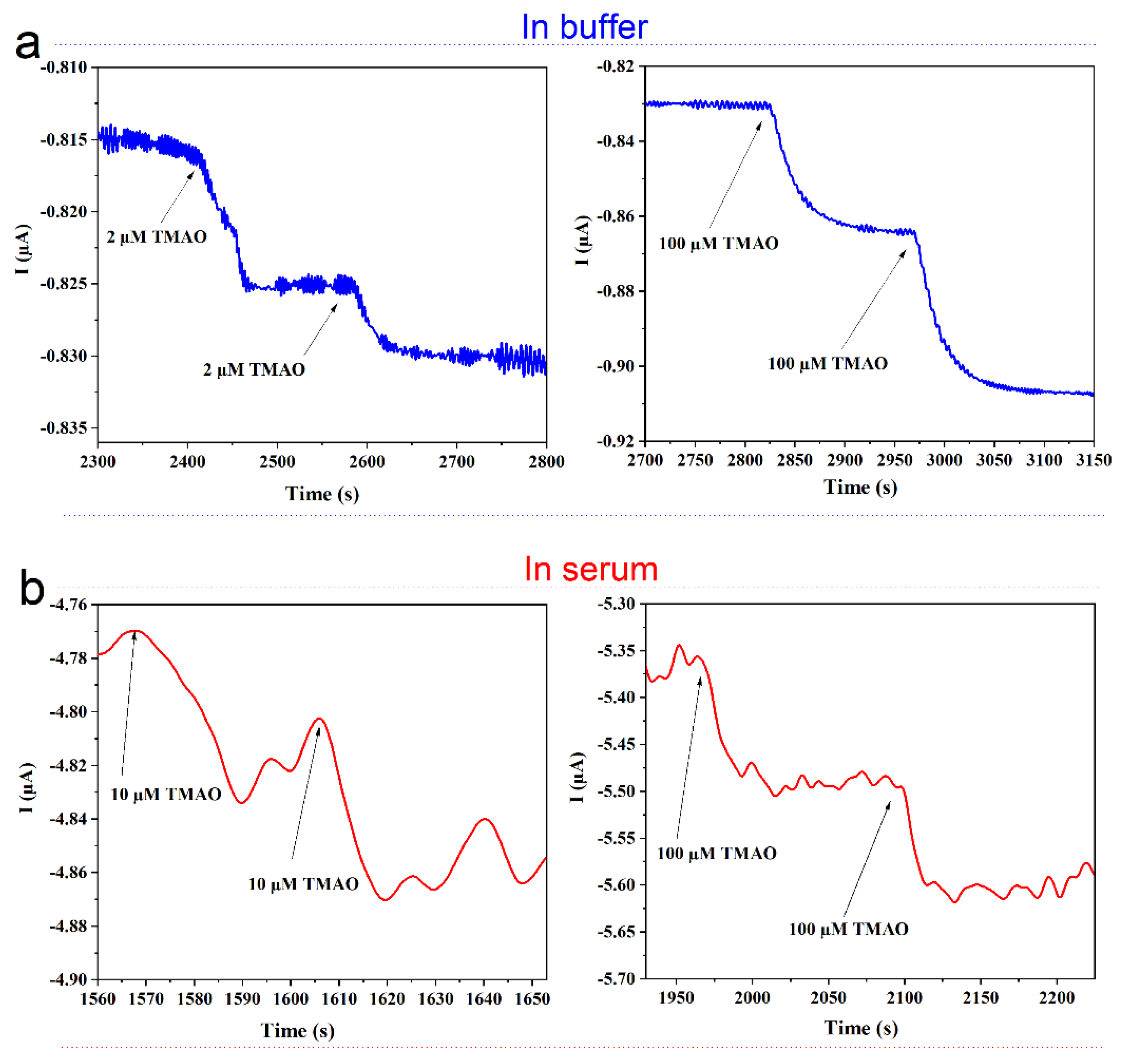
3.6. Sensor Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-oxide: The good, the bad and the unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.C.; Hullar, M.A.J.; Randolph, T.W.; Franke, A.A.; Monroe, K.R.; Cheng, I.; Wilkens, L.R.; Shepherd, J.A.; Madeleine, M.M.; le Marchand, L.; et al. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am. J. Clin. Nutr. 2020, 111, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Mafune, A.; Iwamoto, T.; Tsutsumi, Y.; Nakashima, A.; Yamamoto, I.; Yokoyama, K.; Yokoo, T.; Urashima, M. Associations among serum trimethylamine-N-oxide (TMAO) levels, kidney function and infarcted coronary artery number in patients undergoing cardiovascular surgery: A cross-sectional study. Clin. Exp. Nephrol. 2016, 20, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.R.; House, J.A.; Ocque, A.J.; Zhang, S.; Johnson, C.; Kimber, C.; Schmidt, K.; Gupta, A.; Wetmore, J.B.; Nolin, T.D.; et al. Serum Trimethylamine-N-Oxide is Elevated in CKD and Correlates with Coronary Atherosclerosis Burden. J. Am. Soc. Nephrol. 2016, 27, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Ulrich, C.M.; Neuhouser, M.L.; Malysheva, O.; Bailey, L.B.; Xiao, L.; Brown, E.C.; Cushing-Haugen, K.L.; Zheng, Y.; Cheng, T.Y.D.; et al. Plasma choline metabolites and colorectal cancer risk in the women’s health initiative observational study. Cancer Res. 2014, 74, 7442–7452. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–65. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of trimethylamine n-oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef]
- Mills, G.A.; Walker, V.; Mughal, H. Quantitative determination of trimethylamine in urine by solid-phase microextraction and gas chromatography-mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1999, 723, 281–285. [Google Scholar] [CrossRef]
- Hatton, A.D.; Gibb, S.W. A technique for the determination of trimethylamine-N-oxide in natural waters and biological media. Anal. Chem. 1999, 71, 4886–4891. [Google Scholar] [CrossRef] [PubMed]
- Cháfer-Pericás, C.; Herráez-Hernández, R.; Campíns-Falcó, P. Selective determination of trimethylamine in air by liquid chromatography using solid phase extraction cartridges for sampling. J. Chromatogr. A 2004, 1042, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, Y.; Zhang, X.; Yang, X. A faster and simpler UPLC-MS/MS method for the simultaneous determination of trimethylamine: N -oxide, trimethylamine and dimethylamine in different types of biological samples. Food Funct. 2019, 10, 6484–6491. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, L.; Li, F. Mechanistic Challenges and Advantages of Biosensor Miniaturization into the Nanoscale. ACS Sens. 2017, 2, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Gamati, S.; Luong, J.H.T.; Mulchandani, A. A microbial biosensor for trimethylamine using Pseudomonas aminovorans cells. Biosens. Bioelectron. 1991, 6, 125–131. [Google Scholar] [CrossRef]
- Lakshmi, G.B.V.S.; Yadav, A.K.; Mehlawat, N.; Jalandra, R.; Solanki, P.R.; Kumar, A. Gut microbiota derived trimethylamine N-oxide (TMAO) detection through molecularly imprinted polymer based sensor. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Mitrova, B.; Waffo, A.F.T.; Kaufmann, P.; Nivol, C.I.; Leimkühler, S.; Wollenberger, U. Trimethylamine N-oxide electrochemical biosensor with a chimeric enzyme. ChemElectroChem 2019, 6, 1732–1737. [Google Scholar] [CrossRef]
- Wissenbach, U.; Ternes, D.; Unden, G. An Escherichia coli mutant containing only demethylmenaquinone, but no menaquinone: Effects on fumarate, dimethylsulfoxide, trimethylamine N-oxide and nitrate respiration. Arch. Microbiol. 1992, 158, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Iobbi-Nivol, C.; Pommier, J.; Simala-Grant, J.; Méjean, V.; Giordano, G. High substrate specificity and induction characteristics of trimethylamine-N-oxide reductase of Escherichia coli. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1996, 1294, 77–82. [Google Scholar] [CrossRef]
- Kaufmann, P.; Duffus, B.R.; Mitrova, B.; Iobbi-Nivol, C.; Teutloff, C.; Nimtz, M.; Jänsch, L.; Wollenberger, U.; Leimkühler, S. Modulating the Molybdenum Coordination Sphere of Escherichia coli Trimethylamine N-Oxide Reductase. Biochemistry 2018, 57, 1130–1143. [Google Scholar] [CrossRef] [PubMed]
- Plumeré, N.; Henig, J.; Campbell, W.H. Enzyme-catalyzed O 2 removal system for electrochemical analysis under ambient air: Application in an amperometric nitrate biosensor. Anal. Chem. 2012, 84, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Katsounaros, I.; Schneider, W.B.; Meier, J.C.; Benedikt, U.; Biedermann, P.U.; Auer, A.A.; Mayrhofer, K.J.J. Hydrogen peroxide electrochemistry on platinum: Towards understanding the oxygen reduction reaction mechanism. Phys. Chem. Chem. Phys. 2012, 14, 7384–7391. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.B.; Kochi, J.K. Direct observation of superoxide electron transfer with viologens by immobilization in zeolite. J. Am. Chem. Soc. 1988, 110, 6586–6588. [Google Scholar] [CrossRef]
- Monteiro, T.; Rodrigues, P.R.; Gonçalves, A.L.; Moura, J.J.G.; Jubete, E.; Añorga, L.; Piknova, B.; Schechter, A.N.; Silveira, C.M.; Almeida, M.G. Construction of effective disposable biosensors for point of care testing of nitrite. Talanta 2015, 142, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Yonehara, H.; Fujii, S.I.; Sato, K.; Abo, M.; Yoshimura, E. Construction of a dimethyl sulfoxide sensor based on dimethyl sulfoxide reductase immobilized on a Au film electrode. Anal. Sci. 2007, 23, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Abo, M.; Okubo, A. Development of dimethyl sulfoxide biosensor using a mediator immobilized enzyme electrode. Analyst 2003, 128, 724–727. [Google Scholar] [CrossRef]
- Monteiro, T.; Gomes, S.; Jubete, E.; Añorga, L.; Silveira, C.M.; Almeida, M.G. A quasi-reagentless point-of-care test for nitrite and unaffected by oxygen and cyanide. Sci. Rep. 2019, 9, 2622. [Google Scholar] [CrossRef]
- Michaelis, L.; Hill, E.S. The viologen indicators. J. Gen. Physiol. 1933, 16, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.L.; Kuhn, A.T. Electrochemistry of the viologens. Chem. Soc. Rev. 1981, 10, 49–82. [Google Scholar] [CrossRef]
- Badalyan, A.; Yang, Z.Y.; Seefeldt, L.C. A Voltammetric Study of Nitrogenase Catalysis Using Electron Transfer Mediators. ACS Catal. 2019, 9, 1366–1372. [Google Scholar] [CrossRef]
- Groß, A.; Richter, M.; Kubinski, D.J.; Visser, J.H.; Moos, R. The effect of the thickness of the sensitive layer on the performance of the accumulating NOx sensor. Sensors 2012, 12, 12329–12346. [Google Scholar] [CrossRef]
- Yamamoto, I.; Okubo, N.; Ishimoto, M. Further characterization of trimethylamine N-oxide reductase from Escherichia coli, a molybdoprotein. J. Biochem. 1986, 99, 1773–1779. [Google Scholar] [CrossRef]
- Oughli, A.A.; Conzuelo, F.; Winkler, M.; Happe, T.; Lubitz, W.; Schuhmann, W.; Rüdiger, O.; Plumeré, N. A Redox Hydrogel Protects the O2-Sensitive [FeFe]-Hydrogenase from Chlamydomonas reinhardtii from Oxidative Damage. Angew. Chem. Int. Ed. 2015, 54, 12329–12333. [Google Scholar] [CrossRef]
- Xu, F.J.; Cai, Q.J.; Li, Y.L.; Kang, E.T.; Neoh, K.G. Covalent immobilization of glucose oxidase on well-defined poly(glycidyl methacrylate)-Si(111) hybrids from surface-initiated atom-transfer radical polymerization. Biomacromolecules 2005, 6, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Ghadermarzi, M.; Moosavi-Movahedi, A.A. The effects of temperature and pH on the kinetics of reactions between catalase and its suicide substrate hydrogen peroxide. Ital. J. Biochem. 1997, 46, 197–205. [Google Scholar] [PubMed]
- Jean, B.; Santini, C.L.; Giordani, R.; Czjzek, M.; Wu, L.F.; Giordano, G. Enzymatic and physiological properties of the tungsten-substituted molybdenum TMAO reductase from Escherichia coli. Mol. Microbiol. 1999, 32, 159–168. [Google Scholar] [CrossRef]
- Bourouina, M.; Ourari, A.; Bourouina-Bacha, S. The effect of membrane permeability on the response of a catechol biosensor. Microchim. Acta 2008, 163, 171–178. [Google Scholar] [CrossRef]
- Baronas, R.; Ivanauskas, F.; Kulys, J. The influence of the enzyme membrane thickness on the response of amperometric biosensors. Sensors 2003, 3, 248–262. [Google Scholar] [CrossRef]
- Kühn, T.; Rohrmann, S.; Sookthai, D.; Johnson, T.; Katzke, V.; Kaaks, R.; von Eckardstein, A.; Müller, D. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin. Chem. Lab. Med. 2017, 55, 261–268. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waffo, A.F.T.; Mitrova, B.; Tiedemann, K.; Iobbi-Nivol, C.; Leimkühler, S.; Wollenberger, U. Electrochemical Trimethylamine N-Oxide Biosensor with Enzyme-Based Oxygen-Scavenging Membrane for Long-Term Operation under Ambient Air. Biosensors 2021, 11, 98. https://doi.org/10.3390/bios11040098
Waffo AFT, Mitrova B, Tiedemann K, Iobbi-Nivol C, Leimkühler S, Wollenberger U. Electrochemical Trimethylamine N-Oxide Biosensor with Enzyme-Based Oxygen-Scavenging Membrane for Long-Term Operation under Ambient Air. Biosensors. 2021; 11(4):98. https://doi.org/10.3390/bios11040098
Chicago/Turabian StyleWaffo, Armel F. T., Biljana Mitrova, Kim Tiedemann, Chantal Iobbi-Nivol, Silke Leimkühler, and Ulla Wollenberger. 2021. "Electrochemical Trimethylamine N-Oxide Biosensor with Enzyme-Based Oxygen-Scavenging Membrane for Long-Term Operation under Ambient Air" Biosensors 11, no. 4: 98. https://doi.org/10.3390/bios11040098
APA StyleWaffo, A. F. T., Mitrova, B., Tiedemann, K., Iobbi-Nivol, C., Leimkühler, S., & Wollenberger, U. (2021). Electrochemical Trimethylamine N-Oxide Biosensor with Enzyme-Based Oxygen-Scavenging Membrane for Long-Term Operation under Ambient Air. Biosensors, 11(4), 98. https://doi.org/10.3390/bios11040098





