Highly Sensitive Electrochemical Aptasensor for Detecting the VEGF165 Tumor Marker with PANI/CNT Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
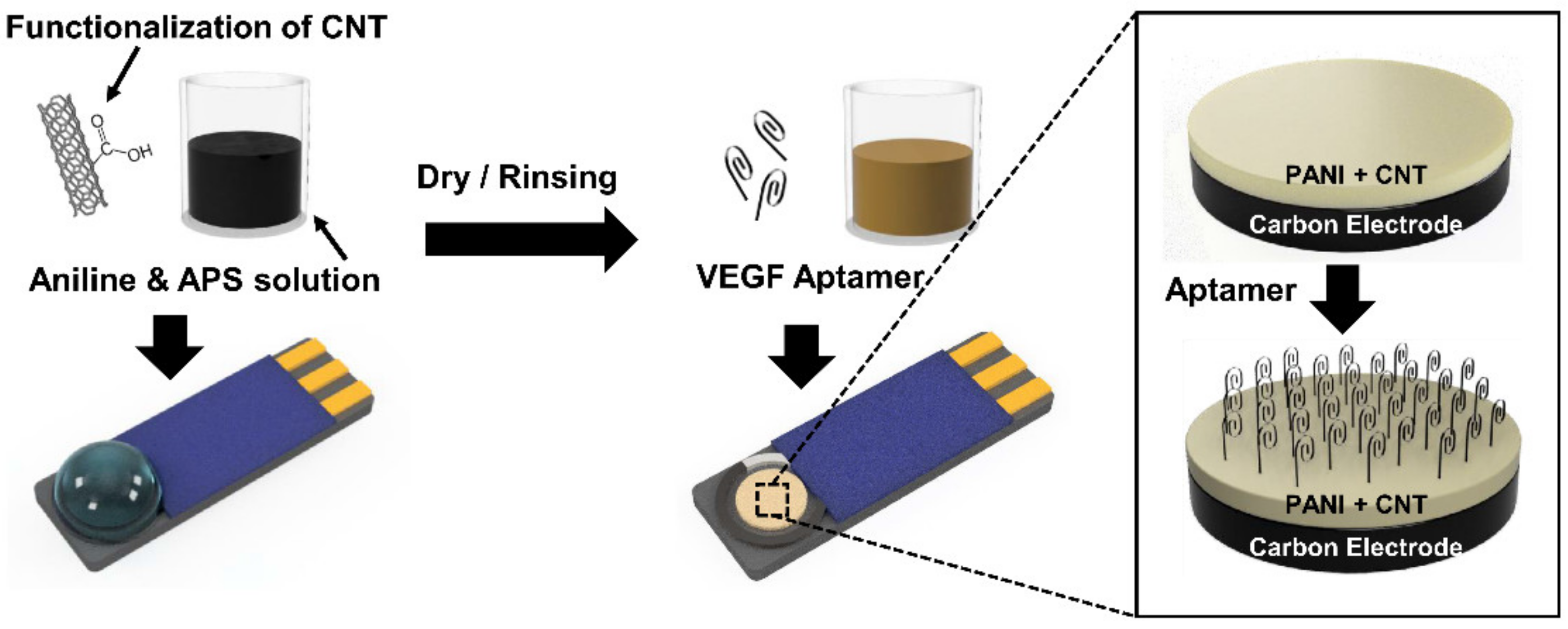
2.2. Fabrication of PANI/CNT Nanocomposite on SPCE
2.3. Immobilization of Aptamer on PANI/CNT Nanocomposite
↓
PANI/CNT nanocomposite − CONH − Anti-VEGF165 RNA aptamer
2.4. Characterization of the Biosensor
2.5. Electrochemical Analysis to Evaluate the Sensitivity of the Biosensor
3. Results and Discussion
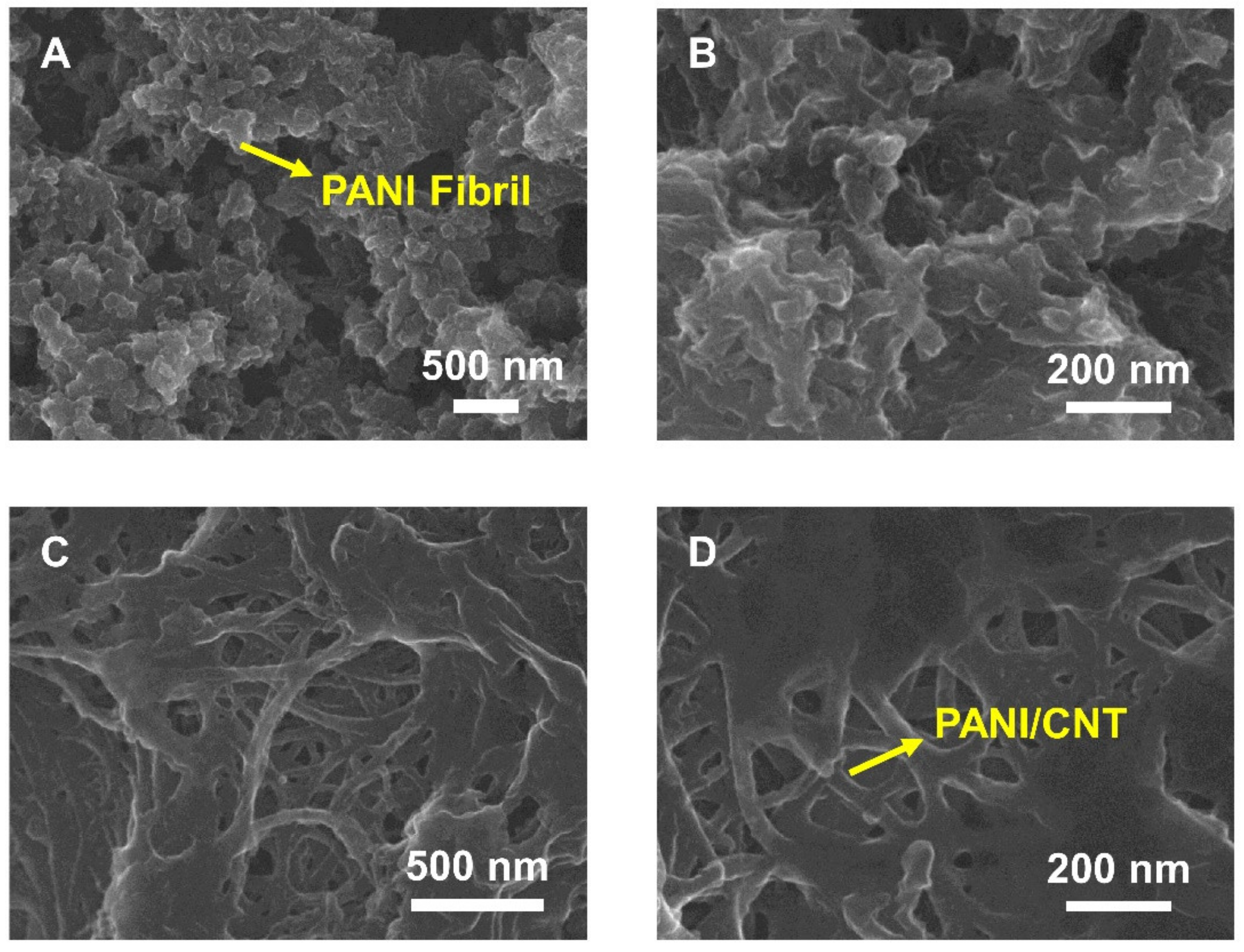
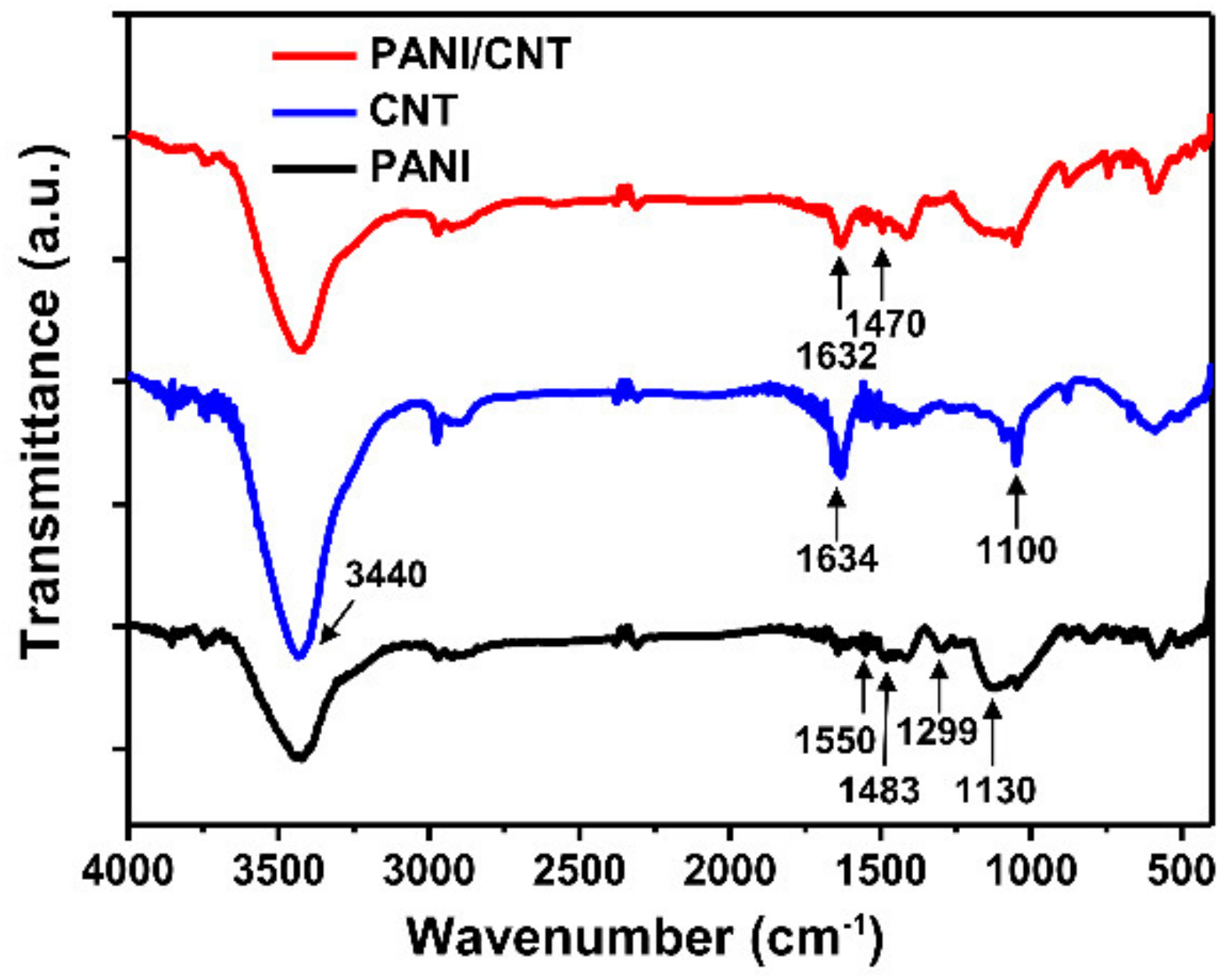
3.1. Characterization of the PANI/CNT Nanocomposite
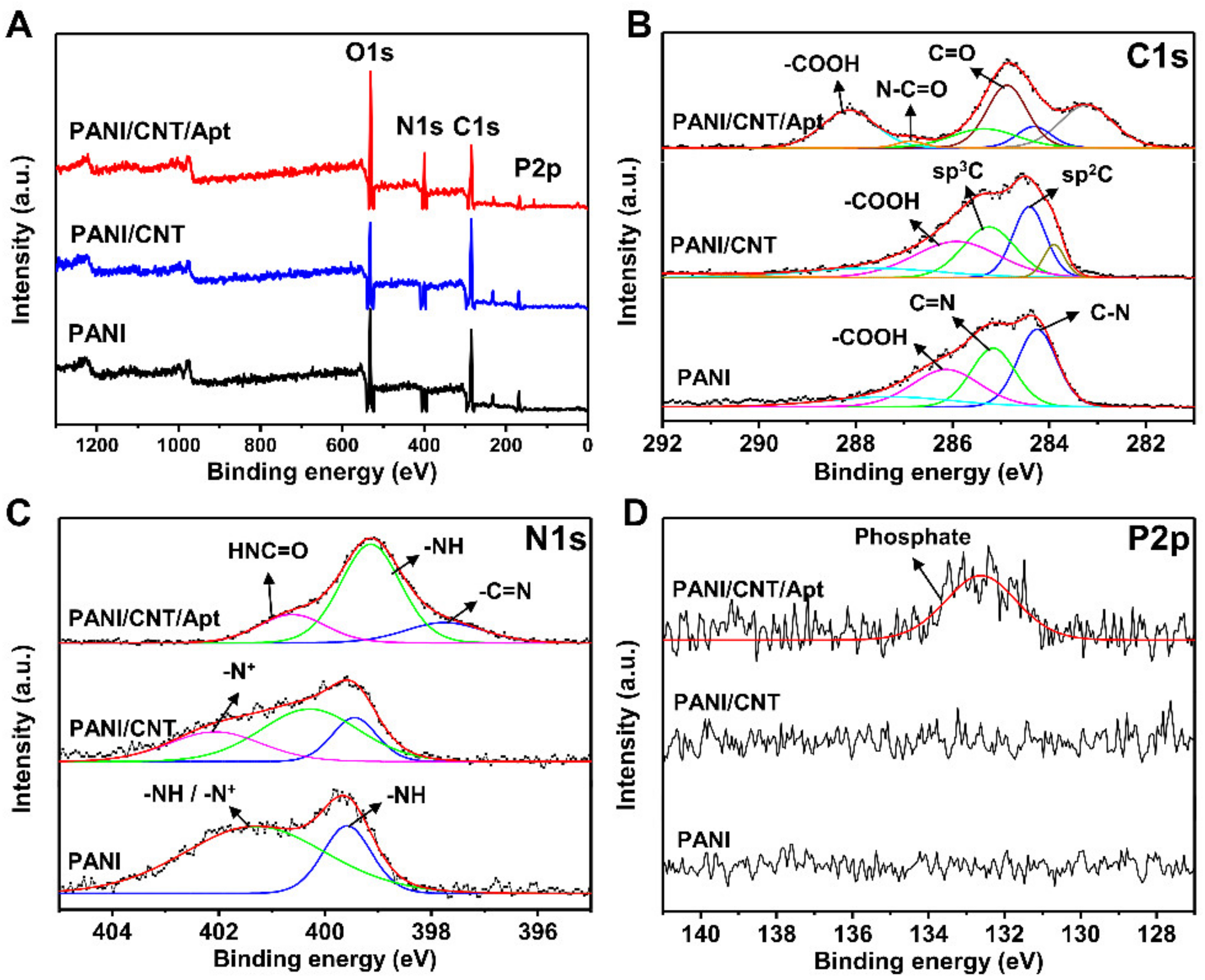
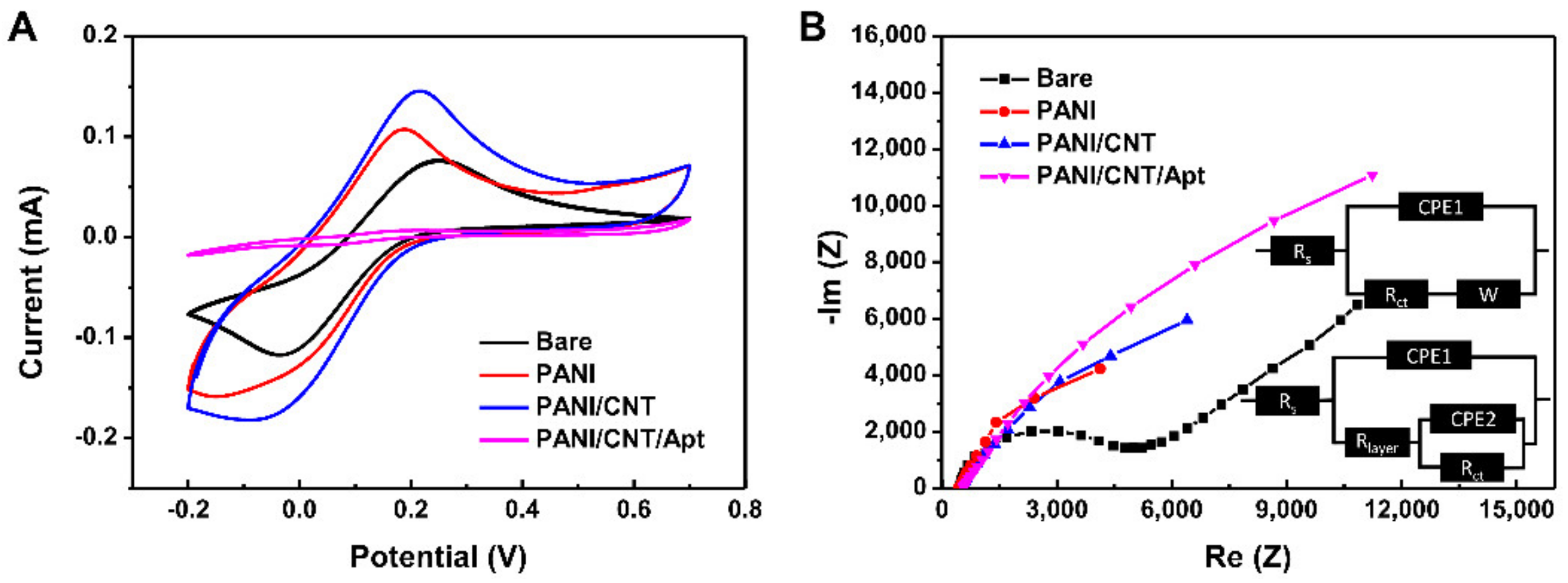
3.2. Characterization of Biosensor Based on SPCE
3.3. Electrochemical Property of the Biosensor Based on an Aptamer with a PANI/CNT Nanocomposite
3.4. VEGF165 Detection and Monitoring
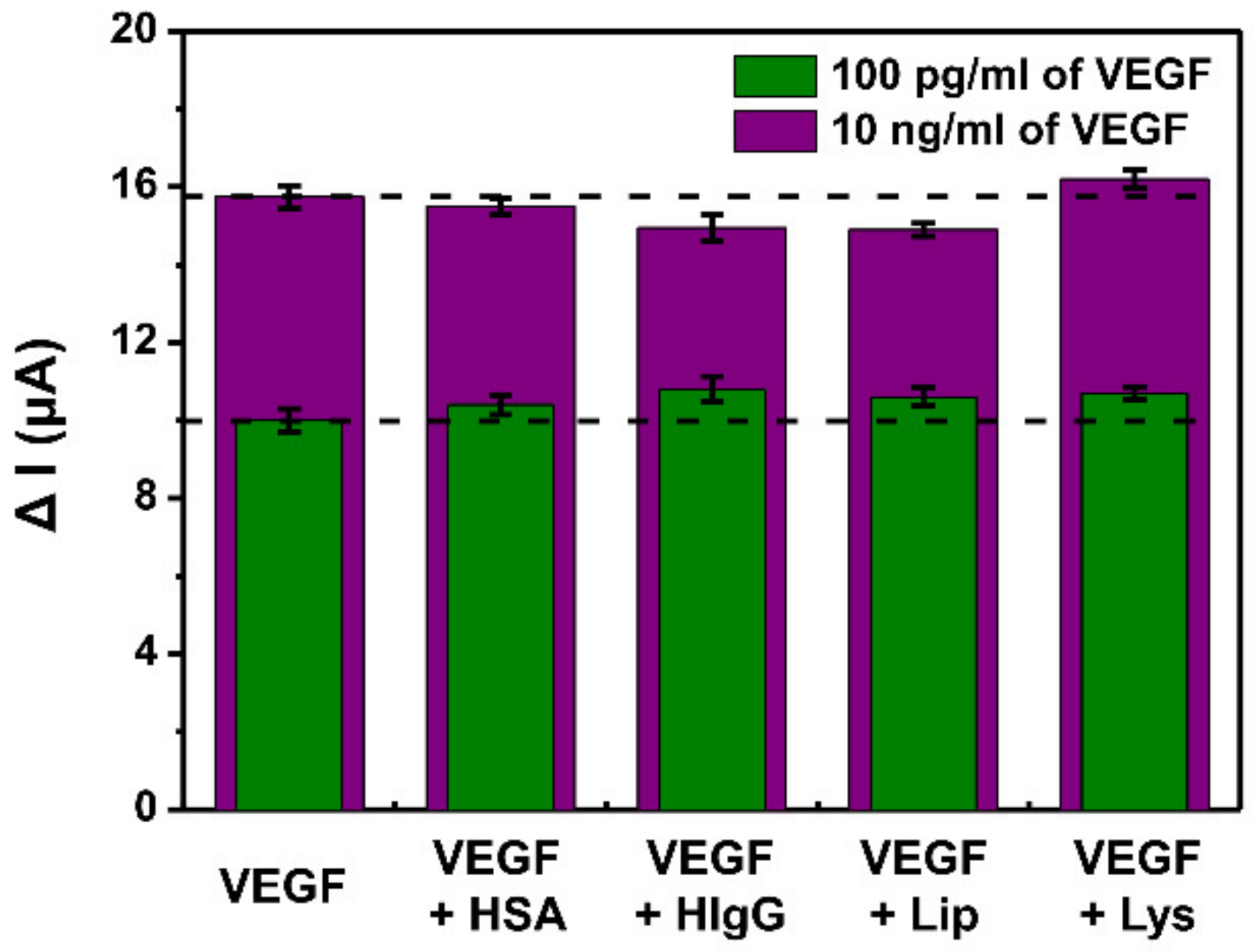
3.5. Selectivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michael, K.; Patricia, A.D. Vascular endothelial growth factor and its receptors. Cytokine Growth Factor Rev. 1996, 7, 259–270. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in signaling and disease: Beyond discovery and development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Bamburowicz-Klimkowska, M.; Poplawska, M.; Grudzinski, I.P. Nanocomposites as biomolecules delivery agents in nanomedicine. J. Nanobiotechnol. 2019, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, R.M.B.; D’Amore, P.A. Transcriptional regulation of vascular endothelial growth factor in cancer. Cytokine Growth Factor Rev. 2005, 16, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Brattström, D.; Bergqvist, M.; Hesselius, P.; Larsson, A.; Wagenius, G.; Brodin, O. Serum VEGF and bFGF adds prognostic information in patients with normal platelet counts when sampled before, during and after treatment for locally advanced non-small cell lung cancer. Lung Cancer 2004, 43, 55–62. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Carenza, E.; Jordan, O.; Segundo, P.M.-S.; Jirik, R.; Starcuk, Z.; Borchard, G.; Rosell, A.; Roig, A. Encapsulation of VEGF165 into magnetic PLGA nanocapsules for potential local delivery and bioactivity in human brain endothelial cells. J. Mater. Chem. B 2015, 3, 2538–2544. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, S.; Jung, K.O.; Song, M.G.; Lee, C.-H.; Chung, S.; Park, J.Y.; Cha, M.G.; Lee, S.G.; Jun, B.-H.; et al. Simultaneous detection of EGFR and VEGF in colorectal cancer using fluorescence-raman endoscopy. Sci. Rep. 2017, 7, 1035. [Google Scholar] [CrossRef]
- Pan, L.-H.; Kuo, S.-H.; Lin, T.-Y.; Lin, C.-W.; Fang, P.-Y.; Yang, H.-W. An electrochemical biosensor to simultaneously detect VEGF and PSA for early prostate cancer diagnosis based on graphene oxide/ssDNA/PLLA nanoparticles. Biosens. Bioelectron. 2017, 89, 598–605. [Google Scholar] [CrossRef]
- Kim, M.; Lezzi, R.; Shim, B.S.; Martin, D.C. Impedimetric biosensors for detecting vascular endothelial growth factor (VEGF) based on poly (3,4-ethylene dioxythiophene) (PEDOT)/gold nanoparticle (AuNP) composites. Front. Chem. 2019, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Park, S.J.; Hong, J.-Y.; Han, A.-R.; Lee, J.S.; Lee, J.S.; Oh, J.H.; Jang, J. Flexible FET-type VEGF aptasensor based on nitrogen-doped graphene converted from conducting polymer. ACS Nano 2012, 6, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Capacitive aptamer–antibody based sandwich assay for the detection of VEGF cancer biomarker in serum. Sens. Actuators B Chem. 2015, 209, 645–651. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Shamsipur, M.; Farzin, L. A high sensitive electrochemical aptasensor for the determination of VEGF165 in serum of lung cancer patient. Biosens. Bioelectron. 2015, 74, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Zhang, Y.S.; Kim, D.-J.; Manbohi, A.; Avci, H.; Silvestri, A.; Aleman, J.; Hu, N.; Kilic, T.; Keung, W.; et al. Aptamer-based microfluidic electrochemical biosensor for monitoring cell-secreted trace cardiac biomarkers. Anal. Chem. 2016, 88, 10019–10027. [Google Scholar] [CrossRef]
- Crulhas, B.P.; Karpik, A.E.; Delella, F.K.; Castro, G.R.; Pedrosa, V.A. Electrochemical aptamer-based biosensor developed to monitor PSA and VEGF released by prostate cancer cells. Anal. Bioanal. Chem. 2017, 409, 6771–6780. [Google Scholar] [CrossRef]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-based biosensors for antibiotic detection: A review. Biosensors 2018, 8, 54. [Google Scholar] [CrossRef]
- Eissa, S.; Siaj, M.; Zourob, M. Aptamer-based competitive electrochemical biosensor for brevetoxin-2. Biosens. Bioelectron. 2015, 69, 148–154. [Google Scholar] [CrossRef]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-based biosensors. Trac. Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Dunn, M.R.; Jimenez, R.M.; Chaput, J.C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 2017, 1, 0076. [Google Scholar] [CrossRef]
- Hong, L.; Zhou, F.; Shi, D.; Zhang, X.; Wang, G. Portable aptamer biosensor of platelet-derived growth factor-BB using a personal glucose meter with triply amplified. Biosens. Bioelectron. 2017, 95, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Kumar, M.; Bansal, K.; Sharma, R.K.; Wangoo, N. Ultrasensitive aptamer biosensor for malathion detection based on cationic polymer and gold nanoparticles. Biosens. Bioelectron. 2016, 85, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, S.; Alam, F.; Pala, N.; Wang, C. A review of electrochemical aptasensors for label-free cancer diagnosis. J. Electrochem. Soc. 2020, 167, 067511. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Yang, Y.; Yuan, Q. Aptamer-functionalized carbon nanomaterials electrochemical sensors for detecting cancer relevant biomolecules. Carbon 2018, 129, 380–395. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, H.; Wang, Z.; Cao, H.; Zhang, K.; Li, X.; Yang, Z. Nanomaterial-based aptamer sensors for arsenic detection. Biosens. Bioelectron. 2020, 148, 111785. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, X.; Nie, T.; Yin, L.; Wang, S.; Zhao, Y.; Wu, H.; Mei, H. A novel electrochemical sensing platform for detection of dopamine based on gold nanobipyramid/multi-walled carbon nanotube hybrids. Anal. Bioanal. Chem. 2020, 412, 2433–2441. [Google Scholar] [CrossRef]
- Landry, M.P.; Ando, H.; Chen, A.Y.; Cao, J.; Kottadiel, V.I.; Chio, L.; Yang, D.; Dong, J.; Lu, T.K.; Strano, M.S. Single-molecule detection of protein efflux from microorganisms using fluorescent single-walled carbon nanotube sensor arrays. Nat. Nanotechnol. 2017, 12, 368–377. [Google Scholar] [CrossRef]
- Jones, L.P.; Stefansson, S.; Kim, M.S.; Ahn, S.N. Comparison of radioimmuno and carbon nanotube field-effect transistor assays for measuring insulin-like growth factor-1 in a preclinical model of human breast cancer. J. Nanobiotechnol. 2011, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Jang, J. Conducting-polymer nanomaterials for high-performance sensor applications: Issues and challenges. Adv. Funct. Mater. 2009, 19, 1567–1576. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Jang, J. A high-performance VEGF aptamer functionalized polypyrrole nanotube biosensor. Biomaterials 2010, 31, 4740–4747. [Google Scholar] [CrossRef]
- Keteklahijani, Y.Z.; Sharif, F.; Roberts, E.P.L.; Sundararaj, U. Enhanced sensitivity of dopamine biosensors: An electrochemical approach based on nanocomposite electrodes comprising polyaniline, nitrogen-doped graphene, and DNA-functionalized carbon nanotubes. J. Electrochem. Soc. 2019, 166, 1415–1425. [Google Scholar] [CrossRef]
- Sen, T.; Mishra, S.; Shimpi, N.G. Synthesis and sensing applications of polyaniline nanocomposites: A review. RSC Adv. 2016, 6, 42196–42222. [Google Scholar] [CrossRef]
- Wan, P.; Wen, X.; Sun, C.; Chandran, B.K.; Zhang, H.; Sun, X.; Chen, X. Flexible transparent films based on nanocomposite networks of polyaniline and carbon nanotubes for high-performance gas sensing. Small 2015, 11, 5409–5415. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wang, W.; Guo, Y.; Liu, G.; Wan, P. Flexible polyaniline/carbon nanotube nanocomposite film-based electronic gas sensors. Sens. Actuators B Chem. 2017, 244, 47–53. [Google Scholar] [CrossRef]
- Hong, M.-S.; Park, Y.; Kim, T.; Kim, K.; Kim, J.-G. Polydopamine/carbon nanotube nanocomposite coating for corrosion resistance. J. Materiomics. 2020, 6, 158–166. [Google Scholar] [CrossRef]
- Kunishima, M.; Kawachi, C.; Hioki, K.; Terao, K.; Tani, S. Formation of carboxamides by direct condensation of carboxylic acids and amines in alcohols using a new alcohol- and water-soluble condensing agent: DMT-MM. Tetrahedron 2001, 57, 1551–1558. [Google Scholar] [CrossRef]
- Kaykha, Y.; Rafizadeh, M. Template synthesis of fibrillar polyaniline complex using a degradable polyelectrolyte. Mater. Chem. Phys. 2019, 229, 98–105. [Google Scholar] [CrossRef]
- Maity, D.; Manoharan, M.; Kumar, R.T.R. Development of the PANI/MWCNT Nanocomposite-Based Fluorescent Sensor for Selective Detection of Aqueous Ammonia. ACS Omega 2020, 5, 8414–8422. [Google Scholar] [CrossRef]
- Chung, S.; Moon, J.-M.; Choi, J.; Hwang, H.; Shim, Y.-B. Magnetic force assisted electrochemical sensor for the detection of thrombin with aptamer-antibody sandwich formation. Biosens. Bioelectron. 2018, 117, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Hong, M.-S.; Park, Y.; Kim, J.G.; Kim, K. Effect of incorporating MoS2 in organic coatings on the corrosion resistance of 316L stainless steel in a 3.5% NaCl solution. Coatings 2019, 9, 45. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kim, K.S.; Kim, C.-J.; Hahn, S.K.; Jo, M.-H. Electrical detection of VEGFs for cancer diagnoses using anti-vascular endotherial growth factor aptamer-modified Si nanowire FETs. Biosens. Bioelectron. 2009, 24, 1801–1805. [Google Scholar] [CrossRef]

| Modified Electrode | Detection Method | Linear Range (pg/mL) | LOD (pg/mL) | Reference |
|---|---|---|---|---|
| GE/GO/ssDNA/PLLA NP | DPV | 50.0–1.0 × 105 | 50 | Pan et al. 2017 [11] |
| PPy-NDFLG-FETs/Apt | FET | 4.5–4.5 × 105 | 4.5 | Kwon et al. 2012 [13] |
| GE/Apt/MB-Abs | nFIS | 5.0–1.0 × 103 | 401.0 | Qureshi et al. 2015 [14] |
| SPE/OMC-Aunano/Apt | EIS | 10.0–300.0 | 1.0 | Tabrizi et al. 2015 [15] |
| GE/Thiolated Apt | SWV | 150.0–1.0 × 105 | 150.0 | Crulhas et al. 2017 [17] |
| CPNT-FETs/Apt | FET | 18.0–18.0 × 107 | 18.0 | Kwon et al. 2010 [32] |
| SiNW-FETs/Apt | FET | 100.0–45.0 × 103 | 100.0 | Lee et al. 2009 [44] |
| SPE/PANI/CNT/Apt | DPV | 0.5–10.0 × 106 | 0.4 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Hong, M.-S.; Lee, W.-H.; Kim, J.-G.; Kim, K. Highly Sensitive Electrochemical Aptasensor for Detecting the VEGF165 Tumor Marker with PANI/CNT Nanocomposites. Biosensors 2021, 11, 114. https://doi.org/10.3390/bios11040114
Park Y, Hong M-S, Lee W-H, Kim J-G, Kim K. Highly Sensitive Electrochemical Aptasensor for Detecting the VEGF165 Tumor Marker with PANI/CNT Nanocomposites. Biosensors. 2021; 11(4):114. https://doi.org/10.3390/bios11040114
Chicago/Turabian StylePark, Yunjeong, Min-Sung Hong, Woo-Hyuk Lee, Jung-Gu Kim, and Kyunghoon Kim. 2021. "Highly Sensitive Electrochemical Aptasensor for Detecting the VEGF165 Tumor Marker with PANI/CNT Nanocomposites" Biosensors 11, no. 4: 114. https://doi.org/10.3390/bios11040114
APA StylePark, Y., Hong, M.-S., Lee, W.-H., Kim, J.-G., & Kim, K. (2021). Highly Sensitive Electrochemical Aptasensor for Detecting the VEGF165 Tumor Marker with PANI/CNT Nanocomposites. Biosensors, 11(4), 114. https://doi.org/10.3390/bios11040114






