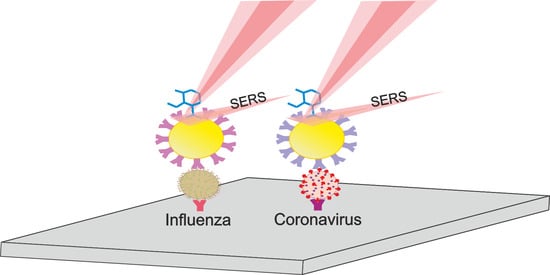
A Review on SERS-Based Detection of Human Virus Infections: Influenza and Coronavirus
Abstract
1. Introduction
2. Principles of SERS
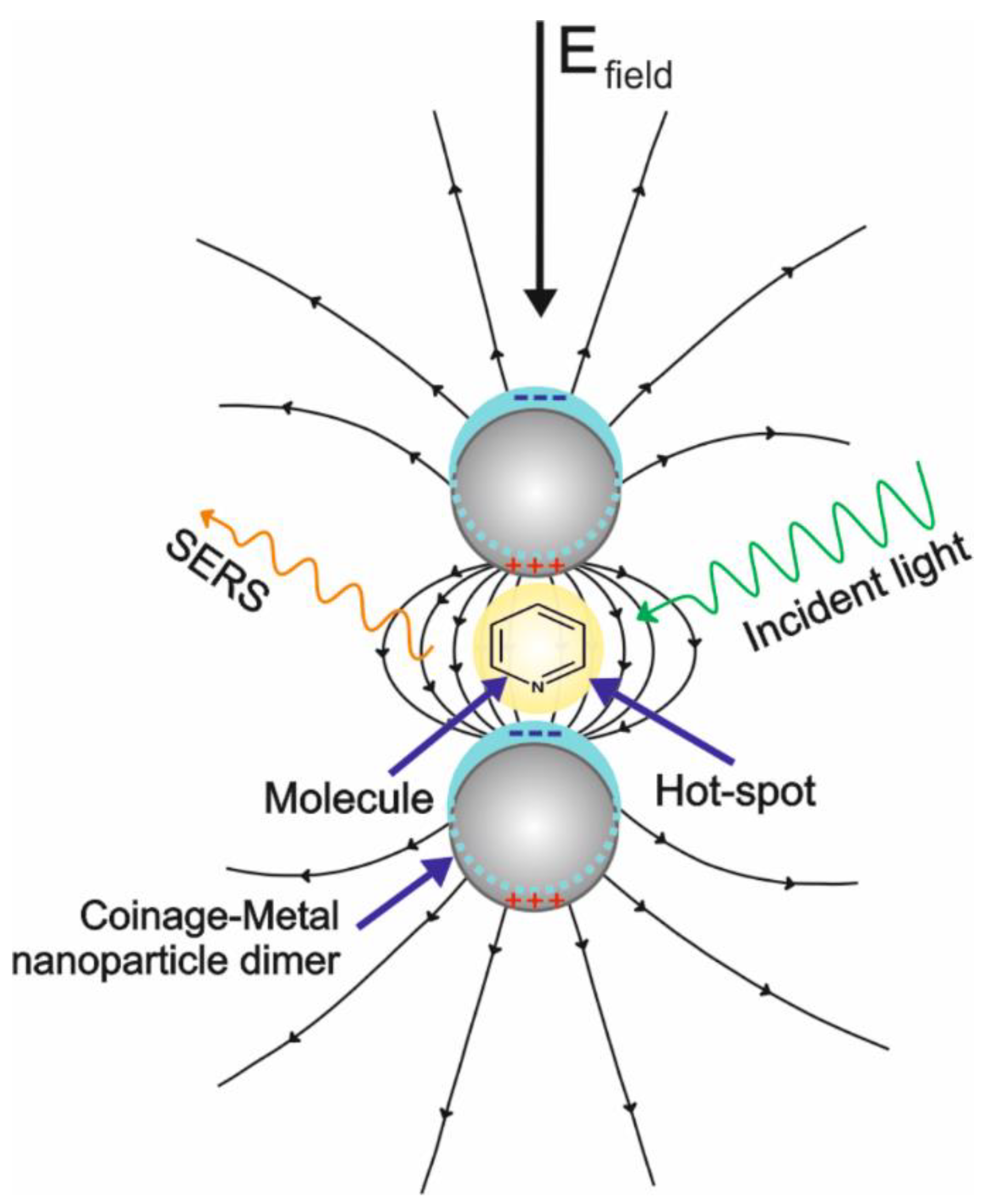
2.1. Enhancement Mechanisms
2.2. SERS Substrates
2.3. SERS Measurements on Solid Substrates
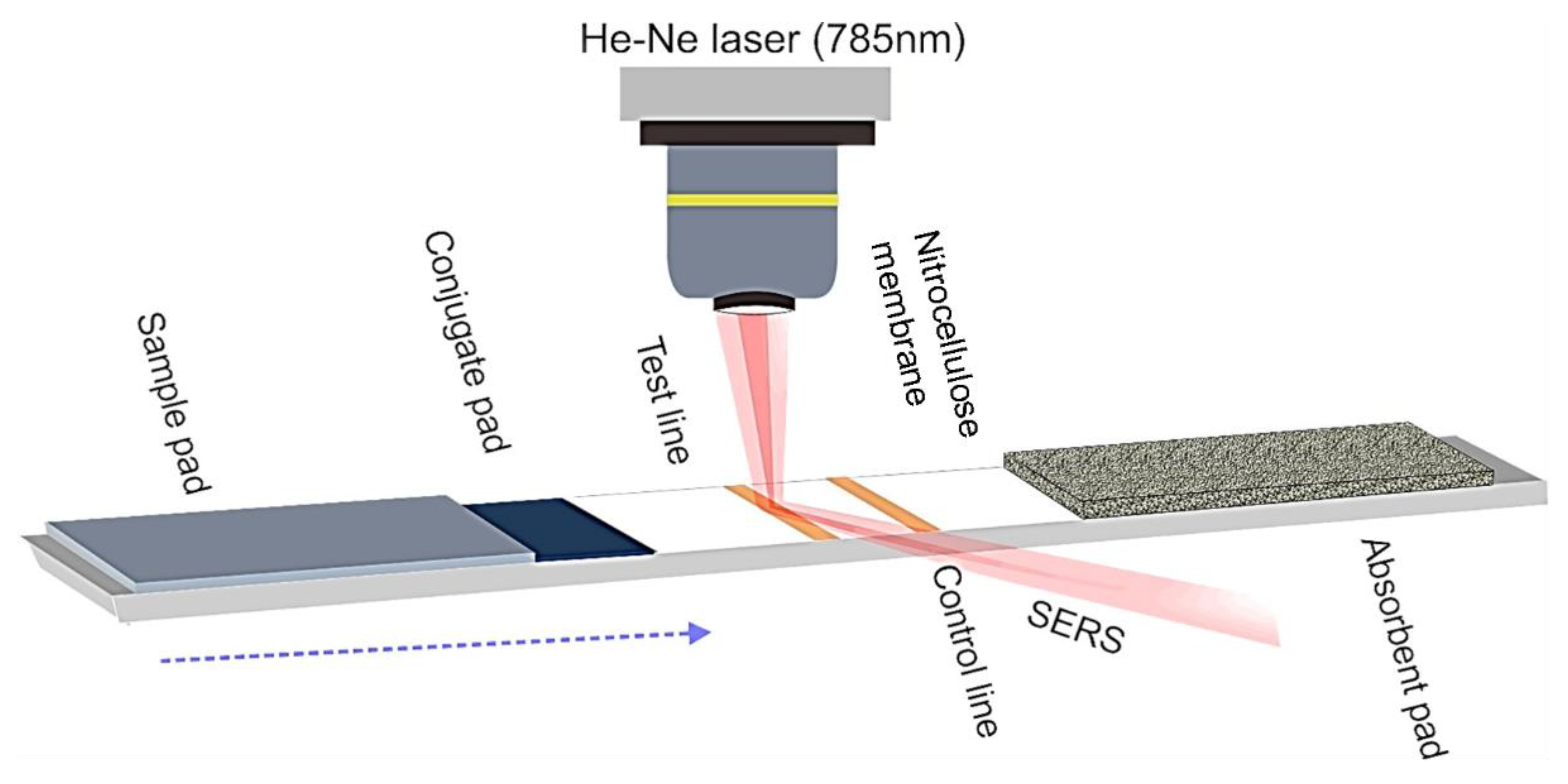
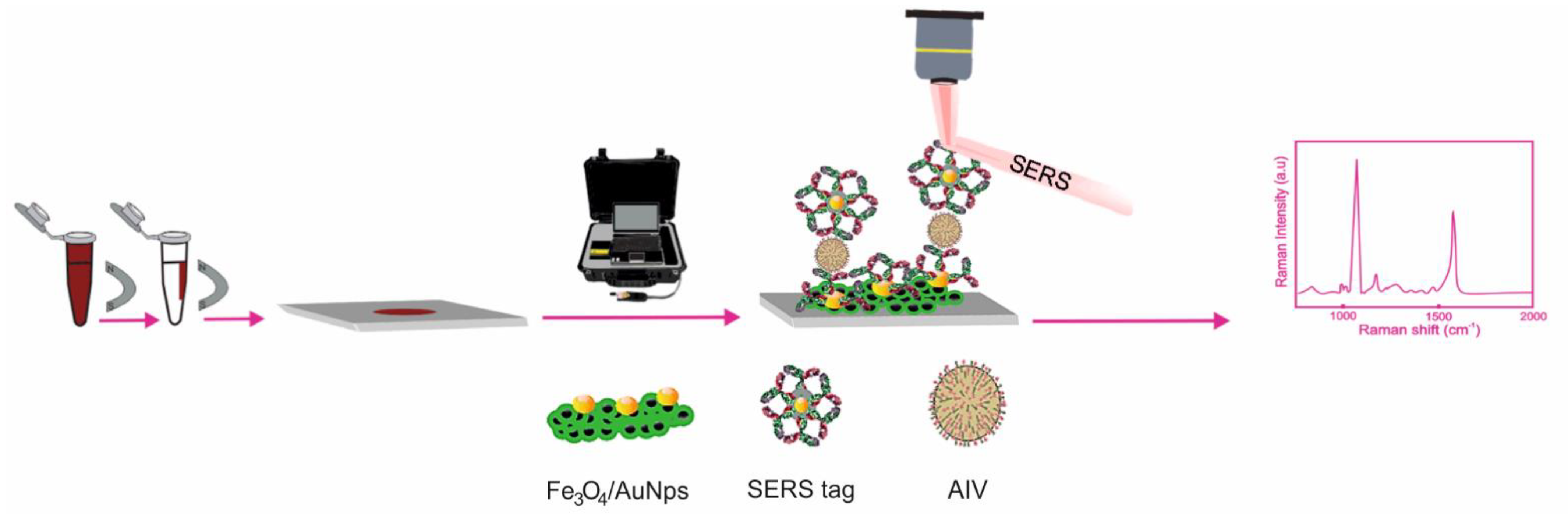
2.4. Detection of Analytes by SERS
2.5. Determination of Viruses by SERS
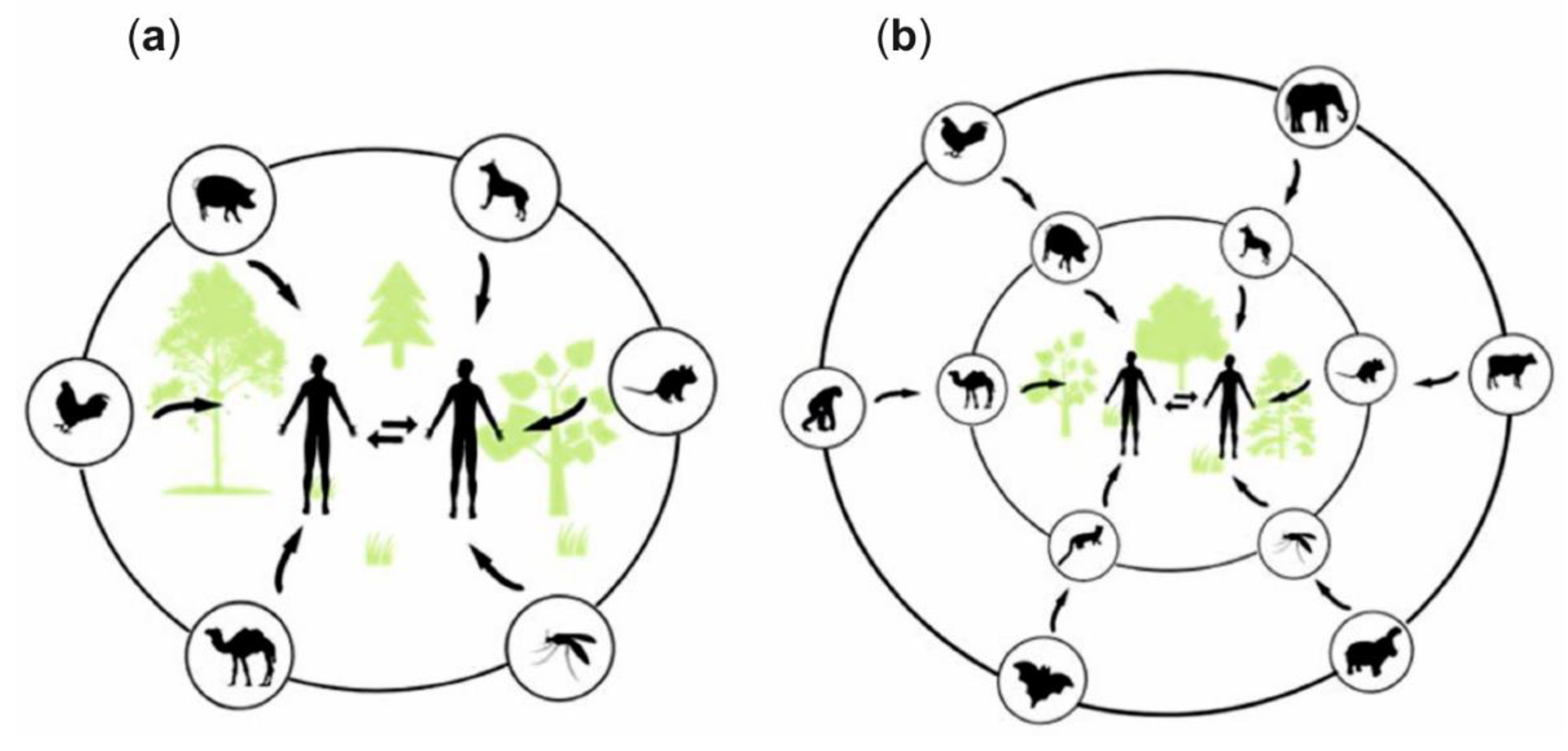
3. Zoonotic Infections
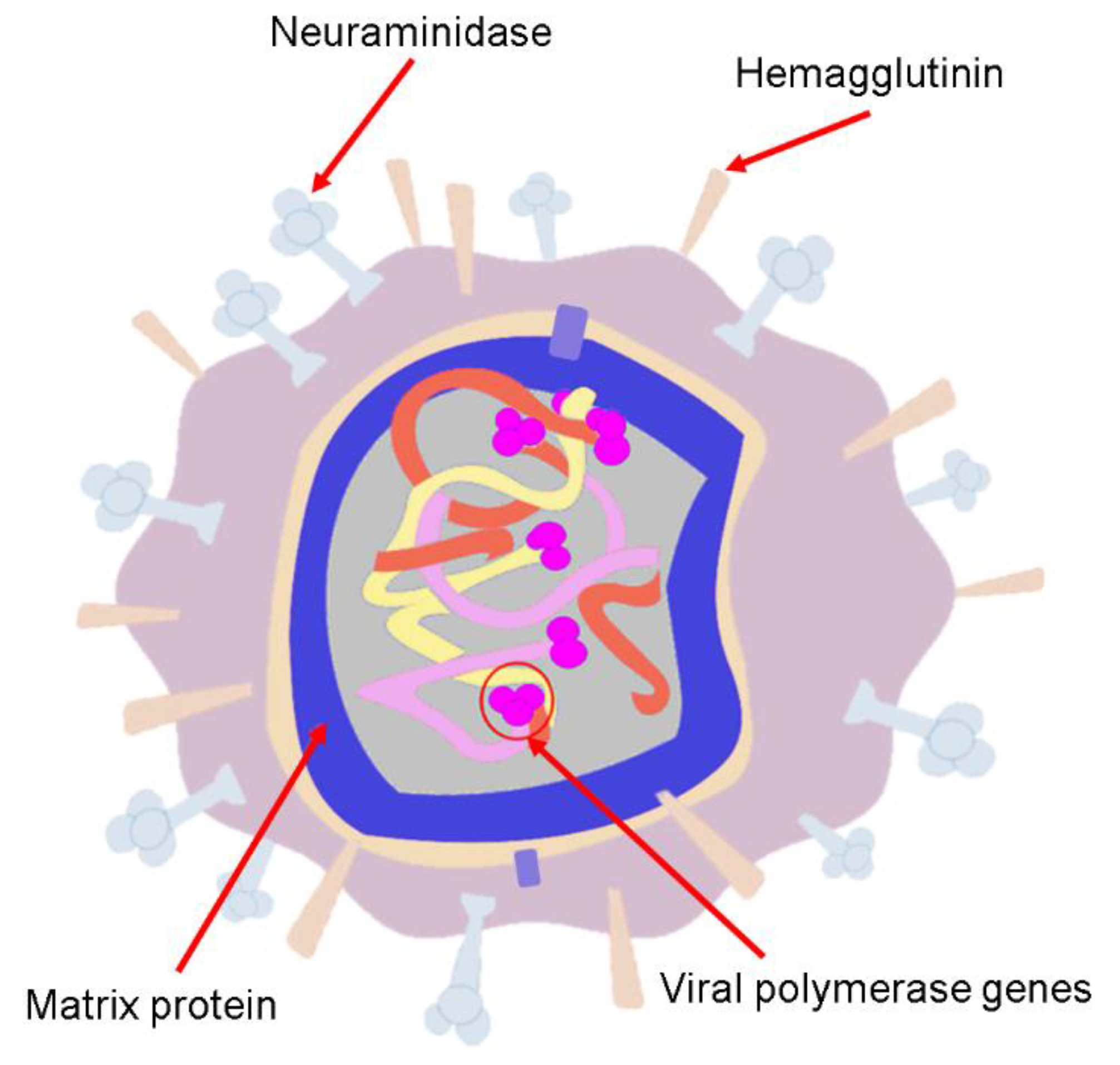
4. Human Influenza Viruses
4.1. Influenza A(H1N1) Virus (Spanish Flu)
SERS Detection of Influenza A(H1N1) Virus
4.2. Influenza A(H7N9) Virus
SERS Detection of A(H7N9)
4.3. Influenza A(H3N2) Virus
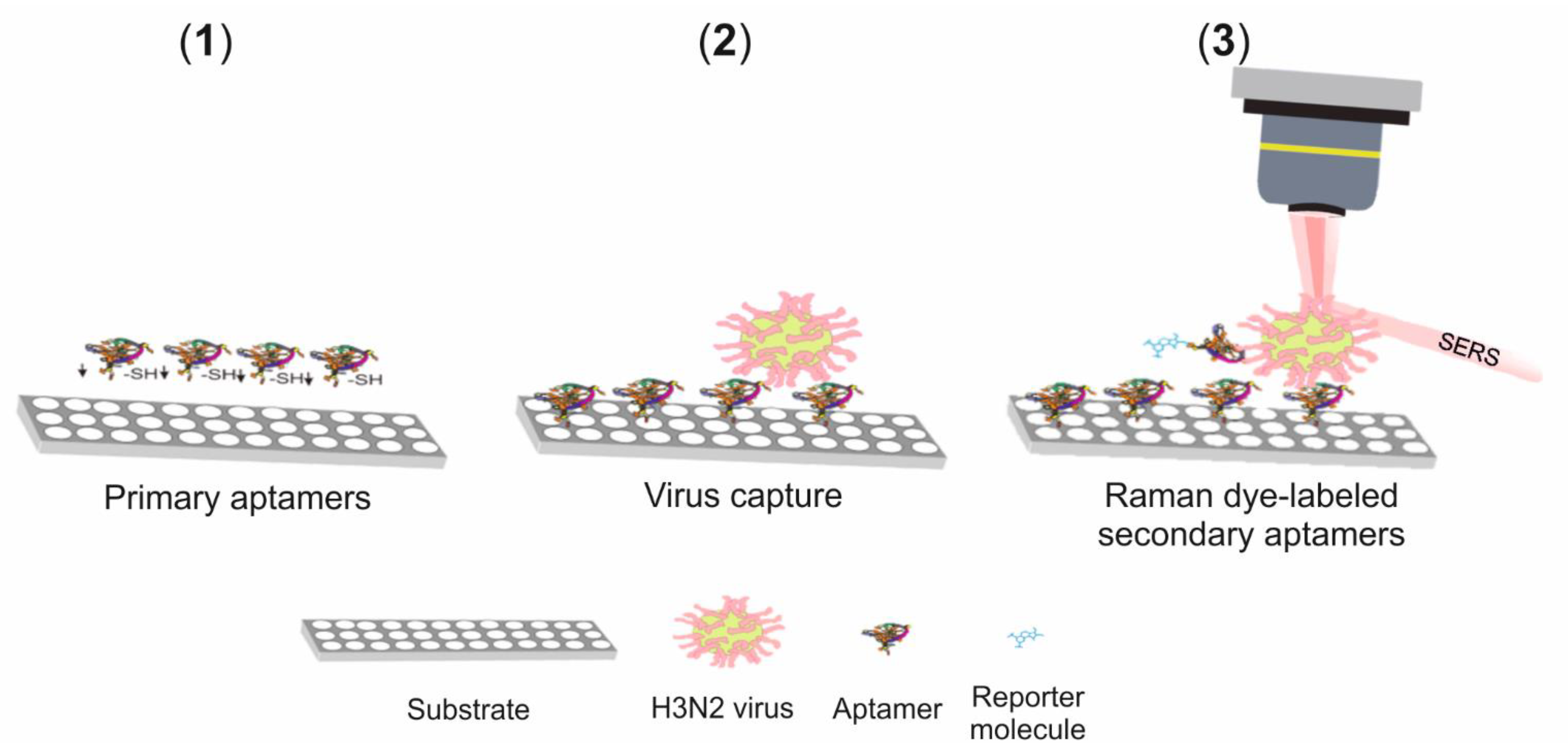
SERS Detection of A(H3N2)
4.4. Influenza A(H5N1)Virus
SERS Detection of A(H5N1)
5. Coronaviruses
5.1. SARS-CoV-2 Virus
SERS Detection of SARS-CoV-2 Virus
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singhal, T. A Review of Coronavirus Disease–2019 (COVID–19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Yao, X.; Zhao, Y.; Wu, J.; Huang, P.; Pan, C.; Liu, S.; Pan, C. Comparative review of respiratory diseases caused by coronaviruses and influenza A viruses during epidemic season. Microb. Infect. 2020, 22, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, O.; Manchanda, V.; Yadav, A.; Sagar, T.; Tuteja, S.; Nagi, N.; Saxena, S. Comparison of two real–time polymerase chain reaction assays for the detection of severe acute respiratory syndrome–CoV–2 from combined nasopharyngeal–throat swabs. Indian J. Med. Microbiol. 2020, 38, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.A.; AL-Samarraie, M.Q.; Ahmed, M.T. Molecular techniques of viral diagnosis. Sci. Arc. 2020, 1, 98–101. [Google Scholar]
- Shahi, S.; Vahed, S.Z.; Fathi, N.; Sharifi, S. Polymerase chain reaction (PCR)–based methods: Promising molecular tools in dentistry. Int. J. Biol. Macromol. 2018, 117, 983–992. [Google Scholar] [CrossRef]
- Kim, G.U.; Kim, M.J.; Ra, S.H.; Lee, J.; Bae, S.; Jung, J.; Kim, S.H. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID–19. Clin. Microbiol. 2020, 26, 948.e1–948.e3. [Google Scholar]
- Barbau-Piednoir, E.; Denayer, S.; Botteldoorn, N.; Dierick, K.; De Keersmaecker, S.C.; Roosens, N.H. Detection and discrimination of five E. coli pathotypes using a combinatory SYBR® Green qPCR screening system. Appl. Microbiol. Biotechnol. 2018, 102, 3267–3285. [Google Scholar] [CrossRef]
- Wu, N.N. (Sensor Division Outstanding Achievement Award Address) Paper-Based Lateral Flow Strips as Point-of-Care Testing Tools for Detection of Biomarkers and Viruses. ECS Meet. Abstr. 2020, 66, 3328. [Google Scholar] [CrossRef]
- Xu, K.; Zhou, R.; Takei, K.; Hong, M. Toward flexible surface-enhanced Raman scattering (SERS) sensors for point-of-care diagnostics. Adv. Sci. 2019, 6, 1900925. [Google Scholar] [CrossRef]
- Chen, H.; Das, A.; Bi, L.; Choi, N.; Moon, J.; Wu, Y.; Park, S.; Choo, J. Recent advances in surface–enhanced Raman scattering–based microdevices for point–of–care diagnosis of viruses and bacteria. Nanoscale 2020, 12, 21560–21570. [Google Scholar] [CrossRef]
- Tran, V.; Walkenfort, B.; König, M.; Salehi, M.; Schlücker, S. Rapid, Quantitative, and Ultrasensitive Point-of-Care Testing: A Portable SERS Reader for Lateral Flow Assays in Clinical Chemistry. Angew. Chem. Int. Ed. 2019, 58, 442–446. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Guo, J.; Ma, X. Nanomaterial Labels in lateral flow immunoassays for point–of–care–testing. J. Mater. Sci. Technol. 2020, 60, 90–104. [Google Scholar] [CrossRef]
- Kim, K.; Kashefi-Kheyrabadi, L.; Joung, Y.; Kim, K.; Dang, H.; Chavan, S.G.; Lee, M.; Choo, J. Recent advances in sensitive surface–enhanced Raman scattering–based lateral flow assay platforms for point–of–care diagnostics of infectious diseases. Sens. Actuators B Chem. 2020, 329, 129214. [Google Scholar] [CrossRef]
- Demirel, G.; Usta, H.; Yilmaz, M.; Celik, M.; Alidagi, H.A.; Buyukserin, F. Surface–enhanced Raman spectroscopy (SERS): An adventure from plasmonic metals to organic semiconductors as SERS platforms. J. Mater. Chem. C 2018, 6, 5314–5335. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, P.; Das, A.; Pathak, C.S. Surface-Enhanced Raman Scattering: Introduction and Applications. In Recent Advances in Nanophotonics-Fundamentals and Applications; IntechOpen: London, UK, 2020; pp. 1–24. [Google Scholar]
- Moore, T.J.; Moody, A.S.; Payne, T.D.; Sarabia, G.M.; Daniel, A.R.; Sharma, B. In vitro and in vivo SERS biosensing for disease diagnosis. Biosensors 2018, 8, 46. [Google Scholar] [CrossRef]
- Panneerselvam, R.; Liu, G.-K.; Wang, Y.-H.; Liu, J.-Y.; Ding, S.-Y.; Li, J.-F.; Wu, D.-Y.; Tian, Z.-Q. Surface–enhanced Raman spectroscopy: Bottlenecks and future directions. Chem. Commun. 2018, 54, 10–25. [Google Scholar] [CrossRef]
- Pérez–Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface–enhanced Raman spectroscopy: Benefits, trade–offs and future developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef]
- Kim, J.; Jang, Y.; Kim, N.-J.; Kim, H.; Yi, G.-C.; Shin, Y.; Kim, M.H.; Yoon, S. Study of chemical enhancement mechanism in nonplasmonic surface enhanced Raman spectroscopy (SERS). Front. Chem. 2019, 7, 582. [Google Scholar] [CrossRef]
- Cui, L.; Wu, D.-Y.; Wang, A.; Ren, B.; Tian, Z.-Q. Charge–transfer enhancement involved in the SERS of adenine on Rh and Pd demonstrated by ultraviolet to visible laser excitation. J. Phys. Chem. C 2010, 114, 16588–16595. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A. Review of SERS substrates for chemical sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Deng, M.; Rao, G.; Yan, Y.; Wu, C.; Jiao, Y.; Deng, A.; Yan, C.; Huang, J.; Wu, S.; et al. High-Performance SERS Substrate Based on Hierarchical 3D Cu Nanocrystals with Efficient Morphology Control. Small 2018, 14, 1802477. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bao, Z.; Wu, Y.; Liu, J. Clean and reproducible SERS substrates for high sensitive detection by solid phase synthesis and fabrication of Ag-coated Fe3O4 microspheres. J. Raman Spectrosc. 2012, 43, 848–856. [Google Scholar] [CrossRef]
- Suresh, V.; Ding, L.; Chew, A.B.; Yap, F.L. Fabrication of large–area flexible SERS substrates by nanoimprint lithography. ACS Appl. Nano Mater. 2018, 1, 886–893. [Google Scholar] [CrossRef]
- Martínez-Torres, P.G.; Martínez-García, M.M.; Cardoso-Ávila, P.E.; Pichardo–Molina, J.L. Facile nanostructured substrate preparation using gold nanocuboids for SERS. Nanomater. Nanotechnol. 2015, 5, 5–12. [Google Scholar] [CrossRef]
- López-Castaños, K.A.; Ortiz-Frade, L.A.; Méndez, E.; Quiroga-González, E.; González-Fuentes, M.A.; Méndez-Albores, A. Indirect Quantification of Glyphosate by SERS Using an Incubation Process With Hemin as the Reporter Molecule: A Contribution to Signal Amplification Mechanism. Front. Chem. 2020, 8, 1209. [Google Scholar] [CrossRef]
- Jahn, I.; Žukovskaja, O.; Zheng, X.-S.; Weber, K.; Bocklitz, T.; Cialla-May, D.; Popp, J. Surface–enhanced Raman spectroscopy and microfluidic platforms: Challenges, solutions and potential applications. Analyst 2017, 142, 1022–1047. [Google Scholar] [CrossRef] [PubMed]
- Grilli, S.; Miccio, L.; Gennari, O.; Coppola, S.; Vespini, V.; Battista, L.; Orlando, P.; Ferraro, P. Active accumulation of very diluted biomolecules by nano–dispensing for easy detection below the femtomolar range. Nat. Commun. 2014, 5, 5314. [Google Scholar] [CrossRef]
- Kumar, S.; Cherukulappurath, S.; Johnson, T.W.; Oh, S.-H. Milimeter–sized suspended plasmonic nanohole arrays for surface–tension–driven flow–through SERS. Chem. Mater. 2014, 26, 6523–6530. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-L.; Gao, Y.; Li, Y.; Li, X.; Zhang, H.; Han, X.X.; Zhao, B.; Su, L. Indirect glyphosate detection based on ninhydrin reaction and surface–enhanced Raman scattering spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 197, 78–82. [Google Scholar] [CrossRef]
- Kearns, H.; Shand, N.; Smith, W.; Faulds, K.; Graham, D. 1064 nm SERS of NIR active hollow gold nanotags. Phys. Chem. Chem. Phys. 2015, 17, 1980–1986. [Google Scholar] [CrossRef]
- Kerr, L.T.; Byrne, H.J.; Hennelly, B.M. Optimal choice of sample substrate and laser wavelength for Raman spectroscopic analysis of biological specimen. Anal. Methods 2015, 7, 5041–5052. [Google Scholar] [CrossRef]
- Kim, N.; Thomas, M.R.; Bergholt, M.S.; Pence, I.J.; Seong, H.; Charchar, P.; Todovora, N.; Nagelkerke, A.; Belessiotis–Richards, A.; Payne, D.J.; et al. Surface enhanced Raman scattering artificial nose for high dimensionality fingerprinting. Nat. Commun. 2020, 11, 207. [Google Scholar] [CrossRef]
- Hubálek, Z. Emerging human infectious diseases: Anthroponoses, zoonoses, and sapronoses. Emerg. Infect. Dis. 2003, 9, 403–404. [Google Scholar] [CrossRef]
- Bueno–Marí, R.; Almeida, A.; Navarro, J.C. Emerging zoonoses: Eco–epidemiology, involved mechanisms, and public health implications. Front. Public Health 2015, 3, 157. [Google Scholar] [PubMed]
- World Health Organization. Available online: http://www.emro.who.int/about-who/rc61/zoonotic-diseases.html (accessed on 4 November 2020).
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Suzán, G.; Marcé, E.; Giermakowski, J.T.; Armién, B.; Pascale, J.; Mills, J.; Ceballos, G.; Gómez, A.; Aguirre, A.A.; Salazar-Bravo, J.; et al. The effect of habitat fragmentation and species diversity loss on hantavirus prevalence in Panama. Ann. N. Y. Acad. Sci. 2008, 1149, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Heeney, J.L. Zoonotic viral diseases and the frontier of early diagnosis, control and prevention. J. Int. Med. 2006, 260, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.G.; Mesquita, J.R.; de São José Nascimento, M.; Ferreira, V.A.M. Viral, host and environmental factors that favor anthropozoonotic spillover of coronaviruses: An opinionated review, focusing on SARS–CoV, MERS–CoV and SARS–CoV–2. Sci. Total Environ. 2020, 750, 141483. [Google Scholar] [CrossRef]
- Cacciò, S.M.; Ryan, U. Molecular epidemiology of giardiasis. Mol. Biochem. Parasitol. 2008, 160, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Witte, W.; Strommenger, B.; Stanek, C.; Cuny, C. Methicillin–resistant Staphylococcus aureus ST398 in humans and animals, Central Europe. Emerg. Infect. Dis. 2007, 13, 255–258. [Google Scholar] [CrossRef]
- Chomel, B.B.; Belotto, A.; Meslin, F.-X. Wildlife, exotic pets, and emerging zoonoses. Emerg. Infect. Dis. 2007, 13, 6–11. [Google Scholar] [CrossRef]
- Rosen, G. A History of Public Health, Revised Expanded ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2015; pp. 169–195. [Google Scholar]
- Johnson, K.M.; Lange, J.V.; Webb, P.A.; Murphy, F.A. Isolation and partial characterization of a new virus causing acute hemorrhagic fever in zaire. Lancet 1977, 309, 569–571. [Google Scholar] [CrossRef]
- Gallo, R.C. The AIDS Virus. Sci. Am. 1987, 256, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Paton, N.I.; Leo, Y.S.; Zaki, S.R.; Auchus, A.P.; Lee, K.E.; Ling, A.E.; Chew, S.K.; Ang, B.; Rollin, P.E.; Umapathi, T.; et al. Outbreak of Nipah–virus infection among abattoir workers in Singapore. Lancet 1999, 354, 1253–1256. [Google Scholar] [CrossRef]
- Lee, L.M.; Henderson, D.K. Emerging viral infections. Curr. Opin. Infect. Dis. 2001, 14, 467–480. [Google Scholar] [CrossRef]
- Rappole, J.H.; Derrickson, S.R.; Hubalek, Z. Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerg. Infect. Dis. 2000, 6, 319–328. [Google Scholar] [CrossRef]
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The challenge of emerging and re–emerging infectious diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Rabozzi, G.; Bonizzi, L.; Crespi, E.; Somaruga, C.; Sokooti, M.; Tabibi, R.; Vellere, F.; Brambilla, G.; Colosio, C. Emerging zoonoses: The “one health approach”. Saf. Health Work 2012, 3, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/polio.html (accessed on 9 November 2020).
- Center for Disease Control and Prevention. Available online: https://www.cdc.gov/measles/about/history.html (accessed on 9 November 2020).
- Center for Disease Control and Prevention. Available online: https://www.cdc.gov/flu/pandemic-resources/pandemic-timeline-1930-and-beyond.htm (accessed on 9 November 2020).
- Center for Disease Control and Prevention. Available online: https://www.cdc.gov/flu/pandemic-resources/1957-1958-pandemic.html (accessed on 9 November 2020).
- Gralinski, L.; Menachery, V.D. Return of the Coronavirus: 2019–nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef]
- Meslin, F.X. Global aspects of emerging and potential zoonoses: A WHO perspective. Emerg. Infect. Dis. 1997, 3, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Shafique, L.; Ihsan, A.; Liu, Q. Evolutionary Trajectory for the Emergence of Novel Coronavirus SARS–CoV–2. Pathogens 2020, 9, 240. [Google Scholar] [CrossRef]
- Gao, G.F. From “A” IV to “Z” IKV: Attacks from emerging and re–emerging pathogens. Cell 2018, 172, 1157–1159. [Google Scholar] [CrossRef]
- Drosten, C.; Günther, S.; Preiser, W.; Van Der Werf, S.; Brodt, H.-R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef]
- Worobey, M.; Holmes, E.C. Evolutionary aspects of recombination in RNA viruses. J. Gen. Virol. 1999, 80, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Garten, R.J.; Davis, C.T.; Russell, C.A.; Shu, B.; Lindstrom, S.; Balish, A.; Sessions, W.M.; Xu, X.; Skepner, E.; Deyde, V.; et al. Antigenic and genetic characteristics of swine–origin 2009 A (H1N1) influenza viruses circulating in humans. Science 2009, 325, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, Y.; Noda, T.; Kawakami, E.; Akkina, R.; Kawaoka, Y. Identification of novel influenza A virus proteins translated from PA mRNA. J. Virol. 2013, 87, 2455–2462. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Bennett, M.; Begon, M.E. Virus zoonoses—A long–term overview. Comp. Immunol. Microbiol. Infect. Dis. 1997, 20, 101–109. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. In Coronaviruses; Humana Press: New York, NY, USA, 2015; pp. 1–23. [Google Scholar]
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; Mchugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019–nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Dawood, F.S.; Iuliano, A.D.; Reed, C.; Meltzer, M.I.; Shay, D.K.; Cheng, P.-Y.; Bandaranayake, D.; Breiman, R.F.; Brooks, W.A.; Buchy, P.; et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: A modelling study. Lancet Infect. Dis. 2012, 12, 687–695. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/spotlight/influenza-are-we-ready (accessed on 15 November 2020).
- Gaur, P.; Munjal, A.; Lal, S.K. Influenza virus and cell signaling pathways. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2011, 17, RA148–RA154. [Google Scholar] [CrossRef]
- Dadonaite, B.; Vijayakrishnan, S.; Fodor, E.; Bhella, D.; Hutchinson, E.C. Filamentous influenza viruses. J. Gen. Virol. 2016, 97, 1755–1764. [Google Scholar] [CrossRef]
- Giocondi, M.-C.; Ronzon, F.; Nicolai, M.C.; Dosset, P.; Milhiet, P.-E.; Chevalier, M.; Le Grimellec, C. Organization of influenza A virus envelope at neutral and low pH. J. Gen. Virol. 2010, 91, 329–338. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Available online: https://espanol.cdc.gov/flu/about/viruses/types.htm (accessed on 17 November 2020).
- Ferguson, L.; Olivier, A.K.; Genova, S.; Epperson, W.B.; Smith, D.R.; Schneider, L.; Barton, K.; McCuan, K.; Webby, R.J.; Wan, X.-F. Pathogenesis of influenza D virus in cattle. J. Virol. 2016, 90, 5636–5642. [Google Scholar] [CrossRef] [PubMed]
- Njouom, R.; Monamele, G.C.; Ermetal, B.; Tchatchouang, S.; Moyo-Tetang, S.; McCauley, J.W.; Daniels, R.S. Detection of influenza C virus infection among hospitalized patients, Cameroon. Emerg. Infect. Dis. 2019, 25, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Li, X.; Goraya, M.U.; Wang, S.; Chen, J.-L. Evolution of influenza a virus by mutation and re–assortment. Int. J. Mol. Sci. 2017, 18, 1650. [Google Scholar] [CrossRef]
- Anhlan, D.; Grundmann, N.; Makalowski, W.; Ludwig, S.; Scholtissek, C. Origin of the 1918 pandemic H1N1 influenza A virus as studied by codon usage patterns and phylogenetic analysis. RNA 2011, 17, 64–73. [Google Scholar] [CrossRef]
- Olson, D.R.; Simonsen, L.; Edelson, P.J.; Morse, S.S. Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc. Natl. Acad. Sci. USA 2005, 102, 11059–11063. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Morens, D.M. 1918 Influenza: The mother of all pandemics. Rev. Biomed. 2006, 17, 69–79. [Google Scholar] [CrossRef]
- Mena, I.; Nelson, M.I.; Quezada-Monroy, F.; Dutta, J.; Cortes-Fernández, R.; Lara-Puente, J.H.; Castro-Peralta, F.; Cunha, L.F.; Trovão, N.S.; Lozano–Dubernard, B.; et al. Origins of the 2009 H1N1 influenza pandemic in swine in Mexico. Elife 2016, 5, e16777. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Available online: https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html#:~:text=From%20April%2012%2C%202009%20to,the%20(H1N1)pdm09%20virus (accessed on 19 November 2020).
- Martínez, A.; Soldevila, N.; Romero-Tamarit, A.; Torner, N.; Godoy, P.; Rius, C.; Jané, M.; Domínguez, À. Risk factors associated with severe outcomes in adult hospitalized patients according to influenza type and subtype. PLoS ONE 2019, 14, e0210353. [Google Scholar] [CrossRef]
- Rewar, S.; Mirdha, D.; Rewar, P. Treatment and prevention of pandemic H1N1 influenza. Ann. Glob. Health 2015, 81, 645–653. [Google Scholar] [CrossRef]
- Dalal, A.; Mohan, H.; Prasad, M.; Pundir, C. Detection methods for influenza A H1N1 virus with special reference to biosensors: A review. Biosci. Rep. 2020, 40, BSR20193852. [Google Scholar]
- Chauhan, N.; Narang, J.; Pundir, S.; Singh, S.; Pundir, C. Laboratory diagnosis of swine flu: A review. Artif. Cells Nanomed. Biotechnol. 2013, 41, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugan, K.; Liao, J.-D.; You, J.-W.; Wu, C.-L. Focused–ion–beam–fabricated Au/Ag multilayered nanorod array as SERS–active substrate for virus strain detection. Sens. Actuators B Chem. 2013, 181, 361–367. [Google Scholar] [CrossRef]
- Chang, C.-W.; Liao, J.-D.; Shiau, A.-L.; Yao, C.-K. Non–labeled virus detection using inverted triangular Au nano–cavities arrayed as SERS–active substrate. Sens. Actuators B Chem. 2011, 156, 471–478. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Liao, J.-D.; Yang, M.-L.; Wu, C.-L. Target–size embracing dimension for sensitive detection of viruses with various sizes and influenza virus strains. Biosens. Bioelectron. 2012, 35, 447–451. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Liao, J.-D.; Ju, Y.-H.; Chang, C.-W.; Shiau, A.-L. Focused ion beam–fabricated Au micro/nanostructures used as a surface enhanced Raman scattering–active substrate for trace detection of molecules and influenza virus. Nanotechnology 2011, 22, 185308. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Nam, J.-S.; Yang, S.-E.; Shin, H.; Jang, Y.-H.; Bae, G.-U.; Kang, T.; Lim, K.-I.; Choi, Y. Identification of newly emerging influenza viruses by surface–enhanced Raman spectroscopy. Anal. Chem. 2015, 87, 11652–11659. [Google Scholar] [CrossRef]
- Moon, J.; Yi, S.Y.; Hwang, A.; Eom, G.; Sim, J.; Jeong, J.; Lim, E.-K.; Chung, B.H.; Kim, B.; Jung, J.; et al. Facile and sensitive detection of influenza viruses using SERS antibody probes. RSC Adv. 2016, 6, 84415–84419. [Google Scholar] [CrossRef]
- Eom, G.; Hwang, A.; Lee, D.K.; Guk, K.; Moon, J.; Jeong, J.; Jung, J.; Kim, B.; Lim, E.-K.; Kang, T. Superb Specific, Ultrasensitive, and Rapid Identification of the Oseltamivir–Resistant H1N1 Virus: Naked–Eye and SERS Dual–Mode Assay Using Functional Gold Nanoparticles. ACS Appl. Bio. Mater. 2019, 2, 1233–1240. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Wang, X.; Wang, K.; Zhu, Y.; Rong, Z.; Wang, W.; Xiao, R.; Wang, S.J. Magnetic SERS strip for sensitive and simultaneous detection of respiratory viruses. ACS Appl. Mater. Interfaces 2019, 11, 19495–19505. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian–origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shi, W.; Shi, Y.; Wang, D.; Xiao, H.; Li, W.; Bi, Y.; Wu, Y.; Li, X.; Yan, J.; et al. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: Phylogenetic, structural, and coalescent analyses. Lancet 2013, 381, 1926–1932. [Google Scholar] [CrossRef]
- Yang, S.; Chen, Y.; Cui, D.; Yao, H.; Lou, J.; Huo, Z.; Xie, G.; Yu, F.; Zheng, S.; Yang, Y.; et al. Avian–origin influenza A (H7N9) infection in influenza A (H7N9)–affected areas of China: A serological study. J. Infect. Dis. 2014, 209, 265–269. [Google Scholar] [CrossRef]
- Lee, D.-H.; Torchetti, M.K.; Killian, M.L.; Berhane, Y.; Swayne, D.E. Highly pathogenic avian influenza A (H7N9) virus, Tennessee, USA, March 2017. Emerg. Infect. Dis. 2017, 23, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Poovorawan, Y.; Pyungporn, S.; Prachayangprecha, S.; Makkoch, J. Global alert to avian influenza virus infection: From H5N1 to H7N9. Pathog. Glob. Health 2013, 107, 217–223. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/influenza/human_animal_interface/faq_H7N9/en/ (accessed on 25 November 2020).
- World Health Organization. Available online: https://www.who.int/influenza/human_animal_interface/avian_influenza/riskassessment_AH7N9_201702/en/ (accessed on 25 November 2020).
- World Health Organization. Available online: https://www.who.int/influenza/human_animal_interface/influenza_h7n9/WHO_H7N9_review_31May13.pdf (accessed on 25 November 2020).
- Xiang, N.; Li, X.; Ren, R.; Wang, D.; Zhou, S.; Greene, C.M.; Song, Y.; Zhou, L.; Yang, L.; Davis, C.T.; et al. Assessing change in avian influenza A (H7N9) virus infections during the fourth epidemic—China, September 2015–August 2016. Morb. Mortal. Wkly. Rep. 2016, 65, 1390–1394. [Google Scholar] [CrossRef]
- Sun, X.; Belser, J.A.; Pappas, C.; Pulit–Penaloza, J.A.; Brock, N.; Zeng, H.; Creager, H.M.; Le, S.; Wilson, M.; Lewis, A.; et al. Risk Assessment of Fifth–Wave H7N9 Influenza A Viruses in Mammalian Models. J. Virol. 2019, 93, e01740-18. [Google Scholar] [CrossRef]
- Xiao, M.; Xie, K.; Dong, X.; Wang, L.; Huang, C.; Xu, F.; Xiao, W.; Jin, M.; Huang, B.; Tang, Y. Ultrasensitive detection of avian influenza A (H7N9) virus using surface–enhanced Raman scattering–based lateral flow immunoassay strips. Anal. Chim. Acta 2019, 1053, 139–147. [Google Scholar] [CrossRef]
- Taranova, N.; Berlina, A.; Zherdev, A.; Dzantiev, B. ‘Traffic light’ immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk. Biosens. Bioelectron. 2015, 63, 255–261. [Google Scholar] [CrossRef]
- Juntunen, E.; Myyryläinen, T.; Salminen, T.; Soukka, T.; Pettersson, K. Performance of fluorescent europium (III) nanoparticles and colloidal gold reporters in lateral flow bioaffinity assay. Anal. Biochem. 2012, 428, 31–38. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Wang, J.; Tang, Z.; Pounds, J.G.; Lin, Y. Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal. Chem. 2010, 82, 7008–7014. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, C.-H.; Chen, J.-J.; Xiong, C.; Chen, Z.; Pang, D.-W.; Zhang, Z.-L. Bifunctional magnetic nanobeads for sensitive detection of avian influenza A (H7N9) virus based on immunomagnetic separation and enzyme–induced metallization. Biosens. Bioelectron. 2015, 68, 586–592. [Google Scholar] [CrossRef]
- Song, C.; Liu, Y.; Jiang, X.; Zhang, J.; Dong, C.; Li, J.; Wang, L. Ultrasensitive SERS determination of avian influenza A H7N9 virus via exonuclease III–assisted cycling amplification. Talanta 2019, 205, 120137. [Google Scholar] [CrossRef]
- Luke, C.J.; Subbarao, K. Vaccines for pandemic influenza. Emerg. Infect. Dis. 2006, 12, 66–72. [Google Scholar] [CrossRef]
- Jester, B.J.; Uyeki, T.M.; Jernigan, D.B. Fifty Years of Influenza A (H3N2) Following the Pandemic of 1968. Am. J. Public Health 2020, 110, 669–676. [Google Scholar] [CrossRef]
- Raghwani, J.; Thompson, R.N.; Koelle, K. Selection on non–antigenic gene segments of seasonal influenza A virus and its impact on adaptive evolution. Virus Evol. 2017, 3, vex034. [Google Scholar] [CrossRef]
- Yamada, A.; Imanishi, J.; Nakajima, E.; Nakajima, K.; Nakajima, S. Detection of influenza viruses in throat swab by using polymerase chain reaction. Microbiol. Immunol. 1991, 35, 259–265. [Google Scholar] [CrossRef]
- Zhang, X.; Dhawane, A.N.; Sweeney, J.; He, Y.; Vasireddi, M.; Iyer, S.S. Electrochemical assay to detect influenza viruses and measure drug susceptibility. Angew. Chem. 2015, 127, 6027–6030. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Kim, J.; Suzuki, T.; Lee, J.; Park, E.Y. Detection of influenza virus using peroxidase-mimic of gold nanoparticles. Biotechnol. Bioeng. 2016, 113, 2298–2303. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Chan, J.Y.; Choo, L.X.; Lim, H.Q.; Mittman, H.; Olivo, M. Plasmonic Contrast Imaging Biosensor for the Detection of H3N2 Influenza Protein–Antibody and DNA–DNA Molecular Binding. IEEE Sens. J. 2019, 19, 11828–11833. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, L.; Zhang, F.; Song, Z.; Hu, Y.; Ji, Y.; Shen, J.; Li, B.; Lu, H.; Yang, H.; et al. A promising magnetic SERS immunosensor for sensitive detection of avian influenza virus. Biosens. Bioelectron. 2017, 89, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Kukushkin, V.I.; Ivanov, N.M.; Novoseltseva, A.A.; Gambaryan, A.S.; Yaminsky, I.V.; Kopylov, A.M.; Zavyalova, E.G. Highly sensitive detection of influenza virus with SERS aptasensor. PLoS ONE 2019, 14, e0216247. [Google Scholar] [CrossRef]
- Xu, X.; Subbarao, K.; Cox, N.J.; Guo, Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: Similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 1999, 261, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.Y.; Chan, P.K.; Peiris, M.; Tsang, D.N.; Que, T.L.; Shortridge, K.F.; Cheung, P.T.; To, W.K.; Ho, E.T.; Sung, R.; et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998, 351, 467–471. [Google Scholar] [CrossRef]
- Chen, H.; Smith, G.; Li, K.; Wang, J.; Fan, X.; Rayner, J.; Vijaykrishna, D.; Zhang, J.; Zhang, L.; Guo, C.; et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: Implications for pandemic control. Proc. Natl. Acad. Sci. USA 2006, 103, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Gauthier–Clerc, M.; Lebarbenchon, C.; Thomas, F.J. Recent expansion of highly pathogenic avian influenza H5N1: A critical review. IBIS 2007, 149, 202–214. [Google Scholar] [CrossRef]
- Claas, E.C.; Osterhaus, A.D.; Van Beek, R.; De Jong, J.C.; Rimmelzwaan, G.F.; Senne, D.A.; Krauss, S.; Shortridge, K.F.; Webster, R.G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 1998, 351, 472–477. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Reid, A.H.; Lourens, R.M.; Wang, R.; Jin, G.; Fanning, T.G. Characterization of the 1918 influenza virus polymerase genes. Nature 2005, 437, 889–893. [Google Scholar] [CrossRef]
- Sims, L.; Domenech, J.; Benigno, C.; Kahn, S.; Kamata, A.; Lubroth, J.; Martin, V.; Roeder, P. Origin and evolution of highly pathogenic H5N1 avian influenza in Asia. Vet. Rec. 2005, 157, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Smith, G.; Zhang, S.; Qin, K.; Wang, J.; Li, K.; Webster, R.; Peiris, J.; Guan, Y. H5N1 virus outbreak in migratory waterfowl. Nature 2005, 436, 191–192. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, H.; Lei, F.; Zhu, Q.; Qin, K.; Zhang, X.-W.; Zhang, X.-I.; Zhao, D.; Wang, G.; Feng, Y.; et al. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science 2005, 309, 1206. [Google Scholar] [CrossRef]
- Peiris, J.M.; De Jong, M.D.; Guan, Y. Avian influenza virus (H5N1): A threat to human health. Clin. Microbiol. Rev. 2007, 20, 243–267. [Google Scholar] [CrossRef]
- De Jong, M.D.; Cam, B.V.; Qui, P.T.; Hien, V.M.; Thanh, T.T.; Hue, N.B.; Beld, M.; Phuong, L.T.; Khanh, T.H.; Chau, N.V.V.; et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 2005, 352, 686–691. [Google Scholar] [CrossRef] [PubMed]
- The Writing Committee of the World Health Organization (WHO) Consultation on Human Influenza A/H5. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 2005, 353, 1374–1385. [Google Scholar] [CrossRef]
- Lee, D.C.; Cheung, C.-Y.; Law, A.H.; Mok, C.K.; Peiris, M.; Lau, A.S. p38 mitogen–activated protein kinase–dependent hyperinduction of tumor necrosis factor alpha expression in response to avian influenza virus H5N1. J. Virol. 2005, 79, 10147–10154. [Google Scholar] [CrossRef]
- Yassine, H.M.; Lee, C.W.; Gourapura, R.; Saif, Y.M. Interspecies and intraspecies transmission of influenza A viruses: Viral, host andenvironmental factors. Anim. Health Res. Rev. 2010, 11, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Cattoli, G.; Fusaro, A.; Monne, I.; Coven, F.; Joannis, T.; Abd El–Hamid, H.S.; Hussein, A.A.; Cornelius, C.; Amarin, N.M.; Mancin, M.; et al. Evidence for differing evolutionary dynamics of A/H5N1 viruses among countries applying or not applying avian influenza vaccination in poultry. Vaccine 2011, 29, 9368–9375. [Google Scholar] [CrossRef] [PubMed]
- García–Sastre, A. Inhibition of interferon–mediated antiviral responses by influenza A viruses and other negative–strand RNA viruses. Virology 2001, 279, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Krug, R.M.; Yuan, W.; Noah, D.L.; Latham, A.G. Intracellular warfare between human influenza viruses and human cells: The roles of the viral NS1 protein. Virology 2003, 309, 181–189. [Google Scholar] [CrossRef]
- Shinya, K.; Ebina, M.; Yamada, S.; Ono, M.; Kasai, N.; Kawaoka, Y. Influenza virus receptors in the human airway. Nature 2006, 440, 435–436. [Google Scholar] [CrossRef]
- Van Riel, D.; Munster, V.J.; De Wit, E.; Rimmelzwaan, G.F.; Fouchier, R.A.; Osterhaus, A.D.; Kuiken, T. H5N1 virus attachment to lower respiratory tract. Science 2006, 312, 399. [Google Scholar] [CrossRef] [PubMed]
- Lien, K.-Y.; Hung, L.-Y.; Huang, T.-B.; Tsai, Y.-C.; Lei, H.-Y.; Lee, G.-B. Rapid detection of influenza A virus infection utilizing an immunomagnetic bead–based microfluidic system. Biosens. Bioelectron. 2011, 26, 3900–3907. [Google Scholar] [CrossRef] [PubMed]
- Oner, A.F.; Bay, A.; Arslan, S.; Akdeniz, H.; Sahin, H.A.; Cesur, Y.; Epcacan, S.; Yilmaz, N.; Deger, I.; Kizilyildiz, B.; et al. Avian influenza A (H5N1) infection in eastern Turkey in 2006. N. Engl. J. Med. 2006, 355, 2179–2185. [Google Scholar] [CrossRef]
- Ng, E.K.; Cheng, P.K.; Ng, A.Y.; Hoang, T.; Lim, W.W. Influenza A H5N1 detection. Emerg. Infect. Dis. 2005, 11, 1303–1305. [Google Scholar] [CrossRef]
- Wang, Y.; Ruan, Q.; Lei, Z.-C.; Lin, S.-C.; Zhu, Z.; Zhou, L.; Yang, C. Highly sensitive and automated surface enhanced Raman scattering–based immunoassay for H5N1 detection with digital microfluidics. Anal. Chem. 2018, 90, 5224–5231. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef]
- Deckert, V.; Deckert-Gaudig, T.; Cialla-May, D.; Popp, J.; Zell, R.; Deinhard-Emmer, S.; Sokolov, A.; Yi, Z.; Scully, M. Laser Spectroscopic Technique for Direct Identification of a Single Virus I: Faster cars. Med. Phys. 2020, 117, 27820–27824. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.–H.; Choi, M.; Ku, K.B.; Lee, C.–S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid detection of COVID–19 causative virus (SARS–CoV–2) in human nasopharyngeal swab specimens using field–effect transistor–based biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.R.; Navas–Martin, S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005, 69, 635–664. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Structure, function, and evolution of coronavirus spike proteins. Ann. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Brian, D.; Baric, R. Coronavirus genome structure and replication. In Coronavirus Replication and Reverse Genetics; Springer: Berlin, Germany, 2005; pp. 1–30. [Google Scholar]
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wang, M.; et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar]
- Guarner, J. Three emerging coronaviruses in two decades: The story of SARS, MERS, and now COVID–19. Am. J. Clin. Pathol. 2020, 153, 420–421. [Google Scholar]
- Liu, S.-L.; Saif, L. Emerging viruses without borders: The Wuhan coronavirus. Viruses 2020, 12, 130. [Google Scholar] [CrossRef]
- Kin, N.; Miszczak, F.; Lin, W.; Gouilh, M.A.; Vabret, A.; Consortium, E. Genomic analysis of 15 human coronaviruses OC43 (HCoV–OC43s) circulating in France from 2001 to 2013 reveals a high intra–specific diversity with new recombinant genotypes. Viruses 2015, 7, 2358–2377. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Weifeng, S.; Jun, L.; Alexander, C.; Jiyong, Z.; Su, S.; Wong, G.; Shi, W.; et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef]
- Forni, D.; Cagliani, R.; Clerici, M.; Sironi, M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017, 25, 35–48. [Google Scholar] [CrossRef]
- Hofmann, H.; Pyrc, K.; Van Der Hoek, L.; Geier, M.; Berkhout, B.; Pöhlmann, S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. USA 2005, 102, 7988–7993. [Google Scholar] [CrossRef]
- Lim, Y.X.; Ng, Y.L.; Tam, J.P.; Liu, D.X. Human coronaviruses: A review of virus–host interactions. Diseases 2016, 4, 26. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P.; Meyer, C.G. The COVID–19 epidemic. Trop. Med. Int. Health 2020, 25, 278–280. [Google Scholar] [CrossRef]
- Lau, S.K.; Woo, P.C.; Li, K.S.; Huang, Y.; Tsoi, H.-W.; Wong, B.H.; Wong, S.S.; Leung, S.-Y.; Chan, K.-H.; Yuen, K.-Y. Severe acute respiratory syndrome coronavirus–like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. USA 2005, 102, 14040–14045. [Google Scholar] [CrossRef]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats are natural reservoirs of SARS–like coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Kan, B.; Wang, M.; Jing, H.; Xu, H.; Jiang, X.; Yan, M.; Liang, W.; Zheng, H.; Wan, K.; Liu, Q.; et al. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus–like virus in palm civets at an animal market and on farms. J. Virol. 2005, 79, 11892–11900. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qi, J.; Yuan, Y.; Xuan, Y.; Han, P.; Wan, Y.; Ji, W.; Li, Y.; Wu, Y.; Wang, J.; et al. Bat origins of MERS–CoV supported by bat coronavirus HKU4 usage of human receptor CD26. Cell Host Microbe 2014, 16, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Osterhaus, A.D.; Fouchier, R.A.; Haagmans, B.L. MERS: Emergence of a novel human coronavirus. Curr. Opin. Virol. 2014, 5, 58–62. [Google Scholar] [CrossRef]
- Al–Hazmi, A. Challenges presented by MERS corona virus, and SARS corona virus to global health. Saudi J. Biol. Sci. 2016, 23, 507–511. [Google Scholar] [CrossRef]
- Ramadan, N.; Shaib, H. Middle East respiratory syndrome coronavirus (MERS–CoV): A review. Germs 2019, 9, 35–42. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID–19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Perlman, S. Another decade, another coronavirus. N. Engl. J. Med. 2020, 382, 760–762. [Google Scholar] [CrossRef]
- Depalo, D. True COVID–19 mortality rates from administrative data. J. Popul. Econ. 2021, 34, 253–274. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Available online: https://www.un.org/en/sections/issues–depth/population/index.html (accessed on 10 January 2021).
- World Health Organization. Available online: https://covid19.who.int/ (accessed on 17 February 2021).
- Shen, K.L.; Yang, Y.H.; Jiang, R.M.; Wang, T.Y.; Zhao, D.C.; Jiang, Y.; Lu, X.-X.; Jin, R.-M.; Zheng, Y.-J.; Xu, B.-P.; et al. Updated diagnosis, treatment and prevention of COVID–19 in children: Experts’ consensus statement (condensed version of the second edition). World J. Pediatr. 2020, 16, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Krammer, F. SARS–CoV–2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS–CoV–2—preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV–19 vaccine (AZD1222) against SARS–CoV–2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2020, 397, 99–111. [Google Scholar] [CrossRef]
- Lobo-Galo, N.; Gálvez-Ruíz, J.C.; Balderrama-Carmona, A.P.; Silva-Beltrán, N.P.; Ruiz-Bustos, E. Recent biotechnological advances as potential intervention strategies against COVID–19. 3 Biotech 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O. The COVID–19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech 2020, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Ceraolo, C.; Giorgi, F.M. Genomic variance of the 2019-nCoV coronavirus. J. Med. Virol. 2020, 92, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Z.; Chen, Z.; Huang, X.; Xu, M.; He, T.; Zhang, Z. The establishment of reference sequence for SARS-CoV-2 and variation analysis. J. Med. Virol. 2020, 92, 667–674. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin–converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Islam, M.R.; Hoque, M.N.; Rahman, M.S.; Alam, A.R.U.; Akther, M.; Puspo, J.A.; Akter, S.; Sultana, M.; Crandall, K.A.; Hossain, M.A. Genome–wide analysis of SARS–CoV–2 virus strains circulating worldwide implicates heterogeneity. Sci. Rep. 2020, 10, 14004. [Google Scholar] [CrossRef]
- Miller, S.; Krijnse-Locker, J. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 2008, 6, 363–374. [Google Scholar] [CrossRef]
- Perlman, S.; Netland, J. Coronaviruses post–SARS: Update on replication and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 439–450. [Google Scholar] [CrossRef]
- Lavine, J.S.; Bjornstad, O.N.; Antia, R. Immunological characteristics govern the transition of COVID–19 to endemicity. Science 2021, 371, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Available online: https://www.cdc.gov/coronavirus/2019-ncov/downloads/community/COVID-19-anterior-self-swab-testing-center.pdf (accessed on 20 January 2021).
- Pachetti, M.; Marini, B.; Benedetti, F.; Giudici, F.; Mauro, E.; Storici, P.; Masciovecchio, C.; Angeletti, S.; Ciccozzi, M.; Gallo, R.C.; et al. Emerging SARS–CoV–2 mutation hot spots include a novel RNA–dependent–RNA polymerase variant. J. Transl. Med. 2020, 18, 179. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor–González, A.; et al. CRISPR–Cas12–based detection of SARS–CoV–2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person–to–person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Ma, R.; Deng, S.; Wang, X.; Zhang, X.; Huang, X.; Liu, Y.; Li, G.; Qu, J.; et al. Ultra–fast and onsite interrogation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS–CoV–2) in environmental specimens via surface enhanced Raman scattering (SERS). medRxiv 2020. [Google Scholar] [CrossRef]


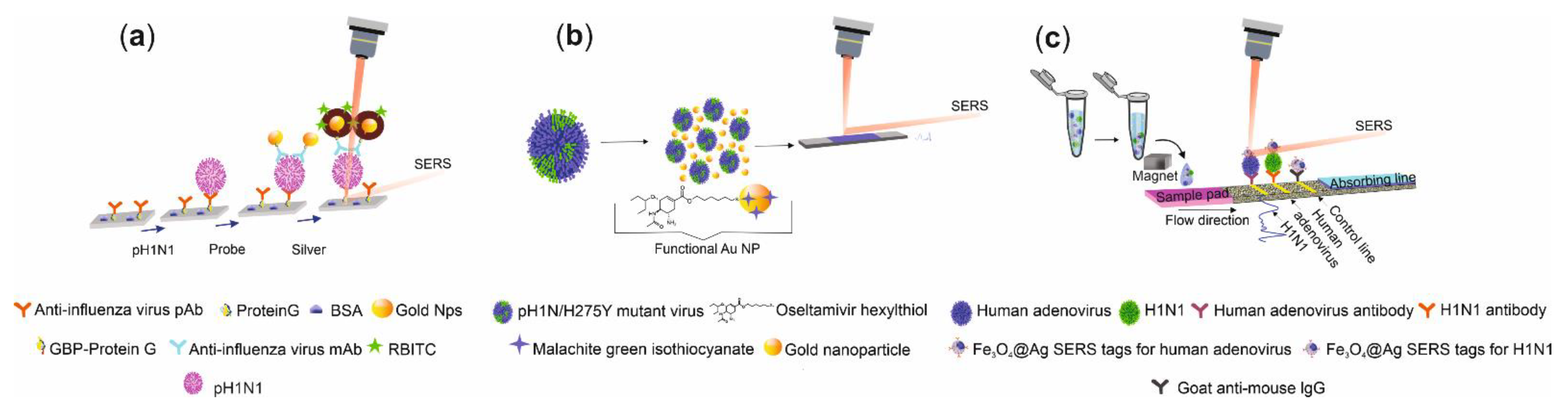

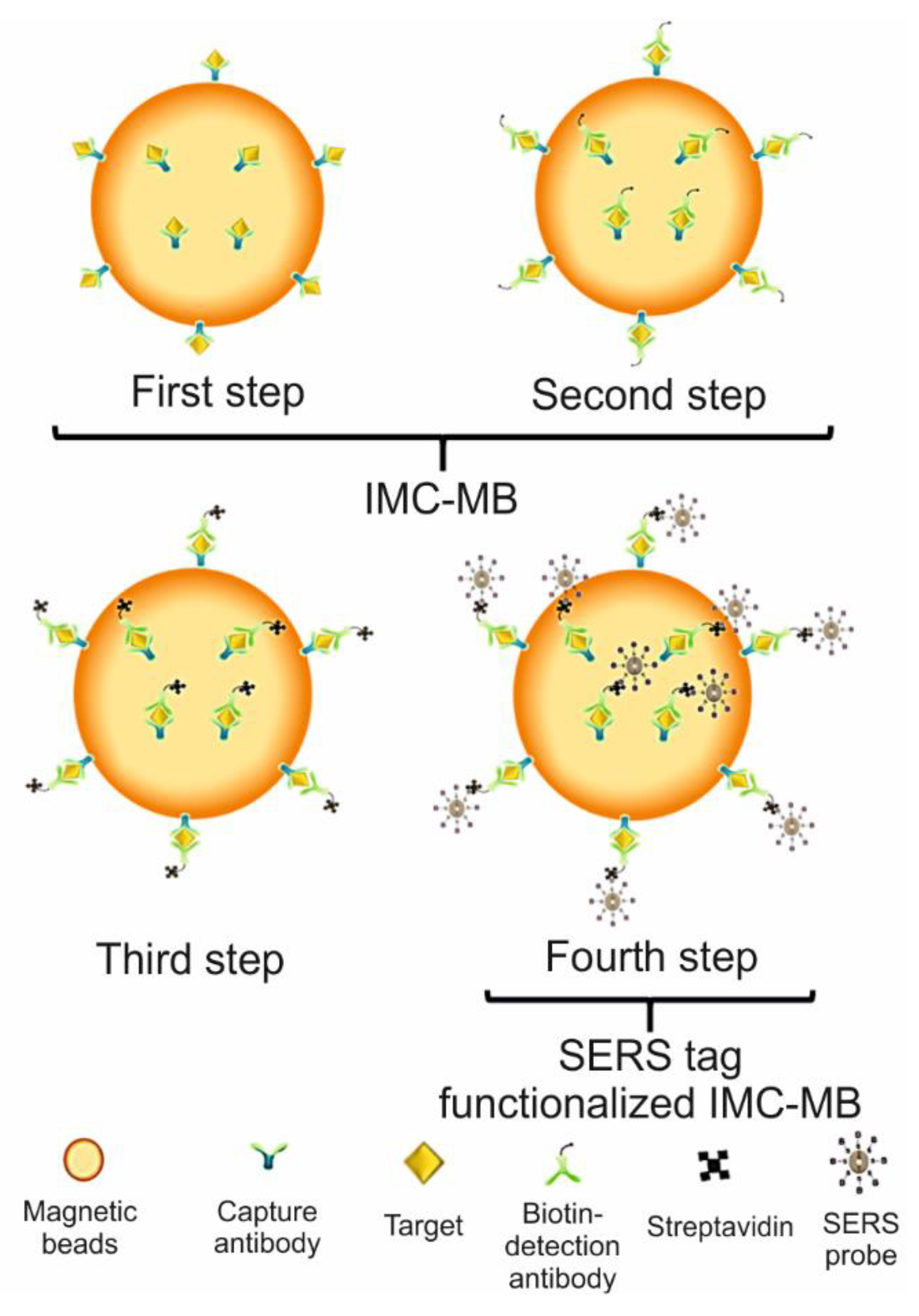
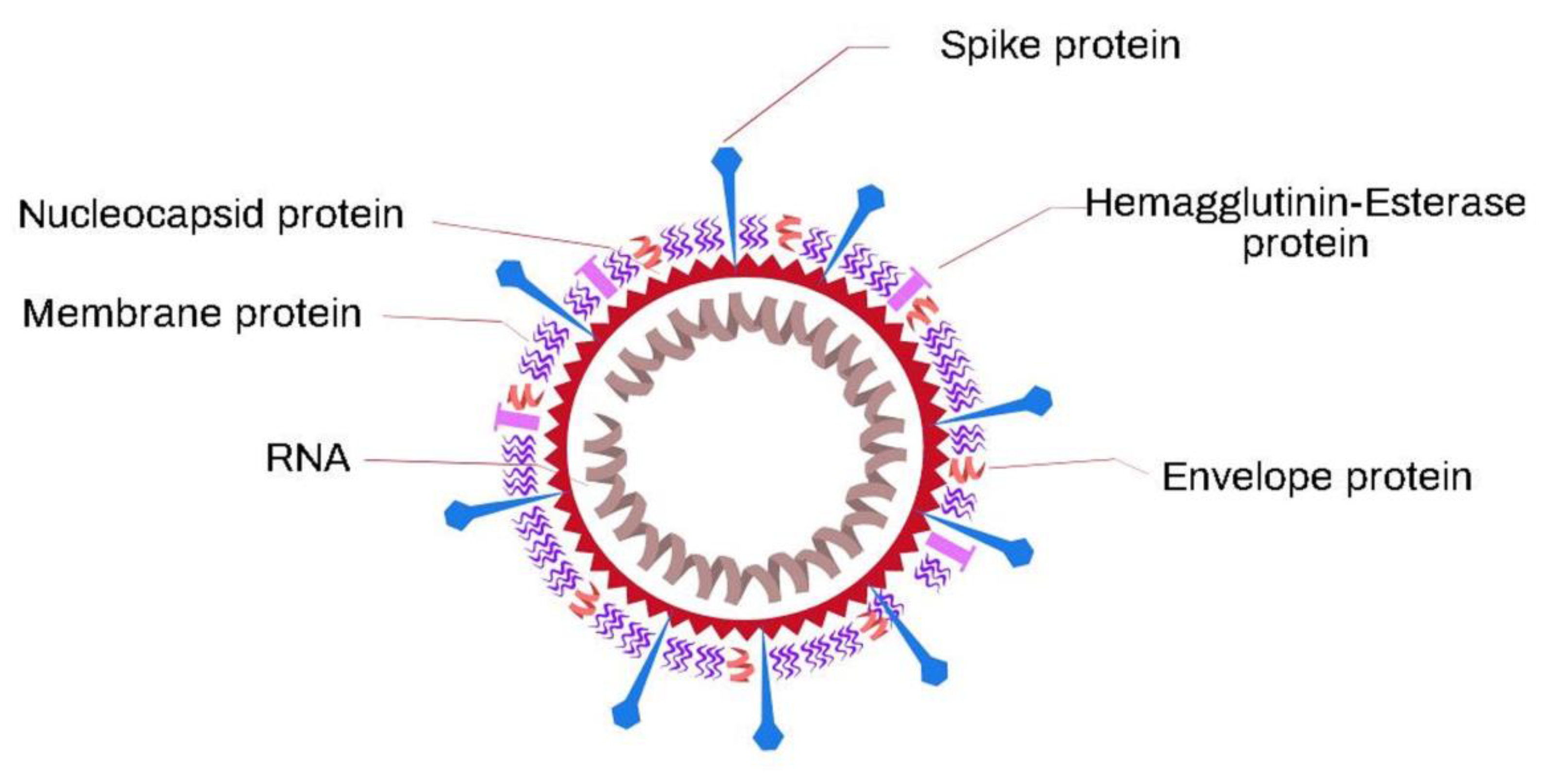
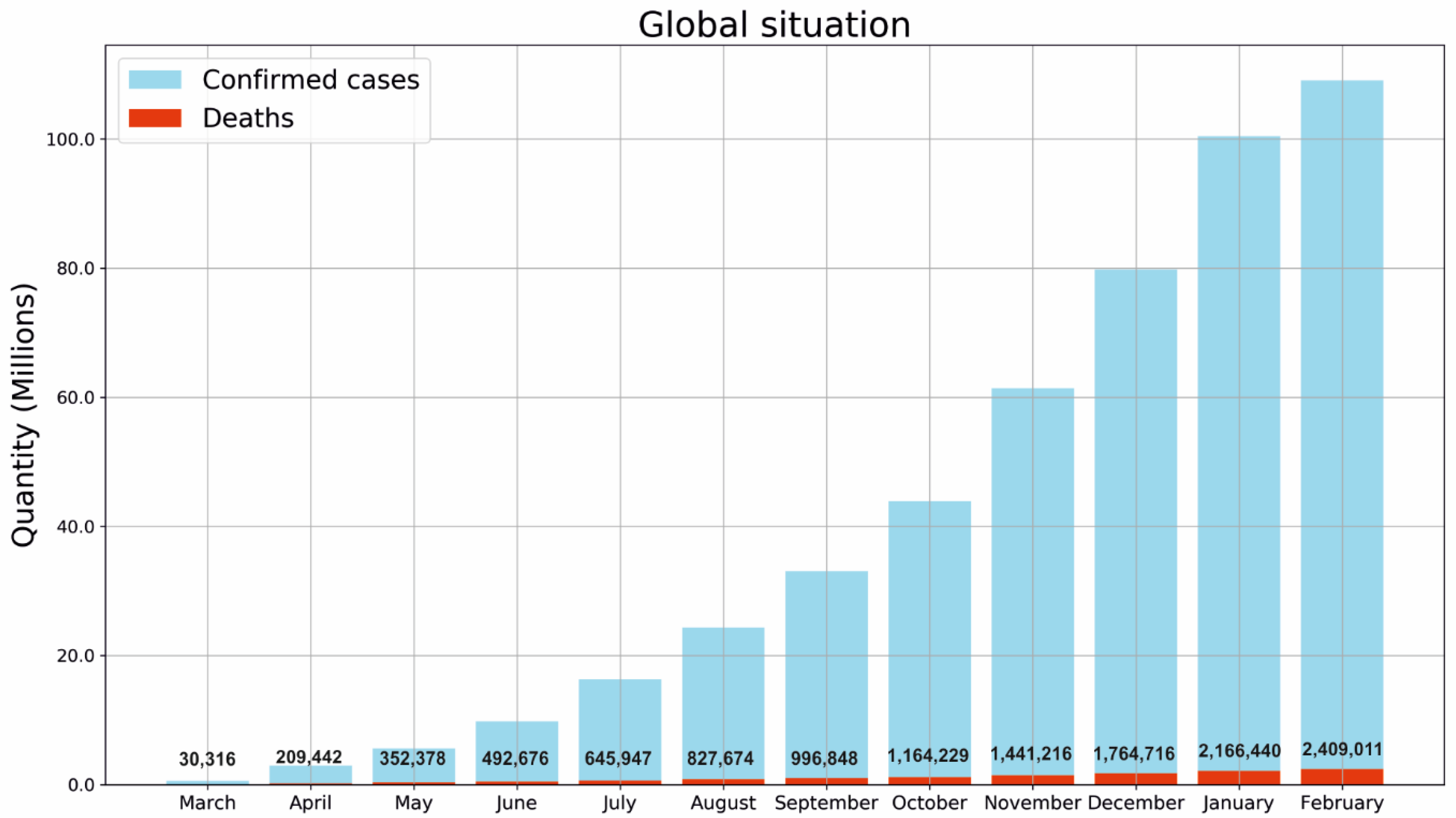

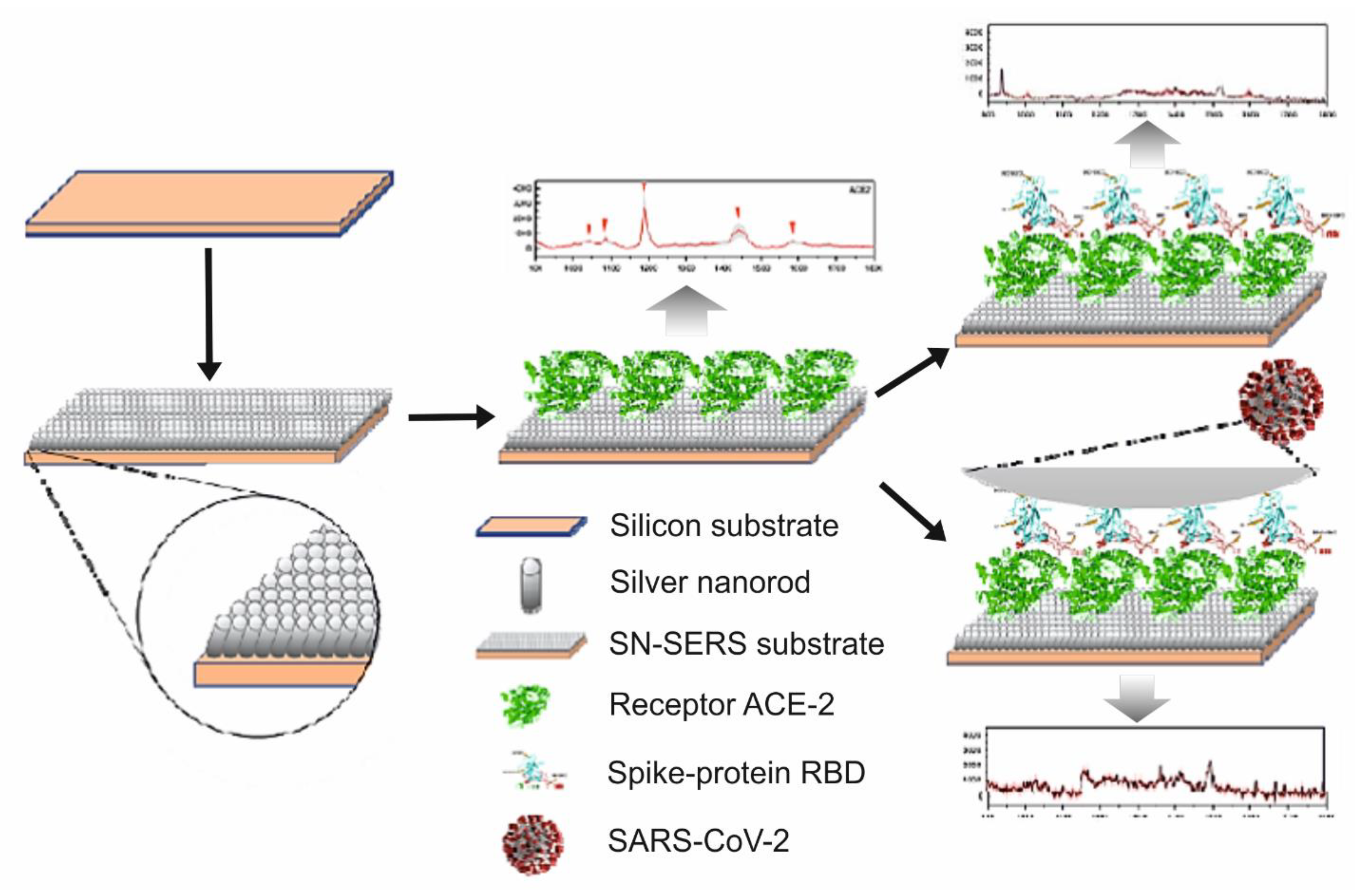
| Name of Flu Virus | Virus Concentration (PFU/mL) | Excitation Laser (nm) | Type of Raman Measurement | SERSS ubstrate | Route of Identification | Ref. |
|---|---|---|---|---|---|---|
| A/WSN/33 (H1N1) † | 1 × 106 | 633 | Wet | Au/Ag multilayered nanorod arrays onto SCS * | Virus entrapment | [88] |
| A/WSN/33 (H1N1) † | 1 × 104 | 633 | Dry | Au substrates onto SCS * | Virus entrapment | [89] |
| A/Taiwan/N39/06 (H1N1) | 1 × 106 | 633 | Wet | Au nanorods onto SCS * | Virus entrapment | [90] |
| A/WSN/33 (H1N1) † | 1 × 104 | 633 | Wet | Au nanorods onto SCS * | Virus entrapment | [91] |
| A/California/04/2009 (H1N1) | – | 785 | Dry | Aggregates of spherical AuNps on cover glass | Virus entrapment | [92] |
| Virus | LOD | Laser (nm) | Strategy | SERS Tag | Capture Substrate | RM | Tracking Band (cm−1) | Selectivity | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| A/CA/07/2009 p(H1N1) | 4.1 × 103 TCID/mL | 632 | Immunoassay | AuNps−Ag−protein G−mAb−RBITC | pAb−Cys3−protein G−glass substrate | RBITC | 1643 | H3N2, H5N2, IBV | [93] |
| p(H1N1)/H275Y mutant | 10 PFU | 633 | Functional nanoparticles | AuNp−OHT and MGITC | – | MGITC | 1616 | p(H1N1) * | [94] |
| A/FM/1/86 (H1N1) | 50 PFU/mL | 785 | Immunoassay | Fe3O4–DTNB/@Ag –DTNB–antibody | pAb–LFIA strip of nitrocellulose membrane | DTNB | 1332 | HAdV, FluB, PIV, RSV | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saviñon-Flores, F.; Méndez, E.; López-Castaños, M.; Carabarin-Lima, A.; López-Castaños, K.A.; González-Fuentes, M.A.; Méndez-Albores, A. A Review on SERS-Based Detection of Human Virus Infections: Influenza and Coronavirus. Biosensors 2021, 11, 66. https://doi.org/10.3390/bios11030066
Saviñon-Flores F, Méndez E, López-Castaños M, Carabarin-Lima A, López-Castaños KA, González-Fuentes MA, Méndez-Albores A. A Review on SERS-Based Detection of Human Virus Infections: Influenza and Coronavirus. Biosensors. 2021; 11(3):66. https://doi.org/10.3390/bios11030066
Chicago/Turabian StyleSaviñon-Flores, Fernanda, Erika Méndez, Mónica López-Castaños, Alejandro Carabarin-Lima, Karen A. López-Castaños, Miguel A. González-Fuentes, and Alia Méndez-Albores. 2021. "A Review on SERS-Based Detection of Human Virus Infections: Influenza and Coronavirus" Biosensors 11, no. 3: 66. https://doi.org/10.3390/bios11030066
APA StyleSaviñon-Flores, F., Méndez, E., López-Castaños, M., Carabarin-Lima, A., López-Castaños, K. A., González-Fuentes, M. A., & Méndez-Albores, A. (2021). A Review on SERS-Based Detection of Human Virus Infections: Influenza and Coronavirus. Biosensors, 11(3), 66. https://doi.org/10.3390/bios11030066