High Channel Temperature Mapping Electronics in a Thin, Soft, Wireless Format for Non-Invasive Body Thermal Analysis
Abstract
:1. Introduction
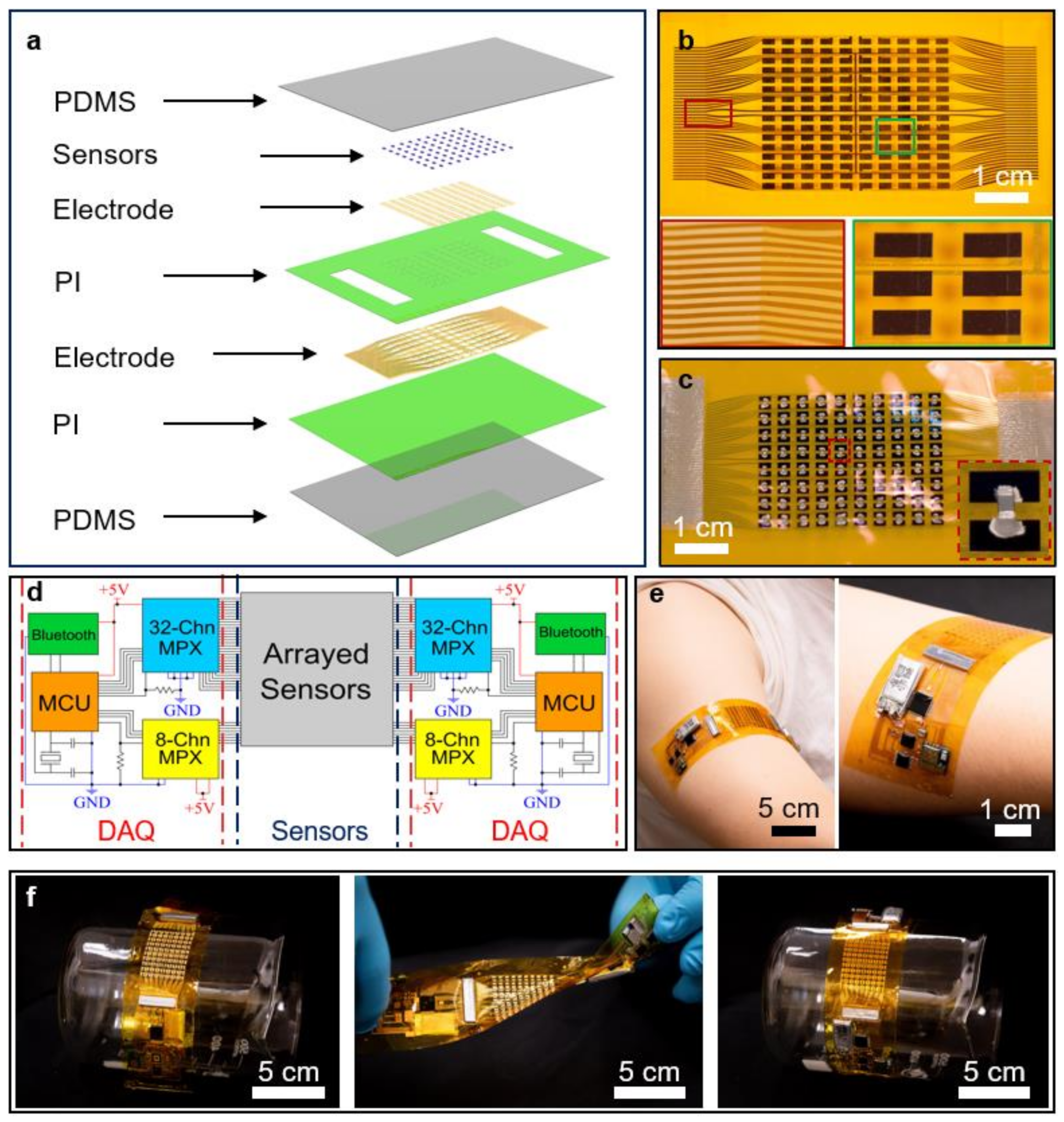
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Assembly of the WH Sensors
4.2. Assembly of the Sensing Panel for the WH Sensors
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kumar, R.; Rajasekaran, M.P. An IoT based patient monitoring system using raspberry Pi. In Proceedings of the 2016 International Conference on Computing Technologies and Intelligent Data Engineering (ICCTIDE’16), Kovilpatti, India, 7–9 January 2016. [Google Scholar]
- Zanjal, S.V.; Talmale, G.R. Medicine reminder and monitoring system for secure health using IOT. Procedia Comput. Sci. 2016, 78, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Kaur, P.; Kumar, R.; Kumar, M. A healthcare monitoring system using random forest and internet of things (IoT). Multimed. Tools Appl. 2019, 78, 19905–19916. [Google Scholar] [CrossRef]
- Abawajy, J.H.; Hassan, M.M. Federated internet of things and cloud computing pervasive patient health monitoring system. IEEE Commun. Mag. 2017, 55, 48–53. [Google Scholar] [CrossRef]
- Hassanalieragh, M.; Page, A.; Soyata, T.; Sharma, G.; Aktas, M.; Mateos, G.; Kantarci, B.; Andreescu, S. Health monitoring and management using Internet-of-Things (IoT) sensing with cloud-based processing: Opportunities and challenges. In Proceedings of the 2015 IEEE International Conference on Services Computing, New York, NY, USA, 27 June–2 July 2015. [Google Scholar]
- Wan, J.; Al-awlaqi, M.A.A.H.; Li, M.; O’Grady, M.; Gu, X.; Wang, J.; Cao, N. Wearable IoT enabled real-time health monitoring system. EURASIP J. Wirel. Commun. Netw. 2018, 2018, 298. [Google Scholar] [CrossRef]
- Talal, M.; Zaidan, A.A.; Zaidan, B.B.; Albahri, A.S.; Alamoodi, A.H.; Albahri, O.S.; Alsalem, M.A.; Lim, C.K.; Tan, K.L.; Shir, W.L.; et al. Smart home-based IoT for real-time and secure remote health monitoring of triage and priority system using body sensors: Multi-driven systematic review. J. Med. Syst. 2019, 43, 42. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Choi, J.; Hourlier-Fargette, A.; Xue, Y.; Chung, H.U.; Lee, J.Y.; Wang, X.; Xie, Z.; Kang, D.; Wang, H.; et al. Relation between blood pressure and pulse wave velocity for human arteries. Proc. Natl. Acad. Sci. USA 2018, 115, 11144–11149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Han, J.; Ahn, C.H. Flexible biosensors on spirally rolled micro tube for cardiovascular in vivo monitoring. Biosens. Bioelectron. 2007, 22, 1988–1993. [Google Scholar] [CrossRef]
- Karpova, E.V.; Shcherbacheva, E.V.; Galushin, A.A.; Vokhmyanina, D.V.; Karyakina, E.E.; Karyakin, A.A. Noninvasive diabetes monitoring through continuous analysis of sweat using flow-through glucose biosensor. Anal. Chem. 2019, 91, 3778–3783. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Madhvapathy, S.R.; Ma, Y.; Patel, M.; Krishnan, S.; Wei, C.; Li, Y.; Xu, S.; Feng, X.; Huang, Y.; Rogers, J.A. Epidermal electronic systems for measuring the thermal properties of human skin at depths of up to several millimeters. Adv. Funct. Mater. 2018, 28, 1802083. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark. Insights 2016, 11, BMI-S38440. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Finkelstein, S.; McVeigh, G.; Morgan, D.; LeMay, L.; Robinson, J.; Mock, J. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension 1995, 26, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Strandness Jr, D.; Schultz, R.D.; Sumner, D.S.; Rushmer, R.F. Ultrasonic flow detection: A useful technic in the evaluation of peripheral vascular disease. Am. J. Surg. 1967, 113, 311–320. [Google Scholar] [CrossRef]
- Lau, E.M.; Manes, A.; Celermajer, D.S.; Galie, N. Early detection of pulmonary vascular disease in pulmonary arterial hypertension: Time to move forward. Eur. Heart J. 2011, 32, 2489–2498. [Google Scholar] [CrossRef]
- Bagavathiappan, S.; Saravanan, T.; Philip, J.; Jayakumar, T.; Raj, B.; Karunanithi, R.; Panicker, T.M.R.; Korath, M.P.; Jagadeesan, K. Infrared thermal imaging for detection of peripheral vascular disorders. J. Med. Phys. Assoc. Med. Phys. India 2009, 34, 43. [Google Scholar]
- Eun, H.C. Evaluation of skin blood flow by laser Doppler flowmetry. Clin. Dermatol. 1995, 13, 337–347. [Google Scholar] [CrossRef]
- Pugsley, M.; Tabrizchi, R. The vascular system: An overview of structure and function. J. Pharmacol. Toxicol. Methods 2000, 44, 333–340. [Google Scholar] [CrossRef]
- Suzuki, K. Overview of deep learning in medical imaging. Radiol. Phys. Technol. 2017, 10, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Antiga, L.; Ene-Iordache, B.; Remuzzi, A. Computational geometry for patient-specific reconstruction and meshing of blood vessels from MR and CT angiography. IEEE Trans. Med. Imaging 2003, 22, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.W.; Goo, J.M. Dual-energy CT: New horizon in medical imaging. Korean J. Radiol. 2017, 18, 555–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bateman, T.M. Advantages and disadvantages of PET and SPECT in a busy clinical practice. J. Nucl. Cardiol. 2012, 19, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, P.K. The first clinical X-ray made in America—100 years. AJR Am. J. Roentgenol. 1995, 164, 241–243. [Google Scholar] [CrossRef]
- Sivasubramanian, M.; Hsia, Y.; Lo, L.-W. Nanoparticle-facilitated functional and molecular imaging for the early detection of cancer. Front. Mol. Biosci. 2014, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Hendee, W.R.; O’Connor, M.K. Radiation risks of medical imaging: Separating fact from fantasy. Radiology 2012, 264, 312–321. [Google Scholar] [CrossRef]
- Fazel, R.; Krumholz, H.M.; Wang, Y.; Ross, J.S.; Chen, J.; Ting, H.H.; Shah, N.D.; Nasir, K.; Einstein, A.J.; Nallamothu, B.K. Exposure to low-dose ionizing radiation from medical imaging procedures. N. Engl. J. Med. 2009, 361, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorfman, A.L.; Fazel, R.; Einstein, A.J.; Applegate, K.E.; Krumholz, H.M.; Wang, Y.; Christodoulou, E.; Chen, J.; Sanchez, R.; Nallamothu, B.K. Use of medical imaging procedures with ionizing radiation in children: A population-based study. Arch. Pediatrics Adolesc. Med. 2011, 165, 458–464. [Google Scholar] [CrossRef] [Green Version]
- Hricak, H.; Brenner, D.J.; Adelstein, S.J.; Frush, D.P.; Hall, E.J.; Howell, R.W.; McCollough, C.H.; Mettler, F.A.; Pearce, M.S.; Suleiman, O.H.; et al. Managing radiation use in medical imaging: A multifaceted challenge. Radiology 2011, 258, 889–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mettler, F.A., Jr.; Mahesh, M.; Bhargavan-Chatfield, M.; Chambers, C.E.; Elee, J.G.; Frush, D.P.; Miller, D.L.; Royal, H.D.; Milano, M.T.; Spelic, D.C.; et al. Patient exposure from radiologic and nuclear medicine procedures in the United States: Procedure volume and effective dose for the period 2006–2016. Radiology 2020, 295, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Linet, M.S.; Slovis, T.L.; Miller, D.L.; Kleinerman, R.; Lee, C.; Rajaraman, P.; Berrington de Gonzalez, A. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J. Clin. 2012, 62, 75–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, E.C. Radiation risk from medical imaging. Mayo Clin. Proc. 2010, 85, 1142–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katholi, R.E.; Taylor, G.J.; McCann, W.P.; Woods, W.T., Jr.; Womack, K.A.; McCoy, C.D.; Katholi, C.R.; Moses, H.W.; Mishkel, G.J.; Lucore, C.L. Nephrotoxicity from contrast media: Attenuation with theophylline. Radiology 1995, 195, 17–22. [Google Scholar] [CrossRef]
- Andreucci, M.; Solomon, R.; Tasanarong, A. Side effects of radiographic contrast media: Pathogenesis, risk factors, and prevention. BioMed Res. Int. 2014, 2014, 741018. [Google Scholar] [CrossRef] [PubMed]
- Beckett, K.R.; Moriarity, A.K.; Langer, J.M. Safe use of contrast media: What the radiologist needs to know. Radiographics 2015, 35, 1738–1750. [Google Scholar] [CrossRef]
- Zhao, Y.; Kim, A.; Wan, G.; Tee, B.C.K. Design and applications of stretchable and self-healable conductors for soft electronics. Nano Converg. 2019, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Cheng, I.-C.; Wagner, S. Overview of flexible electronics technology. In Flexible Electronics; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–28. [Google Scholar]
- Nie, B.; Liu, S.; Qu, Q.; Zhang, Y.; Zhao, M.; Liu, J. Bio-inspired Flexible Electronics for Smart E-skin. Acta Biomater. 2021. [Google Scholar] [CrossRef]
- Nathan, A.; Ahnood, A.; Cole, M.T.; Lee, S.; Suzuki, Y.; Hiralal, P.; Bonaccorso, F.; Hasan, T.; Garcia-Gancedo, L.; Dyadyusha, A.; et al. Flexible electronics: The next ubiquitous platform. Proc. IEEE 2012, 100, 1486–1517. [Google Scholar] [CrossRef]
- Gao, W.; Ota, H.; Kiriya, D.; Takei, K.; Javey, A. Flexible electronics toward wearable sensing. Acc. Chem. Res. 2019, 52, 523–533. [Google Scholar] [CrossRef]
- Nag, A.; Mukhopadhyay, S.C.; Kosel, J. Wearable flexible sensors: A review. IEEE Sens. J. 2017, 17, 3949–3960. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Xie, Z.; Yu, Y.; Lee, J.; Vazquez-Guardado, A.; Luan, H.; Ruban, J.; Ning, X.; Akhtar, A.; Li, D.; et al. Skin-integrated wireless haptic interfaces for virtual and augmented reality. Nature 2019, 575, 473–479. [Google Scholar] [CrossRef]
- Song, E.; Xie, Z.; Bai, W.; Luan, H.; Ji, B.; Ning, X.; Xia, Y.; Baek, J.M.; Lee, Y.; Avila, R.; et al. Miniaturized electromechanical devices for the characterization of the biomechanics of deep tissue. Nat. Biomed. Eng. 2021, 5, 759–771. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, H.; Zhao, L.; Liu, S.; Yao, K.; Li, D.; Yiu, C.; Gao, S.; Avila, R.; Chirarattananon, P.; et al. Electronic skin from high-throughput fabrication of intrinsically stretchable lead zirconate titanate elastomer. Research 2020, 2020, 1085417. [Google Scholar] [CrossRef]
- Wu, M.; Yao, K.; Li, D.; Huang, X.; Liu, Y.; Wang, L.; Song, E.; Yu, J.; Yu, X. Self-Powered Skin Electronics for Energy Harvesting and Healthcare Monitoring. Mater. Today Energy 2021, 21, 100786. [Google Scholar] [CrossRef]
- Wong, T.H.; Yiu, C.K.; Zhou, J.; Song, Z.; Liu, Y.; Zhao, L.; Yao, K.; Park, W.; Yoo, W.; Song, E.; et al. Tattoo-like epidermal electronics as skin sensors for human-machine interfaces. Soft Sci. 2021, 1, 10. [Google Scholar]
- Han, S.; Kim, J.; Won, S.M.; Ma, Y.; Kang, D.; Xie, Z.; Lee, K.-T.; Chung, H.U.; Banks, A.; Min, S.; et al. Battery-free, wireless sensors for full-body pressure and temperature mapping. Sci. Transl. Med. 2018, 10, eaan4950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, K.E.; Ma, Y.; Krishnan, S.; Wei, C.; Capua, D.; Xue, Y.; Xu, S.; Xie, Z.; Won, S.M.; Tian, L.; et al. Advanced approaches for quantitative characterization of thermal transport properties in soft materials using thin, conformable resistive sensors. Extrem. Mech. Lett. 2018, 22, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yu, L.; Yeo, J.C.; Lim, C.T. Flexible hybrid sensors for health monitoring: Materials and mechanisms to render wearability. Adv. Mater. 2020, 32, 1902133. [Google Scholar] [CrossRef]
- Wang, S.; Chinnasamy, T.; Lifson, M.A.; Inci, F.; Demirci, U. Flexible substrate-based devices for point-of-care diagnostics. Trends Biotechnol. 2016, 34, 909–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanu, A.; Casula, G.; Cosseddu, P.; Lai, S.; Martines, L.; Pani, D.; Bonfiglio, A. Flexible and wearable monitoring systems for biomedical applications in organic flexible electronics: Fundamentals, devices, and applications. In Organic Flexible Electronics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 599–625. [Google Scholar]
- Qaiser, N.; Al-Modaf, F.; Khan, S.M.; Shaikh, S.F.; El-Atab, N.; Hussain, M.M. A Robust Wearable Point-of-Care CNT-Based Strain Sensor for Wirelessly Monitoring Throat-Related Illnesses. Adv. Funct. Mater. 2021, 31, 2103375. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Cai, S.; Han, Z.; Liu, X.; Wang, F.; Cao, Y.; Wang, Z.; Li, H.; Chen, Y.; et al. Flexible hybrid electronics for digital healthcare. Adv. Mater. 2020, 32, 902062. [Google Scholar] [CrossRef]
- Yu, X.; Wang, H.; Ning, X.; Sun, R.; Albadawi, H.; Salomao, M.; Silva, A.C.; Yu, Y.; Tian, L.; Koh, A.; et al. Needle-shaped ultrathin piezoelectric microsystem for guided tissue targeting via mechanical sensing. Nat. Biomed. Eng. 2018, 2, 165–172. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Xu, G.; Chen, C.; Song, G.; Dong, Z.; Lin, L.; Wang, Y.; Xu, Z.; Yu, M.; et al. Low-Cost and Scalable Platform with Multiplexed Microwell Array Biochip for Rapid Diagnosis of COVID-19. Research 2021, 2021, 2813643. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, J.; Liu, Y.; Wong, T.; Su, J.; Yao, K.; Zhou, J.; Huang, Y.; Li, H.; Li, D.; et al. Epidermal self-powered sweat sensors for glucose and lactate monitoring. Bio-Des. Manuf. 2021. [Google Scholar] [CrossRef]
- Wu, M.; Gao, Z.; Yao, K.; Hou, S.; Liu, Y.; Li, D.; He, J.; Huang, X.; Song, E.; Yu, J.; et al. Thin, soft, skin-integrated foam-based triboelectric nanogenerators for tactile sensing and energy harvesting. Mater. Today Energy 2021, 20, 100657. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, S.; Zhang, S.; Li, Y.; Qu, Z.; Chen, Y.; Lu, B.; Wang, X.; Feng, X. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 2017, 3, e1701629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michard, F. Hemodynamic monitoring in the era of digital health. Ann. Intensive Care 2016, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Etemadi, M.; Inan, O.T.; Heller, J.A.; Hersek, S.; Klein, L.; Roy, S. A wearable patch to enable long-term monitoring of environmental, activity and hemodynamics variables. IEEE Trans. Biomed. Circuits Syst. 2015, 10, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, S.; Zeng, J.; Ren, X.; Chee, A.J.Y.; Yiu, B.Y.S.; Chung, W.C.; Yang, Y.; Yu, A.C.H.; Roberts, R.C.; et al. High sensitivity, wearable, piezoresistive pressure sensors based on irregular microhump structures and its applications in body motion sensing. Small 2016, 12, 3827–3836. [Google Scholar] [CrossRef] [PubMed]
- Shaltis, P.; Reisner, A.; Asada, H. Calibration of the photoplethysmogram to arterial blood pressure: Capabilities and limitations for continuous pressure monitoring. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006. [Google Scholar]
- Yang, T.; Jiang, X.; Zhong, Y.; Zhao, X.; Lin, S.; Li, J.; Li, X.; Xu, J.; Li, Z.; Zhu, H. A wearable and highly sensitive graphene strain sensor for precise home-based pulse wave monitoring. ACS Sens. 2017, 2, 967–974. [Google Scholar] [CrossRef]
- Cheng, W.; Yu, L.; Kong, D.; Yu, Z.; Wang, H.; Ma, Z.; Wang, Y.; Wang, J.; Pan, L.; Shi, Y. Fast-response and low-hysteresis flexible pressure sensor based on silicon nanowires. IEEE Electron Device Lett. 2018, 39, 1069–1072. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, P.; Chou, J.B.; Xu, R.; Zhao, R.; Hart, A.J.; Kim, S.-G. Extremely elastic wearable carbon nanotube fiber strain sensor for monitoring of human motion. ACS Nano 2015, 9, 5929–5936. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, Y.; Zhao, Y.; Mao, L.; Gao, L.; Pan, W.; Zhang, Y.; Liu, P. Highly stretchable strain sensor based on SWCNTs/CB synergistic conductive network for wearable human-activity monitoring and recognition. Smart Mater. Struct. 2017, 26, 095017. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Huang, Q.; Yuan, Z.; Zhao, Q.; Wang, S.; Zhang, Y.; Tai, H. A do-it-yourself approach to achieving a flexible pressure sensor using daily available materials. J. Mater. Chem. C 2021, 9, 13659–13667. [Google Scholar] [CrossRef]
- Dias, D.; Cunha, J.P.S. Wearable health devices—Vital sign monitoring, systems and technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Chou, E.-F.; Le, J.; Wong, S.; Chu, M.; Khine, M. Soft wearable pressure sensors for beat-to-beat blood pressure monitoring. Adv. Healthc. Mater. 2019, 8, 1900109. [Google Scholar] [CrossRef]
- Bagavathiappan, S.; Saravanan, T.; Philip, J.; Jayakumar, T.; Raj, B.; Karunanithi, R.; Panicker, T.M.; Korath, P.; Jagadeesan, K. Investigation of peripheral vascular disorders using thermal imaging. Br. J. Diabetes Vasc. Disease 2008, 8, 102–104. [Google Scholar] [CrossRef]
- Van de Staak, W.; Brakkee, A.; de Rijke-Herweijer, H. Measurements of the thermal conductivity of the skin as an indication of skin blood flow. J. Investig. Dermatol. 1968, 51, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Sivakorn, C.; Schultz, M.J.; Dondorp, A.M. How to monitor cardiovascular function in critical illness in resource-limited settings. Curr. Opin. Crit. Care 2021, 27, 274–281. [Google Scholar] [CrossRef]
- Shin, J.; Jeong, B.; Kim, J.; Nam, V.B.; Yoon, Y.; Jung, J.; Hong, S.; Lee, H.; Eom, H.; Yeo, J.; et al. Sensitive wearable temperature sensor with seamless monolithic integration. Adv. Mater. 2020, 32, 1905527. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, D.; Tang, L.; Song, X.; Luo, W.; Chu, J.; Gao, T.; Shi, H.; Du, C. Wearable temperature sensor based on graphene nanowalls. RSC Adv. 2015, 5, 25609–25615. [Google Scholar] [CrossRef]
- He, Y.; Li, W.; Han, N.; Wang, J.; Zhang, X. Facile flexible reversible thermochromic membranes based on micro/nanoencapsulated phase change materials for wearable temperature sensor. Appl. Energy 2019, 247, 615–629. [Google Scholar] [CrossRef]
- Lin, L.; Chen, Y.Y.; Zhang, X.X.; Wang, X.D. Optimization of geometry and flow rate distribution for double-layer microchannel heat sink. Int. J. Therm. Sci. 2014, 78, 158–168. [Google Scholar] [CrossRef]
- Pottler, K.; Sippel, C.M.; Beck, A.; Fricke, J. Optimized finned absorber geometries for solar air heating collectors. Sol. Energy 1999, 67, 35–52. [Google Scholar] [CrossRef]
- Rafati, M.; Hamidi, A.; Niaser, M.S. Application of nanofluids in computer cooling systems (heat transfer performance of nanofluids). Appl. Therm. Eng. 2012, 45, 9–14. [Google Scholar] [CrossRef]
- Jorfeldt, L.; Juhlin-Dannfelt, A.; Pernow, B.; Wassen, E. Determination of human leg blood flow: A thermodilution technique based on femoral venous bolus injection. Clin. Sci. Mol. Med. 1978, 54, 517–523. [Google Scholar] [CrossRef]
- Wei, K.; Jayaweera, A.R.; Firoozan, S.; Linka, A.; Skyba, D.M.; Kaul, S. Basis for detection of stenosis using venous administration of microbubbles during myocardial contrast echocardiography: Bolus or continuous infusion? J. Am. Coll. Cardiol. 1998, 32, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Divers, S.M.; Hernandez-Divers, S.J.; Wyneken, J. Angiographic, anatomic and clinical technique descriptions of a subcarapacial venipuncture site for chelonians. J. Herpetol. Med. Surg. 2002, 12, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Pinsky, M.R.; Payen, D. Functional hemodynamic monitoring. Crit. Care 2005, 9, 566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, W.T.; Perl, L. Implantable hemodynamic monitoring for heart failure patients. J. Am. Coll. Cardiol. 2017, 70, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yao, K.; Gao, Z.; Liu, Y.; Yu, X. Recent progress of skin-integrated electronics for intelligent sensing. Light Adv. Manuf. 2021, 2, 4. [Google Scholar]
- Liu, Y.; Zhao, L.; Avila, R.; Yiu, C.; Wong, T.; Chan, Y.; Yao, K.; Li, D.; Zhang, Y.; Li, W.; et al. Epidermal electronics for respiration monitoring via thermo-sensitive measuring. Mater. Today Phys. 2020, 13, 100199. [Google Scholar] [CrossRef]
- Wang, C.; Cai, M.; Hao, Z.; Nie, S.; Liu, C.; Du, H.; Wang, J.; Chen, W.; Song, J. Stretchable, multifunctional epidermal sensor patch for surface electromyography and strain measurements. Adv. Intell. Syst. 2021, 2100031. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Liu, I.-S. On Fourier’s law of heat conduction. Contin. Mech. Thermodyn. 1990, 2, 301–305. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, W.; Yiu, C.; Liu, Y.; Wong, T.H.; Huang, X.; Zhou, J.; Li, J.; Yao, K.; Huang, Y.; Li, H.; et al. High Channel Temperature Mapping Electronics in a Thin, Soft, Wireless Format for Non-Invasive Body Thermal Analysis. Biosensors 2021, 11, 435. https://doi.org/10.3390/bios11110435
Park W, Yiu C, Liu Y, Wong TH, Huang X, Zhou J, Li J, Yao K, Huang Y, Li H, et al. High Channel Temperature Mapping Electronics in a Thin, Soft, Wireless Format for Non-Invasive Body Thermal Analysis. Biosensors. 2021; 11(11):435. https://doi.org/10.3390/bios11110435
Chicago/Turabian StylePark, Wooyoung, Chunki Yiu, Yiming Liu, Tsz Hung Wong, Xingcan Huang, Jingkun Zhou, Jian Li, Kuanming Yao, Ya Huang, Hu Li, and et al. 2021. "High Channel Temperature Mapping Electronics in a Thin, Soft, Wireless Format for Non-Invasive Body Thermal Analysis" Biosensors 11, no. 11: 435. https://doi.org/10.3390/bios11110435
APA StylePark, W., Yiu, C., Liu, Y., Wong, T. H., Huang, X., Zhou, J., Li, J., Yao, K., Huang, Y., Li, H., Li, J., Jiao, Y., Shi, R., & Yu, X. (2021). High Channel Temperature Mapping Electronics in a Thin, Soft, Wireless Format for Non-Invasive Body Thermal Analysis. Biosensors, 11(11), 435. https://doi.org/10.3390/bios11110435






