B-Type Natriuretic Peptide as a Significant Brain Biomarker for Stroke Triaging Using a Bedside Point-of-Care Monitoring Biosensor
Abstract
1. Introduction
2. B-Type Natriuretic Peptide Expression in Stroke Pathology
2.1. Association with Increased Mortality in Stroke
2.2. Correlation with Cardioembolic Stroke
2.3. Indication on Second Stroke Recurrence
| Stroke Pathology | B-Type Natriuretic Peptide Level | Classification Performance | Study | Ref. |
|---|---|---|---|---|
| Increased Mortality | BNP = 118.2 vs. 60.9 pg/mL; p < 0.001) | BNP > 65.3 pg/mL; OR = 1.97; p = 0.034) | Montaner J. et al., 2012 | [102] |
| NT-proBNP levels in the deceased group = 3280 pg/mL | Significantly (p < 0.001) higher than in the survival group = 926.30 pg/mL | Chen X. et al., 2012 | [101] | |
| BNP/NT-proBNP = 255.78 pg/mL (95% CI 105.10–406.47, p = 0.001) | BNP = OR 2.30, 95% CI 1.32–4.01 and NT-proBNP OR = 2.63, 95% CI 1.75–3.94 | García-Berrocoso T. et al., 2013 | [97] | |
| BNP > 100.0 pg/mL | BNP OR = 3.94, 95% CI, 2.31–6.73, p < 0.001 | Shibazaki K. et al., 2013 | [100] | |
| BNP = 284.16 ± 382.79 pg/mL at presentation and 273.78 ± 451.91 pg/mL at 72 h | BNP = 25.29 ± 13.47 pg/mL in healthy individuals as control group | Sayan S. and Kotan D. 2016 | [106] | |
| Cardioembolic Etiology | BNP = OR 15.8 (95% CI: 9.92–25.20) | Sensitivity = 78% (95% CI: 71%–87%) and specificity = 83% (95% CI: 77%–87%) | Yang H.-l. et al., 2014 | [118] |
| BNP = 366.6 pg/mL in cardioembolic patients | Non-cardioembolic = 105.6 pg/mL; p < 0.01) | Kawase S. et al., 2015 | [120] | |
| BNP > 66.5 pg/mL | Sensitivity of 75.56% and a specificity of 87.40% | Wu Z. et al., 2015 | [121] | |
| Elevated BNP levels were observed in 75% of cardioembolic stroke patients | Elevated BNP levels were observed in 45.8% of small artery disease patients, 43.1% of larger artery atherosclerosis patients and 34.5% of stroke of undetermined etiology | Chaudhuri J. R. et al., 2015 | [122] | |
| BNP AUC-ROC = 0.81 | Net reclassification improvement = 0.968, p < 0.0001, integrated discrimination improvement = 0.039, p < 0.05 | Nakamura M. et al., 2018 | [135] | |
| Stroke Recurrence | BNP > 300.0 pg/mL | Sensitivity = 80% and specificity = 73% | Shibazaki K. et al., 2014 | [142] |
| BNP > 255.0 pg/mL | Sensitivity = 76% and specificity = 60% | Shibazaki K. et al., 2014 | [143] | |
| NT-proBNP > 800.0 pg/mL | Sensitivity = 64% and specificity = 79% | Rodríguez-Castro E. et al., 2020 | [145] |
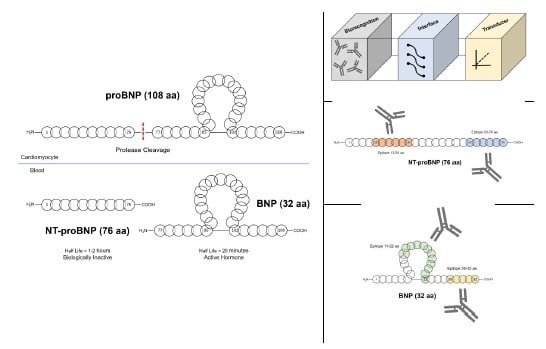
3. Detection of B-Type Natriuretic Peptides
3.1. Point-of-Care Biosensor Platform
3.2. Attractive Epitopes for The Detection of NT-proBNP
3.3. Sandwich Immunoassay Formats for the Detection of BNP
3.4. Commercial Immunossays
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Strong, K.; Mathers, C.; Bonita, R. Preventing stroke: Saving lives around the world. Lancet Neurol. 2007, 6, 182–187. [Google Scholar] [CrossRef]
- Hawkes, M.A.; Rabinstein, A.A. Acute ischemic stroke. In Neurological Emergencies: A Practical Approach; Rabinstein, A.A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 171–188. [Google Scholar] [CrossRef]
- American Heart Association and American Stroke Association Linking Policy. Stroke. 2016. Available online: https://www.stroke.org/en/about-stroke/types-of-stroke (accessed on 1 June 2020).
- Maldonado, S.T.; Riles, S.T. Stroke and Transient Ischemic Attack. In ACS Surgery: Principles and Practice; WebMD, Inc.: New York, NY, USA, 2007; Volume 6, pp. 1–6. [Google Scholar]
- Horgan, N.F.; O’regan, M.; Cunningham, C.J.; Finn, A.M. Recovery after stroke: A 1-year profile. Disabil. Rehabil. 2009, 31, 831–839. [Google Scholar] [CrossRef]
- Bhavna, J. Stroke Diagnostics and Therapeutics: Global Markets; BCC Research: Wellesley, MA, USA, 2015. [Google Scholar]
- Heron, N.; Kee, F.; Donnelly, M.; Cupples, M.E. Systematic review of rehabilitation programmes initiated within 90 days of a transient ischaemic attack or ‘minor’stroke: A protocol. BMJ Open 2015, 5, e007849. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.C.; Rothwell, P.M.; Nguyen-Huynh, M.N.; Giles, M.F.; Elkins, J.S.; Bernstein, A.L.; Sidney, S. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007, 369, 283–292. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Lin, X.-L.; Lu, H.-K.; Liang, X.-Y.; Fan, L.-J.; Liu, X.-T. Lifestyles correlate with stroke recurrence in Chinese inpatients with first-ever acute ischemic stroke. J. Neurol. 2019, 266, 1194–1202. [Google Scholar] [CrossRef]
- MacKay-Lyons, M.; Gubitz, G.; Giacomantonio, N.; Wightman, H.; Marsters, D.; Thompson, K.; Blanchard, C.; Eskes, G.; Thornton, M. Program of rehabilitative exercise and education to avert vascular events after non-disabling stroke or transient ischemic attack (PREVENT Trial): A multi-centred, randomised controlled trial. BMC Neurol. 2010, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.B.; Adams, R.; Alberts, M.J.; Appel, L.J.; Brass, L.M.; Bushnell, C.D.; Culebras, A.; Degraba, T.J.; Gorelick, P.B.; Guyton, J.R.; et al. Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council: Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: The American Academy of Neurology affirms the value of this guideline. Stroke 2006, 37, 1583–1633. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Coull, A.J.; Silver, L.E.; Fairhead, J.F.; Giles, M.F.; Lovelock, C.E.; Redgrave, J.N.; Bull, L.M.; Welch, S.J.; Cuthbertson, F.C.; et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 2005, 366, 1773–1783. [Google Scholar] [CrossRef]
- Chen, R.L.; Balami, J.S.; Esiri, M.M.; Chen, L.K.; Buchan, A.M. Ischemic stroke in the elderly: An overview of evidence. Nat. Rev. Neurol. 2010, 6, 256–265. [Google Scholar] [CrossRef]
- Warlow, C.P.; van Gijn, J.; Dennis, M.; Wardlaw, J.; Bamford, J.; Hankey, G.; Sandercock, P.; Rinkle, G.; Langhorne, P.; Sudlow, C.; et al. Stroke: Practical Management, 3rd ed.; Blackwell Publishing: Oxford, UK, 2008. [Google Scholar]
- Allender, S.; Scarborough, P.; Peto, V.; Rayner, M.; Leal, J.; Luengo-Fernandez, R.; Gray, A. European Cardiovascular Disease Statistics; European Heart Network: Brussels, UK, 2008; pp. 1–112. [Google Scholar]
- National Audit Office, Department of Health. Reducing Brain Damage: Faster Access to Better Stroke Care; National Audit Office: London, UK, 2005.
- Wojner-Alexandrov, A.W.; Alexandrov, A.V.; Rodriguez, D.; Persse, D.; Grotta, J.C. Houston paramedic and emergency stroke treatment and outcomes study (HoPSTO). Stroke 2005, 36, 1512–1518. [Google Scholar] [CrossRef]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Davalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, P.; Wardlaw, J.M.; Lindley, R.I.; Dennis, M.; Cohen, G.; Murray, G.; Innes, K.; Venables, G.; Czlonkowska, A.; Kobayashi, A.; et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): A randomised controlled trial. Lancet 2012, 379, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Kuhrij, L.S.; Marang-van de Mheen, P.J.; van den Berg-Vos, R.M.; de Leeuw, F.-E.; Nederkoorn, P.J. Determinants of extended door-to-needle time in acute ischemic stroke and its influence on in-hospital mortality: Results of a nationwide Dutch clinical audit. BMC Neurol. 2019, 19, 265. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Neurological Disorders; Stroke rt-PA Stroke Study Group. Tissue Plasminogen Activator for Acute Ischemic Stroke. N. Engl. J. Med. 1995, 333, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; Del Zoppo, G.; Alberts, M.J.; Bhatt, D.L.; Brass, L.; Furlan, A.; Grubb, R.L.; Higashida, R.T.; Jauch, E.C.; Kidwell, C. Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007, 38, 1655–1711. [Google Scholar]
- Sukumaran, M.; Cantrell, D.R.; Ansari, S.A.; Huryley, M.; Shaibani, A.; Potts, M.B. Stroke patient workflow optimization. Endovasc Today 2019, 18, 46–50. [Google Scholar]
- Audebert, H.J.; Saver, J.L.; Starkman, S.; Lees, K.R.; Endres, M. Prehospital stroke care: New prospects for treatment and clinical research. Neurology 2013, 81, 501–508. [Google Scholar] [CrossRef]
- Leys, D.; Ringelstein, E.B.; Kaste, M.; Hacke, W. Facilities available in European hospitals treating stroke patients. Stroke 2007, 38, 2985–2991. [Google Scholar] [CrossRef]
- Fladt, J.; Meier, N.; Thilemann, S.; Polymeris, A.; Traenka, C.; Seiffge, D.J.; Sutter, R.; Peters, N.; Gensicke, H.; Flückiger, B. Reasons for Prehospital Delay in Acute Ischemic Stroke. J. Am. Heart Assoc. 2019, 8, e013101. [Google Scholar] [CrossRef]
- Sharobeam, A.; Jones, B.; Walton-Sonda, D.; Lueck, C.J. Factors delaying intravenous thrombolytic therapy in acute ischaemic stroke: A systematic review of the literature. J. Neurol. 2020, 1–12. [Google Scholar] [CrossRef]
- Tai, Y.J.; Yan, B. Minimising time to treatment: Targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern. Med. J. 2013, 43, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Evenson, K.R.; Rosamond, W.D.; Morris, D.L. Prehospital and in-hospital delays in acute stroke care. Neuroepidemiology 2001, 20, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Prim. 2019, 5, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, C.S.; Chalela, J.A.; Saver, J.L.; Starkman, S.; Hill, M.D.; Demchuk, A.M.; Butman, J.A.; Patronas, N.; Alger, J.R.; Latour, L.L. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 2004, 292, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Garrison, M.E.; Chou, T.M.; Hyde, G.M.; Schaer, A.K. Methods and Systems for Treatment of Acute Ischemic Stroke. U.S. Patent US20130281788A1, 24 October 2013. [Google Scholar]
- Ferro, J.M.; Massaro, A.R.; Mas, J.L. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010, 9, 1085–1096. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V.; et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Goldstein, L.B.; Bertels, C.; Davis, J.N. Interrater reliability of the NIH stroke scale. Arch. Neurol. 1989, 46, 660–662. [Google Scholar] [CrossRef]
- Tamar, A.; Malin, R.; Katharina, S.S. NIHSS is not enough for cognitive screening in acute stroke: A cross-sectional, retrospective study. Sci. Rep. 2020, 10, 534. [Google Scholar]
- Hand, P.J.; Kwan, J.; Lindley, R.I.; Dennis, M.S.; Wardlaw, J.M. Distinguishing between stroke and mimic at the bedside: The brain attack study. Stroke 2006, 37, 769–775. [Google Scholar] [CrossRef]
- Hemmen, T.M.; Meyer, B.C.; McClean, T.L.; Lyden, P.D. Identification of nonischemic stroke mimics among 411 code strokes at the University of California, San Diego, Stroke Center. J. Stroke Cerebrovasc. Dis. 2008, 17, 23–25. [Google Scholar] [CrossRef]
- Deng, Y.Z.; Reeves, M.J.; Jacobs, B.S.; Birbeck, G.L.; Kothari, R.U.; Hickenbottom, S.L.; Mullard, A.J.; Wehner, S.; Maddox, K.; Majid, A. IV tissue plasminogen activator use in acute stroke: Experience from a statewide registry. Neurology 2006, 66, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.L.; Leathley, M.J.; Jones, S.P.; Ford, G.A.; Quinn, T.; Sutton, C.J. Training emergency services’ dispatchers to recognise stroke: An interrupted time-series analysis. BMC Health Serv. Res. 2013, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Beal, C.C.; Ogola, G.; Allen, L. Validity and Reliability of the Responses to Ischemic Stroke Symptoms Questionnaire. J. Neurosci. Nurs. 2019, 51, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Stroke Prevention and Care in France: Report for the Minister of Health and Sport. 2009. Available online: https://www1.health.gov.au/internet/main/publishing.nsf/Content/For+Health+Professionals-1 (accessed on 1 June 2020).
- Teo, K.; Slark, J. A systematic review of studies investigating the care of stroke survivors in long-term care facilities. Disabil. Rehabil. 2016, 38, 715–723. [Google Scholar] [CrossRef]
- Greenberg, E.; Treger, I.; Ring, H. Post-Stroke Follow-Up at the Rehabilitation Center Outpatient Clinic. Sat 2004, 28, 19. [Google Scholar]
- Jauch, E.C.; Lindsell, C.; Broderick, J.; Fagan, S.C.; Tilley, B.C.; Levine, S.R. Association of serial biochemical markers with acute ischemic stroke: The National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke 2006, 37, 2508–2513. [Google Scholar] [CrossRef]
- Glickman, S.W.; Phillips, S.; Anstrom, K.J.; Laskowitz, D.T.; Cairns, C.B. Discriminative capacity of biomarkers for acute stroke in the emergency department. J. Emerg. Med. 2011, 41, 333–339. [Google Scholar] [CrossRef]
- Chollet, F.; Albucher, J.-F. Strategies to augment recovery after stroke. Curr. Treat. Options Neurol. 2012, 14, 531–540. [Google Scholar] [CrossRef]
- Ng, G.J.L.; Quek, A.M.L.; Cheung, C.; Arumugam, T.V.; Seet, R.C.S. Stroke biomarkers in clinical practice: A critical appraisal. Neurochem. Int. 2017, 107, 11–22. [Google Scholar] [CrossRef]
- Simpkins, A.N.; Janowski, M.; Oz, H.S.; Roberts, J.; Bix, G.; Doré, S.; Stowe, A.M. Biomarker Application for Precision Medicine in Stroke. Transl. Stroke Res. 2020, 11, 615–627. [Google Scholar] [CrossRef]
- Menosi Gualandro, D.; Twerenbold, R.; Boeddinghaus, J.; Nestelberger, T.; Puelacher, C.; Müller, C. Biomarkers in cardiovascular medicine: Towards precision medicine. Swiss Med. Wkly. 2019, 149, w20125. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, D.; Eltzov, E.; Seet, R.; Marks, R.S.; Tok, A.I.Y. Point-of-care-testing in acute stroke management: An unmet need ripe for technological harvest. Biosensors 2017, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.J.; Drozdowska, B.A. Stroke prediction and the future of prognosis research. Nat. Rev. Neurol. 2019, 15, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Abdelmalak, B.; Farag, E. Neurological biomarkers in the perioperative period. Br. J. Anaesth. 2011, 107, 844–858. [Google Scholar] [CrossRef]
- Seet, R.C.S.; Lee, C.-Y.J.; Chan, B.P.L.; Sharma, V.K.; Teoh, H.-L.; Venketasubramanian, N.; Lim, E.C.H.; Chong, W.-L.; Looi, W.-F.; Huang, S.-H. Oxidative damage in ischemic stroke revealed using multiple biomarkers. Stroke 2011, 42, 2326–2329. [Google Scholar] [CrossRef]
- Laskowitz, D.T.; Kasner, S.E.; Saver, J.; Remmel, K.S.; Jauch, E.C. Clinical usefulness of a biomarker-based diagnostic test for acute stroke: The Biomarker Rapid Assessment in Ischemic Injury (BRAIN) study. Stroke 2009, 40, 77–85. [Google Scholar] [CrossRef]
- Yokobori, S.; Hosein, K.; Burks, S.; Sharma, I.; Gajavelli, S.; Bullock, R. Biomarkers for the clinical differential diagnosis in traumatic brain injury—A systematic review. CNS Neurosci. Ther. 2013, 19, 556–565. [Google Scholar] [CrossRef]
- Mir, I.N.; Chalak, L.F. Serum biomarkers to evaluate the integrity of the neurovascular unit. Early Hum. Dev. 2014, 90, 707–711. [Google Scholar] [CrossRef]
- Peplow, P.V.; Martinez, B.; Dambinova, S.A. Stroke Biomarkers; Humana: New York, NY, USA, 2020. [Google Scholar]
- Kamtchum-Tatuene, J.; Jickling, G.C. Blood Biomarkers for Stroke Diagnosis and Management. Neuromol. Med. 2019, 21, 344–368. [Google Scholar] [CrossRef]
- Sharp, F.; Jickling, G.C. Biomarkers for the Diagnosis of Lacunar Stroke. U.S. Patent US10196690B2, 5 February 2019. [Google Scholar]
- Ford, B.D.; Ford, G. Biomarkers for Stroke. U.S. Patent US20190004065A1, 3 January 2019. [Google Scholar]
- Augello, C.J.; Noll, J.M.; Distel, T.J.; Wainright, J.D.; Stout, C.E.; Ford, B.D. Identification of novel blood biomarker panels to detect ischemic stroke in patients and their responsiveness to therapeutic intervention. Brain Res. 2018, 1698, 161–169. [Google Scholar] [CrossRef]
- Makris, K.; Haliassos, A.; Chondrogianni, M.; Tsivgoulis, G. Blood biomarkers in ischemic stroke: Potential role and challenges in clinical practice and research. Crit. Rev. Clin. Lab. Sci. 2018, 55, 294–328. [Google Scholar] [CrossRef] [PubMed]
- Ng, G.J.; Ng, M.Y.; Quek, A.M.; Seet, R.C. Resolving Difficult Case Scenarios by Incorporating Stroke Biomarkers in Clinical Decision-making. In Acute Brain Impairment: Scientidic Discoveries and Translational Research; Peplow, P.V., Dambinova, S.A., Gennarelli, T.A., Martinez, B., Eds.; Royal Society of Chemistry: London, UK, 2017; pp. 289–314. [Google Scholar]
- Misra, S.; Kumar, A.; Kumar, P.; Yadav, A.K.; Mohania, D.; Pandit, A.K.; Prasad, K.; Vibha, D. Blood-based protein biomarkers for stroke differentiation: A systematic review. Proteom. Clin. Appl. 2017, 11, 1700007. [Google Scholar] [CrossRef] [PubMed]
- LaMont, J.; Mc Connell, I.; Fitzgerald, P. Biomarker-Based Methods and Biochips for Aiding the Diagnosis of Stroke. U.S. Patent 15/457,297, 29 June 2017. [Google Scholar]
- Montaner, J.; Ramiro, L.; Simats, A.; Tiedt, S.; Makris, K.; Jickling, G.C.; Debette, S.; Sanchez, J.-C.; Bustamante, A. Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke. Nat. Rev. Neurol. 2020, 16, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, H.; Woźniak, A.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. The Role of Selected Pro-Inflammatory Cytokines in Pathogenesis of Ischemic Stroke. Clin. Interv. Aging 2020, 15, 469. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.J.; Liu, W.; Timmins, G.; Pan, R. Blood Biomarker for Early Blood Brain Barrier Disruption in Ischemic Stroke. U.S. Patent US10591491B1, 17 March 2020. [Google Scholar]
- Gąsiorek, P.; Banach, M.; Sakowicz, A.; Głąbiński, A.; Sosnowska, B.; Maciejewski, M.; Bielecka-Dabrowa, A. The potential role of inflammation in cryptogenic stroke. Adv. Med. Sci. 2019, 64, 381–387. [Google Scholar] [CrossRef]
- Lee, N.S.; Daniels, L.B. Natriuretic peptide use in screening in the community. In Cardiac Biomarkers; Springer: Berlin/Heidelberg, Germany, 2016; pp. 181–193. [Google Scholar]
- Balion, C.; McKelvie, R.; Don-Wauchope, A.C.; Santaguida, P.L.; Oremus, M.; Keshavarz, H.; Hill, S.A.; Booth, R.A.; Ali, U.; Brown, J.A. B-type natriuretic peptide-guided therapy: A systematic review. Heart Fail. Rev. 2014, 19, 553–564. [Google Scholar] [CrossRef]
- Pemberton, C.J.; Charles, C.J.; Richards, A.M. Chapter 1—Cardiac natriuretic peptides. In Endocrinology of the Heart in Health and Disease; Schisler, J.C., Lang, C.H., Willis, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 3–39. [Google Scholar] [CrossRef]
- Mukoyama, M.; Nakao, K.; Hosoda, K.; Suga, S.; Saito, Y.; Ogawa, Y.; Shirakami, G.; Jougasaki, M.; Obata, K.; Yasue, H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J. Clin. Investig. 1991, 87, 1402–1412. [Google Scholar] [CrossRef]
- Fu, S.; Ping, P.; Zhu, Q.; Ye, P.; Luo, L. Brain Natriuretic Peptide and Its Biochemical, Analytical, and Clinical Issues in Heart Failure: A Narrative Review. Front. Physiol. 2018, 9, 692. [Google Scholar] [CrossRef]
- Mair, J. Biochemistry of B-type natriuretic peptide–where are we now? Clin. Chem. Lab. Med. 2008, 46, 1507–1514. [Google Scholar] [CrossRef]
- Atisha, D.; Bhalla, M.A.; Morrison, L.K.; Felicio, L.; Clopton, P.; Gardetto, N.; Kazanegra, R.; Chiu, A.; Maisel, A.S. A prospective study in search of an optimal B-natriuretic peptide level to screen patients for cardiac dysfunction. Am. Heart J. 2004, 148, 518–523. [Google Scholar] [CrossRef]
- Sedlakova, E.; Prusik, Z.; Škopková, J.; Barth, T.; Kluh, I.; Cort, J.H. Isolation of a tridecapeptide from natriuretic fractions of bovine posterior pituitary. Eur. J. Clin. Investig. 1974, 4, 285–292. [Google Scholar] [CrossRef]
- Nakagawa, M.; Tanaka, I.; Suga, S.I.; Ogawa, Y.; Tamura, N.; Goto, M.; Sugawara, A.; Yoshimasa, T.; Itoh, H.; Mukoyama, M.; et al. Preparation of a monoclonal antibody against mouse brain natriuretic peptide (BNP) and tissue distribution of BNP in mice. Clin. Exp. Pharmacol. Physiol. 1995, 22, S186–S187. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.J.; Yandle, T.G.; Nicholls, M.G.; Richards, A.M.; Espiner, E.A. The amino-terminal portion of pro-brain natriuretic peptide (Pro-BNP) circulates in human plasma. Biochem. Biophys. Res. Commun. 1995, 214, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Jourdain, P.; Maisel, A.; Dahlstrom, U.; Follath, F.; Isnard, R.; Luchner, A.; McDonagh, T.; Mair, J.; Nieminen, M.; et al. Clinical applications of B-type natriuretic peptide (BNP) testing. Eur. Heart J. 2003, 24, 1710–1718. [Google Scholar] [CrossRef]
- De Bold, A.J. Atrial natriuretic factor of the rat heart. Studies on isolation and properties. Proc. Soc. Exp. Biol. Med. 1982, 170, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, Y.; Nakao, K.; Mukoyama, M.; Saito, Y.; Ogawa, Y.; Shiono, S.; Inouye, K.; Yoshida, N.; Imura, H. Isolation and sequence determination of human brain natriuretic peptide in human atrium. FEBS Let. 1990, 259, 341–345. [Google Scholar] [CrossRef]
- Hino, J.; Tateyama, H.; Minamino, N.; Kangawa, K.; Matsuo, H. Isolation and identification of human brain natriuretic peptides in cardiac atrium. Biochem. Biophys. Res. Commun. 1990, 167, 693–700. [Google Scholar] [CrossRef]
- Ala-Kopsala, M.; Ruskoaho, H.; Leppaluoto, J.; Seres, L.; Skoumal, R.; Toth, M.; Horkay, F.; Vuolteenaho, O. Single assay for amino-terminal fragments of cardiac A-and B-type natriuretic peptides. Clin. Chem. 2005, 51, 708–718. [Google Scholar] [CrossRef]
- Lewin, J.; Ledwidge, M.; O’Loughlin, C.; McNally, C.; McDonald, K. Clinical deterioration in established heart failure: What is the value of BNP and weight gain in aiding diagnosis? Eur. J. Heart Failure 2005, 7, 953–957. [Google Scholar] [CrossRef]
- Kangawa, K.; Matsuo, H. Purification and complete amino acid sequence of α-human atrial natriuretic polypeptide (α-hANP). Biochem. Biophys. Res. Commun. 1984, 118, 131–139. [Google Scholar] [CrossRef]
- Kambayashi, Y.; Nakao, K.; Itoh, H.; Hosoda, K.; Saito, Y.; Yamada, T.; Mukoyama, M.; Arai, H.; Shirakami, G.; Suga, S.; et al. Isolation and sequence determination of rat cardiac natriuretic peptide. Biochem. Biophys. Res. Commun. 1989, 163, 233–240. [Google Scholar] [CrossRef]
- Schellenberger, U.; O’Rear, J.; Guzzetta, A.; Jue, R.A.; Protter, A.A.; Pollitt, N.S. The precursor to B-type natriuretic peptide is an O-linked glycoprotein. Arch. Biochem. Biophys. 2006, 451, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Sudoh, T.; Fukuda, K.; Kangawa, K.; Minamino, N.; Matsuo, H. Identification of alpha atrial natriuretic peptide and in porcine brain. Biochem. Biophys. Res. Commun. 1987, 149, 1055–1062. [Google Scholar] [CrossRef]
- Sudoh, T.; Kangawa, K.; Minamino, N.; Matsuo, H. A new natriuretic peptide in porcine brain. Nature 1988, 332, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Kreiger, G. A basic guide to understanding plasma B-type natriuretic peptide in the diagnosis of congestive heart failure. Medsurg Nurs. 2007, 16, 75–79. [Google Scholar]
- Ray, P.; Delerme, S.; Jourdain, P.; Chenevier-Gobeaux, C. Differential diagnosis of acute dyspnea: The value of B natriuretic peptides in the emergency department. QJM Int. J. Med. 2008, 101, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Andritsos, M.; Singh, N.; Patel, P.; Sinha, A.; Fassl, J.; Wyckoff, T.; Riha, H.; Roscher, C.; Subramaniam, B.; Ramakrishna, H.; et al. The year in cardiothoracic and vascular anesthesia: Selected highlights from 2010. J. Cardiothorac. Vasc. Anesth. 2011, 25, 6–15. [Google Scholar] [CrossRef]
- Cowie, M.R. Recent developments in the management of heart failure. Practitioner 2012, 256, 25–30. [Google Scholar] [CrossRef]
- Oremus, M.; McKelvie, R.; Don-Wauchope, A.; Santaguida, L.P.; Ali, U.; Balion, C.; Hill, S.; Booth, R.; Brown, A.J.; Bustamam, A.; et al. A systematic review of BNP and NT-proBNP in the management of heart failure: Overview and methods. Heart Fail. Rev. 2014, 19, 413–419. [Google Scholar] [CrossRef]
- García-Berrocoso, T.; Giralt, D.; Bustamante, A.; Etgen, T.; Jensen, J.K.; Sharma, J.C.; Shibazaki, K.; Saritas, A.; Chen, X.; Whiteley, W.N.; et al. B-type natriuretic peptides and mortality after stroke. Neurology 2013, 81, 1976–1985. [Google Scholar] [CrossRef]
- Shibazaki, K.; Kimura, K. Diagnosis and treatment of acute ischemic stroke. Brain Nerve 2013, 65, 1023–1030. [Google Scholar] [PubMed]
- Jickling, G.C.; Foerch, C. Predicting stroke mortality. Neurology 2013, 81, 1970–1971. [Google Scholar] [CrossRef] [PubMed]
- Shibazaki, K.; Kimura, K.; Sakai, K.; Fujii, S.; Aoki, J.; Saji, N. Brain Natriuretic Peptide on Admission as a Biological Marker of Long-Term Mortality in Ischemic Stroke Survivors. Eur. Neurol. 2013, 70, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhan, X.; Chen, M.; Lei, H.; Wang, Y.; Wei, D.; Jiang, X. The prognostic value of combined NT-pro-BNP levels and NIHSS scores in patients with acute ischemic stroke. Intern. Med. 2012, 51, 2887–2892. [Google Scholar] [CrossRef]
- Montaner, J.; García-Berrocoso, T.; Mendioroz, M.; Palacios, M.; Perea-Gainza, M.; Delgado, P.; Rosell, A.; Slevin, M.; Ribó, M.; Molina, C.A.; et al. Brain Natriuretic Peptide Is Associated with Worsening and Mortality in Acute Stroke Patients but Adds No Prognostic Value to Clinical Predictors of Outcome. Cerebrovasc. Dis. 2012, 34, 240–245. [Google Scholar] [CrossRef]
- Li, J.; Gu, C.; Li, D.; Chen, L.; Lu, Z.; Zhu, L.; Huang, H. Effects of serum N-terminal pro B-type natriuretic peptide and D-dimer levels on patients with acute ischemic stroke. Pak. J. Med Sci. 2018, 34, 994. [Google Scholar] [CrossRef]
- Hess, G.; Horsch, A.; Zdunek, D. Troponin and Bnp Based Diagnosis of Risk Patients and Cause of Stroke. U.S. Patent US20180196066A1, 12 July 2018. [Google Scholar]
- Tu, W.-J.; Ma, G.-Z.; Ni, Y.; Hu, X.-S.; Luo, D.-Z.; Zeng, X.-W.; Liu, Q.; Xu, T.; Yu, L.; Wu, B. Copeptin and NT-proBNP for prediction of all-cause and cardiovascular death in ischemic stroke. Neurology 2017, 88, 1899–1905. [Google Scholar] [CrossRef]
- Sayan, S.; Kotan, D. Levels of brain natriuretic peptide as a marker for the diagnosis and prognosis of acute ischemic stroke. Arch. Med. Sci. Atheroscler. Dis. 2016, 1, e16. [Google Scholar] [CrossRef]
- Bunevicius, A.; Kazlauskas, H.; Raskauskiene, N.; Mickuviene, N.; Ndreu, R.; Corsano, E.; Bunevicius, R. Role of N-terminal pro-B-type natriuretic peptide, high-sensitivity C-reactive protein, and inteleukin-6 in predicting a poor outcome after a stroke. Neuroimmunomodulation 2015, 22, 365–372. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Veronesi, G.; Costanzo, S.; Zeller, T.; Schnabel, R.B.; de Curtis, A.; Salomaa, V.; Borchini, R.; Ferrario, M.; Giampaoli, S. NT-proBNP (N-Terminal Pro-B-Type Natriuretic Peptide) and the Risk of Stroke: Results from the BiomarCaRE Consortium. Stroke 2019, 50, 610–617. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Caplan, L.R.; Donnan, G.A.; Hennerici, M.G. Classification of stroke subtypes. Cerebrovasc. Dis. 2009, 27, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Mac Grory, B.; Flood, S.P.; Apostolidou, E.; Yaghi, S. Cryptogenic Stroke: Diagnostic Workup and Management. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 77. [Google Scholar] [CrossRef]
- Petty, G.W.; Brown, R.D., Jr.; Whisnant, J.P.; Sicks, J.R.D.; O’Fallon, W.M.; Wiebers, D.O. Ischemic stroke subtypes: A population-based study of incidence and risk factors. Stroke 1999, 30, 2513–2516. [Google Scholar] [CrossRef] [PubMed]
- Lodder, J.; Bamford, J.M.; Sandercock, P.A.G.; Jones, L.N.; Warlow, C.P. Are hypertension or cardiac embolism likely causes of lacunar infarction? Stroke 1990, 21, 375–381. [Google Scholar] [CrossRef]
- Grau, A.J.; Weimar, C.; Buggle, F.; Heinrich, A.; Goertler, M.; Neumaier, S.; Glahn, J.; Brandt, T.; Hacke, W.; Diener, H.-C. Risk Factors, Outcome, and Treatment in Subtypes of Ischemic Stroke. Ger. Stroke Data Bank 2001, 32, 2559–2566. [Google Scholar] [CrossRef]
- Lee, L.J.; Kidwell, C.S.; Alger, J.; Starkman, S.; Saver, J.L. Impact on stroke subtype diagnosis of early diffusion-weighted magnetic resonance imaging and magnetic resonance angiography. Stroke 2000, 31, 1081–1089. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Landau, W.M.; Nassief, A. Editorial comment—Time to burn the TOAST. Stroke 2005, 36, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, P. Patent foramen ovale and the risk of stroke: Smoking gun guilty by association? Heart 2005, 91, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-l.; Lin, Y.-P.; Long, Y.; Ma, Q.-l.; Zhou, C. Predicting Cardioembolic Stroke with the B-Type Natriuretic Peptide Test: A Systematic Review and Meta-analysis. J. Stroke Cerebrovasc. Dis. 2014, 23, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Llombart, V.; Antolin-Fontes, A.; Bustamante, A.; Giralt, D.; Rost Natalia, S.; Furie, K.; Shibazaki, K.; Biteker, M.; Castillo, J.; Rodríguez-Yáñez, M.; et al. B-Type Natriuretic Peptides Help in Cardioembolic Stroke Diagnosis. Stroke 2015, 46, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Kawase, S.; Kowa, H.; Suto, Y.; Fukuda, H.; Kusumi, M.; Nakayasu, H.; Nakashima, K. Plasma Brain Natriuretic Peptide is a Marker of Prognostic Functional Outcome in Non–Cardioembolic Infarction. J. Stroke Cerebrovasc. Dis. 2015, 24, 2285–2290. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, M.; He, M.; Zeng, H.; Tan, F.; Li, K.; Chen, S.; Han, Q.; Wang, Q. Validation of the use of B-type natriuretic peptide point-of-care test platform in preliminary recognition of cardioembolic stroke patients in the ED. Am. J. Emerg. Med. 2015, 33, 521–526. [Google Scholar] [CrossRef]
- Chaudhuri, J.R.; Sharma, V.K.; Mridula, K.R.; Balaraju, B.; Bandaru, V.C.S.S. Association of Plasma Brain Natriuretic Peptide Levels in Acute Ischemic Stroke Subtypes and Outcome. J. Stroke Cerebrovasc. Dis. 2015, 24, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, I.M.; Cojocaru, M.; Sapira, V.; Ionescu, A.; Bârlan, S.; Tacu, N. Could pro-BNP, uric acid, bilirubin, albumin and transferrin be used in making the distinction between stroke subtypes? Rom. J. Intern. Med. 2013, 51, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, L.; Guo, Q.; He, M.; Li, K. Experiences and the use of BNP POCT platform on suspected ischemic stroke patients in the emergency department setting. Clin. Neurol. Neurosurg. 2014, 123, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Hajsadeghi, S.; Kashani Amin, L.; Bakhshandeh, H.; Rohani, M.; Azizian, A.R.; Jafarian Kerman, S.R. The diagnostic value of N-terminal pro-brain natriuretic peptide in differentiating cardioembolic ischemic stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2013, 22, 554–560. [Google Scholar] [CrossRef]
- Kara, K.; Gronewold, J.; Neumann, T.; Mahabadi, A.A.; Weimar, C.; Lehmann, N.; Berger, K.; Kalsch, H.I.; Bauer, M.; Broecker-Preuss, M.; et al. B-type natriuretic peptide predicts stroke of presumable cardioembolic origin in addition to coronary artery calcification. Eur. J. Neurol. 2014, 21, 914–921. [Google Scholar] [CrossRef]
- Maruyama, K.; Shiga, T.; Iijima, M.; Moriya, S.; Mizuno, S.; Toi, S.; Arai, K.; Ashihara, K.; Abe, K.; Uchiyama, S. Brain natriuretic peptide in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014, 23, 967–972. [Google Scholar] [CrossRef]
- Qihong, G.; Zhixin, W.; Mingfeng, H.; Lianhong, Y.; Wenchong, X. Experiences and the use of BNP POCT platform on suspected stroke patients by a Chinese emergency department. Ann. Indian Acad. Neurol. 2014, 17, 243–244. [Google Scholar] [CrossRef]
- Sakai, K.; Shibazaki, K.; Kimura, K.; Aoki, J.; Kobayashi, K.; Fujii, S.; Okada, Y. Brain natriuretic peptide as a predictor of cardioembolism in acute ischemic stroke patients: Brain natriuretic peptide stroke prospective study. Eur. Neurol. 2013, 69, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Shibazaki, K.; Kimura, K.; Iguchi, Y.; Okada, Y.; Inoue, T. Plasma brain natriuretic peptide can be a biological marker to distinguish cardioembolic stroke from other stroke types in acute ischemic stroke. Intern. Med. 2009, 48, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Tanaka, R.; Yamashiro, K.; Shimada, Y.; Kuroki, T.; Hira, K.; Urabe, T.; Hattori, N. Impact of BNP on cryptogenic stroke without potential embolic sources on transesophageal echocardiography. J. Neurol. Sci. 2015, 359, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Otaki, Y.; Watanabe, T.; Sato, N.; Shirata, T.; Tsuchiya, H.; Wanezaki, M.; Tamura, H.; Nishiyama, S.; Arimoto, T.; Takahashi, H. Direct comparison of prognostic ability of cardiac biomarkers for cardiogenic stroke and clinical outcome in patients with stroke. Heart Vessel. 2019, 34, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Sun, H.; Xie, L.; Zhu, Y.; Feng, Y. Detection of cardioembolic stroke with B-type natriuretic peptide or N-terminal pro-BNP: A comparative diagnostic meta-analysis. Int. J. Neurosci. 2018, 128, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Lee, D.-G.; Lim, D.-S.; Hong, S.; Park, J.-S. Difference in the prognostic significance of N-terminal pro-B-type natriuretic peptide between cardioembolic and noncardioembolic ischemic strokes. Dis. Markers 2015, 2015, 597570. [Google Scholar] [CrossRef]
- Nakamura, M.; Ishibashi, Y.; Tanaka, F.; Omama, S.; Onoda, T.; Takahashi, T.; Takahashi, S.; Tanno, K.; Ohsawa, M.; Sakata, K. Ability of B-Type Natriuretic Peptide Testing to Predict Cardioembolic Stroke in the General Population―Comparisons with C-Reactive Protein and Urinary Albumin―. Circ. J. 2018, 82, 1017–1025. [Google Scholar] [CrossRef]
- Zhixin, W.U.; Yingying, L.I.; Liang, S.; Qing, F.; Lei, J.; Chen, J.; Mingfeng, H.E.; Kuangyi, L.I. The value of using B-type natriuretic peptide and D-dimer in preliminary recognition of cardioembolic stroke patients. J. Pract. Med. 2018, 34, 44–48. [Google Scholar]
- Lee, S.-J.; Lee, D.-G.; Chung, T.-I. N-terminal pro-B-type natriuretic peptide predicts long-term mortality but not stroke recurrence in acute ischemic stroke patients with a cardioembolic source (P1. 051). Neurology 2015, 84 (Suppl. S14), P1.051. [Google Scholar]
- Hosomi, N.; Yoshimoto, T.; Kanaya, Y.; Neshige, S.; Hara, N.; Himeno, T.; Kono, R.; Takeshima, S.; Takamatsu, K.; Ota, T. Brain natriuretic peptide and particular left ventricle segment asynergy associated with cardioembolic stroke from old myocardial infarction. J. Stroke Cerebrovasc. Dis. 2016, 25, 1165–1171. [Google Scholar] [CrossRef]
- Mohamed, W.S.; Abd ElGawad, E.A.; ElMotayam, A.S.E.; Fathy, S.E. Cardio embolic stroke and blood biomarkers: Diagnosis and predictors of short-term outcome. Egypt. J. Neurol. Psychiatry Neurosurg. 2019, 55, 68. [Google Scholar] [CrossRef]
- Okada, Y.; Terakawa, Y.; Murata, T.; Nakamura, K.; Shimotake, K.; Murata, H.; Ohata, K. Ability of NT-pro-BNP to Diagnose Cardioembolic Etiology in Patients with Acute Ischemic Stroke. Osaka City Med. J. 2016, 62, 95–102. [Google Scholar]
- Inoue, S.; Murakami, Y.; Sano, K.; Katoh, H.; Shimada, T. Atrium as a source of brain natriuretic polypeptide in patients with atrial fibrillation. J. Card Fail. 2000, 6, 92–96. [Google Scholar] [CrossRef]
- Shibazaki, K.; Kimura, K.; Aoki, J.; Sakai, K.; Saji, N.; Uemura, J. Brain natriuretic peptide level on admission predicts recurrent stroke after discharge in stroke survivors with atrial fibrillation. Clin. Neurol. Neurosurg. 2014, 127, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Shibazaki, K.; Kimura, K.; Aoki, J.; Sakai, K.; Saji, N.; Uemura, J. Plasma Brain Natriuretic Peptide as a Predictive Marker of Early Recurrent Stroke in Cardioembolic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2014, 23, 2635–2640. [Google Scholar] [CrossRef] [PubMed]
- Mortezabeigi, H.R.; Taghizadeh, A.; Talebi, M.; Amini, K.; Goldust, M. ABCD2 score and BNP level in patients with TIA and cerebral stroke. Pak. J. Biol. Sci. 2013, 16, 1393–1397. [Google Scholar] [CrossRef]
- Rodríguez-Castro, E.; Hervella, P.; López-Dequidt, I.; Arias-Rivas, S.; Santamaría-Cadavid, M.; López-Loureiro, I.; da Silva-Candal, A.; Pérez-Mato, M.; Sobrino, T.; Campos, F. NT-pro-BNP: A novel predictor of stroke risk after transient ischemic attack. Int. J. Cardiol. 2020, 298, 93–97. [Google Scholar] [CrossRef]
- Cummins, B.M.; Ligler, F.S.; Walker, G.M. Point-of-care diagnostics for niche applications. Biotechnol. Adv. 2016, 34, 161–176. [Google Scholar] [CrossRef]
- Yoo, E.H.; Lee, S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Vasan, A.S.; Mahadeo, D.M.; Doraiswami, R.; Huang, Y.; Pecht, M. Point-of-care biosensor system. Front. Biosci. 2013, 5, 39–71. [Google Scholar] [CrossRef]
- Shah, D.; Maghsoudlou, D. Enzyme-linked immunosorbent assay (ELISA): The basics. Br. J. Hosp. Med. 2016, 77, C98–C101. [Google Scholar] [CrossRef]
- Price, C.P. Point of care testing. BMJ Br. Med J. 2001, 322, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Eltzov, E.; Cosnier, S.; Marks, R.S. Biosensors based on combined optical and electrochemical transduction for molecular diagnostics. Expert. Rev. Mol. Diagn. 2011, 11, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Eltzov, E.; Marks, R.S. Whole-cell aquatic biosensors. Anal. Bioanal. Chem. 2011, 400, 895–913. [Google Scholar] [CrossRef]
- St John, A.; Price, C.P. Existing and Emerging Technologies for Point-of-Care Testing. Clin. Biochem. Rev. 2014, 35, 155–167. [Google Scholar]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab. Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef]
- Paul, S. Biomedical Engineering and Its Applications in Healthcare; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- McMullan, J.T.; Knight, W.A.; Clark, J.F.; Beyette, F.R.; Pancioli, A. Time-critical neurological emergencies: The unfulfilled role for point-of-care testing. Int. J. Emerg. Med. 2010, 3, 127–131. [Google Scholar] [CrossRef]
- Rooney, K.D.; Schilling, U.M. Point-of-care testing in the overcrowded emergency department—Can it make a difference? Crit. Care 2014, 18, 692. [Google Scholar] [CrossRef]
- Harpaz, D.; Eltzov, E.; Seet, R.C.S.; Marks, R.S.; Tok, A.I.Y. Rapid Point-of-Care-Tests for Stroke Monitoring. Org. Bioelectron. Life Sci. Healthc. 2019, 56, 263–314. [Google Scholar]
- Ala-Kopsala, M.; Moilanen, A.-M.; Rysä, J.; Ruskoaho, H.; Vuolteenaho, O. Characterization of Molecular Forms of N-Terminal B-Type Natriuretic Peptide in Vitro. Clin. Chem. 2010, 56, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Ordonez-Llanos, J.; Collinson, P.O.; Christenson, R.H. Amino-Terminal Pro–B-Type Natriuretic Peptide: Analytic Considerations. Am. J. Cardiol. 2008, 101 (Suppl. S3), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Panteghini, M.; Ravkilde, J.; Mair, J.; Wu, A.H.B.; Tate, J.; Pagani, F.; Christenson, R.H.; Jaffe, A.S. Quality Specifications for B-Type Natriuretic Peptide Assays. Clin. Chem. 2005, 51, 486–493. [Google Scholar] [CrossRef]
- Clerico, A.; Emdin, M. Diagnostic Accuracy and Prognostic Relevance of the Measurement of Cardiac Natriuretic Peptides: A Review. Clin. Chem. 2004, 50, 33–50. [Google Scholar] [CrossRef]
- Apple, F.S. Standardization of Cardiac Markers. Scand. J. Clin. Lab. Investig. 2005, 65, 107–111. [Google Scholar] [CrossRef]
- Sinawang, P.D.; Harpaz, D.; Fajs, L.; Seet, R.C.S.; Tok, A.I.Y.; Marks, R.S. Electrochemical impedimetric detection of stroke biomarker NT-proBNP using disposable screen-printed gold electrodes. Eurobiotech. J. 2017, 1, 165–176. [Google Scholar] [CrossRef]
- Harpaz, D.; Koh, B.; Marks, R.S.; Seet, R.; Abdulhalim, I.; Tok, A.I.Y. Point-of-Care surface plasmon resonance biosensor for stroke biomarkers NT-proBNP and S100β using a functionalized gold chip with specific antibody. Sensors 2019, 19, 2533. [Google Scholar] [CrossRef]
- Harpaz, D.; Koh, B.; Seet, R.C.S.; Abdulhalim, I.; Tok, A.I.Y. Functionalized silicon dioxide self-referenced plasmonic chip as point-of-care biosensor for stroke biomarkers NT-proBNP and S100β. Talanta 2020, 212, 120792. [Google Scholar] [CrossRef]
- Hunt, P.; Espiner, E.; Nicholls, M.; Richards, A.; Yandle, T. The role of the circulation in processing pro-brain natriuretic peptide (proBNP) to amino-terminal BNP and BNP-32. Peptides 1997, 18, 1475–1481. [Google Scholar] [CrossRef]
- Goetze, J.P. Biochemistry of pro-B-type natriuretic peptide-derived peptides: The endocrine heart revisited. Clin. Chem. 2004, 50, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Seferian, K.R.; Tamm, N.N.; Semenov, A.G.; Tolstaya, A.A.; Koshkina, E.V.; Krasnoselsky, M.I.; Postnikov, A.B.; Serebryanaya, D.V.; Apple, F.S.; Murakami, M.M. Immunodetection of glycosylated NT-proBNP circulating in human blood. Clin. Chem. 2008, 54, 866–873. [Google Scholar] [CrossRef]
- Ala-Kopsala, M.; Magga, J.; Peuhkurinen, K.; Leipälä, J.; Ruskoaho, H.; Leppaluoto, J.; Vuolteenaho, O. Molecular heterogeneity has a major impact on the measurement of circulating N-terminal fragments of A-and B-type natriuretic peptides. Clin. Chem. 2004, 50, 1576–1588. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Talwar, S.; Squire, I.B.; Davies, J.E.; Ng, L.L. An immunoluminometric assay for N-terminal pro-brain natriuretic peptide: Development of a test for left ventricular dysfunction. Clin. Sci. 1999, 96, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.; Borgya, A.; Gallusser, A.; Huber, E.; Krueger, K.; Rollinger, W.; Schenk, J. Development of a novel, N-terminal-proBNP (NT-proBNP) assay with a low detection limit. Scand. J. Clin. Lab. Investig. 1999, 59, 177–181. [Google Scholar] [CrossRef]
- Campbell, D.J.; Mitchelhill, K.I.; Schlicht, S.M.; Booth, R.J. Plasma amino-terminal pro-brain natriuretic peptide: A novel approach to the diagnosis of cardiac dysfunction. J. Card. Fail. 2000, 6, 130–139. [Google Scholar] [CrossRef]
- Squire, I.B.; O’Brien, R.J.; Demme, B.; Davies, J.E.; Ng, L.L. N-terminal pro-atrial natriuretic peptide (N-ANP) and N-terminal pro-B-type natriuretic peptide (N-BNP) in the prediction of death and heart failure in unselected patients following acute myocardial infarction. Clin. Sci. 2004, 107, 309–316. [Google Scholar] [CrossRef]
- Goetze, J.P.; Kastrup, J.; Pedersen, F.; Rehfeld, J.F. Quantification of pro-B-type natriuretic peptide and its products in human plasma by use of an analysis independent of precursor processing. Clin. Chem. 2002, 48, 1035–1042. [Google Scholar] [CrossRef]
- Mueller, T.; Gegenhuber, A.; Poelz, W.; Haltmayer, M. Comparison of the Biomedica NT-proBNP enzyme immunoassay and the Roche NT-proBNP chemiluminescence immunoassay: Implications for the prediction of symptomatic and asymptomatic structural heart disease. Clin. Chem. 2003, 49, 976–979. [Google Scholar] [CrossRef]
- Hammerer-Lercher, A.; Ludwig, W.; Falkensammer, G.; MuLler, S.; Neubauer, E.; Puschendorf, B.; Pachinger, O.; Mair, J. Natriuretic peptides as markers of mild forms of left ventricular dysfunction: Effects of assays on diagnostic performance of markers. Clin. Chem. 2004, 50, 1174–1183. [Google Scholar] [CrossRef]
- Tamm, N.N.; Seferian, K.R.; Semenov, A.G.; Mukharyamova, K.S.; Koshkina, E.V.; Krasnoselsky, M.I.; Postnikov, A.B.; Serebryanaya, D.V.; Apple, F.S.; Murakami, M.M. Novel immunoassay for quantification of brain natriuretic peptide and its precursor in human blood. Clin. Chem. 2008, 54, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Tetin, S.Y.; Ruan, Q.; Saldana, S.C.; Pope, M.R.; Chen, Y.; Wu, H.; Pinkus, M.S.; Jiang, J.; Richardson, P.L. Interactions of two monoclonal antibodies with BNP: High resolution epitope mapping using fluorescence correlation spectroscopy. Biochemistry 2006, 45, 14155–14165. [Google Scholar] [CrossRef] [PubMed]
- Balion, C.; Don-Wauchope, A.; Hill, S.; Santaguida, P.L.; Booth, R.; Brown, J.A.; Oremus, M.; Ali, U.; Bustamam, A.; Sohel, N. Use of Natriuretic Peptide Measurement in the Management of Heart Failure; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2013.
- Maalouf, R.; Bailey, S. A review on B-type natriuretic peptide monitoring: Assays and biosensors. Heart Fail. Rev. 2016, 21, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Del Ry, S.; Giannessi, D.; Clerico, A. Plasma brain natriuretic peptide measured by fully-automated immunoassay and by immunoradiometric assay compared. Clin. Chem. Lab. Med. 2001, 39, 446–450. [Google Scholar]
- Clerico, A.; Iervasi, G.; Del Chicca, M.G.; Emdin, M.; Maffei, S.; Nannipieri, M.; Sabatino, L.; Forini, F.; Manfredi, C.; Donato, L. Circulating levels of cardiac natriuretic peptides (ANP and BNP) measured by highly sensitive and specific immunoradiometric assays in normal subjects and in patients with different degrees of heart failure. J. Endocrinol. Investig. 1998, 21, 170–179. [Google Scholar] [CrossRef]
- Fellner, S.; Hentze, S.; Kempin, U.; Richter, E.; Rocktäschel, J.; Langer, B. Analytical evaluation of a BNP assay on the new point-of-care platform respons® IQ. Pract. Lab. Med. 2015, 2, 15–21. [Google Scholar] [CrossRef]
- Ishida, J.; Suzuki, T.; Aizawa, K.; Sawaki, D.; Nagai, R. Comparison of analytical performance of two single-step measurement devices of B-type natriuretic peptide. Int. Heart J. 2012, 53, 320–323. [Google Scholar] [CrossRef][Green Version]
- Yasukawa, K.; Hisajima, K.; Imai, K.; Soga, Y. Introduction of Rapidtip BNP and Rapidpia—A clinical significance of rapid measurement BNP for home medicine care. Gan Kagaku Ryoho. Cancer Chemother. 2009, 36, 135–137. [Google Scholar]
- Denny, N.; Lasserson, D.; Price, P.C.; Heneghan, C.; Thompson, M.; Plüddemann, A. Diagnostic Technology: Point of Care B-Type Natriuretic Peptide Testing; The School for Primary Care Research Is a Partnership between the Universities of Birmingham, Bristol, Manchester and Oxford and Is Part of the National Institute for Health Research; Primary Care Diagnostic Horizon Scanning Centre Oxford: Oxford, UK, 2011; Available online: https://www.community.healthcare.mic.nihr.ac.uk/files/reports-and-resources/horizon-scanning-report0019-bnp.pdf (accessed on 1 June 2020).



| 510(K) Number | Product | Company | First Approval Date | Update Approval Date |
|---|---|---|---|---|
| K873133 | BNP-AMYLASE TEST | SCLAVO INC. | 10/6/1987 | |
| K032335 | MAS CARDIOIMMUNE PROBNP | MEDICAL ANALYSIS SYSTEMS INC. | 8/19/2003 | |
| K033606 | ABBOTT AXSYM B-TYPE NATRIURETIC PEPTIDE (BNP) MICROPARTICLE ENZYME IMMUNOASSAY (MEIA) TEST | AXIS-SHIELD DIAGNOSTICS LTD. | 1/30/2004 | |
| K043584 | LIQUICHEK BNP CONTROL | BIO-RAD LABORATORIES INC. | 2/8/2005 | |
| K051265 | ADVIA IMMUNO MODULAR SYSTEM (IMS) B-TYPE NATRIURETIC PEPTIDE (BNP) ASSAY | BAYER HEALTHCARE LLC | 6/23/2003 | 6/13/2005 |
| K052789 | TRIAGE BNP TEST FOR BECKMAN COULTER IMMUNOASSAY SYSTEMS MODEL 98200 | BIOSITE INCORPORATED | 2/29/2000 | 1/23/2006 |
| K051596 | STATUS FIRST CHF (CONGESTIVE HEART FAILURE) NT-PROBNP MODEL 20204 | NANOGEN INC. | 3/13/2006 | |
| K060964 | ARCHITECT BNP ASSAY MODEL 8K28 | FUJIREBIO DIAGNOSTICS INC. | 5/25/2006 | |
| K053597 | I-STAT B-TYPE NATRIURETIC PEPTIDE (BNP) | I-STAT CORPORATION | 7/21/2006 | |
| K071834 | STRATUS CS ACUTE CARE NT-PROBNP TESTPAK MODEL CPBNPM | DADE BEHRING INC. | 2/15/2005 | 8/17/2007 |
| K072189 | PATHFAST NTPROBNP AND D-DIMER TESTS | MITSUBISHI KAGAKU IATRON | 2/5/2008 | |
| K073091 | VIDAS NT-PROBNP ASSAY MODEL 30449 | BIOMERIEUX INC. | 2/29/2008 | |
| K080578 | DIMENSION VISTA N-TERMINAL PRO-BRAIN NATRIURETIC PEPTIDE (PBNP) FLEX REAGENT CARTRIDGE (K6423A) DIMENSION VISTA | SIEMENS HEALTHCARE DIAGNOSTICS INC. | 7/20/2004 | 5/16/2008 |
| K063662 | RAMP NT-PROBNP ASSAY | RESPONSE BIOMEDICAL CORP. | 7/21/2008 | |
| K092649 | ELECSYS PROBNP II STAT IMMUNOASSAY AND ELECSYS PROBNP II CALSET MODELS 05390109-160 04842472-190 | ROCHE DIAGNOSTICS CORP. | 11/19/2002 | 2/4/2010 |
| Number | Acronym | Full Term |
|---|---|---|
| 1 | Ab | Antibody |
| 2 | AF | Atrial Fibrillation |
| 3 | ANP | Atrial Natriuretic Peptide |
| 4 | AUC | Area Under the Curve |
| 5 | BNP | B-type Natriuretic Peptide |
| 6 | CI | Confidence Interval |
| 7 | CT | Computed Tomography |
| 8 | ED | Emergency Department |
| 9 | EDTA | Ethylenediaminetetraacetic Acid |
| 10 | ELISA | Enzyme-Linked Immunosorbent Assay |
| 11 | FDA | Food and Drug Administration |
| 12 | HF | Heart Failure |
| 13 | LOD | Limit Of Detection |
| 14 | LTC | Long-Term Care |
| 15 | MAB | Monoclonal Antibody |
| 16 | MRI | Magnetic Resonance Imaging |
| 17 | NIHSS | National Institutes of Health Stroke Scale |
| 18 | NT-proBNP | N-Terminal pro-B-type Natriuretic Peptide |
| 19 | OR | Odds Ratio |
| 20 | POC | Point-Of-Care |
| 21 | ROC | Receiver Operating Characteristic |
| 22 | TIA | Transient Ischemic Attack |
| 23 | TOAST | Trial of Org 10,172 in Acute Stroke Treatment |
| 24 | tPA | Tissue Plasminogen Activator |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harpaz, D.; Seet, R.C.S.; Marks, R.S.; Tok, A.I.Y. B-Type Natriuretic Peptide as a Significant Brain Biomarker for Stroke Triaging Using a Bedside Point-of-Care Monitoring Biosensor. Biosensors 2020, 10, 107. https://doi.org/10.3390/bios10090107
Harpaz D, Seet RCS, Marks RS, Tok AIY. B-Type Natriuretic Peptide as a Significant Brain Biomarker for Stroke Triaging Using a Bedside Point-of-Care Monitoring Biosensor. Biosensors. 2020; 10(9):107. https://doi.org/10.3390/bios10090107
Chicago/Turabian StyleHarpaz, Dorin, Raymond C. S. Seet, Robert S. Marks, and Alfred I. Y. Tok. 2020. "B-Type Natriuretic Peptide as a Significant Brain Biomarker for Stroke Triaging Using a Bedside Point-of-Care Monitoring Biosensor" Biosensors 10, no. 9: 107. https://doi.org/10.3390/bios10090107
APA StyleHarpaz, D., Seet, R. C. S., Marks, R. S., & Tok, A. I. Y. (2020). B-Type Natriuretic Peptide as a Significant Brain Biomarker for Stroke Triaging Using a Bedside Point-of-Care Monitoring Biosensor. Biosensors, 10(9), 107. https://doi.org/10.3390/bios10090107