Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review
Abstract
1. Introduction
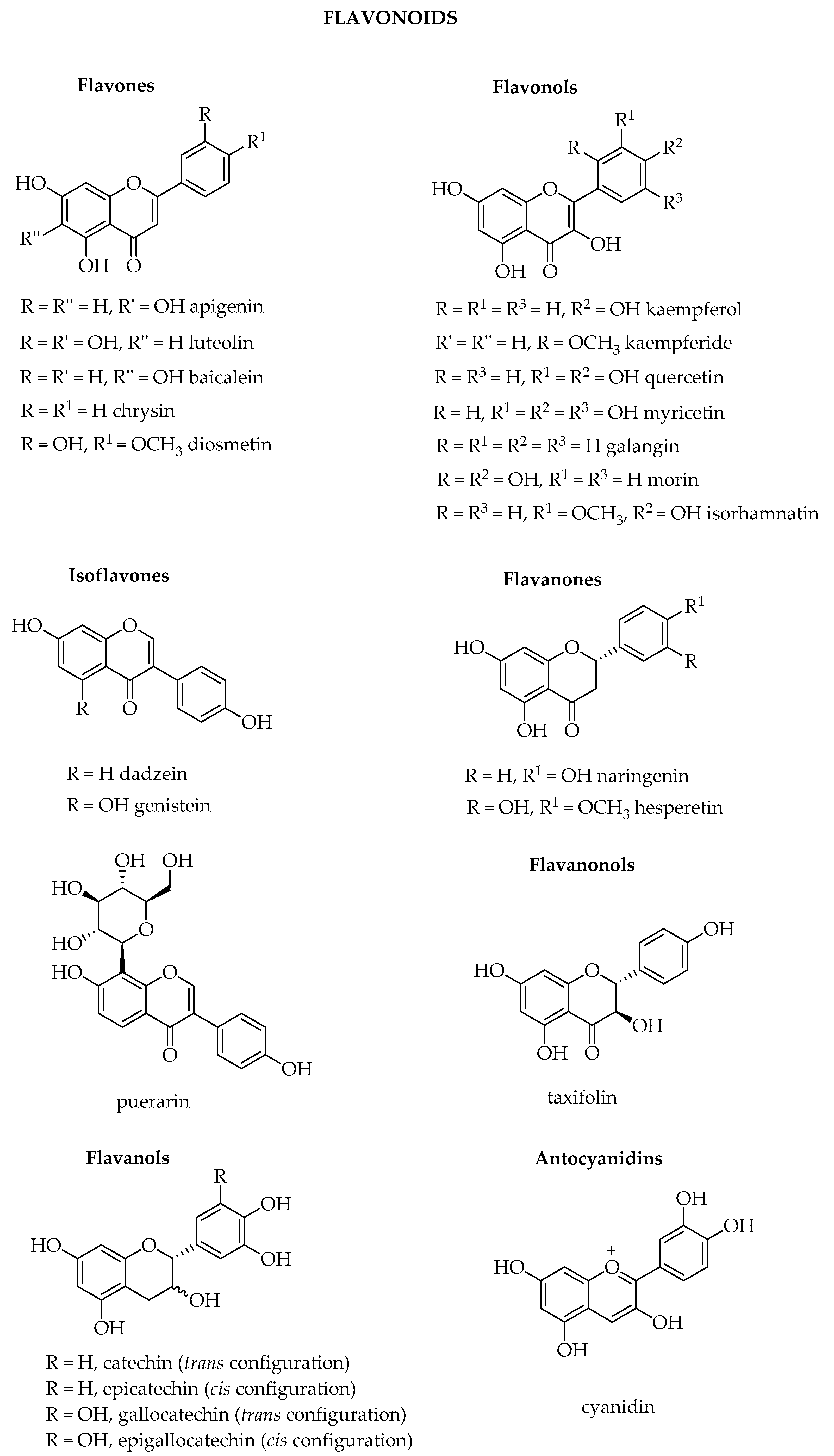
1.1. Flavonoids
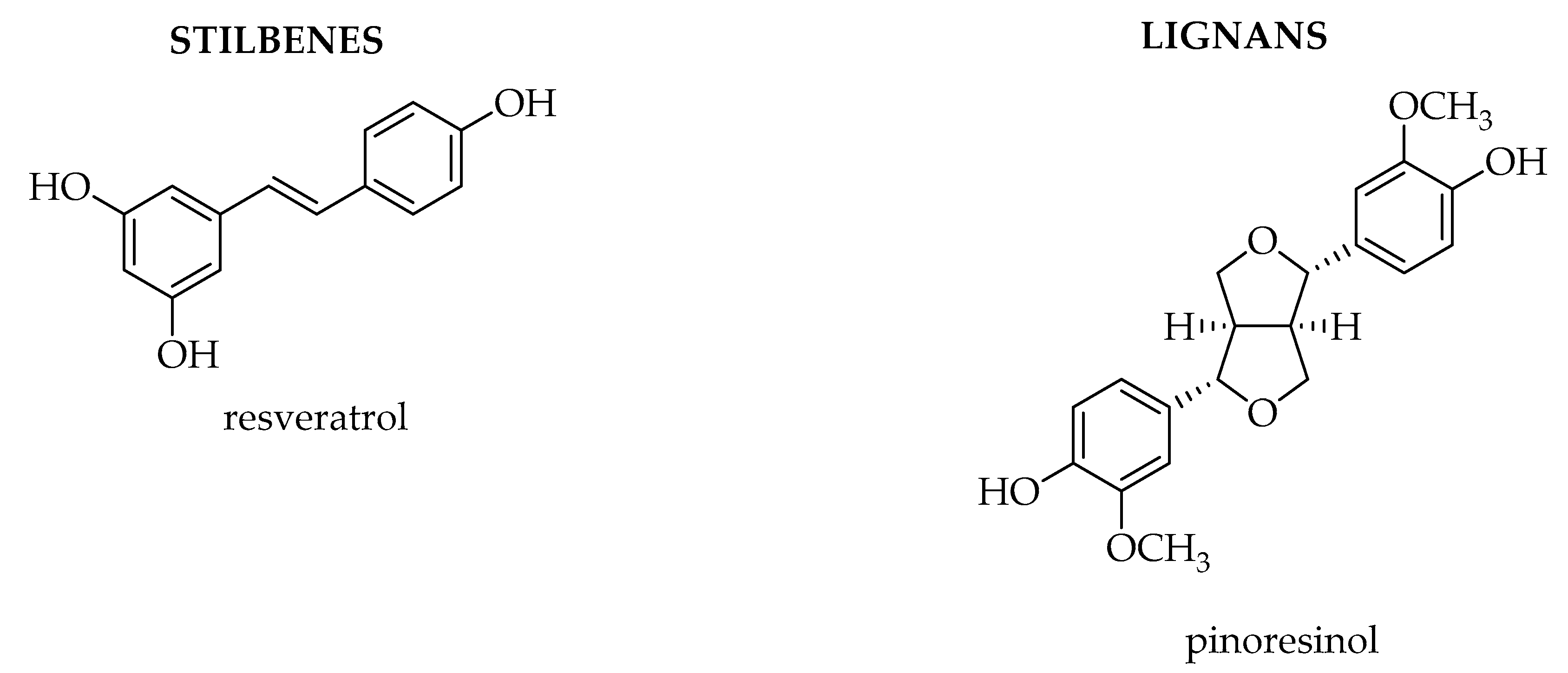
1.2. Stilbenes and Lignans
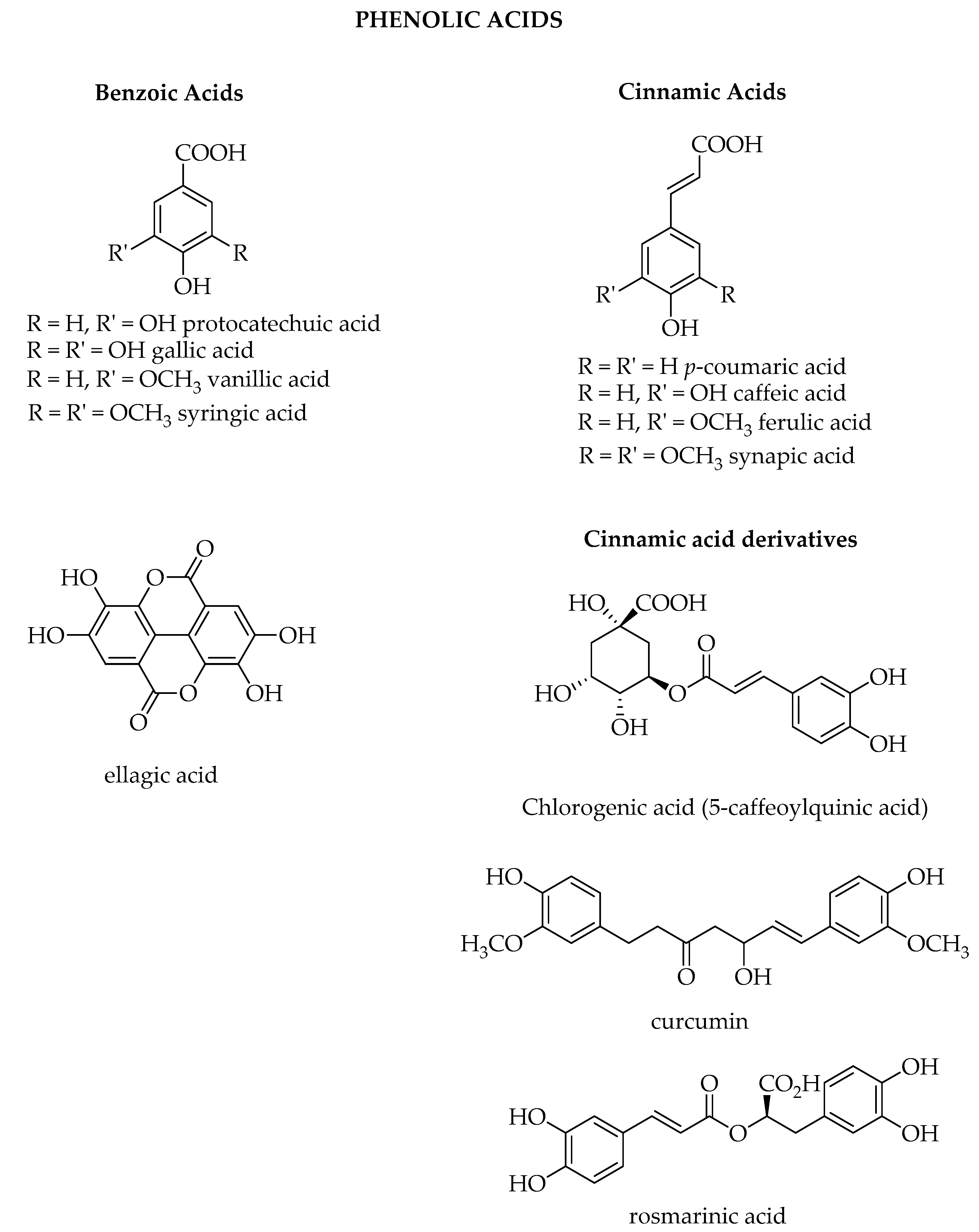
1.3. Phenolic Acids
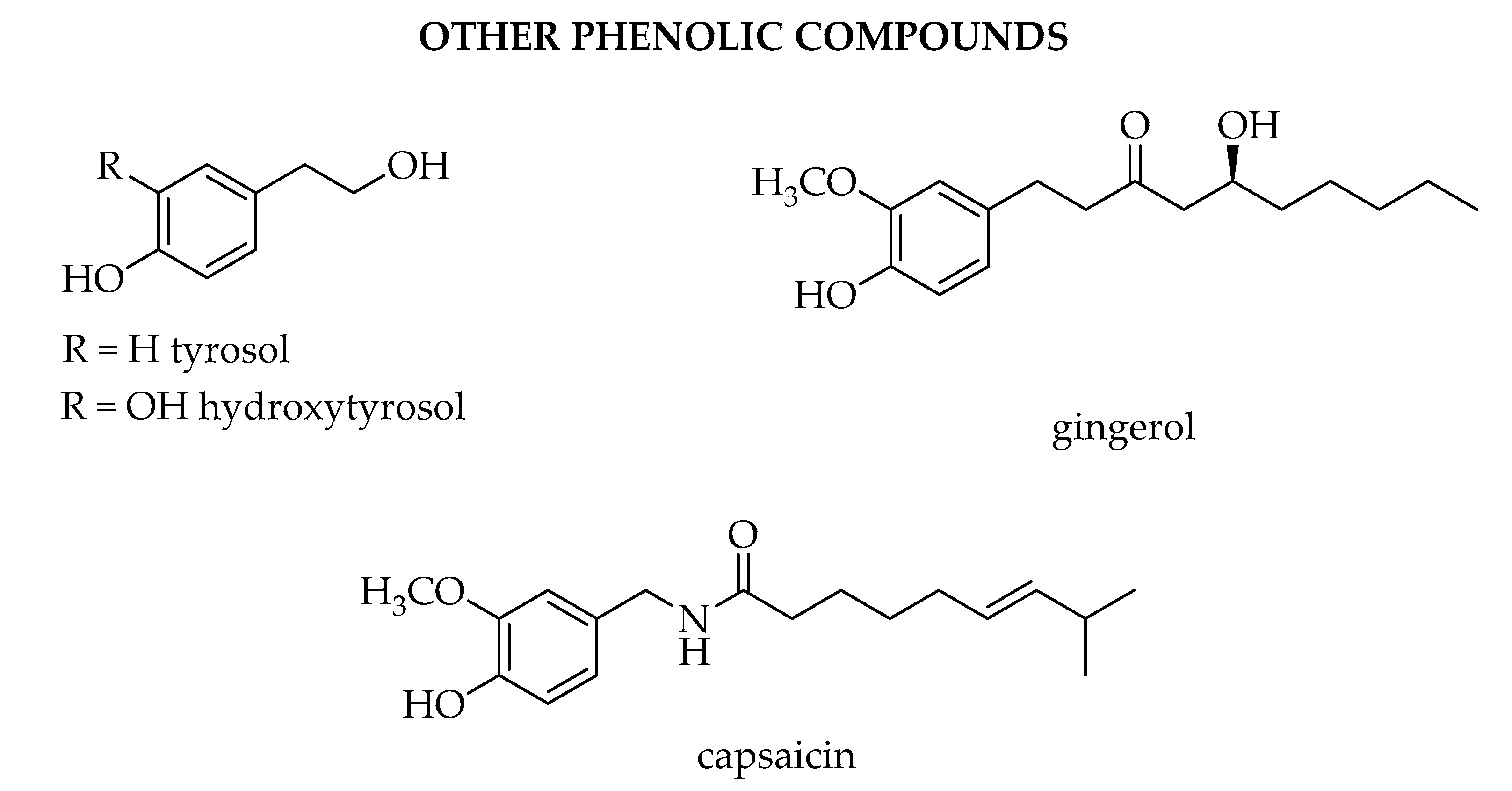
1.4. Other Phenolic Compounds
1.5. Analysis of Phenolic Compounds and Aim of this Review
- To analyze the occurrence of specific recognition elements in sensing systems for phenolic compounds. To this end, we deal with biosensors and we include in this category also sensors based on designed, artificial biomimetic receptors as imprinted polymers. Such materials are in fact comprised in the definition of biosensors by the main journals, including this one. The question is: does the use of a specific recognition element lead to significant advantages in comparison to chemical sensors lacking designed complementarity although prepared with advanced and highly sophisticated materials? The main focus here is therefore on selectivity, obtained with, or without, the use of biological or biomimetic receptors, as enzymes, functional receptors, proteins, peptides, nucleic acids, and imprinted polymeric materials.
- To offer a comprehensive collection of data and references to the reader interested in the available methods for the detection of a specific compound. Here the focus is not only on selectivity and detectability, but rather on the ability of the sensors to operate in the real environment. Special attention is given to the systems whose analytical performance has been validated on real samples by comparison with a reference analytical methodology (most commonly a liquid chromatography-mass spectrometry method but not rarely also a capillary electrophoresis (CE) one), thus evaluating also matrix effects.
2. Electrochemical Sensors
2.1. Electrochemical Sensors without Recognition Elements
2.1.1. Carbon-Based Materials
2.1.2. Composites of Carbon Materials and Inorganic Components
2.1.3. Polymeric Materials
2.1.4. Metal Organic Frameworks and Covalent Organic Frameworks
2.1.5. Photoelectrochemical Systems
| Sensing System (Electrochemical Technique) | Target | LOD | Linear Range | Non Interfering Related Compounds (Interfering Related Compounds) | Recovery | Reference Method | Real Samples (Sample Preparation) | Ref. |
|---|---|---|---|---|---|---|---|---|
| SWCNT-SubPc (DPV) | catechin | 13 nM | 0.1–1.5 μM | rutin, 6-methoxy flavone, gallic acid, caffeic acid | 96.5–98% | no | green, rosehip fruit, Turkish and Indian black tea (EtOH ext.) | [50] |
| Pt/MnO2/f-MWCNT/GCE (CV) | catechin | 0.02 μM | 2–950 μM | rutin, quercetin, caffeic acid, catechol | 99.2–101% | no | red wine, black tea, and green tea samples (not reported) | [51] |
| Nanocarbon-GCE, Nanodiamond-GCE, Graphene-GCE (CV, SWV, Amp) | catechol, hydroquinone, cresol, phenol | 0.04–0.11 μM (nanocarbon); 0.10–0.2 μM (graphene), 0.12–0.43 μM (nanodiamond) | Up to 100 μM hydroquinone (nanodiamond) | Nanocarbon electrode distinguishes catechol and hydroquinone | 101% (catechol) and 102% (hydroquinone) (nanocarbon, river water) | no | river water (nanocarbon electrode: no pretreatment requested), tea (water inf.) | [36] |
| Nafion/ER-GO/GCE (SW-AdSV) | caffeic acid | 0.091 μM | 0.1–10 μM | p-coumaric acid, sinapic acid, ferulic acid, gallic acid, catechin (chlorgenic acid at same concentration as caffeic acid caused a positive interference of 14%) | 97–98% | HPLC | white wine (dil.) | [66] |
| GR/bmim+Br− PE and GR/bmim+Cl−PE (DPV) | caffeic acid | 5 μM (G/bmim+Cl−PE) and 18 μM (G/bmim+Br−PE) | 0.025–2.00 mM | Many polyphenols and flavonoids compounds (permeability and perm-selectivity test) | 97.2–99.7% | HPLC on spiked plasma samples | human plasma (no pretreatment requested) | [42] |
| Pd–Au/PEDOT/rGO/GCE (DPV) | caffeic acid | 0.37 nM | 0.001–55 μM | catechol, p-coumaric acid, gallic acid, vanillic acid, sinapic acid, ferulic acid | 97.8–103.8% | no | red wine (dil.) | [71] |
| GR/CuO@Cu-BTC/GCE (DPV) | caffeic acid | 7.0 nM | 0.020–10.0 μM, | catechol, lemon yellow | 95.91–104.60% calculated based on HPLC values | HPLC | red wine (dil.) | [78] |
| Cu2S NDs@GOS NC/SPCE (Amp) | caffeic acid | 0.22 nM | 0.055–2455 μM | gallic acid, dopamine, hydroquinone, catechol, epinephrine, norepinephrine, resorcinol | 96.18–99.43% calculated based on HPLC values | HPLC | red wine, soft drinks (dil.) | [55] |
| F-GO/GCE (DPV) | caffeic acid | 0.018 μM | 0.5–100.0 μM | p-coumaric acid, hydroquinone, ferulic acid, gallic acid | n.d. | 1 sample of 4 compared with HPLC result | wine (no pretreatment requested) | [43] |
| NCG electrode (ChAmp) | caffeic acid | 43 μM | 50 μM–1mM | results not shown for interferences studies performed on matrix | n.d. | HPLC-MS | berries and chokeberries (MeOH + 2% formic acid ext.) | [38] |
| CB-SPE (SWV) | caffeic acid, catechol, gallic acid, tyrosol | 0.1 μM (catechol), 0.8 μM (caffeic acid) 1 μM (gallic acid), and 2 μM (tyrosol) | 1–50 μM (catechol), 1–50 μM (caffeic acid), 10–100 μM (gallic acid), 10–100 μM (tyrosol) | Tested amongst target molecules: the sensor distinguishes mono and diphenols | n.d. | no | no | [45] |
| TiO2/CNTs/CdTeQDs/FTO (photoelectrochemistry) | caffeic acid | 0.15 μM | 0.5–360 μM | chlorgenic acid, gallic acid, vanillic acid, ferulic acid, quercetin, caffeic ethyl ester | 96.2–101.3%, | no | soluble coffee (water dispersion) and tea sachets (water inf.) | [79] |
| SPE-CB/MoS2 (DPV) | catechins | LOD ≤ 0.17 μM | 0.12–25 μM | n.d. | 94–103% | Folin Ciocalteu, ABTS | cocoa (DMSO ext.) | [62] |
| MWCNTs/SPE (CV, DPV) | chlorgenic acid | 0.34 μM | 0.48 μM–45 μM | n.d. | 94.74–106.65% | HPLC | coffee beans (hexane 1:6 w/v in Soxhlet extraction system + water microwave assisted ext.) | [48] |
| (CS/MWCNTs)6/GCE (DPV) | chlorgenic acid | 11.6 nM | 0.02–225 μM | other compounds not similar to the target | 99.33–109.0% | HPLC (based on standard addition method) | human serum (dil.) | [49] |
| PASA/GCE (CV) | chlorgenic acid | 40 nM | 0.4 μM–12 μM | other compounds not similar to the target | 96.3–102.8% | HPLC | pharmaceutical products (no pretreatment requested) and honeysuckle (EtOH ext. + dil.) | [70] |
| TAPB-DMTP-COFs/AuNPs/GCE (DPV) | chlorgenic acid | 9.5 nM | 10 nM–40 μM | dopamine, L-dopa, hydroquinone, catechol, thymol, rutin, quercetin, caffeic acid, gallic acid, vanillic acid | 99.2–102.5%, | HPLC | coffee, apple, honeysuckle (not reported) | [77] |
| alumina microfiber-modified CPE (DPV) | chlorgenic acid | 14 nM | 28 nM and 5.6 μM | other compounds not similar to the target | n.d. | HPLC | honeysuckle (EtOH ext.) and soft drinks (fil.) | [63] |
| MOF/TiO2/GCE (DPV) | chlorgenic acid | 7 nM | 0.01–15 μM | catechol, dopamine, hydroquinone, paracetamol, caffeic acid, rutin, ferulic acid, gallic acid, vanillic acid | 96.0–102.0% | no | coffee and tea (not reported) | [80] |
| SPE (coarsely stepped cyclic SWV) | capsaicin | 1.98 μM | 0–5000 μM | n.d. | n.d. | no | chili derived sauces (EtOH ext.) | [37] |
| MWCNT-BPPGE and (MWCNT-SPE) | capsaicin | 0.31 μM (0.45 μM) | 0.5–60 mM (0.5–35mM) | n.d. | n.d. | correlation with the average Scoville unit values reported in the literature for these sauces | hot pepper sauces (EtOH ext.) | [47] |
| MCFs/CPE (DPV) | capsaicin | 0.08 μM | 0.76–11.65 μM | catechol, p-chlorophenol | 96–101.1% | HPLC | hot pepper powder | [83] |
| SnO2-PDDA-GR/GCE (LSV) | daidzein | 6.7 nM | 0.02−1.0 μM | other compounds not similar to the target | 97.81–103.30% | HPLC | pueraria lobata (EtOH ext.) | [75] |
| ERGO/GCE (DPV) | ferulic acid | 20.6 nM | 0.085–38.9 μM | other compounds not similar to the target | 99.77–101.73% (A.sinensis); 95.72–104.51% (biological samples) | HPLC (A. sinensis) | A. sinensis (70% EtOH reflux ext. + dil.), urine and plasma (dil.) | [40] |
| CPE/MBIBr/NiO-SWCNTs (SWV) | ferulic acid (and butylated hydroxytoluene) | 20.0 nM (ferulic acid) | 0.06–900.0 μM (ferulic acid) | other compounds not similar to the target | n.d. | HPLC | corn milk (hot water), wheat flour (sulfuric acid dissolution + hot water ext.), corn cider (fil.) | [52] |
| MgO/SWCNTs-[Bmim][Tf2N]-CPE (DPV) | ferulic acid (and sulfite) | 3.0 nM | 0.009–450 μM | other compounds not similar to the target | 99.17–101.6% (ferulic acid) | HPLC | red wine (fil.) and white rice (50% EtOH+ sulfuric acid dissolution) | [53] |
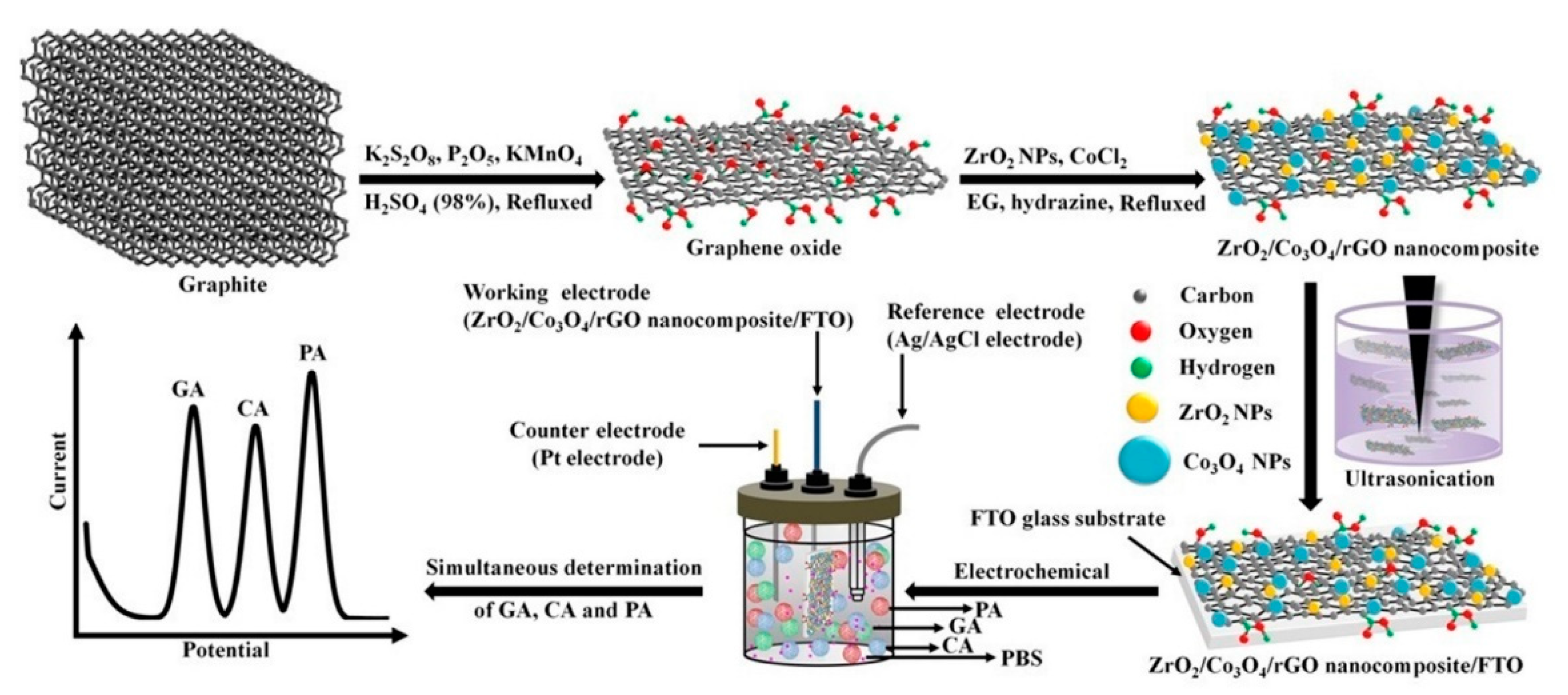
| ZrO2/Co3O4/rGO nanocomposite/FTO (DPV) | gallic acid, caffeic acid, protocatechuic acid | 1.56 nM (gallic acid), 0.62 nM (caffeic acid), 1.35 nM (protocatechuic acid) | 6.24–477.68 nM (gallic acid); 2.48–524.90 nM (caffeic acid); 5.40–424.96 nM (protocatechuic acid) | gentisic acid, sinapic acid, vanillin, p-coumaric acid, vanillic acid, p-hydroxybenzoic acid, vitamin B1, vitamin B2, morin hydrated, rutin, ellagic acid | 95.4–100% (gallic acid); 96.1–99.5% (caffeic acid), 95.5–101.0% (protocatechuic acid) | no | fruit juice (fil. + dil.), rice (85% MeOH ext. + dil.) and tea samples (inf.+fil. +dil.) | [39] |
| PDDA-GR-Pt/GCE (SWV) | gallic acid | 7 nM | 0.03–1 μM | other compounds not similar to the target | 99.8–102.3% | HPLC | Jianmin Yanhou tablets (MeOH ext.), Cortex moutan (MeOH ext. + dil.) and green tea beverage (no pretreatment requested) | [76] |
| PLM/MWCNT/GCE (DPV, Amp) | gallic acid | 3.1 nM (DPV) and 0.5 nM (Amp) | DPV: (4.0 nM–20.0 μM); Amp: (2.0 nM–12.0 μM) | (100 times higher concentrations of caffeic acid, gallic acid oxidation peak current was increased about 22%.) | 96.74–101.49% | LC-MS/MS | black and green tea (inf.+ dil.), red wine (dil.) | [67] |
| heterostructured Bi2MoO6/Bi2S3 nanobelts (photoelectrochemistry) | gallic acid | n.d. | 24.88–348.84 μM | discrimination of gallic acid from other antioxidant compounds (+)-Catechin hydrate, caffeic acid, chlorgenic acid, (-)-Epicatechin, myricetin | 99.58%–101.37% | HPLC | rose oral liquid and pomegranate enrich blood syrup (not reported) | [84] |
| AuMCs/SF-GR/GCE (DPV) | gallic acid (uric acid) | 10.7 nM (0.12 μM uric acid) | 0.05–8.0 μM gallic acid (0.2–50.0 μM uric acid) | other compounds not similar to the target (some polyphenolic compounds with ortho-diphenol groups at B-ring could interfere) | 96.0–102.4% (gallic acid) (97.0–102.4% UA) in urine; 96–100.8% in black tea and Cortex moutan | HPLC | urine (dil.), Cortex moutan (MeOH ext.), black tea (inf. + dil.) | [41] |
| CoPC modified SPCE (SWV) | genistein | 1.5 µM | 2.5–150 µM | other compounds not similar to the target | 99.98-104.68% | no | Derris scandens extracts (EtOH ext.) | [65] |
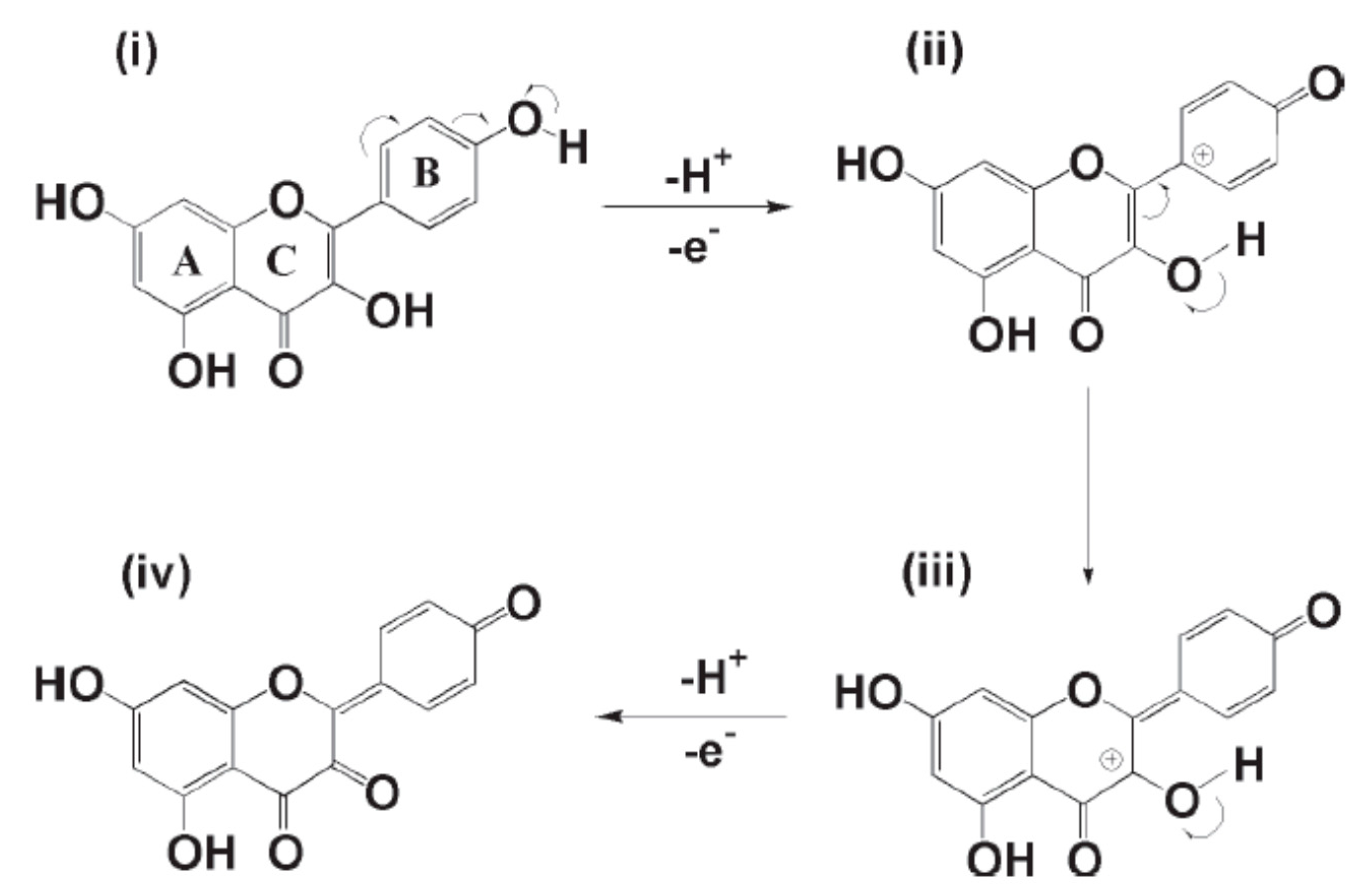
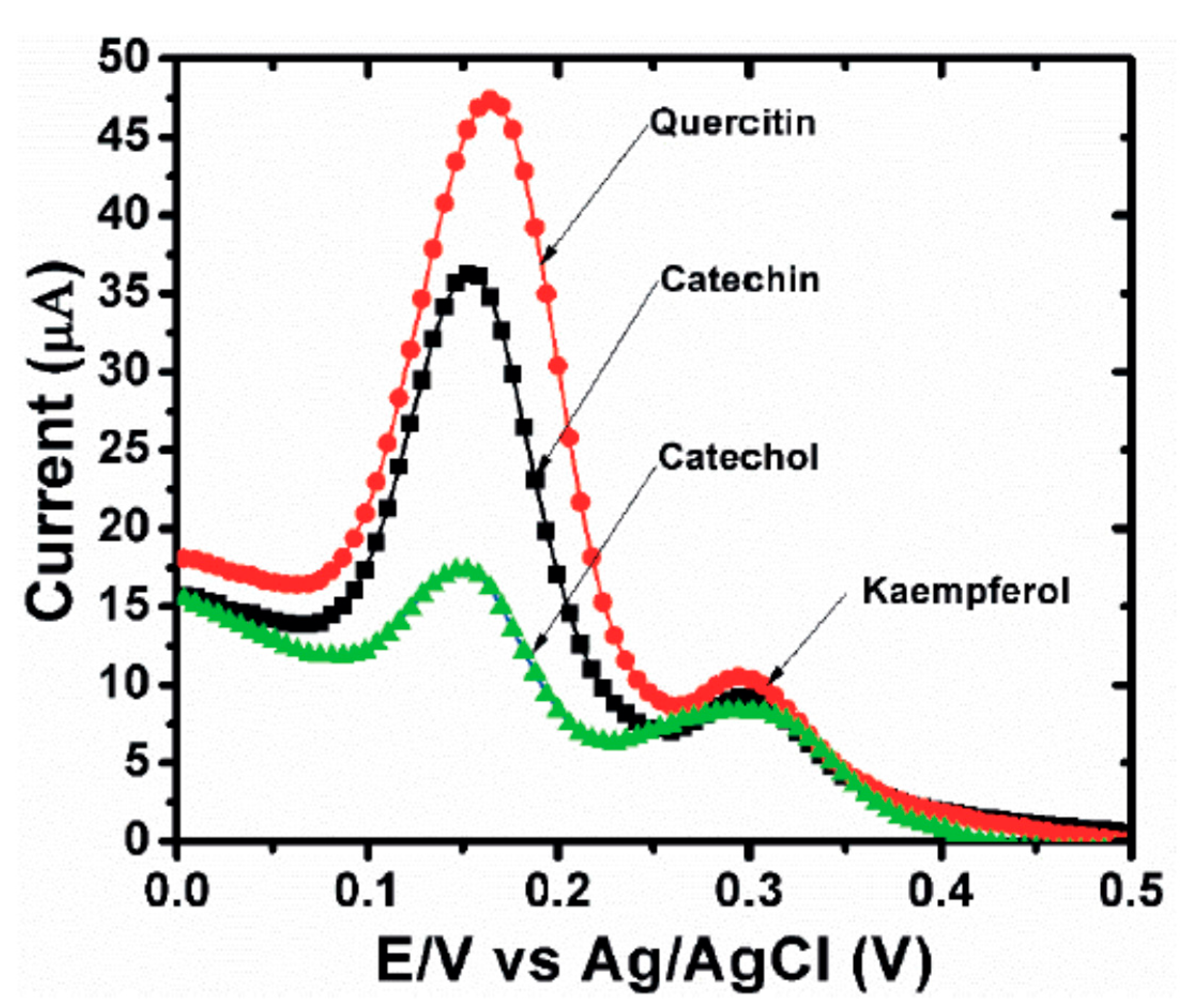
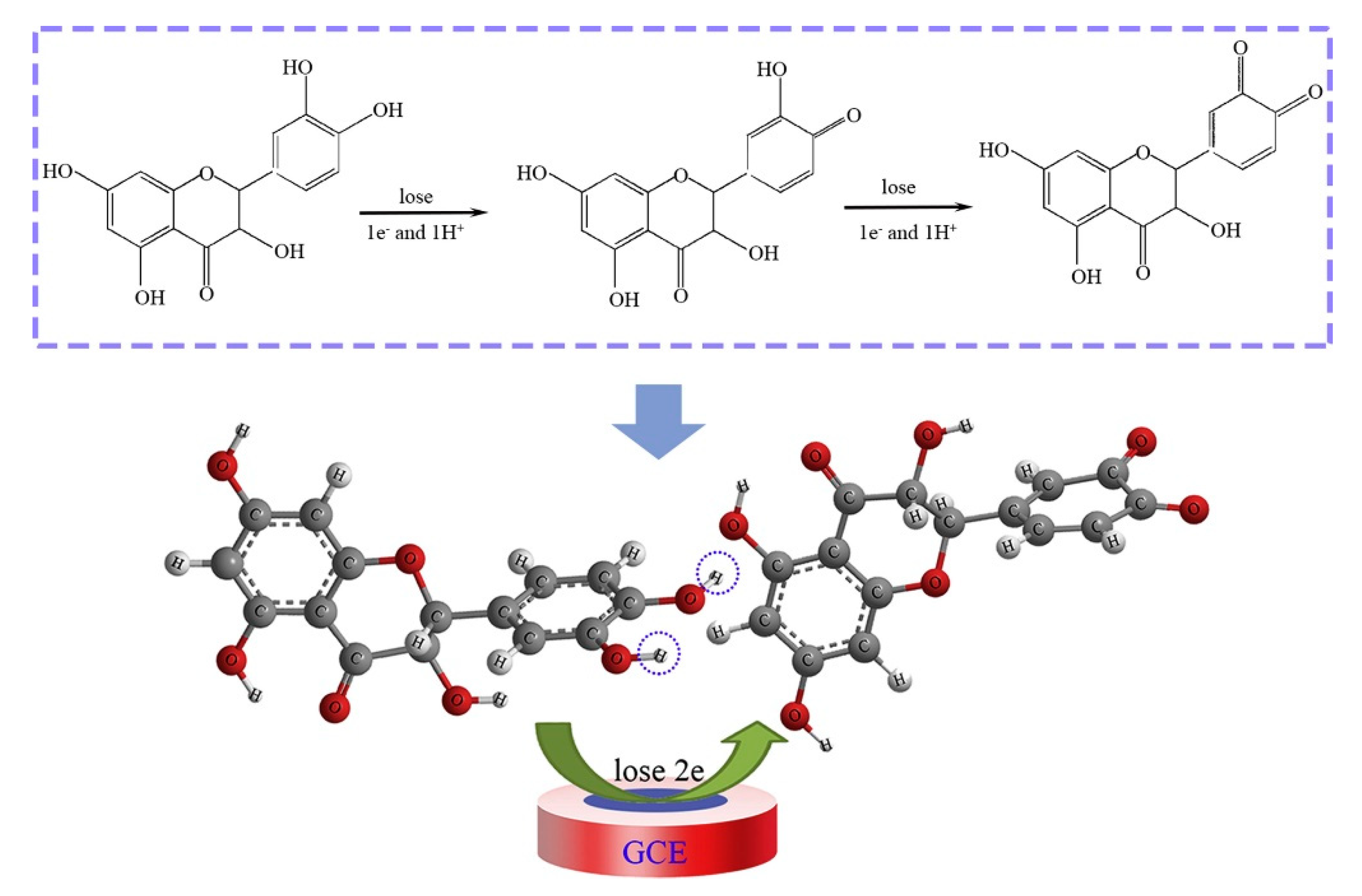
| Fe2 O3 NPs/MWCNTs/GCE (DPV) | kaempferol | 0.53 μM | 1–300 μM | quercetin, catechin, CC | 99.55% average | no | broccoli (EtOH ext.) | [54] |
| Pt-BPC/CILE (CV, DPV) | luteolin | 2.6 nM | 0.008–100.0 μM | quercetin, baicatin, rutin | 98.33–103.75% | no | Duyiwei capsule (EtOH ext.) | [58] |
| AuNCs/CILE (DPV) | luteolin | 0.4 nM | 1–1000 nM | quercetin, baicalein | 95.0–96.7% | no | Duyiwei capsules (EtOH ext.) | [59] |
| Cu1Co3 @ NPCP | luteolin | 0.08 nM | 0.2 nM to 2.5 μM, | other compounds not similar to the target | 99.6–102.2% | no | human serum samples (not reported) | [60] |
| MWCNTs/PEDOT–Au/GCE (CV, SWV) | luteolin | 0.22 nM | 0.001–15 μM | curcumin, quercetin, rutin, myricitrin, diosmetin | 99–103% | no | human serum samples (not reported) | [72] |
| NIPA/AA-MWCNTs-GCE (DPV) | luteolin/baicalein | 0.0145 nM/0.0444 nM | 0.0001–1.5 mM/0.005–35 mM | other compounds not similar to the target | 93.3–106.6% | no | peanuts shell, tomato (EtOH ext.) | [73] |
| CPPI-TiO2/CdS/FTO (photoelectrochemistry) | naringin | 0.03 μM | 1–332 μM | hesperidin, flavone, gallic acid, quercetin, naringenin | 97.8–99.6% | no | orange, lemon, tangerine juice (dil.) | [85] |
| SNO NRs/GCE | quercetin | 1.98 nM | 0.01–68.53 μM | rutin | 86–99.6% | no | apple and grape juice (dil.) | [64] |
| Fe3O @ SiO2-PANI-Au nanocomposite/GCE | quercetin | 3.8 nM | 0.01–15 μM | other compounds not similar to the target | 96–102% | HPLC | human serum and urine samples, tea, radish leaves, and apple juice samples (not reported) | [74] |
| ZnO/CNS/MCPE (DPV) | quercetin | 0.04 μM | 0.166–3.63 μM | rutin | 90.8–113.0% | no | onion and honey buckwheat (dil. PBS) | [56] |
| PB-rGO/TCD/AuNPs (CV, DPV) | quercetin | 1.83 nM | 0.005–0.4 μM | morin, galangin, resveratrol, baicalin, rutin | 95–104.3% | no | apple juice, red wine and honeysuckle (fil. + dil.) | [57] |
| PPy @ ZIF-8 (DPV) | quercetin | 7 nM | 0.01–150 μM | hyperin, delphindin, catechin hydrate; rutin, luteolin, kaempferol | 99.1–102.6% | no | human blood plasma (dil. + PBS + ACN) | [82] |
| poly(gallic acid)/MWCNT/GCE (DPV) | quercetin | 54 nM | 0.075–100 μM | rutin, vanillin, syringaldehyde, gallic acid, ferulic acid, p-coumaric acid, sinapic acid | 97.6–101% | UV | medicinal herbs extract (water inf. or dec.) | [68] |
| Er-BTC | quercetin/luteolin | 0.22 nM/0.14 nM | 0.5–100 nM/0.5–80 nM | other compounds not similar to the target | n.d. | HPLC | drink and tea samples (fil. + dil.) | [81] |
| GCE/PoPD/Pt (DPV and ChAmp) | rosmarinic acid, protocatechuic acid | ChAmp: 0.5 μM (rosmarinic acid) and 0.6 μM (protocatechuic acid); DPV: 0.7 μM (rosmarinic acid, protocatechuic acid) | ChAmp: 1–55 μM (rosmarinic acid) and 1–60 μM (protocatechuic acid); DPV: 2–10 μM (rosmarinic acid) and 1–35 μM | caffeic acid, p-coumaric acid, chlorgenic acid, gallic acid, 2,5-dihydroxybenzoic acid, rutin | n.d. | HPLC | rosemary and melissa extracts (water inf.) | [69] |
| MoS2/ANC/GCE (DPV) | taxifolin | 0.3 nM | 1 nM–1 μM | n.d. | 98.9–100.5% | no | fructus polygoni orientalis (MeOH ext.) | [61] |
2.2. Electrochemical Sensors Equipped with Recognition Elements
2.2.1. Enzymes
Tyrosinase
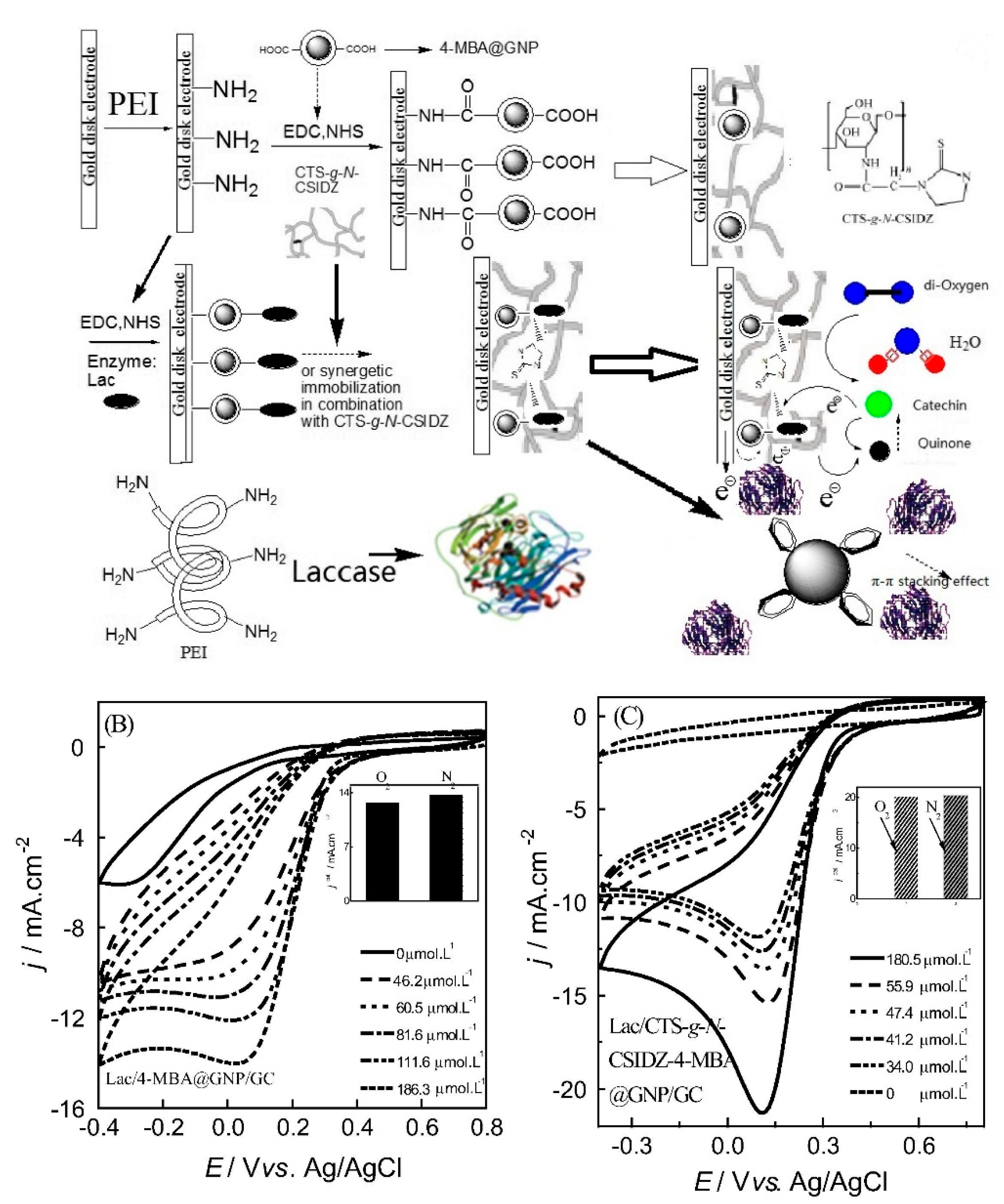
Laccases
Peroxidases
Multiple Enzyme Systems
Enzymes Inhibited by Phenolic Compounds
2.2.2. Functional Receptors of Phenolic Compounds
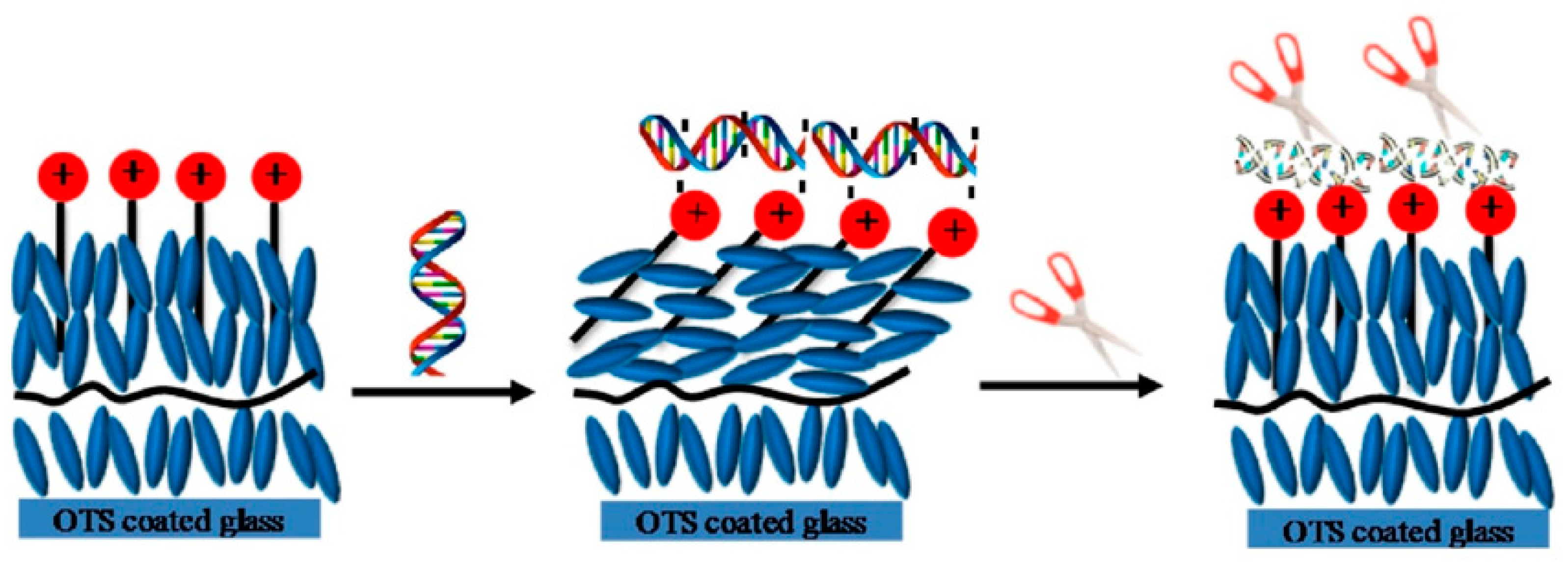
2.2.3. Nucleic Acids
2.2.4. Synthetic Enzymes and Receptor Mimics
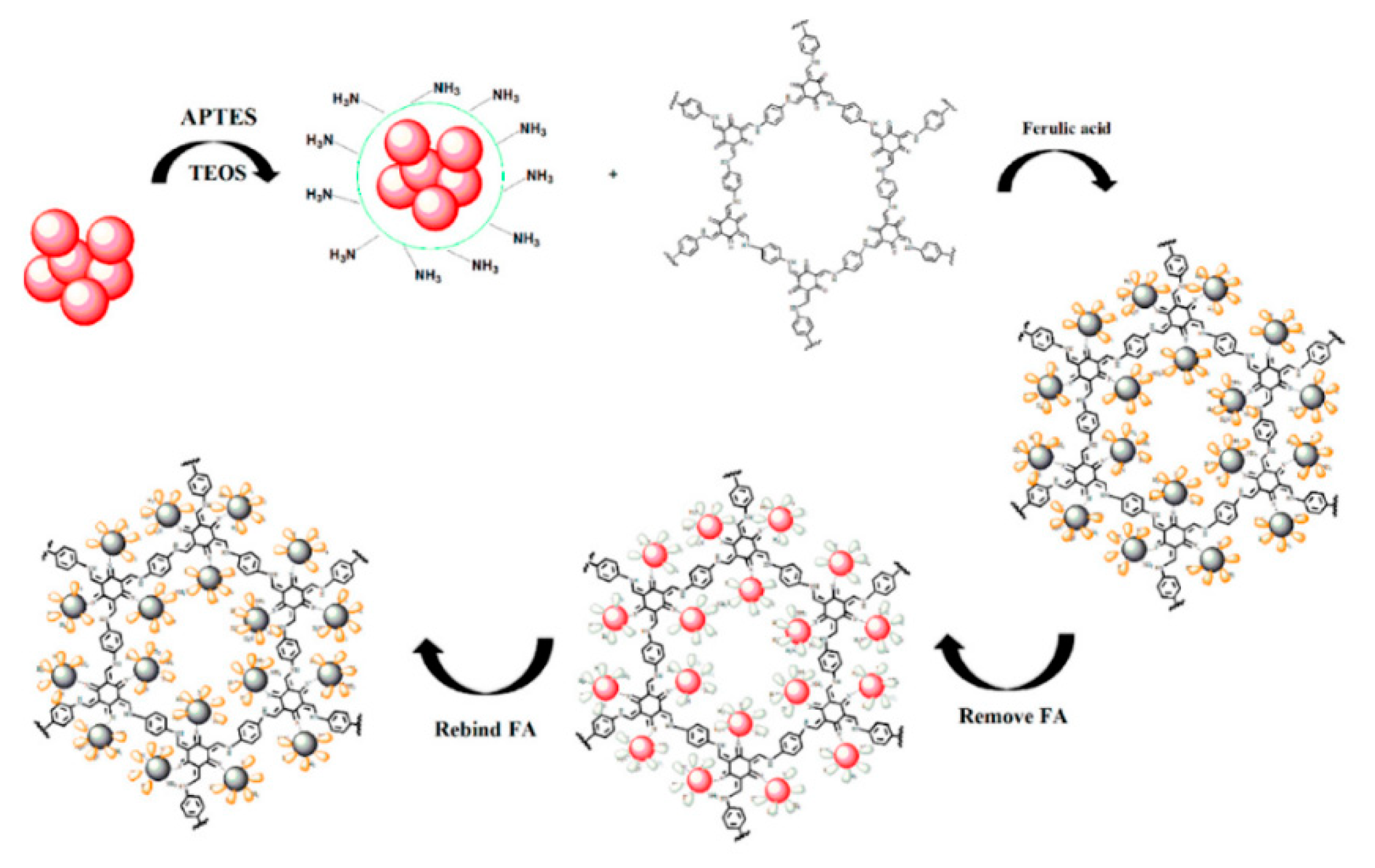
2.2.5. Imprinted Polymeric Materials
| Sensing System | Target | LOD | Linear Range | Non Interfering Related Compounds (Interfering Related Compounds) | Recovery | Reference Method | Real Samples (Sample Preparation) | Ref. |
|---|---|---|---|---|---|---|---|---|
| tyrosinase/CPE (Amp) | a selectivity study | n.d. | n.d. | resveratrol, genistein, and quercetin compared with synthetic estrogens, bisphenol A, nonylphenol, and diethylstilbestrol. | n.d. | ASTM method 9065 | no | [86] |
| Guanine or adenine deposed on the GCE as probes of phenolic anti-oxidant activity | ascorbic acid, gallic acid, caffeic acid, coumaric acid, resveratrol | 1.65 μM, 0.53 μM, 0.33 μM, 0.49 μM, 0.31 μM | 2.8–14.2 μM for arachidic acid; 0.44–2.2 μM for resveratrol | fully studied between the targets | always within +/−6% | no | 43 samples of different flavored water (dil. PBS) | [108] |
| Lac-based sensor (CV) | catechin | 16 nM | 8.7 μM–146.0 μM | phenols and polyphenolic compounds | n.d. | HPLC | real sample from industrial sewage (not reported) | [91] |
| green bean tissue homogenate (source of peroxidase) immobilized on chemically crosslinked chitin CPE (SWV) | caffeic acid | 2.0 μM | 20 μM–200 μM | ferulic acid, vanillic acid, syringic acid, gallic acid, p-coumaric acid, phenol, guaiacol, benzoic acid, (only catechin and hydroquinone produced a response) | 91.0–103.1% | CE | white wine (dil.) | [96] |
| MIS (TEOS-PTEOS-3 APTMS) Au electrode (DPV) | caffeic acid | 0.15 μM | 0.500–60.0 μM | cinnamic acid, ferulic acid, p-coumaric acid, vanillic acid, gallic acid, 1-hydroxy-2-naphthoic acid | 97.4–102.3%, | HPLC | red and white wines (dil.) | [115] |
| laccase on polydopamine/GCE or graphite electrode (SWV) | caffeic acid, rosmarinic acid, gallic acid | 0.14 μM (caffeic acid), 0.09 μM (rosmarinic acid), 0.29 μM (gallic acid) | 1–50 μM (caffeic acid), 1–20 μM (rosmarinic acid), 1–150 μM (gallic acid) | n.d. | n.d. | HPLC | chestnut shell extract, one sample compared with HPLC quite far (not reported) | [89] |
| CSPE/Tyr/gallic acid (Amp, CV) | Catechins | 0.03 μM, | 0.05-80 mM | epicatechin, epicatechin-3-gallate, gallocatechin, epigallocatechin, gallocatechingallate, epigallocatechin-3-gallate | 90–96% | HPLC | black and green teas (water inf.) | [87] |
| graphite oxide, PtNPs, BOT and laccase (SWV) | chlorgenic acid | LOD: 0.18 μM | 0.56–7.3 μM | ferulic acid, p-coumaric acid, caffeic acid | n.d. | HPLC | coffee (Water inf.) | [90] |
| bean sprout homogenate immobilized in chitosan microspheres (I) and silica (II) (SWV) | chlorgenic acid | 0.8 μM (biosensor I) and 0.85 μM (biosensor II) | 4.89 μM–0.32 mM (I) and 4.89 μM–48.5 μM (II) | The biosensors are sensitive to: chlorgenic acid (100%), catechol (90.5%), hydroquinone (75.0%), adrenaline (72.0%), rosmarinic acid (55.0%), caffeic acid (32.0%), adrenaline (30.0%) and l-dopa (25.0%). Esculetin, epigallocatechin gallate, ferulic acid, gallic acid, guaiacol, luteolin, p-coumaric acid, syringic acid, tannic acid, vanillic acid did not produce any response | 96.5–102.6% (I) and 91.3–115.5% (II) | CE | 4 coffee samples (water inf.) | [97] |
| Ir-BMI.PF6 and polyphenol oxidase—CS CPE (SWV) | chlorgenic acid | 0.915 μM | 3.48–49.5 μM | (caffeic acid causes a weak interference) | 93.2–105.7%. | CE | coffee (water dispersion) | [99] |
| tetranuclear copper (II) complex which mimics the active site of catechol oxidase (SWV) | chlorgenic acid | 0.8 μM | 5.0 μM–0.145 mM | The sensor was sensitive to rosmarinic acid (100%), catechol (92.1%), chlorgenic acid (80.5%), hydroquinone (78.0%), adrenaline (71.0%), l-dopa (22.5%) and caffeic acid (12.0%). Carbidopa, epigallocatechin gallate, ferulic acid, gallic acid, guaiacol, luteolin, p-coumaric acid, syringic acid, tannic acid, vanillic acid did not produce any response | 93.2–106.1% | CE | coffee (water inf.) | [110] |
| MIS (TEOS, PTEOS, APTMS) Au electrode (DPV) | chlorgenic acid | 0.15 μM | 0.5 μM–14 μM | caffeic acid, gallic acid, vanillic acid, catechol | 94.3–107.9% | no | coffee, tea samples (water inf.) | [114] |
| MIS (TEOS, PTEOS, APTMS)/MWCNT-VTMS/GCE (DPV) | chlorgenic acid | 0.032 μM | 0.08 μM to 100 μM | gallic acid, caffeic acid | 99.3–108.6% | no | coffee (water inf.), tomato, apple (Dil.) | [116] |
| MIPpy/PGE (pencil graphite electrode) (potentiometric) | chlorgenic acid | 1μM | 1 μM–10 mM | quinic acid, caffeic acid | nd | HPLC | coffee (water inf.+ dil.) | [117] |
| cyclic peptide CWWEVITFFKEC designed in silico (DPV and fluorescence) | chlorgenic acid (and caffeic acid) | n.d. | n.d. | ferulic acid, p-coumaric acid | n.d. | no | no | [112] |
| horseradish peroxidase enzyme (Amp) | capsaicin | 1.94 μM | 2.5–99.0 μM | catechol, phenol, guaiacol, 2.4-dimethylphenol, 3-chlorophenol, 3,4-dimethylphenol, 2-aminophenol, 4-chloro-3-methylphenol and resorcinol | 98–102.0% | HPLC | chili samples (EtOH ext.) | [100] |
| immobilized horseradish peroxidase (Amp) | capsaicin | 0.39 μM | 0.75–24.94 μM | phenolic compounds (data not shown) | >95% | HPLC | chili fruit (EtOH ext.) | [101] |
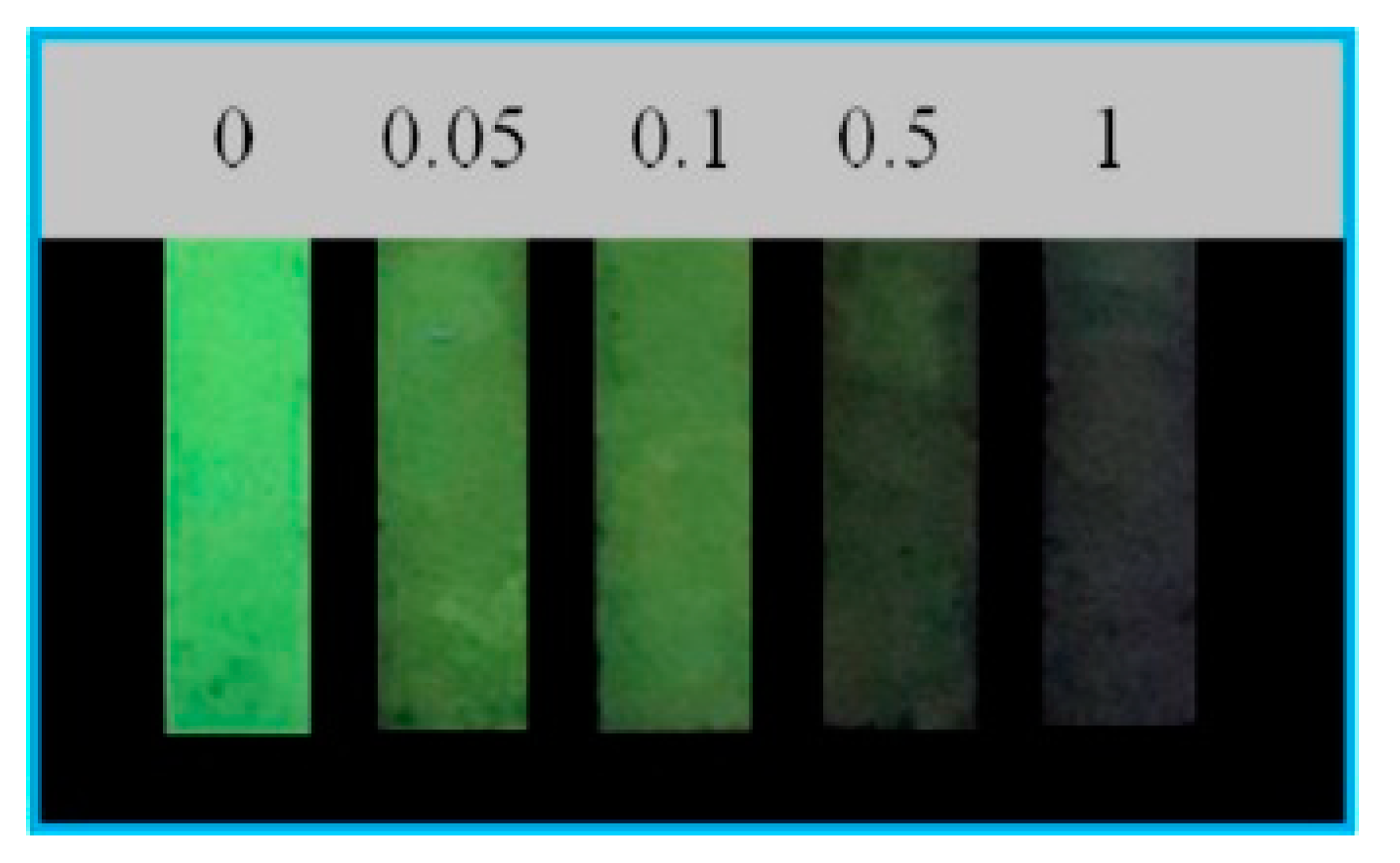
| MIP (imprinted zein)/Fe3O4 NPs/GCE | curcumin | 10 nM | 100 nM–100 μM | n.d. | n.d. | HPLC on 3 samples | potato chips (EtOH ext.) | [113] |
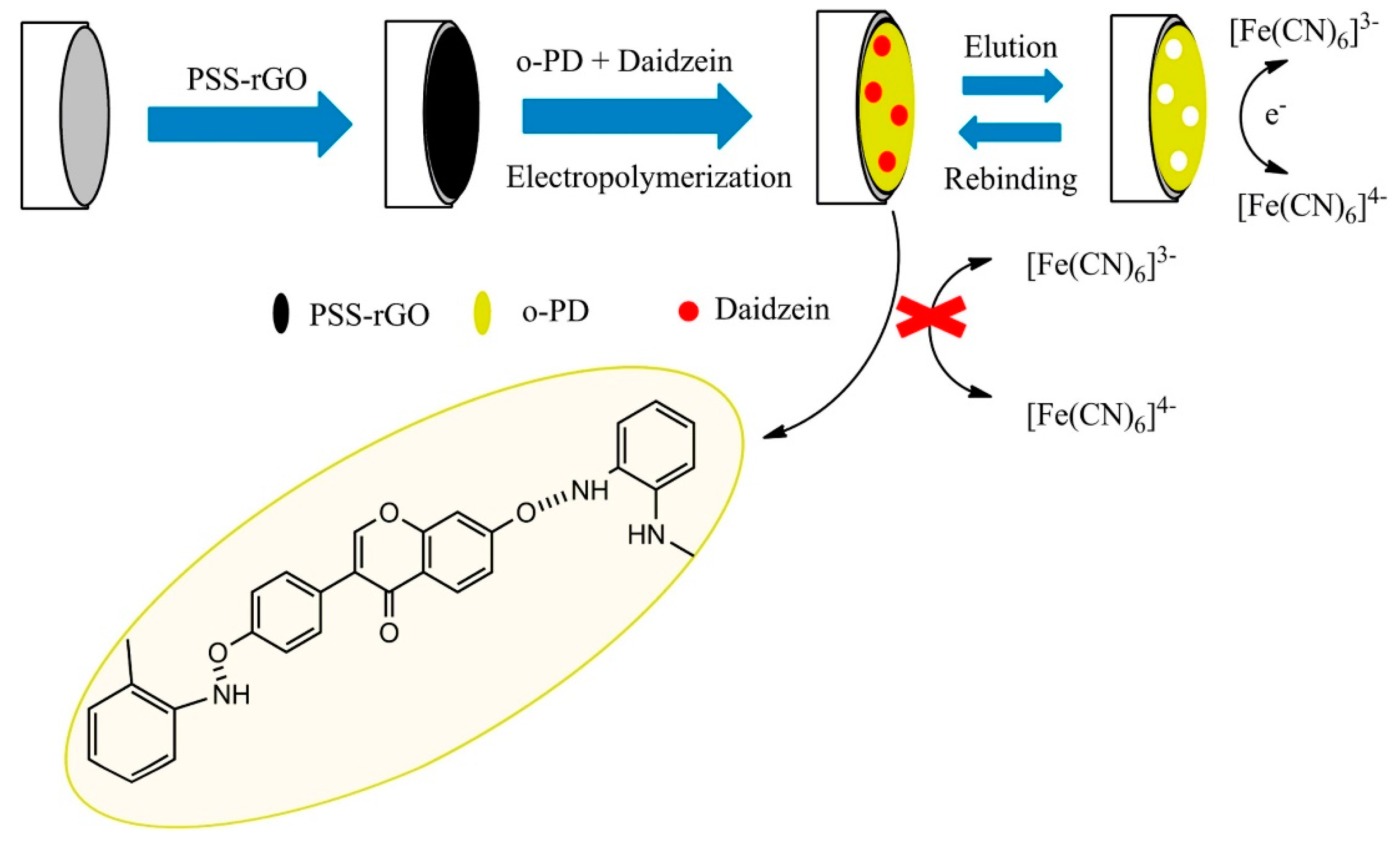
| MIP/PSS-rGO/GCE | daidzein | 0.5 nM | 1.0–20.0 nM | puerarin, quercetin, genistein and chrysin | 106.4–111.7%, | HPLC | pueraria lobata (EtOH ext.) | [119] |
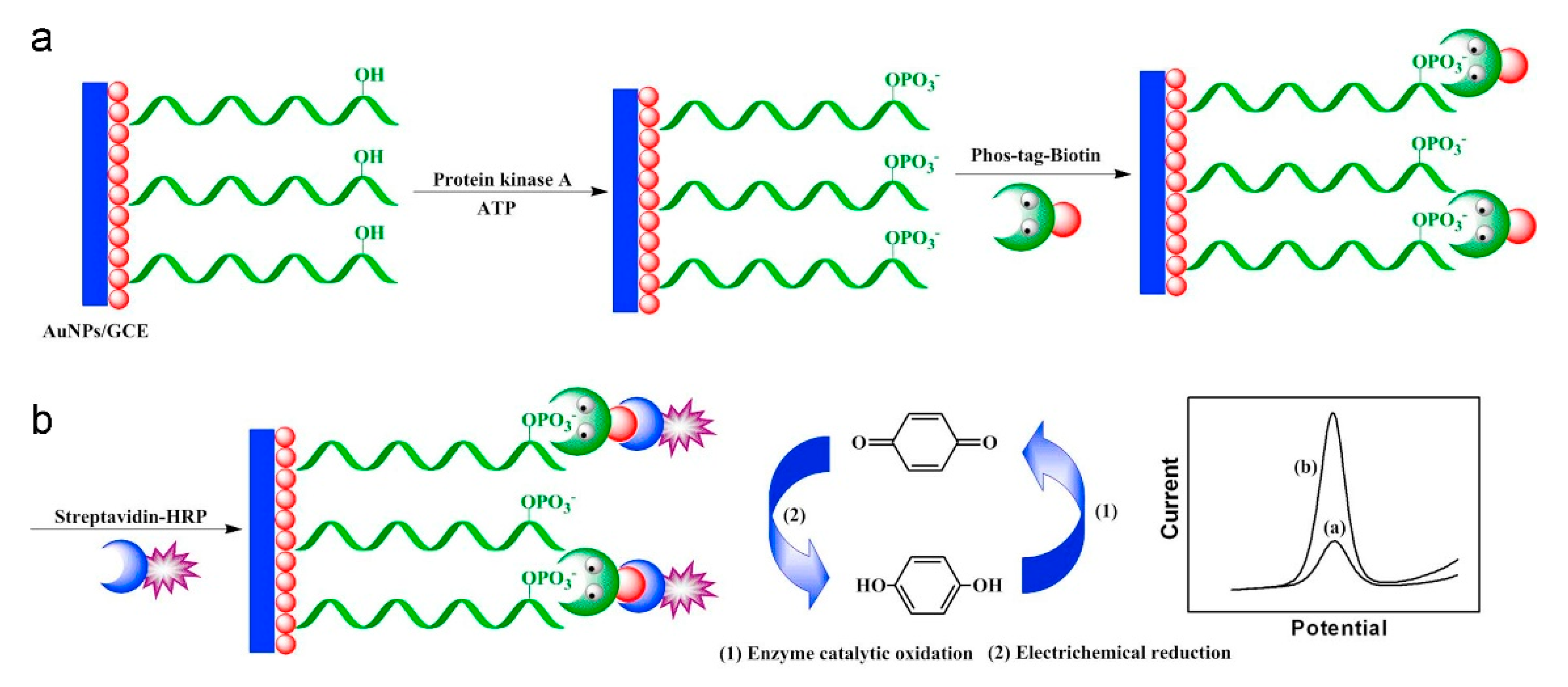
| Protein kinase and immobilized peptide substrate–AuNPs GCE | ellagic acid | 500 nM | 1–100 μM | sensitive to any inhibitor of protein kinase | n.d. | no | not reported | [104] |
| ethylenediamine-Co complex (CV, Amp) | ellagic acid | 35 pM | 0.1–929 mM | dopamine, acetaminophen, catechol, hydroquinone, gallic acid. | in raspberry and strawberry juice. 10 mM found + 10 added. 101% avg. | no | no | [111] |
| Bioelectronic tongue | ferulic acid, gallic acid, SA | n.d. | n.d. | n.d. | average values of 103%, 103% and 106% for ferulic acid, gallic acid, SA respectively | no | spiked beer samples (no pretreatment requested) | [102] |
| taste-bud tissues of SD rats/GCE (stripped rat mucosa) (Amp) | gingerol | 1 nM | 2–30 nM | capsaicin (more sensitive) | n.d. | no | no | [105] |
| αVβ3 integrin (CV and DPV) | gingerol | 260 nM | 0.85–20 mM | resveratrol, genistein, and quercetin compared with synthetic estrogens, bisphenol A, nonylphenol, and diethylstilbestrol. | n.d. | no | ginger ethanolic extract (dil. PBS) | [106] |
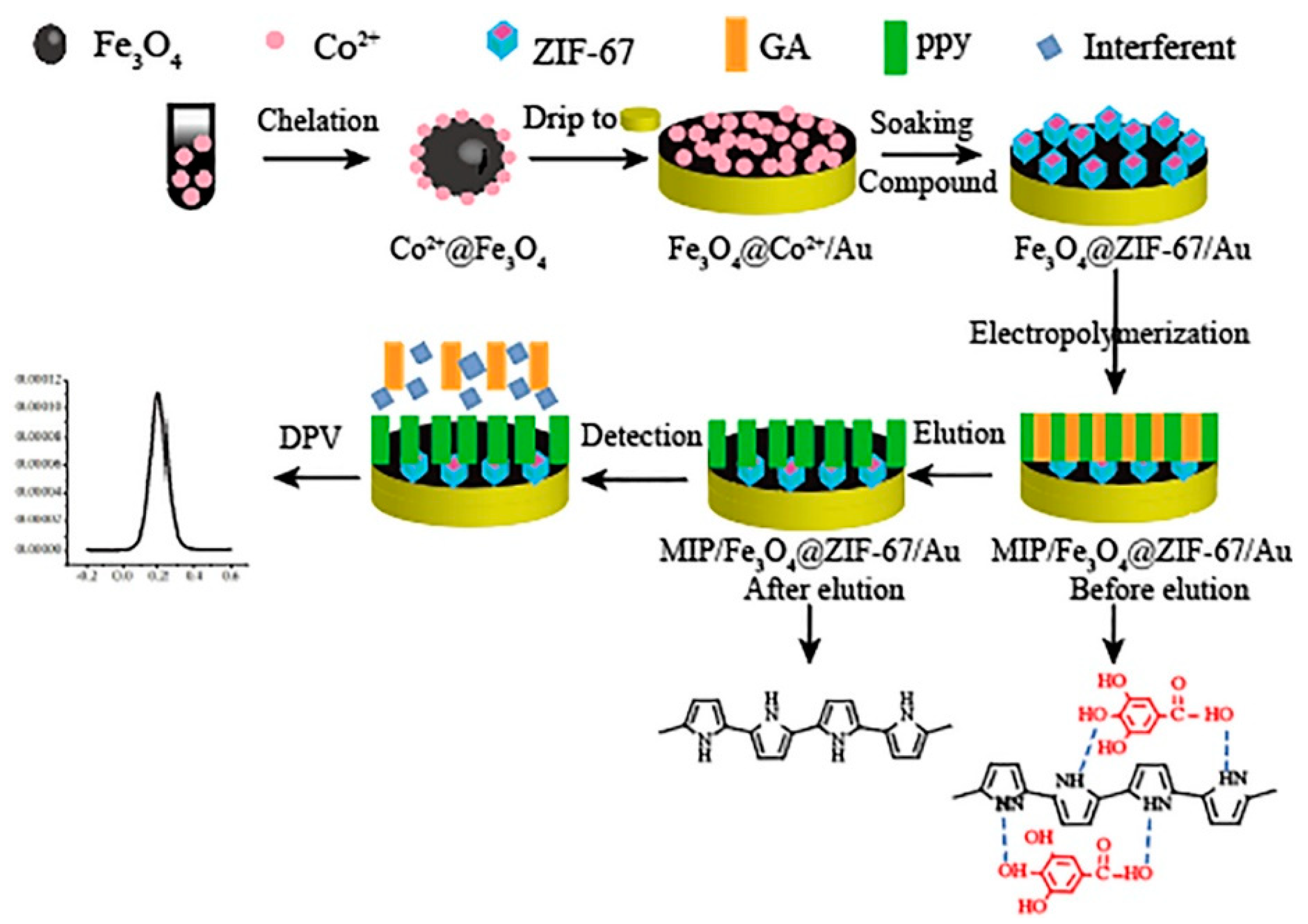
| MIPpy/Fe3O4 @ ZIF-67/Au (DPV) | gallic acid | 0.297 pM | 6–600 pM | p-hydroxybenzoic acid, tannic acid, salicylic acid | 89.5–118.4% | UV-Vis | black and green tea (dil.) | [118] |
| MIP (MAA, EGDMA) -MWCNT–CPE (DPV) | gallic acid | 47.0 nM | 0.12–380.0 μM | other compounds not similar to the target | 98.1–103.3% | no | four different commercial juices (dil.) | [122] |
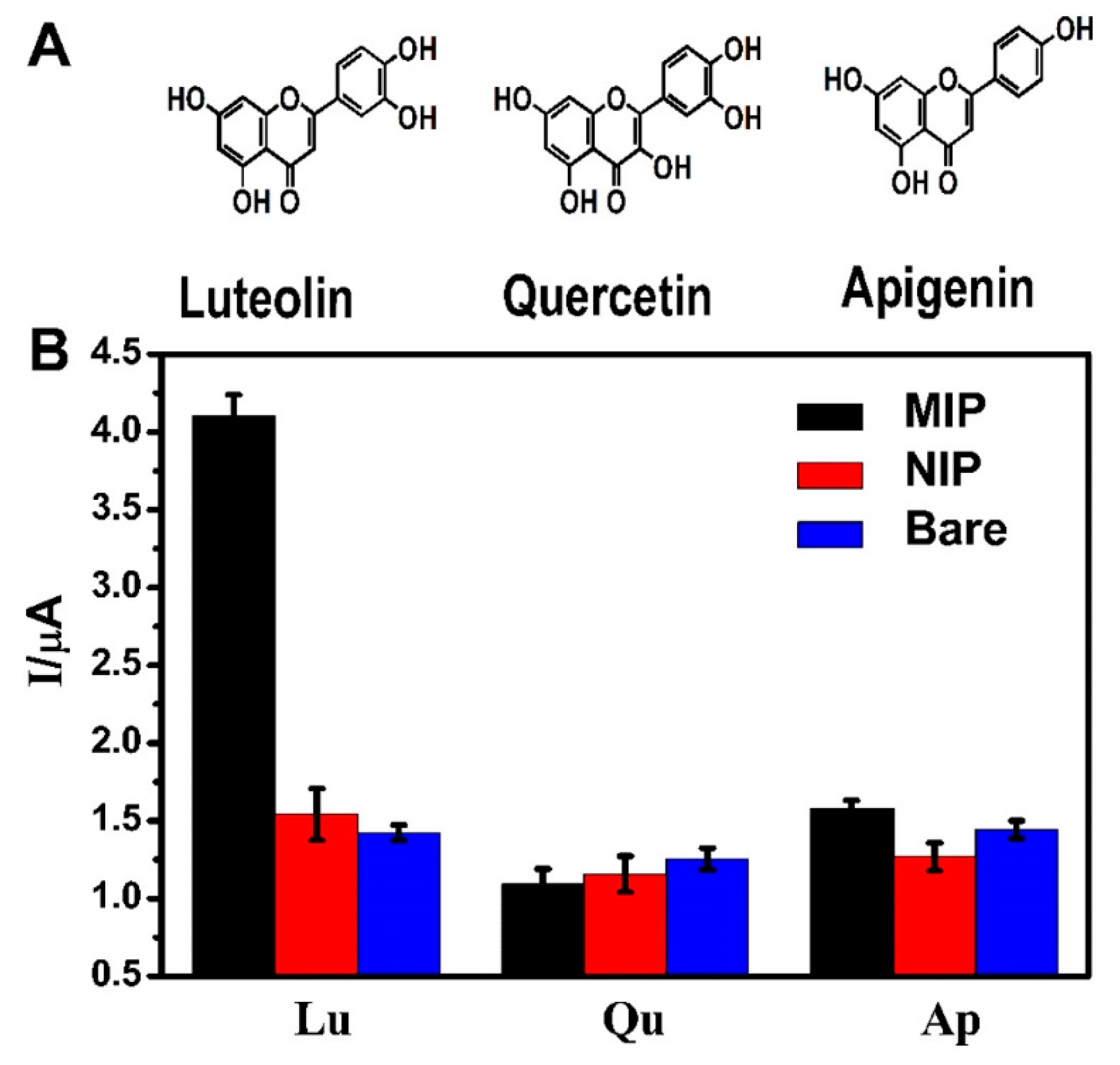
| MIP/ITO (DPV) | luteolin | 24 nM | 50 nM–30 μM | quercetin, apigenin | 96.0–105.2% | HPLC | Duyiwei capsules (EtOH ext.) | [121] |
| MIP/Pd/pGN-CNTs/GCE (DPV) | quercetin | 5.0 nM | 0.01–0.50 μM | other compounds not similar to the target | 90–104% | no | Pule’an tablets, honeysuckle juice and red wine (EtOH ext.) | [120] |
| tissue from the pine nuts of Araucaria angustifolia (containing peroxidase)-CS–IL CPE (SWV) | rosmarinic acid | 72.5 nM | 900 nM–4.5 mM | n.d. | 97–109% | CE | about 10 plant extracts (not reported) | [98] |
| CPE modified with chitosan and CNTs covered with DNA (CV) | rosmarinic acid | 0.014 μM | 0.040–1.5 μM | n.d. | n.d. | HPLC | rosemary extract (water ext.) | [107] |
| Fe(III)Zn(II) complex which mimics the active site of red kidney bean purple acid phosphatase (SWV) | rosmarinic acid | 2.3 mM | 29.8–383 mM | caffeic acid, eriodictyol-7-O-glucoside, hesperetin, hesperidin, m-coumaric acid, naringenin, naringin | 90–97% | CE | lemon balm plant extracts (not reported) | [109] |
| Laccase/Pt-Ag, AgCl electrode base (Amp) | resveratrol | 1 mM | 2–14 mM | caffeic acid, gallic acid, catechin, rutin, quercetin, malvidin | yes, bad in wine samples due to matrix effects. Solid phase extraction required before analysis | no | wine, with large interferences | [88] |
| peroxidase basic isoenzymes—ferrocene CPE (Amp) | resveratrol | 0.83 μM | 1–25 mM | n.d. | n.d. | no | no | [95] |
| bioelectronic tongue based on tyrosinase and laccase | vanillic acid, catechol, caffeic acid, hydroquinone, gallic acid, pyrogallol | 10–100 nM | n.d. | n.d. | n.d. | no | discrimination of musts according to their Total Polyphenolic Index (dil.) | [103] |
3. Optical and Fluorimetric Sensors
3.1. Optical and Fluorimetric Systems without Recognition Elements
3.2. Optical Sensors Exploiting Biomimetic Receptors
3.2.1. Imprinted Polymers
3.2.2. Nucleic Acids
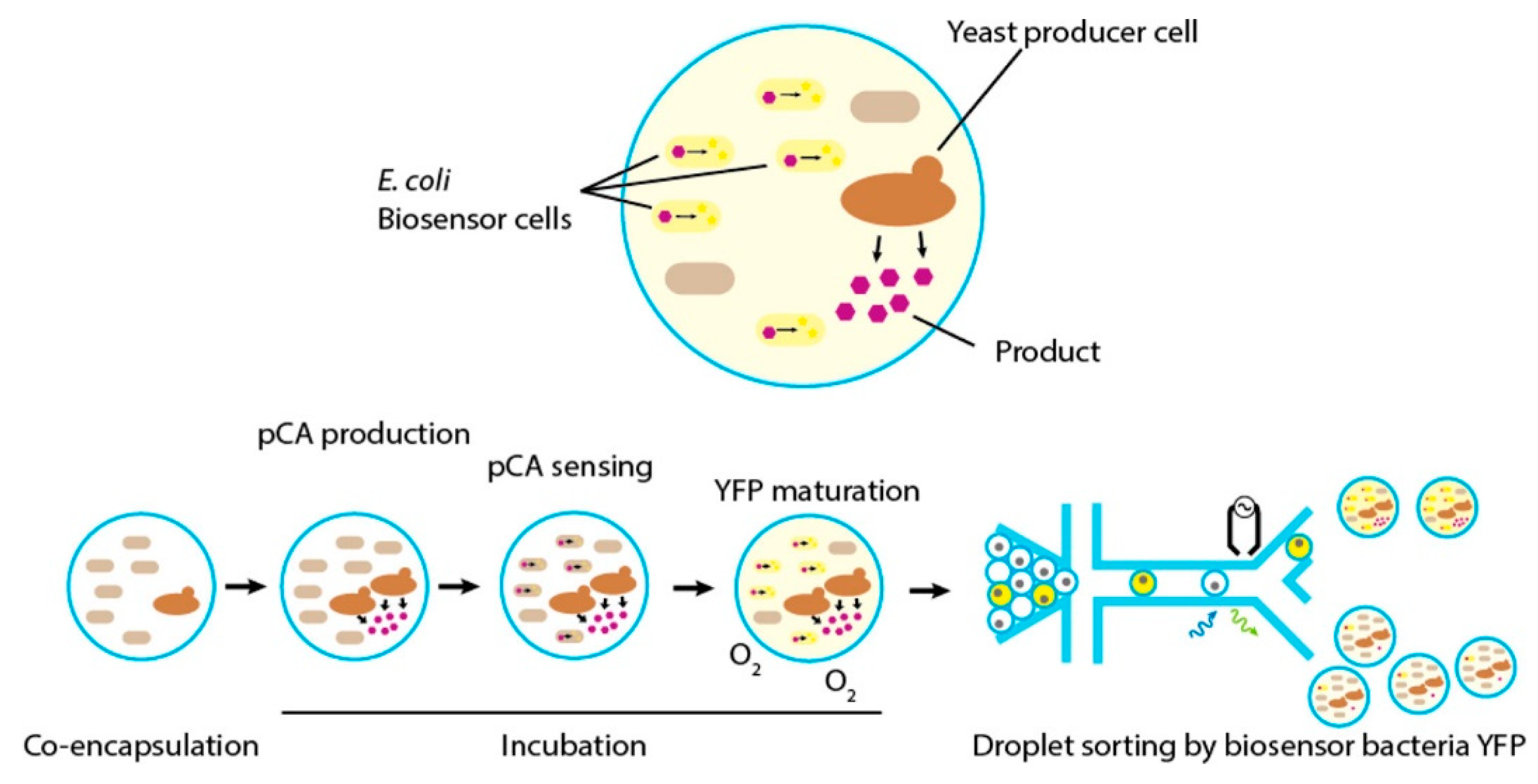
3.3. Whole Cells Optical Sensors
4. Gravimetric Sensors
| Sensing System | Target | LOD | Linear Range | Non Interfering Related Compounds (Interfering Related Compounds) | Recovery | Reference Method | Real Samples (Sample Preparation) | Ref. |
|---|---|---|---|---|---|---|---|---|
| QDs-Fe2+ (FL) | caffeic acid | 63 nM | 0.14–1.4 μM | sinapoyl thiocyanate; sinapic acid; p-coumaric acid; cinnamic acid; pyrogallic acid; syringic acid; ferulic acid, tannins, (only EDTA and especially citric acid, giving a response higher than that of caffeic acid can interfere) | 90.3–99.3% | no | rapeseed samples (MeOH/water 70:30 v/v ext.+ MeOH ext.) | [125] |
| CDs @ MIS (APTES, TEOS) (FL) | caffeic acid | 0.11 μM | 0.5–200 μM | other compounds not similar to the target | 98.4–107.6% | no | spiked human plasma (ACN removal of proteins) | [139] |
| potassium ferricyanide K3[Fe(CN)6] and Fe (III) (UV-Vis) | chlorgenic acid | n.d. | 28 μM and 2.3 mM | n.d. | no | HPLC | fermentation broth (EtOH ext.) and fruits (fractionated ext.) | [124] |
| CDs (FL) | chlorgenic acid | 45 nM | 0.15–60 μM | it seems to be quite selective to caffeic acid, ferulic acid, p-coumaric acid, quercetin | 97.67–101.75% | no | honeysuckle (50% MeOH ext.+ dil.) | [126] |
| QDs, N,S co-doped (FL) | curcumin | 40 nM | 0.15–18 mM | yes, tested on 10 small molecules and 12 salts | 98–102% in urine samples | no | human urine samples (not reported) | [128] |
| QDs, P,N,B co-doped (FL) | curcumin | 68 nM | 0.15–1.5 mM | 10 small molecules, 12 ions and hydrogen peroxide. | 95–10% in spike samples (tap water and mineral water) | no | no | [129] |
| 2-aminoethyl diphenyl borate, which reacts selectively with the 1,3-diketone moiety of curcumin (FL) | curcumin | about 0.44 nM | n.d. | n.d. | no | no | no | [130] |
| MIP (AA, TEOS) grafted on MWCNTs (UV-Vis) | curcumin | 76 pM | 0.27 nM–3.26 μM | n.d. | 94–107% in spiked samples | no | samples of curry, ginger and turmeric powders, spiked human plasma (MeOH/DMSO ext.) | [136] |
| MIP (MAA, magnetic) (UV-Vis) | curcumin | 3.56 μM | n.d. | on 5 curcumin-related compounds | in curry powder, ginger powder and fresh ginger; 79–88% | no | curry and ginger | [137] |
| MIP NPs (iron oxide, TEOS, acryloyl cyclodextrin, NIPAM) (no sensing system) | curcumin | n.d. | n.d. | n.d. | n.d. | no | no | [138] |
| TLC + quantification through paper containing FC reagent (colorimetric) | ferulic acid | 36 μM | 0.1–0.72 mM | other compounds not similar to the target | n.d. | HPLC-UV | 3 cosmetic samples (no pretreatment requested) | [123] |
| CdTe-QDs @ MIS (APTES and TEOS) (FL) | ferulic acid | 4.4 nM | 10–515 nM | chlorgenic acid, 4-hydroxybenzoic acid, caffeic acid, p-coumaric acid, vanillic acid, protocatechuic aldehyde | 91.8–110.3% | Comparison between fluorescence quenching and HPLC results after MIP extraction | pineapple juice and apple juice (fil.) | [140] |
| MIS (APTES, TEOS) based on QD-grafted COFs (FL and solid phase extraction material) | ferulic acid | 26 nM (FL) and 15 nM (HPLC/MS) | 0.15 μM–0.31 mM (FL); 0.1 μM–0.1 mM (HPLC/MS) | cinnamic acid, syringic acid, caffeic acid | 88–114% (FL) and 90–97% (HPLC/MS) | Comparison between fluorescence quenching and HPLC-MS results after extraction with MIPs | highland barley bran, wheat bran, corn silk, and vinasse (60% acetone ext.) | [142] |
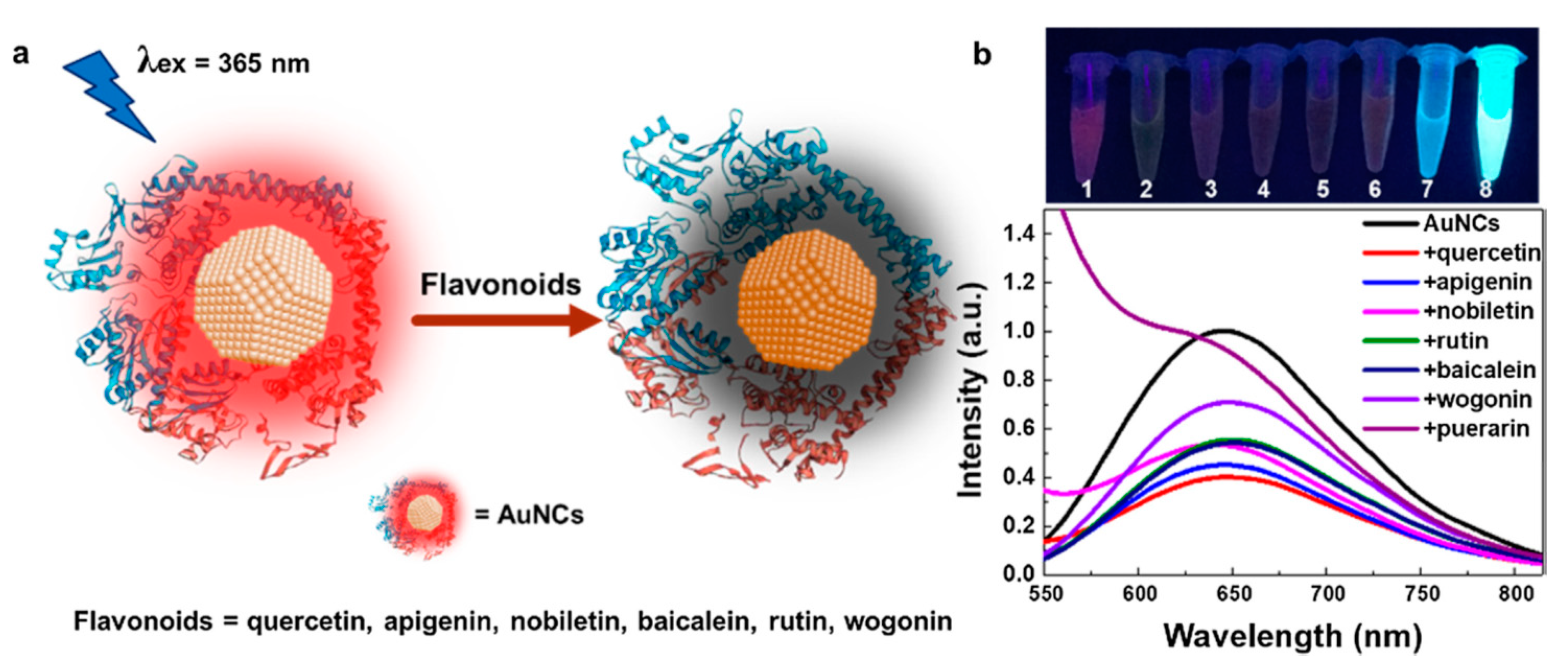
| BSA-AuNCs (FL) | flavonoids (quercetin, apigenin, nobiletin, rutin, baicalein, wogonin, and puerarin) | 1.44–5.07 mg/mL | 0–0.2 mg/mL | other compounds not similar to the target | 70.9–139% | no | serum, plasma, urine (not reported) | [135] |
| CQDs (FL) | myricetin | 18.4 nM | 1–80 μM | chlorgenic acid, chrysophanol, rutin, daidzein, ferulic acid | 97.5–105% | no | red wine, human serum (dil. ACN) | [127] |
| TEM DTAB/DNA (polarized optical microscope) | myricetin | n.d. | n.d. | other compounds not similar to the target | n.d. | no | no | [144] |
| Terbium-complex Tb(pzda)3(NO3)3 ∙ nH2O (Electrochemiluminescence) | protocatechuic acid | 0.085 nM | 0.13 nM–0.38 mM | Gallic acid trimethyl ether, trimebutine, 2-(dimethylamino)-2-phenylbutyl 3,4,5-trimethoxybenzoate maleate, curcumin, Epinephrine bitartrate, gallic acid, 3-Hydroxy-4-methoxybenzoic acid, Homovanillic acid, ferulic acid | nd | no | no | [134] |
| E. Coli biosensor producing GFP (FL) | protocatechuic acid | n.d. | sigmoidal response curve with upper and lower limits at 2000 μM and 4 μM, respectively | whole-cell biosensor either shows very weak response or poor sensitivity toward other closely related benzyl family molecules like 2-hydroxy benzoate (salicylate), 4-hydroxy benzoate, vanillic acid and vanillin | n.d. | no | no | [145] |
| CdTe-QDs @ MIS (APTES, TEOS) (FL) | p-coumaric acid | 41 nM | 122 nM–6.1 μM | ferulic acid, caffeic acid, cinnamic acid, chlorgenic acid, 4-hydroxybenzoic acid, vanillic acid | 92.7–106.0%, | no | pineapple juice and kiwi juice (fil.) | [141] |
| E. Coli biosensor producing YFP (FL) | p-coumaric acid | n.d. | 0.1–1 mM | cinnamic acid, caffeic acid, phloretic acid | n.d. | no | used to discriminate yeast p-coumaric acid-producing cells | [146] |
| CCP-treated C-dots (FL) | quercetin | 0.5 μM | 2.4–119 μM | other compounds not similar to the target | n.d. | no | Citrus reticulata cv. Chachiensis (EtOH ext.) | [131] |
| (MOF)-{[Tb3(CBA)2(HCOO)(μ3-OH)4(H2O)]·2H2O·0.5 DMF}n (colorimetric luminescence) | quercetin | 0.76 μM | 0−993 μM | apigenin, isorhamnetin, hesperidin, catechin, catechol, resorcin, hydroquinone | n.d. | HPLC-MS | onionskin and apple peel samples (MeOH ext.) | [132] |
| CDs@MOF@MIP (FL) | quercetin | 2.9 nM | 0 μM–50.0 μM | isorhamnetin, epicatechin, daidzein, rutin | n.d. | HPLC | Ginkgo biloba extract capsules (MeOH aq. Ext.) | [133] |
| MIPs (4-VP, DVB, fluorescein) (FL) | tyrosol, hydroxytyrosol, and oleuropein | <1 pM | 1 pM–100 nM | n.d. | n.d. | HPLC | real olive leaves extracts (water inf. + dil.) | [143] |
5. Conclusions and Future Perspectives
Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AdSV | adsorptive stripping voltammetry |
| Amp | amperometry |
| ACN | acetonitrile |
| AOC | antioxidant capacity |
| APTMS | (3-aminopropyl)trimethoxysilane |
| APTES | 3-(aminopropyl) triethoxysilane |
| ATP | adenosine triphosphate |
| BSA | bovine serum albumin |
| CB | carbon black |
| CD | carbon dot |
| CE | capillary electrophoresis |
| ChAmp | chronoamperometry |
| COF | covalent organic framework |
| CPE | carbon paste electrode |
| CPP | capacitively-coupled plasma |
| CQD | carbon quantum dot |
| CV | cyclic voltammetry |
| DPV | differential pulse voltammetry |
| EDC | N-Ethyl-N-dimethylamminopropyl carbodiimide |
| EGDMA | ethylene glycol dimethacrylate |
| ERGO | electrochemically reduced graphene oxide |
| FC | Folin—Ciocalteau |
| FTO | fluorine doped tin oxide |
| fMIP | fluorescent molecularly imprinted polymer |
| GCE | glassy carbon electrode |
| GFP | green fluorescent protein |
| GO | graphene oxide |
| GR | graphene |
| HPLC | high performance liquid chroma |
| HRP | horseradish peroxidase |
| IL | ionic liquid |
| LOD | limit of detection |
| LSV | linear sweep cyclic voltammetry |
| MIP | molecularly imprinted polymer |
| MIPpy | molecularly imprinted polypyrrole |
| MIS | molecularly imprinted siloxane |
| MOF | metal organic framewrok |
| MWCNT | multi-walled carbon nanotube |
| NP | nanoparticle |
| PB | phosphate buffer |
| PBS | phosphate buffer saline |
| PDDA | poly (diallyldimethylammonium) chloride |
| PEDOT | poly(3,4-ethylene)dioxythiophene |
| PTEOS | phenyltriethoxysilane |
| QD | quantum dot |
| rGO | reduced graphene oxide |
| SPE | screen-printed electrode |
| SW-AdSV | square wave adsorptive stripping voltammetry |
| SWCNT | single-walled carbon nanotube |
| SWV | square wave voltammetry |
| TEM | transmission electron microscope |
| TEOS | tetraethyl orthosilicate |
| YFP | yellow fluorescent protein |
References
- Cutrim, C.S.; Cortez, M.A.S. A review on polyphenols: Classification, beneficial effects and their application in dairy products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Proper. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Laganà, P.; Anastasi, G.; Marano, F.; Piccione, S.; Singla, R.K.; Dubey, A.K.; Delia, S.; Coniglio, M.A.; Facciolà, A.; Di Pietro, A.; et al. Phenolic Substances in Foods: Health Effects as Anti-Inflammatory and Antimicrobial Agents. J. AOAC Int. 2019, 102, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Malireddy, S.; Kotha, S.R.; Secor, J.D.; Gurney, T.O.; Abbott, J.L.; Maulik, G.; Maddipati, K.R.; Parinandi, N.L. Phytochemical Antioxidants Modulate Mammalian Cellular Epigenome: Implications in Health and Disease. Antioxid. Redox Sign. 2012, 17, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E. Diet components can suppress inflammation and reduce cancer risk. Nutr. Res. Pract. 2014, 8, 233–240. [Google Scholar] [CrossRef]
- Delgado, A.M.; Issaoui, M.; Chammem, N. Analysis of Main and Healthy Phenolic Compounds in Foods. J. AOAC Int. 2019, 102, 1356–1363. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.; Casati, P. Flavonoids: Biosynthesis, biological functions and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar]
- Bie, B.; Sun, J.; Guo, Y.; Li, J.; Jiang, W.; Yang, J.; Huang, C.; Li, Z. Baicalein: A review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed. Pharmacother. 2017, 93, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Gaggeri, R.; Rossi, D.; Collina, S.; Mannucci, B.; Baierl, M.; Juza, M. Quick development of an analytical enantioselective high performance liquid chromatography separation and preparative scale-up for the flavonoid Naringenin. J. Chromatogr. A 2011, 1218, 5414–5422. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yao, S.; Dai, X.; Yin, Q.; Liu, Y.; Jiang, X.; Wu, Y.; Qian, Y.; Pang, Y.; Gao, L.; et al. Identification of UDP-glycosyltransferases involved in the biosynthesis of astringent taste compounds in tea (Camellia sinensis). J. Exp. Bot. 2016, 67, 2285–2297. [Google Scholar] [CrossRef]
- Wang, X.; Guo, L.; Gao, L.; Shi, X.; Zhao, X.; Ma, X.; Xia, T.; Wang, Y. Molecular Evidence for Catechin Synthesis and Accumulation in Tea Buds (Camellia sinensis). J. Agric. Food Chem. 2018, 66, 63–69. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health Benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Karas, D.; Ulrichová, J.; Valentová, K. Galloylation of polyphenols alters their biological activity. Food Chem. Toxicol. 2017, 105, 223–240. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef]
- Ortiz, A.L.G.; Berti, F.; Sánchez, W.S.; Navarini, L.; Colomban, S.; Crisafulli, P.; Forzato, C. Distribution of p-coumaroylquinic acids in commercial Coffea spp. of different geographical origin and in other wild coffee species. Food Chem. 2019, 286, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Ortiz, A.L.; Berti, F.; Navarini, L.; Crisafulli, P.; Colomban, S.; Forzato, C. Aqueous extracts of walnut (Juglans regia L.) leaves: Quantitative analyses of hydroxycinnamic and chlorogenic acids. J. Chromatogr. Sci. 2018, 56, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Sinisi, V.; Stevaert, A.; Berti, F.; Forzato, C.; Benedetti, F.; Navarini, L.; Camps, A.; Persoons, L.; Vermeire, K. Chlorogenic compounds from coffee beans exert activity against respiratory viruses. Planta Med. 2017, 83, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Akshay, K.; Swathi, K.; Bakshi, V.; Boggula, N. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. J. Drug Deliv. Ther. 2019, 9, 323–330. [Google Scholar]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.T. Capsaicin-the major bioactive ingredient of chili peppers: Bio-efficacy and delivery systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef]
- Tôrres de Lima, R.M.; Campinho dos Reis, A.; Pereira Melo de Menezes, A.-A.; de Oliveira Santos, J.V.; Gomes de Oliveira Filho, J.W.; de Oliveira Ferreira, J.R.; Oliveira Barros de Alencar, M.V.; Oliveira Ferreira da Mata, A.M.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Machado, N.; Sobreira, C.; Domínguez-Perles, R.; Gomes, S.; Rosa, E.; Barros, A.I.R.N.A. Critical review on the significance of olive phytochemicals in plant physiology and human health. Molecules 2017, 22, 1986. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Vidrevich, M. Antioxidants: Terminology, methods, and future considerations. Antioxidants 2019, 8, 297. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Ge, L.; Li, S.P.; Lisak, G. Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2020, 179, 112913. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ji, J.; Sun, Z.; Shen, P.; Sun, X. Recent advances in electrochemical biosensors for antioxidant analysis in foodstuff. Trends Anal. Chem. 2020, 122, 115718. [Google Scholar] [CrossRef]
- Saikrithika, S.; Senthil Kumar, A. Electrochemical detections of tea polyphenols: A review. Electroanalysis 2020, 32, 1–19. [Google Scholar] [CrossRef]
- Della Pelle, F.; Compagnone, D. Nanomaterials in sensing and biosensing of phenolic compounds and related antioxidant capacity in food. Sensors 2018, 18, 462. [Google Scholar] [CrossRef]
- Sánchez-Arribas, A.; Martínez-Fernández, M.; Chicharro, M. The role of electroanaytical techniques in analysis of polyphenols in wine. Trends Anal. Chem. 2012, 34, 78–96. [Google Scholar] [CrossRef]
- Jiang, L.; Santiago, I.; Foord, J. Nanocarbon and nanodiamond for high performance phenolics sensing. Commun. Chem. 2018, 1, 43. [Google Scholar] [CrossRef]
- Søpstad, S.; Imenes, K.; Johannessen, E.A. Hybrid electrochemical sensor platform for capsaicin determination using coarsely stepped cyclic square wave voltammetry. Biosens. Bioelectron. 2019, 130, 374–381. [Google Scholar]
- Albu, C.; Eremia, S.A.; Veca, M.L.; Avram, A.; Popa, R.C.; Pachiu, C.; Romanitan, C.; Kusko, M.; Gavrila, R.; Radoi, A. Nano-crystalline graphite film on SiO2: Electrochemistry and electro-analytical application. Electrochim. Acta 2019, 303, 284–292. [Google Scholar] [CrossRef]
- Puangjan, A.; Chaiyasith, S. An efficient ZrO2/Co3O4/reduced graphene oxide nanocomposite electrochemical sensor for simultaneous determination of gallic acid, caffeic acid and protocatechuic acid natural antioxidants. Electrochim. Acta 2016, 211, 273–288. [Google Scholar] [CrossRef]
- Liu, L.; Gou, Y.; Gao, X.; Zhang, P.; Chen, W.; Feng, S.; Hu, F.; Li, Y. Electrochemically reduced graphene oxide-based electrochemical sensor for the sensitive determination of ferulic acid in A. sinensis and biological samples. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 227–233. [Google Scholar] [CrossRef]
- Liang, Z.; Zhai, H.; Chen, Z.; Wang, H.; Wang, S.; Zhou, Q.; Huang, X. A simple, ultrasensitive sensor for gallic acid and uric acid based on gold microclusters/sulfonate functionalized graphene modified glassy carbon electrode. Sens. Actuat. B Chem. 2016, 224, 915–925. [Google Scholar] [CrossRef]
- Valentini, F.; Roscioli, D.; Carbone, M.; Conte, V.; Floris, B.; Bauer, E.M.; Ditaranto, N.; Sabbatini, L.; Caponetti, E.; Chillura-Martino, D. Graphene and ionic liquids new gel paste electrodes for caffeic acid quantification. Sens. Actuat. B Chem. 2015, 212, 248–255. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Sidhureddy, B.; Thiruppathi, A.R.; Chen, A. Sensitive electrochemical detection of caffeic acid in wine based on fluorine-doped graphene oxide. Sensors 2019, 19, 1604. [Google Scholar] [CrossRef] [PubMed]
- Muzyka, K.; Sun, J.; Fereja, T.H.; Lan, Y.; Zhang, W.; Xu, G. Boron-doped diamond: Current progress and challenges in view of electroanalytical applications. Anal. Methods 2019, 11, 397–414. [Google Scholar] [CrossRef]
- Talarico, D.; Arduini, F.; Constantino, A.; Del Carlo, M.; Compagnone, D.; Moscone, D.; Palleschi, G. Carbon black as successful screen-printed electrode modifier for phenolic compound detection. Electrochem. Commun. 2015, 60, 78–82. [Google Scholar] [CrossRef]
- Vashist, S.K.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.; Sheu, F.S. Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol. Adv. 2011, 29, 169–188. [Google Scholar] [CrossRef]
- Kachoosangi, R.T.; Wildgoose, G.G.; Compton, R.G. Carbon nanotube-based electrochemical sensors for quantifying the “heat” of chilli peppers: The absorptive stripping voltammetric determination of capsaicin. Analyst 2008, 133, 888. [Google Scholar] [CrossRef]
- Ma, X.; Yang, H.; Xiong, H.; Li, X.; Gao, J.; Gao, Y. Electrochemical behavior and determination of chlorogenic acid based on multi-walled carbon nanotubes modified screen-printed electrode. Sensors 2016, 16, 1797. [Google Scholar] [CrossRef]
- Cheng, W.; Huang, J.; Liu, C.; Zeng, Q.; Tong, Y.; Wang, L.; Cheng, F. High sensitivity chlorogenic acid detection based on multiple layer-by-layer self-assembly films of chitosan and multi-walled carbon nanotubes on a glassy carbon electrode. RSC Adv. 2017, 7, 6950–6956. [Google Scholar] [CrossRef]
- Şenocak, A.; Basova, T.; Demirbas, E.; Durmuş, M. Direct and fast electrochemical determination of catechin in tea extracts using SWCNT-Subphthalocyanine hybrid material. Electroanalysis 2019, 31, 1697–1707. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Madhu, R.; Chen, S.-M.; Veeramani, V.; Sivakumar, M.; Suk Huh, Y.; Han, Y.-K. Facile synthesis of MnO2/carbon nanotubes decorated with a nanocomposite of Pt nanoparticles as a new platform for the electrochemical detection of catechin in red wine and green tea samples. J. Mater. Chem. B 2015, 3, 6285–6292. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Farahmandfar, R.; Hosseinpour, R.; Alizadeh, J.; Abbaspourrad, A. Determination of ferulic acid in the presence of butylated hydroxytoluene as two phenolic antioxidants using a highly conductive food nanostructure electrochemical sensor. Chem. Pap. 2019, 73, 2441–2447. [Google Scholar] [CrossRef]
- Zabihpour, T.; Shahidi, S.A.; Karimi-Maleh, H.; Ghorbani-HasanSaraei, A. An ultrasensitive electroanalytical sensor based on MgO/SWCNTs-1-Butyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide paste electrode for the determination of ferulic acid in the presence sulfite in food samples. Microchem. J. 2020, 154, 104572. [Google Scholar] [CrossRef]
- Jiyane, N.; Sabela, M.I.; Kanchi, S.; Mdluli, P.S.; Xhakaza, M.; Arodola, O.A.; Bisetty, K. MWCNTs-Fe2O3 nanoparticle nanohybrid-based highly sensitive electrochemical sensor for the detection of kaempferol in broccoli samples. Turk. J. Chem. 2019, 43, 1229–1243. [Google Scholar] [CrossRef]
- Chen, T.W.; Rajaji, U.; Chen, S.M.; Govindasamy, M.; Selvin, S.S.P.; Manavalan, S.; Arumugam, R. Sonochemical synthesis of graphene oxide sheets supported Cu2S nanodots for high sensitive electrochemical determination of caffeic acid in red wine and soft drinks. Compos. Part B-Eng. 2019, 158, 419–427. [Google Scholar] [CrossRef]
- Saritha, D.; Koirala, A.R.; Venu, M.; Dinneswara Reddy, G.; Vijaya Bhaskar Reddy, A.; Sitaram, B.; Madhavi, G.; Aruna, K. A simple, highly sensitive and stable electrochemical sensor for the detection of quercetin in solution, onion and honey buckwheat using zinc oxide supported on carbon nanosheet (ZnO/CNS/MCPE) modified carbon paste electrode. Electrochim. Acta 2019, 313, 523–531. [Google Scholar] [CrossRef]
- Zhou, Z.; Gu, C.; Chen, C.; Zhao, P.; Xie, Y.; Fei, J. An ultrasensitive electrochemical sensor for quercetin based on 1-pyrenebutyrate functionalized reduced oxide graphene/mercapto-β-cyclodextrin/Au nanoparticles composite film. Sens. Actuat. B-Chem. 2019, 288, 88–95. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, H.; Xie, H.; Luo, G.; Niu, Y.; Zhang, S.; Li, G.; Sun, W. Platinum nanoparticles decorating a biomass porous carbon nanocomposite-modified electrode for the electrocatalytic sensing of luteolin and application. RSC Adv. 2019, 9, 33607–33616. [Google Scholar] [CrossRef]
- Li, X.; Zou, R.; Niu, Y.; Sun, W.; Shao, T.; Chen, X. Gold nanocage-based electrochemical sensing platform for sensitive detection of luteolin. Sensors 2018, 18, 2309. [Google Scholar] [CrossRef]
- Feng, X.; Yin, X.; Bo, X.; Guo, L. An ultrasensitive luteolin sensor based on MOFs derived CuCo coated nitrogen-doped porous carbon polyhedron. Sens. Actuat. B-Chem. 2019, 281, 730–738. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Dong, C.; Shi, J.; Sun, Y.; Ye, B.; Xu, Y. Molybdenum sulfide-based electrochemical platform for high sensitive detection of taxifolin in Chinese medicine. Anal. Chim. Acta 2020, 1099, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Della Pelle, F.; Rojas, D.; Scroccarello, A.; Del Carlo, M.; Ferraro, G.; Di Mattia, C.; Martuscelli, M.; Escarpa, A.; Compagnone, D. High-performance carbon black/molybdenum disulfide nanohybrid sensor for cocoa catechins determination using an extraction-free approach. Sens. Actuat. B-Chem. 2019, 296, 126651. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.; Chen, P.; Sun, D. Surface enhancement of porous alumina microfibers toward electrochemical sensing of chlorogenic acid. Microchem. J. 2019, 145, 801–805. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Sangili, A.; Chen, S.-M.; Veerakumar, P.; Lin, K.-C. Sr-Doped NiO3 nanorods synthesized by a simple sonochemical method as excellent materials for voltammetric determination of quercetin. New J. Chem. 2020, 44, 2821. [Google Scholar] [CrossRef]
- Traipop, S.; Chuanuwatanakul, S.; Chailapakul, O.; Punrat, E. Facile and fast detection of genistein in derris scandens by square wave voltammetry using a Cobalt(II) phthalocyanine-modified screen-printed electrochemical sensor. Curr. Anal. Chem. 2020, 16, 341–348. [Google Scholar] [CrossRef]
- Filik, H.; Çetintaş, G.; Avan, A.A.; Aydar, S.; Koç, S.N.; Boz, İ. Square-wave stripping voltammetric determination of caffeic acid on electrochemically reduced graphene oxide–Nafion composite film. Talanta 2013, 116, 245–250. [Google Scholar] [CrossRef]
- Koçak, Ç.C.; Karabiberoğlu, Ş.U.; Dursun, Z. Highly sensitive determination of gallic acid on poly (l-Methionine)-carbon nanotube composite electrode. J. Electroanal. Chem. 2019, 853, 113552. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kozlova, E.; Budnikov, H. Poly(gallic acid)/MWNT-modified electrode for the selective and sensitive voltammetric determination of quercetin in medicinal herbs. J. Electroanal. Chem. 2018, 821, 73–81. [Google Scholar] [CrossRef]
- Özdokur, K.V.; Koçak, Ç.C. Simultaneous determination of rosmarinic acid and protocatechuic acid at poly (o-Phenylenediamine)/Pt nanoparticles modified glassy carbon electrode. Electroanalysis 2019, 31, 2359–2367. [Google Scholar] [CrossRef]
- Chao, M.; Ma, X. Voltammetric determination of chlorogenic acid in pharmaceutical products using poly (aminosulfonic acid) modified glassy carbon electrode. J. Food Drug Anal. 2014, 22, 512–519. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, B.; Gao, Y.; Yang, T.; Yue, R.; Xu, J.; Gao, L. Facile one-pot preparation of Pd–Au/PEDOT/graphene nanocomposites and their high electrochemical sensing performance for caffeic acid detection. RSC Adv. 2016, 6, 89157–89166. [Google Scholar] [CrossRef]
- Cheng, W.; Zeng, P.; Ma, C.; Peng, H.; Yang, J.; Huang, J.; Zhang, M.; Cheng, F. Electrochemical sensor for sensitive detection of luteolin based on multi-walled carbon nanotubes/poly(3,4-ethylenedioxythiophene)–gold nanocomposites. New J. Chem. 2020, 44, 1953–1961. [Google Scholar] [CrossRef]
- Ma, Y.; Kong, Y.; Xu, J.; Deng, Y.; Lu, M.; Yu, R.; Yuan, M.; Li, T.; Wang, J. Carboxyl hydrogel particle film as a local pH buffer for voltammetric determination of luteolin and baicalein. Talanta 2020, 208, 120373. [Google Scholar] [CrossRef]
- Saljooqi, A.; Shamspur, T.; Mostafavi, A. Fe3O4@SiO2-PANI-Au Nanocomposite prepared for electrochemical determination of quercetin in food samples and biological fluids. Electroanalysis 2020, 32, 581–587. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, L.; Duan, Y.; Zou, L.; Ye, B. Facile synthesized SnO2 decorated functionalized graphene modified electrode for sensitive determination of daidzein. Talanta 2017, 168, 1–9. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Zhang, Y.; Zou, L.; Li, G.; Ye, B. Highly sensitive determination of gallic acid based on a Pt nanoparticle decorated polyelectrolyte-functionalized graphene modified electrode. Anal. Methods-UK 2016, 8, 8474–8482. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Huang, W.; Wang, Y.; Hu, X. A novel AuNPs-doped COFs composite as electrochemical probe for chlorogenic acid detection with enhanced sensitivity and stability. Sens. Actuat. B-Chem. 2018, 276, 362–369. [Google Scholar] [CrossRef]
- Tu, X.; Xie, Y.; Gao, F.; Ma, X.; Lin, X.; Huang, X.; Qu, F.; Ping, L.; Yu, Y.; Lu, L. Self-template synthesis of flower-like hierarchical graphene/copper oxide@ copper (II) metal-organic framework composite for the voltammetric determination of caffeic acid. Microchim. Acta 2020, 187, 1–8. [Google Scholar] [CrossRef]
- Botelho, C.N.; das Mercês Pereira, N.; Silva, G.G.; de Menezes, A.S.; Bezerra, C.W.B.; Damos, F.S.; Luz, R.D.C.S. Photoelectrochemical-assisted determination of caffeic acid exploiting a composite based on carbon nanotubes, cadmium telluride quantum dots, and titanium dioxide. Anal. Methods-UK 2019, 11, 4775–4784. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Hu, X.; Yu, H. Highly stable and ultrasensitive chlorogenic acid sensor based on metal—Organic frameworks/titanium dioxide nanocomposites. Analyst 2016, 141, 4647–4653. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, W.; Wu, K. Morphology-controlled electrochemical sensing of erbium benzene-tricarboxylic acid frameworks for azo dyes and flavonoids. Sens. Actuat. B-Chem. 2020, 304, 127370. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; Chen, K.; Zhang, T.; Wang, Y.; Wang, J. Facile fabrication of electrochemical sensor based on novel core-shell PPy@ZIF-8 structures: Enhanced charge collection for quercetin in human plasma Samples. Sens. Actuat. B-Chem. 2019, 290, 434–442. [Google Scholar] [CrossRef]
- Xue, Z.; Hu, C.; Rao, H.; Wang, X.; Zhou, X.; Liu, X.; Lu, X. A novel electrochemical sensor for capsaicin based on mesoporous cellular foams. Anal. Methods 2015, 7, 1167. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Wang, D.; Ni, S.; Han, D.; Wang, W.; Niu, L. Tailoring heterostructured Bi2MoO6/Bi2S3 nanobelts for highly selective photoelectrochemical analysis of gallic acid at drug level. Biosens. Bioelectron. 2017, 94, 107–114. [Google Scholar] [CrossRef]
- Sousa, C.S.; Lima, K.C.M.S.; Botelho, C.N.; Pereira, N.M.; Fernandes, R.N.; Silva, G.G.; Damos, F.S.; Luz, R.C.S. Photoelectrochemical sensor for determination of naringin (the 7-O-glycoside derivative of the flavanone naringenin with the disaccharide neohesperidose) at low oxidation potential using a modified FTO electrode with cadmium sulfide and titanium dioxide sensitized with chloroprotoporphyrin IX iron(III). J. Solid State Electrochem. 2020, 24, 1715. [Google Scholar] [CrossRef]
- Andreescu, S.; Sadik, O.A. Correlation of analyte structures with biosensor responses using the detection of phenolic oestrogens as a model. Anal. Chem. 2004, 76, 552–560. [Google Scholar] [CrossRef]
- Nadifiyine, S.; Calas-Blanchard, C.; Amine, A.; Marty, J.-L. Tyrosinase biosensor used for the determination of catechin derivatives in tea: Correlation with HPLC/DAD method. Food Nutr. Sci. 2013, 4, 108–118. [Google Scholar] [CrossRef]
- Gomes, S.A.S.S.; Nogueira, J.M.F.; Rebelo, M.J.F. An amperometric biosensor for polyphenolic compounds in red wine. Biosens. Bioelectron. 2016, 20, 1211–1216. [Google Scholar] [CrossRef]
- Almeida, L.C.; Correia, R.D.; Squillaci, G.; Morana, A.; La Cara, F.; Correia, J.P.; Viana, A.S. Electrochemical deposition of bio-inspired laccase-polydopamine films for phenolic sensors. Electrochim. Acta 2019, 319, 462–471. [Google Scholar] [CrossRef]
- Salamanca-Neto, C.A.R.; Marcheafave, G.G.; Scremin, J.; Barbosa, E.C.; Camargo, P.H.; Dekker, R.F.H.; Scarminio, I.S.; Barbosa-Dekker, A.M.; Sartori, E.R. Chemometric-assisted construction of a biosensing device to measure chlorogenic acid content in brewed coffee beverages to discriminate quality. Food Chem. 2020, 315, 126306. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, H.; Zhang, Q.; Bai, X.; Liu, C.; Zhang, Y.H. Direct electron transfer and sensing performance for catechin of nano-gold particles-polymer nano-composite with immobilized Laccase. Chem. Phys. Lett. 2016, 658, 259–269. [Google Scholar] [CrossRef]
- Leite, O.D.; Lupetti, K.O.; Fatibello-Filho, O.; Vieira, I.C.; de M Barbosa, A. Synergic effect studies of the bi-enzymatic system laccase–peroxidase in a voltammetric biosensor for catecholamines. Talanta 2003, 59, 889–896. [Google Scholar] [CrossRef]
- Vieira, I.C.; Lupetti, K.O.; Fatibello-Filho, O. Determination of paracetamol in pharmaceutical products using a carbon paste biosensor modified with crude extract of zucchini (Cucurbita pepo). Quim. Nova 2003, 26, 39–43. [Google Scholar] [CrossRef]
- de Souza Ribeiro, F.A.; Tarley, C.R.T.; Borges, K.B.; Pereira, A.C. Development of a square wave voltammetric method for dopamine determination using a biosensor based on multiwall carbon nanotubes paste and crude extract of Cucurbita pepo L. Sens. Actuat. B-Chem. 2013, 185, 743–754. [Google Scholar] [CrossRef]
- Granero, A.M.; Fernández, H.; Agostini, E.; Zón, M.A. An amperometric biosensor for trans-resveratrol determination in aqueous solutions by means of carbon paste electrodes modified with peroxidase basic isoenzymes from Brassica napus. Electroanalysis 2008, 20, 858–864. [Google Scholar] [CrossRef]
- Fernandes, S.C.; de Oliveira, I.R.W.Z.; Vieira, I.C. A green bean homogenate immobilized on chemically crosslinked chitin for determination of caffeic acid in white wine. Enzym. Microb. Technol. 2007, 40, 661–668. [Google Scholar] [CrossRef]
- Moccelini, S.K.; Spinelli, A.; Vieira, I.C. Biosensors based on bean sprout homogenate immobilized in chitosan microspheres and silica for determination of chlorogenic acid. Enzym. Microb. Technol. 2008, 43, 381–387. [Google Scholar] [CrossRef]
- dos Santos Maguerroski, K.; Fernandes, S.C.; Franzoi, A.C.; Vieira, I.C. Pine nut peroxidase immobilized on chitosan crosslinked with citrate and ionic liquid used in the construction of a biosensor. Enzym. Microb. Technol. 2009, 44, 400–405. [Google Scholar] [CrossRef]
- Fernandes, S.C.; Moccelini, S.K.; Scheeren, C.W.; Migowski, P.; Dupont, J.; Heller, M.; Micke, G.A.; Vieira, I.C. Biosensor for chlorogenic acid based on an ionic liquid containing iridium nanoparticles and polyphenol oxidase. Talanta 2009, 79, 222–228. [Google Scholar] [CrossRef]
- Mohammad, R.; Ahmad, M.; Heng, L.Y. An amperometric biosensor utilizing a ferrocene-mediated horseradish peroxidase reaction for the determination of capsaicin (chili hotness). Sensors 2013, 13, 10014–10026. [Google Scholar] [CrossRef]
- Mohammad, R.; Ahmad, M.; Heng, L.Y. Amperometric capsaicin biosensor based on covalent immobilization of horseradish peroxidase (HRP) on acrylic microspheres for chilli hotness determination. Sens. Actuat. B-Chem. 2017, 241, 174–181. [Google Scholar] [CrossRef]
- Cetó, X.; Céspedes, F.; del Valle, M. Assessment of individual polyphenol content in beer by means of a voltammetric bioelectronic tongue. Electroanalysis 2013, 25, 68–76. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; De Saja, J.A.; Rodriguez-Mendez, M.L. Bioelectronic tongue based on lipidic nanostructured layers containing phenol oxidases and lutetium bisphthalocyanine for the analysis of grapes. Biosens. Bioelectron. 2014, 57, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, M.; Li, B.; Yang, Z.; Zhou, Y.; Ai, S. A sensitive electrochemical biosensor for detection of protein kinase A activity and inhibitors based on Phos-tag and enzymatic signal amplification. Biosens. Bioelectron. 2015, 63, 26–32. [Google Scholar] [CrossRef]
- Qiao, L.; Jiao, L.; Pang, G.; Xie, J. A novel pungency biosensor prepared with fixing taste-bud tissue of rats. Biosens. Bioelectron. 2015, 68, 454–461. [Google Scholar] [CrossRef]
- Uliana, C.V.; de Oliveira, C.R.; Cominetti, M.R.; Faria, R.C. Label-free evaluation of small-molecule–protein interaction using magnetic capture and electrochemical detection. Anal. Bioanal. Chem. 2019, 411, 2111–2119. [Google Scholar] [CrossRef]
- Mohamadi, M.; Mostafavi, A.; Torkzadeh-Mahani, M. Voltammetric determination of rosmarinic acid on chitosan/carbon nanotube composite-modified carbon paste electrode covered with DNA. J. Electrochem. Soc. 2015, 162, B344–B349. [Google Scholar] [CrossRef]
- Barroso, M.F.; Delerue-Matos, C.; Oliveira, M.B.P.P. Electrochemical evaluation of total antioxidant capacity of beverages using a purine-biosensor. Food Chem. 2012, 132, 1055–1062. [Google Scholar] [CrossRef]
- Santhiago, M.; Peralta, R.A.; Neves, A.; Micke, G.A.; Vieira, I.C. Rosmarinic acid determination using biomimetic sensor based on purple acid phosphatase mimetic. Anal. Chim. Acta 2008, 613, 91–97. [Google Scholar] [CrossRef]
- De Carvalho, M.L.; Santhiago, M.; Peralta, R.A.; Neves, A.; Micke, G.A.; Vieira, I.C. Determination of chlorogenic acid in coffee using a biomimetic sensor based on a new tetranuclear copper (II) complex. Talanta 2008, 77, 394–399. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Kokulnathan, T.; Chen, S.-M.; Chen, T.-W.; Tseng, T.-W.; Liu, X.; Liao, W.C. A highly selective and sensitive detection of Ellagic acid by using ethylenediamine ligand based cobalt (II) complex modified glassy carbon electrode. Int. J. Electrochem. Sci. 2017, 12, 6829–6841. [Google Scholar] [CrossRef]
- Del Carlo, M.; Capoferri, D.; Gladich, I.; Guida, F.; Forzato, C.; Navarini, L.; Compagnone, D.; Laio, A.; Berti, F. In silico design of short peptides as sensing elements for phenolic compounds. ACS Sens. 2016, 1, 279–286. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Z.-F.; Tan, L.; Wang, S.-X.; Wang, C.-Z.; Zhang, J.-W.; Zhou, L.-D.; Zhang, Q.-H.; Yuan, C.-S. Rapid measurements of curcumin from complex samples coupled with magnetic biocompatibility molecularly imprinted polymer using electrochemical detection. J. Sep. Sci. 2020, 43, 1173–1182. [Google Scholar] [CrossRef]
- Santos, W.D.J.R.; Santhiago, M.; Yoshida, I.V.P.; Kubota, L.T. Novel electrochemical sensor for the selective recognition of chlorogenic acid. Anal. Chim. Acta 2011, 695, 44–50. [Google Scholar] [CrossRef]
- Leite, F.R.F.; Santos, W.D.J.R.; Kubota, L.T. Selective determination of caffeic acid in wines with electrochemical sensor based on molecularly imprinted siloxanes. Sensor Actuat B-Chem 2014, 193, 238–246. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Miguel, E.M.; Silva, J.D.S.; da Silva, C.B.; Goulart, M.O.; Kubota, L.T.; Gonzaga, F.B.; Santos, W.J.R.; Lima, P.R. Application of a nanostructured platform and imprinted sol-gel film for determination of chlorogenic acid in food samples. Talanta 2016, 156, 119–125. [Google Scholar] [CrossRef]
- Koirala, K.; Sevilla, F.B., III; Santos, J.H. Biomimetic potentiometric sensor for chlorogenic acid based on electrosynthesized polypyrrole. Sens. Actuat. B-Chem. 2016, 222, 391–396. [Google Scholar] [CrossRef]
- Ye, C.; Chen, X.; Xu, J.; Xi, H.; Wu, T.; Deng, D.; Zhang, J.; Huang, G. Highly sensitive detection to gallic acid by polypyrrole-based MIES supported by MOFs-Co2+@ Fe3O4. J. Electroanal. Chem. 2020, 859, 113839. [Google Scholar] [CrossRef]
- Liang, Y.; Qu, C.; Yang, R.; Qu, L.; Li, J. Molecularly imprinted electrochemical sensor for daidzein recognition and detection based on poly(sodium 4-styrenesulfonate)functionalized graphene. Sens. Actuat. B-Chem. 2017, 251, 542–550. [Google Scholar] [CrossRef]
- Yang, L.; Xu, B.; Ye, H.; Zhao, F.; Zeng, B. A novel quercetin electrochemical sensor based on molecularly imprinted poly(para-aminobenzoic acid) on 3D Pd nanoparticles-porous graphene-carbon nanotubes composite. Sens. Actuat. B-Chem. 2017, 251, 601–608. [Google Scholar] [CrossRef]
- Wei, M.; Geng, X.; Liu, Y.; Long, H.; Du, J. A novel electrochemical sensor based on electropolymerized molecularly imprinted polymer for determination of luteolin. J. Electroanal. Chem. 2019, 842, 184–192. [Google Scholar] [CrossRef]
- Shojaei, S.; Nasirizadeh, N.; Entezam, M.; Koosha, M.; Azimzadeh, M. An electrochemical nanosensor based on molecularly imprinted polymer (MIP) for detection of gallic acid in fruit juices. Food Anal. Methods 2016, 9, 2721–2731. [Google Scholar] [CrossRef]
- Tee-ngam, P.; Nunant, N.; Rattanarat, P.; Siangproh, W.; Chailapakul, O. Simple and rapid determination of ferulic acid levels in food and cosmetic samples using paper-based platforms. Sensors 2013, 13, 13039–13053. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, Z.; Tian, Z.; Sun, J.; Li, Y.; Fan, X. Validation of spectrophotometric determination of chlorogenic acid in fermentation broth and fruits. Food Chem. 2019, 278, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Shi, J.; Huang, F.; Zheng, M.; Deng, Q. Quantum dots-based label-free fluorescence sensor for sensitive and non-enzymatic detection of caffeic acid. Talanta 2015, 141, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, L.; Yuan, Y.; Pan, S.; Yang, J.; Yan, J.; Zhang, H.; Sun, Q.; Hu, X. A portable synthesis of water-soluble carbon dots for highly sensitive and selective detection of chlorogenic acid based on inner filter effect. Spectrochim. Acta A 2018, 189, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Mi, Z.; Guo, Z.; Wang, J.; Feng, F. A label-free fluorescent sensor based on carbon quantum dots with enhanced sensitive for the determination of myricetin in real samples. Microchem. J. 2020, 157, 104956. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, H.; He, L.; Pan, S.; Liu, H.; Hu, X. One-pot hydrothermal synthesis of nitrogen and sulfur co-doped carbon dots and their application for sensitive detection of curcumin and temperature. Microchem. J. 2019, 146, 300–308. [Google Scholar] [CrossRef]
- Wu, B.; Liu, X.; Shi, X.; Han, W.; Wang, C.; Jiang, L. Highly photoluminescent and temperature sensitive P, N, B-co-doped carbon quantum dots and their highly sensitive recognition for curcumin. RSC Adv. 2019, 9, 8340. [Google Scholar] [CrossRef]
- Kang, Y.Y.; Jung, H.; Yu, G.; Chong, Y.; Mok, H. Complexation of curcumin with 2-aminoethyl diphenyl borate and implications for spatiotemporal fluorescence monitoring. Int. J. Pharmaceut. 2016, 515, 669–676. [Google Scholar] [CrossRef]
- Ke, C.-B.; Lu, T.-L.; Chen, J.-L. Capacitively coupled plasma discharge of ionic liquid solutions to synthesize carbon dots as fluorescent sensors. Nanomaterials 2018, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-Q.; Yuan, K.; Qiao, W.-Z.; Shi, Y.; Dong, J.; Gao, H.-L.; Yang, X.-P.; Cui, J.-Z.; Zhao, B. Water Stable [Tb4] Cluster-based metal−organic framework as sensitive and recyclable luminescence sensor of quercetin. Anal. Chem. 2019, 91, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Pan, M.; Fang, G.; Wang, S. Carbon dots embedded metal-organic framework@molecularly imprinted nanoparticles for highly sensitive and selective detection of quercetin. Sens. Actuat. B-Chem. 2019, 286, 321–327. [Google Scholar] [CrossRef]
- Xu, X.; Qin, X.; Wang, L.; Wang, X.; Lu, J.; Qiu, X.; Zhu, Y. Lanthanide terbium complex: Synthesis, electrochemiluminescence (ECL) performance, and sensing application. Analyst 2019, 144, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Su, Y.; Huang, F.-Q.; Zuo, Q.; Yang, L.; Li, J.; Zhao, L.; Qi, L.-W. A simple and rapid fluorescent approach for flavonoids sensor based on gold nanoclusters. J. Colloid Interface Sci. 2019, 539, 175–183. [Google Scholar] [CrossRef]
- Bahrani, S.; Ghaedi, M.; Mansoorkhani, M.J.K.; Ostovan, A. A highly selective nanocomposite based on MIP for curcumin trace levels quantification in food samples and human plasma following optimization by central composite design. J. Chrom. B 2017, 1040, 129–135. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, L.; Gao, X.; Wang, Y.; Lu, H.; Tang, Y.; Li, J. Magnetic molecularly imprinted polymers for spectrophotometric quantification of curcumin in food. Food Chem. 2016, 202, 309–315. [Google Scholar] [CrossRef]
- Sedghi, R.; Yassari, M.; Heidari, B. Thermo-responsive molecularly imprinted polymer containing magnetic nanoparticles: Synthesis, characterization and adsorption properties for curcumin. Colloids Interface B 2018, 162, 154–162. [Google Scholar] [CrossRef]
- Xu, X.; Xu, G.; Wei, F.; Cen, Y.; Shi, M.; Cheng, X.; Chai, Y.; Sohail, M.; Hu, Q. Carbon dots coated with molecularly imprinted polymers: A facile bioprobe for fluorescent determination of caffeic acid. J. Colloid. Interface Sci. 2018, 529, 568–574. [Google Scholar] [CrossRef]
- Wang, Z.; Long, R.; Peng, M.; Li, T.; Shi, S. Molecularly imprinted polymers-coated CdTe quantum dots for highly sensitive and selective fluorescent determination of ferulic acid. J. Anal. Methods Chem. 2019, 2019, 1505878. [Google Scholar] [CrossRef]
- Long, R.; Li, T.; Tong, C.; Wu, L.; Shi, S. Molecularly imprinted polymers coated CdTe quantum dots with controllable particle size for fluorescent determination of p-coumaric acid. Talanta 2019, 196, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Liu, H. A Novel fluorescence and SPE adsorption nanomaterials of molecularly imprinted polymers based on quantum dot-grafted covalent organic frameworks for the high selectivity and sensitivity detection of ferulic acid. Nanomaterials 2019, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Stavro Santarosa, A.; Berti, F.; Tommasini, M.; Calabretti, A.; Forzato, C. Signal-on fluorescent imprinted nanoparticles for sensing of phenols in aqueous olive leaves extracts. Nanomaterials 2020, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Park, S.Y. Liquid crystal-Based DNA biosensor for myricetin detection. Sens. Actuat. B-Chem. 2016, 233, 559–565. [Google Scholar] [CrossRef]
- Jha, R.K.; Kern, T.L.; Fox, D.T.; Strauss, C.E.M. Engineering an Acinetobacter regulon for biosensing and high-throughput enzyme screening in E. coli via flow cytometry. Nucleic Acids Res. 2014, 42, 8150–8160. [Google Scholar] [CrossRef]
- Siedler, S.; Khatri, N.K.; Zsohar, A.; Kjærbølling, I.; Vogt, M.; Hammar, P.; Nielsen, C.F.; Marienhagen, J.; Sommer, M.O.A.; Joensson, H.N. Development of a bacterial biosensor for rapid screening of yeast p-coumaric acid production. ACS Synth. Biol. 2017, 6, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Gültekin, A.; Karanfil, G.; Kuş, M.; Sönmezoğlu, S.; Say, R. Preparation of MIP-based QCM nanosensor for detection of caffeic acid. Talanta 2014, 119, 533–537. [Google Scholar] [CrossRef] [PubMed]
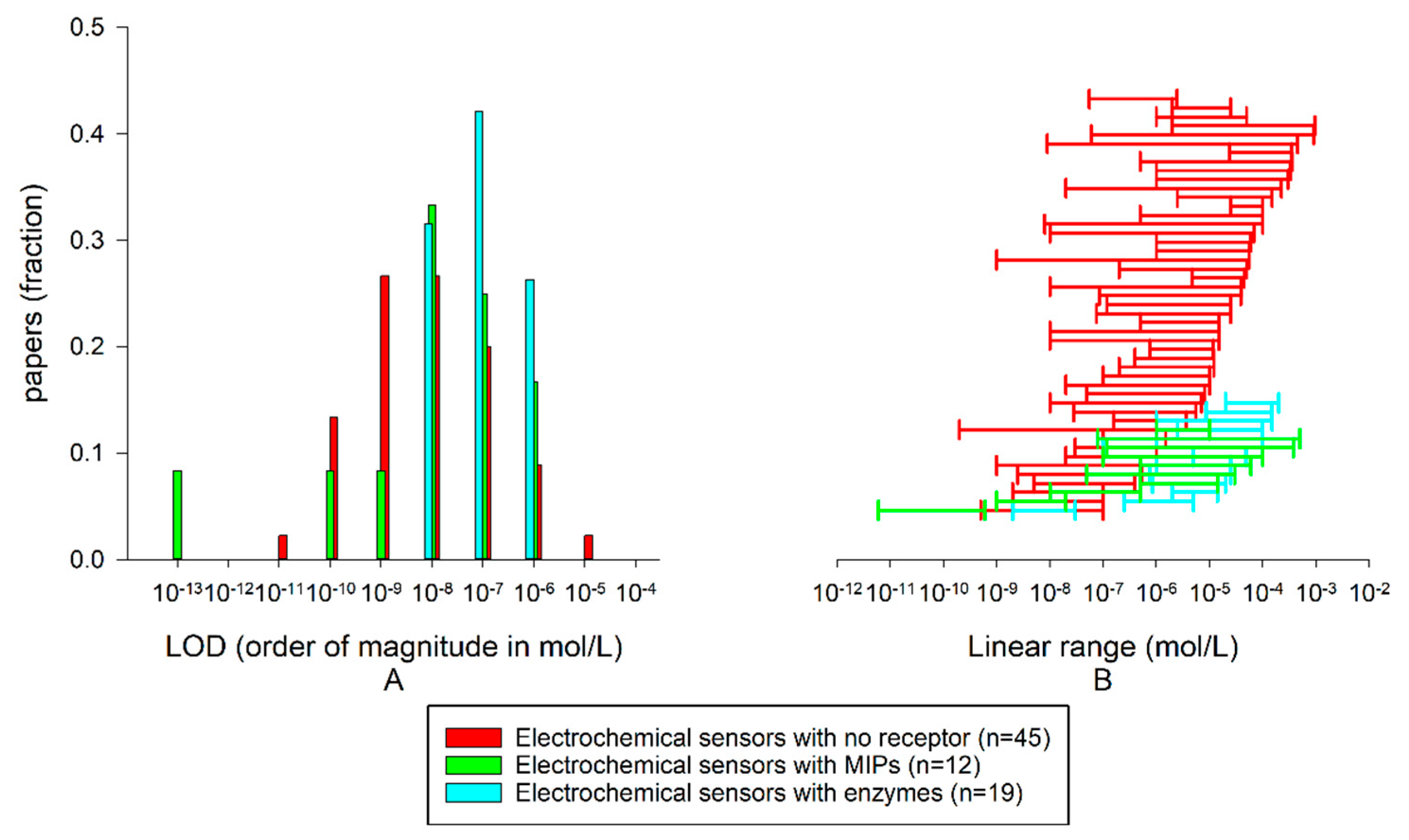
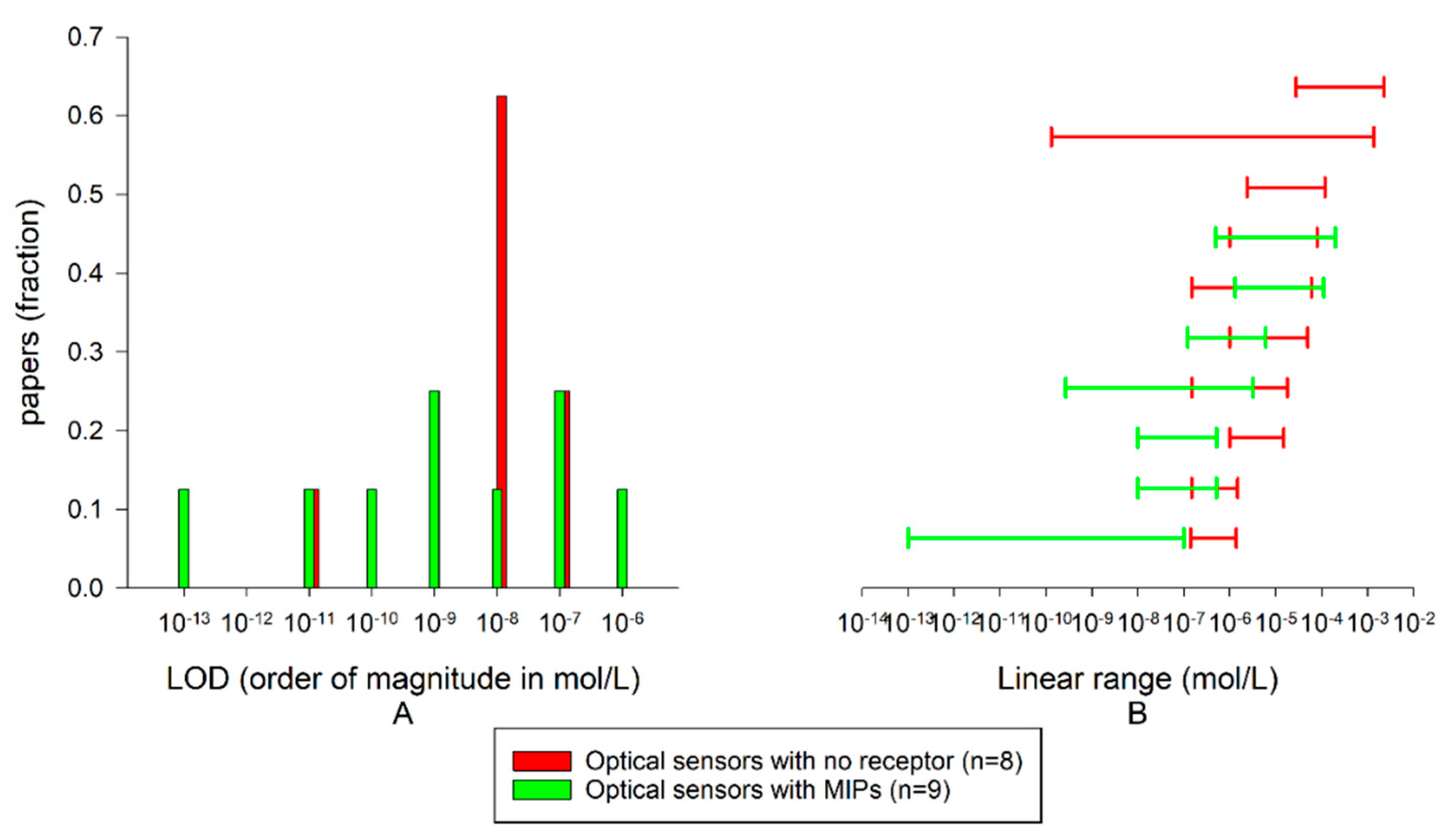
| EL. Sensors without Recognition Elements | EL. Sensors with Enzymes | EL. Sensors with MIPs | Opt. Sensors without Recognition Elements | Opt. Sensors with MIPs | |
|---|---|---|---|---|---|
| Median LOD | 16 nM n = 45 | 500 nM n = 19 | 32 nM n = 12 | 63 nM n = 8 | 23 nM n = 9 |
| Median log(Cmax-Cmin) | 2.31 | 1.37 | 2.43 | 1.80 | 1.92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forzato, C.; Vida, V.; Berti, F. Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors 2020, 10, 105. https://doi.org/10.3390/bios10090105
Forzato C, Vida V, Berti F. Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors. 2020; 10(9):105. https://doi.org/10.3390/bios10090105
Chicago/Turabian StyleForzato, Cristina, Veronica Vida, and Federico Berti. 2020. "Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review" Biosensors 10, no. 9: 105. https://doi.org/10.3390/bios10090105
APA StyleForzato, C., Vida, V., & Berti, F. (2020). Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors, 10(9), 105. https://doi.org/10.3390/bios10090105







