Investigation of Photoplethysmography Behind the Ear for Pulse Oximetry in Hypoxic Conditions with a Novel Device (SPYDR)
Abstract
1. Introduction
2. Materials and Methods
2.1. Arterial Blood Gas Analysis
2.2. Reduced Oxygen Breathing Device (ROBD) Testing
2.3. Statistical Analysis
3. Results
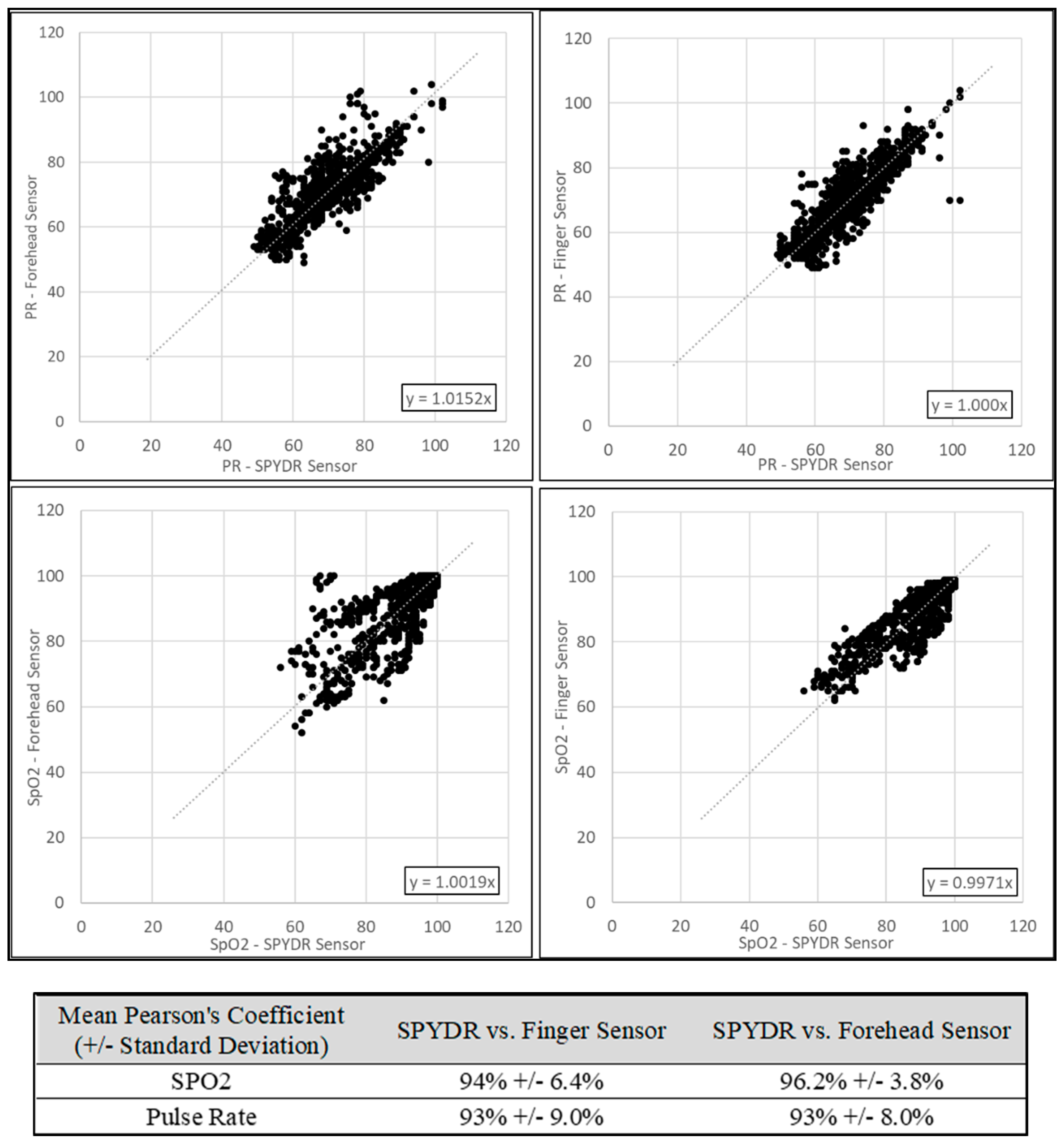
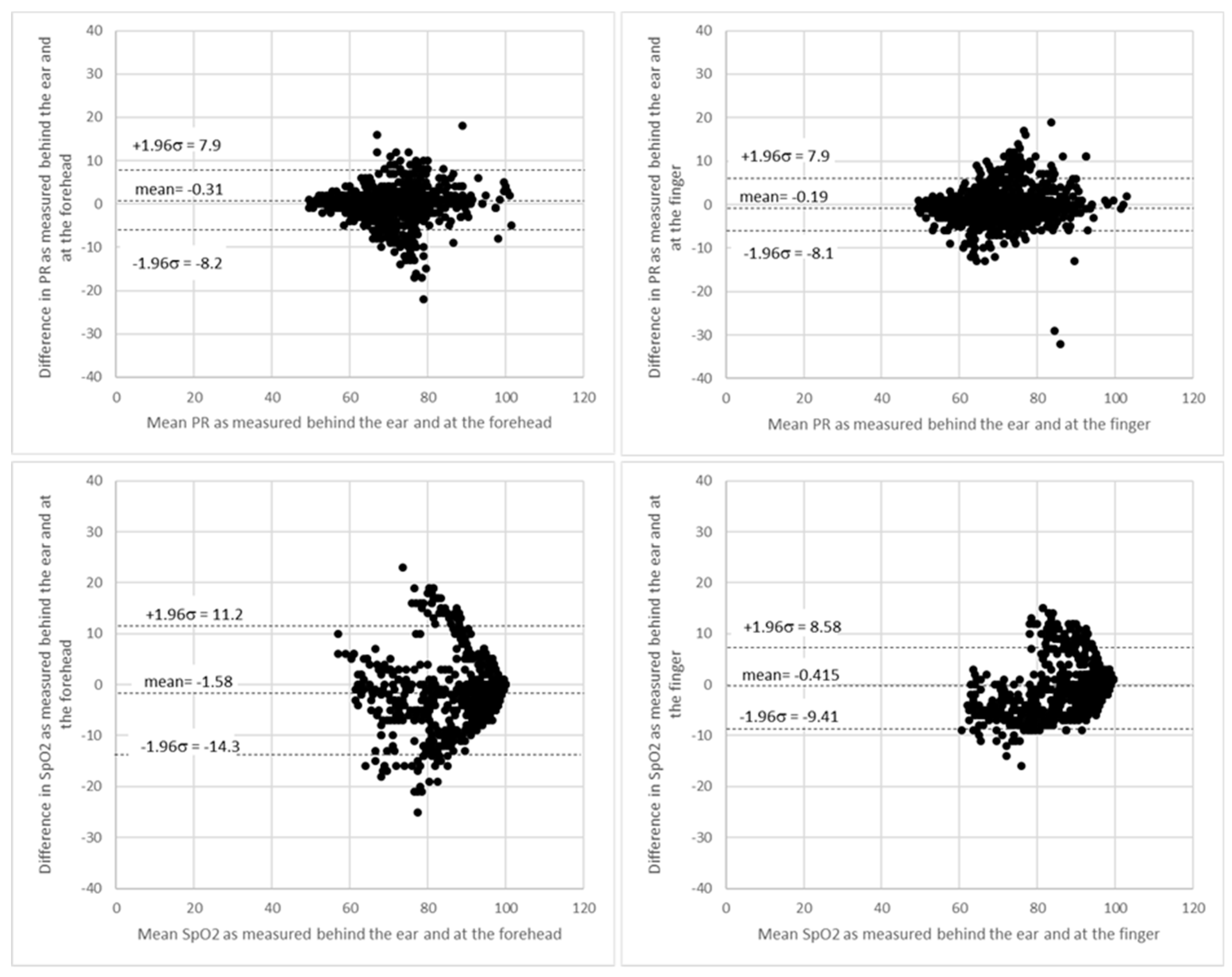
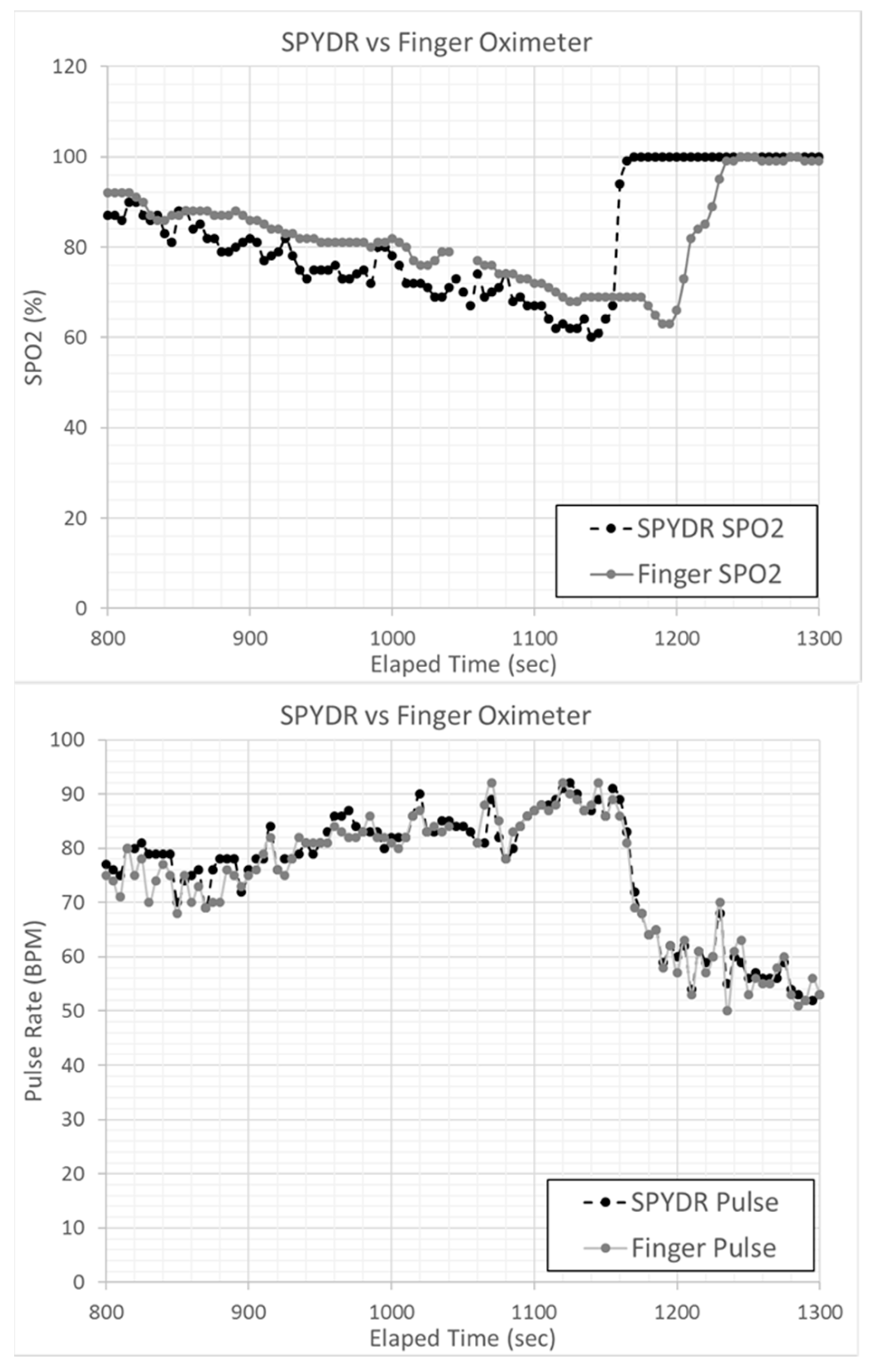
3.1. Arterial Blood Gas Analysis Results
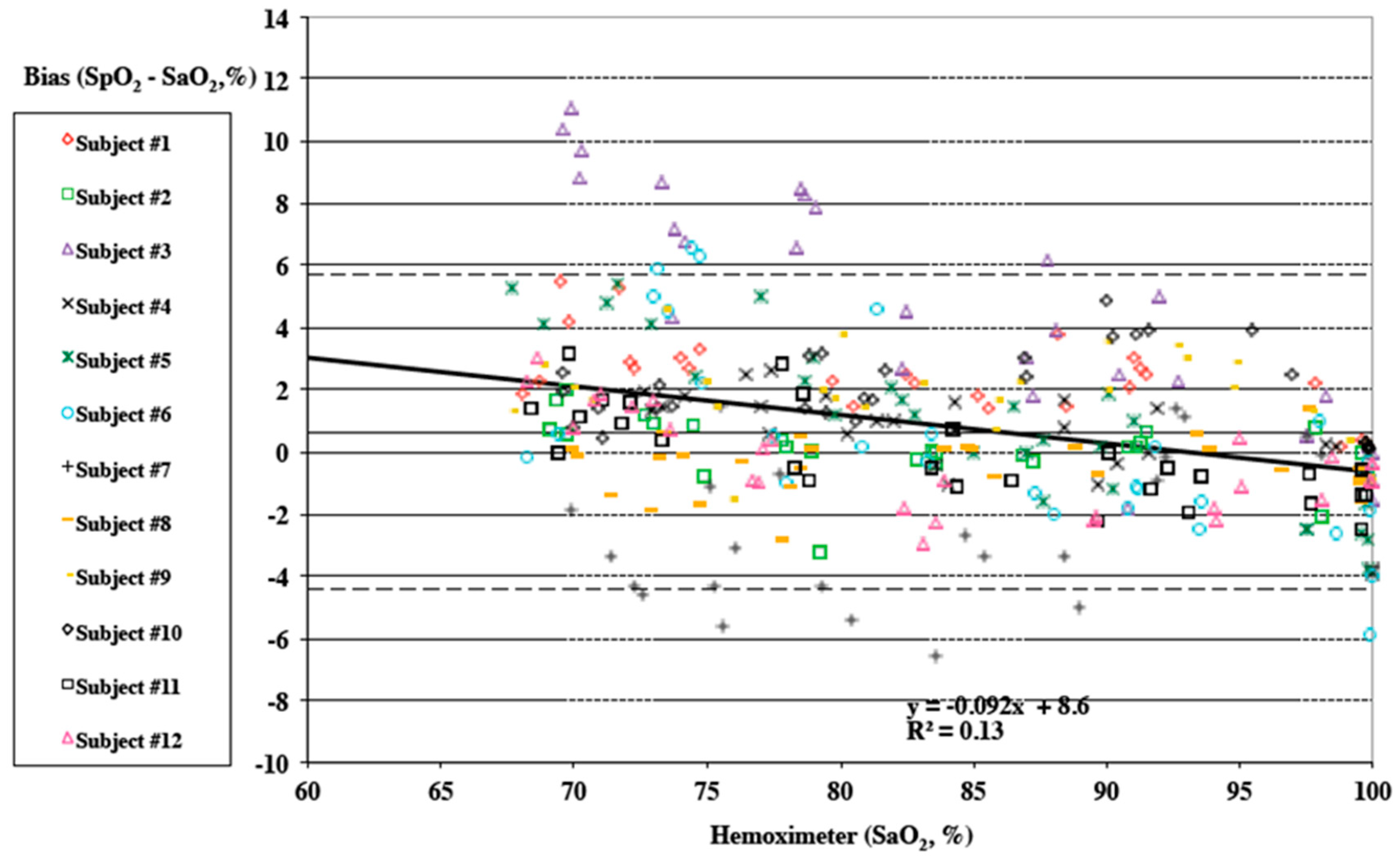
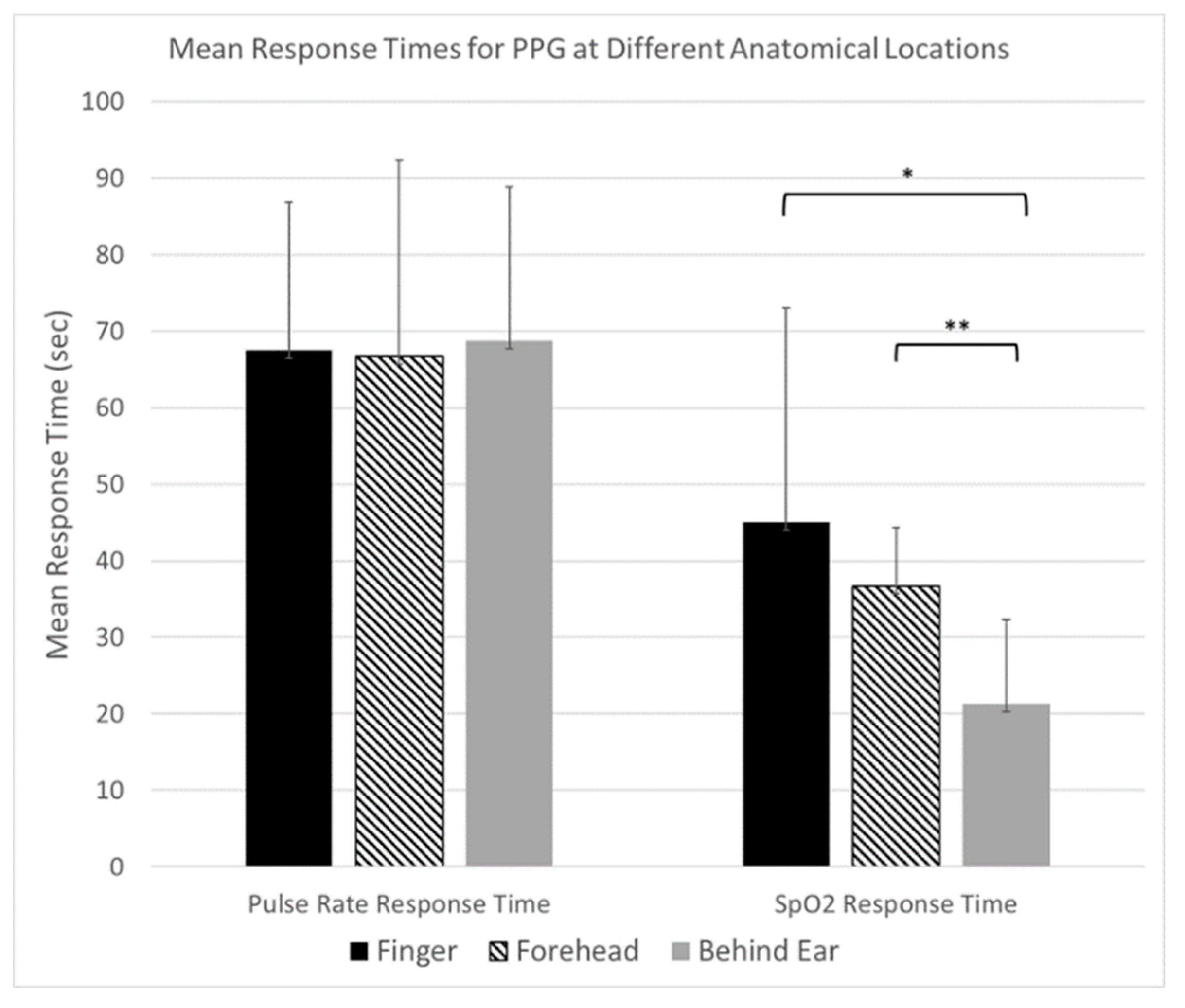
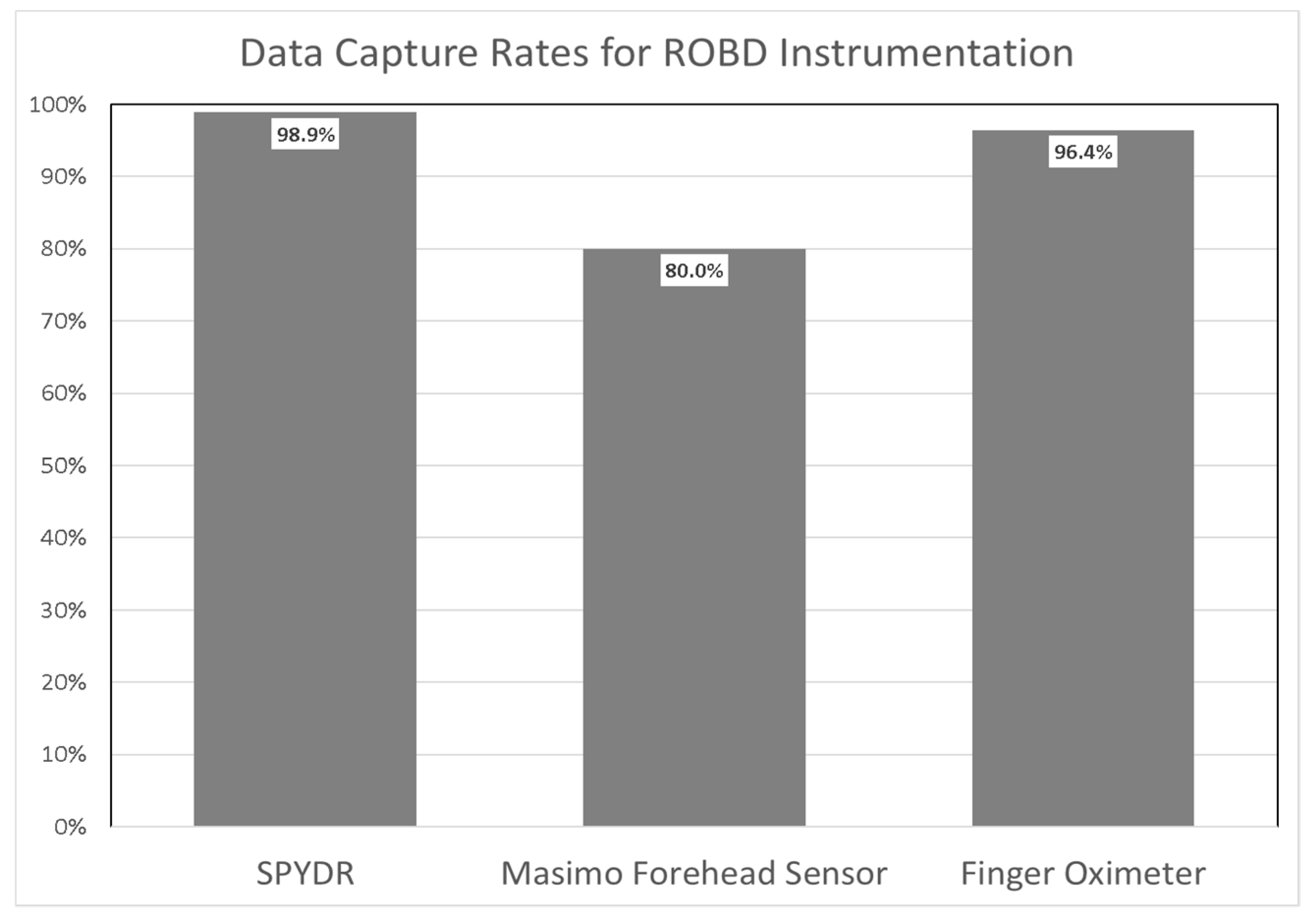
3.2. ROBD Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Penzel, T.; Schöbel, C.; Fietze, I. New technology to assess sleep apnea: Wearables, smartphones, and accessories. F1000Research 2018, 7, 413. [Google Scholar] [CrossRef]
- Jubran, A. Pulse oximetry. Crit. Care 2015, 19, 272. [Google Scholar] [CrossRef]
- Chan, E.D.; Chan, M.M.; Chan, M.M. Pulse oximetry: Understanding its basic principles facilitates appreciation of its limitations. Respir. Med. 2013, 107, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Ghamari, M.; Castaneda, D.; Esparza, A.; Soltanpur, C.; Nazeran, H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Bentham, M.; Stansby, G.; Allen, J. Innovative Multi-Site Photoplethysmography Analysis for Quantifying Pulse Amplitude and Timing Variability Characteristics in Peripheral Arterial Disease. Dis. 2018, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T. Current progress of photoplethysmography and SPO2 for health monitoring. Biomed. Eng. Lett. 2019, 9, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef]
- Mancini, D.M.; Bolinger, L.; Li, H.; Kendrick, K.; Chance, B.; Wilson, J.R. Validation of near-infrared spectroscopy in humans. J. Appl. Physiol. 1994, 77, 2740–2747. [Google Scholar] [CrossRef]
- Kamal, A.; Harness, J.; Irving, G.; Mearns, A. Skin photoplethysmography—A review. Comput. Methods Programs Biomed. 1989, 28, 257–269. [Google Scholar] [CrossRef]
- Mendelson, Y.; Ochs, B.D. Noninvasive pulse oximetry utilizing skin reflectance photoplethysmography. IEEE Trans. Biomed. Eng. 1988, 35, 798–805. [Google Scholar] [CrossRef]
- Hernando, A.; Peláez-Coca, M.D.; Lozano, M.T.; Lazaro, J.; Gil, E. Finger and forehead PPG signal comparison for respiratory rate estimation. Physiol. Meas. 2019, 40, 095007. [Google Scholar] [CrossRef] [PubMed]
- Longmore, S.; Lui, G.; Naik, G.R.; Breen, P.; Jalaludin, B.B.; Gargiulo, G.D. A Comparison of Reflective Photoplethysmography for Detection of Heart Rate, Blood Oxygen Saturation, and Respiration Rate at Various Anatomical Locations. Sensors 2019, 19, 1874. [Google Scholar] [CrossRef] [PubMed]
- Sahni, R. Noninvasive Monitoring by Photoplethysmography. Clin. Perinatol. 2012, 39, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Cooper, R.; Hosanee, M.; Welykholowa, K.; Kyriacou, P.A.; Zheng, D.; Allen, J.; Abbott, D.; Lovell, N.H.; Fletcher, R.; et al. Multi-Site Photoplethysmography Technology for Blood Pressure Assessment: Challenges and Recommendations. J. Clin. Med. 2019, 8, 1827. [Google Scholar] [CrossRef] [PubMed]
- Bickler, P.; Feiner, J.R.; Severinghaus, J.W. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiol. 2005, 102, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Shafqat, K.; Langford, R.M.; Pal, S.K.; Kyriacou, P.A. Estimation of Venous oxygenation saturation using the finger Photoplethysmograph (PPG) waveform. In 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2012; Volume 2012, pp. 2905–2908. [Google Scholar] [CrossRef]
- Spigulis, J.; Gailite, L.; Lihachev, A.; Erts, R. Simultaneous recording of skin blood pulsations at different vascular depths by multiwavelength photoplethysmography. Appl. Opt. 2007, 46, 1754–1759. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Rocha, C.; Ferreira, H.A.; Silva, H. Different lasers reveal different skin microcirculatory flowmotion—Data from the wavelet transform analysis of human hindlimb perfusion. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Kvernebo, K.; Megerman, J.; Hamilton, G.; Abbott, W.M. Response of skin photoplethysmography, laser Doppler flowmetry and transcutaneous oxygen tensiometry to stenosis-induced reductions in limb blood flow. Eur. J. Vasc. Surg. 1989, 3, 113–120. [Google Scholar] [CrossRef]
- Nilsson, L.; Goscinski, T.; Kalman, S.; Johansson, A.; Lindberg, L.-G. Combined photoplethysmographic monitoring of respiration rate and pulse: A comparison between different measurement sites in spontaneously breathing subjects. Acta Anaesthesiol. Scand. 2007, 51, 1250–1257. [Google Scholar] [CrossRef]
- Budidha, K.; Kyriacou, P.A. Investigation of photoplethysmography and arterial blood oxygen saturation from the ear-canal and the finger under conditions of artificially induced hypothermia. In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2015; Volume 2015, pp. 7954–7957. [Google Scholar] [CrossRef]
- Budidha, K.; Kyriacou, P.A. In vivo investigation of ear canal pulse oximetry during hypothermia. J. Clin. Monit. 2017, 32, 97–107. [Google Scholar] [CrossRef]
- Ross, E.M.; Matteucci, M.J.; Shepherd, M.; Barker, M.; Orr, L. Measuring Arterial Oxygenation in a High Altitude Field Environment: Comparing Portable Pulse Oximetry With Blood Gas Analysis. Wilderness Environ. Med. 2013, 24, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Venema, B.; Blanik, N.; Blazek, V.; Gehring, H.; Opp, A.; Leonhardt, S. Advances in Reflective Oxygen Saturation Monitoring With a Novel In-Ear Sensor System: Results of a Human Hypoxia Study. IEEE Trans. Biomed. Eng. 2012, 59, 2003–2010. [Google Scholar] [CrossRef]
- Venema, B.; Schiefer, J.; Blazek, V.; Blanik, N.; Leonhardt, S. Evaluating Innovative In-Ear Pulse Oximetry for Unobtrusive Cardiovascular and Pulmonary Monitoring During Sleep. IEEE J. Transl. Eng. Heal. Med. 2013, 1, 2700208. [Google Scholar] [CrossRef] [PubMed]
- Pertzov, B.; Brachfeld, E.; Unterman, A.; Gershman, E.; Abdel-Rahman, N.; Rosengarten, O.; Kramer, M.R. Significant Delay in the Detection of Desaturation between Finger Transmittance and Earlobe Reflectance Oximetry Probes during Fiberoptic Bronchoscopy: Analysis of 104 Cases. Lung 2018, 197, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Hülsbusch, M.; Starke, D.; Leonhardt, S. In-Ear Heart Rate Monitoring Using a Micro-Optic Reflective Sensor. In 2006 International Conference of the IEEE Engineering in Medicine and Biology Society; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2007; Volume 2007, pp. 1375–1378. [Google Scholar] [CrossRef]
- Passler, S.; Müller, N.; Senner, V. In-Ear Pulse Rate Measurement: A Valid Alternative to Heart Rate Derived from Electrocardiography? Sensors 2019, 19, 3641. [Google Scholar] [CrossRef]
- Yuan, H.; Poeggel, S.; Newe, T.; Lewis, E.; Viphavakit, C.; Leen, G. An Experimental Study of the Effects of External Physiological Parameters on the Photoplethysmography Signals in the Context of Local Blood Pressure (Hydrostatic Pressure Changes). Sensors 2017, 17, 556. [Google Scholar] [CrossRef] [PubMed]
- Elgendi, M. On the analysis of fingertip photoplethysmogram signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef]
- Branson, R.; Mannheimer, P. Forehead oximetry in critically ill patients: The case for a new monitoring site. Respir. Care Clin. 2004, 10, 359–367. [Google Scholar] [CrossRef]
- Grubb, M.; Carpenter, J.; Crowe, J.; Teoh, J.; Marlow, N.; Ward, C.; Mann, C.; Sharkey, D.; Hayes-Gill, B.R. Forehead reflectance photoplethysmography to monitor heart rate: Preliminary results from neonatal patients. Physiol. Meas. 2014, 35, 881–893. [Google Scholar] [CrossRef]
- Sun, S.; Peeters, W.H.; Bezemer, R.; Long, X.; Paulussen, I.; Aarts, R.M.; Noordergraaf, G.J. Finger and forehead photoplethysmography-derived pulse-pressure variation and the benefits of baseline correction. J. Clin. Monit. 2018, 33, 65–75. [Google Scholar] [CrossRef]
- Majeed, I.A.; Jos, S.; Arora, R.; Choi, K.; Bae, S. Motion Artifact Removal of Photoplethysmogram (PPG) Signal. In 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2019; pp. 5576–5580. [Google Scholar] [CrossRef]
- Tautan, A.-M.; Young, A.; Wentink, E.; Wieringa, F. Characterization and reduction of motion artifacts in photoplethysmographic signals from a wrist-worn device. In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2015; Volume 2015, pp. 6146–6149. [Google Scholar] [CrossRef]
- Da He, D.; Winokur, E.S.; Heldt, T.; Sodini, C.G. The ear as a location for wearable vital signs monitoring. In 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2010; Volume 2010, pp. 6389–6392. [Google Scholar] [CrossRef]
- Sausen, K.P.; A Bower, E.; E Stiney, M.; Feigl, C.; Wartman, R.; Clark, J.B. A closed-loop reduced oxygen breathing device for inducing hypoxia in humans. Aviat. Space, Environ. Med. 2003, 74, 1190–1197. [Google Scholar]
- Bland, J.M.; Altman, U.G. Agreement Between Methods of Measurement with Multiple Observations Per Individual. J. Biopharm. Stat. 2007, 17, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Abay, T.Y.; Kyriacou, P.A. Reflectance Photoplethysmography as Noninvasive Monitoring of Tissue Blood Perfusion. IEEE Trans. Biomed. Eng. 2015, 62, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Wijshoff, R.W.C.G.R.; Mischi, M.; Veen, J.; Van Der Lee, A.M.; Aarts, R.M. Reducing motion artifacts in photoplethysmograms by using relative sensor motion: Phantom study. J. Biomed. Opt. 2012, 17, 117007. [Google Scholar] [CrossRef] [PubMed]
- Bickler, P.E.; Feiner, J.R.; Lipnick, M.S.; Batchelder, P.; MacLeod, D.B.; Severinghaus, J.W. Effects of Acute, Profound Hypoxia on Healthy Humans. Anesthesia Analg. 2017, 124, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Budidha, K.; Kyriacou, P.A. The human ear canal: Investigation of its suitability for monitoring photoplethysmographs and arterial oxygen saturation. Physiol. Meas. 2014, 35, 111–128. [Google Scholar] [CrossRef] [PubMed]
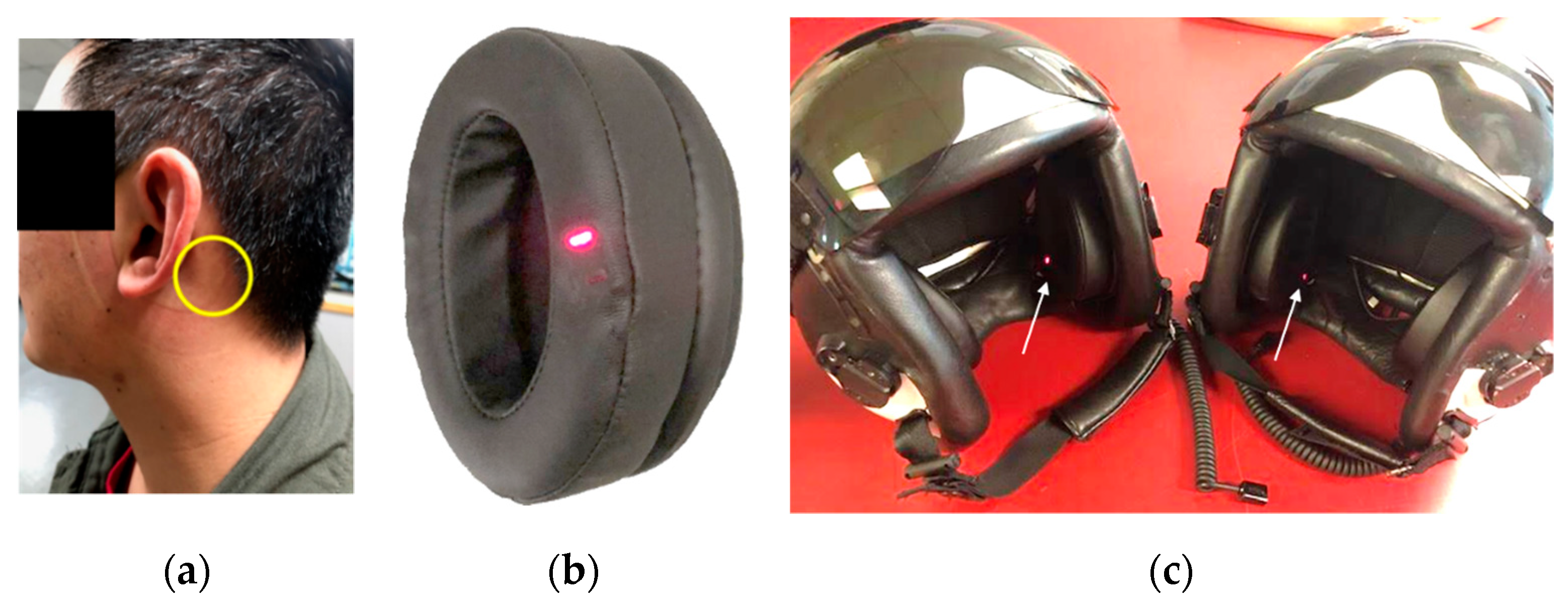

| Test #1 | Test #2 | Test #3 |
|---|---|---|
| 8000 MSL | 8000 MSL | 8000 MSL |
| 10,000 MSL | 10,000 MSL | 10,000 MSL |
| 14,000 MSL | 14,000 MSL | 14,000 MSL |
| 17,500 MSL | 17,500 MSL | 17,500 MSL |
| Sea Level | 14,000 MSL | 21,000 MSL |
| 10,000 MSL | 25,000 MSL | |
| 8000 MSL | 100% Oxygen | |
| Sea Level |
| SPYDR | Finger Sensor A | Finger Sensor B | |
|---|---|---|---|
| Mean | 0.61 | 0.66 | 0.61 |
| Count | 314 | 308 | 308 |
| Missing Data | 8 | 14 | 14 |
| Standard Deviation | 2.54 | 1.6 | 1.28 |
| Standard Error | 0.14 | 0.09 | 0.07 |
| 95% Confidence Interval | 0.28 | 0.18 | 0.14 |
| Limits of Agreement | −4.44 to 5.67 | −2.52 to 3.83 | −1.93 to 3.14 |
| Maximum | 9.7 | 5.8 | 4.5 |
| Minimum | −6.6 | −4.7 | −4.2 |
| Root Mean Square | 2.61 | 1.72 | 1.41 |
| SPYDR Bias | ||||
|---|---|---|---|---|
| Hemoximeter Range | 70–80% | 80–90% | 90–100% | 70–100% |
| Mean | 1.64 | 0.37 | −0.12 | 0.61 |
| Count | 109 | 79 | 126 | 314 |
| Missing Data | 0 | 1 | 7 | 8 |
| Standard Deviation | 3.02 | 2.25 | 1.9 | 2.54 |
| Standard Error | 0.29 | 0.25 | 0.17 | 0.14 |
| 95% Confidence Interval | 0.57 | 0.5 | 0.34 | 0.28 |
| Limits of Agreement | −4.47 to 7.74 | −4.16 to 4.90 | −3.91 to 3.67 | −4.44 to 5.67 |
| Maximum | 9.7 | 6.2 | 5 | 9.7 |
| Minimum | −5.6 | −6.6 | −5.9 | −6.6 |
| Root Mean Square | 3.42 | 2.26 | 1.9 | 2.61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradke, B.; Everman, B. Investigation of Photoplethysmography Behind the Ear for Pulse Oximetry in Hypoxic Conditions with a Novel Device (SPYDR). Biosensors 2020, 10, 34. https://doi.org/10.3390/bios10040034
Bradke B, Everman B. Investigation of Photoplethysmography Behind the Ear for Pulse Oximetry in Hypoxic Conditions with a Novel Device (SPYDR). Biosensors. 2020; 10(4):34. https://doi.org/10.3390/bios10040034
Chicago/Turabian StyleBradke, Brian, and Bradford Everman. 2020. "Investigation of Photoplethysmography Behind the Ear for Pulse Oximetry in Hypoxic Conditions with a Novel Device (SPYDR)" Biosensors 10, no. 4: 34. https://doi.org/10.3390/bios10040034
APA StyleBradke, B., & Everman, B. (2020). Investigation of Photoplethysmography Behind the Ear for Pulse Oximetry in Hypoxic Conditions with a Novel Device (SPYDR). Biosensors, 10(4), 34. https://doi.org/10.3390/bios10040034





