Electrochemical Sensing of Serotonin by a Modified MnO2-Graphene Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of Graphene Oxide (GO) and MnO2-Graphene Composite
2.3. MnO2-Graphene Composite Modified Electrode
2.4. Instruments and Measurements
3. Results
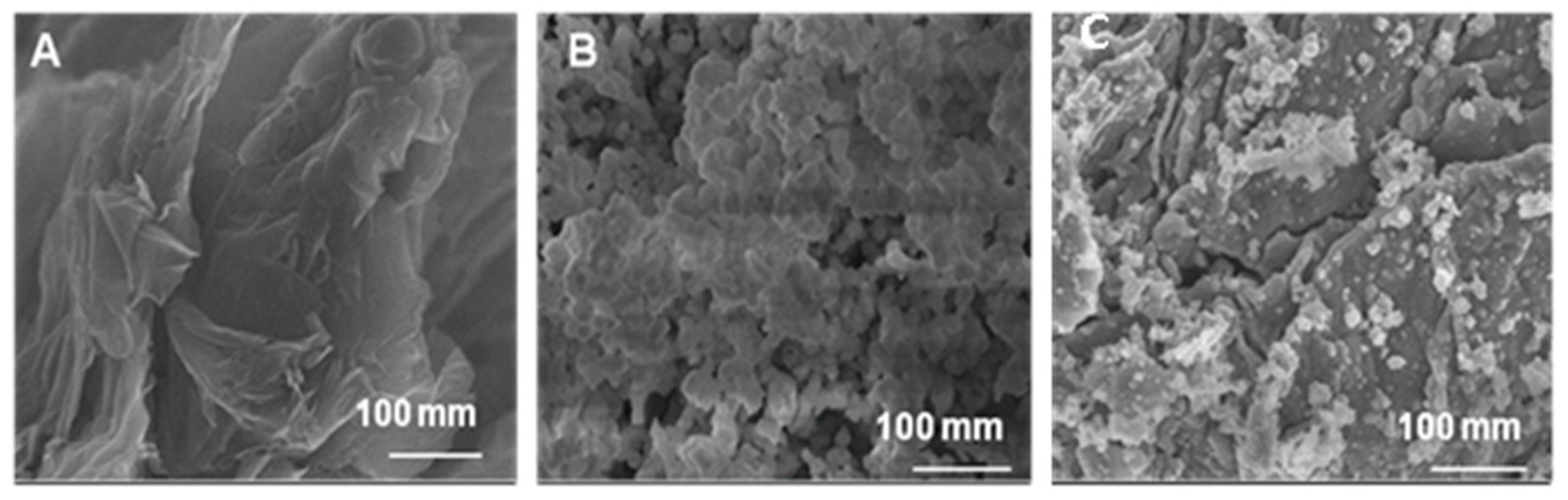
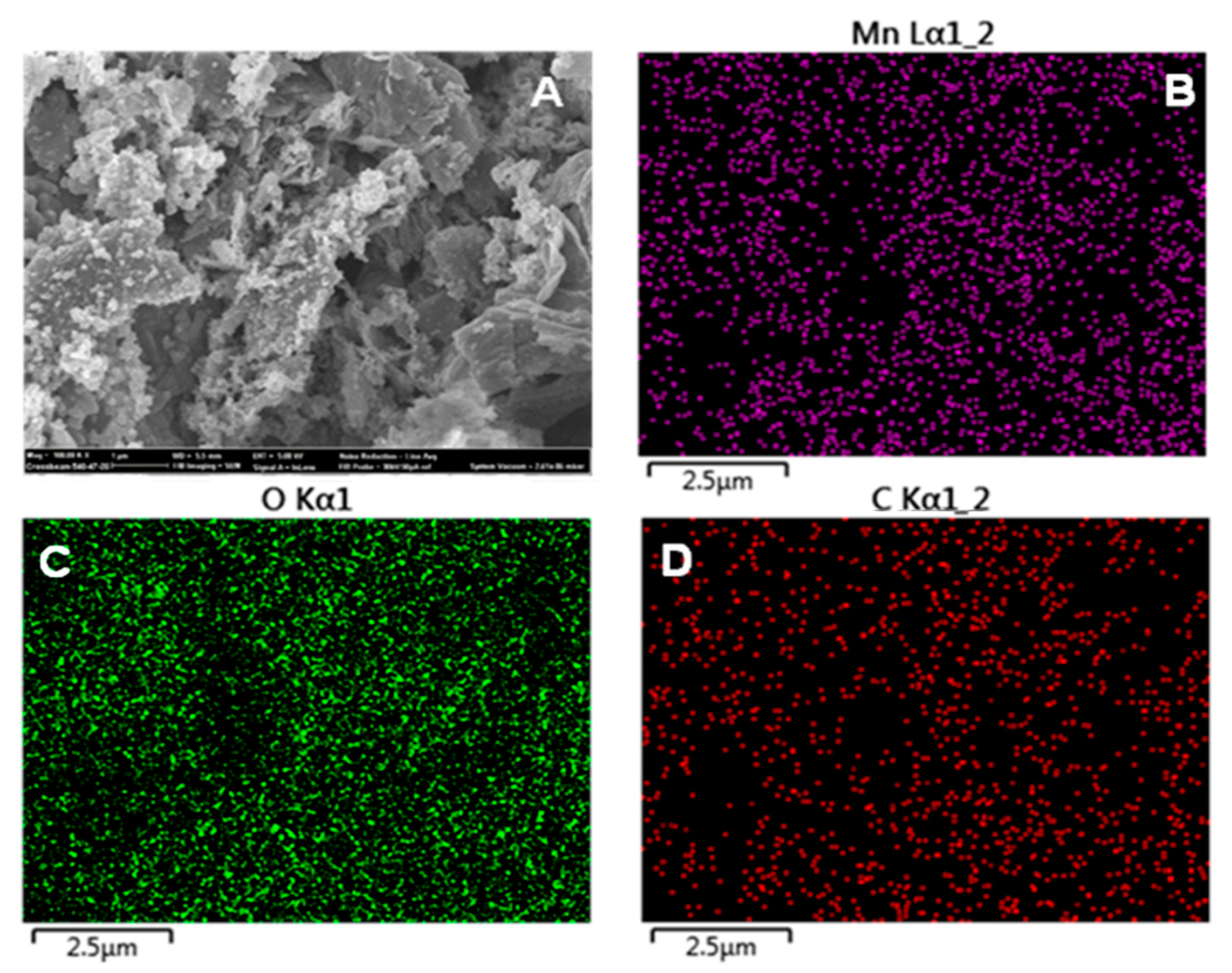
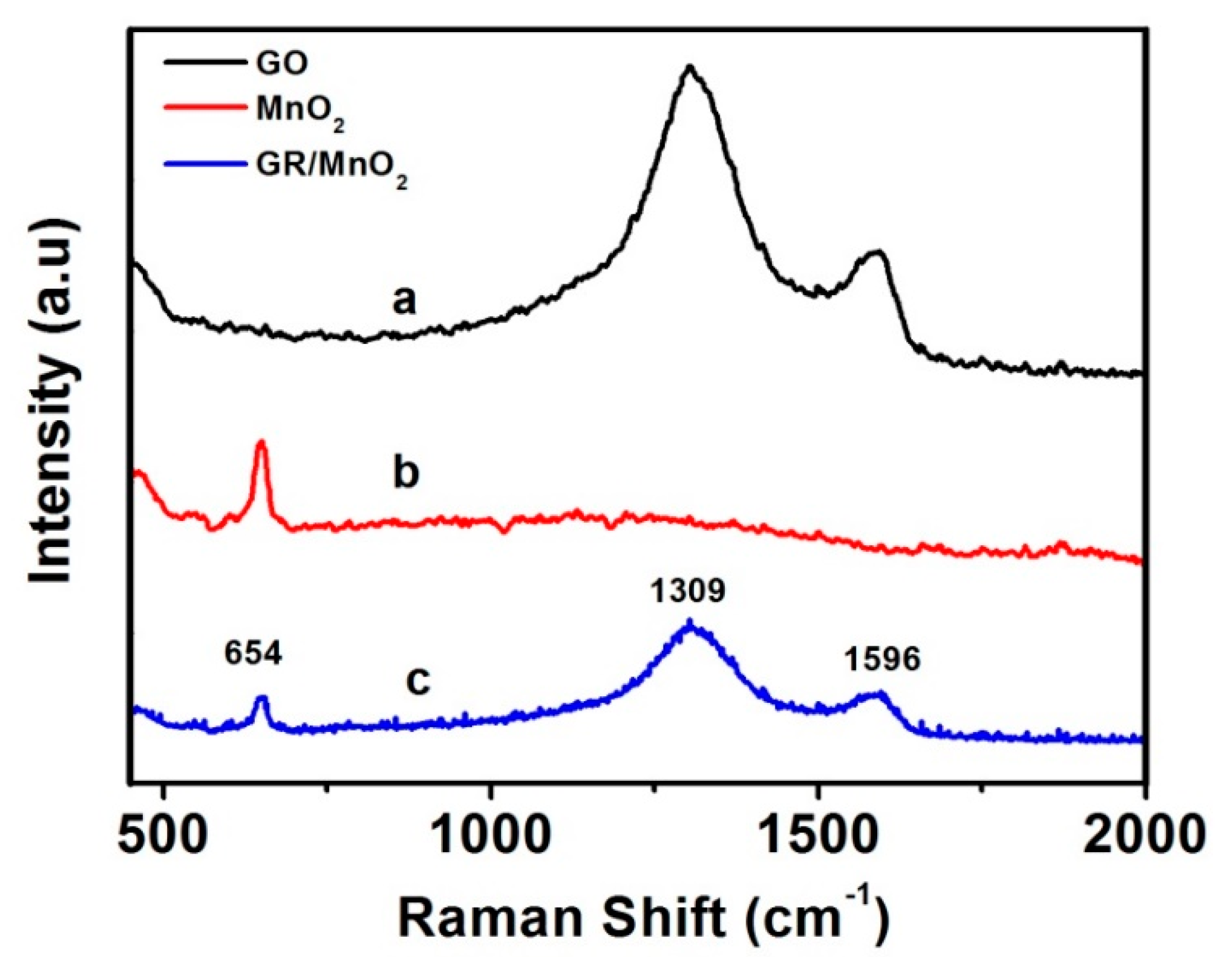
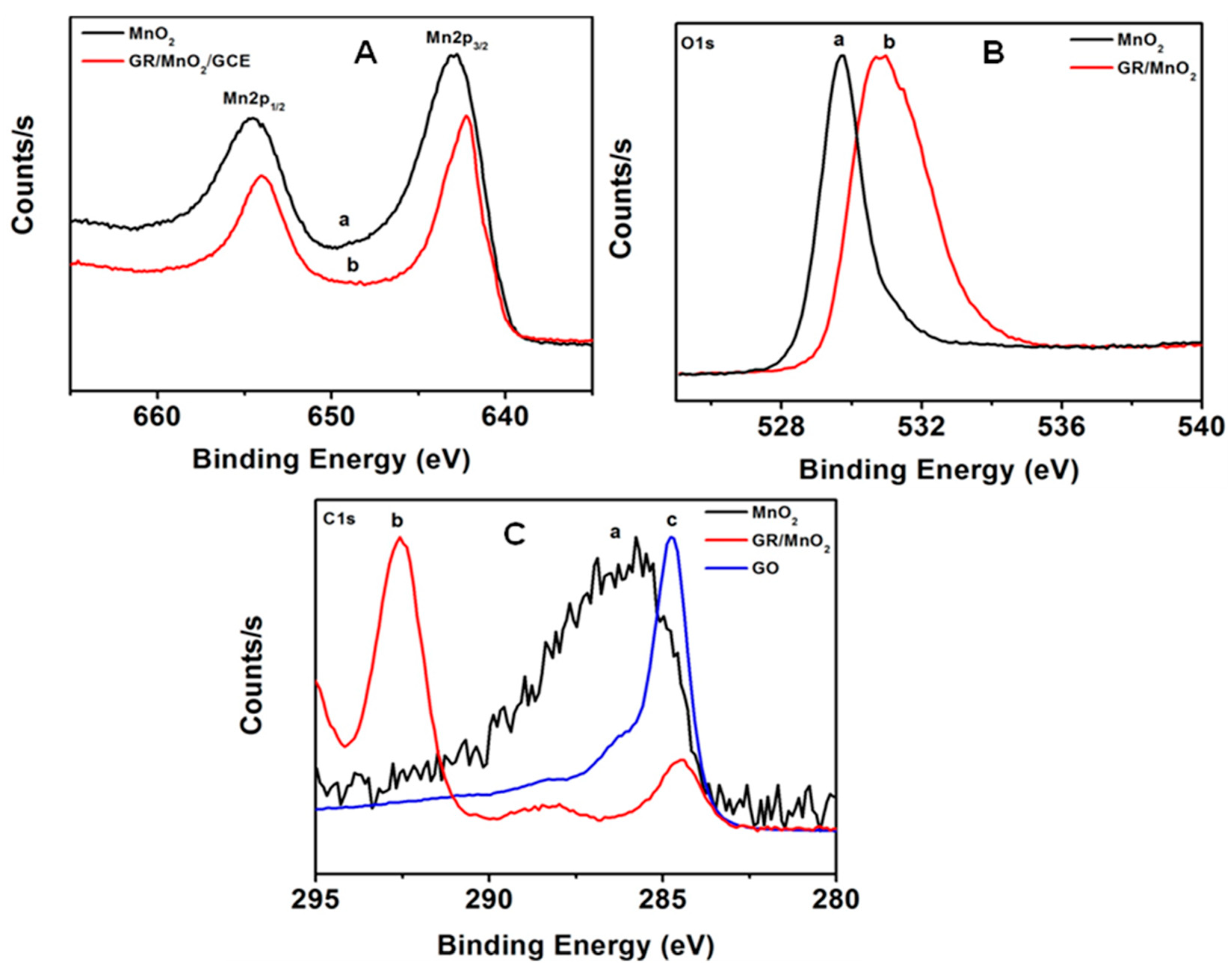
3.1. Morphological, Raman and XPS Studies
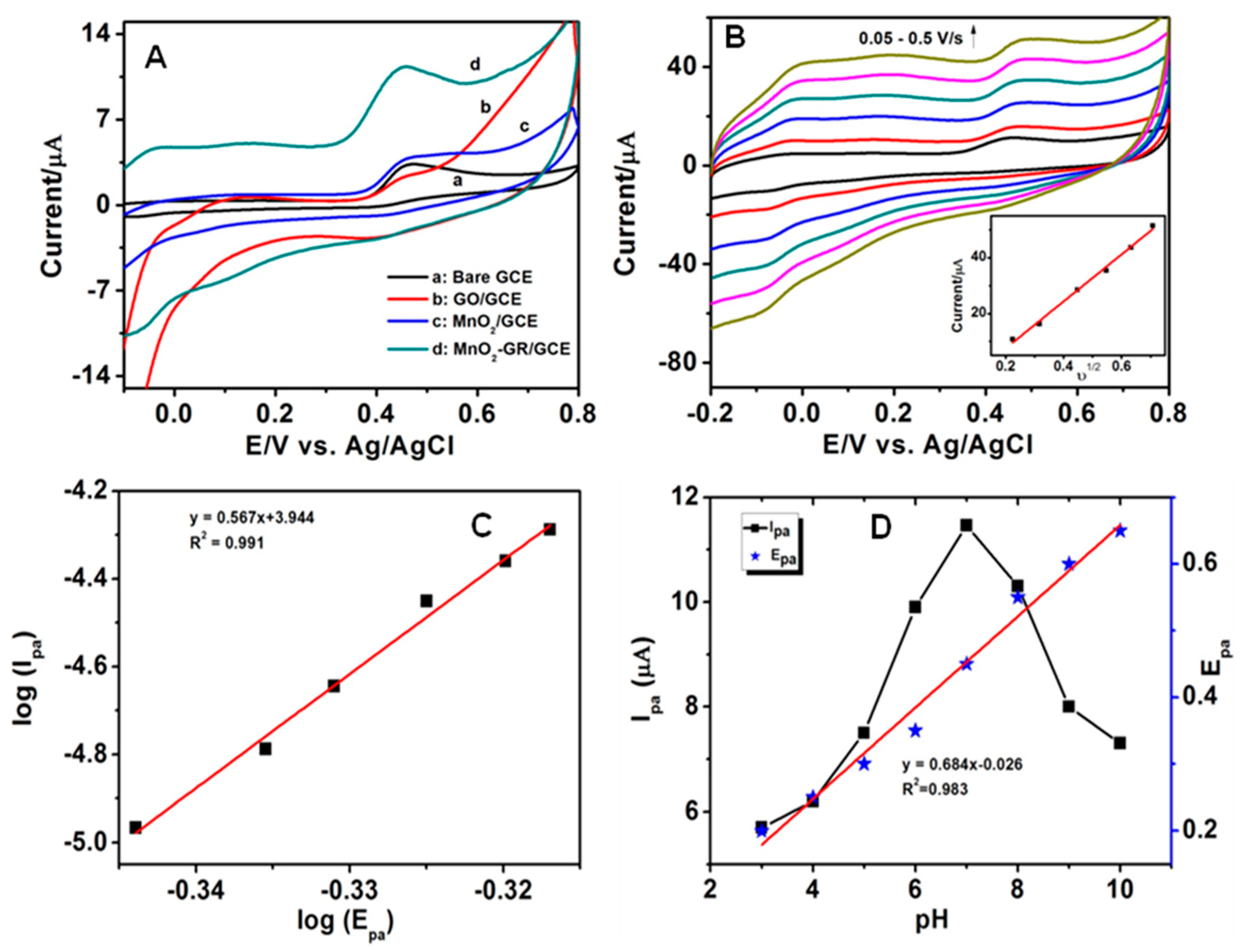
3.2. Electrochemical Behaviour of SE at the MnO2-GR Modified GCE
3.3. Effect of Scan Rate and Mechanisms for Electrode Reaction of SE
3.4. Effect of pH Value
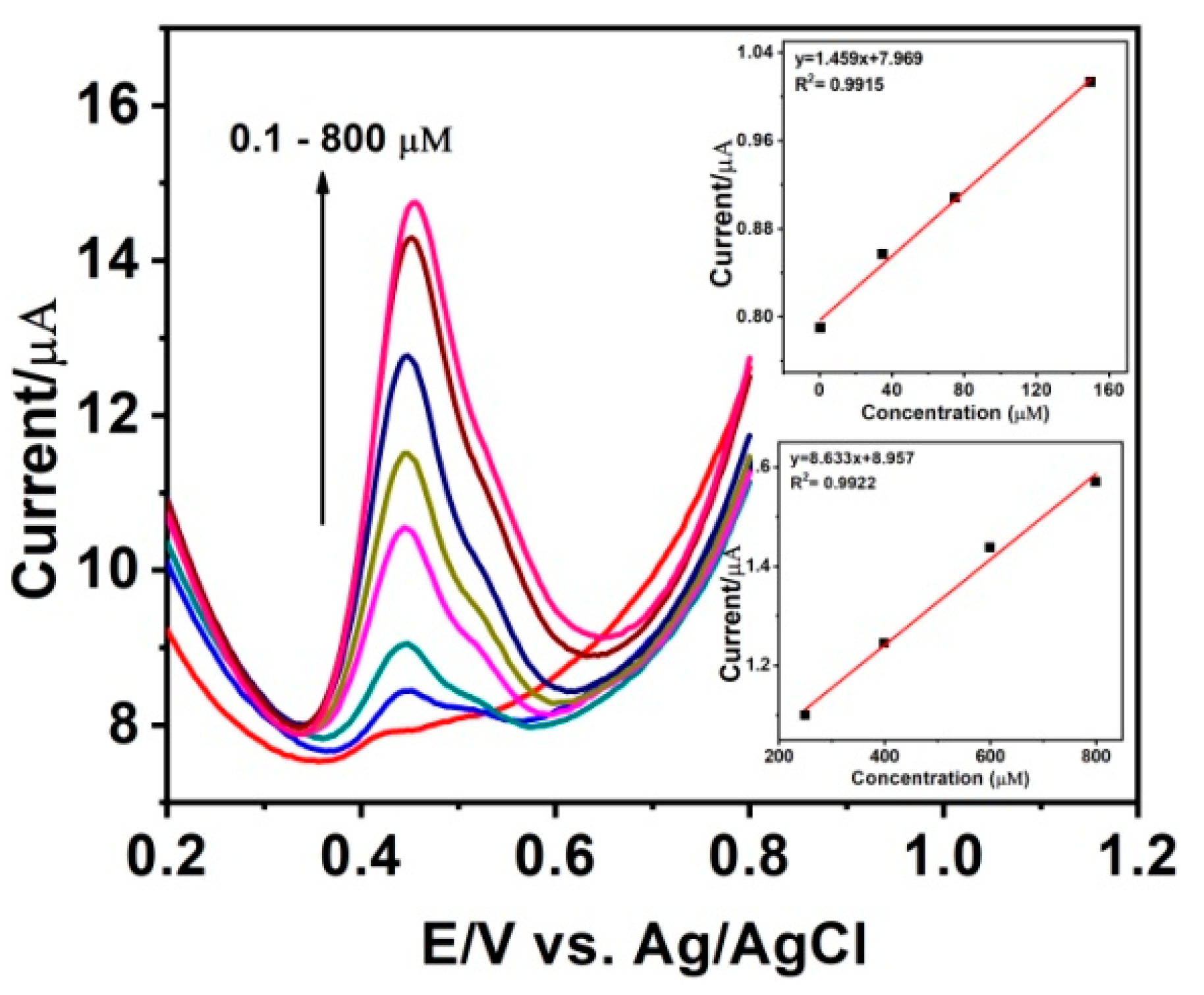
3.5. Electrochemical Determination of SE at the MnO2-GR Modified Electrode
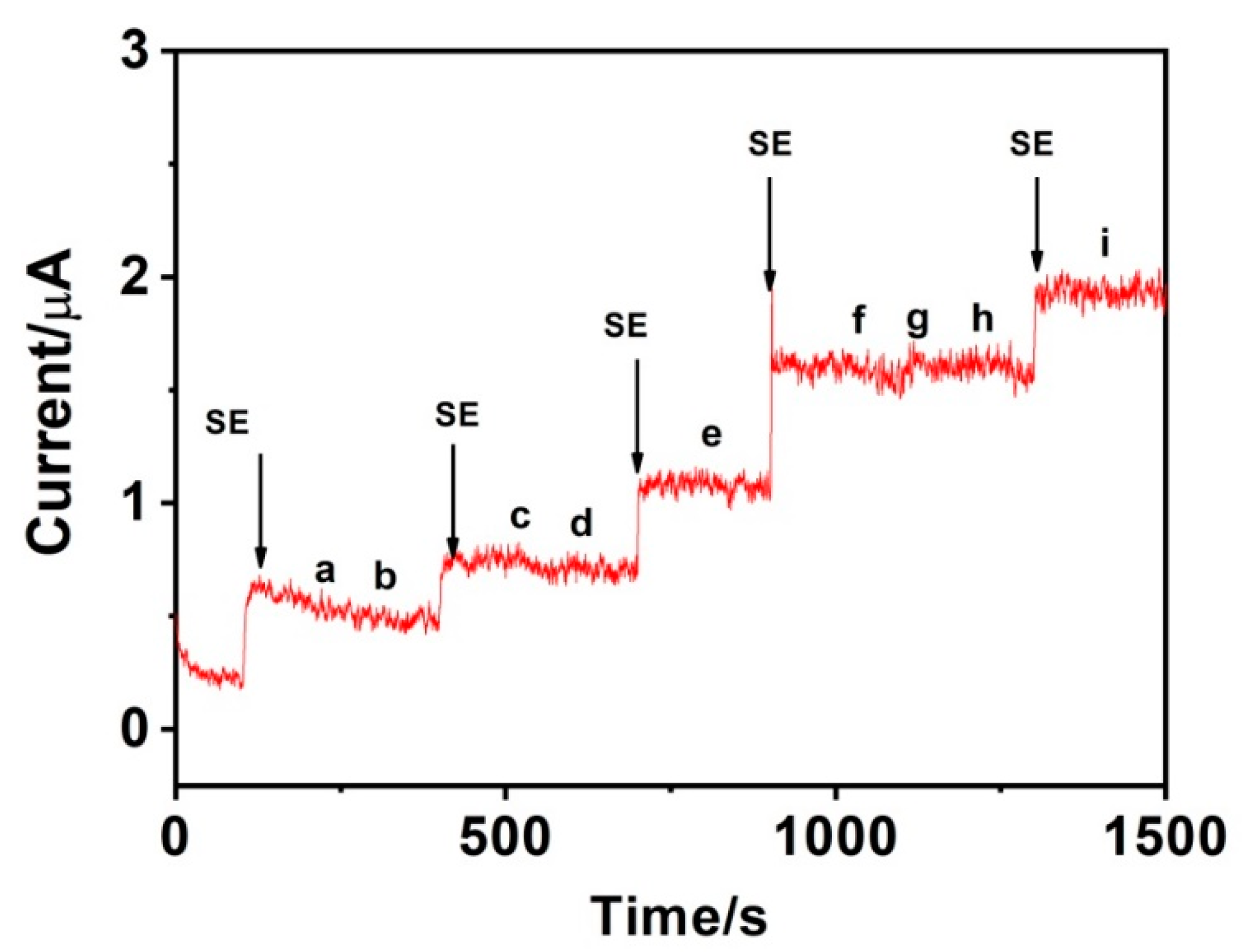
3.6. Selectivity, Stability and Reproducibility of the MnO2-GR/GCE
3.7. Real sample Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Tsay, H.J.; Su, W.H.; Chueh, F.Y. Changes in extracellular serotonin in rat hypothalamus affect thermoregulatory function. Am. J. Phys. 1998, 274, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef]
- Bin, H.-H.; Keattch, O.; Yeoman, M.; Covill, D.; Patel, B. Three-dimensional-printed electrochemical sensor for simultaneous dual monitoring of serotonin overflow and circular muscle contraction. Anal. Chem. 2018, 18, 12014–12020. [Google Scholar]
- Samaranayake, S.; Abdalla, A.; Robke, R.; Nijhout, H.F.; Reed, M.-C.; Best, F.; Hashem, P. voltammetric and mathematical analysis of histaminergic modulation of serotonin in the mouse hypothalamus. J. Neurochem. 2016, 138, 374–383. [Google Scholar] [CrossRef]
- Mumtaz, M.; Narasimhachari, N.; Friedel, R.O.; Pandey, G.N.; Davis, J.M. Evaluation of fluorometric assay methods for serotonin in platelets. Plasma and whole blood samples by comparison with GC–MS-SIM technique. Res. Commun. Chem. Pathol. Pharm. 1982, 36, 45–60. [Google Scholar]
- Umeda, S.; Stagliano, G.W.; Borenstein, M.R.; Raffa, R.B. A reverse-phase HPLC and fluorescence detection method for measurement of 5-hydroxytryptamine (serotonin) in planaria. J. Pharm. Toxicol. Methods 2005, 51, 73–76. [Google Scholar] [CrossRef]
- Peterson, Z.D.; Lee, M.L.; Graves, S.W. Determination of serotonin and its precursors in human plasma by capillary electrophoresis-electrospray ionization-time-of-flight mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 810, 101–110. [Google Scholar] [CrossRef]
- Chauveau, J.; Fert, V.; Morel, A.M.; Delagee, M.A. Rapid and specific enzyme immunoassay of serotonin. Clin. Chem. 1991, 37, 1178–1184. [Google Scholar] [CrossRef]
- De Irazu, S.; Unceta, N.; Carmen Sampedro, M.; Aranzazu Goicolea, M.; Barrio, R.J. Multimembrane carbon fiber microelectrodes for amperometric determination of serotonin in human urine. Analyst 2001, 126, 495–500. [Google Scholar] [CrossRef]
- Manciu, F.S.; Manciu, M.; Ciubuc, J.D.; Sundin, E.M.; Ochoa, K.; Eastman, M.; Durrer, W.G.; Guerrero, J.; Lopez, B.; Subedi, M.; et al. Simultaneous detection of dopamine and serotonin—A comparative experimental and theoretical study of neurotransmitter interactions. Biosensors 2019, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Mahato, K.; Purohit, B.; Bhardwaj, K.; Jaiswal, A.; Chandra, P. Novel electrochemical biosensor for serotonin detection based on gold nanoparticles decorated reduced graphene oxide in biological fluids and in vitro model. Biosens. Bioelectron. 2019, 142, 111502. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-S.; Lee, K.-L.; You, J.-M.; Jeong, H.; Jeon, S. Electrochemical biosensor for simultaneous determination of dopamine and serotonin based on electrochemically reduced GO porphyrin, Sens. Actuators 2014, 190, 886. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.S.; Ebenso, E.E. Electrochemical determination of serotonin in urine samples based on metal oxide nanoparticles/MWCNT on modified glassy carbon electrode. Sens. Bio Sens. Res. 2017, 13, 17–27. [Google Scholar] [CrossRef]
- Anithaa, A.C.; Asokan, K.; Sekar, C. Highly sensitive and selective serotonin sensor based on gamma ray irradiated tungsten trioxide nanoparticles. Sens. Actuators B Chem. 2017, 238, 667–675. [Google Scholar] [CrossRef]
- Ran, G.; Chen, X. Xia, Electrochemical detection of serotonin based on a poly(bromocresol green) film and Fe3O4 nanoparticles in a chitosan matrix. RSC Adv. 2017, 7, 184. [Google Scholar] [CrossRef]
- Xue, C.; Wang, X.; Zhu, W.; Han, Q.; Zhu, C.; Hong, J.; Zhou, X.; Jiang, H. Electrochemical serotonin sensing interface based on double-layered membrane of reduced graphene oxide/polyaniline nanocomposites and molecularly imprinted polymers embedded with gold nanoparticles. Sens. Actuators B Chem. 2014, 196, 57–63. [Google Scholar] [CrossRef]
- Thanh, T.-D.; Balamurugan, J.; Hien, H.-V.; Kim, N.-H.; Lee, J.-H. A Novel sensitive sensor for serotonin based on high-quality of Au-Ag nanoalloy encapsulated graphene electrocatalyst. Biosen. Bioelectron. 2017, 96, 186–193. [Google Scholar] [CrossRef]
- Kumara Swamy, B.-E.; Venton, B.-J. Carbon nanotube-modified microelectrodes for simultaneous detection of dopamine and serotonin in vivo. Analyst 2007, 132, 876–884. [Google Scholar] [CrossRef]
- Shen, M.; Zhu, S.J.; Liu, X.; Fu, X.; Huo, W.C.; Liu, X.L.; Chen, Y.X.; Shan, Q.Y.; Yao, H.-C.; Zhang, Y.X. Phase and morphology controlled polymorphic MnO2 nanostructures for electrochemical energy storage. Cryst. Eng. Comm. 2019, 21, 5322–5331. [Google Scholar] [CrossRef]
- Qu, Q.T.; Zhang, P.; Wang, B.; Chen, Y.H.; Tian, S.; Wu, Y.P.; Holze, R. Electrochemical performance of MnO2 nanorods in neutral aqueous electrolytes as a cathode for asymmetric supercapacitors. J. Phys. Chem. C 2009, 113, 14020. [Google Scholar] [CrossRef]
- Sudhan, N.; Lavanya, N.; Leonardi, S.G.; Neri, G.; Sekar, C. Monitoring of chemical risk factors for Sudden Infant Death Syndrome (SIDS) by hydroxyapatite-graphene-MWCNT composite-based sensors. Sensors 2019, 19, 3437. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Zhang, Z.; Zhen, M.; Wang, F. MnO2 nanotubes with graphene assistance as low cost counter electrode materials in dye sensitized solar cells. RSC Adv. 2016, 13, 10938. [Google Scholar] [CrossRef]
- Lavanya, N.; Fazio, E.; Neri, F.; Bonavita, A.; Leonardi, S.G.; Neri, G.; Sekar, C. Simultaneous electrochemical determination of epinephrine and uric acid in the presence of ascorbic acid using SnO2/graphene nanocomposite modified glassy carbon electrode. Sens. Actuators B Chem. 2015, 221, 1412–1422. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Wei, T.; Qian, W.; Zhang, M.; Wei, F. Fast and reversible surface redox reaction of graphene–MnO2 composites as supercapacitor electrodes. Carbon 2010, 48, 3825–3833. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.; Jaroniec, M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Feng, X.; Yan, Z.; Chen, N.; Zhang, Y.; Ma, Y.; Liu, X.; Fan, Q.; Wang, L.; Huang, W. The synthesis of shape-controlled MnO2/graphene composites via a facile one-step hydrothermal method and their application in supercapacitors. J. Mater. Chem. A 2013, 1, 1281. [Google Scholar] [CrossRef]
- Lavanya, N.; Sekar, C. SnO2-SnS2 nanocomposite as electrocatalyst for simultaneous determination of depression biomarkers serotonin and tryptophan. J. Electroana. Chem. 2019, 840, 1–9. [Google Scholar]
- Jin, G.; Lin, X.; Gong, J. Novel choline and acetylcholine modified glassy carbon electrodes for simultaneous determination of dopamine, serotonin and ascorbic acid. J. Electroanal. Chem. 2004, 569, 135–142. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kim, D.; Jeon, S. Electrochemical determination of serotonin on glassy carbon electrode modified with various graphene nanoparticles. Sens. Actuators 2012, 174, 285. [Google Scholar] [CrossRef]


| Electrode | Method | Linear Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|
| a WO3/GCE | DPV | 0.01–600 | 0.001 | [15] |
| b Fe2O3-CHI/GCE | DPV | 0.1–100 | 0.08 | [16] |
| c GR-PANI/GCE | DPV | 0.2–10 | 0.011 | [17] |
| d Au-Ag/GCE | DPV | 0.002–4.8 | 0.0016 | [18] |
| e SnO2-SnS2/GCE | SWV | 0.1–700 | 0.045 | [28] |
| g Acetylcholine/GCE | DPV | 0.8–200 | 0.8 | [29] |
| f GR-NS/GCE | DPV | 1-100 | 0.038 | [30] |
| h MnO2-GR/GCE | SWV | 0.1–800 | 0.01 | This work |
| Sample | SE Concentration (μM) | Recovery (n = 3) (%) | |
|---|---|---|---|
| Added | Found | ||
| 1 | 1 | 0.9 | 101.3 |
| 2 | 5 | 5.2 | 97.9 |
| 3 | 10 | 10.3 | 98.3 |
| 4 | 15 | 14.6 | 102.6 |
| 5 | 20 | 20.3 | 101.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nehru, L.; Chinnathambi, S.; Fazio, E.; Neri, F.; Leonardi, S.G.; Bonavita, A.; Neri, G. Electrochemical Sensing of Serotonin by a Modified MnO2-Graphene Electrode. Biosensors 2020, 10, 33. https://doi.org/10.3390/bios10040033
Nehru L, Chinnathambi S, Fazio E, Neri F, Leonardi SG, Bonavita A, Neri G. Electrochemical Sensing of Serotonin by a Modified MnO2-Graphene Electrode. Biosensors. 2020; 10(4):33. https://doi.org/10.3390/bios10040033
Chicago/Turabian StyleNehru, Lavanya, Sekar Chinnathambi, Enza Fazio, Fortunato Neri, Salvatore Gianluca Leonardi, Anna Bonavita, and Giovanni Neri. 2020. "Electrochemical Sensing of Serotonin by a Modified MnO2-Graphene Electrode" Biosensors 10, no. 4: 33. https://doi.org/10.3390/bios10040033
APA StyleNehru, L., Chinnathambi, S., Fazio, E., Neri, F., Leonardi, S. G., Bonavita, A., & Neri, G. (2020). Electrochemical Sensing of Serotonin by a Modified MnO2-Graphene Electrode. Biosensors, 10(4), 33. https://doi.org/10.3390/bios10040033








