Competitive HRP-Linked Colorimetric Aptasensor for the Detection of Fumonisin B1 in Food based on Dual Biotin-Streptavidin Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
2.2. Preparation of the Complementary Strand-HRP Sensing Probe
2.3. Preparation of a Streptavidin-Coated Microplate
2.4. Detection Procedure
2.5. Preparation and Measurement of Food Samples
3. Results and Discussion
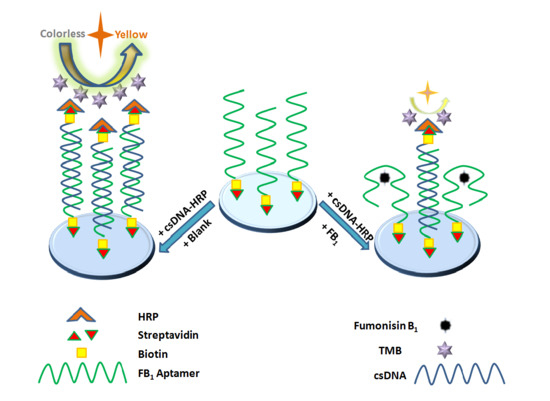
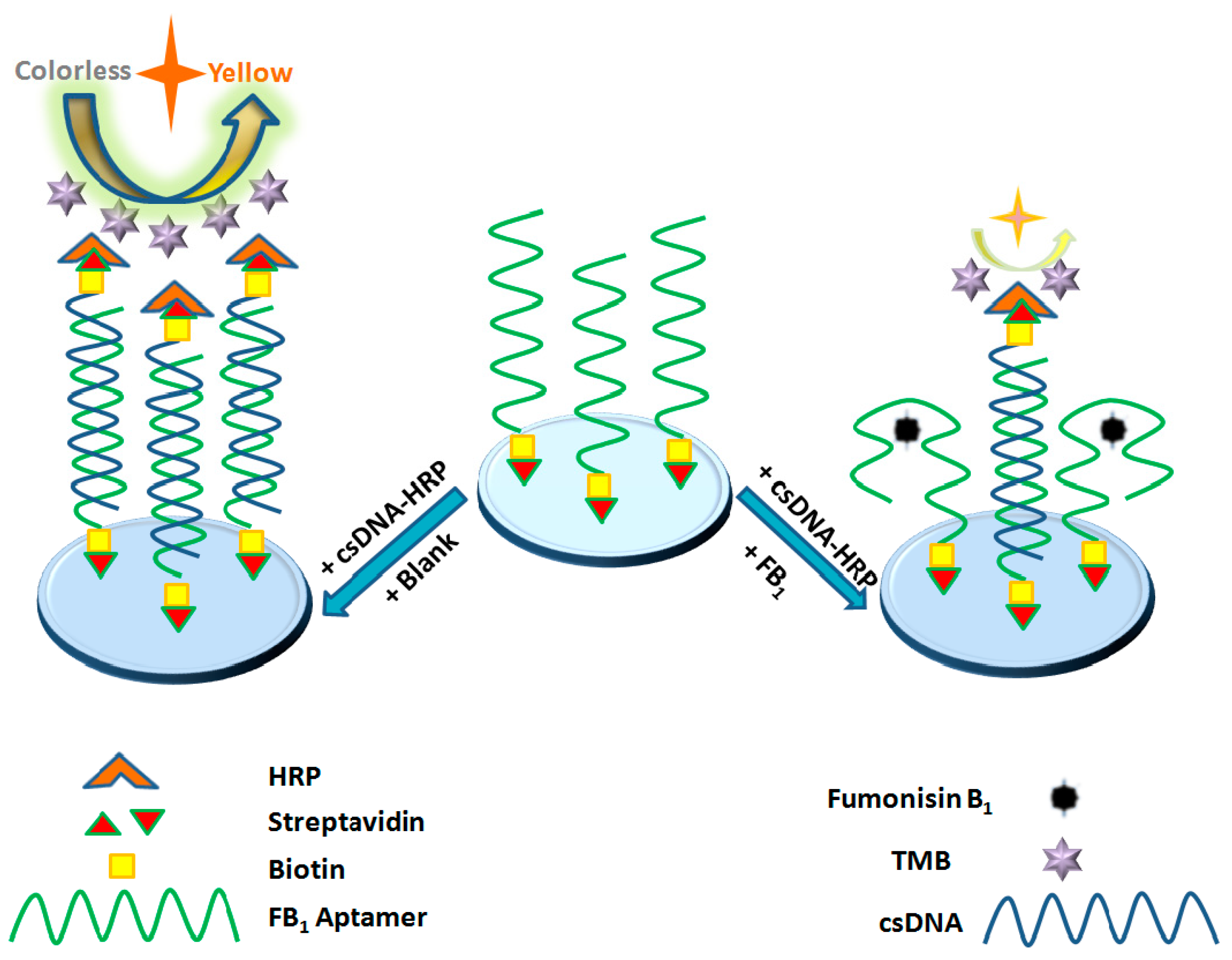
3.1. Principle of the Detection of FB1 by the Aptasensor
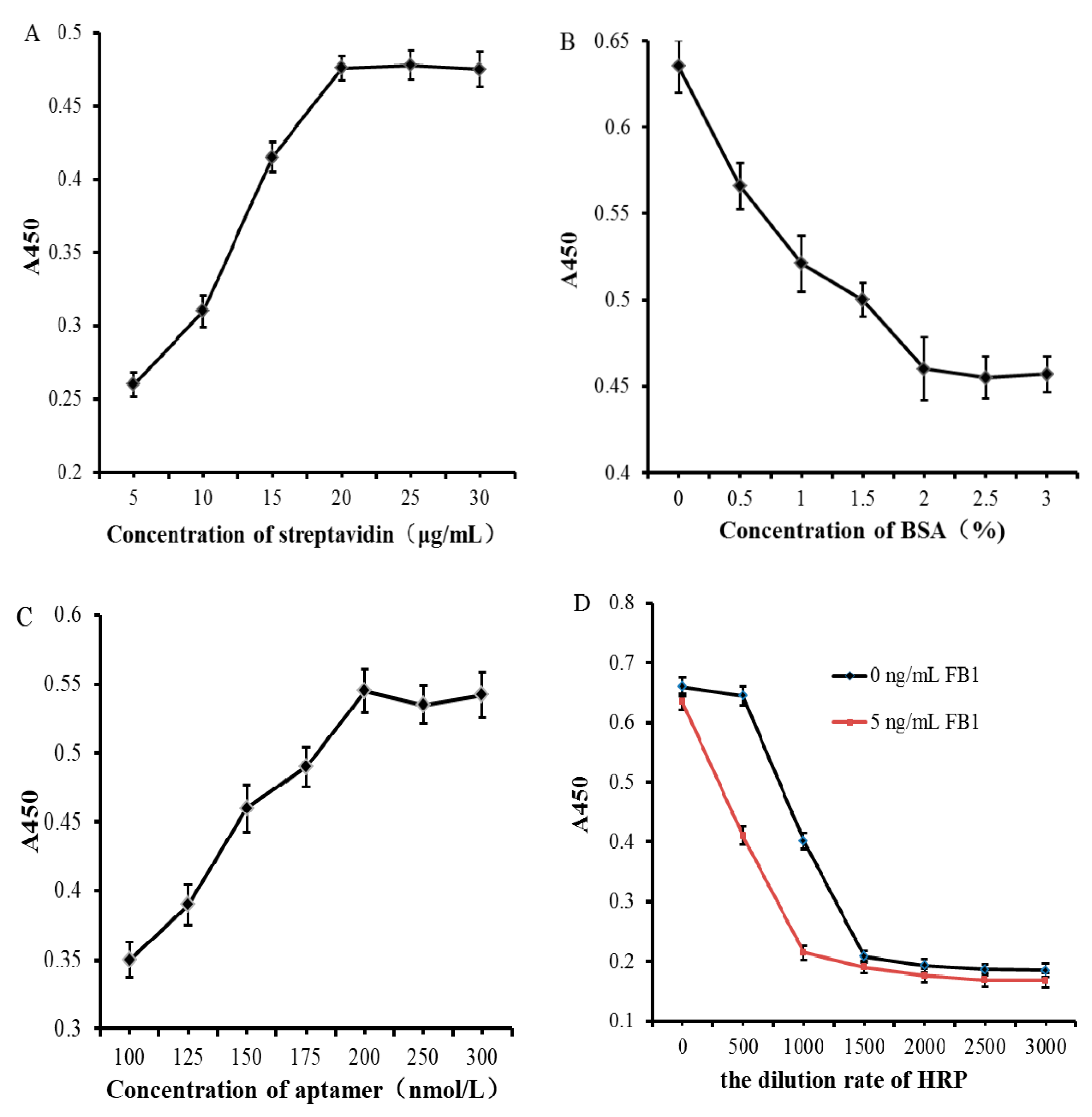
3.2. Optimization of Test Conditions
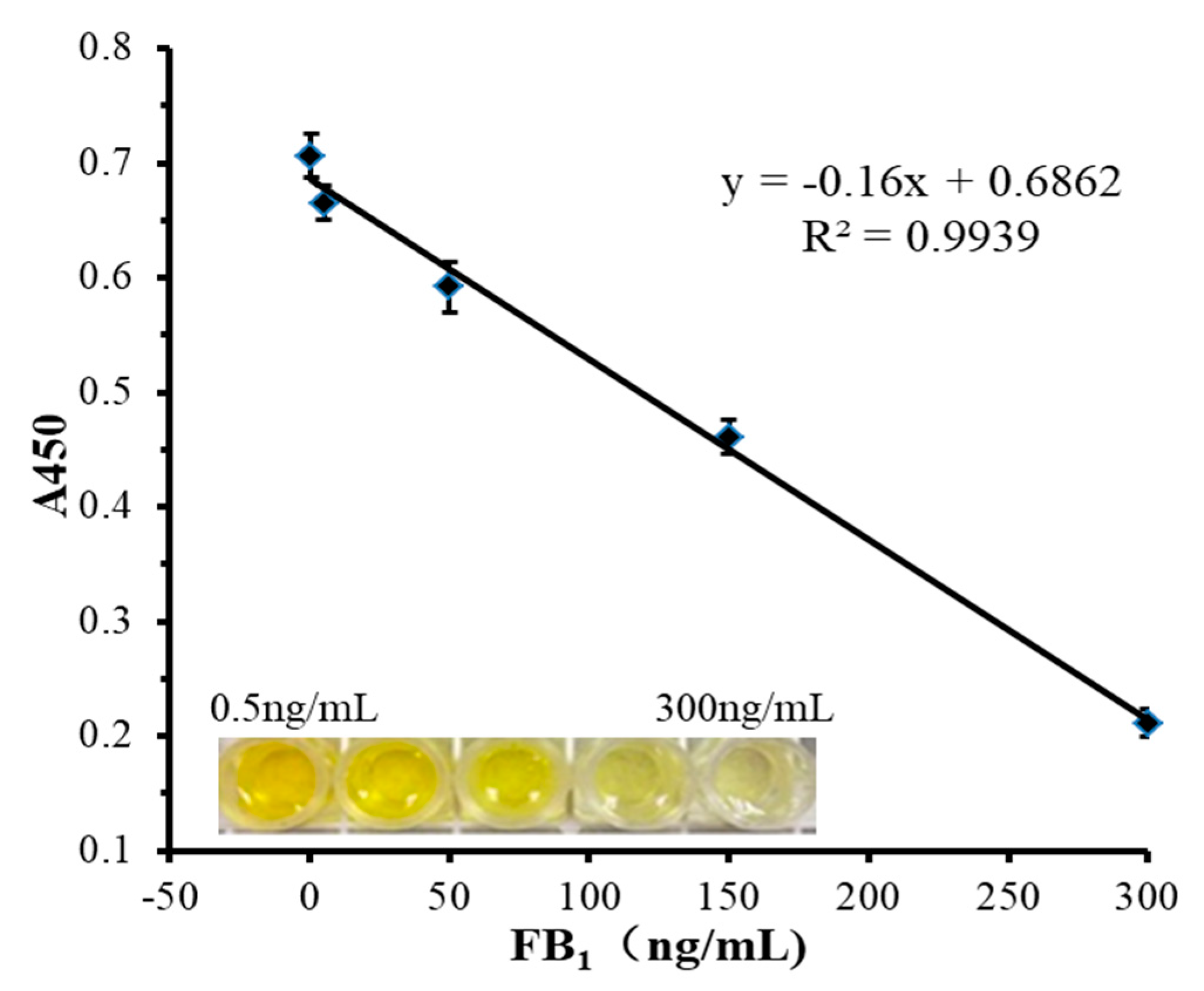
3.3. Detection Performances
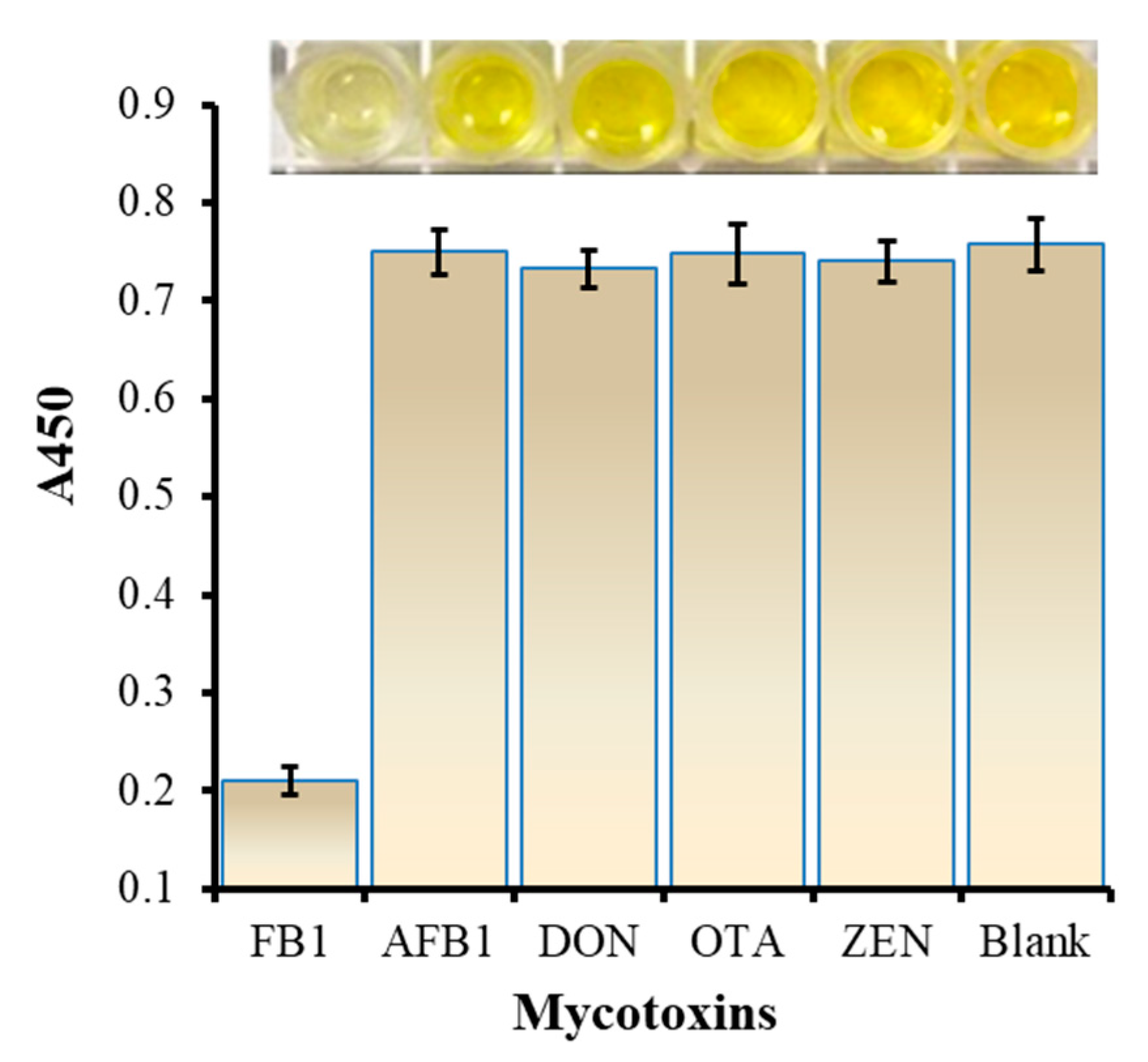
3.4. Analysis for the Specificity
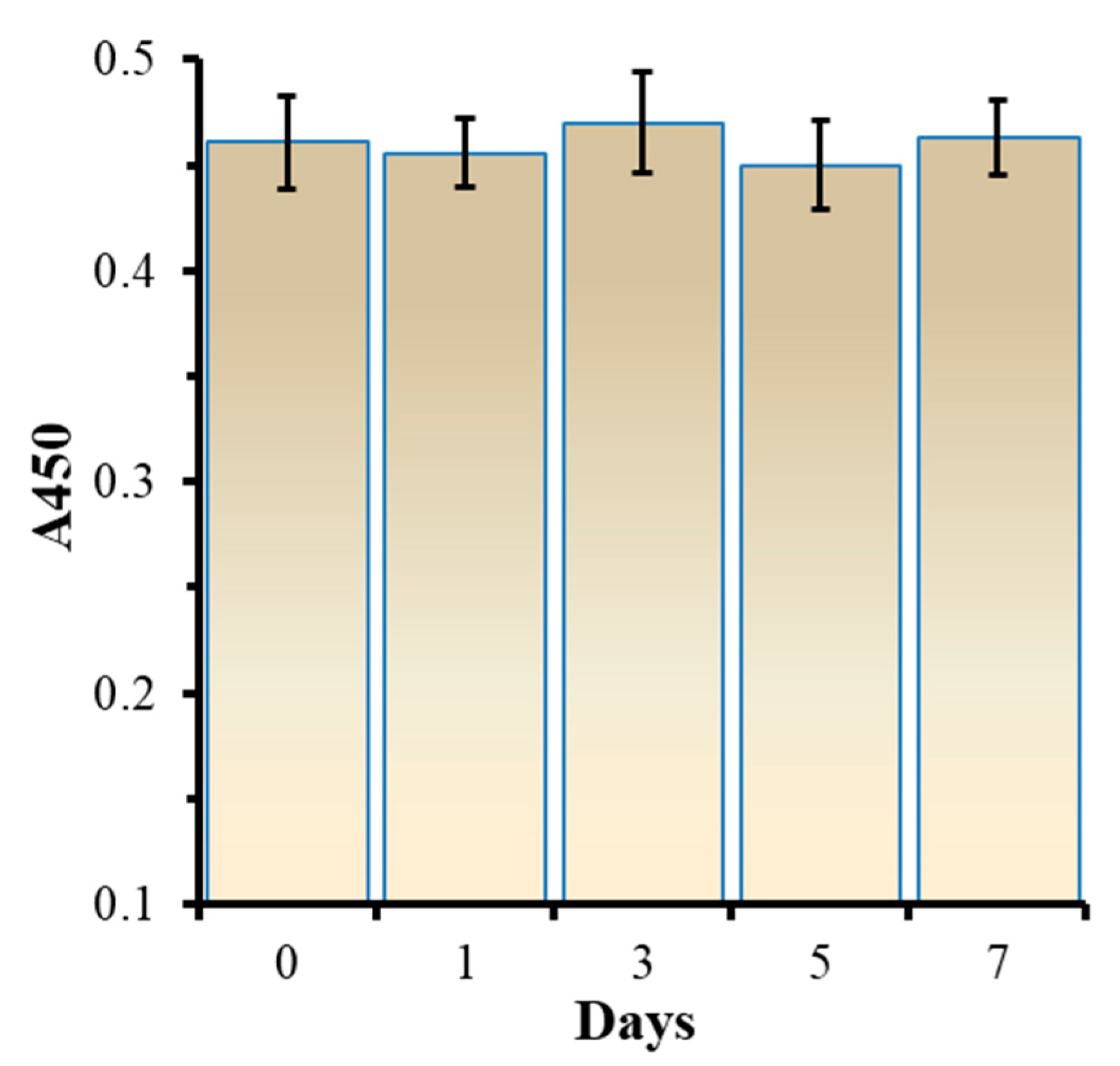
3.5. Analysis for the Stability
3.6. Determination of FB1 in Spiked Food Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rheeder, J.P.; Marasas, W.F.; Vismer, H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef] [PubMed]
- Hinojo, M.J.; Medina, A.; Valle-Algarra, F.M.; Gimeno-Adelantado, J.V.; Jimenez, M.; Mateo, R. Fumonisin production in rice cultures of Fusarium verticillioides under different incubation conditions using an optimized analytical method. Food Microbiol. 2006, 23, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, B.T.; Tiamiyu, L.O.; Ayuba, V.O.; Cheikyula, J.O. Effects of dietary fumonisin B1 on haematology and growth performance of the clariid fish Heterobranchus longifilis. J. Agric. Vet. Sci. 2016, 9, 26–33. [Google Scholar]
- Domijan, A.M.; Abramov, A.Y. Fumonisin B1 inhibits mitochondrial respiration and deregulates calcium homeostasis—Implication to mechanism of cell toxicity. Int. J. Biochem. Cell Biol. 2011, 43, 897–904. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Kellerman, T.S.; Gelderblom, W.C.; Thiel, P.G.; Van der Lugt, J.J.; Coetzer, J.A. Leukoencephalomalacia in a horse induced by fumonisin B₁ isolated from Fusarium moniliforme. Onderstepoort J. vet. Res. 1988, 55, 197–203. [Google Scholar]
- Murashiki, T.C.; Chidewe, C.; Benhura, M.A.; Maringe, D.T.; Dembedza, M.P.; Manema, L.R.; Nyanga, L.K. Levels and daily intake estimates of aflatoxin B1 and fumonisin B1 in maize consumed by rural households in Shamva and Makoni districts of Zimbabwe. Food Control 2017, 72, 105–109. [Google Scholar] [CrossRef]
- Parsons, M.W.; Munkvold, G.P. Effects of planting date and environmental factors on Fusarium ear rot symptoms and fumonisin B1 accumulation in maize grown in six North American locations. Plant Pathol. 2012, 61, 1130–1142. [Google Scholar] [CrossRef]
- Creppy, E.E. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002, 127, 19–28. [Google Scholar] [CrossRef]
- Hu, L.; Liu, H.; Yang, J.; Wang, C.; Wang, Y.; Yang, Y.; Chen, X. Free and hidden fumonisins in raw maize and maize-based products from China. Food Addit. Contam. Part B 2019, 12, 90–96. [Google Scholar] [CrossRef]
- Savi, G.D.; Piacentini, K.C.; Marchi, D.; Scussel, V.M. Fumonisins B1 and B2 in the corn-milling process and corn-based products, and evaluation of estimated daily intake. Food Addit. Contam. Part A 2016, 33, 339–345. [Google Scholar]
- Sugita-Konishi, Y.; Nakajima, M.; Tabata, S.; Ishikuro, E.; Tanaka, T.; Norizuki, H.; Kumagai, S. Occurrence of aflatoxins, ochratoxin A, and fumonisins in retail foods in Japan. J. Food Prot. 2006, 69, 1365–1370. [Google Scholar] [CrossRef]
- da Silva, J.B.; Pozzi, C.R.; Mallozzi, M.A.; Ortega, E.M.; Corrêa, B. Mycoflora and occurrence of aflatoxin B1 and fumonisin B1 during storage of Brazilian sorghum. J. Agric. Food Chem. 2000, 48, 4352–4356. [Google Scholar] [CrossRef] [PubMed]
- Balendres, M.A.O.; Karlovsky, P.; Cumagun, C.J.R. Mycotoxigenic Fungi and Mycotoxins in Agricultural Crop Commodities in the Philippines: A Review. Foods 2019, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, K.C.; Rocha, L.O.; Fontes, L.C.; Carnielli, L.; Reis, T.A.; Corrêa, B. Mycotoxin analysis of industrial beers from Brazil: The influence of fumonisin B1 and deoxynivalenol in beer quality. Food Chem. 2017, 218, 64–69. [Google Scholar] [CrossRef]
- Pascari, X.; Ramos, A.J.; Marín, S.; Sanchís, V. Mycotoxins and beer. Impact of beer production process on mycotoxin contamination. A review. Food Res. Int. 2018, 103, 121–129. [Google Scholar] [CrossRef]
- Peters, J.; Van Dam, R.; Van Doorn, R.; Katerere, D.; Berthiller, F.; Haasnoot, W.; Nielen, M.W. Mycotoxin profiling of 1000 beer samples with a special focus on craft beer. PLoS ONE 2017, 12, e0185887. [Google Scholar] [CrossRef]
- Campone, L.; Rizzo, S.; Piccinelli, A.L.; Celano, R.; Pagano, I.; Russo, M.; Rastrelli, L. Determination of mycotoxins in beer by multi heart-cutting two-dimensional liquid chromatography tandem mass spectrometry method. Food Chem. 2020, 318, 126496. [Google Scholar] [CrossRef]
- Dragan, Y.P.; Bidlack, W.R.; Cohen, S.M.; Goldsworthy, T.L.; Hard, G.C.; Howard, P.C.; Voss, K.A. Implications of apoptosis for toxicity, carcinogenicity, and risk assessment: Fumonisin B1 as an example. Toxicol. Sci. 2001, 61, 6–17. [Google Scholar] [CrossRef]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Huang, T.; Yu, J.; Wang, J.S. Fumonisin B1 contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China. Food Addit. Contam. 2007, 24, 181–185. [Google Scholar] [CrossRef]
- Khan, R.B.; Phulukdaree, A.; Chuturgoon, A.A. Fumonisin B1 induces oxidative stress in oesophageal (SNO) cancer cells. Toxicon 2018, 141, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.S.; Wink, M. Exposure, occurrence, and chemistry of fumonisins and their cryptic derivatives. Compr. Rev. Food Sci. Food Saf. 2018, 17, 769–791. [Google Scholar] [CrossRef]
- Shephard, G.S.; Sewram, V. Determination of the mycotoxin fumonisin B1 in maize by reversed-phase thin-layer chromatography: A collaborative study. Food Addit. Contam. 2004, 21, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Gazzotti, T.; Lugoboni, B.; Zironi, E.; Barbarossa, A.; Serraino, A.; Pagliuca, G. Determination of fumonisin B1 in bovine milk by LC–MS/MS. Food Control 2009, 20, 1171–1174. [Google Scholar] [CrossRef]
- Roscoe, V.; Lombaert, G.A.; Huzel, V.; Neumann, G.; Melietio, J.; Kitchen, D.; Scott, P.M. Mycotoxins in breakfast cereals from the Canadian retail market: A 3-year survey. Food Addit. Contam. 2008, 25, 347–355. [Google Scholar] [CrossRef]
- Ling, S.; Pang, J.; Yu, J.; Wang, R.; Liu, L.; Ma, Y.; Wang, S. Preparation and identification of monoclonal antibody against fumonisin B1 and development of detection by Ic-ELISA. Toxicon 2014, 80, 64–72. [Google Scholar] [CrossRef]
- Lu, L.; Seenivasan, R.; Wang, Y.C.; Yu, J.H.; Gunasekaran, S. An electrochemical immunosensor for rapid and sensitive detection of mycotoxins fumonisin B1 and deoxynivalenol. Electrochim. Acta 2016, 213, 89–97. [Google Scholar] [CrossRef]
- Shu, M.; Xu, Y.; Liu, X.; Li, Y.; He, Q.; Tu, Z.; Hammock, B.D. Anti-idiotypic nanobody-alkaline phosphatase fusion proteins: Development of a one-step competitive enzyme immunoassay for fumonisin B1 detection in cereal. Anal. Chim. Acta 2016, 11924, 53–59. [Google Scholar] [CrossRef]
- Peltomaa, R.; Benito-Peña, E.; Barderas, R.; Sauer, U.; Gonzalez Andrade, M.; Moreno-Bondi, M.C. Microarray-based immunoassay with synthetic mimotopes for the detection of fumonisin B1. Anal. Chem. 2017, 89, 6216–6223. [Google Scholar] [CrossRef]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Zhang, B. Recent advances in SELEX technology and aptamer applications in biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef]
- Teller, C.; Shimron, S.; Willner, I. Aptamer−dnazyme hairpins for amplified biosensing. Anal. Chem. 2009, 81, 9114–9119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Huang, P.J.J.; Ding, J.; Liu, J. Aptamer-based biosensors for biomedical diagnostics. Analyst 2014, 139, 2627–2640. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv. 2019, 37, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Gawande, B.N.; Rohloff, J.C.; Carter, J.D.; von Carlowitz, I.; Zhang, C.; Schneider, D.J.; Janjic, N. Selection of DNA aptamers with two modified bases. Proc. Natl. Acad. Sci. USA 2017, 114, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Farzin, L.; Shamsipur, M.; & Sheibani, S. A review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals. Talanta 2017, 174, 619–627. [Google Scholar] [CrossRef]
- Teng, J.; Yuan, F.; Ye, Y.; Zheng, L.; Yao, L.; Xue, F.; Chen, W.; Li, B. Aptamer-based technologies in foodborne pathogen detection. Front. Microbiol. 2016, 7, 1426. [Google Scholar] [CrossRef]
- Zhang, L.; Dou, X.W.; Zhang, C.; Logrieco, A.F.; Yang, M.H. A review of current methods for analysis of mycotoxins in herbal medicines. Toxins 2018, 10, 65. [Google Scholar] [CrossRef]
- Sheng, L.; Lu, Y.; Deng, S.; Liao, X.; Zhang, K.; Ding, T.; Li, J. A transcription aptasensor: Amplified, label-free and culture-independent detection of foodborne pathogens via light-up RNA aptamers. Chem. Commun. 2019, 55, 10096–10099. [Google Scholar] [CrossRef]
- Lv, L.; Cui, C.; Liang, C.; Quan, W.; Wang, S.; Guo, Z. Aptamer-based single-walled carbon nanohorn sensors for ochratoxin A detection. Food Control 2016, 60, 296–301. [Google Scholar] [CrossRef]
- McKeague, M.; Bradley, C.R.; Girolamo, A.D.; Visconti, A.; Miller, J.D.; DeRosa, M.C. Screening and initial binding assessment of fumonisin B1 aptamers. Int. J. Mol. Sci. 2010, 11, 4864–4881. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.; Zhang, W.; Leng, Y.; Xiong, Y. A colorimetric immunoassay based on glucose oxidase-induced AuNP aggregation for the detection of fumonisin B1. Talanta 2018, 186, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, X.; Zhang, X.; Qing, Y.; Luo, M.; Liu, X.; Qiu, J. A highly sensitive electrochemical immunosensor for fumonisin B1 detection in corn using single-walled carbon nanotubes/chitosan. Electroanalysis 2015, 27, 2679–2687. [Google Scholar] [CrossRef]
- Ling, S.; Wang, R.; Gu, X.; Wen, C.; Chen, L.; Chen, Z.; Wang, S. Rapid detection of fumonisin B1 using a colloidal gold immunoassay strip test in corn samples. Toxicon 2015, 108, 210–215. [Google Scholar] [CrossRef] [PubMed]
| Detection Method | LOD (ng/mL) | Linear Range (ng/mL) | Reference |
|---|---|---|---|
| SPE based Electrochemical Immunosensor | 4.2 | 200–4500 | [27] |
| Chemiluminescence ELISA | 0.35 | 0.93–7.73 | [28] |
| Microarray-Based Immunoassay | 11.1 | 17.3–79.6 | [29] |
| AuNPs based dc-pELISA | 3.07 | 3.125–25 | [41] |
| Nanotubes based Electrochemical sensor | 0.002 | 0.01–1000 | [42] |
| Colloidal gold immunoassay | 2.5 | 2.5–10 | [43] |
| Aptamer based colorimetric assay | 0.3 | 0.5–300 | this work |
| Number | Background (ng/mL) | Added (ng/mL) | Found (ELISA) (ng/mL) | Spike Recovery (ELISA) (%) | Found (ng/mL) | Spike Recovery (%) | |
|---|---|---|---|---|---|---|---|
| beer | 1 | 0 | 10 | 9.140 | 91.40 | 9.010 | 90.10 |
| 2 | 0 | 50 | 50.68 | 101.4 | 51.08 | 102.2 | |
| 3 | 0 | 200 | 197.4 | 98.69 | 197.2 | 98.60 | |
| Number | Background (µg/kg) | Added (µg/kg) | Found (ELISA) (µg/kg) | Spike Recovery (ELISA) (%) | Found (µg/kg) | Spike Recovery (%) | |
|---|---|---|---|---|---|---|---|
| corn | 1 | 0 | 10 | 9.260 | 92.60 | 9.180 | 91.80 |
| 2 | 0 | 50 | 52.21 | 104.4 | 52.60 | 105.2 | |
| 3 | 0 | 200 | 206.3 | 103.1 | 207.3 | 103.7 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Z.; Zhou, Y.; Li, X.; Wang, Z. Competitive HRP-Linked Colorimetric Aptasensor for the Detection of Fumonisin B1 in Food based on Dual Biotin-Streptavidin Interaction. Biosensors 2020, 10, 31. https://doi.org/10.3390/bios10040031
Tao Z, Zhou Y, Li X, Wang Z. Competitive HRP-Linked Colorimetric Aptasensor for the Detection of Fumonisin B1 in Food based on Dual Biotin-Streptavidin Interaction. Biosensors. 2020; 10(4):31. https://doi.org/10.3390/bios10040031
Chicago/Turabian StyleTao, Zui, You Zhou, Xiang Li, and Zhouping Wang. 2020. "Competitive HRP-Linked Colorimetric Aptasensor for the Detection of Fumonisin B1 in Food based on Dual Biotin-Streptavidin Interaction" Biosensors 10, no. 4: 31. https://doi.org/10.3390/bios10040031
APA StyleTao, Z., Zhou, Y., Li, X., & Wang, Z. (2020). Competitive HRP-Linked Colorimetric Aptasensor for the Detection of Fumonisin B1 in Food based on Dual Biotin-Streptavidin Interaction. Biosensors, 10(4), 31. https://doi.org/10.3390/bios10040031