Recyclable SERS-Based Immunoassay Guided by Photocatalytic Performance of Fe3O4@TiO2@Au Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
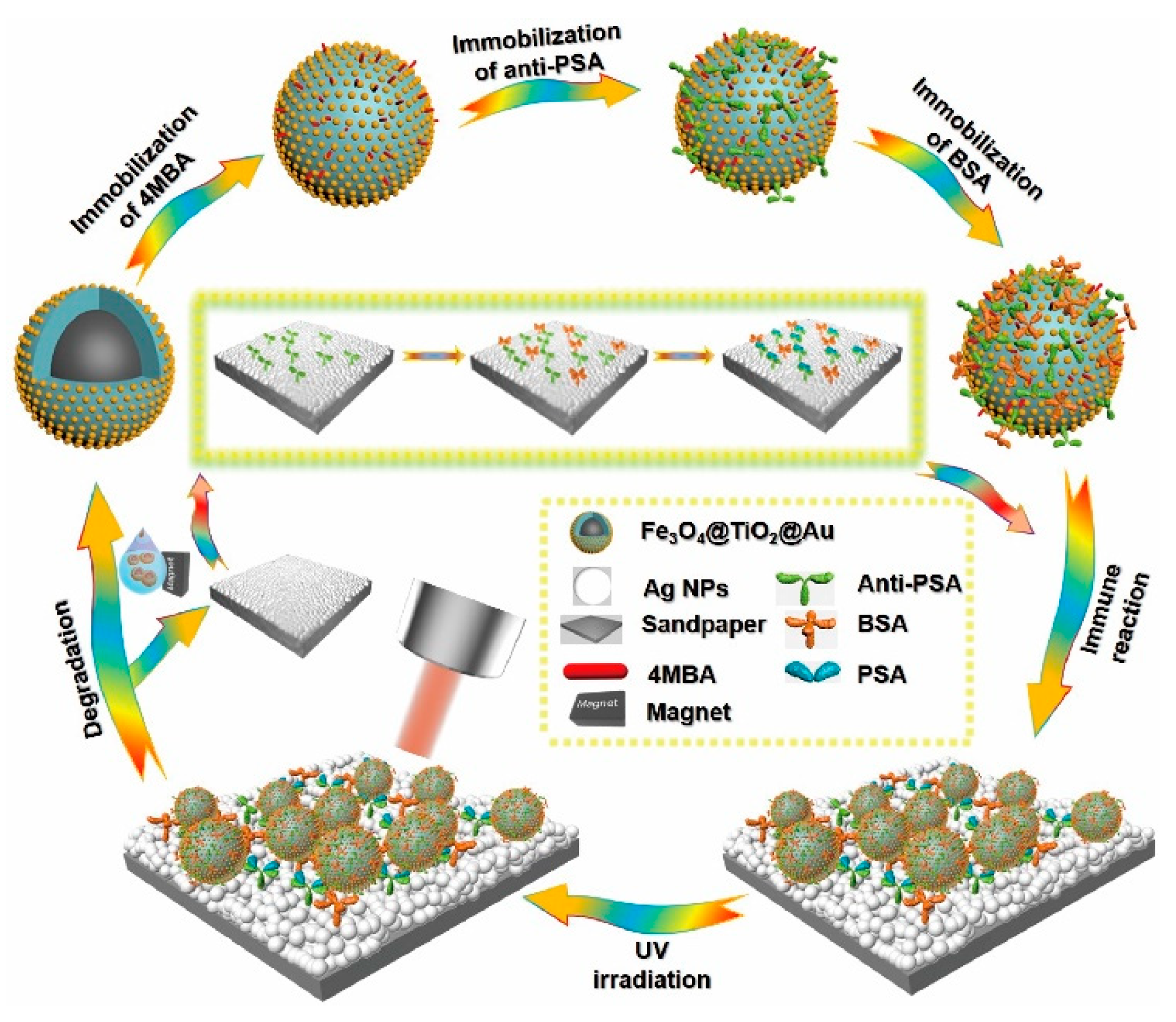
2.2. Preparation of the Fe3O4@TiO2@Au Immune Probes
2.2.1. Synthesis of Fe3O4 NPs
2.2.2. Synthesis of Fe3O4@TiO2 NPs
2.2.3. Fabrication of Fe3O4@TiO2@Au Nanocomposites
2.2.4. Preparation of the Immune Probes
2.3. Preparation of the SERS-Active Immune Substrate
2.4. Recyclable Sandwich Immunoassay
2.5. Instruments
3. Results and Discussion
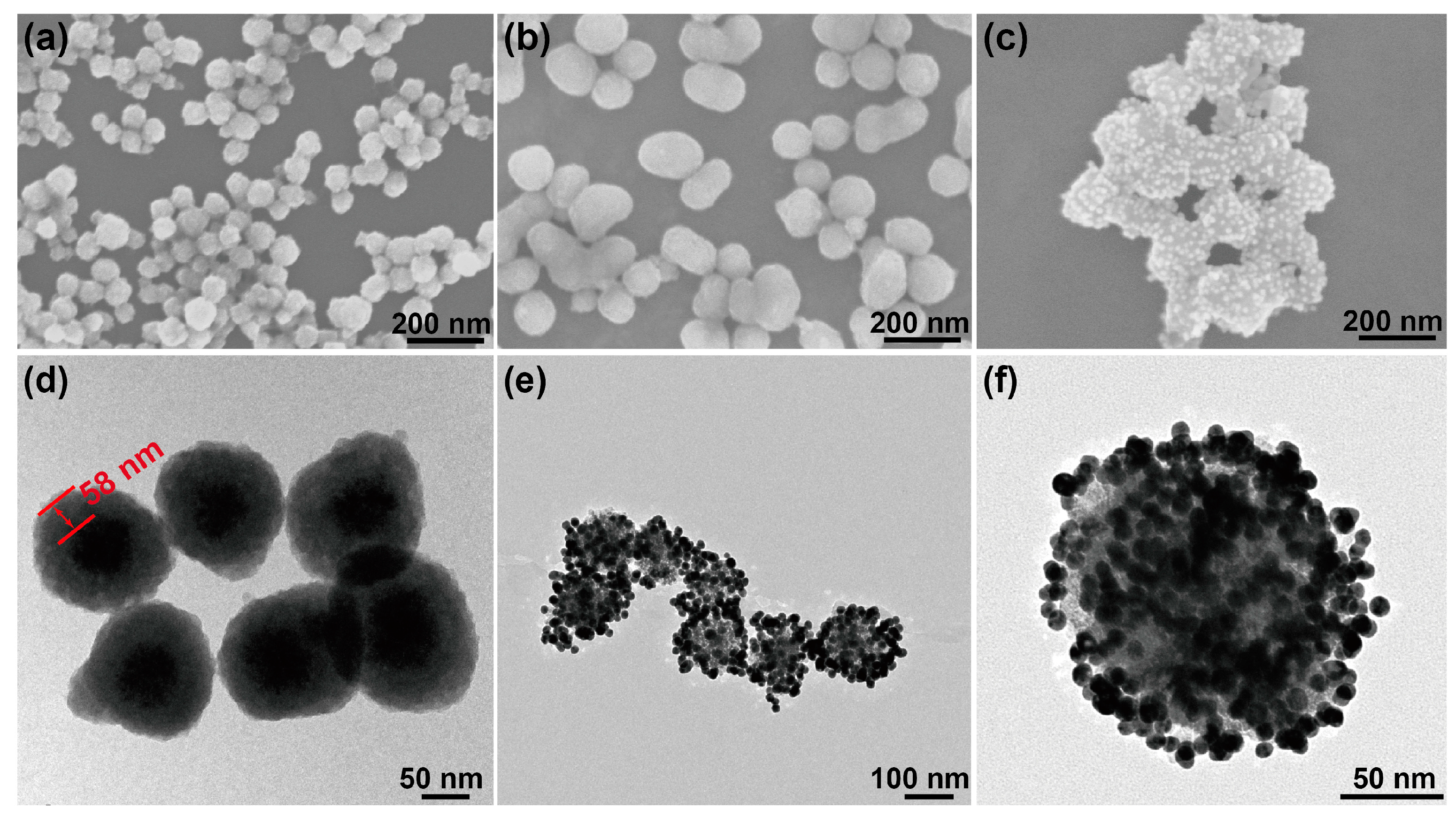
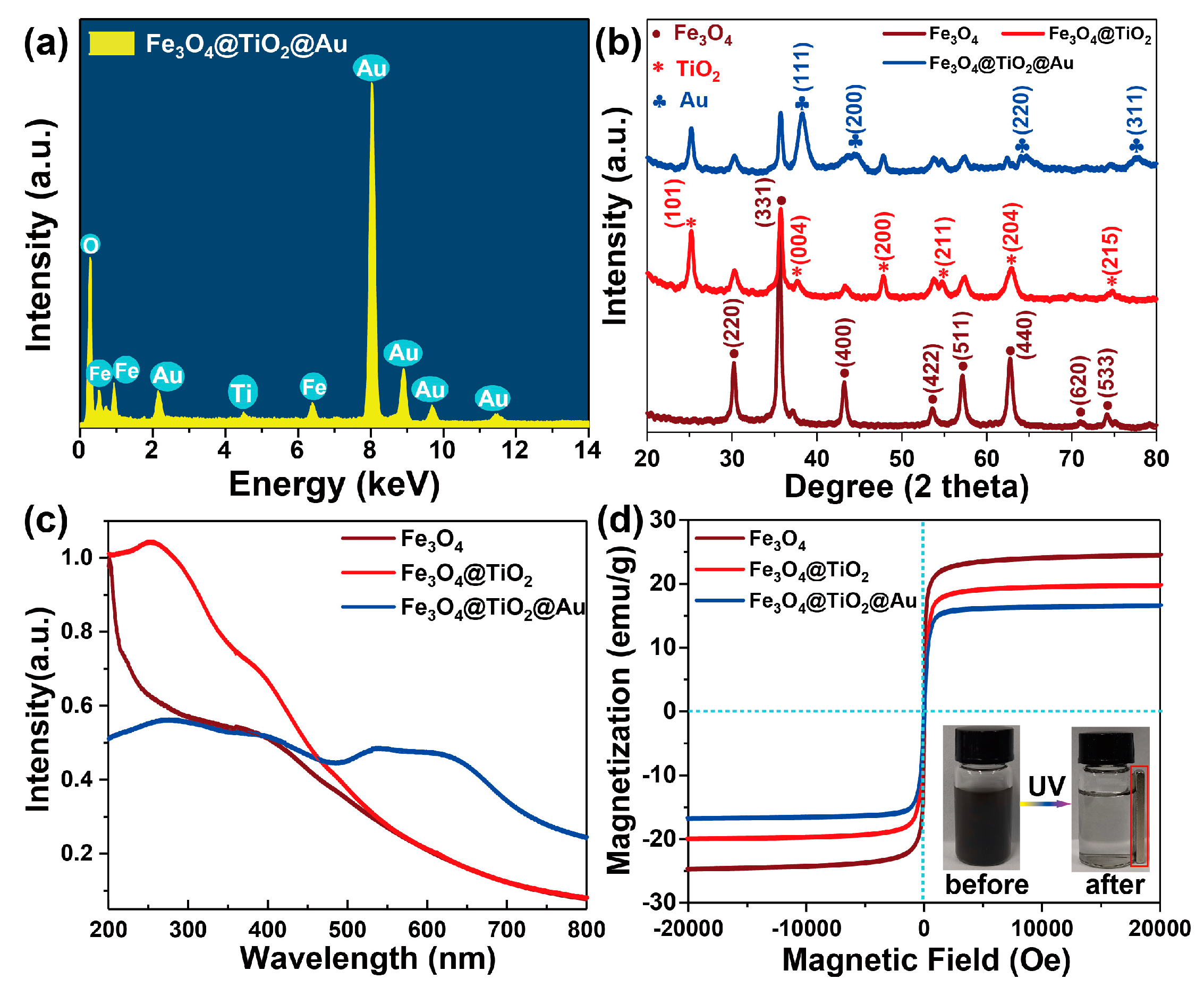
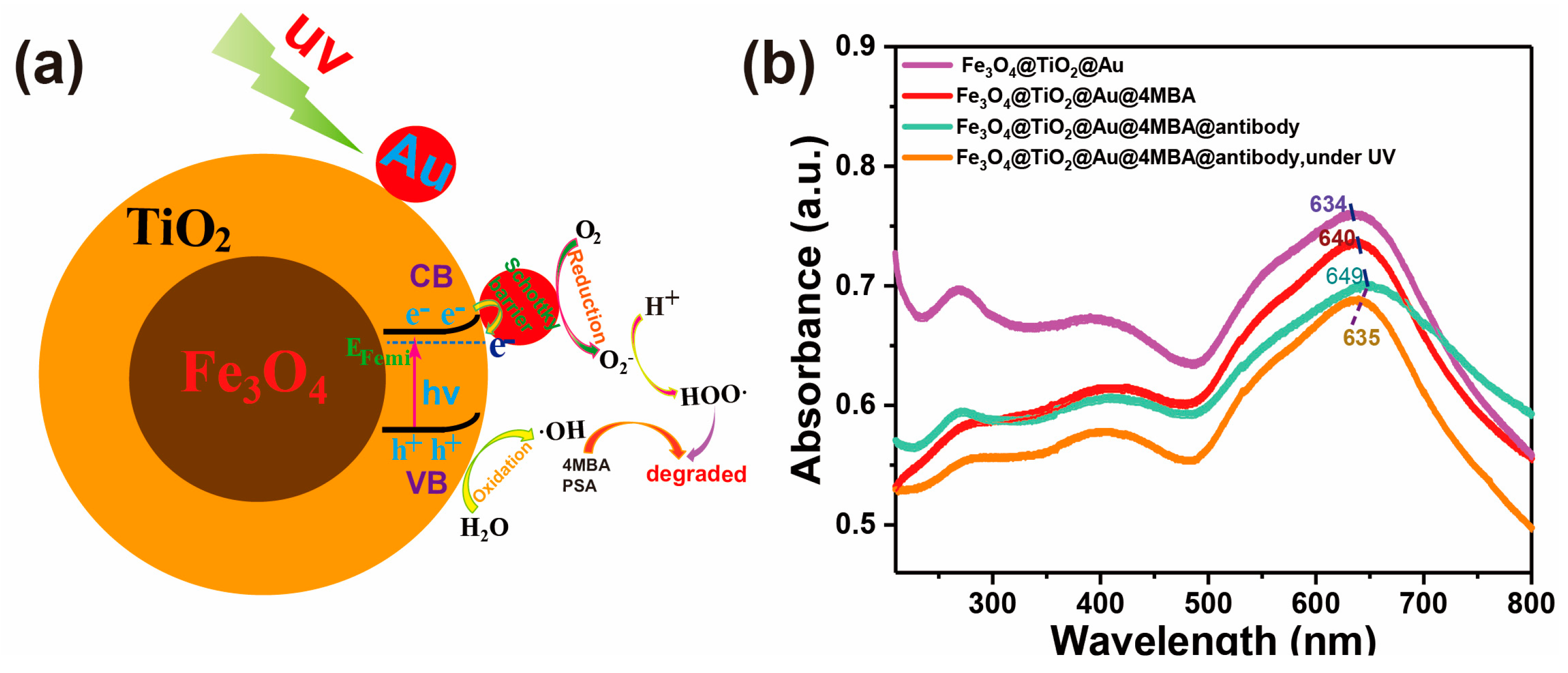
3.1. Characterization of the Fe3O4@TiO2@Au Immune Probe
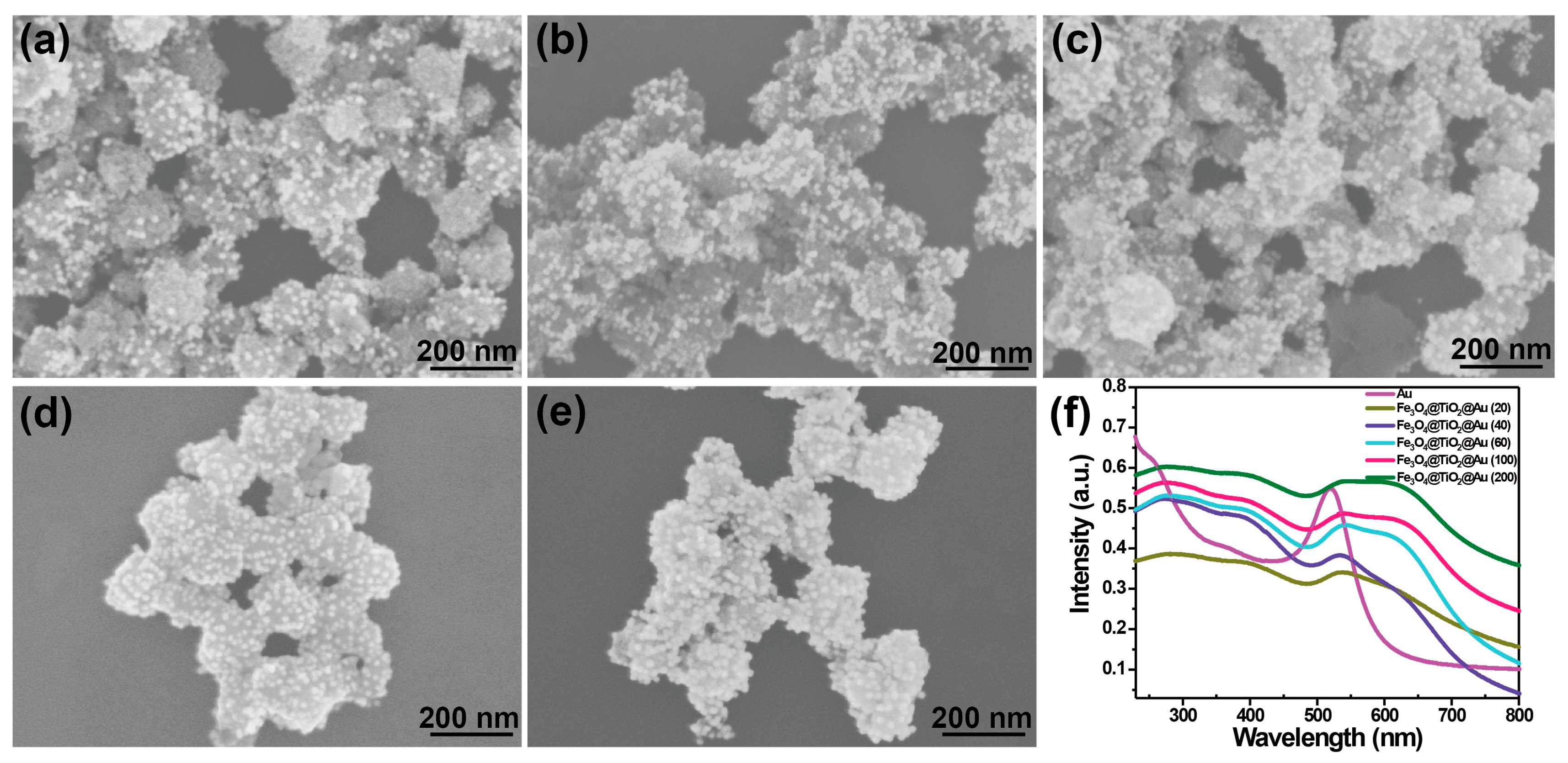
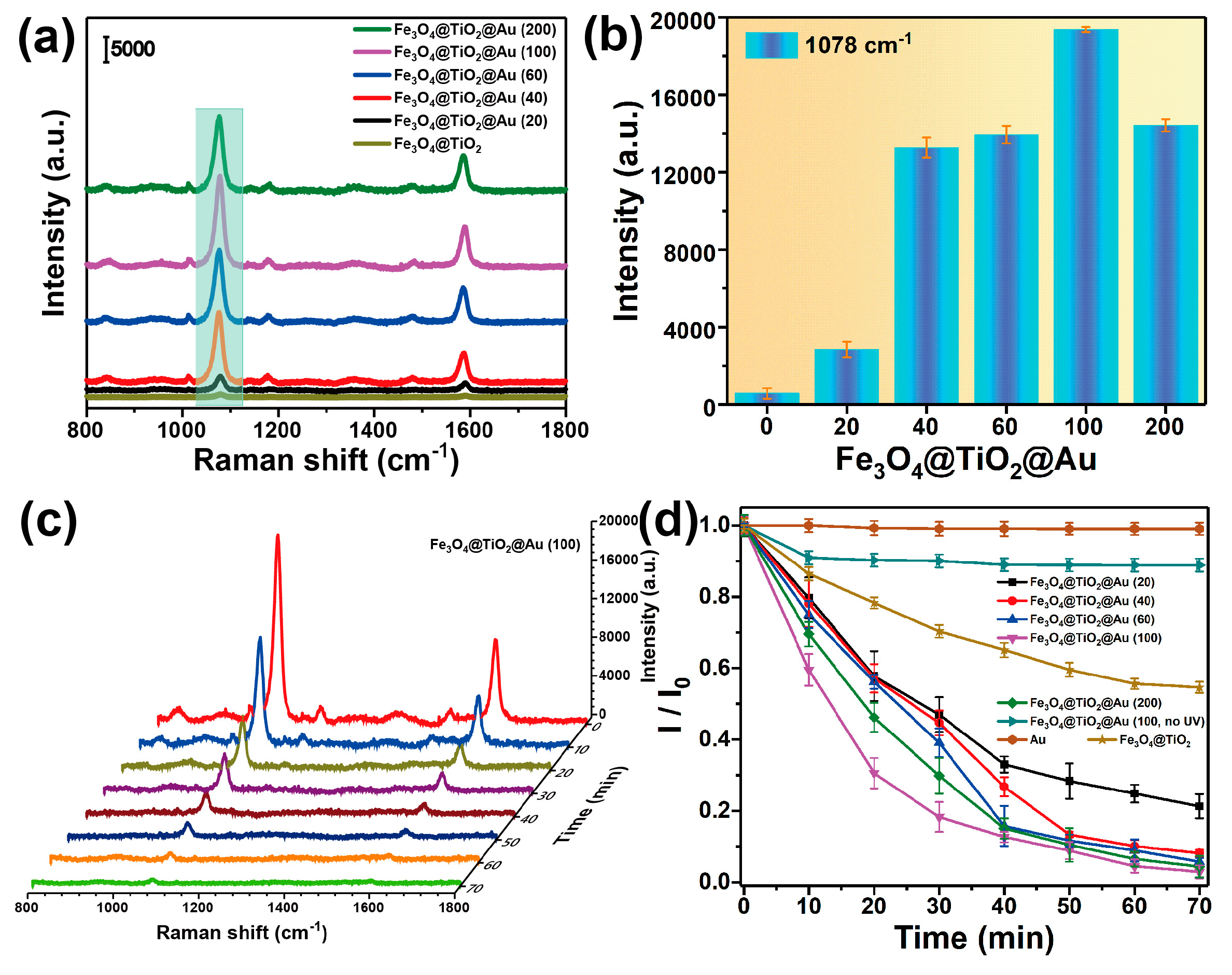
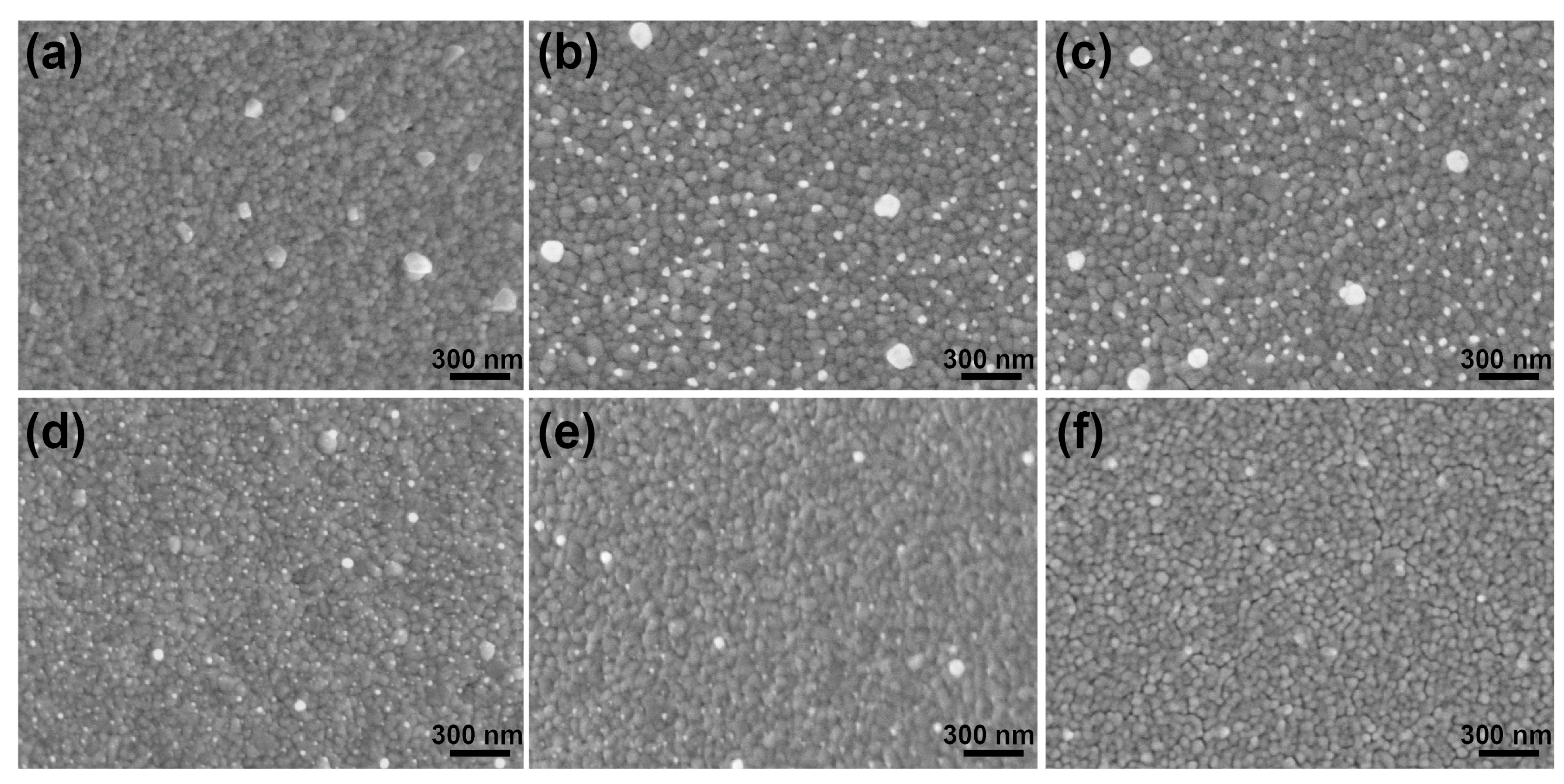
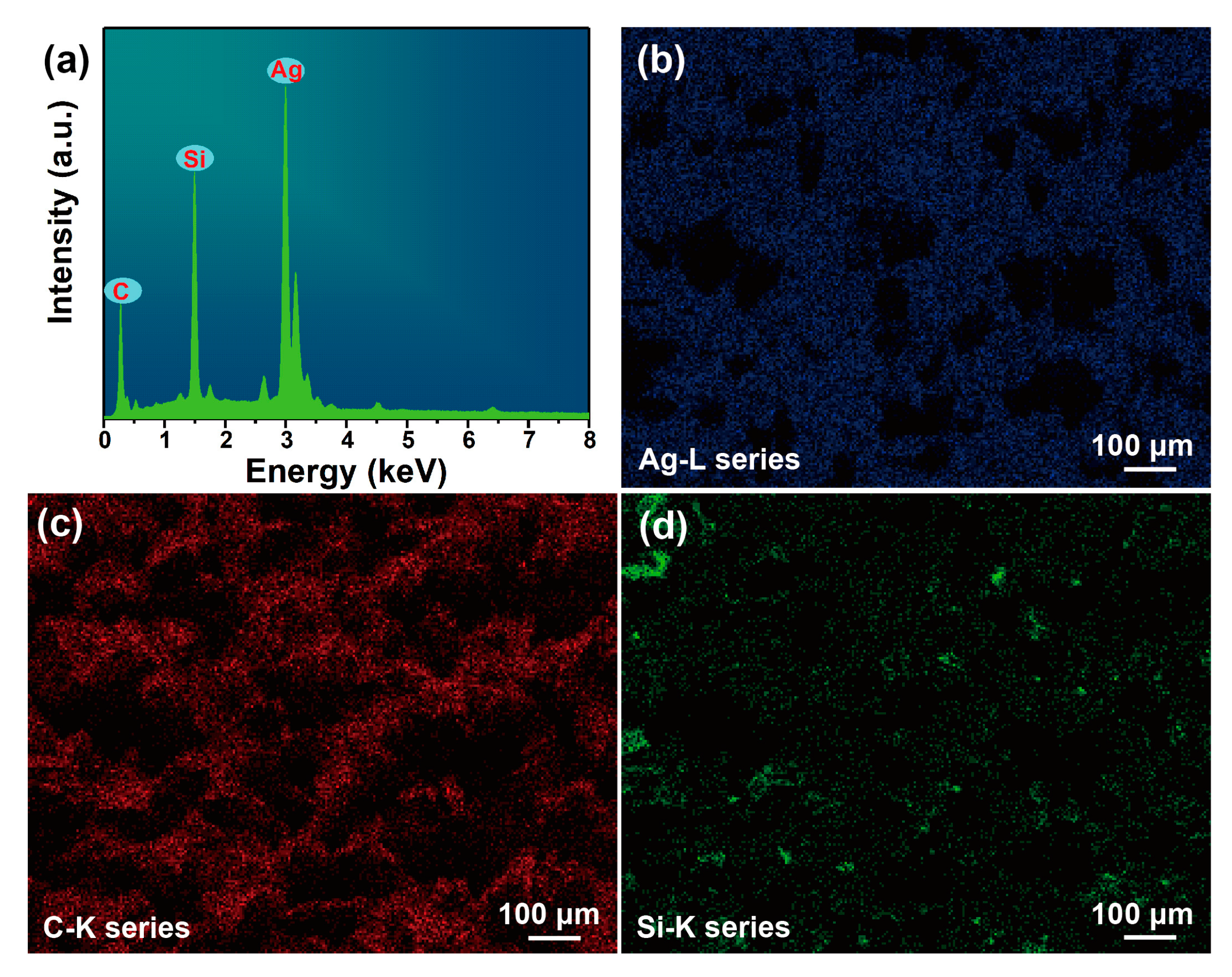
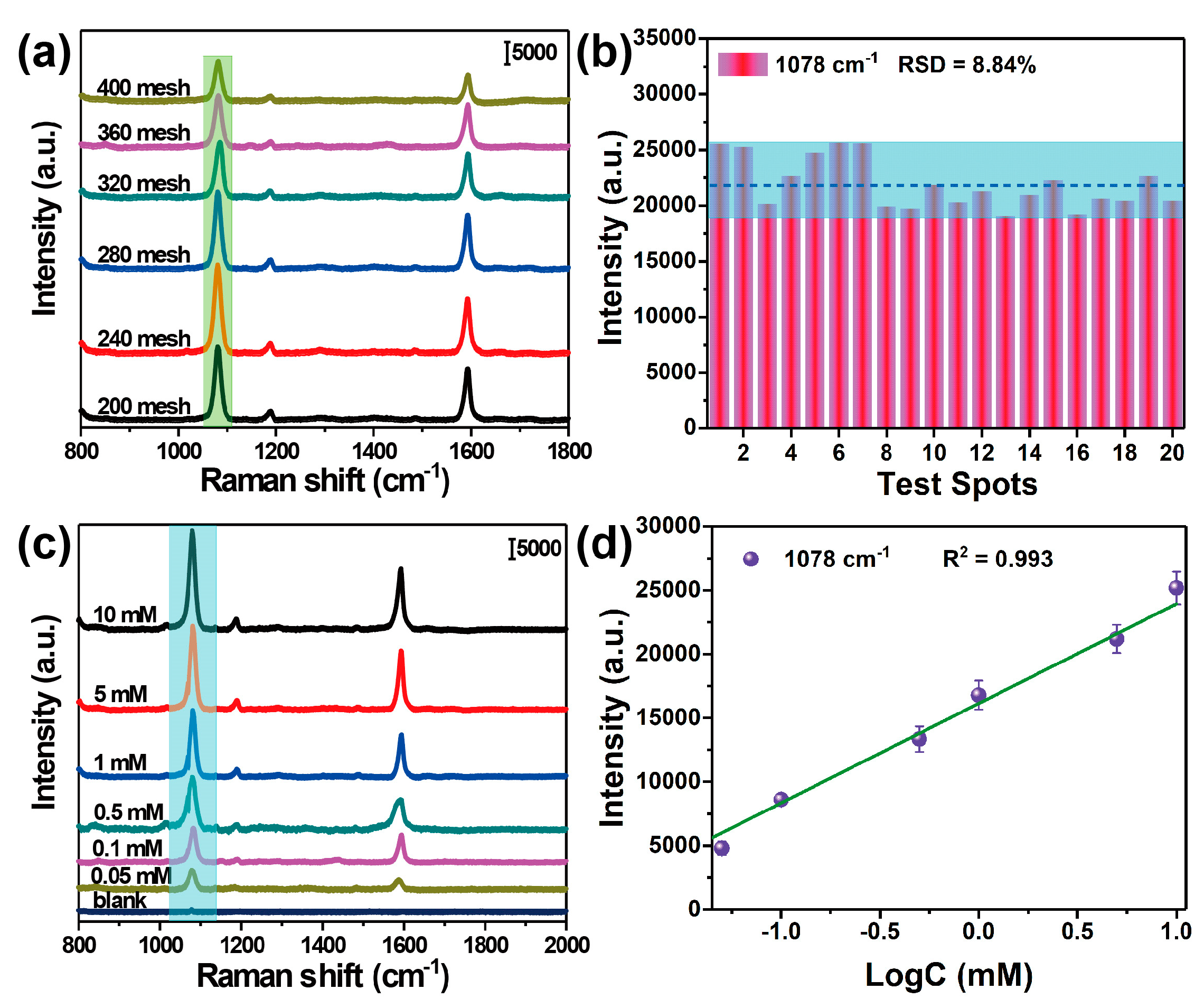
3.2. Characterization of the SERS-Active Substrate
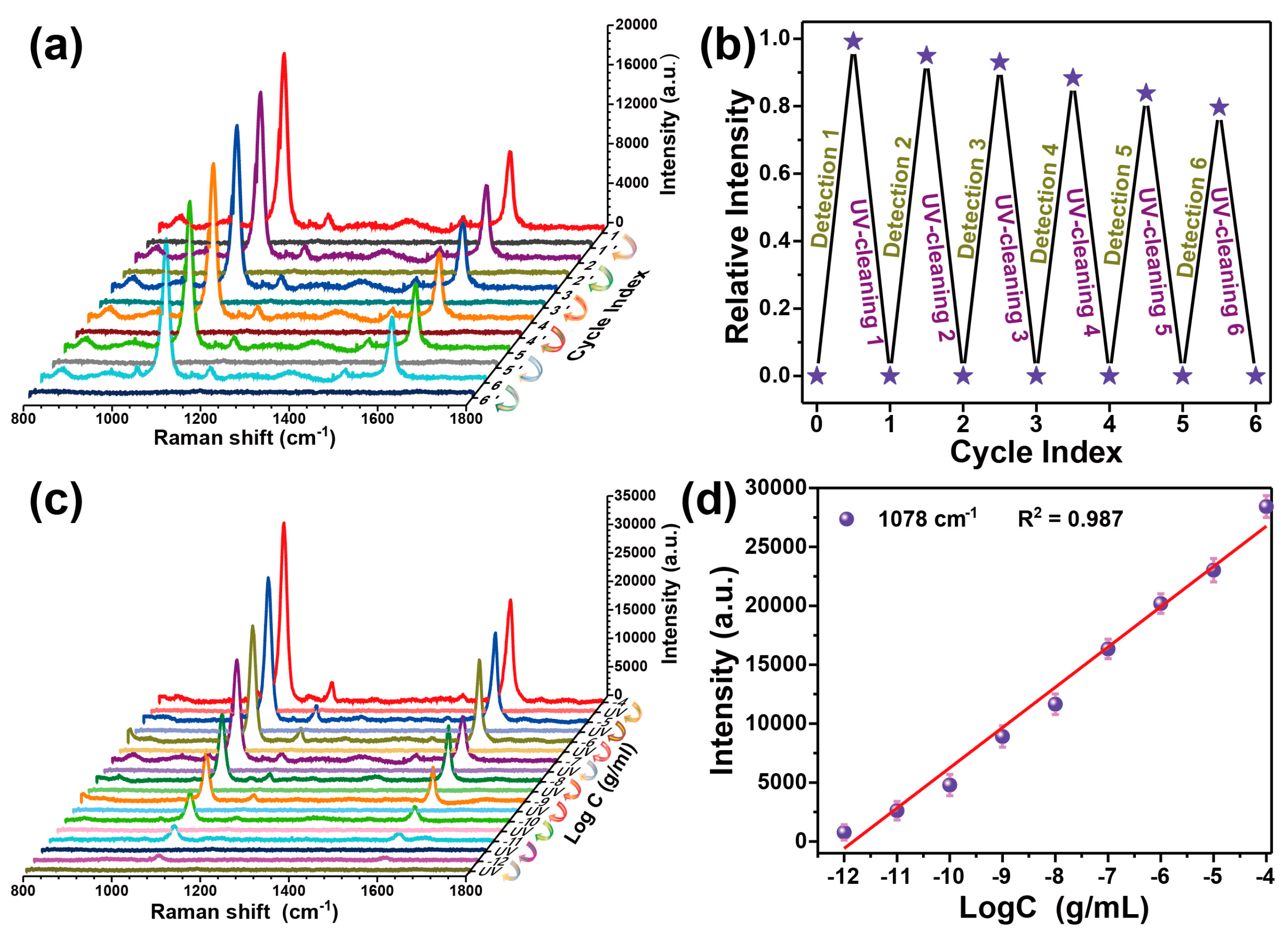
3.3. Recyclable SERS-Based Immunoassay of PSA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.; Bray, F. International Variation in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2012, 61, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA-Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Lilja, H.; Ulmert, D.; Vickers, A. Prostate-Specific Antigen and Prostate Cancer: Prediction, Detection and Monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhen, S.J.; Li, Y.F.; Huang, C.Z. Silver Nanoparticles Deposited on Graphene Oxide for Ultrasensitive Surface-Enhanced Raman Scattering Immunoassay of Cancer Biomarker. Nanoscale 2018, 10, 11942–11947. [Google Scholar] [CrossRef]
- Nolan, J.P.; Duggan, E.; Liu, E.; Condello, D.; Dave, I.; Stoner, S.A. Single Cell Analysis Using Surface Enhanced Raman Scattering (SERS) Tags. Methods 2012, 57, 272–279. [Google Scholar] [CrossRef]
- Fu, X.; Wen, J.; Li, J.; Lin, H.; Liu, Y.; Zhuang, X.; Tian, C.; Chen, L. Highly Sensitive Detection of Prostate Cancer Specific PCA3 Mimic DNA Using SERS-Based Competitive Lateral Flow Assay. Nanoscale 2019, 11, 15530–15536. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, G.; Tian, X.; Tang, S.; Zhou, J.; Feng, Y.; Chen, H. Construction of Long Narrow Gaps in Ag Nanoplates. J. Am. Chem. Soc. 2018, 140, 15560–15563. [Google Scholar] [CrossRef]
- Nowicka, A.B.; Czaplicka, M.; Kowalska, A.A.; Szymborski, T.; Kamińska, A. Flexible PET/ITO/Ag SERS Platform for Label-Free Detection of Pesticides. Biosensor 2019, 9, 111. [Google Scholar] [CrossRef]
- Rigó, I.; Veres, M.; Váczi, T.; Holczer, E.; Hakkel, O.; Deák, T.; Fürjes, P. Preparation and Characterization of Perforated SERS Active Array for Particle Trapping and Sensitive Molecular Analysis. Biosensor 2019, 9, 93. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, B.; Chen, L. SERS Tags: Novel Optical Nanoprobes for Bioanalysis. Chem. Rev. 2012, 113, 1391–1428. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, S.; Wu, L.; Zhu, D.; Cui, Y. SERS-Activated Platforms for Immunoassay: Probes, Encoding Methods, and Applications. Chem. Rev. 2017, 117, 7910–7963. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liu, B.; Ni, H.; Chang, N.; Luan, C.; Ge, Q.; Dong, J.; Zhao, X.W. Vertical Flow Assays Based on Core-Shell SERS Nanotags for Multiplex Prostate Cancer Biomarker Detection. Analyst 2019, 144, 4051–4059. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Choi, N.; Wang, R.; Lee, S.; Moon, K.C.; Yoon, S.Y.; Chen, L.; Choo, J. Simultaneous Detection of Dual Prostate Specific Antigens Using Surface-Enhanced Raman Scattering-Based Immunoassay for Accurate Diagnosis of Prostate Cancer. ACS Nano 2017, 11, 4926–4933. [Google Scholar] [CrossRef]
- Rizzo, L. Inactivation and Injury of Total Coliform Bacteria after Primary Disinfection of Drinking Water by TiO2 Photocatalysis. J. Hazard. Mater. 2009, 165, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.; Sun, D.; Yang, Z.; Xinga, Z.; Li, Z.; Zhu, Q.; Zhoua, W. Bifunctional Nest-like Self-Floating Microreactor for Enhanced Photothermal Catalysis and Biocatalysis. Environ. Sci. Nano 2019, 6, 3551–3559. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, P.; Zhang, F.; Liu, H.; Fan, J.; Liu, X.; Yang, G.; Jiang, L.; Wang, S. A Self-Cleaning TiO2 Nanosisal-like Coating toward Disposing Nanobiochips of Cancer Detection. ACS Nano 2015, 9, 9284–9291. [Google Scholar] [CrossRef]
- Song, Y.Y.; Schmidt-Stein, F.; Berger, S.; Schmuki, P. TiO2 Nano Test Tubes as a Self-Cleaning Platform for High-Sensitivity Immunoassays. Small 2010, 6, 1180–1184. [Google Scholar] [CrossRef]
- Chokkareddy, R.; Bhajanthri, N.K.; Redhi, G.G. An Enzyme-Induced Novel Biosensor for the Sensitive Electrochemical Determination of Isoniazid. Biosensor 2017, 7, 21. [Google Scholar] [CrossRef]
- Bhirud, A.P.; Sathaye, S.D.; Waichal, R.P.; Ambekar, J.D.; Park, C.J.; Kale, B.B. In-situ Preparation of N-TiO2/Graphene Nanocomposite and its Enhanced Photocatalytic Hydrogen Production by H2S Splitting under Solar Light. Nanoscale 2015, 7, 5023–5034. [Google Scholar] [CrossRef]
- Gainanova, A.; Kuz’micheva, G.; Khramov, E.; Chumakov, R.; Zybinskiy, A.; Yashina, N. The Role of Composition and Structure of Vanadium-Doped Nanosized Titanium (IV) Oxides (Anatase and ƞ-phase) in the Realization of Photocatalytic, Adsorption and Bactericidal Properties. New J. Chem. 2018, 42, 13025–13037. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Quan, X.; Yu, H.; Zhao, H.; Zhang, Y. Fabrication of TiO2−Pt Coaxial Nanotube Array Schottky Structures for Enhanced Photocatalytic Degradation of Phenol in Aqueous Solution. J. Phys. Chem. C 2008, 112, 9285–9290. [Google Scholar] [CrossRef]
- Christopher, P.; Ingram, D.B.; Linic, S. Enhancing Photochemical Activity of Semiconductor Nanoparticles with Optically Active Ag Nanostructures: Photochemistry Mediated by Ag Surface Plasmons. J. Phys. Chem. C 2010, 114, 9173–9177. [Google Scholar] [CrossRef]
- Halim, N.H.A.; Heng, L.Y.; Marugan, R.S.P.M.; Hashim, U. Mediatorless Impedance Studies with Titanium Dioxide Conjugated Gold Nanoparticles for Hydrogen Peroxide Detection. Biosensor 2017, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Sellappan, R.; Nielsen, M.G.; González-Posada, F.; Vesborg, P.C.K.; Chorkendorff, I.; Chakarov, D. Effects of Plasmon Excitation on Photocatalytic Activity of Ag/TiO2 and Au/TiO2 Nanocomposites. J. Catal. 2013, 307, 214–221. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, H.D.; Krishnan, K.M. In Vivo Delivery, Pharmacokinetics, Biodistribution and Toxicity of Iron Oxide Nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [PubMed]
- Ankamwar, B.; Lai, T.C.; Huang, J.H.; Liu, R.S.; Hsiao, M.; Chen, C.H.; Hwu, Y. Biocompatibility of Fe3O4 Nanoparticles Evaluated by in Vitro Cytotoxicity Assays using Normal, Glia and Breast Cancer Cells. Nanotechnology 2010, 21, 075102. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Yang, X.; Zhou, Y.; Yao, Y.; Li, C. Multifunctional Fe3O4@TiO2@Au magnetic microspheres as recyclable substrates for surface-enhanced Raman scattering. Nanoscale 2014, 6, 5971–5979. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Deng, Y.; Zou, Y.; Li, C.; Guo, X.; Xiong, L.; Gao, Y.; Li, F.; Zhao, D. Highly Water-Dispersible Biocompatible Magnetite Particles with Low Cytotoxicity Stabilized by Citrate Groups. Angew. Chem. Int. Ed. 2009, 48, 5875–5879. [Google Scholar] [CrossRef]
- Ma, W.F.; Zhang, Y.; Li, L.L.; You, L.J.; Zhang, P.; Zhang, Y.T.; Li, J.M.; Yu, M.; Guo, J.; Lu, H.J.; et al. Tailor-Made Magnetic Fe3O4@mTiO2 Microspheres with a Tunable Mesoporous Anatase Shell for Highly Selective and Effective Enrichment of Phosphopeptides. ACS Nano 2012, 6, 3179–3188. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Zhang, R.; Yang, J.; Tan, X.; Cui, Y. Highly Sensitive Immunoassay Based on Raman Reporter-Labeled Immuno-Au Aggregates and SERS-Active Immune Substrate. Biosens. Bioelectron. 2009, 25, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, J.; Feng, Z.; Wang, F.; Xie, S.; Bu, S. Immunoassay for Tumor Markers in Human Serum Based on Si Nanoparticles and SiC@Ag SERS-Active Substrate. Analyst 2016, 141, 2534–2541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, F.; Yang, W.; Guo, M.; Wang, X.; Zhang, B.; Tang, J. A Facile One-pot Method to High-Quality Ag-Graphene Composite Nanosheets for Efficient Surface-Enhanced Raman Scattering. Chem. Commun. 2011, 47, 6440. [Google Scholar] [CrossRef] [PubMed]
- Indrasekara, A.S.D.S.; Paladini, B.J.; Naczynski, D.J.; Starovoytov, V.; Moghe, P.V.; Fabris, L. Dimeric Gold Nanoparticle Assemblies as Tags for SERS-Based Cancer Detection. Adv. Heal. Mater. 2013, 2, 1370–1376. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Luo, C.; Liu, Z.; Chen, Y.; Dong, S.; Jiang, C.; Yang, S.; Wang, F.; Xiao, X. Volume-Enhanced Raman Scattering Detection of Viruses. Small 2019, 15, 1805516. [Google Scholar] [CrossRef]
- Michaelson, H.B. The Work Function of the Elements and its Periodicity. J. Appl. Phys. 1977, 48, 4729. [Google Scholar] [CrossRef]
- Tan, T.; Yip, A.C.; Beydoun, D.; Amal, R. Effects of Nano-Ag Particles Loading on TiO2 Photocatalytic Reduction of Selenate Ions. Chem. Eng. J. 2003, 95, 179–186. [Google Scholar] [CrossRef]
- Zhang, Q.; Lima, D.Q.; Lee, I.; Zaera, F.; Chi, M.; Yin, Y. A Highly Active Titanium Dioxide Based Visible-Light Photocatalyst with Nonmetal Doping and Plasmonic Metal Decoration. Angew. Chem. Int. Ed. 2011, 50, 7088–7092. [Google Scholar] [CrossRef]
- Kazuma, E.; Tatsuma, T. In Situ Nanoimaging of Photoinduced Charge Separation at the Plasmonic Au Nanoparticle-TiO2 Interface. Adv. Mater. Interfaces 2014, 1, 1400066. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Li, X.; Zeng, G.; Wang, D.; Niu, C.G.; Zhao, J.; An, H.; Xie, T.; Deng, Y. Hierarchical Assembly of Graphene-Bridged Ag3PO4/Ag/BiVO4 (040) Z-Scheme Photocatalyst: An Efficient, Sustainable and Heterogeneous Catalyst with Enhanced Visible-Light Photoactivity towards Tetracycline Degradation under Visible Light Irradiation. Appl. Catal. B Environ. 2017, 200, 330–342. [Google Scholar] [CrossRef]
- Wu, C.Y.; Tu, K.J.; Deng, J.P.; Lo, Y.S.; Wu, C.H. Markedly Enhanced Surface Hydroxyl Groups of TiO2 Nanoparticles with Superior Water-Dispersibility for Photocatalysis. Materials 2017, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, G. Green Synthesis of a Bifunctional Fe–Montmorillonite Composite during the Fenton Degradation Process and its Enhanced Adsorption and Heterogeneous Photo-Fenton Catalytic Properties. RSC Adv. 2016, 6, 2537–2545. [Google Scholar] [CrossRef]
- Liang, Z.; Zhou, J.; Petti, L.; Shao, L.; Jiang, T.; Qing, Y.; Xie, S.; Wu, G.; Mormile, P. SERS-Based Cascade Amplification Bioassay Protocol of miRNA-21 by Using Sandwich Structure with Biotin–Streptavidin System. Analyst 2019, 144, 1741–1750. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Wang, F.; Jia, Z.; Zhou, J.; Jiang, T.; Petti, L.; Chen, Y.; Xiong, Q.; Wang, X. Classification Analyses for Prostate Cancer, Benign Prostate Hyperplasia and Healthy Subjects by SERS-Based Immunoassay of Multiple Tumour Markers. Talanta 2018, 188, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, B.-B.; Liu, X.Q.; Zhang, Y.; Xu, Y.; Chen, Q.D.; Sun, H.B. Highly Efficient SERS Test Strips. Chem. Commun. 2012, 48, 5913. [Google Scholar] [CrossRef]
- Fan, M.; Andrade, G.F.; Brolo, A.G. A Review on the Fabrication of Substrates for Surface Enhanced Raman Spectroscopy and their Applications in Analytical Chemistry. Anal. Chim. Acta 2011, 693, 7–25. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Su, S.; Xu, T.; Zhong, Y.; Zapien, J.A.; Li, J.; Fan, C.; Lee, S.T. Silicon Nanowires-based Highly-Efficient SERS-Active Platform for Ultrasensitive DNA Detection. Nano Today 2011, 6, 122–130. [Google Scholar] [CrossRef]
- Wang, A.J.; Lv, J.J.; Zhou, D.L.; Weng, X.; Qin, S.F.; Feng, J.J. Facile Synthesis of Ultrathin Worm-like Au Nanowires for Highly Active SERS Substrates. New J. Chem. 2014, 38, 3395–3400. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Wang, M.; Petti, L.; Jiang, T.; Jia, Z.; Xie, S.; Zhou, J. SERS-based Multiplex Immunoassay of Tumor Markers Using Double SiO2@Ag Immune Probes and Gold-Film Hemisphere Array Immune Substrate. Colloids Surfaces A: Physicochem. Eng. Asp. 2018, 546, 48–58. [Google Scholar] [CrossRef]
- Boqué, R.; Heyden, Y.V. The Limit of Detection. LC GC Eur. 2009, 22, 82–85. [Google Scholar]
- Wang, W.; Zhang, Y.; Wang, J.; Shi, X.; Ye, J. Determination of β-agonists in Pig Feed, Pig Urine and Pig Liver Using Capillary Electrophoresis with Electrochemical Detection. Meat Sci. 2010, 85, 302–305. [Google Scholar] [CrossRef] [PubMed]

| SERS Probes | Zeta Potential/mV |
|---|---|
| Fe3O4 | −1.4 ± 0.1 |
| Fe3O4@TiO2 | −22.9 ± 0.4 |
| Fe3O4@TiO2@NH2 | 25.4 ± 0.5 |
| Fe3O4@TiO2@Au | 7.8 ± 0.8 |
| Fe3O4@TiO2@Au@4MBA | −17.9 ± 1.2 |
| Fe3O4@TiO2@Au@4MBA@anti-PSA | −20.2 ± 1.1 |
| Fe3O4@TiO2@Au@4MBA@anti-PSA, under UV | 4.8 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Liu, H.; Tian, Y.; Gu, C.; Zhao, Z.; Zeng, S.; Jiang, T. Recyclable SERS-Based Immunoassay Guided by Photocatalytic Performance of Fe3O4@TiO2@Au Nanocomposites. Biosensors 2020, 10, 25. https://doi.org/10.3390/bios10030025
Du Y, Liu H, Tian Y, Gu C, Zhao Z, Zeng S, Jiang T. Recyclable SERS-Based Immunoassay Guided by Photocatalytic Performance of Fe3O4@TiO2@Au Nanocomposites. Biosensors. 2020; 10(3):25. https://doi.org/10.3390/bios10030025
Chicago/Turabian StyleDu, Yuanyuan, Hongmei Liu, Yiran Tian, Chenjie Gu, Ziqi Zhao, Shuwen Zeng, and Tao Jiang. 2020. "Recyclable SERS-Based Immunoassay Guided by Photocatalytic Performance of Fe3O4@TiO2@Au Nanocomposites" Biosensors 10, no. 3: 25. https://doi.org/10.3390/bios10030025
APA StyleDu, Y., Liu, H., Tian, Y., Gu, C., Zhao, Z., Zeng, S., & Jiang, T. (2020). Recyclable SERS-Based Immunoassay Guided by Photocatalytic Performance of Fe3O4@TiO2@Au Nanocomposites. Biosensors, 10(3), 25. https://doi.org/10.3390/bios10030025






