A Label-Free Immunosensor Based on Graphene Oxide/Fe3O4/Prussian Blue Nanocomposites for the Electrochemical Determination of HBsAg
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. Preparation of Fe3O4/GO Nanocomposites
2.4. Preparation of PB/Fe3O4/GO Nanocomposites
2.5. Fabrication of the Immunosensor
2.6. Electrochemical Measurements
3. Results and Discussion
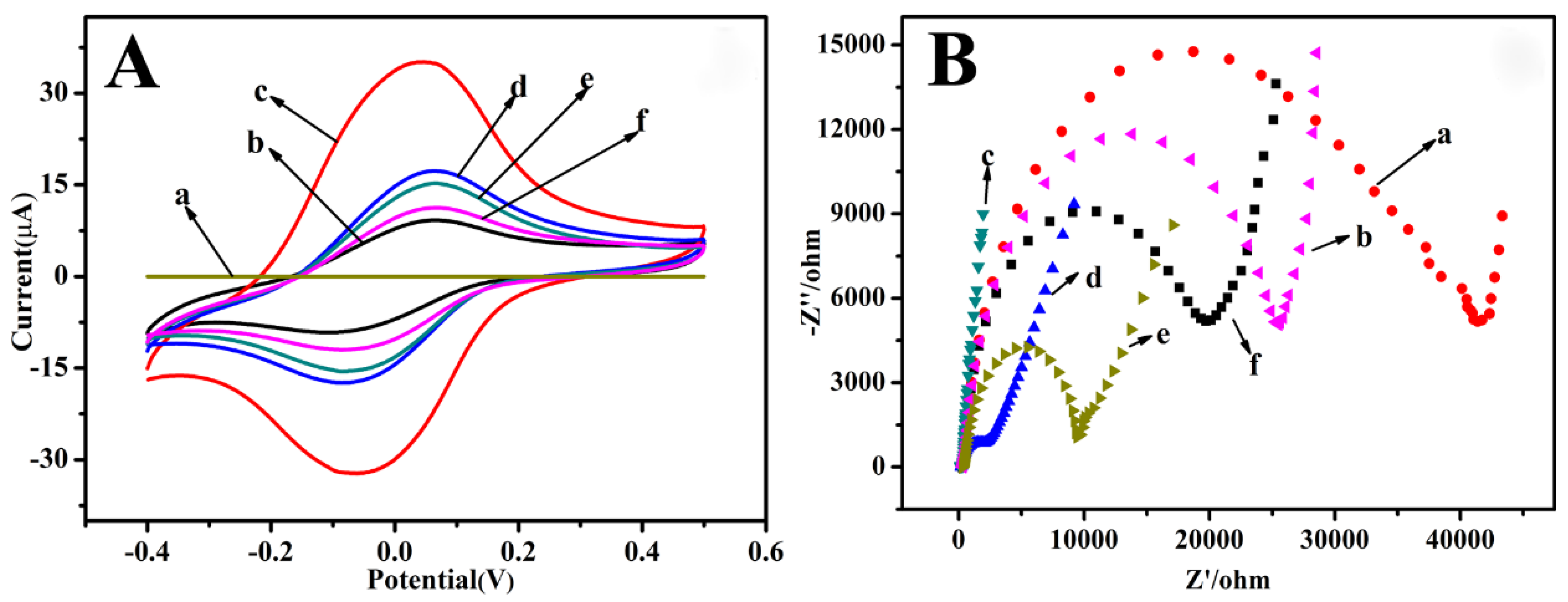
3.1. Electrochemical Characterization of the Immunosensor
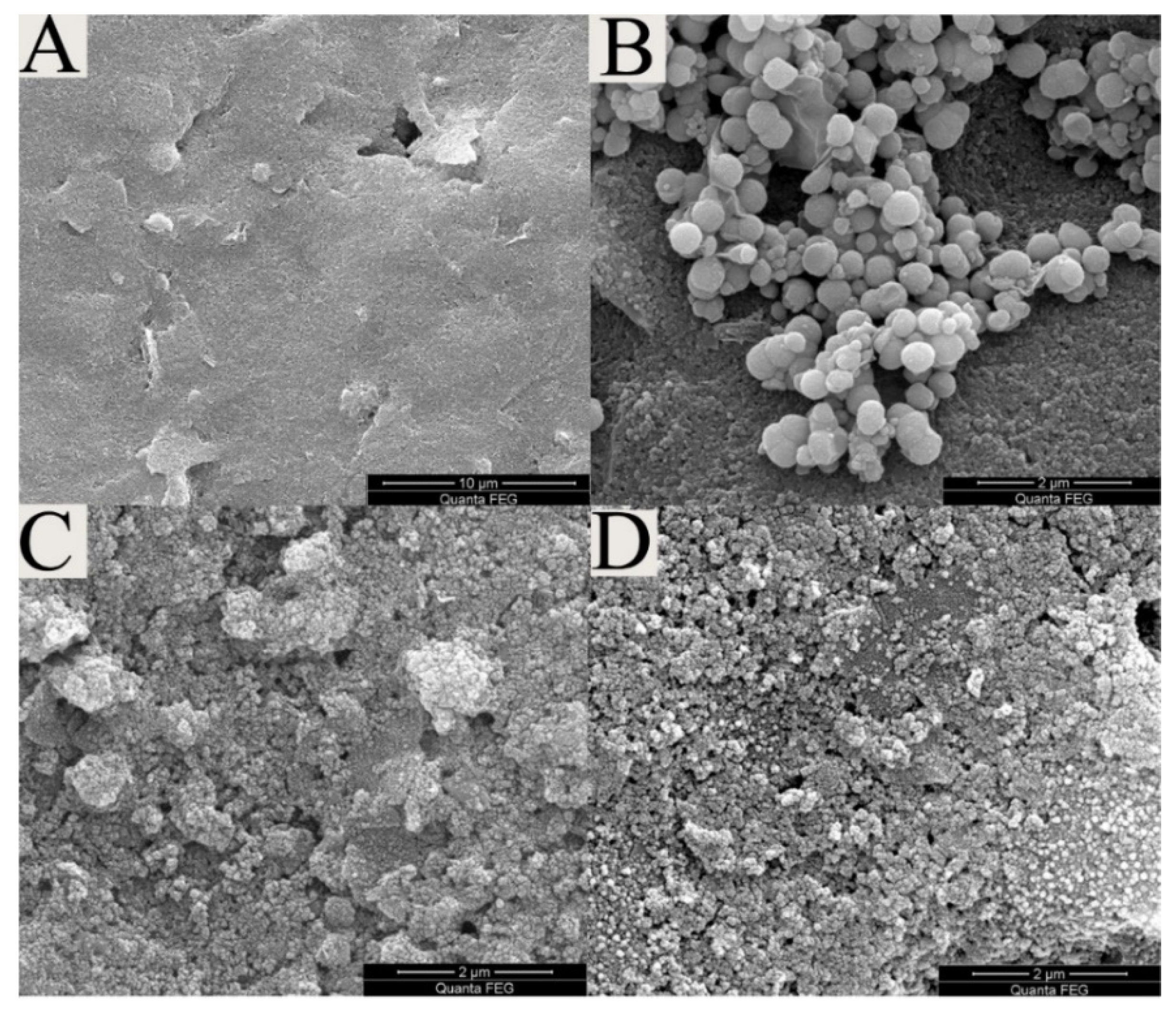
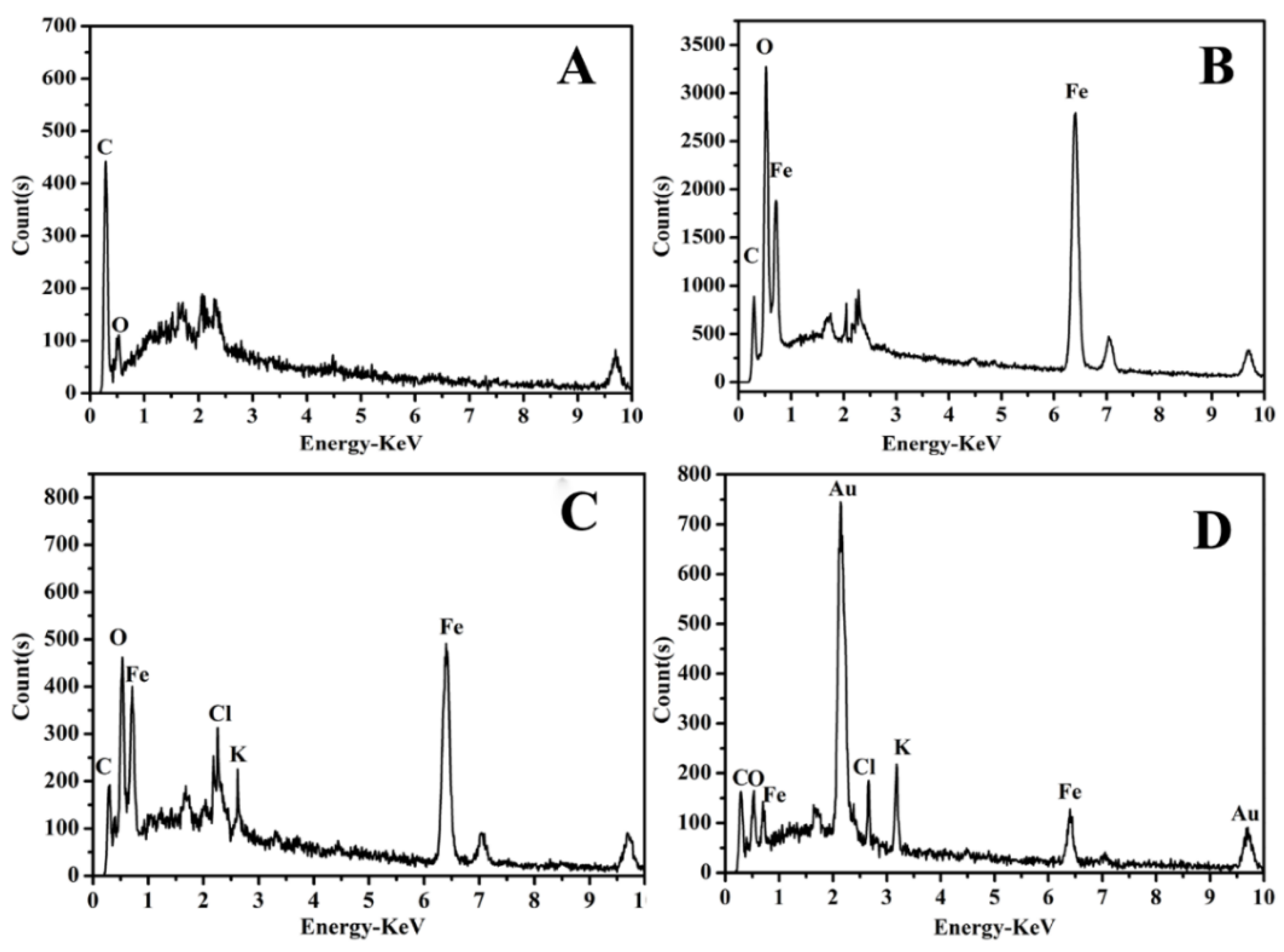
3.2. Characterization of the Modified SPEs
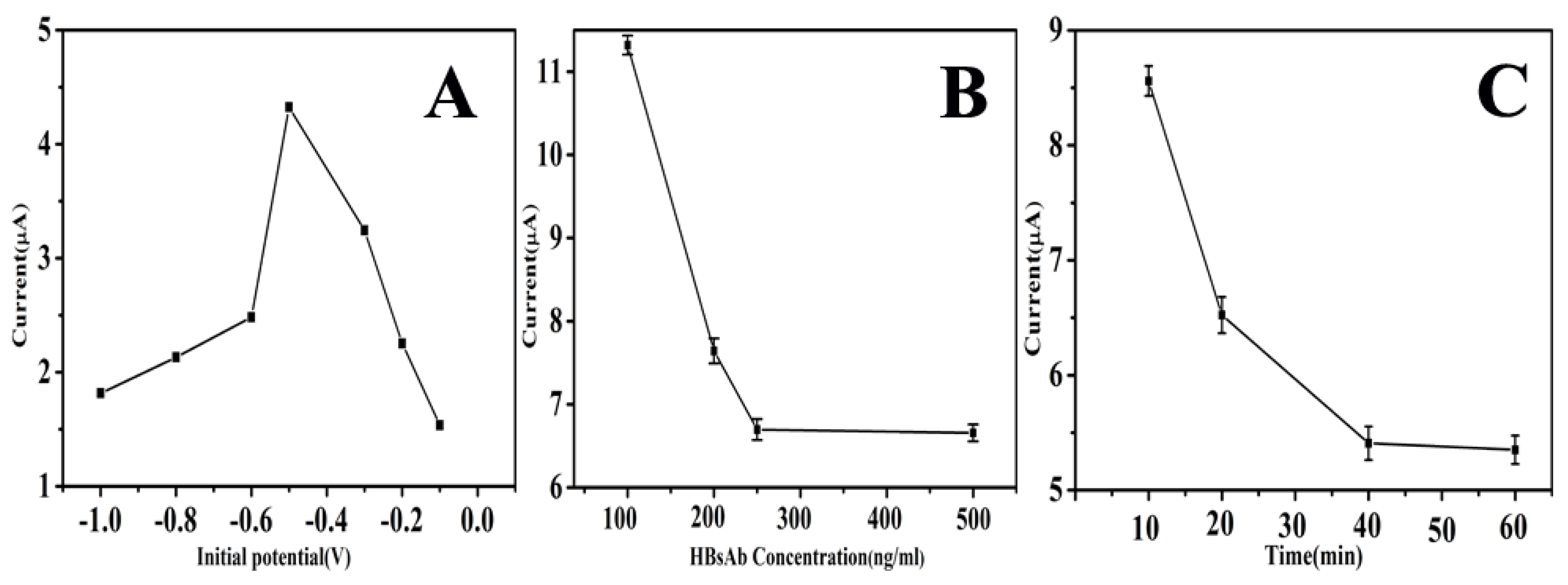
3.3. Optimization of Experimental Condition
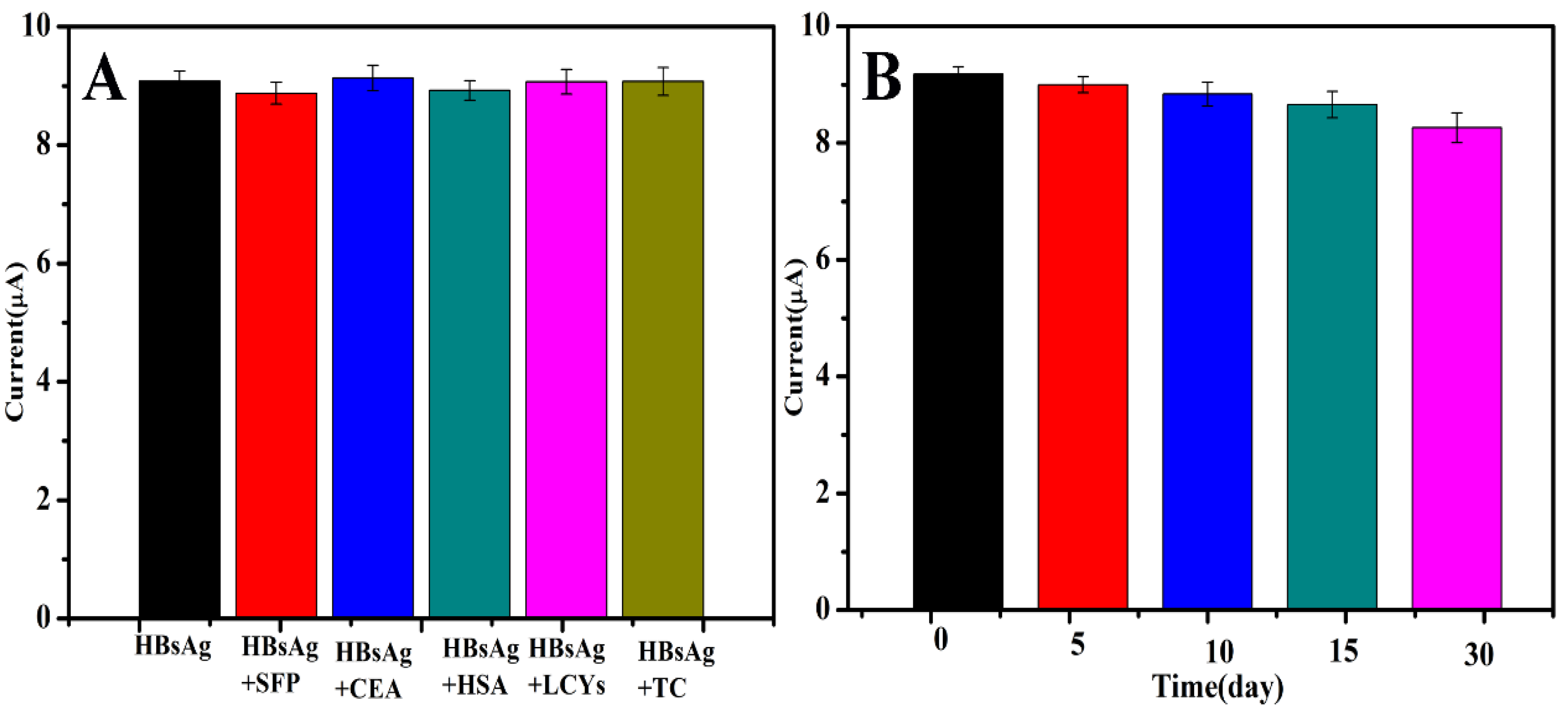
3.4. Selectivity, Stability and Repeatability of the Immunosensor
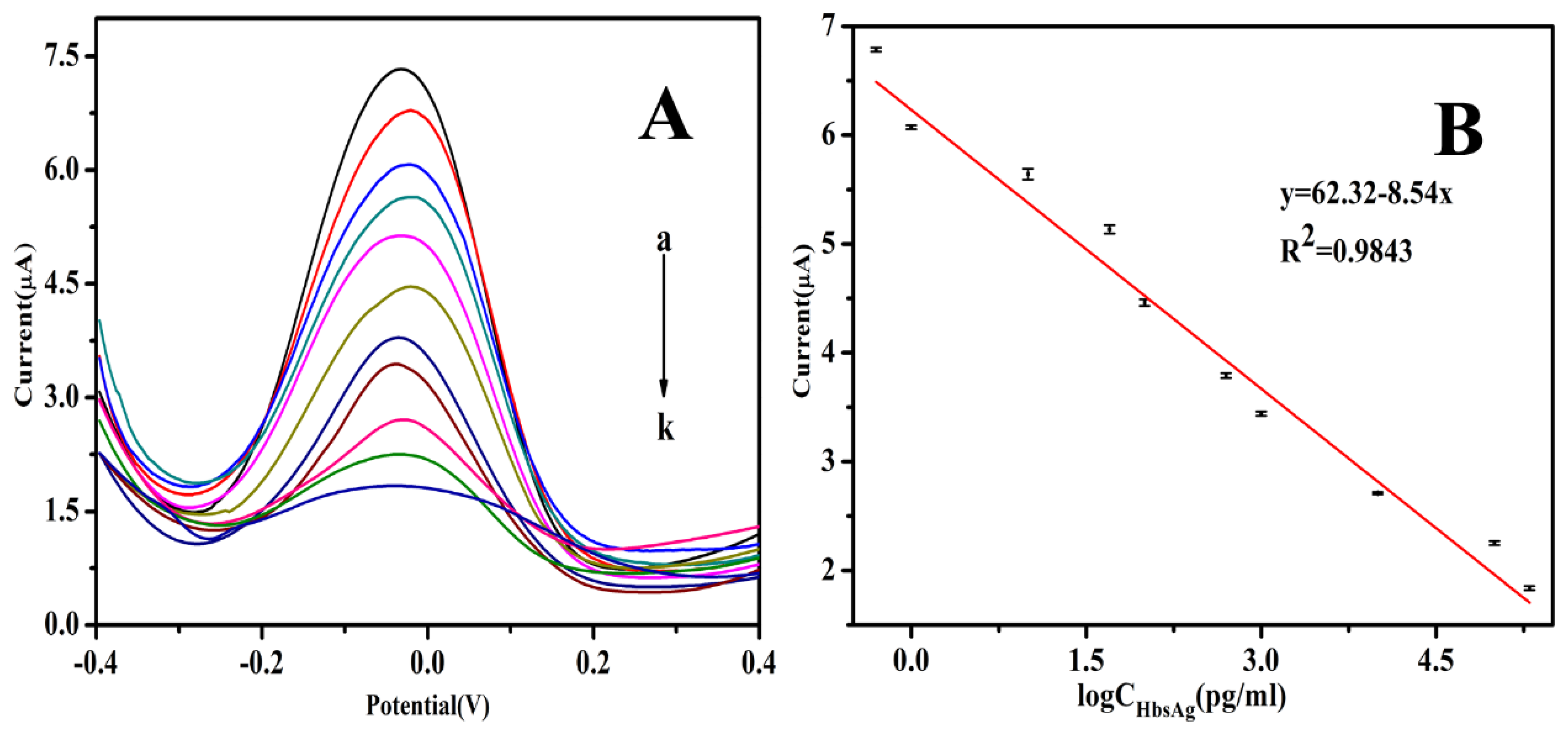
3.5. Analytical Performance
3.6. Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valaydon, Z.S.; Locarnini, S.A. The virological aspects of hepatitis B. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Elisabetta, F. Hepatitis B: Epidemiology and prevention in developing countries. World J. Hepatol. 2012, 4, 48–54. [Google Scholar]
- D’Souza, R.; Foster, G.R. Diagnosis and treatment of chronic hepatitis B. J. R. Soc. Med. 2004, 97, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Martinotpeignoux, M.; Carvalhofilho, R.; Lapalus, M.; Lada, O.; Batrla, R.; Krause, F.; Asselah, T.; Marcellin, P. Hepatitis B surface antigen serum level is associated with fibrosis severity in treatment-naïve, e antigen-positive patients. J. Hepatol. 2013, 58, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Ganem, D.; Prince, A.M. Hepatitis B virus infection—Natural history and clinical consequences. N. Engl. J. Med. 2004, 350, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Weinian, Z.; Shigui, R. Modeling the transmission dynamics and control of hepatitis B virus in China. J. Theor. Biol. 2017, 262, 330–338. [Google Scholar]
- Gyss, C.; Bourdillon, C. Enzymatic electrocatalysis as a strategy for electrochemical detection in heterogeneous immunoassays. Anal. Chem. 1987, 59, 2350–2355. [Google Scholar] [CrossRef]
- Qiao, Y.H.; Ze-Liang, G.U.; Jian, Z. Development and application of TRFIA for hepatitis B surface antibody. Label. Immunoass. Clin. Med. 2007, 14, 24–26. [Google Scholar]
- Rodrigues, C.; Deshmukh, M.; Jacob, T.; Nukala, R.; Menon, S.; Mehta, A. Significance of HBV DNA by PCR over serological markers of HBV in acute and chronic patients. Indian J. Med. Microbiol. 2001, 19, 141–144. [Google Scholar]
- MacGregor, I.; Hope, J.; Barnard, G.; Kirby, L. Application of a time-resolved fluoroimmunoassay for the analysis of normal prion protein in human blood and its components. Vox Sang. 1999, 77, 88–96. [Google Scholar] [CrossRef]
- Song, Y.; Luo, Y.; Zhu, C.; Li, H.; Du, D.; Lin, Y. Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens. Bioelectron. 2016, 76, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Manju, R.; Vu, H.; Savay, S.; Chea, K.; Toda, K. Results from nationwide hepatitis B serosurvey in Cambodia using simple and rapid laboratory test: Implications for national immunization program. Am. J. Trop. Med. Hyg. 2009, 81, 252–257. [Google Scholar]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2017, 98, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Ekins, R.P. Ligand assays: From electrophoresis to miniaturized microarrays. Clin. Chem. 1998, 44, 2015–2030. [Google Scholar] [CrossRef]
- Ricci, F.; Adornetto, G.; Palleschi, G. A review of experimental aspects of electrochemical immunosensors. Electrochim. Acta 2012, 84, 74–83. [Google Scholar] [CrossRef]
- Skládal, P. Advances in electrochemical immunosensors. Electroanalysis 2010, 9, 737–745. [Google Scholar] [CrossRef]
- Vetterl, V.; Papadopoulos, N.; Dražan, V.; Strašák, L.; Hasoň, S.; Dvořák, J. Nucleic acid sensing by impedance measurements. Electrochim. Acta 2000, 45, 2961–2971. [Google Scholar] [CrossRef]
- Fang, L.; Yuqi, Y.; Hua, C.; Di, Y.; Zhiping, B. Label-Free electrochemiluminescence immunosensor for cardiac troponin I using luminol functionalized gold nanoparticles as a sensing platform. Analyst 2013, 138, 1844–1850. [Google Scholar]
- Holford, T.R.J.; Frank, D.; Higson, S.P.J. Recent trends in antibody based sensors. Biosens. Bioelectron. 2012, 34, 12–24. [Google Scholar] [CrossRef]
- Makaraviciute, A.; Ramanaviciene, A. Site-Directed antibody immobilization techniques for immunosensors. Biosens. Bioelectron. 2013, 50, 460–471. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Zheng, S. A novel and label-free immunosensor for bisphenol A using rutin as the redox probe. Talanta 2016, 160, 241–246. [Google Scholar] [CrossRef]
- Xiang, Q.; Gao, Y.; Liu, J.Q.; Wang, K.Q.; Tang, J.; Yang, M.; Wang, S.P.; Wang, W.L. Development of nanomaterials electrochemical biosensor and its applications. Adv. Mater. Res. 2011, 418–420, 2082–2085. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Jin, W.; Maduraiveeran, G.; Jin, W. Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications. Trends Environ. Anal. Chem. 2017, 13, 10–23. [Google Scholar] [CrossRef]
- Kim, K.S.; Yue, Z.; Jang, H.; Sang, Y.L.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-Scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Tang, L.; Ying, W.; Li, Y.; Feng, H.; Li, J. Preparation, structure, and electrochemical properties of reduced graphene sheet films. Adv. Funct. Mater. 2010, 19, 2782–2789. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Hong, W.; Liu, J.J.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Li, H.; He, J.; Li, S.; Turner, A.P.F. Electrochemical immunosensor with N-doped graphene-modified electrode for label-free detection of the breast cancer biomarker CA 15-3. Biosens. Bioelectron. 2013, 43, 25–29. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Z.; Liu, N.; Ma, Z. A label-free immunosensor based on graphene nanocomposites for simultaneous multiplexed electrochemical determination of tumor markers. Biosens. Bioelectron. 2014, 53, 160–166. [Google Scholar] [CrossRef]
- Salamon, J.; Sathishkumar, Y.; Ramachandran, K.; Yang, S.L.; Dong, J.Y.; Kim, A.R.; Kumar, G.G. One-Pot synthesis of magnetite nanorods/graphene composites and its catalytic activity toward electrochemical detection of dopamine. Biosens. Bioelectron. 2015, 64, 269–276. [Google Scholar] [CrossRef]
- Nourani, S.; Ghourchian, H.; Boutorabi, S.M. Magnetic nanoparticle-based immunosensor for electrochemical detection of hepatitis B surface antigen. Anal. Biochem. 2013, 441, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Qiao, X.; Wang, H.; Sun, Z.; Hong, C. A sandwich-type electrochemical immunosensor for carcinoembryonic antigen based on signal amplification strategy of optimized ferrocene functionalized Fe3O4@SiO2 as labels. Biosens. Bioelectron. 2016, 79, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Yitao, H.; Pei, L.; Yiting, X.; Hao, L.; Zhiling, S.; Zhou, N.; Zhuo, C.; Shouzhuo, Y. Fluorescent nanosensor for probing histone acetyltransferase activity based on acetylation protection and magnetic graphitic nanocapsules. Small 2015, 11, 877–885. [Google Scholar]
- Robin, M.B. The color and electronic configurations of prussian blue. Inorg. Chem. 1962, 1, 1095. [Google Scholar] [CrossRef]
- Itaya, K.; Uchida, I. Nature of intervalence charge-transfer bands in Prussian blues. Inorg. Chem. 1986, 25, 389–392. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, G.; Huang, H.; Wang, L. Graphene-Prussian blue/gold nanoparticles based electrochemical immunoassay of carcinoembryonic antigen. Anal. Methods 2011, 3, 2082–2087. [Google Scholar] [CrossRef]
- Nirala, N.R.; Pandey, S.; Bansal, A.; Singh, V.K.; Mukherjee, B.; Saxena, P.S.; Srivastava, A. Different shades of cholesterol: Gold nanoparticles supported on MoS2 nanoribbons for enhanced colorimetric sensing of free cholesterol. Biosens. Bioelectron. 2015, 74, 207–213. [Google Scholar] [CrossRef]
- Sun, D.; Li, H.; Li, M.; Li, C.; Qian, L.; Yang, B. Electrochemical immunosensors with AuPt-vertical graphene/glassy carbon electrode for alpha-fetoprotein detection based on label-free and sandwich-type strategies. Biosens. Bioelectron. 2019, 132, 68–75. [Google Scholar] [CrossRef]
- Huang, K.J.; Niu, D.J.; Xie, W.Z.; Wang, W. A disposable electrochemical immunosensor for carcinoembryonic antigen based on nano-Au/multi-walled carbon nanotubes-chitosans nanocomposite film modified glassy carbon electrode. Anal. Chim. Acta 2010, 659, 102–108. [Google Scholar] [CrossRef]
- Baniukevic, J.; Kirlyte, J.; Ramanavicius, A.; Ramanaviciene, A. Application of oriented and random antibody immobilization methods in immunosensor design. Sens. Actuators B Chem. 2013, 189, 217–223. [Google Scholar] [CrossRef]
- Omidfar, K.; Khorsand, F.; Azizi, M.D. New analytical applications of gold nanoparticles as label in antibody based sensors. Biosens. Bioelectron. 2013, 43, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Ruiyi, L.; Juanjuan, Z.; Zhouping, W.; Zaijun, L.; Junkang, L.; Zhiguo, G.; Guangli, W. Novel graphene-gold nanohybrid with excellent electrocatalytic performance for the electrochemical detection of glucose. Sens. Actuators B Chem. 2015, 208, 421–428. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Dan, W.; Ma, H.; Qin, W. Label-Free electrochemical immunosensor based on flower-like Ag/MoS2/rGO nanocomposites for ultrasensitive detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2017, 255, 125–132. [Google Scholar] [CrossRef]
- Yaoguang, W.; Guanhui, Z.; Yong, Z.; Xuehui, P.; Wei, C. Sandwich-Type electrochemical immunosensor for CEA detection based on Ag/MoS2@Fe3O4 and an analogous ELISA method with total internal reflection microscopy. Sens. Actuators B Chem. 2018, 561–569. [Google Scholar]
- Zhijiang, Z.L.; Huang, R.; He, N.; Wang, T.; Su, E.; Deng, Y. Selection of HBsAg-specific DNA aptamers based on carboxylated magnetic nanoparticles and their application in the rapid and simple detection of hepatitis B virus infection. ACS Appl. Mater. Interfaces 2015, 7, 11215–11223. [Google Scholar]
- Tian, L.; Li, L.; Li, Y.; Xue, F.; Wei, C. A novel label-free electrochemical immunosensor for the detection of hepatitis B surface antigen. Anal. Methods 2016, 8, 7380–7386. [Google Scholar] [CrossRef]
- Zhao, F.; Bai, Y.; Zeng, R.; Cao, L.; Zhu, J.; Han, G.; Chen, Z. An Electrochemical immunosensor with graphene-oxide-ferrocene-based nanocomposites for hepatitis B surface antigen detection. Electroanalysis 2018, 30, 1–8. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.Y.; Shuklab, S.; Hongc, S.B.; Heoa, N.S.; Bajpaib, V.K.; Chund, H.S.; Joe, C.; Choic, B.G.; Huha, Y.S.; et al. Heteroassembled gold nanoparticles with sandwich-immunoassay LSPR chip format for rapid and sensitive detection of hepatitis B virus surface antigen (HBsAg). Biosens. Bioelectron. 2018, 107, 118–122. [Google Scholar] [CrossRef]
- Qiu, J.D.; Liang, R.-P.; Wang, R.; Fan, L.-X.; Chen, Y.-W.; Xia, X.-H. A label-free amperometric immunosensor based on biocompatible conductive redox chitosan-ferrocene/gold nanoparticles matrix. Biosens. Bioelectron. 2009, 25, 852–857. [Google Scholar] [CrossRef]
- Alizadeh, N.; Hallaj, R.; Salimi, A. Dual amplified electrochemical immunosensor for hepatitis B virus surface antigen detection using hemin/G-quadruplex immobilized onto Fe3O4-AuNPs or (hemin-amino-rGO-Au) nanohybrid. Electroanalysis 2018, 30, 402–414. [Google Scholar] [CrossRef]
- Akkapinyo, C.; Khownarumit, P.; Waraho-Zhmayev, D.; Poo-Arporn, R.P. Development of a multiplex immunochromatographic strip test and ultrasensitive electrochemical immunosensor for hepatitis B virus screening. Anal. Chim. Acta 2020, 1095, 162–171. [Google Scholar] [CrossRef] [PubMed]


| Immunosensor | Linear Range | Detection Limit (ng/mL) | Reference |
|---|---|---|---|
| Fe3O4@SiO2/MNPs/SELEX | 1–200 | 0.1 | [45] |
| Ab1@Ni AuPt-NGs/GCE | 0.001–80 | 0.00031 | [46] |
| GO-Fc-CS/Au NPs/GE | 0.05–150 | 0.01 | [47] |
| Nafion/gelatin/Au NPs/PDE | 4–800 | 1.3 | [48] |
| Chitosan-ferrocene/gold nanoparticles | 0.5–305 | 0.016 | [49] |
| EDC/NHS | 5–3000 | 2.1 | [50] |
| hemin-rGO/Au NPs | 0.0001–1 | 0.00001 | [51] |
| GO/Fe3O4/PB@AuNPs | 0.0005–200 | 0.00016 | This work |
| Sample No. | Proposed Method (ng/mL) (n = 5) | Reference Method (ng/mL) | Relative Error (%) |
|---|---|---|---|
| 1 | 5.7 × 10−3 | 5 × 10−3 | 14 |
| 2 | 0.121 | 0.118 | 2.54 |
| 3 | 9.27 | 9.4 | 1.38 |
| 4 | 98.21 | 96.35 | 1.94 |
| 5 | 125.96 | 129.63 | −2.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Xiao, H.; Cao, L.; Chen, Z. A Label-Free Immunosensor Based on Graphene Oxide/Fe3O4/Prussian Blue Nanocomposites for the Electrochemical Determination of HBsAg. Biosensors 2020, 10, 24. https://doi.org/10.3390/bios10030024
Wei S, Xiao H, Cao L, Chen Z. A Label-Free Immunosensor Based on Graphene Oxide/Fe3O4/Prussian Blue Nanocomposites for the Electrochemical Determination of HBsAg. Biosensors. 2020; 10(3):24. https://doi.org/10.3390/bios10030024
Chicago/Turabian StyleWei, Shanshan, Haolin Xiao, Liangli Cao, and Zhencheng Chen. 2020. "A Label-Free Immunosensor Based on Graphene Oxide/Fe3O4/Prussian Blue Nanocomposites for the Electrochemical Determination of HBsAg" Biosensors 10, no. 3: 24. https://doi.org/10.3390/bios10030024
APA StyleWei, S., Xiao, H., Cao, L., & Chen, Z. (2020). A Label-Free Immunosensor Based on Graphene Oxide/Fe3O4/Prussian Blue Nanocomposites for the Electrochemical Determination of HBsAg. Biosensors, 10(3), 24. https://doi.org/10.3390/bios10030024




