A Disposable Passive Microfluidic Device for Cell Culturing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
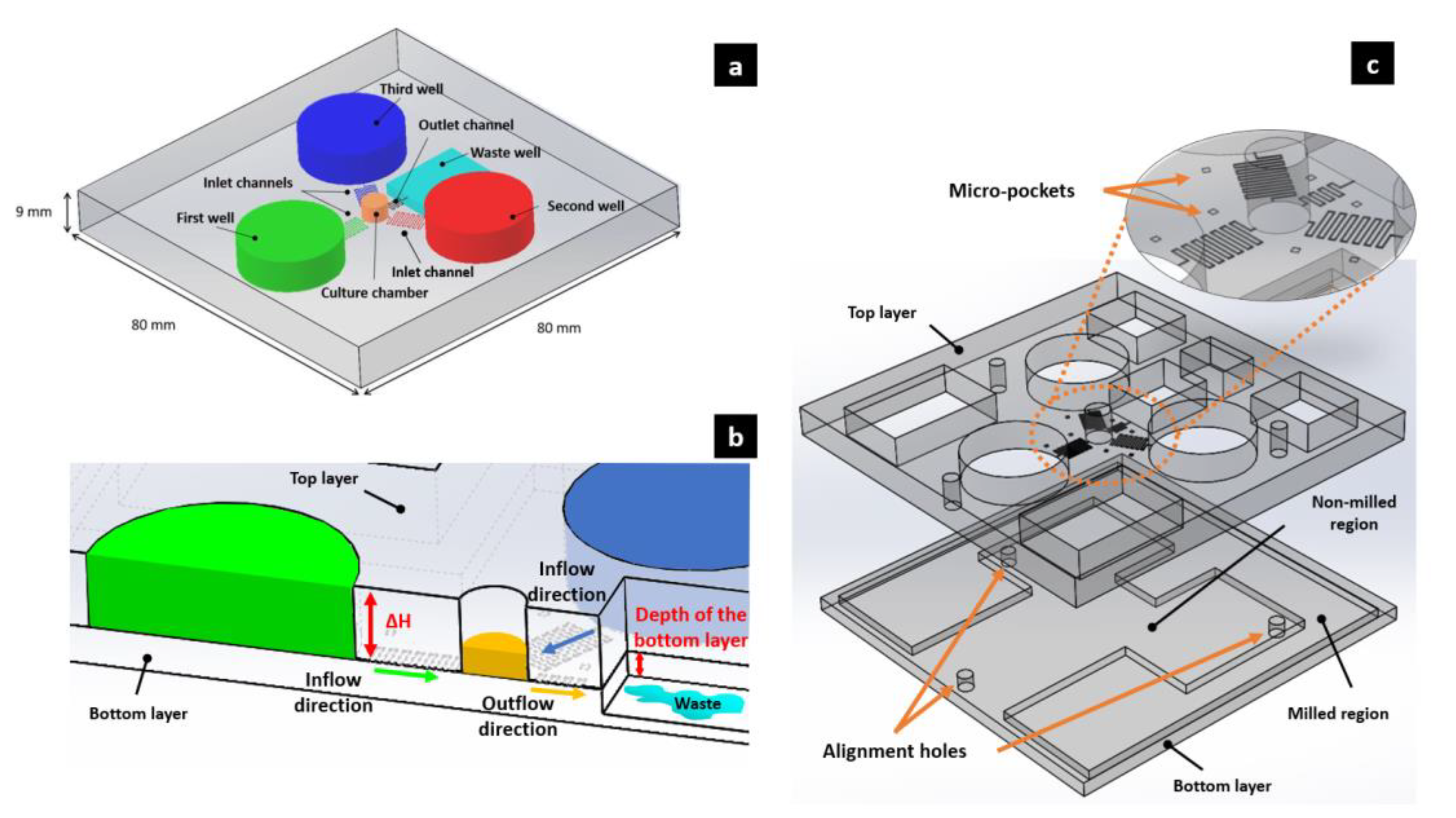
2.2. Working Principles of the Device
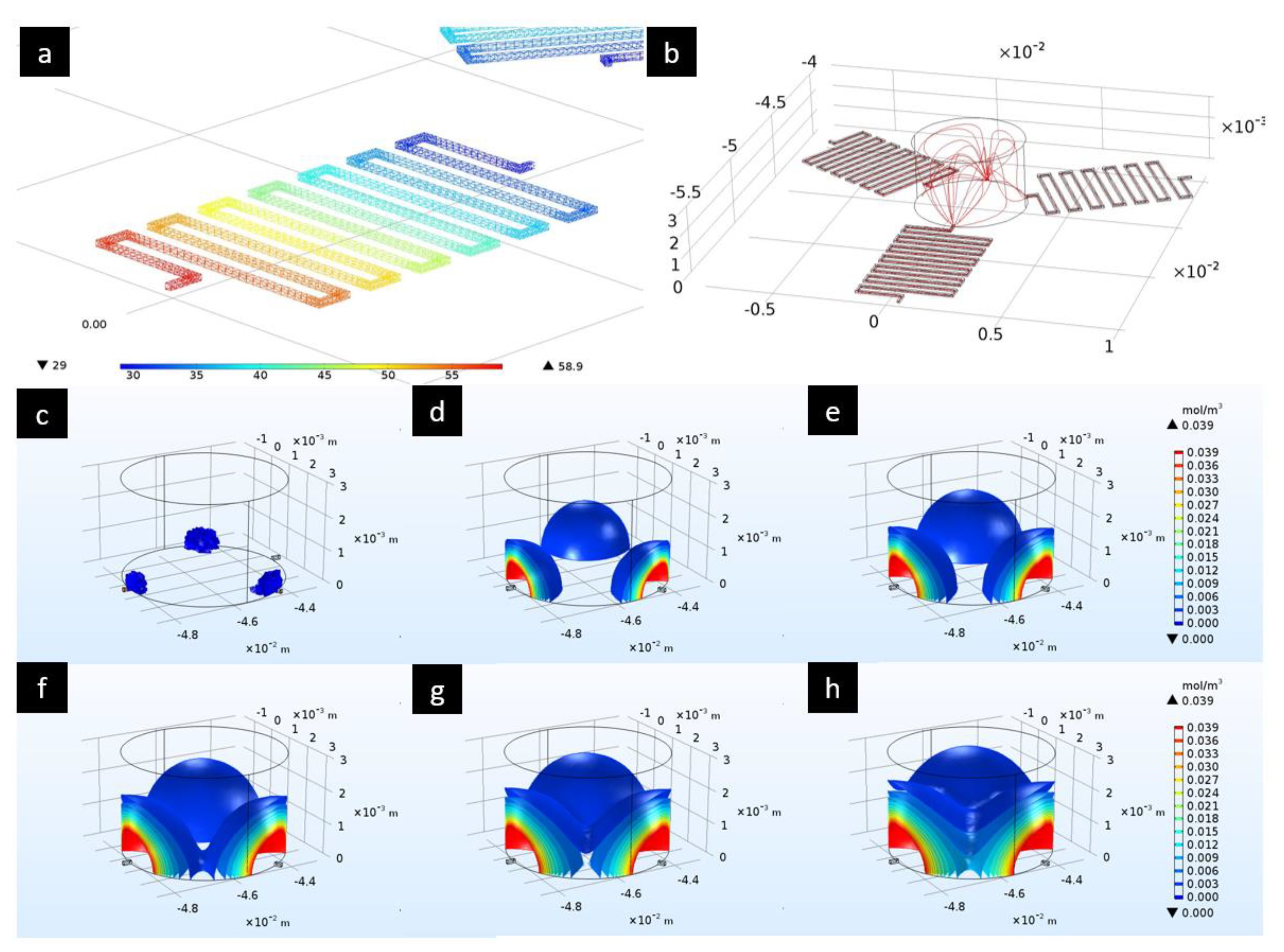
2.3. Flow and Pressure Drop Simulations
2.4. Device Manufacturing
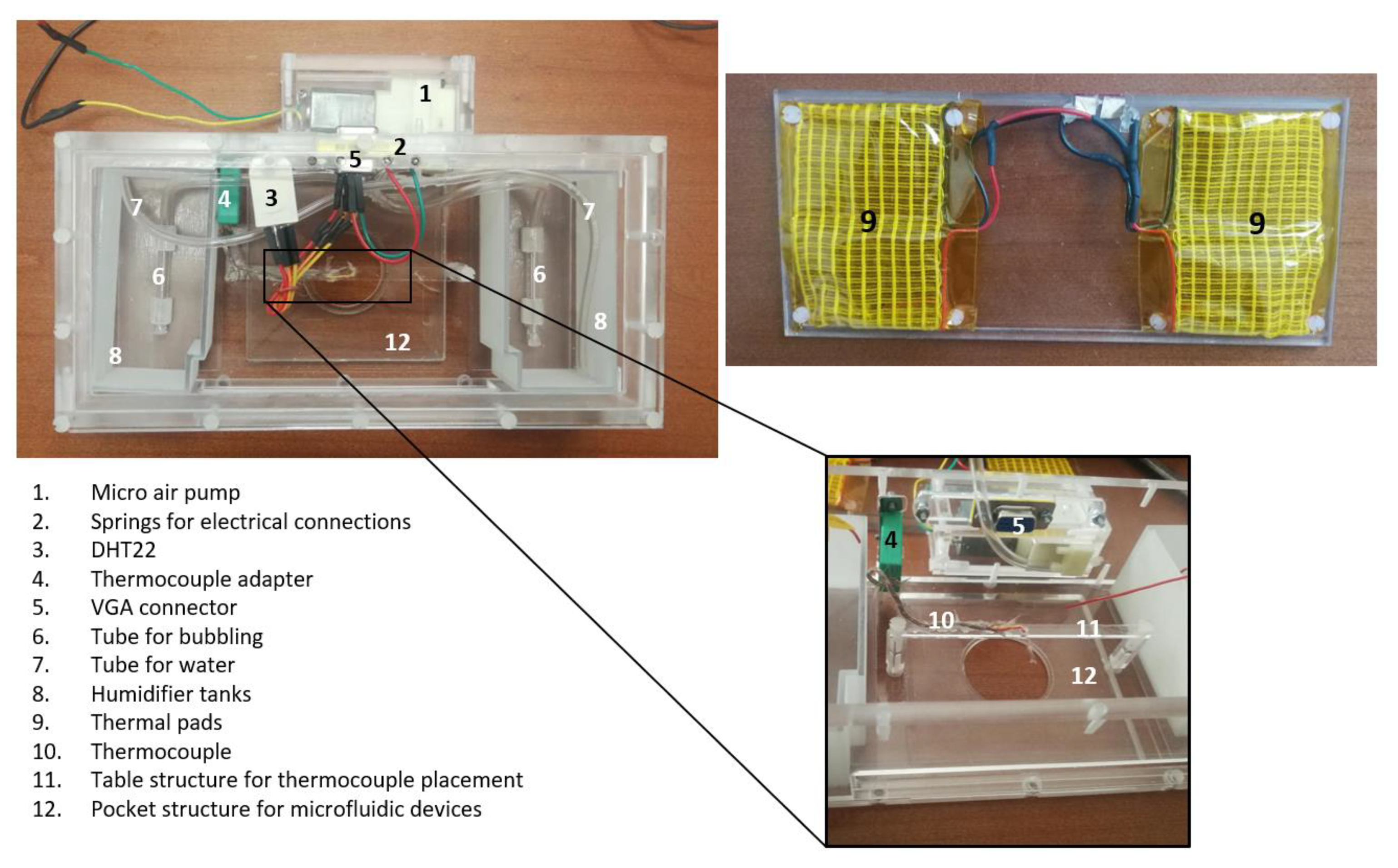
2.5. Experimental Setup
2.6. Device Experimental Characterization
2.7. Device Experimental Validation
- Priming of the channels with culture medium using suction tube under biological hood, thus preventing air bubbles;
- Filling of the reservoirs with culture medium (circa 2 mL for each);
- Suction of any volume accumulated in the culture chamber in the previous phases;
- Loading of cells inside the culture chamber at the concentration of circa 62,500/mL. The culture chamber was filled with circa 59 μL of fluid containing circa 3700 cells;
- Priming of the waste reservoir with 200 μL of culture medium to overcome hydrophobic forces.
3. Results
3.1. Flow and Pressure Drop Simulations
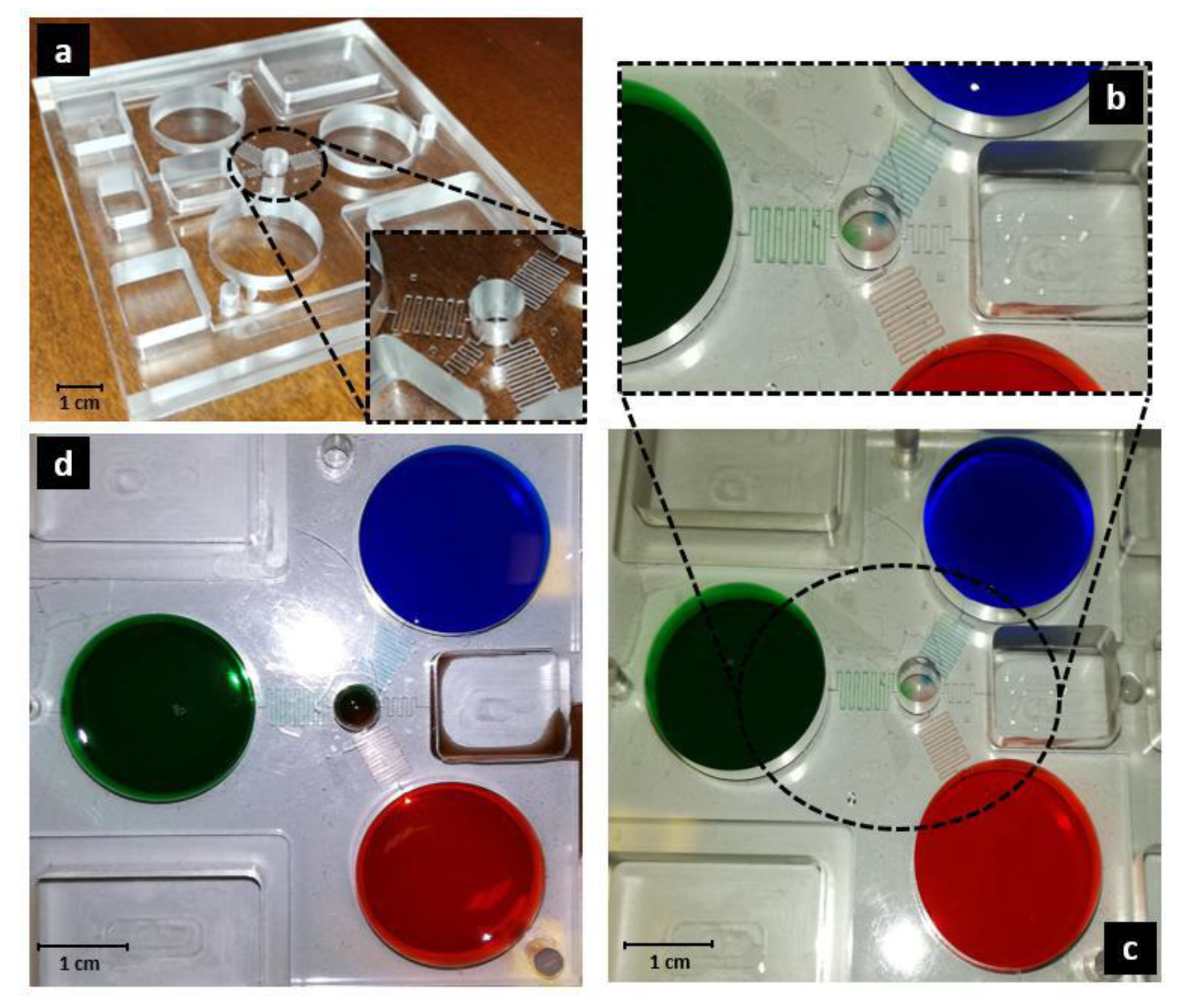
3.2. Device Manufacturing
3.3. Device Experimental Characterization and Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Halldorsson, S.; Lucumi, E.; Gomez-Sjoberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Toh, Y.C.; Voldman, J.; Yu, H. A practical guide to microfluidic perfusion culture of adherent mammalian cells. Lab Chip 2007, 7, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Perozziello, G.; Candeloro, P.; Gentile, F.; Nicastri, A.; Perri, A.; Coluccio, M.L.; Adamo, A.; Pardeo, F.; Catalano, R.; Parrotta, E.; et al. Microfluidics & nanotechnology: Towards fully integrated analytical devices for the detection of cancer biomarkers. RSC Adv. 2014, 4, 55590–55598. [Google Scholar]
- Wu, J.; Wu, X.; Lin, F. Recent developments in microfluidics-based chemotaxis studies. Lab Chip 2013, 13, 2484–2499. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Pui, T.S.; Rahman, A.R.; Kang, Y. 3D numerical simulation of a coulter counter array with analysis of electrokinetic forces. Electrophoresis 2013, 34, 417–424. [Google Scholar] [CrossRef]
- Perozziello, G.; Candeloro, P.; De Grazia, A.; Esposito, F.; Allione, M.; Coluccio, M.; Tallerico, R.; Valpapuram, I.; Tirinato, L.; Das, G.; et al. Microfluidic device for continuous single cells analysis via Raman spectroscopy enhanced by integrated plasmonic nanodimers. Opt. Express 2016, 24, A180. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Ai, Y. On-Demand Lensless Single Cell Imaging Activated by Differential Resistive Pulse Sensing. Anal. Chem. 2015, 87, 6516–6519. [Google Scholar] [CrossRef]
- Simone, G.; Malara, N.; Trunzo, V.; Perozziello, G.; Neuzil, P.; Francardi, M.; Roveda, L.; Renne, M.; Prati, U.; Mollace, V.; et al. Protein-carbohydrate complex reveals circulating metastatic cells in a microfluidic assay. Small 2013, 9, 2152–2161. [Google Scholar] [CrossRef]
- Simone, G.; Perozziello, G.; Sardella, G.; Disegna, I.; Tori, S.; Manaresi, N.; Medoro, G. A microvalve for hybrid microfluidic systems. Microsyst. Technol. 2010, 16, 1269–1276. [Google Scholar] [CrossRef]
- Perozziello, G.; Bundgaard, F.; Geschke, O. Fluidic interconnections for microfluidic systems: A new integrated fluidic interconnection allowing plug ‘n’play functionality. Sens. Actuators B Chem. 2008, 130, 947–953. [Google Scholar] [CrossRef]
- Snakenborg, D.; Perozziello, G.; Klank, H.; Geschke, O.; Kutter, J. Direct milling and casting of polymer-based optical waveguides for improved transparency in the visible range. J. Micromech. Microeng. 2006, 16, 375. [Google Scholar] [CrossRef]
- Xu, D.; Huang, X.; Guo, J.; Ma, X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens. Bioelectron. 2018, 110, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Perozziello, G.; Catalano, R.; Francardi, M.; Rondanina, E.; Pardeo, F.; Angelis, F.D.; Malara, N.; Candeloro, P.; Morrone, G.; Fabrizio, E.D. A microfluidic device integrating plasmonic nanodevices for Raman spectroscopy analysis on trapped single living cells. Microelectron. Eng. 2013, 111, 314–319. [Google Scholar] [CrossRef]
- Guo, J.; Li, C.M.; Kang, Y. PDMS-film coated on PCB for AC impedance sensing of biological cells. Biomed. Microdevices 2014, 16, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Simone, G.; Perozziello, G. UV/Vis Visible Optical Waveguides Fabricated Using Organic-Inorganic Nanocomposite Layers. J. Nanosci. Nanotechnol. 2011, 11, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ai, Y.; Cheng, Y.; Li, C.M.; Kang, Y.; Wang, Z. Volumetric measurement of human red blood cells by MOSFET-based microfluidic gate. Electrophoresis 2015, 36, 1862–1865. [Google Scholar] [CrossRef]
- Abaci, H.E.; Devendra, R.; Smith, Q.; Gerecht, S.; Drazer, G. Design and development of microbioreactors for long-term cell culture in controlled oxygen microenvironments. Biomed. Microdevices 2012, 14, 145–152. [Google Scholar] [CrossRef]
- Dertinger, S.K.W.; Chiu, D.T.; Jeon, N.L.; Whitesides, G.M. Generation of Gradients Having Complex Shapes Using Microfluidic Networks. Anal. Chem. 2001, 73, 1240–1246. [Google Scholar] [CrossRef]
- Jeon, N.L.; Dertinger, S.K.W.; Chiu, D.T.; Choi, I.S.; Stroock, A.D.; Whitesides, G.M. Generation of Solution and Surface Gradients Using Microfluidic Systems. Langmuir 2000, 16, 8311–8316. [Google Scholar] [CrossRef]
- Somaweera, H.; Haputhanthri, S.O.; Ibraguimov, A.; Pappas, D. On-chip gradient generation in 256 microfluidic cell cultures: Simulation and experimental validation. Analyst 2015, 140, 5029–5038. [Google Scholar] [CrossRef]
- Tai, C.H.; Hsiung, S.K.; Chen, C.Y.; Tsai, M.L.; Lee, G.B. Automatic microfluidic platform for cell separation and nucleus collection. Biomed. Microdevices 2007, 9, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Perozziello, G.; Møllenbach, J.; Laursen, S.; Di Fabrizio, E.; Gernaey, K.; Krühne, U. Lab on a chip automates in vitro cell culturing. Microelectron. Eng. 2012, 98, 655–658. [Google Scholar] [CrossRef][Green Version]
- Perozziello, G.; Candeloro, P.; Gentile, F.; Coluccio, M.L.; Tallerico, M.; De Grazia, A.; Nicastri, A.; Perri, A.M.; Parrotta, E.; Pardeo, F.; et al. A microfluidic dialysis device for complex biological mixture SERS analysis. Microelectron. Eng. 2015, 144, 37–41. [Google Scholar] [CrossRef]
- Simone, G.; Perozziello, G. Ca2 Mediates the Adhesion of Breast Cancer Cells in Self-Assembled Multifunctional Microfluidic Chip Prepared with Carbohydrate Beads. Micro Nanosyst. 2010, 2, 261–268. [Google Scholar] [CrossRef]
- Keramas, G.; Perozziello, G.; Geschke, O.; Christensen, C.B.V. Development of a multiplex microarray microsystem. Lab Chip 2004, 4, 152–158. [Google Scholar] [CrossRef]
- Perozziello, G.; La Rocca, R.; Cojoc, G.; Liberale, C.; Malara, N.; Simone, G.; Candeloro, P.; Anichini, A.; Tirinato, L.; Gentile, F.; et al. Microfluidic devices modulate tumor cell line susceptibility to NK cell recognition. Small 2012, 8, 2886–2894. [Google Scholar] [CrossRef]
- Coluccio, M.L.; D’Attimo, M.A.; Cristiani, C.M.; Candeloro, P.; Parrotta, E.; Dattola, E.; Guzzi, F.; Cuda, G.; Lamanna, E.; Carbone, E.; et al. A Passive Microfluidic Device for Chemotaxis Studies. Micromachines 2019, 10, 551. [Google Scholar] [CrossRef]
- Jaccard, N.; Macown, R.J.; Super, A.; Griffin, L.D.; Veraitch, F.S.; Szita, N. Automated and online characterization of adherent cell culture growth in a microfabricated bioreactor. J. Lab. Autom. 2014, 19, 437–443. [Google Scholar] [CrossRef][Green Version]
- Woodruff, K.; Maerkl, S.J. A High-Throughput Microfluidic Platform for Mammalian Cell Transfection and Culturing. Sci. Rep. 2016, 6, 23937. [Google Scholar] [CrossRef]
- Li, R.; Lv, X.; Hasan, M.; Xu, J.; Xu, Y.; Zhang, X.; Qin, K.; Wang, J.; Zhou, D.; Deng, Y. A Rapidly Fabricated Microfluidic Chip for Cell Culture. J. Chromatogr. Sci. 2015, 54, 523–530. [Google Scholar] [CrossRef]
- Laser, D.J.; Santiago, J.G. A review of micropumps. J. Micromech. Microeng. 2004, 14, R35–R64. [Google Scholar] [CrossRef]
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering Flows in Small Devices: Microfluidics Toward a Lab-on-a-Chip. Annu. Rev. Fluid Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef]
- Moore, T.A.; Young, E.W. Single cell functional analysis of multiple myeloma cell populations correlates with diffusion profiles in static microfluidic coculture systems. Biomicrofluidics 2016, 10, 044105. [Google Scholar] [CrossRef]
- Jang, M.; Kleber, A.; Ruckelshausen, T.; Betzholz, R.; Manz, A. Differentiation of the human liver progenitor cell line (HepaRG) on a microfluidic-based biochip. J. Tissue Eng. Regen. Med. 2019, 13, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Goral, V.N.; Zhou, C.; Lai, F.; Yuen, P.K. A continuous perfusion microplate for cell culture. Lab Chip 2013, 13, 1039–1043. [Google Scholar] [CrossRef]
- Goral, V.N.; Tran, E.; Yuen, P.K. A pump-free membrane-controlled perfusion microfluidic platform. Biomicrofluidics 2015, 9, 054103. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.J.Y.; Chong, L.H.; Jin, L.; Singh, P.K.; Lee, P.S.; Yu, H.; Ananthanarayanan, A.; Leo, H.L.; Toh, Y.C. A pump-free microfluidic 3D perfusion platform for the efficient differentiation of human hepatocyte-like cells. Biotechnol. Bioeng. 2017, 114, 2360–2370. [Google Scholar] [CrossRef]
- Jeong, G.S.; Oh, J.; Kim, S.B.; Dokmeci, M.R.; Bae, H.; Lee, S.-H.; Khademhosseini, A. Siphon-driven microfluidic passive pump with a yarn flow resistance controller. Lab Chip 2014, 14, 4213–4219. [Google Scholar] [CrossRef]
- Simone, G.; Neuzil, P.; Perozziello, G.; Francardi, M.; Malara, N.; Di Fabrizio, E.; Manz, A. A facile in situ microfluidic method for creating multivalent surfaces: Toward functional glycomics. Lab Chip 2012, 12, 1500–1507. [Google Scholar] [CrossRef]
- Snakenborg, D.; Perozziello, G.; Geschke, O.; Kutter, J. A fast and reliable way to establish fluidic connections to planar microchips. J. Micromech. Microeng. 2006, 17, 98. [Google Scholar] [CrossRef]
- Bundgaard, F.; Perozziello, G.; Geschke, O. Rapid prototyping tools and methods for all-topas (R) cyclic olefin copolymer fluidic microsystems. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2006, 220, 1625–1632. [Google Scholar] [CrossRef]
- Simone, G.; Perozziello, G.; Battista, E.; De Angelis, F.; Candeloro, P.; Gentile, F.; Malara, N.; Manz, A.; Carbone, E.; Netti, P.; et al. Cell rolling and adhesion on surfaces in shear flow. A model for an antibody-based microfluidic screening system. Microelectron. Eng. 2012, 98, 668–671. [Google Scholar] [CrossRef]
- Kang, W.; Giraldo-Vela, J.P.; Nathamgari, S.S.; McGuire, T.; McNaughton, R.L.; Kessler, J.A.; Espinosa, H.D. Microfluidic device for stem cell differentiation and localized electroporation of postmitotic neurons. Lab. Chip 2014, 14, 4486–4495. [Google Scholar] [CrossRef] [PubMed]
- Ramji, R.; Wong, V.; Chavali, A.; Gearhart, L.; Miller-Jensen, K. A passive-flow microfluidic device for imaging latent HIV activation dynamics in single T cells. Integr. Biol. 2015, 7, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Perozziello, G.; Simone, G.; Geschke, O.; Di Fabrizio, E. Theory. In Microfluidic System Interfacing—An Onverview on Packaging of Microfluidic Systems, How to Interface Them Fluidically, Electrically and Optically, 1st ed.; LAP LAMBERT Academic Publishing GmbH & Co. KG: Saarbrücken, Germany, 2011; pp. 16–19. [Google Scholar]
- Stein, W.D.; Litman, T. Simple diffusiion of nonelectrolytes and ions. In Channels, Carriers and Pumps. An Introduction to Membrane Transport, 2nd ed.; Elsevier-Academic Press: Cambridge, MA, USA, 2015; pp. 37–81. [Google Scholar]
- Smits, A.J.; Lim, T. Dye and smoke visualization. In Flow Visualization, Techniques and Examples, 2nd ed.; Imperial College Press: London, UK, 2000; pp. 43–45. [Google Scholar]
- Dalby, M.J.; Di Silvio, L.; Harper, E.J.; Bonfield, W. Initial interaction of osteoblasts with the surface of a hydroxyapatite-poly (methylmethacrylate) cement. Biomaterials 2001, 22, 1739–1747. [Google Scholar] [CrossRef]
- Saito, T.; Kin, Y.; Koshino, T. Osteogenic response of hydroxyapatite cement implanted into the femur of rats with experimentally induced osteoporosis. Biomaterials 2002, 23, 2711–2716. [Google Scholar] [CrossRef]
- Zebarjad, S. A Study on Mechanical Properties of PMMA/Hydroxyapatite Nanocomposite. Engineering 2011, 3, 795–801. [Google Scholar] [CrossRef]
- Lei, K.F.; Chang, C.-H.; Chen, M.-J. Paper/PMMA Hybrid 3D Cell Culture Microfluidic Platform for the Study of Cellular Crosstalk. ACS Appl. Mater. Interfaces 2017, 9, 13092–13101. [Google Scholar] [CrossRef]
- Shinzato, S.; Kobayashi, M.; Mousa, W.F.; Kamimura, M.; Neo, M.; Kitamura, Y.; Kokubo, T.; Nakamura, T. Bioactive polymethyl methacrylate-based bone cement: Comparison of glass beads, apatite- and wollastonite-containing glass-ceramic, and hydroxyapatite fillers on mechanical and biological properties. J. Biomed. Mater. Res. 2000, 51, 258–272. [Google Scholar] [CrossRef]
- Maricevich, J.; Cezar-Junior, A.B.; de Oliveira-Junior, E.X.; Veras, E.S.J.A.M.; da Silva, J.V.L.; Nunes, A.A.; Almeida, N.S.; Azevedo-Filho, H.R.C. Functional and aesthetic evaluation after cranial reconstruction with polymethyl methacrylate prostheses using low-cost 3D printing templates in patients with cranial defects secondary to decompressive craniectomies: A prospective study. Surg. Neurol. Int. 2019, 10, 1. [Google Scholar] [CrossRef]
- Santavirta, S.; Takagi, M.; Gómez-Barrena, E.; Nevalainen, J.; Lassus, J.; Salo, J.; Konttinen, Y.T. Studies of host response to orthopedic implants and biomaterials. J. Long Term Eff. Med. Implants 1999, 9, 67–76. [Google Scholar] [PubMed]
- Tan, H.-Y.; Loke, W.-K.; Nguyen, N.-T. A reliable method for bonding polydimethylsiloxane (PDMS) to polymethylmethacrylate (PMMA) and its application in micropumps. Sens. Actuators B Chem. 2010, 151, 133–139. [Google Scholar] [CrossRef]
- Murat, S.; Alp, G.; Alatalı, C.; Uzun, M. In Vitro Evaluation of Adhesion of Candida albicans on CAD/CAM PMMA-Based Polymers. J. Prosthodont. 2018, 28, e873–e879. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, D.; Jiang, Y.H.; Yao, W.J.; Wang, X.J.; Wei, X.C.; Gao, J.; Xie, L.D.; Yan, Z.Y.; Wen, Z.Y.; et al. Influence of expressed TRAIL on biophysical properties of the human leukemic cell line Jurkat. Cell Res. 2004, 14, 161–168. [Google Scholar] [CrossRef][Green Version]
- Cramer Morales, K.; Heer, C.; Mapuskar, K. SOD2 targeted gene editing by CRISPR/Cas9 yields human cells devoid of MnSOD. Free Radic. Biol. Med. 2015, 89, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Puthli, A.; Tiwari, R.; Mishra, K. Bystander response triggered by doxorubicin-killed dead cells contributes to acquire drug resistance but increasing radiosensitivity in vitro. J. Radiat. Cancer Res. 2016, 7, 103. [Google Scholar]
- Wu, F.Y.; Fan, J.; Tang, L.; Zhao, Y.M.; Zhou, C.C. Atypical chemokine receptor D6 inhibits human non-small cell lung cancer growth by sequestration of chemokines. Oncol. Lett. 2013, 6, 91–95. [Google Scholar] [CrossRef][Green Version]
- Gonzalez, C.; Touraud, E.; Spinelli, S.; Thomas, O. Organic constituents. In UV-visible Spectrophotometry of Water and Wastewater, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 27, pp. 54–59. [Google Scholar]
- Sundarakrishnan, A.; Herrero Acero, E.; Coburn, J.; Chwalek, K.; Partlow, B.; Kaplan, D.L. Phenol red-silk tyrosine cross-linked hydrogels. Acta Biomater. 2016, 42, 102–113. [Google Scholar] [CrossRef]

| Width (mm) | Height (mm) | Length (mm) | Diameter (mm) | Volume (μL) | Flow Rate (μL/h) | |
|---|---|---|---|---|---|---|
| Total dimensions | 80 | 9 | 80 | |||
| Inlet channel* | 0.1 | 0.1 | 60 | 6.207 (−7%/day) | ||
| Outlet channel | 0.1 | 0.1 | 20 | 18.61 (−7%/day) | ||
| Reservoirs | 6 | 20 | 1884 | |||
| Culture chamber | 3 | 5 | 58.875 | |||
| Reservoirs total filling volume | 5652 | |||||
| Predicted total flow rate | 18.61 (−7%/day) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzzi, F.; Candeloro, P.; Coluccio, M.L.; Cristiani, C.M.; Parrotta, E.I.; Scaramuzzino, L.; Scalise, S.; Dattola, E.; D’Attimo, M.A.; Cuda, G.; et al. A Disposable Passive Microfluidic Device for Cell Culturing. Biosensors 2020, 10, 18. https://doi.org/10.3390/bios10030018
Guzzi F, Candeloro P, Coluccio ML, Cristiani CM, Parrotta EI, Scaramuzzino L, Scalise S, Dattola E, D’Attimo MA, Cuda G, et al. A Disposable Passive Microfluidic Device for Cell Culturing. Biosensors. 2020; 10(3):18. https://doi.org/10.3390/bios10030018
Chicago/Turabian StyleGuzzi, Francesco, Patrizio Candeloro, Maria Laura Coluccio, Costanza Maria Cristiani, Elvira Immacolata Parrotta, Luana Scaramuzzino, Stefania Scalise, Elisabetta Dattola, Maria Antonia D’Attimo, Giovanni Cuda, and et al. 2020. "A Disposable Passive Microfluidic Device for Cell Culturing" Biosensors 10, no. 3: 18. https://doi.org/10.3390/bios10030018
APA StyleGuzzi, F., Candeloro, P., Coluccio, M. L., Cristiani, C. M., Parrotta, E. I., Scaramuzzino, L., Scalise, S., Dattola, E., D’Attimo, M. A., Cuda, G., Lamanna, E., Passacatini, L. C., Carbone, E., Krühne, U., Di Fabrizio, E., & Perozziello, G. (2020). A Disposable Passive Microfluidic Device for Cell Culturing. Biosensors, 10(3), 18. https://doi.org/10.3390/bios10030018









