Abstract
Milk is a source of essential nutrients for infants and adults, and its production has increased worldwide over the past years. Despite developments in the dairy industry, premature spoilage of milk due to the contamination by Bacillus cereus continues to be a problem and causes considerable economic losses. B. cereus is ubiquitously present in nature and can contaminate milk through a variety of means from the farm to the processing plant, during transport or distribution. There is a need to detect and quantify spores directly in food samples, because B. cereus might be present in food only in the sporulated form. Traditional microbiological detection methods used in dairy industries to detect spores show limits of time (they are time consuming), efficiency and sensitivity. The low level of B. cereus spores in milk implies that highly sensitive detection methods should be applied for dairy products screening for spore contamination. This review describes the advantages and disadvantages of classical microbiological methods used to detect B. cereus spores in milk and milk products, related to novel methods based on molecular biology, biosensors and nanotechnology.
1. Introduction
The dairy industry has a long tradition of safeguarding the safety and quality of consumer milk, based on two main processes: cooling of the raw milk to temperatures below 7–10 °C until processing and heating the milk in a dairy plant. Production of different milk products demands various heating processes to be applied (pasteurization, ultra-high temperature (UHT) treatment, drying). The shelf life of pasteurized milk is mainly determined by the presence and growth of Gram-positive, rod-shaped aerobic endospore formers, of which members of the Bacillus cereus group are the most important. Thermophilic endospores resist the pasteurization process and may revive by germination and outgrowth and produce spoilage enzymes (proteases, lipases and phospholipases) in the pasteurized milk leading to off-flavors [1,2]. In addition, B. cereus is a well-known foodborne pathogen. Because of these risks, the dairy industry must constantly optimize and improve the processes that result in products that meet business and consumer demands and which can be exported over long distances and sometimes in unfavorable storage conditions without loss of quality. Despite further developments in the dairy industry in the last century, premature spoilage of milk continues to be a problem and causes considerable environmental and economic losses that are linked to the direct costs of recalls of products and indirectly linked by the image damage to the companies concerned. If the recall concerns a product containing milk powder, the recall costs may be greater than for consumer milk [3].
Bacillus cereus is a Gram-positive, rod-shaped, motile, spore-forming opportunistic pathogen that is commonly found in soil, air, grains, rice (row and cooked), vegetables, meat and milk due to the bacterial capability to grow at temperatures from 4 °C to 50 °C and resist heat and chemicals [4]. Some species from the Bacillus cereus group, also known as Bacillus cereus sensu lato (s.l.), cause foodborne outbreaks in humans [5]. The group, as reported by Liu et al. [6], comprised until recently of nine officially closely related species: B. anthracis, B. cereus sensu stricto (s.s.), B. thuringiensis, B. mycoides, B. pseudomycoides, B. weihenstephanensis, B. cytotoxicus, B. toyonensis and B. wiedmannii. From 2017 onwards, the B. cereus group has been expanded with another nine new species, which were all isolated from marine sediments: B. paranthracis, B. pacificus, B. tropicus, B. albus, B. mobilis, B. luti, B. proteolyticus, B. nitratireducens and B. paramycoides [7]. Three new species have been described based on a whole genomic sequencing comparison and could potentially also belong to the B. cereus group, but they have not (yet) been validly described according to bacterial nomenclature: B. gaemokensis, B. manliponensis (DSMZ, L.I. German Collection of Microorganisms and Cell Cultures GmbH, Inhoffenstraβe 7B, 38124, Braunschweig, Germany) and B. bingmayongensis (TEDA. School of Biological Sciences and Biotechnology Nankai University, Tianjin, China). In routine practice, it is difficult to distinguish between several of the different species of the B. cereus group, and therefore exact speciation is not often done, but rather a general allocation is given to the species group.
B. cereus s.s. causes mild to serious food poisoning, mainly through secretion of enterotoxin, causing diarrhea and emetic toxins [8], but also through some still non-elucidated mechanisms [9]. It was also found to be the etiologic agent of systemic and local infections in immunologically compromised and vulnerable individuals [5]. In same rare cases, B. cereus causes toxic shock syndrome in the central nervous system [5,10,11,12]. B. anthracis is responsible for causing a lethal disease anthrax in humans and animals.
B. thuringiensis is considered safe for humans, which enables commercialization and use of specific marketed strains as a bio-pesticide for pest controls as it causes lethal infections in insects with toxins, called Cry proteins [13]. B. thuringiensis has been reported in some cases of foodborne outbreaks, but it developed because classical routine detection methods did not allow to distinguish between B. cereus and B. thuringiensis [14,15]. The Cry genes encoded for the toxin show a high level of polymorphisms, which makes the differentiation of B. thuringiensis from other B. cereus strains difficult to define. However, the contribution of B. thuringiensis to foodborne outbreaks is still controversial but may be underestimated. Indeed, some B. thuringiensis strains used as biopesticide can produce modest levels of enterotoxins [14].
The psychrotolerant species B. weihenstephanensis and B. mycoides were, until recently, not considered foodborne pathogens. However, it has been demonstrated that B. weihenstephanensis can also produce emetic toxin [16]. It was also reported that some B. pseudomycoides isolates, which are considered as harmless microorganisms, may be potentially cytotoxic and possibly pathogenic [17,18]. B. toyonensis is used as probiotic and feed additive. In a recent study on B. cereus group isolates from spices, it was demonstrated that besides B. thuringiensis, some B. weihenstephanensis and B. toyonensis-like isolates can potentially produce enterotoxins [19]. Furthermore, B. pseudomycoides isolates were found to be potentially cytotoxic [18]. B. cytotoxicus associated with specific food products is the only thermotolerant member of the B. cereus group (it grows between 20 °C and 50 °C). It was shown to produce the highly toxic CytK1 variant, which was responsible for a deadly outbreak [20]. For the other most recent members of the B. cereus group, nothing is known about their pathogenic potential.
Consuming food contaminated by B. cereus may lead to gastrointestinal diseases, characterized by abdominal pain and non-bloody diarrhea that occur 4–16 h after eating. These symptoms are caused by the bacterial production of diarrheal toxins hemolysin BL, Hbl [21], nonhemolytic enterotoxin Nhe [21] and cytotoxin CytK [22] in the human small intestine. All three enterotoxins are cytotoxic and cell membrane pore-forming toxins [23,24]. Emetic disease may occur within 0.5–5 h after eating contaminated food as an acute attack of nausea, vomiting and profuse abdominal cramping due to a thermo- and acidic-stable non-ribosomal peptide, cereulide [25,26]. The mechanism of action of cereulide is not well documented but seems to be receptor-mediated [27,28]. In contrast to less heat-stable Hbl, Nhe and CytK toxins, which are produced in the small intestine and cause diarrhea as a result of a toxico-infection, cereulide can be present in contaminated foods and cause intoxication as it is resistant to heat, acid and proteolysis [29,30]. Specific peptides of the polypeptide toxins Hbl and Nhe can be easily detected through commercial immunological kits [31], while HPLC (High-Performance Liquid Chromatography) or mass spectroscopy are need for the other toxins [32,33,34].
B. cereus may survive under non-favorable conditions, such as under starvation or in a reduced oxygen or dry environment, for many years in the form of spores. Spores easily spread and can resist ow and high temperatures, desiccation, disinfectant agents, ionization, radiation and ultraviolet light. B. cereus spores are found in all categories of food (such as meat products, dairy products, vegetables, and rice) as they are widely distributed in soil, air, water, plants and animals. Milk and dairy products are particularly at risk because spores survive in pasteurized and other heat-treated milk products, such as milk powder and cheese. Fortunately, ultrahigh heat temperature (UHT) treatment, which is commonly used to produce consumer milk effectively, kills B. cereus spores [35]. Reconstituted infant foods are considered to be a high risk due to infant susceptibility to enteric bacterial pathogens because of their underdeveloped immune and metabolic systems. Powered infant formula, commercialized as a breast milk substitute, and powered follow-up formula, commercialized as an addition to breast milk, cannot be sterilized using current technologies because of the complex processing [15,36]. In this review, we summarize the contamination, prevention and detection of spores of B. cereus in milk and dairy products. We particularly focus on new analytical strategies to sense spore-contaminated milk.
2. Origin of Milk Contamination
B. cereus is widely distributed in nature and can contaminate foods primarily through soil and air [37,38]. Soil contains 50–380,000 CFU (colony-forming unit)/g and air <100 CFU/m3 of B. cereus spores [39]. The composition of the Bacillus microbiota in raw milk exhibits seasonal differences, with higher B. cereus spore counts in summer [39]. This is largely attributed to soil as a major source of B. cereus spores, to which cows are more exposed while pasturing during summer. During winter months, feed and bedding are considered the main contamination sources, as the cows are housed indoor in that period. These general observations were confirmed in a study conducted in Belgium, in which organic dairy farms were compared with conventional farms. Members of the B. cereus group were isolated more frequently in the late summer/autumn period and were also more frequently obtained in milk from organic dairy farms. This could be linked to more general outdoor grazing of cows on the organic farms compared to the conventional farms [40]. Depending on the country studied, seasonal changes in the composition of the B. cereus group population have been or have not been observed in raw milk, e.g., in Sweden higher psychrotrophic B. cereus levels were found in summer compared to winter [41], while in Japan, such a seasonal variation in psychrotrophic spore levels was not found [42].
The prevalence rate of B. cereus in raw milk is rather high, but usually at very low levels (<100 CFU/mL of raw milk) [39], although there probably exists farm differences depending on hygienic levels and housing systems of dairy farms. In the Netherlands, the average is 1.2 log spores of B. cereus per liter of raw bulk tank milk, and a maximum B. cereus spore limit in farm tank milk of 3 log spores per liter is required to achieve a shelf life for pasteurized milk of at least seven days [43]. Different types of cheeses in Turkey were positive for B. cereus at a rate of 10.4% and with counts up to 3.8 × 105 CFU/g [44]. In fresh ricotta cheese, about 16% of the samples were positive for B. cereus, but at very low concentrations (<10 CFU/g) [45]. In recent studies on UHT-milk, B. cereus was found, which is unexpected in this type of product [46,47]. Possible explanations are an inadequate control of the UHT process or post-heat treatment contamination. Post-production, powders can be stored for extended periods and, in the absence of water, bacterial metabolic activity and growth is limited. Thus, drying milk prevents spoilage and product defects. However, under these conditions, bacterial spores can remain dormant until more favorable conditions are encountered, upon which germination and outgrowth can proceed [48,49].
Because of B. cereus ubiquitous presence in soil, water, and food processing environments, food contamination seems to be inevitable [50]. Although B. cereus spores have been detected in almost all food, they are mainly contaminants of raw foods, such as fresh vegetables and fruits, seeds, and raw milk. Spores of B. cereus can contaminate raw milk if hygienic milking conditions are not fully respected, but also via contaminated milking equipment or during transport from the farm to the dairy plants.
Contamination of milk powder for infants is one of the problems to be solved. It is interesting to note that the spore-forming bacterial flora present in the raw milk is different from the bacterial spore-forming flora present in the milk powders, in consequence of the industrial process used [51].
3. Legislation
B. cereus was among the primary microbes associated with baby food contamination, as reported by FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), and the third agent responsible for collective foodborne infections in Europe [38]. In 2005, EFSA (European Food Safety Authority) estimated that B. cereus accounts for 1.3% of all bacterial foodborne diseases [38]. In 2016, the European Union reported that 5.5% of outbreaks caused by a foodborne pathogen are attributed to Bacillus toxins as a causal agent [15]. Some dehydrated foods, including dried infant formulae and dried dietary foods, are consumed by potentially fragile consumers; therefore, the numbers of Bacillus cereus spores in these products should be as low as possible during processing, and good practices should be designed to reduce delay between preparation and consumption. Dried infant formulae and dried dietary foods for special medical purposes intended for infants below six months of age are the only food products for which there are official criteria in the EU for B. cereus: The target limit is 50 CFU/g with a tolerance limit of 500 CFU/g in a sampling plan comprising five sample units, of which one sample unit may be between both limits [52].
Foodborne diseases due to B. cereus are usually related to the presence of high numbers of cells or spores/g in the range of 105–108 in ingested food, although the amount regarded as hazardous can decrease to 103–104 cells or spores/g [38]. In several countries like Belgium, action limits (>105 CFU/g or CFU/mL) are in place based on the EFSA recommendations. Spores of B. cereus are usually present in low numbers in raw milk (< 1 and up to 10–100 spores/mL), while the finished products, such as pasteurized milk, milk powder or cheeses, can contain about 102–103 spores/g during shelf life [43]. As explained above, the content of B. cereus in milk and dairy products may increase due to milk contamination with bacteria from soil, air or water and through storage abuse conditions.
4. Spores of B. cereus
To resist to environmental stress, an adaptive strategy of Bacillus cells is to transform into spores, which are called endospores because they are produced within a mother cell [53]. In the sporulation form, bacterium may stay dormant without nutrients for an undefined period but will germinate and again become a vegetative cell when conditions change to favorable. It is interesting to note that some food treatments, such as a sub-lethal heat exposure (at 65 to 80 °C), can act as triggers for germination [54]. B. cereus spores have great ability to survive at conditions like an unfavorable pH (from 1 to 5.2), a high temperature shock (such as 95 °C, for 2 min), or under antibiotic treatments (such as ampicillin, cephalothin and oxacillin), which, in contrast, their vegetative form cannot survive [4,31].
The process of bacterial differentiation from cell to spore starts upon phosphorylation of the key transcriptional regulator, Spo0A [55]. For that, the starvation signals induce auto-phosphorylation of Spo0F kinase, followed by the phosphorylation of Spo0B kinase and Spo0A [56]. Phosphorylated Spo0A modifies expression of more than 500 genes [57], among which are some other regulators. About 100 genes are essential for the sporulation process [58,59,60,61,62]. The mineral composition of media may have a significant effect on sporulation. Manganese is especially an essential element for sporulation in the genus Bacillus [63]. The formation of endospores starts by an asymmetric cell division and DNA segregation (Figure 1a). Segregated DNA becomes separated from the rest of the cell by a double layer septum that is formed around it. This structure is called forespore (Figure 1a). Calcium dipicolinate (2, 6-pyridinedicarboxylic acid) stabilizes forespore formation and structure. Next, the peptidoglycan cortex is synthetized between the two layers and a spore coat is formed at the outside of the forespore. Finally, maturation of endospore and mother cell lysis enables spore release. The whole process takes around 8 h. Endospores contain several tick layers (Figure 1b). The endospores are resistant to higher temperature mainly due to their electronegative peptidoglycan cortex layer. The inner peptidoglycan layer surrounding the core enables endospore resistance to UV light and various bactericide chemicals. The inner membrane is surrounded by the germ cell wall, which becomes the membrane of the vegetative cell after germination [64]. Spores also contain some proteins, such as proteases, lyases, endonucleases and heat shock proteins that also contribute to the resistance of spores in unfavorable conditions [65].
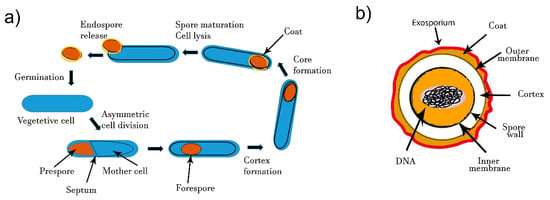
Figure 1.
(a) Formation of spores by endospore-forming B. cereus. Upon unfavorable environmental conditions, the vegetative cell differentiates and enables spore morphogenesis. Mature spores are released and will germinated to give rise to a vegetative cell under favorable conditions. (b) Schematic diagram of bacterial spore structure.
Resistance of Bacillus cereus spores to external conditions indicates that their detection by an internal biomarker that should be extracted would be difficult. The more adapted analytical methods for spores detection in dairy products would be based on detection of spore’ surface epitopes or a surface structure. Spore preparation in controled laboratory conditions have shown that physicochemical propertis depend on sporulation medium and condition [66].
Various methods are used to induce B. cereus sporulation in the laboratory. For this, the bacterium is allowed to multiply to the wanted growth level (usually to mid-exponential phase), and then cells are exposed to a stress that induces their transformation. For instance, B. cereus cells can be transferred by plating from a rich broth, such as brain-heart infusion (BHI) that support bacterial proliferation, to a nutrition agar complemented with 0.05 g/L MnSO4. To avoid starvation in the presence of Mn2+-ions, 90–95% of B. cereus cells sporulate within few days incubation at 30 °C or 37 °C [67]. Spores formed by various species from the B. cereus group have a similar size (about 1 µm) and morphology. However, the structure of the superficial layer, named the exosporium, can be modified to some extent by growing the medium or sporulation condition. Overall, findings obtained in laboratory conditions indicate the high risk associated with the presence of B. cereus spores in thermally processed and packaged milk products that may have high salt concentration, absence of oxygen, or are conserved at refrigerated temperatures.
5. Detection Methods
5.1. Classical Methods
Conventional detection of B. cereus spores or cells involves plate count method analysis and toxin detection by commercial immunological kits or a combination of liquid chromatography and mass spectrometry (Figure 2). In addition, PCR-based methods are frequently used to evidence the toxin genes. Official methods to enumerate and detect the B. cereus group are ISO 7932 (for direct plate counting) and ISO 21,871 (for detection and counting of low numbers through the most probable number). Both methods give no indication of the ability of the bacteria to produce toxins, nor do they differentiate B. cereus sensu stricto from other bacteria of the B. cereus group; therefore, the result is presented as “presumptive Bacillus cereus”. Genetic molecular methods detect the presence of certain genes, so enable discrimination of B. cereus from other bacilli. Methods for detection of toxins and strain characterization for the production of toxins are not standardized. As a result, no official method is available for routine use to screen food. However, some advanced methods are under investigation [34,68].
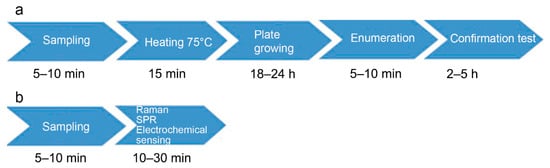
Figure 2.
Envisioned spore diagnostic workflow by a classical method (a) and biosensors (b).
The traditional microbiological methods for general detection of spores include pour-plating on plate count agar and microscopic observation. B. anthracis and B. thuringiensis, which are subtyped in serotypes, are also detected by strain iso-typing using specific antibodies. As for the detection of spores of other aerobic spore-forming bacteria, the standard plating procedure to specifically detect spores of B. cereus considers the thermal treatment of the homogenized sample solution at 75 °C for 15 min before plating 1 mL of the solution on the specific medium MYP ((Mannitol Egg Yolk Polymyxin Agar) and confirmation of suspected colonies by a hemolysis test [68]. This thermal treatment kills the vegetative forms, allowing the spore, a resistant form, to grow on nutrients. By plating serial dilutions of the treated homogenate, it is possible to evaluate the spore concentration. In that way, the method quantifies the survival of B. cereus spores after heat treatment of food products.
Official plating methods of general spore or specific B. cereus detection or enumeration described above require at least two-day incubation to provide results. However, endospores can be detected in more rapid manners (Figure 2). One of the rapid methods that has been used for many years to quantify spores in a bacterial culture is based on the utilization of a hemocytometer. For instance, malachite green at 0.5% water solution colors spores and allows their visualization under simple optic microscopy. This method, proposed by Kiozuka and Tochikubo in 1991, was useful for dormant spore detection [69]. However, there is no evidence that it can be applied for food analysis.
5.2. PCR and LAMP
Traditional techniques can lead to misidentification as there is a high phenotypic similarity among the B. cereus group species in terms of pathogenicity, morphology of colonies, mobility and growth kinetics or sporulation efficiency. Molecular techniques are an alternative to classical methods, although it is difficult to extract DNA from spores that are highly resistant against chemical or enzymatic lysis. Nevertheless, various methods can be used to release DNA, e.g., sonication or mechanical lysis with glass beads. Some commercial kits for spore disruption are also available [70]. However, in many diagnostic protocols, spores are allowed to germinate and DNA is extracted from lysed vegetative cells. Martinez–Blanch et al. described a quantitative real-time PCR to evaluate spore concentration, using as a target the pc-plc gene encoding for phosphatidylcholine-specific phospholipase involved in the hemolysis activity [71]. The protocol allowed the detection limit for B. cereus in artificially contaminated reconstituted infant formula of about 4 spores per reaction or 60 spores per mL, using the curves obtained with an efficiency of 1.09. In another study, the Ruggedized Advanced Pathogen Identification Device (RAPID) was employed to detect spores of Bacillus anthracis in raw milk, with the limit of detection (LoD) down to 2500 spores/mL [72]. The protocol consisted of the utilization of a commercially available DNA extraction kit, portable equipment for real-time PCR and commercially available primers and probes, developed to detect either the protective antigen gene or the lethal factor gene with the RAPID [72].
Since milk typically contains a too low concentration of spores to be directly tested by a PCR method, a time-consuming microbiological enrichment step is needed prior to detection in routine analysis. In addition, milk contains a high concentration of ions and fats that have been shown to inhibit real-time PCR. To overcome problems of low spore and high ion and fat concentrations, Fisher et al. proposed an aptamer-based trapping protocol for milk analysis [73]. Magnetic capture is an excellent strategy for extracting and concentrating bacteria or spores from complex food matrices and/or highly diluted solutions [74]. The spore trapping process was based on the use of aptamer-functionalized silica magnetic beads for the specific capture and concentration before detection by specific real-time PCR. The aptamers were designed to specifically detect B. cereus spores with high affinity (KD in the nM range). Such aptamer-decorated magnetic beads successfully trapped spores from milk with different fat contents, showing enrichment factors up to six-fold. This strategy improved the limits of detection of the subsequent PCR test. Indeed, after several washing steps with pure water, trapped spores were analyzed by a highly specific real-time PCR assay to gain a detection limit of 103 CFU/mL for B. cereus in milk. However, this is still not a very sensitive detection limit, since it is not sufficient for raw milk where the contamination level is still much lower. Other molecules, such as the bacteriophage cell wall-binding domain, can be used to pre-concentrate B. cereus from complex food matrices [75]. Some rapid tests for detection of B. anthracis spores based on nucleic acid detection showed a very low limit of detection (LoD) of 1 to 30 spores per reaction [76,77,78]. However, to reach such a low limit of detection, a clean and concentrated starting material is needed, which can hardly be the case with contaminated milk and dairy products.
An alternative nucleic acid amplification technology is loop-mediated isothermal amplification (LAMP), which works at an isothermal temperature, which is advantageous for rapid and point-of-care portable detection systems [34]. A rotate and react SlipChip (RnR-SlipChip) was developed for simultaneous visual detection of multiple bacterial pathogens by LAMP, including B. cereus [79]. After sample loading, one-step rotation allowed immediate mixing and reaction of target bacteria with the reagents on the chip, allowing visual identification in 60 min with a success rate of 100%. It has to be further investigated if this technology can be adapted to also detect spores of B. cereus.
5.3. Dipicolinic Acid Detection
The spore-specific dipicolinic acid, present as a Ca2+ chelate complex, is a unique and characteristic biomarker for endospores of B. cereus, but also for other bacterial spores produced by other aerobic spore-forming species and Clostridium, but not in spores formed by other organisms, such as molds. Dipicolinic acid makes 5% to 15% of the total dry mass of the spores and may be released during heating or hydrolysis [80]. Dipicolinic acid detection provides good estimation of bacterial spore content in suspicious samples and could be used for a rapid screening of milk and dairy products. In the literature, different techniques have been described for dipicolinic acid detection. Several optical methods, such as spectrophotometry, Raman/surface-enhanced Raman scattering (SERS) and infrared spectroscopies, fluorescence and photoluminescence, have been applied to dipicolinic acid detection. Rosen proposed to color bacterial endospores by a suspension of terbium chloride, which reacts with the calcium dipicolinate [81]. Formed terbium-(III)-dipicolinate anion produces photoluminescence that can be easily detected. The LoD obtained using 31.4 µM TbCl3 b was 4.4 × 105 CFU/mL. Gültekin et al. [82] described the elaboration of molecular imprinted polymer (MIP)-nanoshells on gold-silver nanoclusters. These MIP-nanosensors were developed with a specific cavity for dipicolinic acid interaction. The binding affinity was investigated by fluorescence and KD was calculated between 5 × 10−8 to 1 × 10−7 M. This strategy allowed B. cereus spore detection via dipicolinic acid quantification in a concentration of 104 CFU/mL. Baig and Chen have used glutathione-capped gold nanoparticles complexed with Ca2+−ions (Ca2+-AuNP@GSH) through GSH-Ca2+ chelation as a sensing agent for dipicolinic acid from complex matrices [83]. In the presence of dipicolinic, Ca2+-AuNP@GSH is dissociated to the Ca2+-dipicolinic complex and AuNP@GSH, which desegregates nanoparticles and induces a color change of the solution. The feasibility of using this sensing method for quantitative detection of dipicolinic acid was demonstrated in lysate samples prepared from the B. cereus spore suspension.
Raman spectroscopy is particularly adapted for dipicolinic acid detection. In SERS, a single spore (or in bulk) in a spatially confined zone of enhanced electromagnetic field generates a highly specific spectral signature within seconds. SERS has many advantages, such as sensitivity, selectivity, no need for biomarker extraction, and no interference from aqueous media that makes it attractive for biosensing applications. Chan et al. described an analytical technique combining optical trapping and confocal Raman spectroscopy to detect and identify a single spore particle. B. cereus spores were identified based on their intrinsic Raman signature, which revealed to be specific [84]. Raman spectroscopy coupled with SERS-active gold nanoparticles was used to detect and discriminate among five Bacillus spores by using the SERS method [85]. In a similar way, peptide-functionalized silver particles embedded in a porous silica sol-gel was used for selective capture and detection of B. cereus spores via dipicolinic acid signature by SERS [86]. In 2013, Cowcher et al. described a SERS portable device for quantitative detection of bacterial spores. The technique was also based on dipicolinic acid detection and quantification and proved specific to B. cereus and B. subtilis spores. An approximated LoD of 1100 spores in a 200 µL sample volume was reached by the technique [87]. These performances are close to those of real-time PCR, as previously described.
Spore content in Bacillus species’ samples was also determined by mass spectrometry, as described by Beverly et al. [88]. The detection of dipicolinic ions at m/z 167 was used to quantify various degrees of sporulation directly in live bacteria samples with good accuracy. Nevertheless, detection methods based on Raman spectroscopy or mass spectroscopy require expensive and sophisticated instrumentations.
The methods for detecting spores by quantifying dipicolinic acid are rarely applied in complex matrices, such as the milk or dairy industry. We should also mention the work of Han et al. [89], who developed a rapid enumeration of spores in raw milk through analyzing dipicolinic acid. In this assay, the concentration of dipicolinic acid in raw milk was obtained by measuring the solution absorbency and converting it into endospore numbers using endospore-forming pure cultures as standards. The LoD was 1.46 × 103 CFU/mL.
5.4. Colorimetric Detection (Lateral Flow)
Quinlan and Foegeding used monoclonal antibodies for spore isotyping [90]. Antibody B183 and B48 reacted very strongly with B. cereus spores, but a light reactivity was also detected for B. subtilis subsp. globigii and B. megaterium spores, indicating cross reaction. Using immunogold staining they showed that both antibodies were bound to surface epitopes of spores. Such antibodies enable detection of whole spores in rapid tests, such as lateral flow immunoassays.
Lateral flow immunoassays attract growing interest for the development of user-friendly rapid tests to monitor food safety. The first principal advantage of lateral flow tests is that they can provide a colorimetric signal that can be assessed by the naked eye without any instrumentation. The second advantage lies in the movement of the sample through the paper carrier of the test, allowing separation of molecules from the sample before detection. This is particularly important regarding the complexity of food matrices and usually requires complicated sample preparation prior to analysis. Wang et al. coupled a super-paramagnetic separation with a lateral flow test to detect B. anthracis spores in water and milk [91]. To adapt the lateral flow test for the detection of large-sized objects, spores were firstly captured by paramagnetic beads, decorated with a specific antibody and then the formed immunocomplexes were deposited on the sample pad of the lateral flow test. Each spore was bound to numerous beads because they presented many surface epitopes. In consequence, immunocomplexes aggregated into large particles that could not flow through the paper stip. Instead, they were retained near the sample pad of the strip and generated a retention line. Super-paramagnetic beads mixed with spore free milk samples migrated over the paper strip, and no retention line was formed.
In another study that combined magnetic separation of spores from water and milk samples with the lateral flow detection, spores were eluted from functionalized paramagnetic beads before detection [92]. Anti-spore antibodies coupled with carboxyl coated magnetic beads captured spores from milk samples within 30 min (Figure 3). The resulting complex was dissociated with formamide-EDTA (2,2′,2″,2‴-(Ethane-1,2-diyldinitrilo)tetraacetic acid ) solution for 30 s in a microwave oven, and then eluted spores were evidenced by the lateral flow test within 10 min.
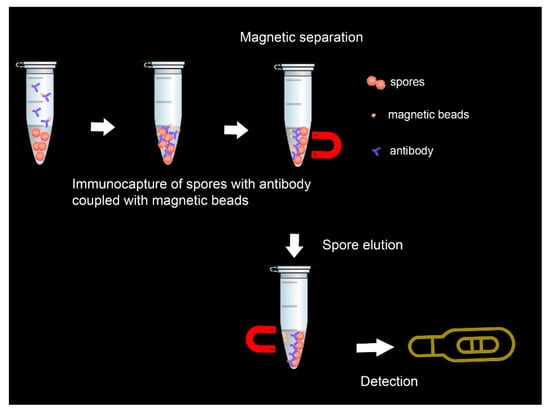
Figure 3.
Illustration of the immunomagnetic lateral flow sensing principle.
Lateral flow assays were developed using different antibodies that recognized high affinity spores of B. anthracis. Both lateral flow set-ups can potentially be extended to spores of other Bacillus cereus species in milk samples. Some of the available antibodies are highly specific, while others can be used for B. cereus sensu lato spore detection. Alternatively, new antibodies should be raised against a soluble exosporium fraction of targeted B. cereus to enable detection of its specific whole spores.
5.5. Biosensors
Due to the low number of recognition elements (such as antibodies, aptamers) that enable efficient and specific targeting of whole bacterial spores, only a few biosensors have been developed in the literature. Biosensors attract considerable attention because of their simplicity, sensitivity and rapidity (Figure 2). Electrochemical biosensors are the most rapidly growing compared to other methods, because they are low cost, sensitive and easy to miniaturize [93,94,95]. In 2012, Bruno and Carrillo reported an aptameric sequence, which specifically binds to spores of B. anthracis and B. cereus [96]. Based on the binding properties of this sequence, a label-free impedimetric aptasensor has been developed recently (Figure 4) [97]. B. cereus spores were detected with a linear range between 104 CFU/mL and 5 × 106 CFU/mL, with a detection limit of 3 × 103 CFU/mL. The selectivity of the sensor proved efficient. The sensor also detected B. subtilis spores but with a slightly lower signal than for B. cereus, while no response was observed in the case of Legionella pneumophila and Salmonella typhimurium bacteria.
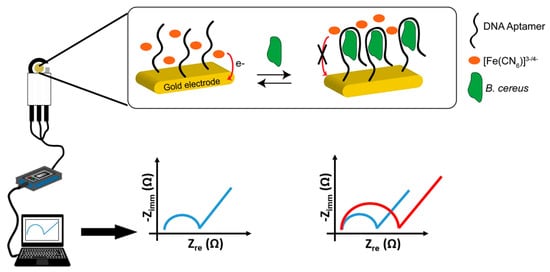
Figure 4.
Illustration of principle for B. cereus spore detection and electrochemical set-up for signal measurement using a miniaturized aptasensor (from Reference [97]).
Surface plasmon resonance (SPR) is a sensitive method well suited to the label-free detection of microorganisms. Wang et al. used this technique for specific detection of B. anthracis spores through the specific recognition of a monoclonal antibody-functionalized sensor [98]. A detection limit as low as 104 CFU/mL was reached in the 40 min analysis. In another study, B. cereus vegetative cells were detected by phage endolysin modified SPR chips with a limit of detection of 102 CFU/mL by using a subtractive inhibition assay [99]. Such subtractive inhibitory assays may potentially increase the sensitivity of spore detection by a SPR sensor.
In view of the literature on spore detection methods, the development of new approaches appears necessary in order to develop more sensitive (LoD < 103 CFU/mL) and specific analytical tools that can differentiate the spore species with good accuracy.
6. Conclusions and Perspectives
To ensure microbiological quality and consumer safety, strict controls and hygienic conditions are observed by the dairy industry, as recommended by guidelines of good dairy farming [100] and hygienic conditions at dairy plants (ISO 8086:2004). The control of environmental parameters (temperature, pH, water activity, salinity, atmosphere, presence of additives) can help the control of B. cereus proliferation in foods. The ability to produce spores make B. cereus capable to escape processing conditions carried out by the food industries to preserve products and to eliminate or reduce the bacterial number in the final product. Heat treatments commonly used by the food industry require longer treatments to efficiently eliminate B. cereus spores [101]. Moreover, depending on the heating conditions, pasteurization can either provoke activation of germination or growth of spores of B. cereus. This may result from the failure to recover or repair non-lethal injuries in this products at this temperature, as can be seen by the steady rate at which the spores died during storage at 7 °C [102]. Unfortunately, high heat procedures cannot be employed for all dairy products, especially not for infantile milk because it may destroy the nutritive quality of the milk. Subsequently, screening of milk and milk products for B. cereus spore contamination is of high importance for the dairy industry, which is looking for new cost-efficient, rapid and easy to use methods.
In this review, we have summarized the main problems that arise from the milk contamination by Bacillus cereus spores and remarkable advances in the development of novel sensitive assays for direct spore detection. Currently used plate-based methods for spore detection in milk and dairy products are time consuming, expensive, and need highly trained personnel. Inherent sensitivity, simplicity, and low cost of novel analytical methods, such as LAMP, electrochemical biosensors and lateral flow tests, start to attract more attention in the dairy industry. However, although novel portable assays display many advantages over currently used methods, they are not easily implementable, partially because only a few are available commercially. Nevertheless, it is evident that portable and cost-efficient biosensors for spore detection will be employed in the future to help milk producers, retailers, authorities and even consumers to increase microbiological quality in dairy products with minimal investment. Actual developments based on the combination of novel recognition elements, analytical technics and novel materials enabled significant improvements in microbiological analysis compared to current analytical systems. However, efforts should be made to take these assays from the laboratory bench to practical applications and mass production. The public and industrial demand for food safety will drive investment into biosensor design, marketing and commercialization.
Author Contributions
All authors contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lücking, G.; Stoeckel, M.; Atamer, Z.; Hinrichs, J.; Ehling-Schulz, M. Characterization of aerobic spore-forming bacteria associated with industrial dairy processing environments and product spoilage. Int. J. Food Microbiol. 2013, 166, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Gopal, N.; Hill, C.; Ross, P.R.; Beresford, T.P.; Fenelon, M.A.; Cotter, P.D. The prevalence and control of Bacillus and related spore-forming bacteria in the dairy industry. Front. Microbiol. 2015, 6, 1418. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.G.; Baglinière, F.; Marchand, S.; Van Coillie, E.; Vanetti, M.C.; De Block, J.; Heyndrickx, M. The biodiversity of the microbiota producing heat-resistant enzymes responsible for spoilage in processed bovine milk and dairy products. Front. Microbiol. 2017, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, Q.; Göker, M.; Meier-Kolthoff, J.P.; Wang, M.; Sun, Y.; Wang, L.; Shao, Z. Genomic insights into the taxonomic status of the Bacillus cereus group. Sci. Rep. 2015, 5, 14082. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Lai, Q.; Zeng, R.; Ye, D.; Xu, J.; Shao, Z. Proposal of nine novel species of the Bacillus cereus group. Int. J. Syst. Evol. Microbiol. 2017, 67, 2499–2508. [Google Scholar] [CrossRef]
- Dierick, K.; Van Coillie, E.; Swiecicka, I.; Meyfroidt, G.; Devlieger, H.; Meulemans, A.; Hoedemaekers, G.; Fourie, L.; Heyndrickx, M.; Mahillon, J. Fatal family outbreak of Bacillus cereus-associated food poisoning. J. Clin. Microbiol. 2005, 43, 4277–4279. [Google Scholar] [CrossRef]
- Jessberger, N.; Kranzler, M.; Da Riol, C.; Schwenk, V.; Buchacher, T.; Dietrich, R.; Ehling-Schulz, M.; Märtlbauer, E. Assessing the toxic potential of enteropathogenic Bacillus cereus. Food Microbiol. 2019, 84, 103276. [Google Scholar] [CrossRef]
- Lund, T.; De Buyser, M.L.; Granum, P.E. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 2000, 38, 254–261. [Google Scholar] [CrossRef]
- Pósfay-Barbe, K.M.; Schrenzel, J.; Frey, J.; Studer, R.; Korff, C.; Belli, D.C.; Parvex, P.; Rimensberger, P.C.; Schäppi, M.G. Food poisoning as a cause of acute liver failure. Pediatric Infect. Dis. J. 2008, 27, 846–847. [Google Scholar] [CrossRef] [PubMed]
- Rishi, E.; Rishi, P.; Sengupta, S.; Jambulingam, M.; Madhavan, H.N.; Gopal, L.; Therese, K.L. Acute postoperative Bacillus cereus endophthalmitis mimicking toxic anterior segment syndrome. Ophthalmology 2013, 120, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Johler, S.; Kalbhenn, E.M.; Heini, N.; Brodmann, P.; Bağcıoğlu, M.; Contzen, M.; Stephan, R.; Ehling-Schulz, M. Enterotoxin production of Bacillus thuringiensis isolates from biopesticides, foods, and outbreaks. Front. Microbiol. 2018, 9, 1915. [Google Scholar] [CrossRef]
- Hazards, E.P.O.B. Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14, e04524. [Google Scholar]
- Thorsen, L.; Hansen, B.M.; Nielsen, K.F.; Hendriksen, N.B.; Phipps, R.K.; Budde, B.B. Characterization of emetic Bacillus weihenstephanensis, a new cereulide-producing bacterium. Appl. Environ. Microbiol. 2006, 72, 5118–5121. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, H.B.; Kim, K.S. Isolation and Characterization of Spore-Forming Bacilli (SFB) from Shepherd’s Purse (Capsella bursa-pastoris). J. Food Sci. 2016, 81, M684–M691. [Google Scholar] [CrossRef]
- Miller, R.A.; Jian, J.; Beno, S.M.; Wiedmann, M.; Kovac, J. Intraclade variability in toxin production and cytotoxicity of Bacillus cereus group type strains and dairy-associated isolates. Appl. Environ. Microbiol. 2018, 84, e02479-17. [Google Scholar] [CrossRef]
- Frentzel, H.; Kraushaar, B.; Krause, G.; Bodi, D.; Wichmann-Schauer, H.; Appel, B.; Mader, A. Phylogenetic and toxinogenic characteristics of Bacillus cereus group members isolated from spices and herbs. Food Control 2018, 83, 90–98. [Google Scholar] [CrossRef]
- Heini, N.; Stephan, R.; Ehling-Schulz, M.; Johler, S. Characterization of Bacillus cereus group isolates from powdered food products. Int. J. Food Microbiol. 2018, 283, 59–64. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Guinebretiere, M.-H.; Monthán, A.; Berge, O.; Fricker, M.; Svensson, B. Toxin gene profiling of enterotoxic and emetic Bacillus cereus. FEMS Microbiol. Lett. 2006, 260, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, A.; Ween, O.; Lund, T.; Hardy, S.P.; Granum, P.E. Genetic and functional analysis of the cytK family of genes in Bacillus cereus. Microbiology 2004, 150, 2689–2697. [Google Scholar] [CrossRef] [PubMed]
- Ramarao, N.; Sanchis, V. The pore-forming haemolysins of Bacillus cereus: A review. Toxins 2013, 5, 1119–1139. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Sarkar, P.K. Bacillus cereus hazard and control in industrial dairy processing environment. Food Control 2016, 69, 20–29. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Vukov, N.; Schulz, A.; Shaheen, R.; Andersson, M.; Märtlbauer, E.; Scherer, S. Identification and partial characterization of the nonribosomal peptide synthetase gene responsible for cereulide production in emetic Bacillus cereus. Appl. Environ. Microbiol. 2005, 71, 105–113. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Frenzel, E.; Gohar, M. Food–bacteria interplay: Pathometabolism of emetic Bacillus cereus. Front. Microbiol. 2015, 6, 704. [Google Scholar] [CrossRef]
- Bauer, T.; Sipos, W.; Stark, T.; Kaeser, T.; Knecht, C.; Brunnthaler, R.; Saalmueller, A.; Hofmann, T.; Ehling-Schulz, M. First insights into within host translocation of the Bacillus cereus toxin cereulide using a porcine model. Front. Microbiol. 2018, 9, 2652. [Google Scholar] [CrossRef]
- Vangoitsenhoven, R.; Rondas, D.; Crèvecoeur, I.; D’Hertog, W.; Baatsen, P.; Masini, M.; Andjelkovic, M.; Van Loco, J.; Matthys, C.; Mathieu, C. Foodborne cereulide causes beta-cell dysfunction and apoptosis. PLoS ONE 2014, 9, e104866. [Google Scholar] [CrossRef]
- Ducrest, P.; Pfammatter, S.; Stephan, D.; Vogel, G.; Thibault, P.; Schnyder, B. Rapid detection of Bacillus ionophore cereulide in food products. Sci. Rep. 2019, 9, 5814. [Google Scholar] [CrossRef]
- Agata, N.; Mori, M.; Ohta, M.; Suwan, S.; Ohtani, I.; Isobe, M. A novel dodecadepsipeptide, cereulide, isolated from Bacillus cereus causes vacuole formation in HEp-2 cells. FEMS Microbiol. Lett. 1994, 121, 31–34. [Google Scholar]
- Ceuppens, S.; Uyttendaele, M.; Drieskens, K.; Heyndrickx, M.; Rajkovic, A.; Boon, N.; Van de Wiele, T. Survival and germination of Bacillus cereus spores without outgrowth or enterotoxin production during in vitro simulation of gastrointestinal transit. Appl. Environ. Microbiol. 2012, 78, 7698–7705. [Google Scholar] [CrossRef]
- Tsilia, V.; Devreese, B.; De Baenst, I.; Mesuere, B.; Rajkovic, A.; Uyttendaele, M.; Van de Wiele, T.; Heyndrickx, M. Application of MALDI-TOF mass spectrometry for the detection of enterotoxins produced by pathogenic strains of the Bacillus cereus group. Anal. Bioanal. Chem. 2012, 404, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Delbrassinne, L.; Andjelkovic, M.; Rajkovic, A.; Dubois, P.; Nguessan, E.; Mahillon, J.; Van Loco, J. Determination of Bacillus cereus Emetic Toxin in food products by means of LC–MS². Food Anal. Methods 2012, 5, 969–979. [Google Scholar] [CrossRef]
- Vidic, J.; Vizzini, P.; Manzano, M.; Kavanaugh, D.; Ramarao, N.; Zivkovic, M.; Radonic, V.; Knezevic, N.; Giouroudi, I.; Gadjanski, I. Point-of-need DNA testing for detection of foodborne pathogenic bacteria. Sensors 2019, 19, 1100. [Google Scholar] [CrossRef] [PubMed]
- Heyndrickx, M.; Scheldeman, P. Bacilli associated with spoilage in dairy products and other food. Appl. Syst. Bacillus Relat. 2002, 64–82. [Google Scholar] [CrossRef]
- FDA/WHO. 2012. Available online: https://apps.who.int/iris/handle/10665/43659 (accessed on 25 February 2020).
- Arnesen, L.P.S.; O’Sullivan, K.; Granum, P.E. Food poisoning potential of Bacillus cereus strains from Norwegian dairies. Int. J. Food Microbiol. 2007, 116, 292–296. [Google Scholar] [CrossRef]
- Authority, E.F.S. Opinion of the Scientific Panel on biological hazards (BIOHAZ) on Bacillus cereus and other Bacillus spp in foodstuffs. EFSA J. 2005, 3, 175. [Google Scholar]
- Christiansson, A.; Bertilsson, J.; Svensson, B. Bacillus cereus spores in raw milk: Factors affecting the contamination of milk during the grazing period. J. Dairy Sci. 1999, 82, 305–314. [Google Scholar] [CrossRef]
- Coorevits, A.; De Jonghe, V.; Vandroemme, J.; Reekmans, R.; Heyrman, J.; Messens, W.; De Vos, P.; Heyndrickx, M. Comparative analysis of the diversity of aerobic spore-forming bacteria in raw milk from organic and conventional dairy farms. Syst. Appl. Microbiol. 2008, 31, 126–140. [Google Scholar] [CrossRef]
- Svensson, B.; Ekelund, K.; Ogura, H.; Christiansson, A. Characterisation of Bacillus cereus isolated from milk silo tanks at eight different dairy plants. Int. Dairy J. 2004, 14, 17–27. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Komori, K.; Uchida, K.; Motoshima, H.; Katano, N. Seasonal variation in spore levels of Bacillus cereus and its psychrotrophic strains in raw milk in Hokkaido, Japan, and evaluation of strain diversity. Int. Dairy J. 2019, 97, 209–215. [Google Scholar] [CrossRef]
- Heyndrickx, M. Dispersal of aerobic endospore-forming bacteria from soil and agricultural activities to food and feed. In Endospore-Forming Soil Bacteria; Springer: Berlin/Heidelberg, Germany, 2011; pp. 135–156. [Google Scholar]
- Yibar, A.; Cetinkaya, F.; Soyutemiz, E.; Yaman, G. Prevalence, enterotoxin production and antibiotic resistance of Bacillus cereus isolated from milk and cheese. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 635–642. [Google Scholar]
- Scatassa, M.L.; Mancuso, I.; Sciortino, S.; Macaluso, G.; Palmeri, M.; Arcuri, L.; Todaro, M.; Cardamone, C. Retrospective study on the hygienic quality of fresh ricotta cheeses produced in Sicily, Italy. Ital. J. Food Saf. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewicz, M.; Marjańska, P.S. Milk-originated Bacillus cereus sensu lato strains harbouring Bacillus anthracis-like plasmids are genetically and phenotypically diverse. Food Microbiol. 2017, 67, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.Q.; de Paiva, E.P.; Rabinovitch, L.; Vivoni, A.M. Molecular Characterization and Risk Assessment of Bacillus cereus Sensu Lato Isolated from Ultrahigh-Temperature and Pasteurized Milk Marketed in Rio de Janeiro, Brazil. J. Food Prot. 2017, 80, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Spore resistance properties. In The bacterial Spore: From Molecules to Systems; American Society of Microbiology: Washington, DC, USA, 2016; pp. 201–215. [Google Scholar]
- Setlow, P. Germination of spores of Bacillus species: What we know and do not know. J. Bacteriol. 2014, 196, 1297–1305. [Google Scholar] [CrossRef]
- Heyndrickx, M. The importance of endospore-forming bacteria originating from soil for contamination of industrial food processing. Appl. Environ. Soil Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Miller, R.A.; Kent, D.J.; Watterson, M.J.; Boor, K.J.; Martin, N.H.; Wiedmann, M. Spore populations among bulk tank raw milk and dairy powders are significantly different. J. Dairy Sci. 2015, 98, 8492–8504. [Google Scholar] [CrossRef]
- 1441/2007, E.C.R.E.N. Amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2007, 322, 23. [Google Scholar]
- Sonenshein, A.L. Control of sporulation initiation in Bacillus subtilis. Curr. Opin. Microbiol. 2000, 3, 561–566. [Google Scholar] [CrossRef]
- Luu, S.; Cruz-Mora, J.; Setlow, B.; Feeherry, F.E.; Doona, C.J.; Setlow, P. The effects of heat activation on Bacillus spore germination, with nutrients or under high pressure, with or without various germination proteins. Appl. Environ. Microbiol. 2015, 81, 2927–2938. [Google Scholar] [CrossRef] [PubMed]
- Hoch, J.A. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu. Rev. Microbiol. 1993, 47, 441–465. [Google Scholar] [CrossRef] [PubMed]
- Burbulys, D.; Trach, K.A.; Hoch, J.A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 1991, 64, 545–552. [Google Scholar] [CrossRef]
- Molle, V.; Fujita, M.; Jensen, S.T.; Eichenberger, P.; González-Pastor, J.E.; Liu, J.S.; Losick, R. The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 2003, 50, 1683–1701. [Google Scholar] [CrossRef] [PubMed]
- Meeske, A.J.; Rodrigues, C.D.; Brady, J.; Lim, H.C.; Bernhardt, T.G.; Rudner, D.Z. High-throughput genetic screens identify a large and diverse collection of new sporulation genes in Bacillus subtilis. PLoS Biol. 2016, 14, e1002341. [Google Scholar] [CrossRef] [PubMed]
- Verplaetse, E.; Slamti, L.; Gohar, M.; Lereclus, D. Two distinct pathways lead Bacillus thuringiensis to commit to sporulation in biofilm. Res. Microbiol. 2017, 168, 388–393. [Google Scholar] [CrossRef]
- Bidnenko, V.; Nicolas, P.; Grylak-Mielnicka, A.; Delumeau, O.; Auger, S.; Aucouturier, A.; Guerin, C.; Francis, R.; Bardowski, J.; Aymerich, S. Termination factor Rho: From the control of pervasive transcription to cell fate determination in Bacillus subtilis. PLoS Genet. 2017, 13, e1006909. [Google Scholar] [CrossRef]
- Soni, A.; Oey, I.; Silcock, P.; Bremer, P. Bacillus spores in the food industry: A review on resistance and response to novel inactivation technologies. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1139–1148. [Google Scholar] [CrossRef]
- Bressuire-Isoard, C.; Bornard, I.; Henriques, A.O.; Carlin, F.; Broussolle, V. Sporulation temperature reveals a requirement for CotE in the assembly of both the coat and exosporium layers of Bacillus cereus spores. Appl. Environ. Microbiol. 2016, 82, 232–243. [Google Scholar] [CrossRef]
- Charney, J.; Fisher, W.; Hegarty, C. Manganese as an essential element for sporulation in the genus Bacillus. J. Bacteriol. 1951, 62, 145. [Google Scholar] [CrossRef]
- Setlow, P. Spore germination. Curr. Opin. Microbiol. 2003, 6, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Abhyankar, W.; Ouwerling, N.; Dekker, H.L.; van Veen, H.; van der Wel, N.N.; Roseboom, W.; de Koning, L.J.; Brul, S.; de Koster, C.G. Bacillus subtilis spore inner membrane proteome. J. Proteome Res. 2016, 15, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.A.; Planchon, S.; Jobin, M.; Schmitt, P. Absence of oxygen affects the capacity to sporulate and the spore properties of Bacillus cereus. Food Microbiol. 2014, 42, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K.; Mishra, A.; Pradhan, A.K. Dynamic predictive model for growth of Bacillus cereus from spores in cooked beans. J. Food Prot. 2017, 81, 308–315. [Google Scholar] [CrossRef]
- Shinagawa, K. Analytical methods for Bacillus cereus and other Bacillus species. Int. J. Food Microbiol. 1990, 10, 125–141. [Google Scholar] [CrossRef]
- Kozuka, S.; Tochikubo, K. Permeability of dormant spores of Bacillus subtilis to malachite green and crystal violet. Microbiology 1991, 137, 607–613. [Google Scholar] [CrossRef]
- Dauphin, L.A.; Moser, B.D.; Bowen, M.D. Evaluation of five commercial nucleic acid extraction kits for their ability to inactivate Bacillus anthracis spores and comparison of DNA yields from spores and spiked environmental samples. J. Microbiol. Methods 2009, 76, 30–37. [Google Scholar] [CrossRef]
- Martinez-Blanch, J.F.; Sanchez, G.; Garay, E.; Aznar, R. Evaluation of a real-time PCR assay for the detection and quantification of Bacillus cereus group spores in food. J. Food Prot. 2010, 73, 1480–1485. [Google Scholar] [CrossRef]
- Perdue, M.L.; Karns, J.; Higgins, J.; Van Kessel, J.A. Detection and fate of Bacillus anthracis (Sterne) vegetative cells and spores added to bulk tank milk. J. Food Prot. 2003, 66, 2349–2354. [Google Scholar] [CrossRef]
- Fischer, C.; Hünniger, T.; Jarck, J.-H.; Frohnmeyer, E.; Kallinich, C.; Haase, I.; Hahn, U.; Fischer, M. Food sensing: Aptamer-based trapping of Bacillus cereus spores with specific detection via real time PCR in milk. J. Agric. Food Chem. 2015, 63, 8050–8057. [Google Scholar] [CrossRef]
- Kotsiri, Z.; Vantarakis, A.; Rizzotto, F.; Kavanaugh, D.; Ramarao, N.; Vidic, J. Sensitive Detection of E. coli in Artificial Seawater by Aptamer-Coated Magnetic Beads and Direct PCR. Appl. Sci. 2019, 9, 5392. [Google Scholar] [CrossRef]
- Park, C.; Kong, M.; Lee, J.-H.; Ryu, S.; Park, S. Detection of Bacillus Cereus Using Bioluminescence Assay with Cell Wall-Binding Domain Conjugated Magnetic Nanoparticles. Biochip J. 2018, 12, 287–293. [Google Scholar] [CrossRef]
- Adone, R.; Pasquali, P.; La Rosa, G.; Marianelli, C.; Muscillo, M.; Fasanella, A.; Francia, M.; Ciuchini, F. Sequence analysis of the genes encoding for the major virulence factors of Bacillus anthracis vaccine strainCarbosap′. J. Appl. Microbiol. 2002, 93, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Hartley, H.A.; Baeumner, A.J. Biosensor for the specific detection of a single viable B. áanthracis spore. Anal. Bioanal. Chem. 2003, 376, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Hoffmaster, A.R.; Fitzgerald, C.C.; Ribot, E.; Mayer, L.W.; Popovic, T. Molecular subtyping of Bacillus anthracis and the 2001 bioterrorism-associated anthrax outbreak, United States. Emerg. Infect. Dis. 2002, 8, 1111. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Z.; Yan, S.; Yin, F.; Feng, X.; Liu, B.-F. Identifying multiple bacterial pathogens by loop-mediated isothermal amplification on a rotate & react slipchip. Sens. Actuators B Chem. 2016, 228, 491–499. [Google Scholar]
- Janssen, F.L.A.J.; Anderson, L.E. Colorimetric assay for dipicolinic acid in bacterial spores. Science 1958, 127, 26–27. [Google Scholar] [CrossRef]
- Rosen, D.L. Airborne bacterial endospores detected by use of an impinger containing aqueous terbium chloride. Appl. Opt. 2006, 45, 3152–3157. [Google Scholar] [CrossRef]
- Gültekin, A.; Diltemiz, S.E.; Ersöz, A.; Sarıözlü, N.Y.; Denizli, A.; Say, R. Gold–silver nanoclusters having dipicolinic acid imprinted nanoshell for Bacillus cereus spores recognition. Talanta 2009, 78, 1332–1338. [Google Scholar] [CrossRef]
- Baig, M.M.F.; Chen, Y.-C. Gold nanoparticle-based colorimetric sensing of dipicolinic acid from complex samples. Anal. Bioanal. Chem. 2018, 410, 1805–1815. [Google Scholar] [CrossRef]
- Chan, J.W.; Esposito, A.; Talley, C.; Hollars, C.; Lane, S.; Huser, T. Reagentless identification of single bacterial spores in aqueous solution by confocal laser tweezers Raman spectroscopy. Anal. Chem. 2004, 76, 599–603. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, Y.; Lin, M.; Mustapha, A.; Wang, Y. Detecting single Bacillus spores by surface enhanced Raman spectroscopy. Sens. Instrum. Food Qual. Saf. 2008, 2, 247. [Google Scholar] [CrossRef]
- Shende, C.; Inscore, F.; Huang, H.; Farquharson, S.; Sengupta, A. Detection of Bacillus spores within 15 minutes by surface-enhanced Raman spectroscopy. In Proceedings Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XIII; SPIE Defense, Security, and Sensing: Baltimore, MD, USA, 2012; p. 83580G. [Google Scholar]
- Cowcher, D.P.; Xu, Y.; Goodacre, R. Portable, quantitative detection of Bacillus bacterial spores using surface-enhanced Raman scattering. Anal. Chem. 2013, 85, 3297–3302. [Google Scholar] [CrossRef]
- Voorhees, K.J.; Hadfield, T.L.; Cody, R.B. Electron monochromator mass spectrometry for the analysis of whole bacteria and bacterial spores. Anal. Chem. 2000, 72, 2428–2432. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, L.W.; Zhen, F.; Yi, H.X.; Du, M.; Zhang, L.L.; Li, Y.H.; Wang, W.J. Dipicolinic acid contents used for estimating the number of spores in raw milk. Adv. Mater. Res. 2011, 183–185, 1467–1471. [Google Scholar] [CrossRef]
- Quinlan, J.; Foegeding, P. Monoclonal antibodies for use in detection of Bacillus and Clostridium spores. Appl. Environ. Microbiol. 1997, 63, 482–487. [Google Scholar] [CrossRef]
- Wang, D.-B.; Tian, B.; Zhang, Z.-P.; Wang, X.-Y.; Fleming, J.; Bi, L.-J.; Yang, R.-F.; Zhang, X.-E. Detection of Bacillus anthracis spores by super-paramagnetic lateral-flow immunoassays based on “Road Closure”. Biosens. Bioelectron. 2015, 67, 608–614. [Google Scholar] [CrossRef]
- Fisher, M.; Atiya-Nasagi, Y.; Simon, I.; Gordin, M.; Mechaly, A.; Yitzhaki, S. A combined immunomagnetic separation and lateral flow method for a sensitive on-site detection of Bacillus anthracis spores–assessment in water and dairy products. Lett. Appl. Microbiol. 2009, 48, 413–418. [Google Scholar] [CrossRef]
- Manzano, M.; Viezzi, S.; Mazerat, S.; Marks, R.S.; Vidic, J. Rapid and label-free electrochemical DNA biosensor for detecting hepatitis A virus. Biosens. Bioelectron. 2018, 100, 89–95. [Google Scholar] [CrossRef]
- Vidic, J.; Manzano, M.; Chang, C.-M.; Jaffrezic-Renault, N. Advanced biosensors for detection of pathogens related to livestock and poultry. Vet. Res. 2017, 48, 11. [Google Scholar] [CrossRef]
- Vizzini, P.; Braidot, M.; Vidic, J.; Manzano, M. Electrochemical and Optical Biosensors for the Detection of Campylobacter and Listeria: An Update Look. Micromachines 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P. Development of aptamer beacons for rapid presumptive detection of Bacillus spores. J. Fluoresc. 2012, 22, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Mazzaracchio, V.; Neagu, D.; Porchetta, A.; Marcoccio, E.; Pomponi, A.; Faggioni, G.; D’Amore, N.; Notargiacomo, A.; Pea, M.; Moscone, D. A label-free impedimetric aptasensor for the detection of Bacillus anthracis spore simulant. Biosens. Bioelectron. 2019, 126, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-B.; Bi, L.-J.; Zhang, Z.-P.; Chen, Y.-Y.; Yang, R.-F.; Wei, H.-P.; Zhou, Y.-F.; Zhang, X.-E. Label-free detection of B. anthracis spores using a surface plasmon resonance biosensor. Analyst 2009, 134, 738–742. [Google Scholar] [CrossRef]
- Kong, M.; Sim, J.; Kang, T.; Nguyen, H.H.; Park, H.K.; Chung, B.H.; Ryu, S. A novel and highly specific phage endolysin cell wall binding domain for detection of Bacillus cereus. Eur. Biophys. J. 2015, 44, 437–446. [Google Scholar] [CrossRef]
- IDF, F.A. Guide to good dairy farming practice. In Animal Production and Health Guidelines; International Dairy Federation and the Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; Volume 8. [Google Scholar]
- Dufrenne, J.; Soentoro, P.; Tatini, S.; Day, T.; Notermans, S. Characteristics of Bacillus cereus related to safe food production. Int. J. Food Microbiol. 1994, 23, 99–109. [Google Scholar] [CrossRef]
- Samapundo, S.; Heyndrickx, M.; Xhaferi, R.; de Baenst, I.; Devlieghere, F. The combined effect of pasteurization intensity, water activity, pH and incubation temperature on the survival and outgrowth of spores of Bacillus cereus and Bacillus pumilus in artificial media and food products. Int. J. Food Microbiol. 2014, 181, 10–18. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).