Rapid Multianalyte Microfluidic Homogeneous Immunoassay on Electrokinetically Driven Beads
Abstract
1. Introduction
2. Materials and Methods
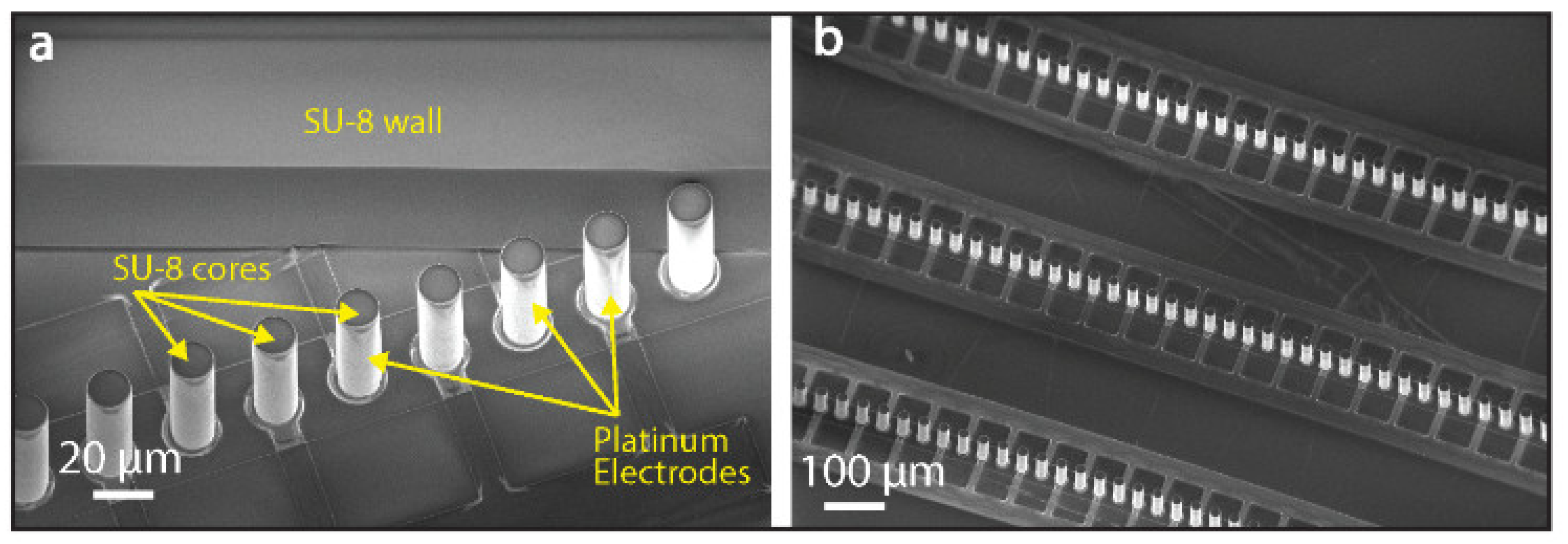
2.1. Chip Microfabrication
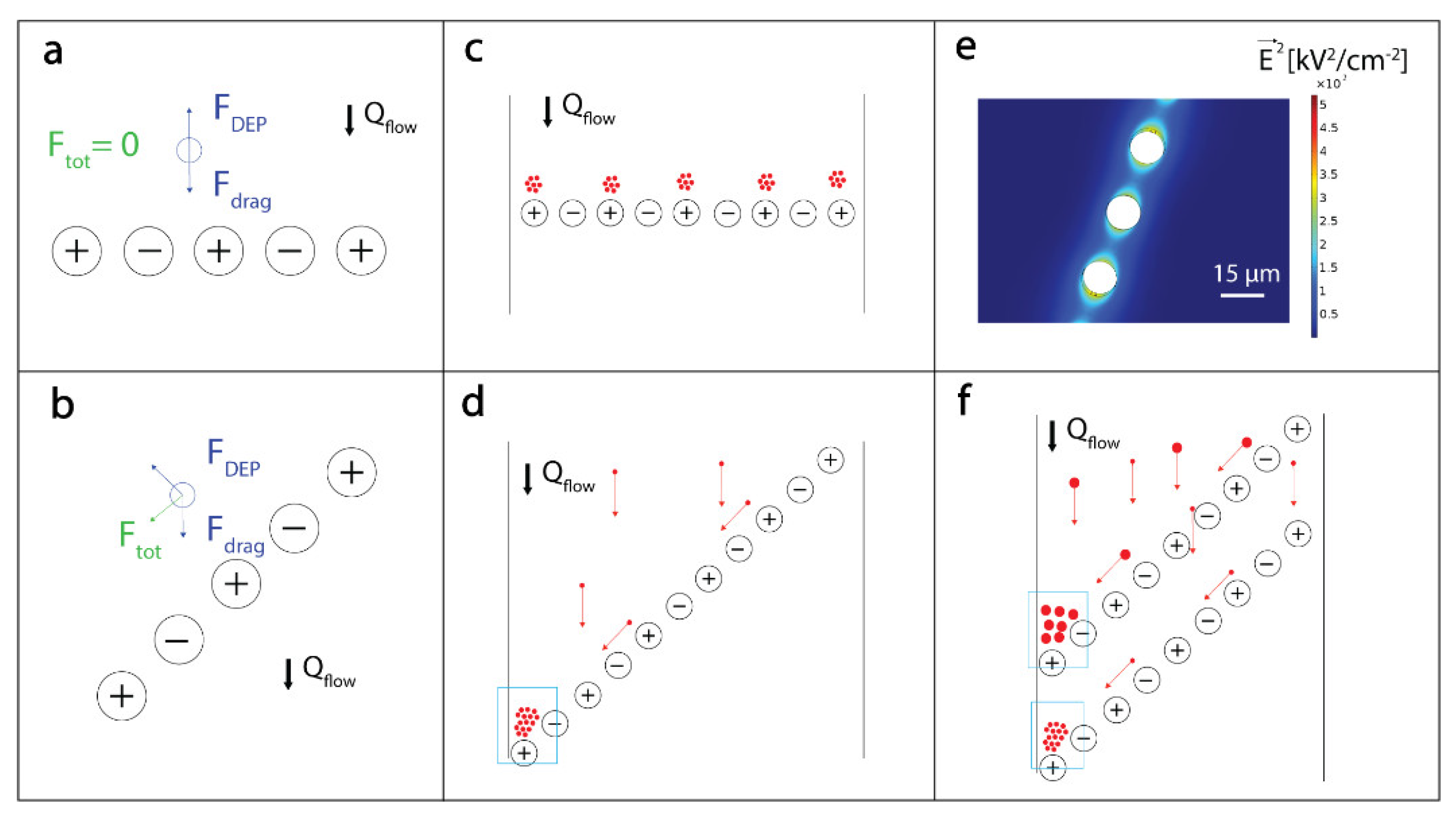
2.2. DEP-Based Manipulation of Beads
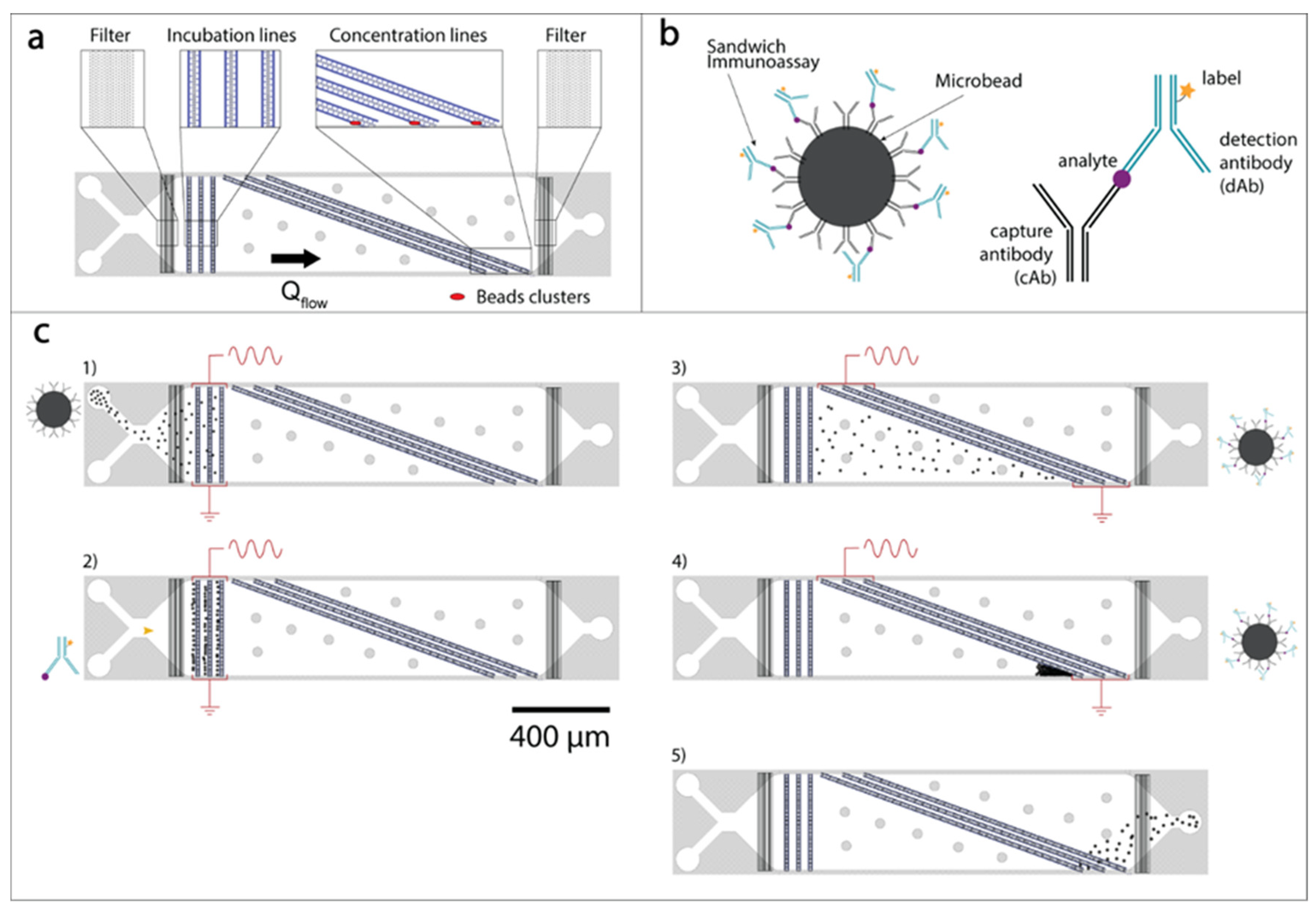
2.3. Microfluidic Analytical Device Operation and Experimental Setup
2.4. Antibodies’ Conjugation to Beads and to Labeling Fluorescent Molecules
2.5. Data Analysis
3. Results and Discussion
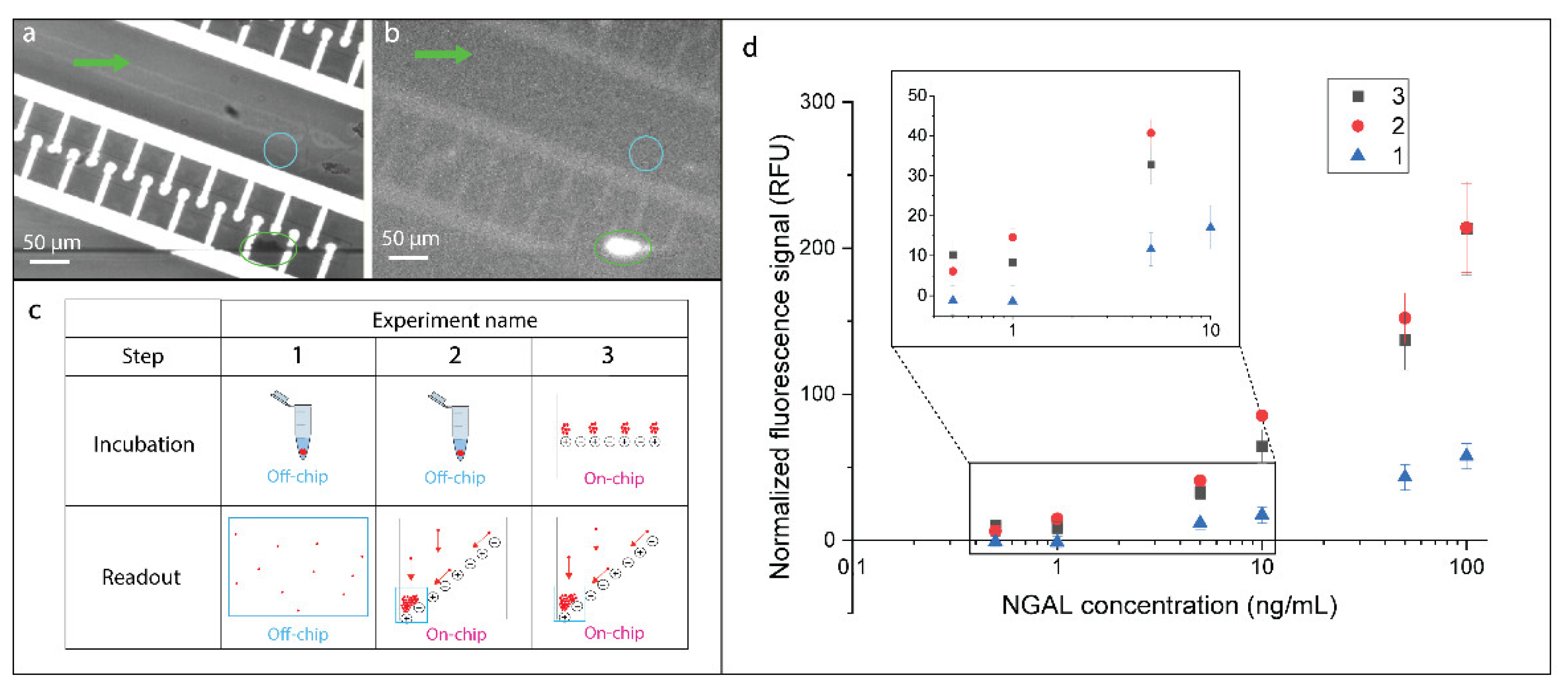
3.1. Immunoassay Incubation On-Chip
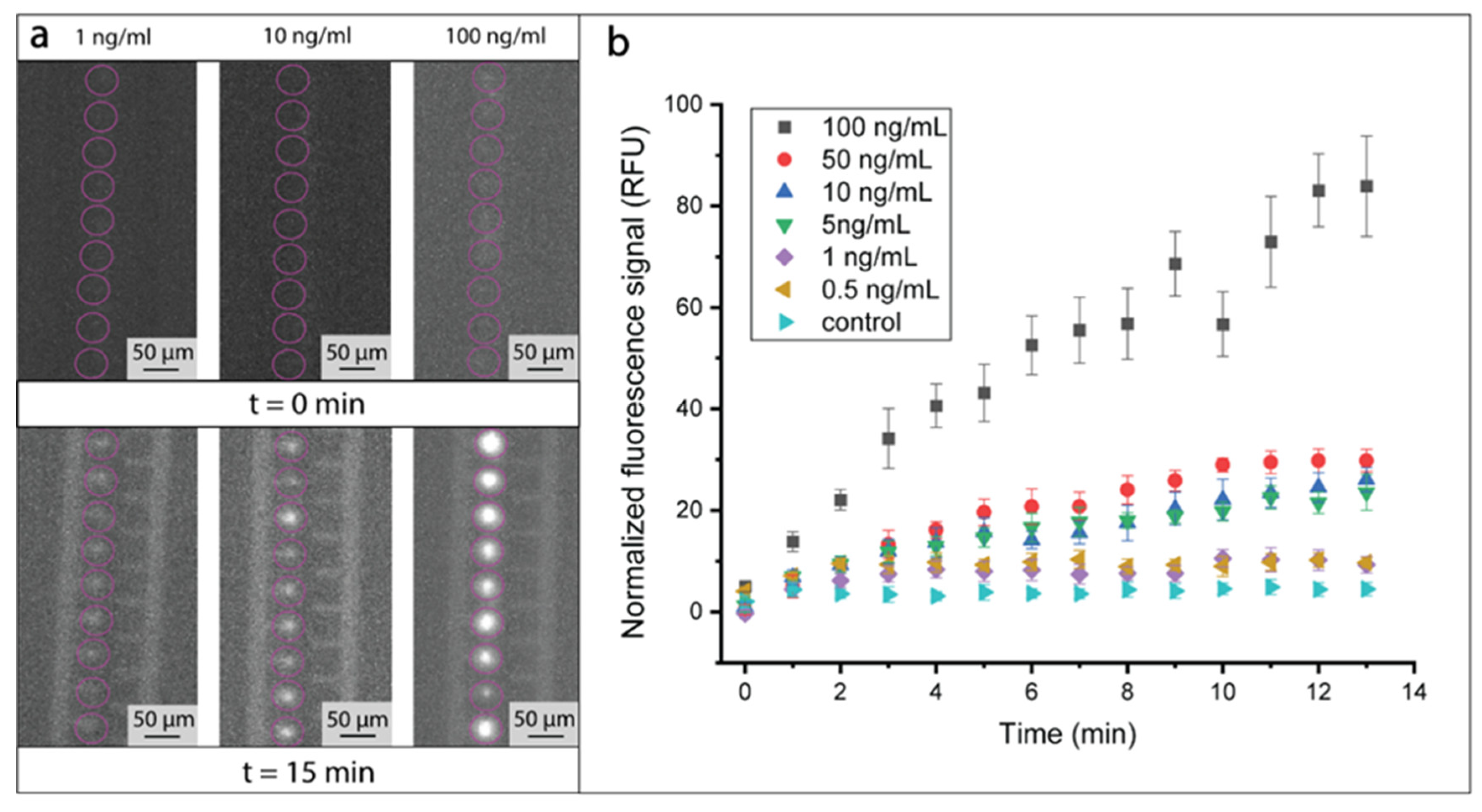
3.2. Immunoassay Performance
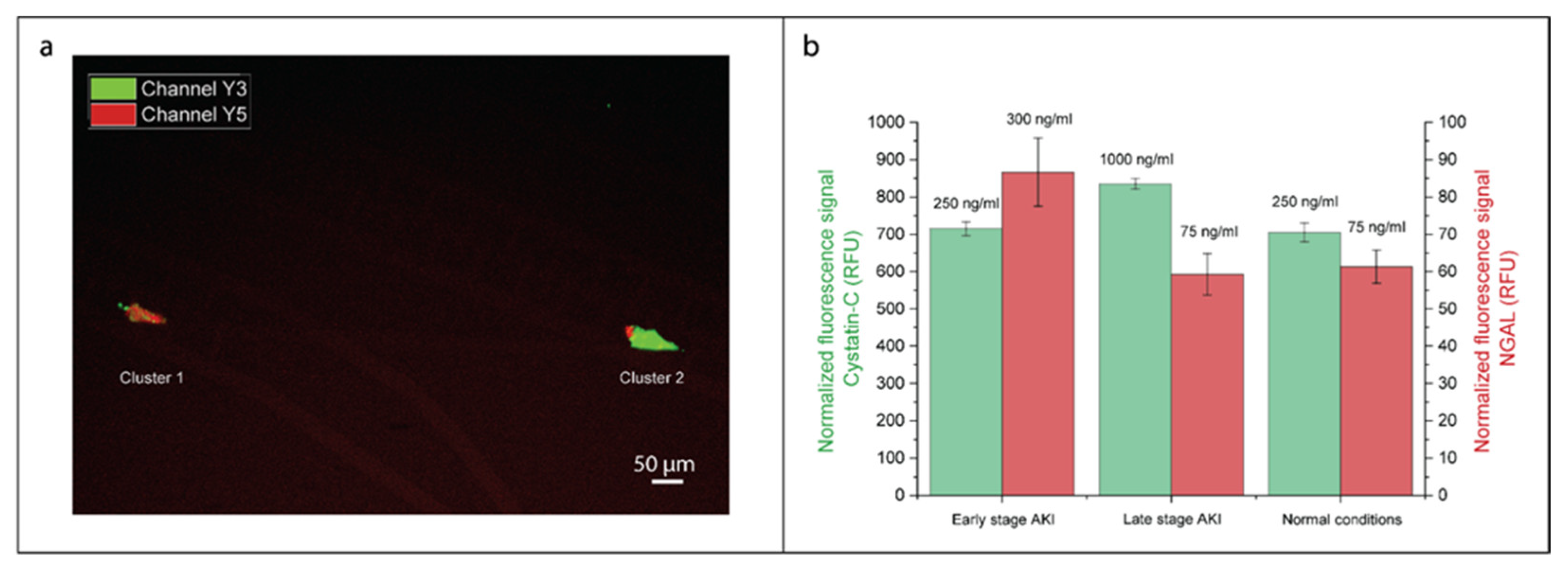
3.3. Simultaneous On-Chip Analysis of AKI Biomarkers NGAL and Cystatin C
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Shad, F.; Smith, M.C. Acute kidney injury: A guide to diagnosis and management. Am. Fam. Physician 2012, 86, 631–639. [Google Scholar] [PubMed]
- Maggi, P.; Montinaro, V.; Mussini, C.; Di Biagio, A.; Bellagamba, R.; Bonfanti, P.; Calza, L.; Cherubini, C.; Corsi, P.; Gargiulo, M.; et al. Novel antiretroviral drugs and renal function monitoring of HIV patients. AIDS Rev. 2014, 16, 144–151. [Google Scholar]
- Wilson, F.P.; Greenberg, J.H. Acute Kidney Injury in Real Time: Prediction, Alerts, and Clinical Decision Support. Nephron 2018, 140, 116–119. [Google Scholar] [CrossRef]
- Ostermann, M.; Joannidis, M. Acute kidney injury 2016: Diagnosis and diagnostic workup. Crit. Care 2016, 20, 299. [Google Scholar] [CrossRef]
- Murty, M.S.N.; Sharma, U.K.; Pandey, V.B.; Kankare, S.B. Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J. Nephrol. 2013, 23, 180–183. [Google Scholar] [CrossRef]
- Urbschat, A.; Obermüller, N.; Haferkamp, A. Biomarkers of kidney injury. Biomarkers 2011, 16, S22–S30. [Google Scholar] [CrossRef]
- Padhy, M.; Kaushik, S.; Girish, M.P.; Mohapatra, S.; Shah, S.; Koner, B.C. Serum neutrophil gelatinase associated lipocalin (NGAL) and cystatin C as early predictors of contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention. Clin. Chim. Acta 2014, 435, 48–52. [Google Scholar] [CrossRef]
- Khawaja, S.; Jafri, L.; Siddiqui, I.; Hashmi, M.; Ghani, F. The utility of neutrophil gelatinase-associated Lipocalin (NGAL) as a marker of acute kidney injury (AKI) in critically ill patients. Biomark. Res. 2019, 4. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin (NGAL). Scand. J. Clin. Lab. Investig. Suppl. 2008, 241, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Armbrecht, L.; Dincer, C.; Kling, A.; Horak, J.; Kieninger, J.; Urban, G. Self-assembled magnetic bead chains for sensitivity enhancement of microfluidic electrochemical biosensor platforms. Lab Chip 2015, 15, 4314–4321. [Google Scholar] [CrossRef] [PubMed]
- Falconnet, D.; She, J.; Tornay, R.; Leimgruber, E.; Bernasconi, D.; Lagopoulos, L.; Renaud, P.; Demierre, N.; van den Bogaard, P. Rapid, Sensitive and Real-Time Multiplexing Platform for the Analysis of Protein and Nucleic-Acid Biomarkers. Anal. Chem. 2015, 87, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yuan, Y.J. A review of polystyrene bead manipulation by dielectrophoresis. Rsc. Adv. 2019, 9, 4963–4981. [Google Scholar] [CrossRef]
- Salari, A.; Thompson, M. Recent advances in AC electrokinetic sample enrichment techniques for biosensor development. Sens. Actuators B Chem. 2018, 255, 3601–3615. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Varhue, W.; Rohani, A.; Liao, K.-T.; Bazydlo, L.A.L.; Chou, C.-F.; Swami, N.S. Ultrafast immunoassays by coupling dielectrophoretic biomarker enrichment in nanoslit channel with electrochemical detection on graphene. Lab Chip 2015, 15, 4563–4570. [Google Scholar] [CrossRef]
- Holmes, D.K.; She, J.L.; Roach, P.; Morgan, H. Bead-based immunoassays using a micro-chip flow cytometer. Lab Chip 2007, 7, 1048–1056. [Google Scholar] [CrossRef]
- Iswardy, E.; Tsai, T.-C.; Cheng, I.-F.; Ho, T.-C.; Perng, G.; Chang, H.-C. A bead-based immunofluorescence-assay on a microfluidic dielectrophoresis platform for rapid dengue virus detection. Biosens. Bioelectron. 2017, 95, 174–180. [Google Scholar] [CrossRef]
- Ramón-Azcón, J.; Kunikata, R.; Sanchez, F.-J.; Marco, M.-P.; Shiku, H.; Yasukawa, T.; Matsue, T. Detection of pesticide residues using an immunodevice based on negative dielectrophoresis. Biosens. Bioelectron. 2009, 24, 1592–1597. [Google Scholar] [CrossRef]
- Park, S.; Yossifon, G. Combining dielectrophoresis and concentration polarization-based preconcentration to enhance bead-based immunoassay sensitivity. Nanoscale 2019. [Google Scholar] [CrossRef]
- Yasukawa, T.; Suzuki, M.; Sekiya, T.; Shiku, H.; Matsue, T. Flow sandwich-type immunoassay in microfluidic devices based on negative dielectrophoresis. Biosens. Bioelectron. 2007, 22, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Thiriet, P.-E.; Pezoldt, J.; Gambardella, G.; Keim, K.; Deplancke, B.; Guiducci, C. Selective Retrieval of Individual Cells from Microfluidic Arrays Combining Dielectrophoretic Force and Directed Hydrodynamic Flow. Micromachines 2020, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, P.R.C.; Vykoukal, J. Particle separation by dielectrophoresis. Electrophoresis 2002, 23, 1973–1983. [Google Scholar] [CrossRef]
- Thiriet, P.-E.; Medagoda, D.; Guiducci, C. Dielectrophoresis Detection. Device. Patent 20178445.1, 5 June 2020. [Google Scholar]
- Nerguizian, V.; Stiharu, I.; Nosayba, A.-A.; Bader, Y.-D.; Alazzam, A. The effect of dielectrophoresis on living cells: Crossover frequencies and deregulation in gene expression. Analyst 2019, 144, 3853–3860. [Google Scholar] [CrossRef]
- Putallaz, L.; van den Bogaard, P.; Laub, P.; Rebeaud, F. Nanofluidics Drives Point-of-care Technology for on the Spot Protein Marker Analysis with Rapid Actionable Results. J. Nanomed. Nanotechnol. 2019, 10. [Google Scholar] [CrossRef]
- Taylor, T.P.; Janech, M.G.; Slate, E.H.; Lewis, E.C.; Arthur, J.M.; Oates, J.C. Overcoming the Effects of Matrix Interference in the Measurement of Urine Protein Analytes. Biomark Insights 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Price, C.P. Point of care testing. BMJ 2001, 322, 1285–1288. [Google Scholar] [CrossRef]
- İçöz, K.; Mzava, O. Detection of Proteins Using Nano Magnetic Particle Accumulation-Based Signal Amplification. Appl. Sci. 2016, 6, 394. [Google Scholar] [CrossRef]
- Jalal, U.M.; Jin, G.J.; Eom, K.S.; Kim, M.H.; Shim, J.S. On-chip signal amplification of magnetic bead-based immunoassay by aviating magnetic bead chains. Bioelectrochemistry 2018, 122, 221–226. [Google Scholar] [CrossRef]
- Yoo, H.; Lee, D.J.; Kim, D.; Park, J.; Chen, X.; Hong, S. Magnetically-focusing biochip structures for high-speed active biosensing with improved selectivity. Nanotechnology 2018, 29, 265501. [Google Scholar] [CrossRef]
- Dittmer, W.U.; de Kievit, P.; Prins, M.W.J.; Vissers, J.L.M.; Mersch, M.E.C.; Martens, M.F.W.C. Sensitive and rapid immunoassay for parathyroid hormone using magnetic particle labels and magnetic actuation. J. Immunol. Methods 2008, 338, 40–46. [Google Scholar] [CrossRef]
- Sreenivasan, K.R. Turbulent mixing: A perspective. Proc. Natl. Acad. Sci. USA 2019, 116, 18175–18183. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Delamarche, E.; Wolf, M.; Hunziker, P. Modeling and Optimization of High-Sensitivity, Low-Volume Microfluidic-Based Surface Immunoassays. Biomed. Microdevices 2005, 7, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Nothing, M.; Coble, B.; Zheng, H.; Park, J.; Im, H.; Weber, G.F.; Castro, C.M.; Swirski, F.K.; Weissleder, R.; et al. Integrated Biosensor for Rapid and Point-Of-Care Sepsis Diagnosis. ACS Nano 2018, 12, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Hang, C.-C.; Yang, J.; Wang, S.; Li, C.-S.; Tang, Z.-R. Evaluation of serum neutrophil gelatinase-associated lipocalin in predicting acute kidney injury in critically ill patients. J. Int. Med. Res. 2017, 45, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Pei, X.; Zhu, B.; Yuan, H.; Zhao, W. Predictive value of serum cystatin C for acute kidney injury in adults: A meta-analysis of prospective cohort trials. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Villa, P.; Jiménez, M.; Soriano, M.-C.; Manzanares, J.; Casasnovas, P. Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit. Care Lond. Engl. 2005, 9, R139–R143. [Google Scholar] [CrossRef]
- Ellington, A.A.; Kullo, I.J.; Bailey, K.R.; Klee, G.G. Antibody-Based Protein Multiplex Platforms: Technical and Operational Challenges. Clin Chem 2010, 56, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kingsmore, S.F. Multiplexed protein measurement: Technologies and applications of protein and antibody arrays. Nat. Rev. Drug Discov. 2006, 5, 310–320. [Google Scholar] [CrossRef]
- Harink, B.; Nguyen, H.; Thorn, K.; Fordyce, P. An open-source software analysis package for Microspheres with Ratiometric Barcode Lanthanide Encoding (MRBLEs). PLoS ONE 2019, 14, e0203725. [Google Scholar] [CrossRef]
- Aira, C.; Ruiz, T.; Dixon, L.; Blome, S.; Rueda, P.; Sastre, P. Bead-Based Multiplex Assay for the Simultaneous Detection of Antibodies to African Swine Fever Virus and Classical Swine Fever Virus. Front. Vet. Sci. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.M.; Rusch, S.; Temiz, Y.; Lovchik, R.D.; Beck, H.P.; Karlen, W.; Delamarche, E. A bead-based immunogold-silver staining assay on capillary-driven microfluidics. Biomed. Microdevices 2018, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Epifania, R.; Soares, R.R.G.; Pinto, I.F.; Chu, V.; Conde, J.P. Capillary-driven microfluidic device with integrated nanoporous microbeads for ultrarapid biosensing assays. Sens. Actuators B Chem. 2018, 265, 452–458. [Google Scholar] [CrossRef]
| DEP Surfing | ELISA | Abbott Architect | Lateral Flow Assay | |
|---|---|---|---|---|
| Limit of detection | Low | Low | Low | Average |
| Total analysis time | Short | Long | Average | Short |
| Sample processing | Limited | Extensive | Extensive | Limited |
| Volume needed | Low | Average | Average | High |
| Multiple analytes | Easy | Difficult | Easy | Average |
| Translation to PoC | Easy | Difficult | Difficult | Easy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiriet, P.-E.; Medagoda, D.; Porro, G.; Guiducci, C. Rapid Multianalyte Microfluidic Homogeneous Immunoassay on Electrokinetically Driven Beads. Biosensors 2020, 10, 212. https://doi.org/10.3390/bios10120212
Thiriet P-E, Medagoda D, Porro G, Guiducci C. Rapid Multianalyte Microfluidic Homogeneous Immunoassay on Electrokinetically Driven Beads. Biosensors. 2020; 10(12):212. https://doi.org/10.3390/bios10120212
Chicago/Turabian StyleThiriet, Pierre-Emmanuel, Danashi Medagoda, Gloria Porro, and Carlotta Guiducci. 2020. "Rapid Multianalyte Microfluidic Homogeneous Immunoassay on Electrokinetically Driven Beads" Biosensors 10, no. 12: 212. https://doi.org/10.3390/bios10120212
APA StyleThiriet, P.-E., Medagoda, D., Porro, G., & Guiducci, C. (2020). Rapid Multianalyte Microfluidic Homogeneous Immunoassay on Electrokinetically Driven Beads. Biosensors, 10(12), 212. https://doi.org/10.3390/bios10120212





