Label-Free and Sensitive Determination of Cadmium Ions Using a Ti-Modified Co3O4-Based Electrochemical Aptasensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
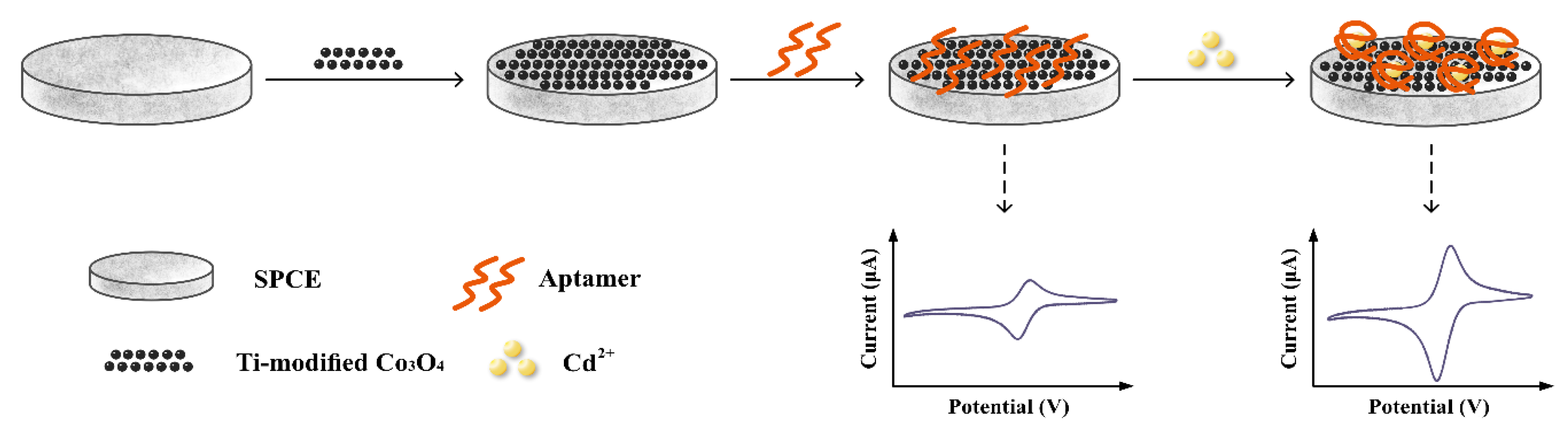
2.3. Aptasensor Fabrication Procedures
2.4. Electrochemical Measurements
2.5. Determination of Cd2+ in Environmental Samples
3. Results and Discussion
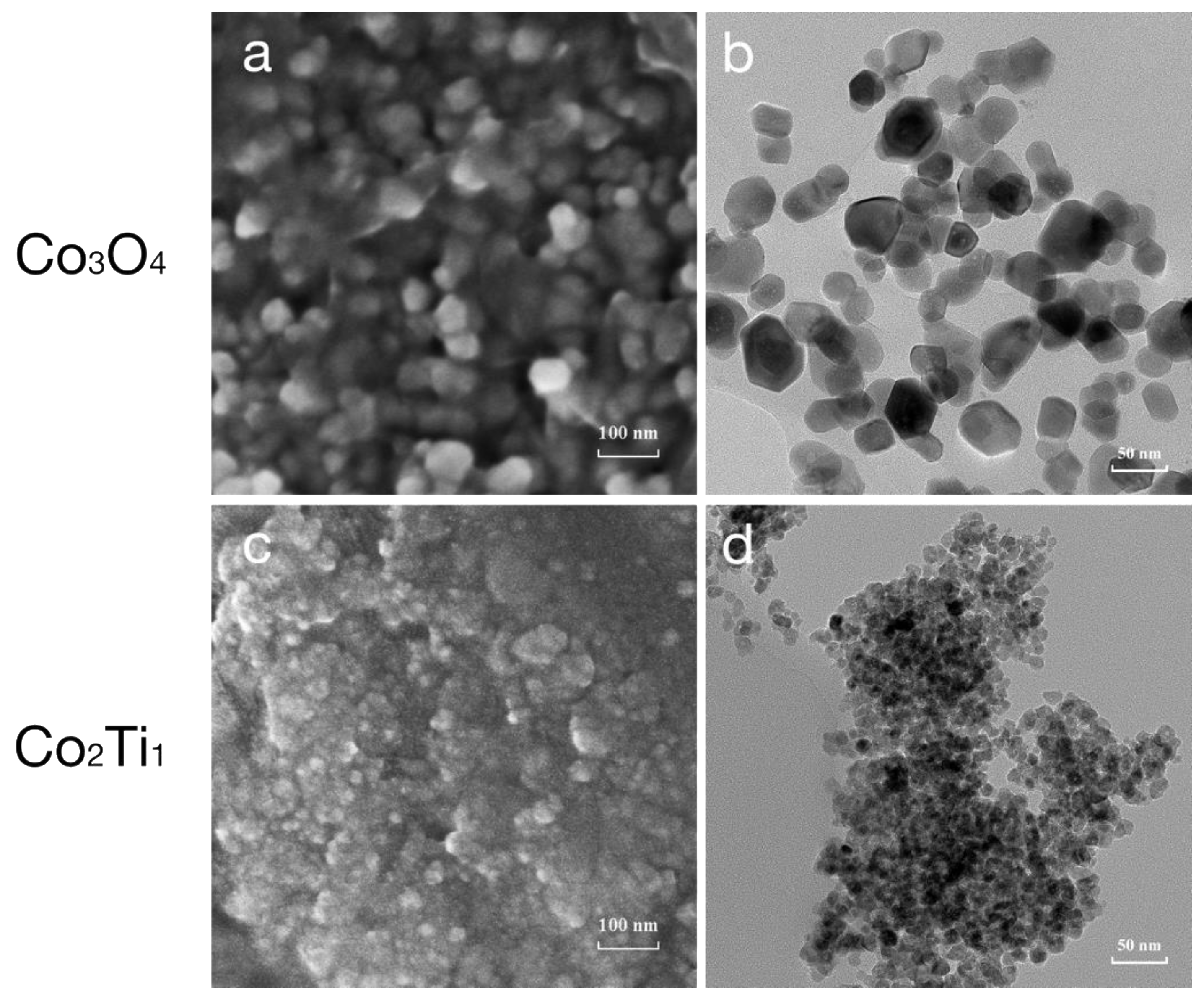
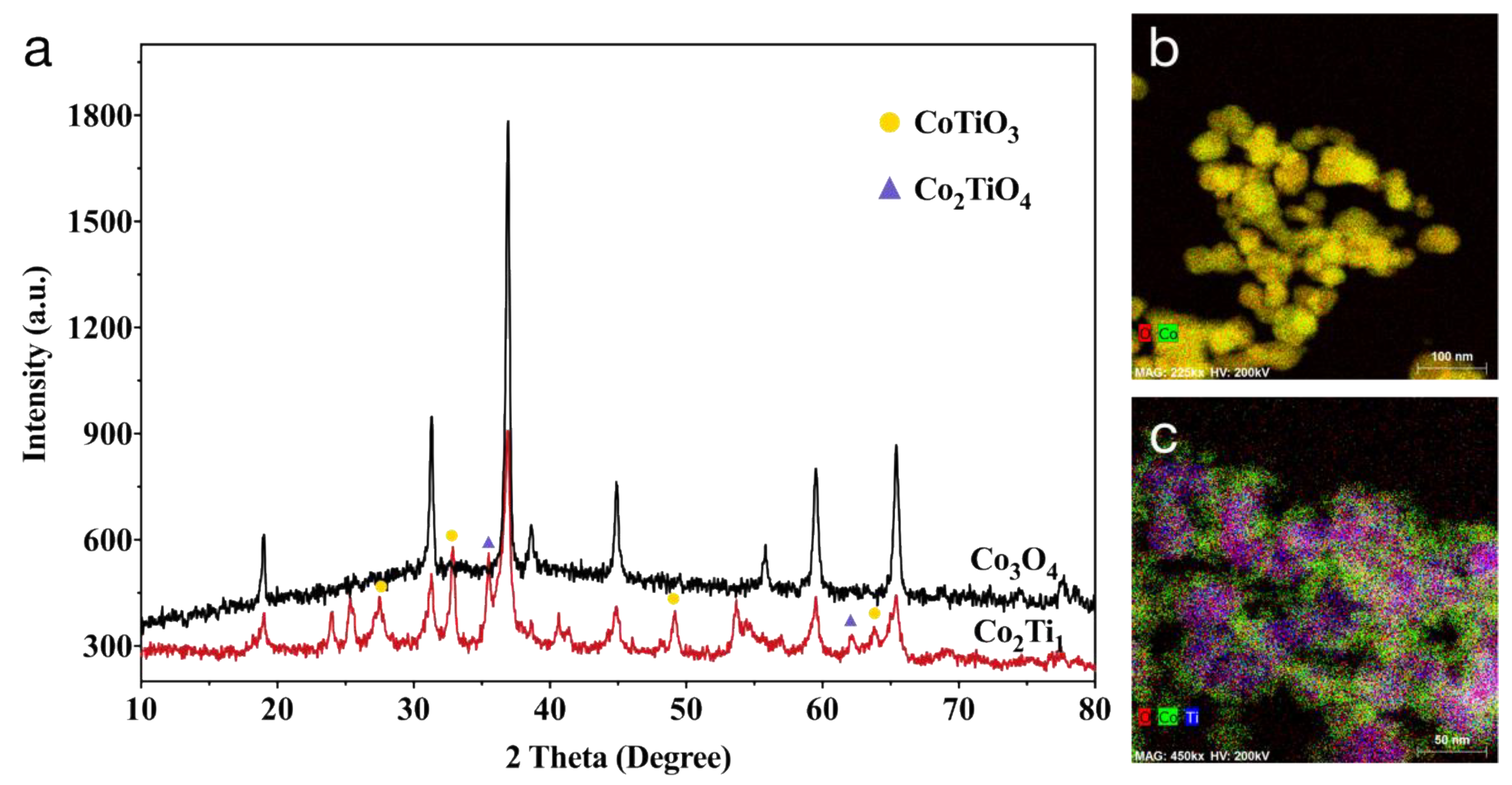
3.1. Characterization of Co3O4 and Co2Ti1
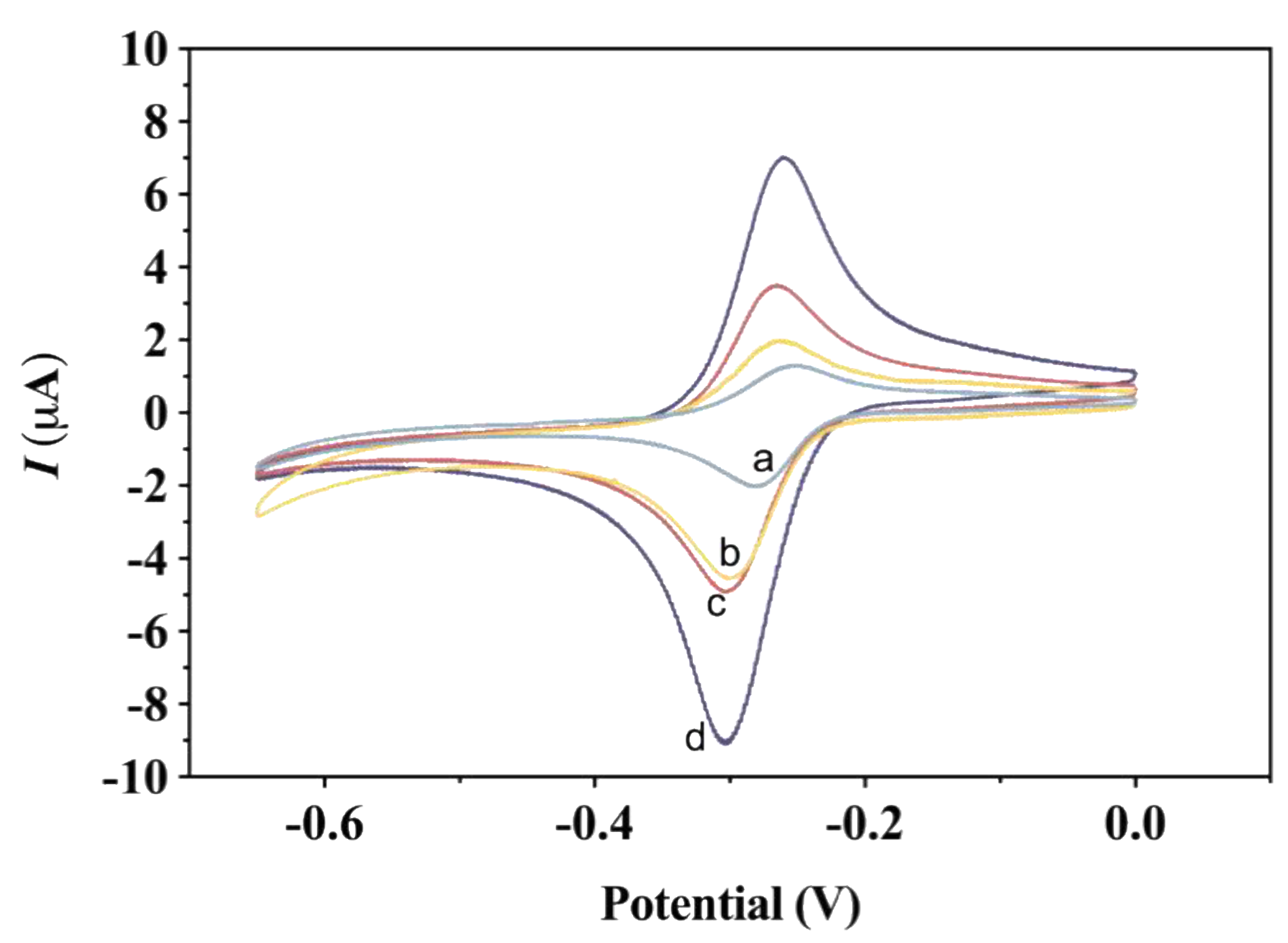
3.2. Electrochemical Characterization of the Aptasensor
3.3. Optimization of Experimental Conditions
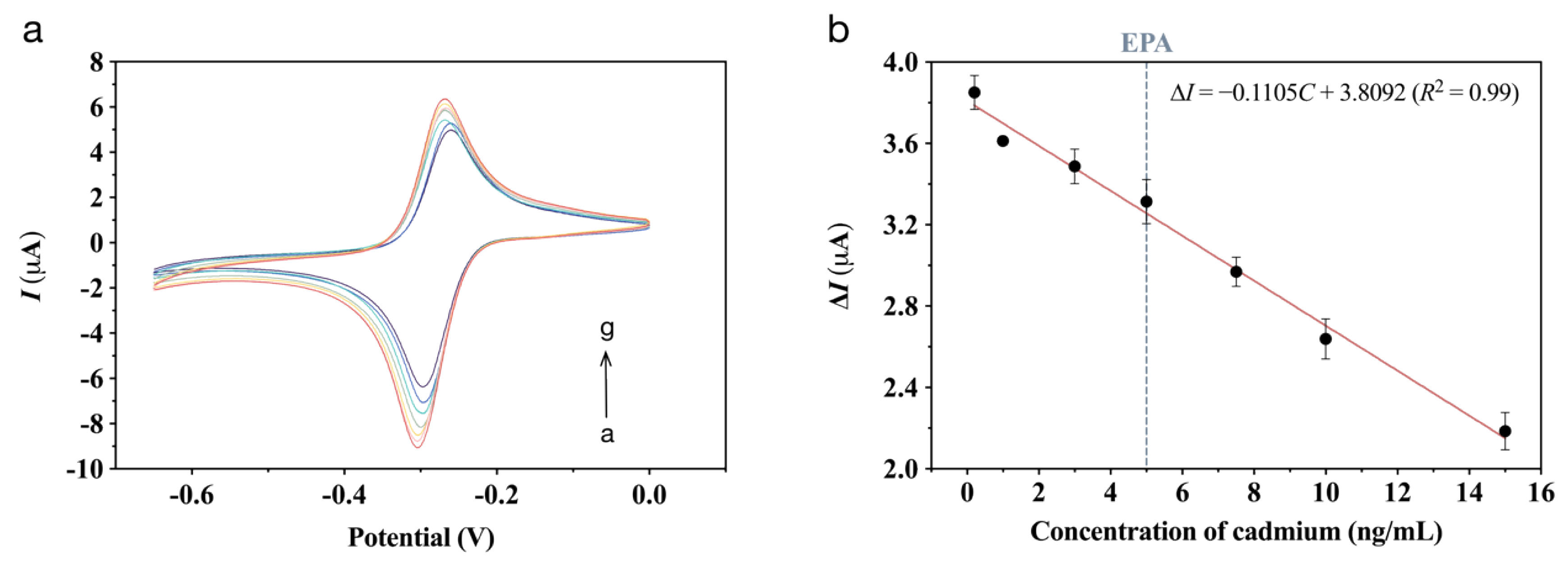
3.4. Analytical Performance
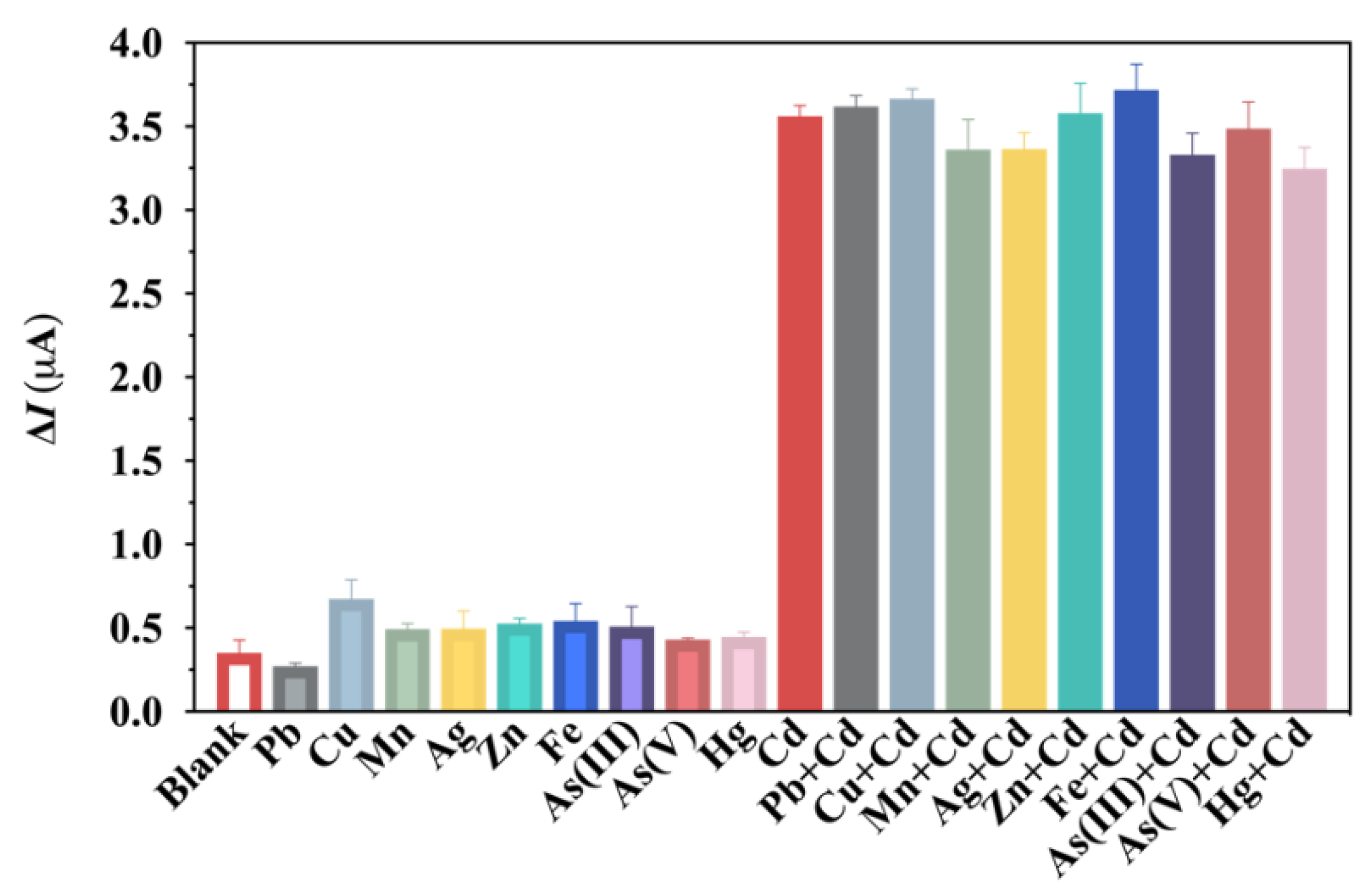
3.5. Selectivity and Reproducibility
3.6. Application in Environmental Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, A.; Soltani-Felehgari, F.; Madrakian, T.; Ghaedi, H.; Rezaeivala, M. Fabrication and application of a new modified electrochemical sensor using nano-silica and a newly synthesized Schiff base for simultaneous determination of Cd2+, Cu2+ and Hg2+ ions in water and some foodstuff samples. Anal. Chim. Acta 2013, 771, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.A.; Blake, D.A. Development and validation of a one-step immunoassay for determination of cadmium in human serum. Anal. Chem. 2002, 74, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.A.; Carrillo, G. Simultaneous determination of arsenic, cadmium, copper, chromium, nickel, lead and thallium in total digested sediment samples and available fractions by electrothermal atomization atomic absorption spectroscopy (ET AAS). Talanta 2012, 97, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Ashrafzadeh Afshar, E.; Taher, M.A.; Fazelirad, H. Ultra-trace determination of thallium(I) using a nanocomposite consisting of magnetite, halloysite nanotubes and dibenzo-18-crown-6 for preconcentration prior to its quantitation by ET-AAS. Microchim. Acta 2017, 184, 791–797. [Google Scholar] [CrossRef]
- Paixao, L.B.; Brandao, G.C.; Araujo, R.G.O.; Korn, M.G.A. Assessment of cadmium and lead in commercial coconut water and industrialized coconut milk employing HR-CS GF AAS. Food Chem. 2019, 284, 259–263. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; Quinaia, S.P.; Felsner, M.L. Fast and direct analysis of Cr, Cd and Pb in brown sugar by GF AAS. Food Chem. 2018, 260, 19–26. [Google Scholar] [CrossRef]
- Nawab, J.; Khan, S.; Xiaoping, W. Ecological and health risk assessment of potentially toxic elements in the major rivers of Pakistan: General population vs. Fishermen. Chemosphere 2018, 202, 154–164. [Google Scholar] [CrossRef]
- Wang, M.; Ma, H.; Chi, Q.; Li, Q.; Li, M.; Zhang, H.; Li, C.; Fang, H. A monolithic copolymer prepared from N-(4-vinyl)-benzyl iminodiacetic acid, divinylbenzene and N,N′-methylene bisacrylamide for preconcentration of cadmium(II) and cobalt(II) from biological samples prior to their determination by ICP-MS. Microchim. Acta 2019, 186, 537. [Google Scholar] [CrossRef]
- Li, Y.; Guo, W.; Hu, Z.; Jin, L.; Hu, S.; Guo, Q. Method Development for Direct Multielement Quantification by LA-ICP-MS in Food Samples. J. Agric. Food Chem. 2019, 67, 935–942. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.J.; Sun, J.F.; Yao, Y.; Liu, H.M.; Huang, J.C.; Guo, Y.M.; Sun, X. Novel electrochemical aptasensor with dual signal amplification strategy for detection of acetamiprid. Sci. Total Environ. 2020, 705, 135905. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Dong, H.; Zhao, Q.; Cheng, S.; Guo, Y.; Sun, X. Fabrication of refreshable aptasensor based on hydrophobic screen-printed carbon electrode interface. Sci. Total Environ. 2020, 712, 136410. [Google Scholar] [CrossRef]
- Cheng, W.; Pan, J.; Yang, J.; Zheng, Z.; Lu, F.; Chen, Y.; Gao, W. A photoelectrochemical aptasensor for thrombin based on the use of carbon quantum dot-sensitized TiO2 and visible-light photoelectrochemical activity. Microchim. Acta 2018, 185, 263. [Google Scholar] [CrossRef]
- He, Y.; Tian, F.; Zhou, J.; Zhao, Q.; Fu, R.; Jiao, B. Colorimetric aptasensor for ochratoxin A detection based on enzyme-induced gold nanoparticle aggregation. J. Hazard. Mater. 2020, 388, 121758. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, G.; Huang, Y.; Yang, S.; Ren, S.; Gao, Z.; Chen, A. Dual-competitive lateral flow aptasensor for detection of aflatoxin B1 in food and feedstuffs. J. Hazard. Mater. 2018, 344, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Song, J.L.; Huang, M.H.; Jiang, N.; Zheng, S.Y.; Mu, T.W.; Meng, L.J.; Liu, Y.B.; Liu, J.Y.; Chen, G. Ultrasensitive detection of amoxicillin by TiO2-g-C3N4@AuNPs impedimetric aptasensor: Fabrication, optimization, and mechanism. J. Hazard. Mater. 2020, 391, 122024. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Y.; Zhang, D.; Yu, M.; Zhan, X.; Zhang, D.; Zhou, P. An electrochemical aptasensor based on gold@polypyrrole composites for detection of lead ions. Microchim. Acta 2018, 185, 545. [Google Scholar] [CrossRef]
- Memon, A.G.; Xing, Y.P.; Zhou, X.H.; Wang, R.Y.; Liu, L.H.; Zeng, S.Y.; He, M.; Ma, M. Ultrasensitive colorimetric aptasensor for Hg2+ detection using Exo-III assisted target recycling amplification and unmodified AuNPs as indicators. J. Hazard. Mater. 2020, 384, 120948. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Cai, Z.X.; Sheng, L.; Ma, M.H.; Wang, X.Y. A magnetic relaxation switching and visual dual-mode sensor for selective detection of Hg2+ based on aptamers modified Au@Fe3O4 nanoparticles. J. Hazard. Mater. 2020, 388, 121728. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y.; Li, L.; Zhu, J.J. A label-free aptasensor for ultrasensitive Pb2+ detection based on electrochemiluminescence resonance energy transfer between carbon nitride nanofibers and Ru(phen)32+. J. Hazard. Mater. 2018, 359, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ding, J.; Liu, J. 2-Aminopurine-modified DNA homopolymers for robust and sensitive detection of mercury and silver. Biosens. Bioelectron. 2017, 87, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Solra, M.; Bala, R.; Wangoo, N.; Soni, G.K.; Kumar, M.; Sharma, R.K. Optical pico-biosensing of lead using plasmonic gold nanoparticles and a cationic peptide-based aptasensor. Chem. Commun. 2019, 56, 289–292. [Google Scholar] [CrossRef]
- Memon, A.G.; Zhou, X.; Liu, J.; Wang, R.; Liu, L.; Yu, B.; He, M.; Shi, H. Utilization of unmodified gold nanoparticles for label-free detection of mercury(II): Insight into rational design of mercury-specific oligonucleotides. J. Hazard. Mater. 2017, 321, 417–423. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, H.; Wang, J.; Xu, L.; Chen, H.; Pei, R. Selection and characterization of DNA aptamers for the development of light-up biosensor to detect Cd(II). Talanta 2016, 154, 498–503. [Google Scholar] [CrossRef]
- Wu, Y.; Zhan, S.; Wang, L.; Zhou, P. Selection of a DNA aptamer for cadmium detection based on cationic polymer mediated aggregation of gold nanoparticles. Analyst 2014, 139, 1550–1561. [Google Scholar] [CrossRef]
- Xu, L.; Liang, J.; Wang, Y.; Ren, S.; Wu, J.; Zhou, H.; Gao, Z. Highly Selective, Aptamer-Based, Ultrasensitive Nanogold Colorimetric Smartphone Readout for Detection of Cd(II). Molecules 2019, 24, 2745. [Google Scholar] [CrossRef]
- Luan, Y.X.; Lu, A.X.; Chen, J.Y.; Fu, H.L.; Xu, L. A Label-Free Aptamer-Based Fluorescent Assay for Cadmium Detection. Appl. Sci. 2016, 6, 432. [Google Scholar] [CrossRef]
- Smart, A.; Crew, A.; Pemberton, R.; Hughes, G.; Doran, O.; Hart, J.P. Screen -printed carbon based biosensors and their applications in agri-food safety. Trac Trends Anal. Chem. 2020, 127, 115898. [Google Scholar] [CrossRef]
- Hughes, G.; Westmacott, K.; Honeychurch, K.; Crew, A.; Pemberton, R.; Hart, J. Recent Advances in the Fabrication and Application of Screen-Printed Electrochemical (Bio)Sensors Based on Carbon Materials for Biomedical, Agri-Food and Environmental Analyses. Biosensors 2016, 6, 50. [Google Scholar] [CrossRef]
- Farzin, L.; Shamsipur, M.; Sheibani, S. A review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals. Talanta 2017, 174, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Ramnani, P.; Saucedo, N.M.; Mulchandani, A. Carbon nanomaterial-based electrochemical biosensors for label-free sensing of environmental pollutants. Chemosphere 2016, 143, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Xu, L.N.; Chen, J. Co3O4 nanomaterials in lithium-ion batteries and gas sensors. Adv. Funct. Mater. 2005, 15, 851–857. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.C.; Xu, H.; Wang, X.W.; Huang, Y.X.; Chan-Park, M.B.; Zhang, H.; Wang, L.H.; Huang, W.; Chen, P. 3D graphene-cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H.M. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef]
- Haldorai, Y.; Kim, J.Y.; Vilian, A.T.E.; Heo, N.S.; Huh, Y.S.; Han, Y.K. An enzyme-free electrochemical sensor based on reduced graphene oxide/Co3O4 nanospindle composite for sensitive detection of nitrite. Sens. Actuators B Chem. 2016, 227, 92–99. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Cheng, C.; Li, H.; Zhang, J.; Gong, H.; Fan, H.J. Co3O4 Nanowire@MnO2 ultrathin nanosheet core/shell arrays: A new class of high-performance pseudocapacitive materials. Adv. Mater. 2011, 23, 2076–2081. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, L.; Lou, X.W. Metal oxide hollow nanostructures for lithium-ion batteries. Adv. Mater. 2012, 24, 1903–1911. [Google Scholar] [CrossRef]
- Lin, H.G.; Ji, X.B.; Chen, Q.Y.; Zhou, Y.K.; Banks, C.E.; Wu, K.B. Mesoporous-TiO2 nanoparticles based carbon paste electrodes exhibit enhanced electrochemical sensitivity for phenols. Electrochem. Commun. 2009, 11, 1990–1995. [Google Scholar] [CrossRef]
- Bao, S.J.; Li, C.M.; Zang, J.F.; Cui, X.Q.; Qiao, Y.; Guo, J. New nanostructured TiO2 for direct electrochemistry and glucose sensor applications. Adv. Funct. Mater. 2008, 18, 591–599. [Google Scholar] [CrossRef]
- Nadzirah, S.; Gopinath, S.C.B.; Parmin, N.A.; Hamzah, A.A.; Mohamed, M.A.; Chang, E.Y.; Dee, C.F. State-of-the-Art on Functional Titanium Dioxide-Integrated Nano-Hybrids in Electrical Biosensors. Crit. Rev. Anal. Chem. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Huang, W.; Qu, Z.; Hu, X.; Yan, N. Catalytic oxidation of dibromomethane over Ti-modified Co3O4 catalysts: Structure, activity and mechanism. J. Colloid Interface Sci. 2017, 505, 870–883. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, M.; Han, A.; Liu, C. Preparation and characterization of Co2TiO4 and doped Co2−xMxTiO4 (M = Zn2+, Ni2+)-coated mica composite pigments. Appl. Phys. A 2016, 122, 670. [Google Scholar] [CrossRef]
- Acharya, T.; Choudhary, R.N.P. Structural, dielectric and impedance characteristics of CoTiO3. Mater. Chem. Phys. 2016, 177, 131–139. [Google Scholar] [CrossRef]
- Huang, K.J.; Wu, Z.W.; Wu, Y.Y.; Liu, Y.M. Electrochemical immunoassay of carcinoembryonic antigen based on TiO2-graphene/thionine/gold nanoparticles composite. Can. J. Chem. Rev. Can. Chim. 2012, 90, 608–615. [Google Scholar] [CrossRef]
- Yang, C.Y.; Wang, Q.; Xiang, Y.; Yuan, R.; Chai, Y.Q. Target-induced strand release and thionine-decorated gold nanoparticle amplification labels for sensitive electrochemical aptamer-based sensing of small molecules. Sens. Actuators B Chem. 2014, 197, 149–154. [Google Scholar] [CrossRef]
- Lotfi Zadeh Zhad, H.R.; Rodríguez Torres, Y.M.; Lai, R.Y. A reagentless and reusable electrochemical aptamer-based sensor for rapid detection of Cd(II). J. Electroanal. Chem. 2017, 803, 89–94. [Google Scholar] [CrossRef]
| Approaches | Linear Range (ng/mL) | LOD (ng/mL) | Reference |
|---|---|---|---|
| fluorometry | 0.00~112.41 | 4.50 | [26] |
| 100.00~10,000.00 | 0.04 | [29] | |
| colorimetry | 1.00~400.00 | 1.00 | [28] |
| 1.12~44.96 | 0.52 | [27] | |
| electrochemistry | 28.10~112.41 | 10.34 | [49] |
| 0.20~15.00 | 0.49 | This work |
| Sample | Background (ng/mL) | Spiked (ng/mL) | Proposed Electrochemical Aptasensor | AAS | ||||
|---|---|---|---|---|---|---|---|---|
| Found (ng/mL) | Recovery (%) | RSD (%) | Found (ng/mL) | Recovery (%) | RSD (%) | |||
| River water | 0.02 | 3.00 | 3.18 | 105.24 | 8.17 | 3.09 | 102.17 | 0.66 |
| 6.00 | 6.12 | 101.73 | 5.75 | 5.99 | 99.48 | 0.46 | ||
| 12.00 | 13.03 | 108.43 | 6.11 | 12.75 | 106.03 | 1.50 | ||
| Tap water | 0.07 | 3.00 | 3.03 | 98.71 | 8.24 | 3.20 | 104.22 | 0.18 |
| 6.00 | 6.22 | 102.43 | 7.43 | 6.29 | 103.69 | 0.82 | ||
| 12.00 | 13.27 | 109.95 | 7.64 | 12.82 | 106.19 | 2.48 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, D.; Ding, J.; Hayat, K.; Yang, X.; Zhan, X.; Zhang, D.; Lu, Y.; Zhou, P. Label-Free and Sensitive Determination of Cadmium Ions Using a Ti-Modified Co3O4-Based Electrochemical Aptasensor. Biosensors 2020, 10, 195. https://doi.org/10.3390/bios10120195
Liu Y, Zhang D, Ding J, Hayat K, Yang X, Zhan X, Zhang D, Lu Y, Zhou P. Label-Free and Sensitive Determination of Cadmium Ions Using a Ti-Modified Co3O4-Based Electrochemical Aptasensor. Biosensors. 2020; 10(12):195. https://doi.org/10.3390/bios10120195
Chicago/Turabian StyleLiu, Yang, Dongwei Zhang, Jina Ding, Kashif Hayat, Xijia Yang, Xuejia Zhan, Dan Zhang, Yitong Lu, and Pei Zhou. 2020. "Label-Free and Sensitive Determination of Cadmium Ions Using a Ti-Modified Co3O4-Based Electrochemical Aptasensor" Biosensors 10, no. 12: 195. https://doi.org/10.3390/bios10120195
APA StyleLiu, Y., Zhang, D., Ding, J., Hayat, K., Yang, X., Zhan, X., Zhang, D., Lu, Y., & Zhou, P. (2020). Label-Free and Sensitive Determination of Cadmium Ions Using a Ti-Modified Co3O4-Based Electrochemical Aptasensor. Biosensors, 10(12), 195. https://doi.org/10.3390/bios10120195





