Drug-Based Gold Nanoparticles Overgrowth for Enhanced SPR Biosensing of Doxycycline
Abstract
1. Introduction
2. Experimental
2.1. Materials
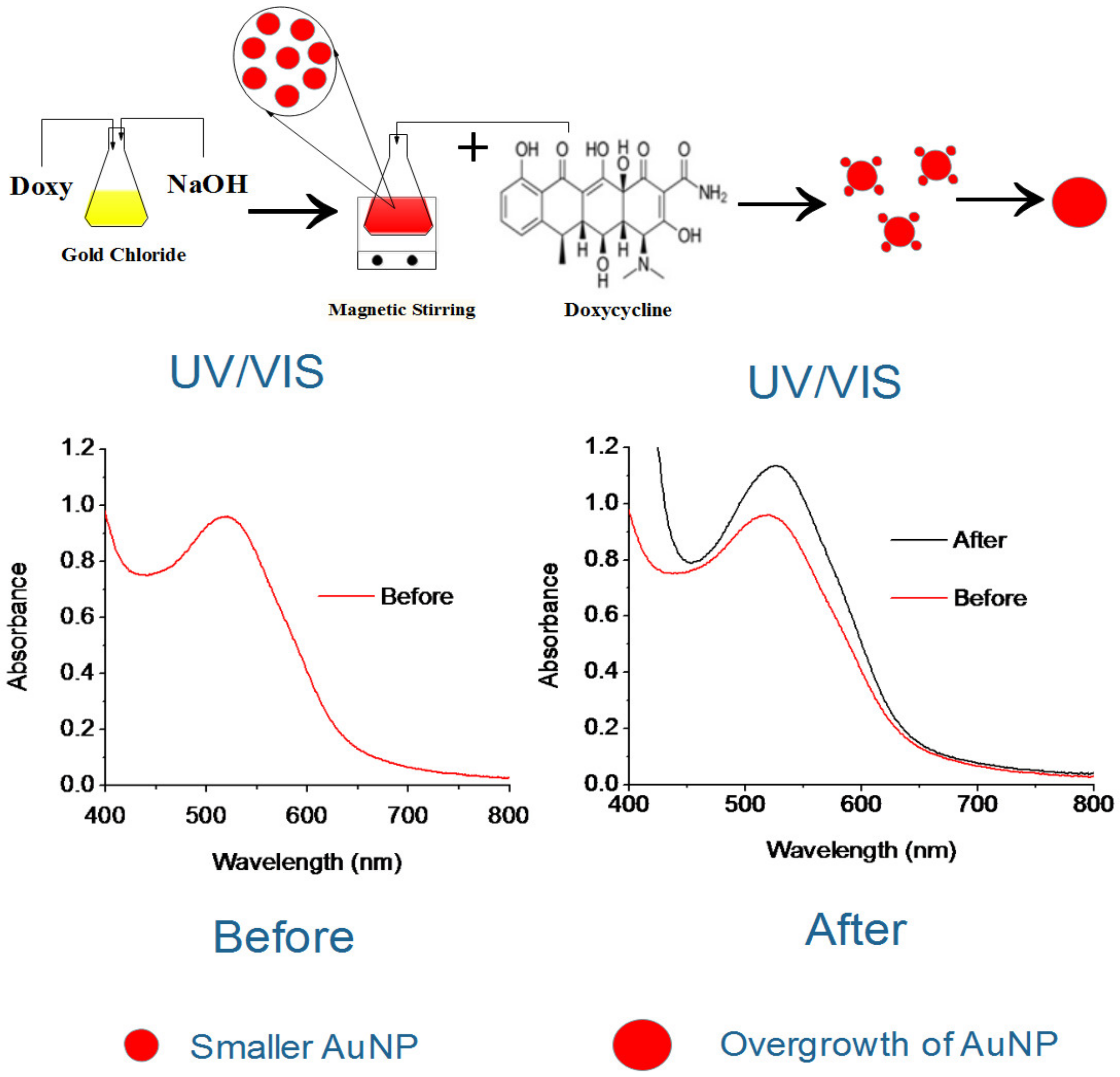
2.2. Preparation of the Doxy-AuNPs
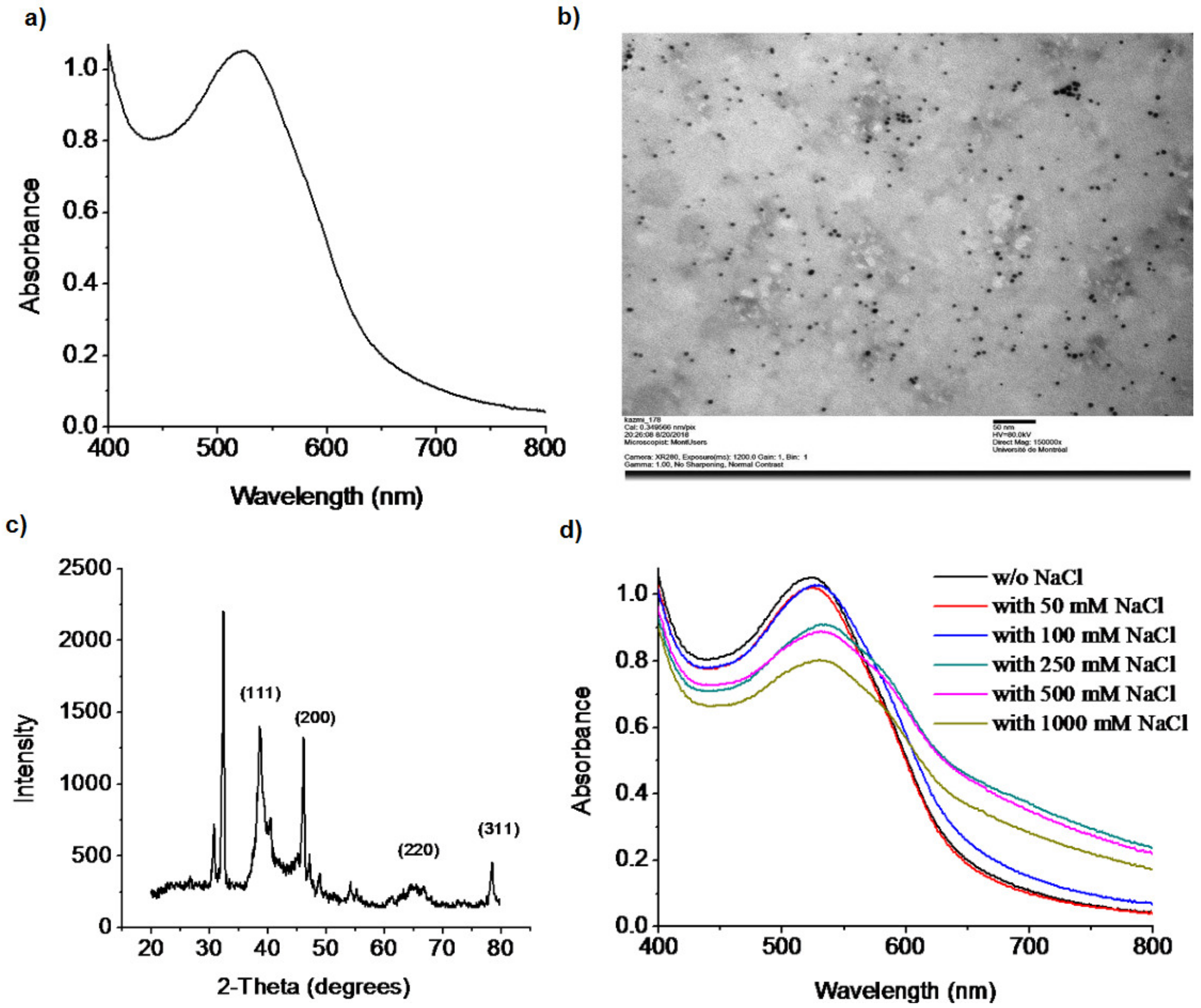
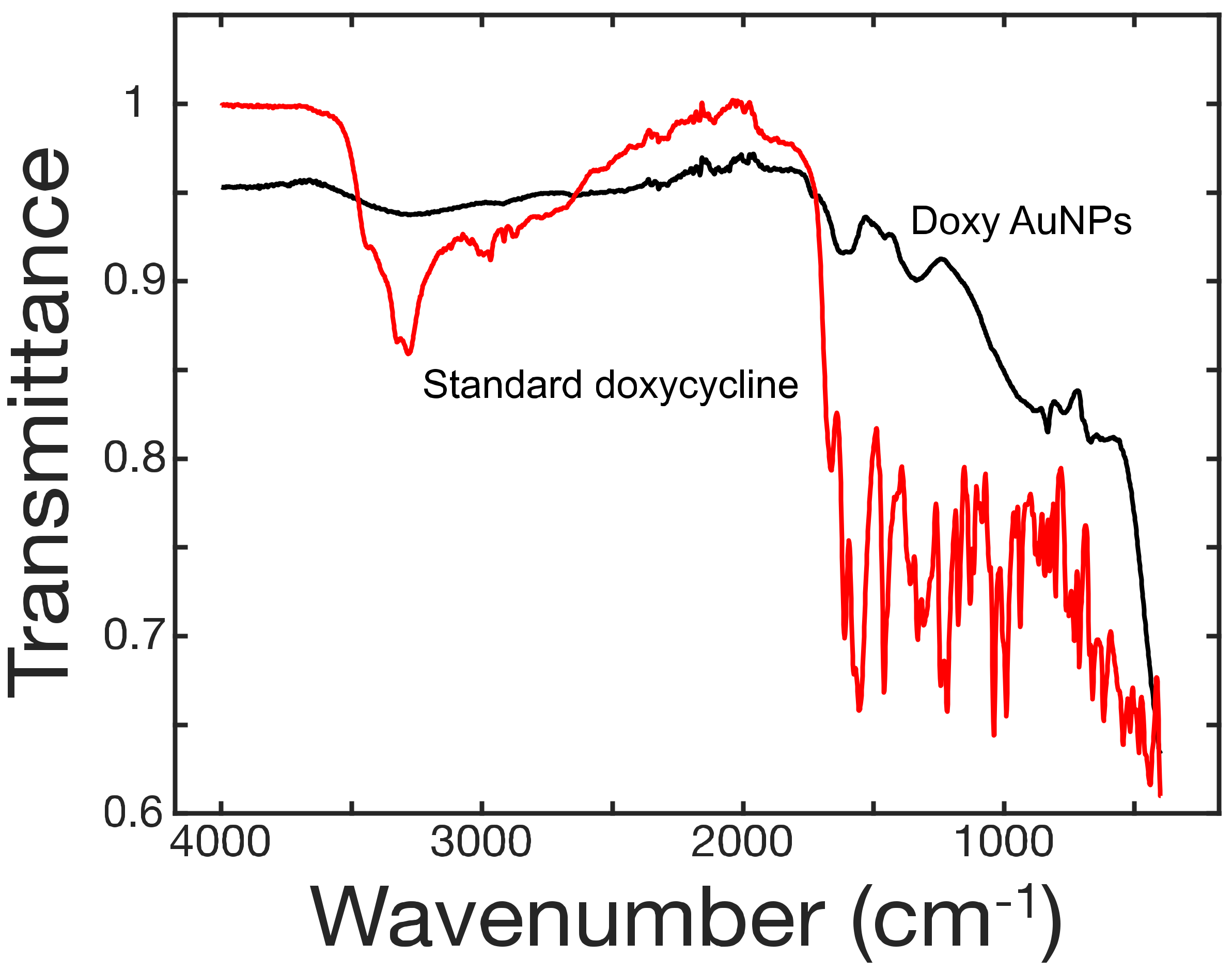
2.3. Characterization of the Doxy-AuNPs
2.4. Fabrication of the SPR Sensor
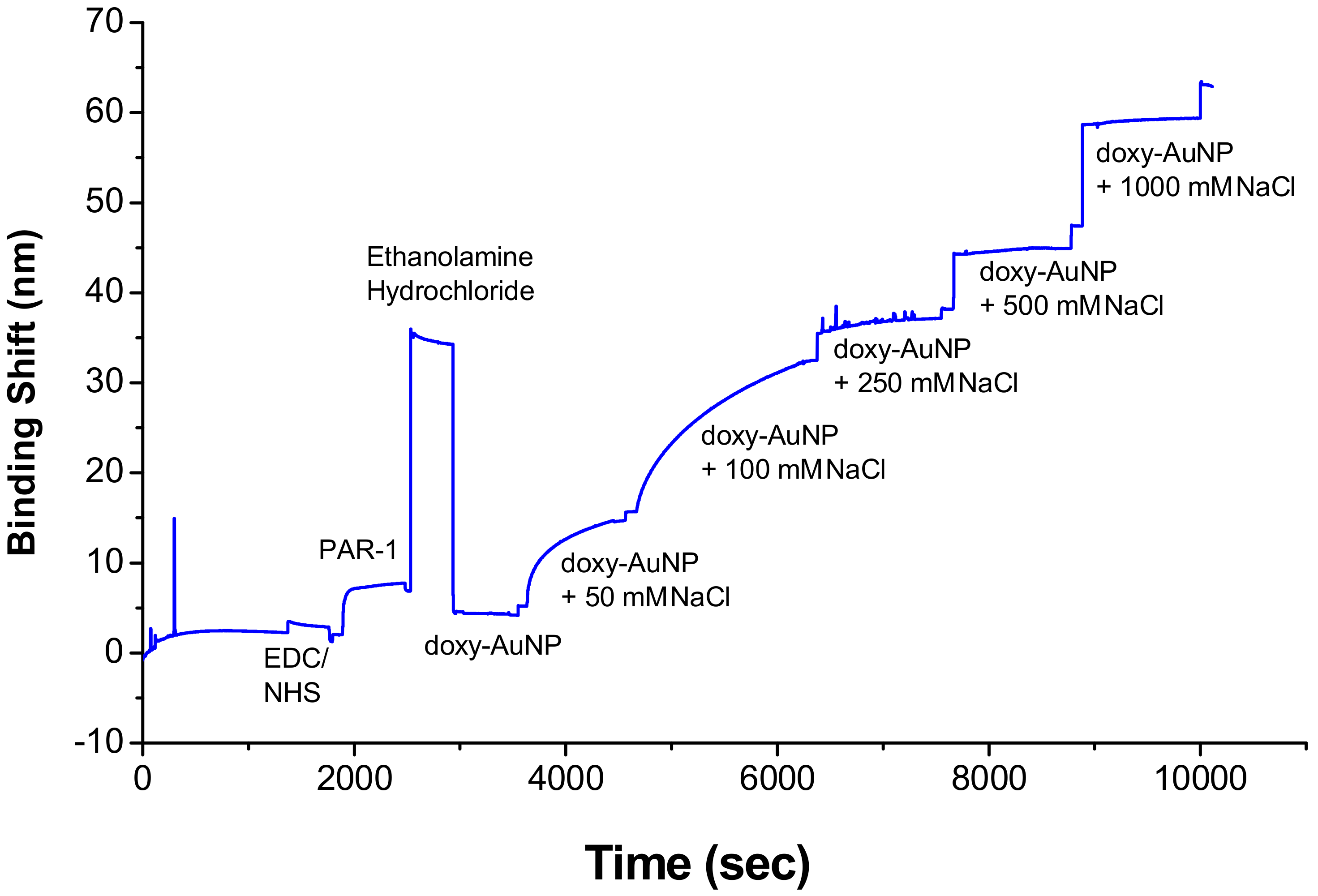
2.5. Immobilization of Receptor on Sensor Surface
2.6. Electrolytic Stability of Doxy-AuNPs
2.7. Sequential Analysis for Determination of Concentration of Doxycycline
3. Results and Discussion
3.1. Strategy of the Assay
3.2. Synthesis and Stability of Doxy-AuNPs
3.3. SPR Analysis for Detection of Doxycycline
3.3.1. Optimization of Electrolytic Conditions of Doxy-AuNPs
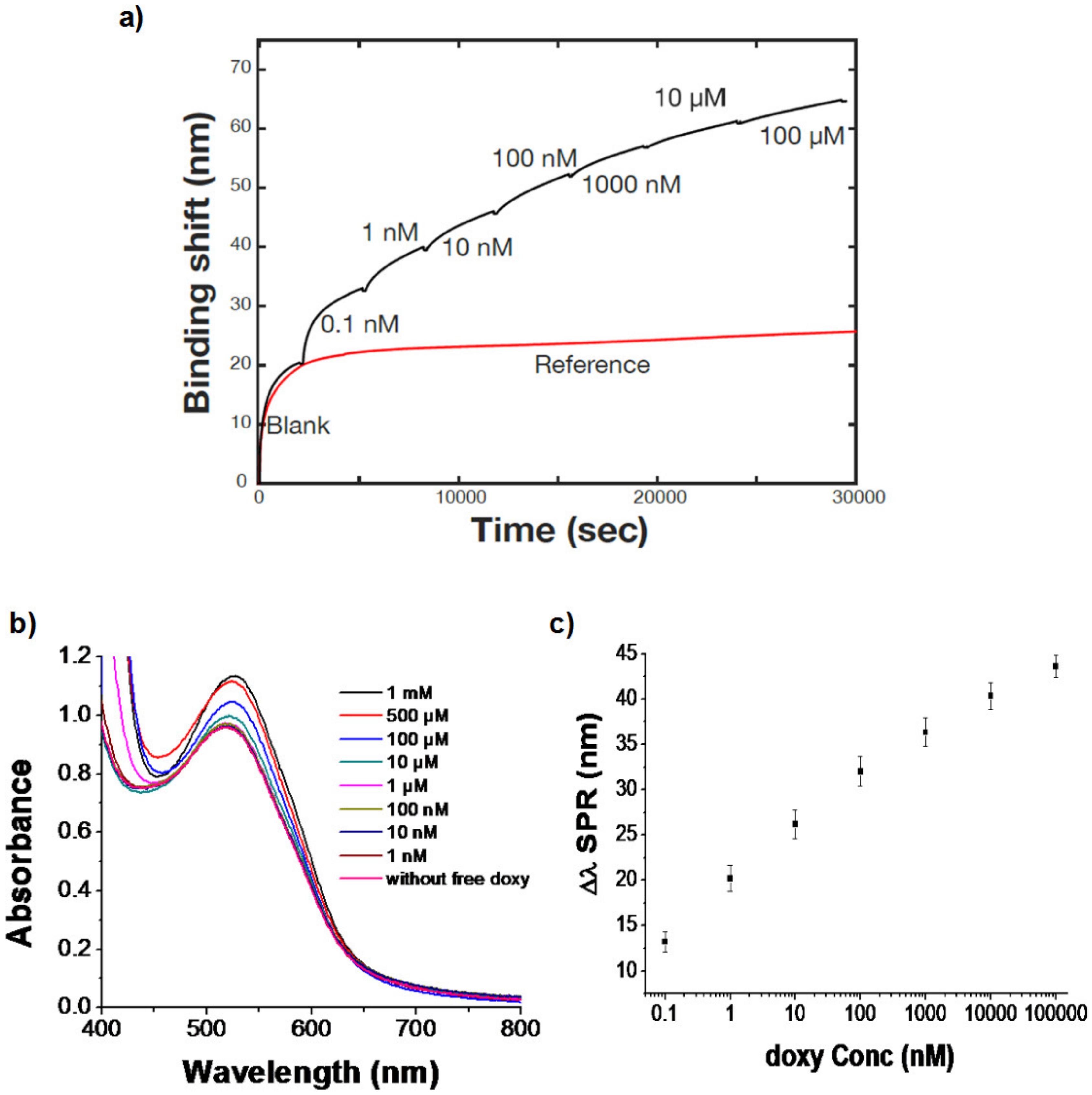
3.3.2. SPR Bioassay for Doxycycline Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.R. Why error reporting systems should be voluntary. Br. Med. J. 2000, 320, 728–729. [Google Scholar] [CrossRef] [PubMed]
- Booth, M.A.; Gowers, S.A.N.; Leong, C.L.; Rogers, M.L.; Samper, I.C.; Wickham, A.P.; Boutelle, M.G. Chemical Monitoring in Clinical Settings: Recent Developments toward Real-Time Chemical Monitoring of Patients. Anal. Chem. 2018, 90, 2–18. [Google Scholar] [CrossRef]
- Haddada, M.B.; Jeannot, K.; Spadavecchia, J. Novel Synthesis and Characterization of Doxycycline-Loaded Gold Nanoparticles: The Golden Doxycycline for Antibacterial Applications. Part. Part. Syst. Charact. 2019, 36, 1800395. [Google Scholar] [CrossRef]
- Liu, X.; Yu, J.; Song, S.; Yue, X.; Li, Q. Protease-activated receptor-1 (PAR-1): A promising molecular target for cancer. Oncotarget 2017, 8, 107334–107345. [Google Scholar] [CrossRef]
- Zhong, W.; Chen, S.; Zhang, Q.; Xiao, T.; Qin, Y.; Gu, J.; Sun, B.; Liu, Y.; Jing, X.; Hu, X.; et al. Doxycycline directly targets PAR1 to suppress tumor progression. Oncotarget 2017, 8, 16829–16842. [Google Scholar] [CrossRef]
- Elzeinová, F.; Pěknicová, J.; Děd, L.; Kubátová, A.; Margaryan, H.; Dorosh, A.; Makovický, P.; Rajmon, R. Adverse effect of tetracycline and doxycycline on testicular tissue and sperm parameters in CD1 outbred mice. Exp. Toxicol. Pathol. 2013, 65, 911–917. [Google Scholar] [CrossRef]
- Sun, T.; Zhao, N.; Ni, C.S.; Zhao, X.L.; Zhang, W.Z.; Su, X.; Zhang, D.F.; Gu, Q.; Sun, B.C. Doxycycline inhibits the adhesion and migration of melanoma cells by inhibiting the expression and phosphorylation of focal adhesion kinase (FAK). Cancer Lett. 2009, 285, 141–150. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Wang, H.; Zhou, Q.; Liang, Y.; Sui, A.; Yao, R.; Zhao, B.; Sun, M. Lentiviral vector-mediated doxycycline-inducible USP39 shRNA or cDNA expression in triple-negative breast cancer cells. Oncol. Rep. 2015, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Fujioka, S.; Iida, T.; Furukawa, K.; Fujita, T.; Yamada, H.; Chiao, P.J.; Yanaga, K. Doxycycline induces apoptosis in PANC-1 pancreatic cancer cells. Anticancer Res. 2009, 29, 3995–4003. [Google Scholar] [PubMed]
- Duivenvoorden, W.C.M.; Popović, S.V.; Lhotük, Š.; Seidlitz, E.; Hirte, H.W.; Tozer, R.G.; Singh, G. Doxycycline decreases tumor burden in a bone metastasis model of human breast cancer. Cancer Res. 2002, 62, 1588–1591. [Google Scholar] [PubMed]
- Hadad, G.M.; El-Gindy, A.; Mahmoud, W.M.M. HPLC and chemometrics-assisted UV-spectroscopy methods for the simultaneous determination of ambroxol and doxycycline in capsule. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Šatínský, D.; Dos Santos, L.M.L.; Sklenářová, H.; Solich, P.; Montenegro, M.C.B.S.M.; Araújo, A.N. Sequential injection chromatographic determination of ambroxol hydrochloride and doxycycline in pharmaceutical preparations. Talanta 2005, 68, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Aboul-Enein, H.Y. Internal solid contact sensor for the determination of doxycycline hydrochloride in pharmaceutical formulation. Talanta 2002, 58, 387–396. [Google Scholar] [CrossRef]
- Abadian, P.N.; Kelley, C.P.; Goluch, E.D. Cellular analysis and detection using surface plasmon resonance techniques. Anal. Chem. 2014, 86, 2799–2812. [Google Scholar] [CrossRef] [PubMed]
- Špringer, T.; Homola, J. Biofunctionalized gold nanoparticles for SPR-biosensor-based detection of CEA in blood plasma. Anal. Bioanal. Chem. 2012, 404, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dou, X.; Zhang, L.; Zhao, X.; Luo, J.; Yang, M. Oriented assembly of surface plasmon resonance biosensor through staphylococcal protein A for the chlorpyrifos detection. Anal. Bioanal. Chem. 2019, 411, 6057–6066. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators 1983, 4, 299–304. [Google Scholar] [CrossRef]
- Masson, J.F. Surface Plasmon Resonance Clinical Biosensors for Medical Diagnostics. ACS Sens. 2017, 2, 16–30. [Google Scholar] [CrossRef]
- Yuan, H.; Ji, W.; Chu, S.; Qian, S.; Wang, F.; Masson, J.F.; Han, X.; Peng, W. Fiber-optic surface plasmon resonance glucose sensor enhanced with phenylboronic acid modified Au nanoparticles. Biosens. Bioelectron. 2018, 117, 637–643. [Google Scholar] [CrossRef]
- Mariani, S.; Minunni, M. Surface plasmon resonance applications in clinical analysis. Anal. Bioanal. Chem. 2014, 406, 2303–2323. [Google Scholar] [CrossRef] [PubMed]
- Bocková, M.; Chadtová Song, X.; Gedeonová, E.; Levová, K.; Kalousová, M.; Zima, T.; Homola, J. Surface plasmon resonance biosensor for detection of pregnancy associated plasma protein A2 in clinical samples. Anal. Bioanal. Chem. 2016, 408, 7265–7269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Bukar, N.; Toulouse, J.L.; Pelechacz, D.; Robitaille, R.; Pelletier, J.N.; Masson, J.F. Miniature multi-channel SPR instrument for methotrexate monitoring in clinical samples. Biosens. Bioelectron. 2015, 64, 664–670. [Google Scholar] [CrossRef]
- Brulé, T.; Granger, G.; Bukar, N.; Deschênes-Rancourt, C.; Havard, T.; Schmitzer, A.R.; Martel, R.; Masson, J.F. A field-deployed surface plasmon resonance (SPR) sensor for RDX quantification in environmental waters. Analyst 2017, 142, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Herranz, S.; Bocková, M.; Marazuela, M.D.; Homola, J.; Moreno-Bondi, M.C. An SPR biosensor for the detection of microcystins in drinking water. Anal. Bioanal. Chem. 2010, 398, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Pilolli, R.; Visconti, A.; Monaci, L. Rapid and label-free detection of egg allergen traces in wines by surface plasmon resonance biosensor. Anal. Bioanal. Chem. 2015, 407, 3787–3797. [Google Scholar] [CrossRef] [PubMed]
- McGrath, T.F.; Elliott, C.T.; Fodey, T.L. Biosensors for the analysis of microbiological and chemical contaminants in food. Anal. Bioanal. Chem. 2012, 403, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Graybill, R.M.; Bailey, R.C. Emerging Biosensing Approaches for microRNA Analysis. Anal. Chem. 2016, 88, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.F.; Wang, W.H.; Hong, Y.W.; Yuan, R.Y.; Chen, K.H.; Huang, Y.W.; Lu, P.L.; Chen, Y.H.; Chen, Y.M.A.; Su, L.C.; et al. Simple Strategy for Rapid and Sensitive Detection of Avian Influenza A H7N9 Virus Based on Intensity-Modulated SPR Biosensor and New Generated Antibody. Anal. Chem. 2018, 90, 1861–1869. [Google Scholar] [CrossRef]
- Jabbari, S.; Dabirmanesh, B.; Arab, S.S.; Amanlou, M.; Daneshjou, S.; Gholami, S.; Khajeh, K. A novel enzyme based SPR-biosensor to detect bromocriptine as an ergoline derivative drug. Sens. Actuators B Chem. 2017, 240, 519–527. [Google Scholar] [CrossRef]
- Carrascosa, L.G.; Calle, A.; Lechuga, L.M. Label-free detection of DNA mutations by SPR: Application to the early detection of inherited breast cancer. Anal. Bioanal. Chem. 2009, 393, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.S.; Erika, G.; Jiří, H. Surface plasmon resonance biosensor for the ultrasensitive detection of bisphenol A. Anal. Bioanal. Chem. 2019, 411, 5655–5658. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, H.S. Aptamer-based Au nanoparticles-enhanced surface plasmon resonance detection of small molecules. Anal. Chem. 2008, 80, 7174–7178. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.A.R.; Qureshi, M.Z.; Ali, S.; Masson, J.F. In Vitro Drug Release and Biocatalysis from pH-Responsive Gold Nanoparticles Synthesized Using Doxycycline. Langmuir 2019, 35, 16266–16274. [Google Scholar] [CrossRef]
- Frederix, F.; Friedt, J.M.; Choi, K.H.; Laureyn, W.; Campitelli, A.; Mondelaers, D.; Maes, G.; Borghst, G. Biosensing Based on Light Absorption of Nanoscaled Gold and Silver Particles. Anal. Chem. 2003, 75, 6894–6900. [Google Scholar] [CrossRef]
- Yockell-Lelièvre, H.; Bukar, N.; McKeating, K.S.; Arnaud, M.; Cosin, P.; Guo, Y.; Dupret-Carruel, J.; Mougin, B.; Masson, J.F. Plasmonic sensors for the competitive detection of testosterone. Analyst 2015, 140, 5105–5111. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Uludag, Y.; Tothill, I.E. Cancer biomarker detection in serum samples using surface plasmon resonance and quartz crystal microbalance sensors with nanoparticle signal amplification. Anal. Chem. 2012, 84, 5898–5904. [Google Scholar] [CrossRef]
- Špringer, T.; Ermini, M.L.; Špačková, B.; Jabloňků, J.; Homola, J. Enhancing sensitivity of surface plasmon resonance biosensors by functionalized gold nanoparticles: Size matters. Anal. Chem. 2014, 86, 10350–10356. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Tyner, K.M.; Dair, B.J.; Deschamps, J.R.; Medintz, I.L. Analyzing nanomaterial bioconjugates: A review of current and emerging purification and characterization techniques. Anal. Chem. 2011, 83, 4453–4488. [Google Scholar] [CrossRef]
- Bolduc, O.R.; Lambert-Lanteigne, P.; Colin, D.Y.; Zhao, S.S.; Proulx, C.; Boeglin, D.; Lubell, W.D.; Pelletier, J.N.; Féthière, J.; Ong, H.; et al. Modified peptide monolayer binding His-tagged biomolecules for small ligand screening with SPR biosensors. Analyst 2011, 136, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Shen, L.; Liu, R.; Luo, Z.; Li, Z. Direct detection of β-agonists by use of gold nanoparticle-based colorimetric assays. Anal. Chem. 2011, 83, 6988–6995. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Javed, S.; Qureshi, M.Z.; Khan, M.U.; Khan, M.S. Synthesis and study of catalytic application of l-methionine protected gold nanoparticles. Appl. Nanosci. 2017, 7, 429–437. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.J.; Gu, M.M.; Zheng, T.T.; Zhu, J.J. Synthesis of gelatin-stabilized gold nanoparticles and assembly of carboxylic single-walled carbon nanotubes/Au composites for cytosensing and drug uptake. Anal. Chem. 2009, 81, 6641–6648. [Google Scholar] [CrossRef] [PubMed]
- Divakaran, D.; Lakkakula, J.R.; Thakur, M.; Kumawat, M.K.; Srivastava, R. Dragon fruit extract capped gold nanoparticles: Synthesis and their differential cytotoxicity effect on breast cancer cells. Mater. Lett. 2019, 236, 498–502. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Gupta, B.D. Influence of ions on the surface plasmon resonance spectrum of a fiber optic refractive index sensor. Sens. Actuators B Chem. 2011, 156, 559–562. [Google Scholar] [CrossRef]
- Bukar, N.; Zhao, S.S.; Charbonneau, D.M.; Pelletier, J.N.; Masson, J.F. Influence of the Debye length on the interaction of a small molecule-modified Au nanoparticle with a surface-bound bioreceptor. Chem. Commun. 2014, 50, 4947–4950. [Google Scholar] [CrossRef]
- Mitchell, J.S.; Wu, Y.; Cook, C.J.; Main, L. Sensitivity enhancement of surface plasmon resonance biosensing of small molecules. Anal. Biochem. 2005, 343, 125–135. [Google Scholar] [CrossRef]
- Shen, L.; Chen, J.; Li, N.; He, P.; Li, Z. Rapid colorimetric sensing of tetracycline antibiotics with in situ growth of gold nanoparticles. Anal. Chim. Acta 2014, 839, 83–90. [Google Scholar] [CrossRef]
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Jeyabaskaran, M.; Rambabu, C.; Sree Janardhanan, V.; Rajinikanth, V.; Pranitha, T.; Dhanalakshmi, B. RP-HPLC method development and validation of doxycycline in bulk and tablet formulation. Int. J. Pharm. Anal. Res. 2014, 3, 397–404. [Google Scholar]
- Adrian, J.; Fernández, F.; Sánchez-Baeza, F.; Marco, M.P. Preparation of antibodies and development of an enzyme-linked immunosorbent assay (ELISA) for the determination of doxycycline antibiotic in milk samples. J. Agric. Food Chem. 2012, 60, 3837–3846. [Google Scholar] [CrossRef] [PubMed]
- Kogawa, A.C.; Salgado, H.R.N. Quantification of doxycycline hyclate in tablets by HPLC-UV method. J. Chromatogr. Sci. 2013, 51, 919–925. [Google Scholar] [CrossRef]
- Selvadurai, M.; Meyyanathan, S.N. Determination of Doxycycline in Human Plasma by Liquid Chromatography-Mass Spectrometry after Liquid-Liquid Extraction and its Application in Human Pharmacokinetics Studies. J. Bioequiv. Availab. 2010, 02, 93–97. [Google Scholar] [CrossRef]
- Ramesh, P.; Basavaiah, K.; Tharpa, K.; Basavaiah, K.; Revanasiddappa, H.D. Development and validation of RP-HPLC method for the determination of doxycycline hyclate in spiked human urine and pharmaceuticals. J. Preclin. Clin. Res. 2010, 4, 101–107. [Google Scholar]
| Ref. | Method | Linearity Range | Limit of Detection (LOD) | |
|---|---|---|---|---|
| As Reported in Paper | Value in mol/L | |||
| Jeyabaskaran et al., 2014 [52] | RP-HPLC | 25–150 µg/mL | 0.02 µg/mL | 3.7 × 10−8 mol/L |
| Adrian et al., 2012 [53] | ELISA | 0.25–6.7 µg/mL | 0.1 µg/L | 1.95 × 10−10 mol/L |
| Kogawa et al., 2012 [54] | HPLC–UV | 50–100 µg/mL | 2.83 µg/mL | 5.2 × 10−6 mol/L |
| Selvadurai et al., 2010 [55] | Liquid chromatography–mass spectrometric (LC-MS) | 0.5–5 µg/mL | 50 ng/mL | 9.2 × 10−8 mol/L |
| Ramesh et al., 2010 [56] | RP-HPLC | 30–300 µg/mL | 0.02 µg/mL | 3.7 × 10−8 mol/L |
| This work | Surface plasmon resonance biosensor | 0.1 nM–100 µM | 7 pM | 7 × 10−12 mol/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazmi, S.A.R.; Qureshi, M.Z.; Masson, J.-F. Drug-Based Gold Nanoparticles Overgrowth for Enhanced SPR Biosensing of Doxycycline. Biosensors 2020, 10, 184. https://doi.org/10.3390/bios10110184
Kazmi SAR, Qureshi MZ, Masson J-F. Drug-Based Gold Nanoparticles Overgrowth for Enhanced SPR Biosensing of Doxycycline. Biosensors. 2020; 10(11):184. https://doi.org/10.3390/bios10110184
Chicago/Turabian StyleKazmi, Syed Akif Raza, Muhammad Zahid Qureshi, and Jean-Francois Masson. 2020. "Drug-Based Gold Nanoparticles Overgrowth for Enhanced SPR Biosensing of Doxycycline" Biosensors 10, no. 11: 184. https://doi.org/10.3390/bios10110184
APA StyleKazmi, S. A. R., Qureshi, M. Z., & Masson, J.-F. (2020). Drug-Based Gold Nanoparticles Overgrowth for Enhanced SPR Biosensing of Doxycycline. Biosensors, 10(11), 184. https://doi.org/10.3390/bios10110184





